Abstract
Objective
The effects of burn injury on cardiovascular responsiveness to vasoactive agents are not well understood. The aims of the present study were to determine whether burn injury alters cardiovascular reactivity to vasoactive drugs in vivo and intrinsic function of isolated mesenteric resistance arteries.
Methods
Anesthetized Sprague-Dawley rats were subjected to sham procedure or 30% total body surface area dorsal scald burn, followed by crystalloid resuscitation (Parkland Formula). At 24h, 72h, 96h, and 168h post-burn rats were re-anesthetized and the mean arterial blood pressure (MAP) responses to various doses of the α1-adrenergic receptor agonist phenylephrine and arginine vasopressin were tested. Mesenteric arteries were harvested from uninjured animals and at 24h and 168h post-burn. The responsiveness of arteries to phenylephrine and arginine vasopressin was tested by pressure myography. Dose response curves were generated and EC50 concentrations, Hill slopes and maximal effects were compared.
Results
The potency of phenylephrine to increase MAP was reduced 2-fold 24h post-burn (p<0.05 vs. sham) and gradually normalized at later time points. The reactivity of isolated arteries to phenylephrine was not significantly altered after burns. The potency of arginine vasopressin to increase MAP and to constrict isolated arteries was increased 2-3-fold at 24h post-burn (p<0.05) and normalized at later time points.
Conclusions
Burn injury differentially regulates vasopressor and blood pressure effects of α-adrenergic and vasopressin receptor agonists. Intrinsic vasopressin receptor reactivity of resistance arteries is sensitized early after burns. These findings will help to optimize resuscitation strategies and vasopressor use in difficult to resuscitate burn patients.
Keywords: resuscitation, arginine vasopressin, alpha-adrenergic receptors, catecholamines, phenylephrine, burn, vasopressor use
Introduction
Severe burn injuries are associated with increases in vascular permeability, which leads to third spacing of fluids into tissues and is most pronounced within the first 24–48 hrs after thermal injury (1). Accordingly, adequate early fluid resuscitation to compensate for intravascular volume deficits and to support organ perfusion is an essential cornerstone in the treatment of the severely burned patient (2–4). The current American Burn Association Consensus Guidelines recommend a crystalloid-based resuscitation strategy with 2–4 mL/kg/body weight/% burned total body surface area (TBSA) during the first 24 hours after injury to achieve resuscitation endpoints, such as urine output and hemodynamics (1).
In patients who fail to meet resuscitation targets despite aggressive crystalloid resuscitation, colloids and/or vasopressors are commonly added at the discretion of the health care provider. More recently, resuscitation algorithms for difficult to resuscitate military and civilian burn patients have been reported, in which arginine vasopressin and the α-adrenergic receptor agonist norepinephrine are proposed as vasopressors for the treatment of persistent hypotension during early burn resuscitation (5–7). While these resuscitation algorithms empirically address the well-recognized risk of fluid overload (over-resuscitation or “fluid creep”), the effects of burn injury on intrinsic vascular function and on the cardiovascular responsiveness to vasoactive agents remain poorly understood (8–12). Such information, however, could provide the pathophysiologic basis for the use of a particular vasoactive drug and guide in the development of resuscitation strategies that are optimized for the specific pathophysiology of the burned patient.
Thus, the aims of the present study were to determine whether burn injury alters cardiovascular reactivity to vasoactive drugs and to delineate whether burn injury affects intrinsic vascular function. To address these aims, we used a well-established rat scald burn model to determine the mean arterial blood pressure response to arginine vasopressin and phenylephrine, a selective α1-adrenergic receptor agonist, in vivo and pressure myography to assess possible burn induced changes in the vasopressor reactivity of isolated mesenteric resistance arteries.
Materials and Methods
Animal Protocol
All procedures were performed according to National Institutes of Health (NIH) Guidelines for Use of Laboratory Animals and approved by the Loyola Institutional Animal Care and Use Committee (IACUC) and the Department of Defense Animal Care and Use Review Office (ACURO). Male Sprague Dawley rats (325–350g body weight, Harlan, Indianapolis, IN) were anesthetized with 2.5% isoflurane (Patterson Veterinary, Devens, MA), shaved, and placed into a template that exposes a dorsal body area corresponding to 30% of their total body surface area. A full thickness burn was then induced by immersion of the dorsal skin into boiling water for 17 seconds, as described previously (13,14). Sham animals were treated as described before, except that their dorsal surface was immersed in tepid water. Animals were then resuscitated with crystalloid solution i.p. using a modified Parkland formula (4mL/kg per percent TBSA, 4mL/kg/%TBSA divided over four equal dosages) over the first 48h after burn injury. All animals at the end of the experimental period were euthanized (isoflurane inhalation, bilateral pneumothorax).
