Abstract
Background
Certain populations with a large proportion of Indigenous American (IA) genetic ancestry may be evolutionarily adapted to traditional diets high in legumes and complex carbohydrates, and may have a detrimental metabolic response to U.S. diets high in refined carbohydrates and added sugars. We tested whether IA ancestry modified the metabolic response to a U.S. versus traditional Mexican diet in a controlled dietary intervention.
Methods
First and second generation Mexican immigrant women (n=53) completed a randomized crossover feeding trial testing the effects of a U.S. versus traditional Mexican diet. The metabolic response to the diets was measured by fasting serum concentrations of glucose, insulin, IGF-1, IGFBP-3, adiponectin, CRP, IL-6, and computed HOMAIR. Blood collected at baseline was used for genotyping and estimation of African, European, and IA ancestries with the use of 214 Ancestry Informative Markers.
Results
The genetic ancestral background was 56% IA, 38% European, and 6% African. Women in the highest IA ancestry tertile (>62%) were shorter in height, less educated and less acculturated to the U.S. lifestyle, and tended to have higher waist-to-hip ratio compared to women in the middle and lowest IA ancestry tertiles, respectively. Compared to the U.S. diet, the traditional Mexican diet tended to reduce glucose, insulin, IGF-1, IGFBP-3, and HOMAIR among women in the middle IA ancestry group (IA ancestry ≤45–62%); while having no effect on biomarkers related to inflammation.
Conclusions
We observed modest interactions between IA ancestry and the metabolic response to a U.S. versus traditional Mexican diet among Mexican immigrant women.
Keywords: Ancestry Informative Markers, Controlled Feeding Trial, Genetic Ancestry, Mexican immigrants, Traditional Mexican diet, U.S. diet
INTRODUCTION
As Mexican immigrants acculturate to the U.S. lifestyle, they tend to transition from consuming traditional Mexican foods to adopting U.S. dietary patterns (1, 2). U.S. diets commonly consumed by the majority of the population are usually high in processed foods, refined carbohydrates, added sugars, and low in plant foods; while traditional Mexican diets are usually high in fruits and vegetables and complex carbohydrates and legumes rich in dietary fiber (2–5). According to the thrifty gene hypothesis (6), populations with a large proportion of Indigenous American (IA) genetic ancestry, may be evolutionarily adapted to diets high in legumes and complex carbohydrates. Consuming an inexpensive and readily available U.S. diet high in processed foods, refined carbohydrates, and added sugars may lead to a detrimental metabolic and/or inflammatory response placing these groups at a disproportionately higher risk of metabolic disease (7–9).
The distribution of genetic ancestry among Mexican immigrants is widely variable, ranging from individuals who are indistinguishable from European ancestry populations to individuals who are indistinguishable from IA populations (e.g., Mayan or Pima) (9–13). In this regard, one of the key questions is the degree to which genetics and environment (e.g., socioeconomic status (SES), diet, and physical activity) contribute to the risk of metabolic disease. Among Mexican immigrants, greater adherence to a U.S. diet has been associated with increased risk of metabolic disease, including, obesity, insulin resistance (IR), systemic inflammation, and breast cancer (3, 5). Whether a greater proportion of IA ancestry in this population modifies the metabolic response to specific dietary patterns kept or adopted by Mexican immigrants has not been previously investigated.
In a randomized controlled feeding trial we found that compared to a U.S. diet, a traditional Mexican diet reduced IR and circulating concentrations of insulin-like growth factors (IGFs) among Mexican immigrant women (14). Building on this research, we aimed to evaluate the interplay between genetic ancestral background and diet-related risk of metabolic disease. We hypothesized that IA genetic ancestral background would modify the metabolic response to a U.S. versus traditional Mexican diet among first and second generation, healthy Mexican immigrant women.
MATERIALS AND METHODS
Subjects and study design
The study participants and study design have been previously described (14). Briefly, 58 healthy, Mexican or Mexican American women (first and second generation), ages 18–45 years, enrolled in the trial. Out of 58 study participants, 53 completed the trial that consisted two 24-day intervention periods, separated by a washout period of 28 days. In one period they consumed a traditional Mexican diet and in the other a typical U.S. diet. The order of the diets was randomized. Exclusion criteria included elevated fasting glucose (≥100 mg/dl), pregnancy, lactation or cessation of menses, BMI <18.5 or >40 kg/m2, smoking, physician-diagnosed disease requiring dietary restrictions or certain medications, or intake of ≥2 alcoholic drinks per day. The Institutional Review Board and Clinical Trials Office of the Fred Hutchinson Cancer Research Center (FHCRC) approved the study and all participants signed written informed consent. The trial was registered at clinicaltrials.gov (identifier: NCT01369173). Participants provided demographic, acculturation, habitual diet and physical activity through self-administered questionnaires and research staff measured height, weight, waist circumference and hip circumference at baseline using standardized protocols (15).
