Abstract
Background
Anti-CD154mAb is a powerful costimulation blockade agent that is efficacious in preventing rejection, even in xenogeneic settings. It has been used in the majority of successful long-term pig-to-nonhuman primate islet transplantation models. However, its clinical use was halted as a result of thromboembolic complications that were also observed in pre-clinical and clinical organ transplantation models.
Methods
An anti-CD154mAb was administered to 14 streptozotocin-induced diabetic cynomolgus monkey recipients of porcine islets, some of which received the agent for many months. Monkeys were monitored for complications, and blood monitoring was carried out frequently. After euthanasia, multiple biopsies of all organs were examined for histological features of thromboembolism.
Results
Anti-CD154mAb prevented rejection of genetically-engineered pig islets in all monkeys. No significant complications were attributable specifically to anti-CD154mAb. There was no evidence of thromboembolism in multiple histological sections from all major organs, including the brain.
Conclusions
Our data suggest that in diabetic monkeys with pig islet grafts, anti-CD154mAb would appear to be an effective and safe therapy, and is not associated with thromboembolic complications.
Keywords: Anti-CD154mAb, Immunosuppression, Islets of Langerhans, xenotransplantation
INTRODUCTION
Costimulation blockade was developed to target (and block) communication along the secondary pathway required for T cell activation (1), and has proven to be a powerful form of immunosuppressive therapy, even in preclinical models of xenotransplantation where the T cell response is generally considered to be stronger than after allotransplantation (2). It is usually directed to block the CD28/B7 and/or CD40/CD154 pathways. Historically, CTLA4-Ig is used to block the CD28/B7 pathway and an anti-CD154 monoclonal antibody (mAb) or anti-CD40mAb to block the CD40/CD154 pathway (1).
The combination of these agents successfully prevented kidney allograft rejection in a nonhuman primate (NHP) model (3). Anti-CD154mAb monotherapy worked well in monkeys with renal (4) or islet (5) allotransplants, whereas CTLA4-Ig monotherapy showed only modest ability to prolong similar allograft function (6). In a 2000 study on the transplantation of porcine peripheral blood progenitor cells into baboons, anti-CD154mAb successfully prevented sensitization to the graft (7). This success in a xenograft model led to anti-CD154mAb being widely used in xenotransplantation studies involving NHPs (2), including those of islet xenotransplantation (8-10).
The broadly powerful immunotherapeutic effects of anti-CD154mAb are likely derived through several mechanisms. It impacts (i) T cells by blocking the secondary signal, and (ii) B cell function through lack of T cell help. It also acts (iii) by blocking soluble CD154 released by platelets (11), and (iv) by its effect on vascular endothelial cells by modulating CD154-mediated adhesion molecules and cytokine receptors (12,13).
Following promising results in animal models, anti-CD154mAb was tested in clinical trials. Phase 1 and 2 trials were conducted (by Biogen, Cambridge, MA) from 1997-1999 in patients with immune thrombocytopenic purpura, systemic lupus erythematosus, factor VIII antibody syndrome, and in patients undergoing renal transplantation. These trials showed efficacy, but were halted when several patients suffered thromboembolic events (14). In Phase 1 and 2 trials conducted (by IDEC, San Diego, CA) from 2001-2002 in patients with systemic lupus erythematosus, the agent proved safe but was not efficacious (15). Clinical use of anti-CD154mAb was put on hold.
Thromboembolic events have also been reported in NHPs in association with use of anti-CD154mAb (16-20). The mechanisms behind these secondary effects appear to involve the formation of immune complexes between anti-CD154mAb and CD154, with subsequent platelet activation via the IgG receptor FcγRIIA, thus triggering coagulation (21). A role for the IgG receptor FcγRIIA on adjacent platelets was also proposed for increasing the risk of thrombosis (22). Anti-CD154mAb may also affect arterial thrombi stabilization (23).