In vivo testing of cardiovascular responsiveness
In vivo testing of the cardiovascular responsiveness to vasoactive drugs was performed as described previously (15). Animals (n = 4–9/group and vasopressor) after sham procedure and at 24h, 72h, 96h, and 168h post-burn were re-anesthetized (2.5% isoflurane) and instrumented with central venous and arterial catheters that were placed into the femoral vessels. Rats were then resuscitated with crystalloids (3–5 mL of Lactated Ringer’s solution) to a mean arterial blood pressure (MAP) ≥ 80mmHg. Hemodynamic stability was assumed when MAP remained above 80 mmHg for 15 min.
Animals received then intravenous bolus injections of nine increasing doses of phenylephrine (2.5 μg/kg – 5 mg/kg, Sigma, St. Louis) or nineteen increasing doses of arginine vasopressin (0.01 pg/kg – 25 μg/kg, Sigma) in 0.5 mL of normal saline in 5 min intervals. MAP was recorded at 10 s intervals for the duration of the experimental period. At the end of the experimental period, animals were euthanized (isoflurane inhalation, bilateral pneumothorax). For each dose of phenylephrine or arginine vasopressin, the area under the MAP curve was calculated with the GraphPad-Prism 6 software and dose response curves were generated.
Pressure Myography
Pressure myography was performed as described previously (16) with slight modifications. In brief, animals were euthanized, the mesentery was immediately removed and placed in 130 mM NaCl, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO47H2O, 14.9 mM NaHCO3, 5.5 mM d-Glucose, 0.026 mM EDTA and 1.16 CaCl2. Third or 4th order mesenteric arteries, which contribute significantly to vascular resistance (17,18), were then dissected free from the mesentery, mounted with 11-0 nylon sutures onto two glass cannulae of a pressure myography system (Model 110P, DMT-USA, Ann Arbor, MI) and pressurized to 80 mmHg. The vessel bath solution and intralumunal solution were as described before. All solutions were aerated with 95% O2 and 5% CO2 for twenty minutes prior to use and the vessel bath solution was continuously aerated during the experiments. The outer diameter (o.d.) of the pressurized artery was then continuously measured and recorded via digital video-edge detection utilizing the MyoVIEW 3.1.1 software (DMT-USA). Increasing concentrations of arginine vasopressin and phenylephrine were then added to the organ bath in seven minute intervals. Vasoconstriction after each dose of vasopressor was calculated as percent of the outer vessel diameter measured in the absence of vasopressors at the beginning of the experiment.
Data analyses and statistics
Data are given as mean ± SEM. Area under curve (AUC) and non-linear regression analyses were calculated using the GraphPad Prism 6 Program (GraphPad Software, LaJolla, CA). Best-fit values were compared with the extra sum-of-squares F test. A two-tailed p<0.05 was considered significant.