All food and beverages were prepared by the FHCRC Human Nutrition Laboratory. Participants came to the study center three times per week for food pick-ups and body weight measures. Participants were instructed to consume only the foods provided and to return any unconsumed food to study staff. Diets were eucaloric (e.g., diets that provided energy content for weight maintenance) and did not differ in macronutrient composition as a percent of total energy (50% from carbohydrates, 35% from fat, and 15% from protein). Experimental diets differed in foods and beverages such that the traditional Mexican diet included corn tortillas, beans, traditional soups, Mexican-mixed dishes, citrus fruits, vegetables, full-fat milk and Mexican cheeses. The U.S. diet, on the other hand, included processed foods, mixed dishes such as mac and cheese and pizza, refined carbohydrates and added sugars and it was based on the proportion of foods and beverages that contributes the most to Americans daily intake as reported in the National Health and Examination Nutrition Survey (NHANES, 2007–2010) (15). Adherence to controlled diets was carefully monitored and participants’ energy intakes were controlled and adjusted as needed to maintain their weight within 3% of baseline measures.
Sample collection and analyses
Blood was collected on the first day and last day of each intervention period after a 12–hour fast by trained research staff. Specimens were locally processed and stored at −80°C until analyses. Glucose was measured on a Roche Module P chemistry autoanalyzer (Roche Diagnostic Inc., Indianapolis, IN) at the Northwest Lipid Research Laboratories (University of Washington, WA). Insulin was measured using a Tosoh 2000 autoanalyzer (Tosoh Biosciences Inc., South San Francisco, CA) at the Diabetes Endocrinology Research Center Immunoassay Laboratory (University of Washington, WA). The rest of the biomarkers assessments and the genotyping were conducted at the FHCRC Biomarker Core Laboratory and the Molecular Epidemiology Laboratories. Immunoassays were used to measured total adiponectin (Total Adiponectin EIA, Aplco), IGF-1 (Human IGF-I Quantikine ELISA, R&D Systems), IGFBP-3 (Human IGFBP-3 Quantikine ELISA, R&D Systems), and IL-6 (Human IL-6 Quantikine HS ELISA, R&D Systems). CRP was measured using CRP (3)-Wide Range reagent (Kamiya Biomedical Company) on Roche Cobas Mira chemistry analyzer with a high sensitivity protocol. The intra-assay CVs were 0.7%, 7.8%, 1.3%, 1.5%, 1.8%, 2.3%, and 3.3% for glucose, insulin, adiponectin, IGF-1, IGFBP-3, IL-6, and CRP, respectively. The details of specimen collection and analysis have been previously described (14).
Genetic ancestry estimation
DNA was extracted from baseline blood using the Qiagen whole blood kit. Genotypes were collected on the Illumina BeadExpress platform using standard protocols. We used a modified version of the AIMs selection algorithm developed by Galanter et al (16, 17), that allows for a 4-way population admixture model, to select a set of 220 AIMs (Supplemental Table 1). African, European, and Indigenous American (IA) ancestry estimates obtained with the AIMs panel were strongly correlated with genome-wide genotype-based estimates in reference samples (Correlation coefficients of 0.88, 0.95, and 0.96 respectively). It is well known that AIMs panels tend to overestimate the influence of minor ancestral components (16, 18). When evaluating the correlation between estimates for individuals who had more than 10% ancestry from a minor component (as estimated by the genome-wide panel), then the correlation between genome-wide estimates and panel estimates increased greatly (>0.90 correlation for African ancestry proportion).
A panel of 214 of the 220 selected AIMs passed QC on the BeadExpress genotyping platform. In DNA samples passing QC, the call rate for the 214 AIMs analyzed averaged 99.7% +/− 0.3%, with a minimum of 97.5% of genotypes called. After Bonferroni correction for 214 tests, none of the AIMs showed significant departure from Hardy Weinberg Equilibrium in either reference ancestral population (669 reference European samples or 131 reference Native American samples). Ancestry was estimated using the ADMIXTURE software package v1.23 (19). The ADMIXTURE algorithm was primed with five EM steps at K=3 populations, and converged rapidly. Bootstrap replication using the default 200 bootstrap replicates yielded a standard error of less than 4% for each ancestry component within an individual. Reference populations were included in the analysis to anchor the inferred ancestries on nominally un-admixed individuals (K=3 ancestral populations). These reference populations included HapMap reference panels (YRI, ASW, HCB, JPT and MXC populations), and indigenous populations from the Americas (Nahua, Quechua, Aymara, Zapoteca, Tepehuano, and Maya) (16).
Statistical Analyses
The power analysis for the detection of the interaction of diet and ancestry was conducted using Quanto (version 1.2.4; 2009; University of Southern California, CA), and the coefficient of the interaction was on the scale of standard deviations of the biomarker measurements per unit increase in the ancestry measurement. Based on the calculation, we would have 80% power to detect changes of 2 standard deviations in the biomarkers. Indigenous American (IA) ancestry tertiles were created as: ≤45% IA ancestry (lowest), >45 to ≤62% IA ancestry (middle), and >62% IA ancestry (highest), respectively. Natural logarithmic transformation was applied to insulin, HOMAIR, adiponectin, hs-CR and IL-6 biomarker concentrations to achieve approximate normality. General linear models (unadjusted) were used to compare means of demographic and baseline characteristics across IA ancestry tertiles for continuous variables and chi-squared tests for categorical variables. General linear models adjusted for age, acculturation and BMI were used to compare baseline biomarker concentrations across IA ancestry tertiles, with the Duncan multiple range tests post hoc whenever the overall test indicated a statistically significant difference between IA ancestry tertiles. Linear mixed models were used to test the effect modification of ancestry on the metabolic response to the U.S. versus traditional Mexican diet, including participant as a random effect while treating diet sequence, feeding period, baseline and washout biomarker concentrations, age, acculturation, and BMI, ancestry (as continuous variable) and the interaction between ancestry and diet variables as fixed effects. Also, linear mixed effects models including participants as random effect and diet sequence, feeding period, baseline and washout biomarker concentrations, age, acculturation, and BMI as fixed effects were used to investigate the biomarkers responses to the diet intervention within each category of IA ancestry tertiles. We examined the presence of potential carry-over effects with the inclusion of the diet sequence variable (in addition to other variables including diet and feeding period) as a fixed effect in the mixed effects models. This variable (sequence) was not found to be significantly associated with the biomarkers responses to the diet intervention and hence, no carry-over effect was detected in our analysis. This might be related to the wash-out period of 28 days between each diet period being probably appropriate enough to minimize the carry-over effects. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC), all tests were two-sided and P values <0.05 were considered statistically significant.