Heparin and other anticoagulants have shown efficacy in reducing anti-CD154mAb-mediated thromboembolism (16-19,24).
The last 10 years have brought forth several successful pre-clinical pig-to-NHP islet xenotransplantation studies, reviewed by Park CG et al (25), including our own (9,10). Successful models have been differentiated by characteristics of the pig islet donor while the studies were almost universal in using costimulation blockade immunosuppression, most frequently based on an anti-CD154mAb (25).
The aim of the present study was to determine whether relatively prolonged administration (up to one year) of an anti-CD154mAb was associated with thromboembolic events in monkeys with porcine islet transplants.
MATERIALS AND METHODS
Porcine islet donors
Islets were harvested from wild-type (WT) pigs (n=2) (Wally Whippo, Enon Valley, PA) or genetically-engineered (GE) pigs with one or more modifications (see Table 1 for information on the genetics of donor pigs); α1,3-galactosyltransferase gene-knockout (GTKO, n=2), transgenic for hCD46 (n=5), 3 GE (GTKO,hCD46,hCD39, n=1), 4 GE (GTKO,hCD46,hCD39,hTFPI, n=2), or 5 GE (GTKO,hCD46,hCD39,hTFPI,CTLA4-Ig n=2) pigs (all from Revivicor, Blacksburg, VA) (9,10).
Table 1.
Post-transplant follow-up of recipient monkeys
| Monkey | Pig donor genetics |
Post-Tx Follow-up (days) |
Presence of intravascular catheters post-Tx (days) |
2nd dose of ATG |
Anti- CD154mAb type |
Anti-CD154mAb maintenance dose (mg/kg/week) |
Anti-CD154mab serum trough levels (μg/mL) mean±SD |
|---|---|---|---|---|---|---|---|
| 1 | GTKO | 8 | 8 | y | ABI793 | 5-15 | 641 |
| 2 | WT | 270 | 21 | y | ABI793 | 5-15 | 1138±440 |
| 3 | GTKO | 70 | 42 | y | ABI793 | 5-15 | 1021±489 |
| 4 | WT | 41 | 41 | y | ABI793 | 5-15 | 1388±578 |
| 5 | hCD46 | 98 | 98 | y | ABI793 | 5-15 | 1075±514 |
| 6 | hCD46 | 131 | 75 | y/n* | ABI793 | 5-15 | 609±165 |
| 7 | hCD46 | 120 | 0 | n | ABI793 | 5-15 | 944±46 |
| 8 | hCD46 | 183 | 40 | n/y* | ABI793 | 5-15 | 1663±184 |
| 9 | hCD46 | 365 | 78 | y | ABI793 | 5-15 | 744±415 |
| 10 | 4-GE | 364 | 29 | y | h5c8 | 25 | n/a |
| 11 | 4-GE | 365 | 16 | y | h5c8 | 25 | n/a |
| 12 | 5-GE | 162 | 14 | y | h5c8 | 25 | n/a |
| 13 | 3-GE | 58 | 21 | n | h5c8 | 25 | n/a |
| 14 | 5-GE | 52 | 21 | y | h5c8 | 25 | n/a |
All monkeys received; Mycophertolate mofetil, Dextran sulfate, Prostacyclicn, Methylprednisolone, Aspirin, Ganciclovir or Valganciclovir, Famotidine, and Heparin as detailed in Table 2.
Monkeys 6,8 received two transplants and two courses of ATG.
n/a = not measured
GE (genetically-engineered)
GTKO (α1,3-galactosyltransferase gene-knockout)
hTFPI (human tissue factor pathway inhibitor)
WT (wild-type)
3-GE (GTKO/hCD46/hCD39)
4-GE (GTKO/hCD46/hTFPI/CTLA4-Ig)
5-GE(GTKO/hCD46/hCD39/hTFPI/CTLA4-Ig)
Diabetic monkey recipients
Diabetes was induced in 14 cynomolgus monkeys (Macaca fascicularis; Three Springs Scientific, Perkasie, PA, and Alpha Genesis, Yemassee, SC) by the administration of streptozotocin, as previously described (10). The monkeys were 2-5yr of age and weighed 3.5±0.6kg.