Results
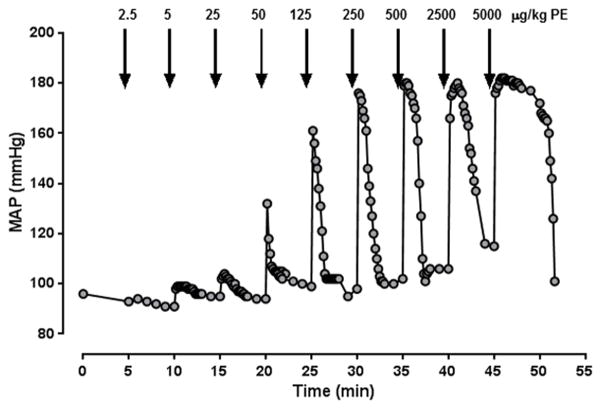
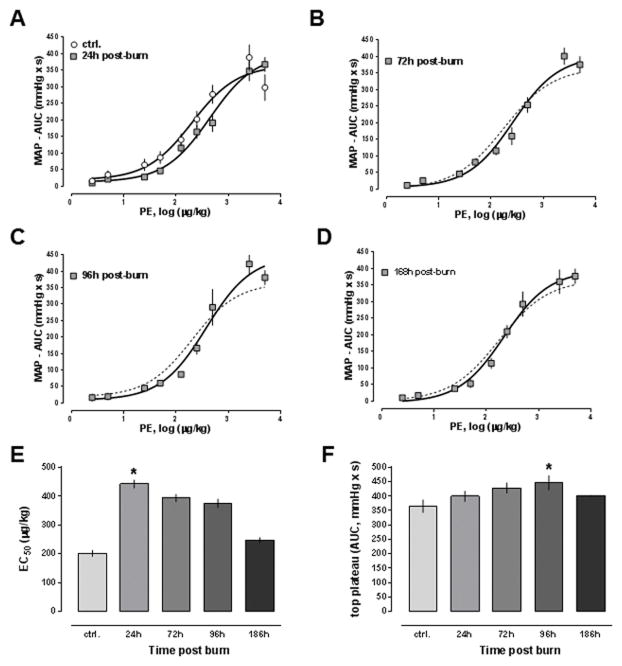
A representative MAP curve from a dose-response experiment with phenylephrine is shown in Fig. 1. As a quantifiable marker of the blood pressure response to phenylephrine or arginine vasopressin, we determined the area under the mean MAP curve for each dose of vasopressor and generated dose-response curves. Fig. 2A–D show the dose-response curves for phenylephrine in animals after sham procedure and in animals at the various time points after burns. The phenylephrine dose-response curve was shifted to the right at 24h post-burn and normalized thereafter. The potency of phenylephrine was significantly reduced 24h post-burn (EC50: sham - 201±11 μg/kg; 24h post-burn – 443±14 μg/kg, p<0.05) and gradually normalized at later time points (393±13 pg/kg, 375±15 pg/kg and 247±9 μg/kg at 72h, 96h and 168h post-burn, respectively, p>0.05 vs. baseline; Fig. 2E). The efficacy of phenylephrine transiently increased by 22% at 96h post-burn (top plateau: sham - 365±22, 96h post-burn - 446±25, p<0.05, Fig. 2F). The Hill slopes of the PE dose response curves were indistinguishable at all time points (p>0.05 vs. sham for all time points, not shown).
Figure 1. Representative mean arterial blood pressure (MAP) responses to increasing doses of phenylephrine.
Arrows indicate the time points of vasopressor administration. The individual doses of phenylephrine (PE, μg/kg) are indicated. For each dose, the area under MAP curve was calculated with the GraphPad Prism 6 Program and used to generate dose-response curves.
Figure 2. Mean arterial blood pressure (MAP) effects of phenylephrine after burn.
Data are mean±SEM. *: p<0.05 vs. sham. A.–D. Dose response curves to phenylephrine after sham procedure (A., n = 8) and 24h (A., n = 7), 72h (B., n = 5), 96h (C., n = 4) and 168h (D., n = 4) post-burn. Animals received increasing doses of phenylephrine and the MAP was monitored continuously, as shown in Fig. 1. For each dose of phenylephrine, the area under the blood pressure curve (AUC, mmHg x s) was calculated and dose response curves were generated with non-linear regression analyses. The dashed line in B.–D. shows the dose response from animals after sham procedure. E. Half-maximal effective concentrations (EC50, μg/kg) from dose response curves shown in A.–D. F. Maximum responses (top plateau, AUC - mmHg x s) of the dose response curves from A.–D.