RESULTS
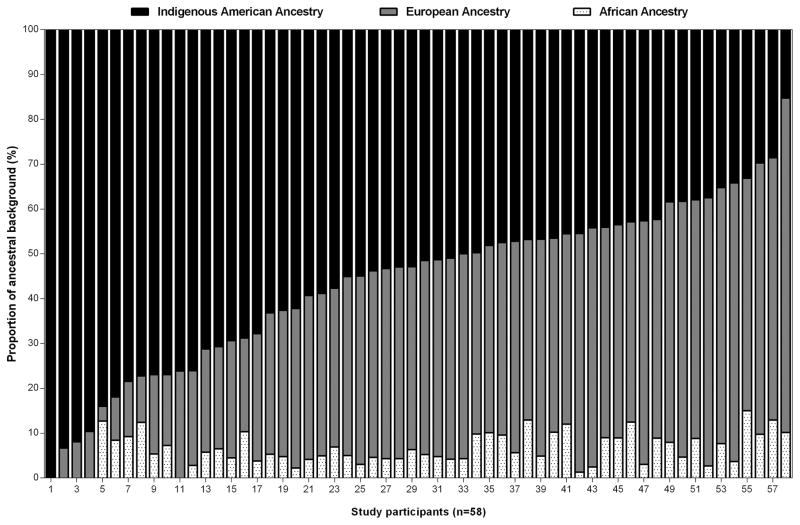
We first examined the overall distribution of African, European, and IA ancestry. In this sample of 58 healthy, first and second generation Mexican immigrant women, the overall distribution of ancestry was 56% IA, 38% European, and 6% African (Figure 1). We then examined the distribution of demographic characteristics and baseline measures for the women who completed the trial (n=53) across IA ancestry tertiles: 45% IA ancestry (lowest), >45 to 62% IA ancestry (middle), and >62% IA ancestry (highest), respectively (Table 1). Women in the highest IA ancestry tertile (>62%) were shorter in height (P<0.05), less educated (P<0.05), and less acculturated to the U.S. lifestyle (P<0.05), and tended to have higher waist-to-hip ratio (P=0.07) compared to women in the middle and lowest IA ancestry tertiles, respectively.
FIGURE 1.
Genetic ancestral background composition for 58 healthy, first and second generation Mexican immigrant women from three populations – Indigenous American (IA), European, and African – with the use of 214 Informative Ancestry Markers (AIMs)
TABLE 1.
Demographic characteristics of 53 healthy, first and second generation Mexican immigrant women across Indigenous American (IA) ancestry tertiles
| IA ancestry tertiles
|
||||
|---|---|---|---|---|
| Lowest IA ≤ 45% (n = 18) |
Middle IA > 45 to ≤62% (n = 17) |
Highest IA > 62% (n = 18) |
P value1 | |
|
| ||||
| Baseline characteristics | N (%) or Mean ± SD | |||
| Age, years | 27 ± 8 | 27 ± 10 | 28 ± 7 | 0.8 |
| Weight, kg | 67.6 ± 11.5 | 65.7 ± 14.0 | 66.2 ± 10.8 | 0.8 |
| Height, cm | 163 ± 5a | 159 ± 6b | 157 ± 6b | 0.005 |
| Body mass index, kg/m2 | 25.4 ± 4.8 | 25.9 ± 5.7 | 26.8 ± 3.9 | 0.6 |
| BMI categories | 0.2 | |||
| Normal weight: ≥18.2–24.9 | 11 (61) | 10 (59) | 6 (33) | |
| Overweight: ≥25–29.9 | 4 (22) | 3 (18) | 9 (50) | |
| Obese: ≥30.0 | 3 (17) | 4 (23) | 3 (17) | |
| Waist circumference, cm | 83.8 ± 11.2 | 81.3 ± 13.8 | 86.8 ± 11.2 | 0.4 |
| Hip circumference, cm | 102 ± 8 | 100 ± 12 | 102 ± 9 | 0.7 |
| Waist to hip ratio (WHR) | 0.82 ± 0.06a | 0.81 ± 0.07a | 0.85 ± 0.06b | 0.07 |
| Education | 0.02 | |||
| High school diploma | 1 (6) | 3 (19) | 7 (47) | |
| Some college or ≥ college | 16 (94) | 13 (81) | 8 (53) | |
| Marital Status | 0.2 | |||
| Married | 4 (22) | 5 (29) | 8 (47) | |
| Single | 14 (78) | 12 (71) | 8 (24) | |
| Employment Status | 0.05 | |||
| Employed | 11 (61) | 7 (41) | 8 (50) | |
| Full time student | 6 (33) | 10 (59) | 4 (25) | |
| Unemployed | 1 (6) | 0 (0) | 4 (25) | |
| Place of birth | 0.2 | |||
| Mexico | 12 (67) | 8 (47) | 13 (72) | |
| United States | 6 (33) | 9 (53) | 5 (28) | |
| Language spoken | 0.06 | |||
| Spanish | 9 (53) | 4 (24) | 11 (61) | |
| English | 8 (47) | 13 (76) | 7 (39) | |
| Language Thought | 0.3 | |||
| Spanish | 1 (6) | 2 (12) | 4 (22) | |
| English | 17 (94) | 15 (88) | 14 (78) | |
| Ethnic identity | 0.2 | |||
| Mexican | 11 (61) | 9 (53) | 14 (78) | |
| Mexican American | 7 (39) | 8 (47) | 4 (22) | |
| Acculturation score | 1.6 ± 1.7a | 2.4 ± 1.5b | 1.1 ± 1.4a | 0.04 |
General linear models for continuous variables and chi-squared tests for categorical variables
Labeled means in a row with differing superscripts letters are significantly different from one another, using general linear models with the Duncan multiple range test (P<0.05).