All animal care was in compliance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 2011), and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Immunosuppressive and adjunctive therapy
The immunosuppressive (and adjunctive drug) regimen was based on anti-CD154mAb and is summarized in Table 2. Anti-CD154mAbs (ABI793 [Novartis Pharma, Basel, Switzerland; n=9] or chimeric h5c8 [NIH NHP Reagent Resource, Boston, MA; n=5]) were used for induction, with the same antibody also being used for maintenance of immunosuppression. Dosage was modulated to maintain serum trough levels in the anticipated range. Trough levels were not measured in 5 monkeys who were, therefore, administered a dose proportionally higher than received by the other monkeys.
Table 2.
immunosuppressive and supportive therapy
| Induction |
| Antithymocyte globulin, i.v. 25mg day −3 (ATG, Thymoglobulin, Genzyme, Cambridge, MA). A second variable dose was administered on day −1 if the CD3+ T cell count remained >500cells/mL. |
| Anti-CD154mAb, i.v. 25mg/kg on days −1, 0, 3, 7, 11 and 15 (ABI793 [n=9], Novartis Pharma, Basel, Switzerland, or chimeric h5c8 [n=5], NIH NHP Reagent Resource, Boston, MA) |
| Maintenance |
| Anti-CD154mAb, i.v. 5–15mg/kg weekly from day 22 to maintain serum trough levels of >500μg/mL. In the absence of trough levels, a dosage of 25mg/kg was administered weekly. |
| Mycophenolate mofetil, p.o. 50-100mg/kg/day to maintain whole blood trough levels of 3-5μg/mL (Cellcept; Roche, Nutley, NJ). |
| Supportive therapy on the day of transplantation |
| Dextran sulfate before islet infusion, i.v. 5mg/kg (Sigma-Aldrich, St. Louis, MO) |
| Prostacyclin, i.v. 20ng/kg/min (Flolan; GlaxoSmithKline, Philadelphia, PA) starting before islet infusion and for additional 3h. |
| Methylprednisolone, i.v. 10mg/kg before islet infusion (SoluMedrol; Pfizer, New York, NY). |
| Supportive therapy during follow-up |
| Aspirin, p.o. 81mg daily starting 1 week before islet transplantation |
| Ganciclovir, i.v. 5mg/kg/day (Cytovene; Roche, Welwyn Garden City, UK) or |
| Valganciclovir, p.o. 15mg/kg x2 daily (Valcyte; Genentech, San Francisco, CA) (to prevent cytomegalovirus reactivation). |
| Famotidine, i.v. 0.25mg/kg/day or p.o. 1mg/kg/day while i.v. catethers were in place (APP Pharmaceuticals, Schaumburg, IL, and Baxter Healthcare, Deerfield, IL) (to prevent peptic ulceration). |
| Heparin, i.v. 10-20U/h while i.v. cathethers were in place (Hospira, Lake Forest, IL). |
Apart from low-dose continuous heparin infusion to maintain i.v. catheter patency (Table 1), the only antiplatelet/anticoagulant/anti-inflammatory agent administered to the monkeys was aspirin (81mg/day) (Table 2).
Monitoring
The monkeys were monitored clinically for signs of infection or thromboembolism. Daily records of the following signs were kept - mobility impairment, lack of coordination, hypotonic limbs, hemiplegia, respiratory dysfunction (shortness of breath), and diarrhea, in addition to lack of appetite and lack of interest in enrichment activities. Blood was collected at least monthly for complete blood counts and tests of renal and hepatic function (Presbyterian Hospital, University of Pittsburgh Medical Center). Blood glucose was monitored at least x2 daily and insulin was administered to maintain the blood glucose <200mg/dL pre-transplant and, as necessary afterwards. The studies were designed for follow-ups of 3, 6 or 12mo, at which time monkeys were electively euthanized (9,10). Serum levels of porcine C-peptide were measured at intervals as an indicator of graft function. If undetectable, the recipient monkey was euthanized. Whole blood trough levels of anti-CD154mAb were measured by competitive inhibition ELISA (26) in 9 of the 14 monkeys. The activated clotting time (ACT) was measured using I-Stat (Abbott, Princeton, NJ) while heparin was being administered.