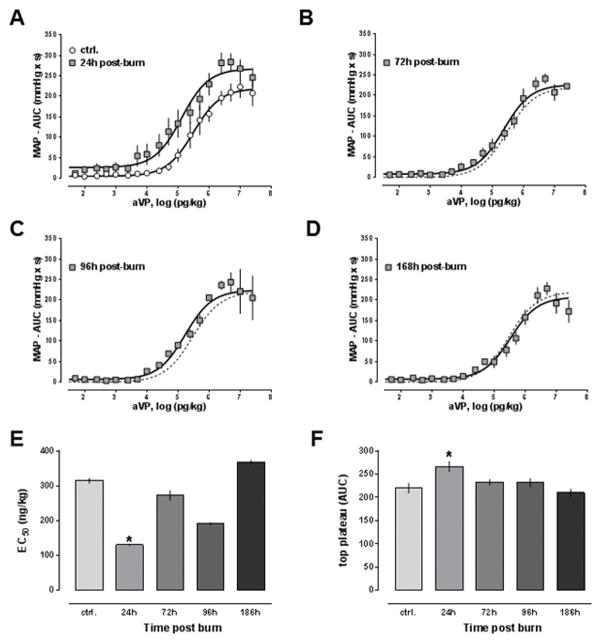
The dose response curves for arginine vasopressin at the various time points are shown in Fig. 3A–D. In contrast to the dose-response curves for phenylephrine, the arginine vasopressin dose-response curve was shifted to the left at 24h post-burn and normalized at subsequent time points. Potency (Fig. 3E) and efficacy (Fig. 3F) of arginine vasopressin were significantly increased 24h post-burn and normalized within 168h post-burn (EC50: sham – 315±7 ng/kg; 24h post-burn – 131±3 ng/kg, p<0.05; top plateau: sham – 220±11 mmHg x s; 24h post-burn – 267±11 mmHg x s, p<0.05). The Hill slopes of the aVP dose-response curves were not significantly altered after burns (p>0.05 vs. sham for all time points, not shown).
Figure 3. Mean arterial blood pressure effects of arginine vasopressin after burn.
Data are mean±SEM. *: p<0.05 vs. sham. A.–D. Dose response curves to arginine vasoprssin after sham procedure (A., n = 6) and 24h (A., n = 9), 72h (B., n = 4), 96h (C., n = 4) and 168h (D., n = 6) post-burn. Animals received increasing doses of arginine vasopressin and the MAP was monitored continuously, as shown in Fig. 1 for an experiment with phenylephrine. For each dose of arginine vasopressin, the area under the blood pressure curve (AUC, mmHg x s) was calculated and dose response curves were generated with non-linear regression analyses. The dashed line in B.–D. shows the dose response from animals after sham procedure. E. Half-maximal effective concentrations (EC50, ng/kg) from dose response curves shown in A.–D. F. Maximum responses (top plateau, AUC - mmHg x s) of the dose response curves from A.–D.
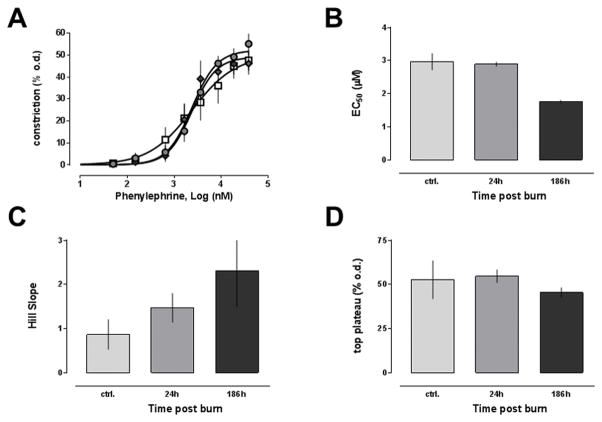
To assess whether the observed changes of the blood pressure response to phenylephrine and arginine vasopressin after burns are accompanied by alterations of intrinsic vascular function, we then utilized pressure myography to test the responsiveness of isolated mesenteric resistance arteries that were pressurized to 80 mmHg. The phenylephrine dose-response curves of mesenteric arteries from uninjured animals and animals at 24h and 168h post-burn are shown Fig. 4A, and the corresponding EC50 concentrations, the top plateau values and Hill slopes are shown in Fig. 4B–D. The reactivity of isolated arteries to phenylephrine stimulation was not significantly altered after burns. Nevertheless, it should be noted that Hill slopes increased from 0.86±0.34 at baseline to 1.47±0.33 and 2.3±0.8 at 24h post-burn and 168h post-burn, respectively. These changes, however, did not reach a level of statistical significance.
Figure 4. Dose response of isolated mesenteric resistance arteries to phenylephrine after burn.
Vascular reactivity of isolated mesenteric arteries to phenylephrine was measured in pressure myography experiments. Data are mean±SEM. A. Vasoconstriction is expressed as % of the outer diameter (% o.d.) measured before phenylephrine administration. Open squares: Arteries from control animals (n = 6). Grey circles: Arteries from animals 24h post-burn (n = 8). Grey diamonds: Arteries from animals 168h post-burn (n = 5). Dose response curves were calculated with non-linear regression analyses. B. Half-maximal effective concentrations (EC50, μM) from A. C. Hill slopes from A. D. Maximum responses (top plateau - % o.d.) from A.