Table 2 shows the baseline (pre-intervention) serum fasting concentrations of biomarkers of metabolic disease risk across IA ancestry tertiles. Women in the highest IA ancestry tertile (>62%) tended to have lower circulating concentrations of IGF-1 (P=0.09), and significantly lower circulating concentrations of IGFBP-3 compared to women in the middle and lowest IA ancestry tertiles, respectively (P<0.05). There was no association of ancestry with the IGF-1/IGFBP-3 ratio and baseline serum concentrations of adiponectin, CRP and IL-6.
TABLE 2.
Baseline serum concentrations of biomarkers of disease risk across Indigenous American (IA) ancestry tertiles
| IA ancestry tertiles
|
|||||
|---|---|---|---|---|---|
| All study participants (n = 53) | Lowest IA ≤ 45% (n = 18) |
Middle IA > 45 to ≤62% (n = 17) |
Highest IA > 62% (n = 18) |
||
|
| |||||
| Biomarkers | Mean ± SEM or Geometric Mean (95% CI) | P value1 | |||
| Glucose,2 mg/dL | 91.9 ± 7.1 | 91.3 ± 7.2 | 89.6 ± 7.9 | 94.6 ± 5.3 | 0.1 |
| Insulin,3 μU/mL | 9.9 (8.6, 11.3) | 9.5 (7.7, 12) | 9.6 (7.7, 12) | 10.5 (8.4, 13) | 0.8 |
| HOMAIR3 | 2.2 (1.9, 2.6) | 2.2 (1.7, 2.7) | 2.1 (1.7, 2.7) | 2.4 (1.9, 3.1) | 0.6 |
| IGF-1,2 ng/mL | 149 ± 45 | 162 ± 53a | 149 ± 55a | 136 ± 41b | 0.09 |
| IGFBP-3,2 ng/mL | 2430 ± 440 | 2600 ± 426a | 2423 ± 451b | 2265 ± 402b | 0.02 |
| IGF-1/ IGFBP-3 | 6.1 ± 1.4 | 6.3 ± 1.3 | 6.1 ± 1.7 | 5.9 ± 1.1 | 0.7 |
| Adiponectin,3 μg/mL | 6.9 (6.3, 7.6) | 7.5 (6.4, 8.9) | 6.6 (5.5, 7.8) | 6.6 (5.6, 7.8) | 0.4 |
| hs-CRP,3 mg/L | 0.9 (0.6, 1.2) | 0.6 (0.4, 0.9) | 1.2 (0.7, 2.0) | 0.9 (0.6, 1.4) | 0.1 |
| IL-6,3 pg/mL | 1.4 (1.2, 1.6) | 1.2 (0.9, 1.5) | 1.5 (1.2, 2.0) | 1.5 (1.1, 1.9) | 0.3 |
Abbreviations: HOMAIR, homeostasis model assessment for insulin resistance; hs-CRP, high-sensitivity C-reactive protein; IA, Indigenous American Ancestry IGF-1, insulin-like growth factor-1; IGFBP-3, IGF-binding protein-3; IL-6, interleukin-6.
General linear regression models were adjusted for age, acculturation, and BMI
Mean ± SEM
Geometric means (95% CI)
Labeled means in a row with differing superscripts letters are significantly different from one another, using general linear models with the Duncan multiple range test (P<0.05).