Necropsy and microscopic examination of major organs
After euthanasia, a full necropsy was performed in all monkeys. All major organs (brain, heart, intestine, kidney, liver, lung) were cut into blocks of approximately 5×5×5mm and macroscopically examined prior to fixation. Areas of ischemic injury, hemorrhage, or other gross pathology could therefore be excluded. Formalin-fixed tissue was embedded in paraffin. Three random biopsies, each from 3-5 areas of the organ, covering approximately 25mm2 were stained with hematoxylin and eosin (H&E) using standard procedures and examined.
Images were captured using a Zeiss Axiovert 200 Microscope (Carl Zeiss Microscopy, Thornwood, NY) and an Olympus BX51 microscope and Nikon D300 camera (B&B Microscopes, Pittsburgh, PA).
RESULTS
Clinical observations
Follow-up after islet transplantation ranged from 8 to 365 days with a mean of 163 days and a median of 125 days (Table 1). Nine monkeys were followed for >3months, 5 for >6months, and 3 for 12months. Of the 12 monkey recipients with graft function warranting follow-up beyond 40 days, the mean follow-up was 187 days with a median of 147 days. Anti-CD154mAb was administered weekly throughout the entire period of follow-up. Serum levels of anti-CD154mAb were between 609-1663μg/mL (mean 1025μg/mL; median 1021μg/mL). Intravascular catheters with auxiliary i.v. heparin infusion remained in situ for between 8 and 98 days in 13 monkeys (mean 39 days; median 29 days) (Table 1).
No monkey suffered a complication attributed to anti-CD154mAb therapy. There were no transient complications during or following i.v. anti-CD154mAb administration (e.g., vomiting, loss of appetite, diarrhea, fever, seizures), and no major clinical complications (e.g., infections, malignancies, or thromboembolism) occurred. In 2 monkeys (15%), we detected evidence of reactivation of cytomegalovirus, despite the fact that all monkeys received prophylaxis in the form of ganciclovir or valganciclovir, but there were no clinical features of cytomegalovirus disease.
Parameters such as white and red blood cell and platelet counts, as well as those of hepatic and renal function did not show significant changes during the post-transplant phase in the study animals (9,10). ACT levels were 127±25s (mean±SD, n=12) while heparin (and aspirin) was being administered.
There was no evidence of thromboembolic events in the monkeys regardless of whether they received a higher or lower dose of anti-CD154mAb (Table 1).
Histopathology of major organs
At necropsy no macroscopic evidence of abnormalities was seen.
Multiple H&E-stained sections from the major organs in all monkeys were examined: no microscopic features of thrombosis or embolism were detected. Figure 1 shows representative images from H&E-stained tissues of two recipients that were followed-up for one year.
Figure 1.