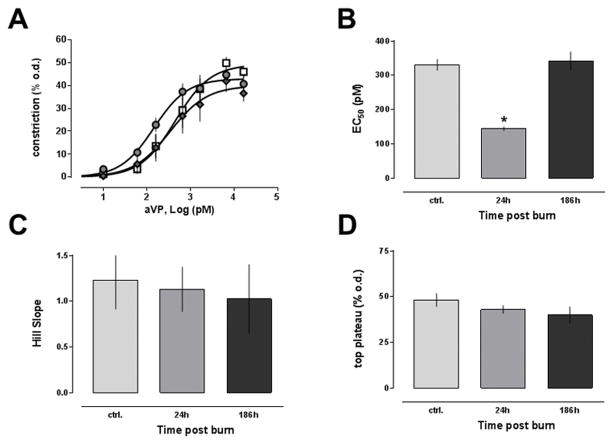
The arginine vasopressin dose-response curves of isolated arteries from uninjured animals and animals after burns are shown in Fig. 5A. Similar to the in vivo blood pressure response to arginine vasopressin, the dose-response of isolated arteries to arginine vasopressin was shifted to the left at 24h post-burn and indistinguishable from uninjured animals at 168h after burns. While the potency of arginine vasopressin to induce vasoconstriction in arteries harvested 24h post-burn was significantly increased (Fig. 5B), efficacy (Fig. 5D) and Hill slopes (Fig. 5C) of the dose response curves were unchanged, as compared with arteries form control animals. The reactivity of arteries from animals 168h post-burn was comparable with those from uninjured animals.
Figure 5. Dose response of isolated mesenteric resistance arteries to arginine vasopressin after burn.
Vascular reactivity of isolated mesenteric arteries to arginine vasopressin was measured in pressure myography experiments. Data are mean±SEM. *: p<0.05 vs. control. A. Vasoconstriction is expressed as % of the outer diameter (% o.d.) measured before phenylephrine administration. Open squares: Arteries from control animals (n = 7). Grey circles: Arteries from animals 24h post-burn (n = 6). Grey diamonds: Arteries from animals 168h post-burn (n = 6). Dose response curves were calculated with non-linear regression analyses. B. Half-maximal effective concentrations (EC50, pM) from A. C. Hill slopes from A. D. Maximum responses (top plateau - % o.d.) from A.
Discussion
In the present study, we provide an evaluation of burn induced changes in systemic cardiovascular responsiveness and intrinsic resistance artery responsiveness to the α1-adrenergic receptor agonist phenylephrine and the vasopressin receptor agonist arginine vasopressin. There are several new findings from the present study. First, burn injuries are associated with alterations in intrinsic vascular function. Second, systemic cardiovascular responsiveness and intrinsic resistance artery responsiveness to arginine vasopressin are increased 24h post-burn, resulting in a 2–3-fold higher potency of the vasoconstrictor and blood pressure effects of arginine vasopressin. Third, the potency of phenylephrine to increase MAP is 2-fold reduced 24h post-burn.
As adverse effects of over-resuscitation are being increasingly recognized in trauma and burn patients, the concept of early pressure support resuscitation in patients requiring excessive amounts of crystalloids and/or colloids has gained particular attention (9–12,19,20). Moreover, a standard low-dose of vasopressin followed by norepinephrine titrated by effect has been incorporated in several published resuscitation algorithms for the difficult to resuscitate hypotensive burn patient (5–7). This treatment strategy has been largely adapted from septic shock patients, in which endogenous arginine vasopressin deficiency contributes to vasodilatory shock and exogenous arginine vasopressin has a significant norepinephrine sparing effect (21–23).
It should be noted, however, that sufficient evidence supporting the use of one vasopressor or any combination of vasopressors over other vasopressors is currently lacking and that early vasopressor use after injury remains controversial (24–29).
Nevertheless, information on early burn induced changes in cardiovascular reactivity to various vasopressors is sparse and, to the best of our knowledge, possible changes in the reactivity of resistance arteries after burns have not been studied previously. Although norepinephrine is used more frequently than phenylephrine in patients, it is a non-selective agonist of α- and β-adrenoceptors (30). As the use of norepinephrine would thus not permit direct conclusions on vascular α1-adrenergic receptor function in vivo and in isolated arteries, we used the selective α1-adrenoceptor agonist phenylephrine in our experiments (30).