Results testing whether IA ancestry modified of the exploratory analysis on the metabolic response to the controlled intervention diets (U.S. versus traditional Mexican diet) stratified by IA ancestry tertile are shown in Table 3. Overall, the effect modification associations observed were modest. Compared to the U.S. diet, the traditional Mexican diet tended to reduce glucose (P=0.08), and insulin concentrations (P<0.05) among women in the middle IA ancestry tertile, and tended to reduce insulin concentrations among women in the lowest IA ancestry tertile (P=0.06). Similarly, compared to the U.S. diet, the traditional Mexican diet significantly reduced HOMAIR (P<0.05) and circulating concentrations of IGFBP-3 (P<0.01) and, while tended to reduce IGF-1 (P=0.06) among women in the middle IA ancestry tertile. We found no significant effect of effect modification of ancestry in the response to the diet intervention diets for IGF-1/IGFBP-3 ratio, and serum concentrations of CRP and IL-6 in any of the IA ancestry strata. Lastly, compared to the U.S. diet, the traditional Mexican diet tended to increase adiponectin concentrations among women in the middle IA ancestry tertile (P=0.07). There was no statistically significant difference in the biomarkers response to the diet intervention among the IA strata. In additional analyses, a cross-product interaction term of IA ancestry (as continuous variable) and diet treatment (U.S. vs. Mexican) were tested in adjusted linear mixed models that also included the main effect variables but we found no statistically significant interaction for any of the biomarkers examined (data not shown).
TABLE 3.
Metabolic response to controlled U.S. versus traditional Mexican diet on serum biomarkers of disease risk stratified by Indigenous American (IA) ancestry tertiles as determined by Ancestry Informative Markers (AIMs)1
| Biomarkers | n | U.S. diet | n | Traditional Mexican diet | Mean Diff. | P for diet effect within tertiles2 |
|---|---|---|---|---|---|---|
| Glucose,3 mg/dL | ||||||
| Lowest IA ancestry ≤45% | 16 | 87 ± 1.3 | 16 | 88 ± 1.4 | −1.6 | 0.30 |
| Middle IA ancestry >45 to ≤62% | 18 | 90 ± 1.9 | 18 | 85 ± 2.0 | 4.0 | 0.08 |
| Highest IA ancestry >62% | 17 | 93 ± 1.8 | 18 | 91 ± 1.8 | 1.6 | 0.13 |
| Insulin,4 μU/mL | ||||||
| Lowest IA ancestry ≤45% | 16 | 8.8 (7.1, 11) | 16 | 7.7 (6.2, 9.6) | 1.1 | 0.06 |
| Middle IA ancestry >45 to ≤62% | 18 | 10.5 (8.8, 12.6) | 18 | 7.4 (6.2, 8.9) | 3.1 | 0.01 |
| Highest IA ancestry >62% | 17 | 8.8 (7.3, 10.7) | 18 | 8.6 (7.2, 10.3) | 0.2 | 0.82 |
| HOMAIR4 | ||||||
| Lowest IA ancestry ≤45% | 16 | 1.9 (1.5, 2.3) | 16 | 1.7 (1.4, 2.1) | 0.2 | 0.12 |
| Middle IA ancestry >45 to ≤62% | 18 | 2.3 (1.9, 2.9) | 18 | 1.5 (1.3, 1.9) | 0.8 | 0.01 |
| Highest IA ancestry >62% | 17 | 2.0 (1.6, 2.5) | 18 | 1.9 (1.6, 2.3) | 0.08 | 0.74 |
| IGF-1,3 ng/mL | ||||||
| Lowest IA ancestry ≤45% | 16 | 160 ± 6.0 | 16 | 160 ± 5.5 | -0.07 | 0.98 |
| Middle IA ancestry >45 to ≤62% | 18 | 152 ± 4.0 | 18 | 142 ± 4.2 | 10.3 | 0.06 |
| Highest IA ancestry >62% | 17 | 135 ± 4.6 | 18 | 130 ± 4.2 | 5.2 | 0.40 |
| IGFBP-3,3 ng/mL | ||||||
| Lowest IA ancestry ≤45% | 16 | 2577 ± 65 | 16 | 2468 ± 67 | 109 | 0.25 |
| Middle IA ancestry >45 to ≤62% | 18 | 2455 ± 52 | 18 | 2327 ± 54 | 128 | 0.004 |
| Highest IA ancestry >62% | 17 | 2255 ± 54 | 18 | 2166 ± 50 | 85 | 0.26 |
| IGF-1/ IGFBP-3 | ||||||
| Lowest IA ancestry ≤45% | 16 | 6.3 ± 0.2 | 16 | 6.6 ± 0.2 | −0.3 | 0.21 |
| Middle IA ancestry >45 to ≤62% | 18 | 6.1 ± 0.5 | 18 | 6.1 ± 0.2 | 0.05 | 0.76 |
| Highest IA ancestry >62% | 17 | 5.8 ± 0.2 | 18 | 5.9 ± 0.2 | −0.1 | 0.61 |
| Adiponectin,4 μg/mL | ||||||
| Lowest IA ancestry ≤45% | 16 | 7.2 (6.7, 7.7) | 16 | 7.0 (6.5, 7.6) | 14.7 | 0.58 |
| Middle IA ancestry >45 to ≤62% | 18 | 6.4 (5.8, 7.0) | 18 | 7.2 (6.6, 7.9) | −8.2 | 0.07 |
| Highest IA ancestry >62% | 17 | 6.3 (5.8, 6.8) | 18 | 6.1 (5.6, 6.5) | 2.1 | 0.34 |
| hs-CRP,4 mg/L | ||||||
| Lowest IA ancestry ≤45% | 16 | 0.5 (0.4, 0.8) | 16 | 0.5 (0.4, 0.8) | −0.002 | 0.98 |
| Middle IA ancestry >45 to ≤62% | 18 | 1.2 (0.6, 2.4) | 18 | 1.0 (0.5, 2.1) | 0.2 | 0.71 |
| Highest IA ancestry >62% | 17 | 1.2 (0.8, 1.9) | 18 | 1.4 (0.9, 2.1) | −0.2 | 0.61 |
| IL-6,4 pg/mL | ||||||
| Lowest IA ancestry ≤45% | 16 | 1.1 (0.9, 1.4) | 16 | 1.2 (0.9, 1.5) | −0.1 | 0.51 |
| Middle IA ancestry >45 to ≤62% | 18 | 1.5 (1.1, 2.2) | 18 | 1.4 (1.0, 1.9) | 0.2 | 0.64 |
| Highest IA ancestry >62% | 17 | 1.6 (1.3, 1.9) | 18 | 1.4 (1.1, 1.7) | 0.2 | 0.36 |
Abbreviations: HOMAIR, homeostasis model assessment for insulin resistance; hs-CRP, high-sensitivity C-reactive protein; IA, Indigenous American Ancestry IGF-1, insulin-like growth factor-1; IGFBP-3, IGF-binding protein-3; IL-6, interleukin-6.