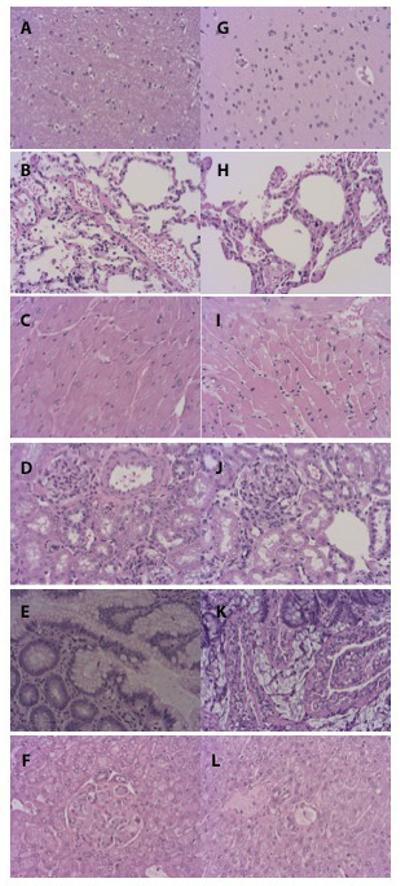
H&E-stained sections of brain (A, G), lung (B, H), heart (C, I), kidney (D, J) intestine (E, K), liver (F, L) from two cynomolgus monkey recipients of pig islets treated with anti-CD154mAb for one year. No histopathological features of thromboembolism were seen in any of the multiple sections examined.
DISCUSSION
Of the several groups that have reported relatively long-term successful pig islet transplantation in diabetic monkeys (25), only one recorded any severe adverse events associated with anti-CD154mAb in recipient NHPs treated with the antibody, affecting 8 of 9 monkeys tested (27). In that study, however, it was difficult to distinguish the specific side-effects of anti-CD154mAb from those of other agents that were administered, e.g., everolimus, which can also increase thrombotic events (28). Previous studies, including relevant work by Cardona et al (29) and Thompson et al (30) with WT as well as GTKO neonatal islets, observed no adverse anti-CD154mAb-derived events. Although follow-up was for <1 year, this finding is significant in view of the fact that neonatal pig islets (that express galactose-α1,3-galactose [Gal]) are more thrombogenic than adult islets (31).
In our own preclinical studies of porcine islet xenotransplantation, an anti-CD154mAb-based immunosuppressive regimen has proved effective in maintaining islet graft function for at least 1year and has been well-tolerated and safe over relatively long periods of administration (9,10).
We have employed both the ABI793 and h5c8 varieties of the monoclonal antibody in cynomolgus monkey and baboon recipients of other porcine organs/tissues. We have reported thrombosis in pig artery patch (17) and organ (18) xenotransplantation models.
Our observations in the present study on the lack of adverse events are relevant in the light of earlier reports of thromboembolism in both pre-clinical NHP studies (16,18-20) and in clinical trials (14) that were halted because of the incidence of this complication. To note, none of those earlier pre-clinical and clinical studies involved islet cells.
In our present study, 14 monkeys were administered anti-CD154mAb during follow-up, with 12 being followed for >45 days, with a median of 187 days in this sub-group. None experienced an adverse event. A WT pig-to-NHP islet transplantation study published by Shin et al (32), which reported use of anti-CD154mAb immunosuppression associated with normoglycemia in 4 of 5 monkeys for >6 months, including one for >600 days, confirmed our own observation of no evidence of thromboembolism or other adverse events (32).
Considering data from both groups reporting this level of long-term success with pig-to-NHP islet transplantation, at least 6 monkeys have now received anti-CD154mAb treatment for >300 days with demonstrated beta cell function and no ill effects associated with its use (9,10,32). Although more definitive evaluation including confirmative diagnostic tools would be beneficial, such a prolonged period of time of treatment is reassuring regarding the lack of adverse events, particularly in the absence of evidence of any histopathological features of thromboembolism in the present study.
What accounts for the difference seen in the incidence of thromboembolism after pig islet xenotransplantation compared with pig solid organ or artery patch xenotransplantation? The answer to this particular question lies beyond the scope of our present study, but we may point to several intriguing possibilities. There are peculiarities in the islet xenotransplantation setting that may prove relevant when compared to whole organ or tissue transplantation. For example, (i) the much smaller size of the islet graft might play a significant role in reducing the antigen load (thus stimulating a much weaker antibody response); (ii) the relative absence of vascular endothelium in the islet graft may be important from the perspective of coagulation dysfunction (almost certainly initiated by anti-pig antibodies); (iii) the invariable administration of a large dose of heparin or dextran sulfate immediately before the islet graft is exposed to the recipient’s blood may prevent thrombosis just as a single dose of ketorolac prior to anti-CD154mAb can prevent thrombosis in a pig artery patch graft model (17) or two doses in a NHP kidney allotransplantation model, despite anti-CD154mAb being administered for weeks or months post-transplantation (19). It may be interesting to note that the dose of anti-CD154mAb used in monkeys is greater proportionally to body weight than doses administered to humans: it would be expected, therefore, that potential ill effects would be more readily observed in the monkeys (14).