It is well established that burn injuries lead to a rapid and pronounced stress response with significantly increased plasma concentrations of endogenous arginine vasopressin and catecholamines (31–33). Furthermore, vasopressin and adrenergic receptors are known to undergo agonist induced internalization (34,35). Thus, the finding that the potency of phenylephrine to increase MAP was reduced 24h after burns is in agreement with a post-burn catecholamine surge. Our observation that the responsiveness of isolated resistance arteries to phenylephrine is not significantly altered at the same time point after burns is consistent with previous observations from aortic ring preparations that were also tested in catecholamine-free organ solutions (36). These data suggest that intrinsic vascular α-adrenergic receptor function is not affected by the thermal injury, despite attenuation of the in vivo blood pressure response to phenylephrine.
Based on the foregoing, it was unexpected that the potency and efficacy of arginine vasopressin to increase MAP was significantly increased at 24h post-burn. As the potency of arginine vasopressin to induce vasoconstriction in isolated arteries was also increased to a comparable degree and at the same time point post-burn, these findings provide initial evidence for burn-induced alterations in the intrinsic function of resistance arteries, which result in a sensitization of vascular smooth muscle vasopressin receptors.
Furthermore, our data show that the sensitivity of the blood pressure response to α-adrenergic and vasopressin receptor stimulation is diametrically opposed early after burns. Our observations are similar, but not identical, to previous findings in a long term rodent model of sepsis, in which the blood pressure response and the efficacy to contract isolated arteries of norepinephrine were reduced in septic animals, whereas the blood pressure response was maintained and the potency of arginine vasopressin to contract arteries was enhanced (37). Several mechanisms could be responsible for the observed changes in vascular α-adrenergic and vasopressin receptor sensitivity after burns or sepsis. One obvious possibility is that burn or sepsis may lead to alterations in receptor expression levels. Alternatively, burn or sepsis may affect vascular α-adrenergic and vasopressin receptor sensitivity through alterations of receptor heteromerization on the cell surface or via interference with their downstream signaling events (15,16). As reliable analyses of receptor densities, heteromeric receptor complexes or receptor signaling properties in intact arteries appear currently impossible, further experimentation with isolated vascular smooth muscle cells is required to test these hypotheses. Such experiments, however, are beyond the scope of the present study. Thus, the exact molecular mechanisms underlying these effects remain to be determined in future studies.
In conclusion, the findings from the present study suggest that burn injury results in a differential regulation of the vasopressor and blood pressure effects of α-adrenergic and vasopressin receptor agonists. While the potency of α-adrenergic receptor agonists to increase MAP is reduced 24h post burn, potency and efficacy of arginine vasopressin are increased. Furthermore, our observations suggest that burn injury sensitizes intrinsic vasopressin receptor reactivity in resistance arteries. The observed changes occur at a critical time period during which stabilization of hemodynamics and maintenance of sufficient urine output are of major clinical relevance. It is possible that the changes in cardiovascular and intrinsic vascular function might be even more pronounced at time points closer to the burn injury than 24 hours. As our observations, however, were not anticipated, we did not include earlier post-burn time points in the initial study design.
Because vascular resistance is inversely proportional to the fourth power of the vessel radius (Hagen-Poiseuille equation), the observed increased potency of arginine vasopressin 24h after burns can explain the therapeutic efficacy of low-dose vasopressin to stabilize hemodynamics in burn patients. Thus, our findings now provide a pathophysiologic rationale for the use of arginine vasopressin in an initial attempt to stabilize hemodynamics in burn patients who remain hypotensive despite crystalloid and/or colloid resuscitation. These data may help to optimize vasopressor use in the difficult to resuscitate burn patient and to reduce the complications associated with fluid overload. Whether early vasopressor support in the difficult to resuscitate burn patient will improve outcomes, however, remains to be determined in prospective clinical trials.
Acknowledgments
This research was made possible by a grant that was awarded and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC), at Fort Detrick, MD, under Contract Number W81XWH1110559. The views, opinions and/or findings contained in this research are those of the author(s) and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy, or decision unless so designated by other documentation. No official endorsement should be made. This work was also supported by the National Institute of General Medical Sciences Awards R01GM107495 and T32GM008750.