All regression models were adjusted for baseline and washout biomarker concentrations, diet sequence, feeding period, age, acculturation and BMI.
P values are from linear mixed models testing the intervention effect of the U.S. versus Traditional Mexican diet within IA ancestry tertiles.
Mean ± SEM
Geometric means (95% CI)
DISCUSSION
In this randomized, crossover feeding trial among first and second generation, healthy Mexican immigrant women, we found a modest effect modification by IA ancestry in the metabolic response to the U.S. versus traditional Mexican diet among women in the middle IA ancestry tertile (>45 to ≤62%). Further, IA ancestry was associated with several baseline demographic and anthropometric characteristics, as well as baseline circulating concentrations of IGF-1 and IGFBP-3, independent of age, BMI, and acculturation status.
In cross-sectional analyses of baseline measures (pre-intervention), we found that women with greater IA ancestry tended to have higher adiposity (waist-to-hip ratio), were more likely to be less educated and less acculturated to the U.S. lifestyle compared to women with greater European ancestry, consistent with previous findings (12, 13, 20). Greater IA ancestry tended to be associated with baseline waist-to-hip ratio, but not with BMI, as previously reported among Hispanic women (12, 13, 21) suggesting a greater contribution of visceral fat to the risk of metabolic disease in this ethnic group. Of particular interest is our finding of an inverse association between IA ancestry and baseline circulating concentrations of IGF-1 and IGFBP-3. Insulin-like growth factors (IGFs) are peptides known to promote cellular proliferation of normal breast cells, and therefore, high circulating concentrations of IGF-1 and IGFBP-3 are associated with increased risk of breast cancer (22–24). This is in agreement with studies by Fejerman et al, in which greater European versus IA ancestry among women of Mexican descent was associated with increased risk of breast cancer (25, 26).
We previously showed that compared to the U.S. diet, the traditional Mexican diet improved insulin sensitivity and reduced circulating concentrations of IGF-1 and IGFBP-3 (14). Building upon these findings, in the present study we found a modest effect modification of IA ancestry in relation to the metabolic response to the intervention diets. This interaction seemed strongest for insulin sensitivity biomarkers and IGFs and only among women in the middle IA ancestry tertile. These findings suggest that genetic ancestral background may play a role in the metabolic response to specific dietary patterns kept or adopted by Mexican immigrants that can lead to future risk of diabetes. Consistent with our results, in a large observational study evaluating the association between genetic ancestry and risk of diabetes in a multi-ethnic cohort of postmenopausal women (n=16,476) who participated in the Women’s Health Initiative, it was found that among Hispanic women, greater IA ancestry was associated with increased risk of diabetes (13). However, since our diet-ancestry findings in this report are limited to women in the middle IA ancestry tertile, it is possible that other factors underlie the observed associations. For example, women in the middle IA ancestry tertile had lower waist-to-hip ratio (non-statistically significant), and were more likely to have more education (P=0.02) and to be more acculturated to the U.S. lifestyle (P=0.04) compared to women in the lowest (≤45%) or highest IA ancestry tertiles (>62%), respectively. It is possible that these differences in SES (higher education status) influence the ancestry-metabolic response association in a manner that could not be captured using these standard methods. Similar results for differences in SES have been shown to be strongly associated with admixture proportions and disease risk in Hispanic populations. For example, in several studies among Hispanics evaluating the association between ancestry and disease risk, adjustments for SES significantly attenuated these associations (13, 27, 28), and in some cases the association become non-significant after adjustments for SES (29).
We found no effect modification of IA ancestry in response to the intervention diets for the inflammatory biomarkers examined, including CRP and IL-6. Although, there was a non-statistically significant increase in adiponectin levels (P=0.07) in the Mexican vs US diet but only among women in the middle IA ancestry group. It is possible that the conditions of weight stability in our study played an important role in the null results. Similar to our results and under conditions of weight stability, others have found no diet-induced effect on inflammatory biomarkers, including CRP and adiponectin (30, 31). On the other hand, others have being able to demonstrate a diet-induced inflammatory response in dietary interventions that were coupled with weight loss. These findings suggests that diet-related changes in inflammatory profiles are greater when coupled with weight loss, in part, due to the strong association between adiposity and inflammation (32, 33).