In the current study, the use of intravascular catheters with continuous infusion of fluids supplemented with very low doses of heparin (approximately 10-20U/h) would have provided some (though minimal) anticoagulant coverage at least during the period the catheters were in place (Table 1). Furthermore, a daily low-dose of aspirin was given to all recipients, which may have imparted sufficient anti-platelet/anti-inflammatory activity to minimize the risk of clotting. Schuler et al (20) observed that in the absence of aspirin, platelet counts fell in cynomolgus monkeys administered anti-CD154mAb, while the addition of aspirin normalized the platelet count. The drop in numbers may be indicative of platelet consumption, which can be associated with thromboembolic events. Monkeys in our study all received a daily dose of aspirin and their platelet counts showed no significant variability during the course of immunosuppressive therapy (Table 3).
Table 3.
Complete blood counts (means and ranges) in monkey recipients followed for at least 3 months after pig islet transplantation
| Monkey | WBC X10E+09/L |
RBC X10E+12/L |
Hematocrit % |
Platelet x10E+09/L |
Platelet volume fL |
|
|---|---|---|---|---|---|---|
| mean±SD (range) | ||||||
| 2 | n=9 | 7.4±2.3 (4.5-10.9) | 3.9±0.6 (2.6-4.4) | 29.3±4.6 (20.2-35.1) | 400±98 (219-537) | 9.1±0.8 (8.3-10.9) |
| 5 | n=9 | 5.0±3.3 (1.2-10.3) | 3.5±0.5 (2.8-4.5) | 28.5±4.2 (19.3-33.4) | 267±81 (156-380) | 9.2±1.1 (7.1-10.3) |
| 6 | n=9 | 4.4±1.7 (1.9-8.1) | 4.0±0.5 (3.2-5.2) | 31.7±4.1 (23.4-39.5) | 250±68 (142-436) | 10.6±0.9 (8.9-12.4) |
| 7 | n=12 | 8.7±3.3 (5.0-15.6) | 4.3±0.3 (3.3-4.7) | 33.8±3.2 (26.0-39.0) | 422±104 (249-580) | 9.0±0.7 (8.3-10.5) |
| 8 | n=6 | 4.5±1.2 (2.8-7.1) | 4.6±0.3 (4.3-4.8) | 33.3±1.5 (30.5-34-7) | 323±71 (230-533) | 10.7±0.7 (9.2-11.6) |
| 9 | n=13 | 6.7±1.5 (1.9-10.7) | 4.5±0.4 (4.1-5.1) | 36.3±3.5 (31.2-41.0) | 321±57 (207-440) | 10.0±0.6 (8.9-11.4) |
| 10 | n=3 | 6.6±1.6 (5.5-8.4) | 5.6±0.1 (5.5-5.6) | 37.0±1.2 (36.7±38.9) | 447±50 (390-476) | 9.0±0.5 (8.6-9.6) |
| 11 | n=7 | 7.0±2.7 (3.0-11.7) | 5.2±0.3 (4.7-5.4) | 37.0±1.8 (34.3±39.1) | 460±95 (292-501) | 8.3±0.4 (7.9-9.0) |
| 12 | n=7 | 8.3±4.1 (9.5-13.8) | 4.4±0.5 (3.6-5.1) | 32.3±4.0 (30.0±38.5) | 439±140 (311-710) | 84-0.4 (8.0-9.2) |
All of the groups that have reported long-term success in the pig-to-NHP islet xenotransplantation model using anti-CD154mAb-based immunosuppressive therapy administered an anti-coagulant (heparin or dextran sulfate) to the recipients at the time of transplantation (9,10,27,29,30,32), but only the groups with the longest follow-ups (>1yr) continued some form of anti-coagulation therapy beyond the time of islet infusion (9,10,32). Our group administered daily aspirin while Shin et al administered Plavix for one month after transplantation. Also of note, of three groups with the most sustained anti-CD154mAb treatment (9,10,27), the only group to report severe events associated with its use was also the only one not to extend anticoagulant coverage beyond the transplant (27). These observations suggest that extended administration of anticoagulants, even in relatively small doses, may be beneficial.