This research has been presented, in part, at the 47th Annual Meeting of the American Burn Association, Chicago, IL, April 2015, and at the 38th Annual Conference on Shock, Denver, Colorado, June 2015.
References
- 1.Pham TN, Cancio LC, Gibran NS American Burn A. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257–266. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 2.Lundy JB, Chung KK, Pamplin JC, Ainsworth CR, Jeng JC, Friedman BC. Update on Severe Burn Management for the Intensivist. J Intensive Care Med. 2015 Jun 24; doi: 10.1177/0885066615592346. pii: 0885066615592346 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Barrow RE, Jeschke MG, Herndon DN. Early fluid resuscitation improves outcomes in severely burned children. Resuscitation. 2000;45:91–96. doi: 10.1016/s0300-9572(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 4.Cancio LC. Initial assessment and fluid resuscitation of burn patients. Surg Clin North Am. 2014;94:741–754. doi: 10.1016/j.suc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Chung KK, Blackbourne LH, Wolf SE, White CE, Renz EM, Cancio LC, Holcomb JB, Barillo DJ. Evolution of burn resuscitation in operation Iraqi freedom. J Burn Care Res. 2006;27:606–611. doi: 10.1097/01.BCR.0000235466.57137.f2. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Hemmila MR, Wahl WL. Early albumin use improves mortality in difficult to resuscitate burn patients. J Trauma Acute Care Surg. 2012;73:1294–1297. doi: 10.1097/TA.0b013e31827019b1. [DOI] [PubMed] [Google Scholar]
- 7.Endorf FW, Dries DJ. Burn resuscitation. Scand J Trauma Resusc Emerg Med. 2011;19:69. doi: 10.1186/1757-7241-19-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruitt BA., Jr Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma. 2000;49:567–568. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Bacomo FK, Chung KK. A primer on burn resuscitation. J Emerg Trauma Shock. 2011;4:109–113. doi: 10.4103/0974-2700.76845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goverman J, Bittner EA, Friedstat JS, Moore M, Nozari A, Ibrahim AE, Sarhane KA, Chang PH, Sheridan RL, Fagan SP. Discrepancy in Initial Pediatric Burn Estimates and Its Impact on Fluid Resuscitation. J Burn Care Res. 2015;36:574–579. doi: 10.1097/BCR.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 11.McBeth PB, Sass K, Nickerson D, Ball CG, Kirkpatrick AW. A necessary evil? Intra-abdominal hypertension complicating burn patient resuscitation. J Trauma Manag Outcomes. 2014;8:12. doi: 10.1186/1752-2897-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen S, Palmieri T, Greenhalgh D. Review of Burn Research for Year 2014. J Burn Care Res. 2015;36:587–594. doi: 10.1097/BCR.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 13.Vana PG, LaPorte HM, Wong YM, Kennedy RH, Gamelli RL, Majetschak M. Proteasome Inhibition After Burn Injury. J Burn Care Res. 2015 Jul 21; doi: 10.1097/BCR.0000000000000280. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong YM, La Porte HM, Szilagyi A, Bach HHt, Ke-He L, Kennedy RH, Gamelli RL, Shankar R, Majetschak M. Activities of nonlysosomal proteolytic systems in skeletal and cardiac muscle during burn-induced hypermetabolism. J Burn Care Res. 2014;35:319–327. doi: 10.1097/BCR.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathi A, Vana PG, Chavan TS, Brueggemann LI, Byron KL, Tarasova NI, Volkman BF, Gaponenko V, Majetschak M. Heteromerization of chemokine (C-X-C motif) receptor 4 with alpha1A/B-adrenergic receptors controls alpha1-adrenergic receptor function. Proc Natl Acad Sci U S A. 2015;112:E1659–1668. doi: 10.1073/pnas.1417564112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach HHt, Wong YM, Tripathi A, Nevins AM, Gamelli RL, Volkman BF, Byron KL, Majetschak M. Chemokine (C-X-C motif) receptor 4 and atypical chemokine receptor 3 regulate vascular alpha(1)-adrenergic receptor function. Mol Med. 2014;20:435–447. doi: 10.2119/molmed.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen KL, Mulvany MJ. Perindopril changes the mesenteric pressure profile of conscious hypertensive and normotensive rats. Hypertension. 1994;23:325–328. doi: 10.1161/01.hyp.23.3.325. [DOI] [PubMed] [Google Scholar]