Our study is not without limitations. The modest sample size may have precluded us from finding a more robust effect modification of genetic ancestry in relation to the metabolic response to the intervention diets. Despite this limitation, a novel contribution of the present study is the use of AIMs to better understand the interplay between genetic ancestral background and diet-related risk of metabolic disease in a population that displays a great degree of genetic admixture.
In conclusion, we observed a modest interaction between IA ancestry and the metabolic response to a U.S. versus traditional Mexican diet among women in the middle IA ancestry tertile, who were also more educated and acculturated to the U.S. when compared to their counterparts. Future experimental and longitudinal studies evaluating the extent by which IA ancestry plays a role in diet-related risk of metabolic disease will be necessary to confirm these results.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute (NCI) grant P50 CA148143 and the Cancer Center Support Grant P30CA 015704. M. Santiago-Torres is currently supported by grant R25 CA094880 from the NCI.
Abbreviations
- AIMs
Ancestry Informative Markers
- CRP
C-reactive protein
- HOMAIR
homeostasis model assessment of insulin resistance
- IA
Indigenous American
- IGF-1
insulin-like growth factor-1
- IGFBP-3
insulin-like growth factor binding protein-3
- IL-6
interleukin-6
- NHW
non-Hispanic white
- SES
socio-economic status
- T2D
type 2 diabetes
- WC
waist circumference
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Author contributions: C.S.C., M.L.N., and J.W.L.; K.L.B., L.L., and A.V. designed the experimental diets; K.L.B., L.L., X.S. implemented the study protocol; C.S.C., M.S.T., J.D.T., and C.Y.W analyzed data; M.S.T. wrote the paper; M.S.T., C.S.C., and M.L.N. had primary responsibility for final content; and all co-authors critically reviewed and revised the manuscript.
Conflict of Interest: The authors declare that they have no conflict of interest
The trial was registered at clinicaltrials.gov (identifier: NCT01369173)
References
- 1.Batis C, Hernandez-Barrera L, Barquera S, Rivera JA, Popkin BM. Food Acculturation Drives Dietary Differences among Mexicans, Mexican Americans, and Non-Hispanic Whites. J Nutr. 2011;141:1898–906. doi: 10.3945/jn.111.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuhouser ML, Thompson B, Solomon CC. Higher fat intake and lower fruit and vegetables intakes are associated with greater acculturation among Mexicans living in Washington state. J Am Diet Assoc. 2004;104:51–7. doi: 10.1016/j.jada.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Santiago-Torres M, Tinker LF, Allison MA, Breymeyer KL, Garcia L, Kroenke CH, et al. Development and Use of a Traditional Mexican Diet Score in Relation to Systemic Inflammation and Insulin Resistance among Women of Mexican Descent. J Nutr. 2015;145:2732–40. doi: 10.3945/jn.115.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtaugh MA, Herrick JS, Sweeney C, Baumgartner KB, Guiliano AR, Byers T, et al. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J Am Diet Assoc. 2007;107:1311–21. doi: 10.1016/j.jada.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Murtaugh MA, Sweeney C, Giuliano AR, Herrick JS, Hines L, Byers T, et al. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr. 2008;87:978–84. doi: 10.1093/ajcn/87.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neel JV. Diabetes mellitus: A "thrifty" genotype rendered detrimental by "progress"? B World Health Organ. 1999;77:694–703. [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon-Larsen P, Harris KM, Ward DS, Popkin BM National Longitudinal Study of Adolescent H. Acculturation and overweight-related behaviors among Hispanic immigrants to the US: the National Longitudinal Study of Adolescent Health. Social science & medicine. 2003;57:2023–34. doi: 10.1016/s0277-9536(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 8.Van Rompay MI, McKeown NM, Castaneda-Sceppa C, Falcon LM, Ordovas JM, Tucker KL. Acculturation and Sociocultural Influences on Dietary Intake and Health Status among Puerto Rican Adults in Massachusetts. J Acad Nutr Diet. 2012;112:64–74. doi: 10.1016/j.jada.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casazza K, Hanks LJ, Beasley TM, Fernandez JR. Beyond Thriftiness: Independent and Interactive Effects of Genetic and Dietary Factors on Variations in Fat Deposition and Distribution Across Populations. Am J Phys Anthropol. 2011;145:181–91. doi: 10.1002/ajpa.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins-Schramm HE, Chima B, Morii T, Wah K, Figueroa Y, Criswell LA, et al. Mexican American ancestry-informative markers: Examination of population structure and marker characteristics in European Americans, Mexican Americans, Amerindians and Asians. Hum Genet. 2004;114:263–71. doi: 10.1007/s00439-003-1058-6. [DOI] [PubMed] [Google Scholar]