Is it possible that the genetic-engineering of the porcine islets may have provided protection against thromboembolic events? The expression of hCD39 and/or human tissue factor pathway inhibitor (hTFPI) in some of the pig donor islet grafts may have protected against thromboembolism (10). The addition of CTLA4-Ig or hCD46, or the deletion of Gal in pig aortic endothelial cells would not be predicted to have had a major impact on thrombosis (33). This is supported by the absence of thromboembolic complications in recipients of WT donor pig islets in our study and, after much longer follow-up, in the study by Shin et al (32). Importantly, in combinations of 5 different genetically-engineered donor islets along with 2 types of anti-CD154mAbs studied (Table 1), the lack of thromboembolic events was general. Based on our data, the specific genetic manipulation does not appear to influence the occurrence of thromboembolic events either negatively or positively. We suggest that the absence of thromboembolism irrespective of the genetic nature of the islet-source pig or of the immunosuppressive regimen strengthens our conclusion that anti-CD154mAb was not thrombogenic under the circumstances of these experiments.
Although alternative costimulation blockade agents have been – or are being – developed, our experience and that of others (32) suggests that any anti-CD154mAb, when given as monotherapy (or combined with other less potent agents), is more potent than others, e.g., CTLA4-Ig (abatacept and belatacept) and anti-CD40mAb, even though the results using this latter agent have been very encouraging following the xenotransplantation of solid pig organs (34,35). Because of their undoubted efficacy, attempts are being made to develop an anti-CD154mAb that successfully blocks the CD40/CD154 pathway, but does not activate platelets (36).
In conclusion, anti-CD154mAb remains one of the most potent anti-rejection drugs with, except for thromboembolism, a good safety profile. Despite earlier reports of its use in clinical trials and NHP organ transplantation being associated with serious adverse events, in our model of pig-to-NHP islet transplantation, it proved an effective and safe agent to administer under a daily regimen of aspirin. Whether this experience is sufficient to consider its use in clinical trials of islet allotransplantation remains uncertain.
Acknowledgements
The authors would like to thank Deborah Potonia in the Pathology Laboratory of Allegheny General Hospital for histological preparation of tissues and H&E staining. Chimeric h5c8 anti-CD154mAb was kindly made available to us by Dr. Keith Reimann through the Nonhuman Primate Reagent Resource funded by the US National Institutes of Health contract HHSN272201300031C. ABI793 monoclonal antibody was a gift from Novartis. Genetically-engineered pigs were provided through Sponsored Research Agreements between Revivicor, Inc., Blacksburg, VA, and the University of Pittsburgh.
Funding: This work was in part supported by Department of Defense grant W81XWH-06-1-0317 (MT), JDRF grant 6-2005-1180 (MT), NIH grants #U19 AI090959-01, #U01 AI068642 and #R21 A1074844 (DKCC), and by Sponsored Research Agreements between Revivicor, Inc., Blacksburg, VA, and the University of Pittsburgh.
Abbreviations
- Gal
galactose-α1,3-galactose
- GE
genetically-engineered
- GTKO
α1,3-galactosyltransferase gene-knockout
- mAb
monoclonal antibody
- NHP
nonhuman primate
- WT
wild-type
Footnotes
Author contributions:
All authors contributed to the writing and editing of the manuscript and are responsible for the final version for publication.