- 18.Dunn WR, Gardiner SM. Structural and functional properties of isolated, pressurized, mesenteric resistance arteries from a vasopressin-deficient rat model of genetic hypertension. Hypertension. 1995;26:390–396. doi: 10.1161/01.hyp.26.3.390. [DOI] [PubMed] [Google Scholar]
- 19.Cohn SM, Blackbourne LH, Landry DW, Proctor KG, Walley KR, Wenzel V. San Antonio Vasopressin in Shock Symposium report. Resuscitation. 2010;81:1473–1475. doi: 10.1016/j.resuscitation.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Anand T, Skinner R. Arginine vasopressin: The future of pressure-support resuscitation in hemorrhagic shock. J Surg Res. 2012;178:321–329. doi: 10.1016/j.jss.2012.02.062. [DOI] [PubMed] [Google Scholar]
- 21.Landry DW, Levin HR, Gallant EM, Ashton RC, Jr, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 22.Landry DW, Levin HR, Gallant EM, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25:1279–1282. doi: 10.1097/00003246-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Cartotto R, McGibney K, Smith T, Abadir A. Vasopressin for the septic burn patient. Burns. 2007;33:441–451. doi: 10.1016/j.burns.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhou F, Mao Z, Zeng X, Kang H, Liu H, Pan L, Hou PC. Vasopressors in septic shock: a systematic review and network meta-analysis. Ther Clin Risk Manag. 2015;11:1047–1059. doi: 10.2147/TCRM.S80060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oba Y, Lone NA. Mortality benefit of vasopressor and inotropic agents in septic shock: a Bayesian network meta-analysis of randomized controlled trials. J Crit Care. 2014;29:706–710. doi: 10.1016/j.jcrc.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Mullner M, Urbanek B, Havel C, Losert H, Waechter F, Gamper G. Vasopressors for shock. The Cochrane database of systematic reviews. 2004:CD003709. doi: 10.1002/14651858.CD003709.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Havel C, Arrich J, Losert H, Gamper G, Mullner M, Herkner H. Vasopressors for hypotensive shock. The Cochrane database of systematic reviews. 2011:CD003709. doi: 10.1002/14651858.CD003709.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D Investigators V. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 29.Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht BG, Moore EE, Maier RV, Nirula R. Early use of vasopressors after injury: caution before constriction. J Trauma. 2008;64:9–14. doi: 10.1097/TA.0b013e31815dd029. [DOI] [PubMed] [Google Scholar]
- 30.Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, Collaborators C. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui M, Kudo T, Kudo M, Ishihara H, Matsuki A. The endocrine response after burns. Agressologie. 1991;32:233–235. [PubMed] [Google Scholar]
- 32.Smith A, Barclay C, Quaba A, Sedowofia K, Stephen R, Thompson M, Watson A, McIntosh N. The bigger the burn, the greater the stress. Burns. 1997;23:291–294. doi: 10.1016/s0305-4179(96)00137-4. [DOI] [PubMed] [Google Scholar]
- 33.Crum R, Bobrow B, Shackford S, Hansbrough J, Brown MR. The neurohumoral response to burn injury in patients resuscitated with hypertonic saline. J Trauma. 1988;28:1181–1187. doi: 10.1097/00005373-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Briner VA, Williams B, Tsai P, Schrier RW. Demonstration of processing and recycling of biologically active V1 vasopressin receptors in vascular smooth muscle. Proc Natl Acad Sci U S A. 1992;89:2854–2858. doi: 10.1073/pnas.89.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahan LC, McKernan RM, Insel PA. Metabolism of alpha- and beta-adrenergic receptors in vitro and in vivo. Annu Rev Pharmacol Toxicol. 1987;27:215–235. doi: 10.1146/annurev.pa.27.040187.001243. [DOI] [PubMed] [Google Scholar]
- 36.Cioffi WG, DeMeules JE, Gamelli RL. Vascular reactivity following thermal injury. Circulatory shock. 1988;25:309–317. [PubMed] [Google Scholar]
- 37.Barrett LK, Orie NN, Taylor V, Stidwill RP, Clapp LH, Singer M. Differential effects of vasopressin and norepinephrine on vascular reactivity in a long-term rodent model of sepsis. Crit Care Med. 2007;35:2337–2343. doi: 10.1097/01.ccm.0000281861.72907.17. [DOI] [PubMed] [Google Scholar]