- 11.Qu HQ, Li Q, Lu Y, Hanis CL, Fisher-Hoch SP, McCormick JB. Ancestral Effect on HOMA-IR Levels Quantitated in an American Population of Mexican Origin. Diabetes Care. 2012;35:2591–3. doi: 10.2337/dc12-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassir R, Qi L, Kosoy R, Garcia L, Allison M, Ochs-Balcom HM, et al. Relationship between adiposity and admixture in African-American and Hispanic-American women. Int J Obesity. 2012;36:304–13. doi: 10.1038/ijo.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi L, Nassir R, Kosoy R, Garcia L, Curb JD, Tinker L, et al. Relationship between diabetes risk and admixture in postmenopausal African-American and Hispanic-American women. Diabetologia. 2012;55:1329–37. doi: 10.1007/s00125-012-2486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santiago-Torres M, Kratz M, Lampe JW, Tapsoba JD, Breymeyer K, Levy L, et al. Metabolic responses to a traditional Mexican compared with a commonly consumed US diet in women of Mexican descent: A randomized crossover feeding trial. Am J Clin Nutr. 2016;103:1–9. doi: 10.3945/ajcn.115.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norgan NG. Anthropometric Standardization Reference Manual - Lohman,Tg, Roche,Af, Martorell,R. Ergonomics. 1988;31:1493–4. [Google Scholar]
- 16.Galanter JM, Fernandez-Lopez JC, Gignoux CR, Barnholtz-Sloan J, Fernandez-Rozadilla C, Via M, et al. Development of a panel of genome-wide ancestry informative markers to study admixture throughout the Americas. PLoS Genet. 2012;8:e1002554. doi: 10.1371/journal.pgen.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daya M, van der Merwe L, Galal U, Moller M, Salie M, Chimusa ER, et al. A panel of ancestry informative markers for the complex five-way admixed South African coloured population. PLoS One. 2013;8:e82224. doi: 10.1371/journal.pone.0082224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avena S, Via M, Ziv E, Perez-Stable EJ, Gignoux CR, Dejean C, et al. Heterogeneity in genetic admixture across different regions of Argentina. PLoS One. 2012;7:e34695. doi: 10.1371/journal.pone.0034695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lins TC, Pires AS, Paula RS, Moraes CF, Vieira RG, Vianna LG, et al. Association of serum lipid components and obesity with genetic ancestry in an admixed population of elderly women. Genet Mol Biol. 2012;35:575. doi: 10.1590/S1415-47572012005000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fejerman L, Stern MC, John EM, Torres-Mejia G, Hines LM, Wolff RK, et al. Interaction between Common Breast Cancer Susceptibility Variants, Genetic Ancestry, and Nongenetic Risk Factors in Hispanic Women. Cancer Epidemiol Biomarkers Prev. 2015;24:1731–38. doi: 10.1158/1055-9965.EPI-15-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldi S, Biessy C, Hernandez M, Lesueur F, dos-Santos-Silva I, Rice MS, et al. Circulating concentrations of insulin-like growth factor-I, insulin-like growth factor-binding protein-3, genetic polymorphisms and mammographic density in premenopausal Mexican women: Results from the ESMaestras cohort. Int J Cancer. 2014;134:1436–44. doi: 10.1002/ijc.28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood TL, Yee D. IGFs and IGFBPs in the normal mammary gland and in breast cancer. J Mammary Gland Biol. 2000;5:1–5. doi: 10.1023/a:1009580913795. [DOI] [PubMed] [Google Scholar]
- 25.Fejerman L, Romieu I, John EM, Lazcano-Ponce E, Huntsman S, Beckman KB, et al. European Ancestry Is Positively Associated with Breast Cancer Risk in Mexican Women. Cancer Epidemiol Biomarkers Prev. 2010;19:1074–82. doi: 10.1158/1055-9965.EPI-09-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fejerman L, Hu DL, Huntsman S, John EM, Stern MC, Haiman CA, et al. Genetic Ancestry and Risk of Mortality among US Latinas with Breast Cancer. Cancer Res. 2013;73:7243–53. doi: 10.1158/0008-5472.CAN-13-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florez JC, Price AL, Campbell D, Riba L, Parra MV, Yu F, et al. Strong association of socioeconomic status with genetic ancestry in Latinos: implications for admixture studies of type 2 diabetes. Diabetologia. 2009;52:1528–36. doi: 10.1007/s00125-009-1412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Marignac VL, Valladares A, Cameron E, Chan A, Perera A, Globus-Goldberg R, et al. Admixture in Mexico City: implications for admixture mapping of type 2 diabetes genetic risk factors. Hum Genet. 2007;120:807–19. doi: 10.1007/s00439-006-0273-3. [DOI] [PubMed] [Google Scholar]
- 29.Piccolo RS, Pearce N, Araujo AB, McKinlay JB. The contribution of biogeographical ancestry and socioeconomic status to racial/ethnic disparities in type 2 diabetes mellitus: results from the Boston Area Community Health Survey. Ann Epidemiol. 2014;24:648–54. doi: 10.1016/j.annepidem.2014.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuhouser ML, Schwarz Y, Wang CC, Breymeyer K, Coronado G, Wang CY, et al. A Low-Glycemic Load Diet Reduces Serum C-Reactive Protein and Modestly Increases Adiponectin in Overweight and Obese Adults. J Nutr. 2012;142:369–74. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS. Effect of dietary n-3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr. 2008;87:347–53. doi: 10.1093/ajcn/87.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 33.Festa A, D'Agostino R, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obesity. 2001;25:1407–15. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.