Rita Bottino contributed to the research design, performance of research and data analysis.
Michael F. Knoll contributed to data analysis.
Joshua Graeme-Wilson contributed to data analysis.
Edwin C. Klein contributed the histopathological analysis.
David Ayares contributed to research design.
Massimo Trucco contributed to research design and data analysis.
David KC Cooper contributed to research design, performance of research, and data analysis.
Disclosures
David Ayares is an employee of Revivicor, Inc. The other authors have no conflicts of interest to disclose
References
- 1.Larsen CP, Knechtle SJ, Adams A, et al. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6:876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DKC, Satyananda V, Ekser B, et al. Progress in pig-to-non-human primate transplantation models: 1998-2013: a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon NS, Chatzipetrou M, Masetti M, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96:8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levisetti MG, Padrid PA, Szot GL, et al. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159:5187–5191. [PubMed] [Google Scholar]
- 7.Bühler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Bühler L, Deng S, O'Neil J, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9:3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 9.van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 10.Bottino R, Wijkstrom M, van der Windt DJ, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14:2275–2287. doi: 10.1111/ajt.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzelarab MB, Ekser B, Isse K, et al. Increased soluble CD154 (CD40 ligand) levels in xenograft recipients correlate with the development of de novo anti-pig IgG antibodies. Transplantation. 2014:502–508. doi: 10.1097/TP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 12.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 13.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 14.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 15.Kalunian KC, Davis JC, Merrill JT, et al. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:3251–3258. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Andrews D, Colvin RB, et al. Thromboembolic complications after treatment with monoclonal antibody against cd40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 17.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knosalla C, Gollackner B, Cooper DKC. Anti-CD154 monoclonal antibody and thromboembolism revisited. Transplantation. 2002;74:416–417. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 19.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 20.Schuler W, Bigaud M, Brinkmann V, et al. Efficacy and safety of ABI793, a novel human anti-human CD154 monoclonal antibody, in cynomolgus monkey renal allotransplantation. Transplantation. 2004;77:717–726. doi: 10.1097/01.tp.0000116563.72763.83. [DOI] [PubMed] [Google Scholar]
- 21.Robles-Carrillo L, Meyer T, Hatfield M, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185:1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 22.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 24.Bühler L, Alwayn IP, Appel JZIII, et al. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71:491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]
- 25.Park CG, Bottino R, Hawthorne WJ. Current status of islet xenotransplantation. Int J Surg. 2015;23(Pt B):261–266. doi: 10.1016/j.ijsu.2015.07.703. [DOI] [PubMed] [Google Scholar]
- 26.Tai HC, Campanile N, Ezzelarab M, et al. Measurement of anti-CD154 monoclonal antibody in primate sera by competitive inhibition ELISA. Xenotransplantation. 2006;13:566–570. doi: 10.1111/j.1399-3089.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 27.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 28.Bass MC, Gerdes VE, Ten Berge IJ, et al. Treatment with everolimus is associated with a procoagulant state. Thromb Res. 2013;132:307–311. doi: 10.1016/j.thromres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 30.Thompson P, Badell IR, Lowe M, et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11:2593–2602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawthorne WJ, Salvaris EJ, Phillips P, et al. Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. Am J Transplant. 2014;14:1300–1309. doi: 10.1111/ajt.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin JS, Kim JM, Kim JS, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15:2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 33.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21:72–83. doi: 10.1111/xen.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohiuddin MM, Singh AK, Corcoran PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation. 2014;21:35–45. doi: 10.1111/xen.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie JH, Yamniuk AP, Borowski V, et al. Engineering of a novel anti-CD40L domain antibody for treatment of autoimmune diseases. J Immunol. 2014;192:4083–4092. doi: 10.4049/jimmunol.1303239. [DOI] [PubMed] [Google Scholar]


