Abstract
Background
Recent reports have demonstrated that binge-like ethanol drinking leads to an increase in hypothalamic orexin (OX) signaling and that suppressing this signaling via systemic administration of an orexin receptor (OXR) antagonist blocks this behavior; however, the specific OX pathways that modulate this behavior remain unknown. The goal of the present study was to further elucidate the role of the OX system in binge-like ethanol drinking using behavioral, molecular, and pharmacological techniques.
Methods
The drinking-in-the-dark (DID) paradigm was used to model binge-like drinking behavior in male C57BL/6J mice. Experiment 1 examined changes in the OX precursor, prepro-orexin, within the hypothalamus following multiple cycle ethanol or sucrose DID using polymerase chain reaction (PCR) analysis. In experiments 2a & 2b, we used site-directed infusion of an OXR antagonist to examine the individual contribution of each OXR subtype within the ventral tegmental area (VTA) and central amygdala (CeA), repectively, in binge-like ethanol or sucrose drinking.
Results
Findings from our PCR study revealed that multiple cycles of binge-like ethanol drinking did not lead to changes in prepro-orexin mRNA as a function of binge-like ethanol drinking. However, data from site-directed pharmacology studies indicate that the OX1R is the predominate receptor subtype within the VTA and CeA that regulates binge-like ethanol drinking. Interestingly, inhibition of OX1Rs did not affect binge-like sucrose intake, which suggests that these OX circuits are specific for ethanol consumption.
Conclusions
As a whole, these data suggest that the VTA and CeA are important regions in which OX regulates binge-like ethanol drinking behavior. Moreover, these findings identify OXR antagonists as a potential treatment option that may be used to ameliorate problematic drinking behavior while leaving responding to natural rewards relatively intact.
Keywords: binge ethanol drinking, drinking in the dark, orexin, hypocretin
1. Introduction
The two peptides of the orexin (OX) system, orexin-A and orexin-B, are cleaved from the precursor, prepro-orexin (de Lecea et al., 1998; Sakurai et al., 1998). Neurons that produce OX are located exclusively within the hypothalamus and send widespread projections throughout the brain where OX peptide released from these fibers act on two receptors, the orexin-1 (OX1R) and orexin-2 receptor (OX2R), to influence a wide array of neurobiological functions. Previous pharmacological studies have shown that signaling onto OXRs plays a role in moderate-level ethanol intake such that ethanol drinking parallels OX signaling as agonists increase ethanol consumption (Barson et al., 2015; Schneider et al., 2007) while antagonists reduce drinking (Jupp et al., 2011; Lawrence et al., 2006; Moorman and Aston-Jones, 2009; Srinivasan et al., 2012); however, its role in excessive ethanol intake remains relatively unexplored.
We and others have recently demonstrated that the OX system participates in binge-like ethanol drinking behavior. Indeed, repeated episodes of binge-like ethanol drinking produce reduced levels of orexin-A protein within the lateral hypothalamus (LH) consistent with increased release from LH neurons. These results suggest that binge-like ethanol drinking is associated with increased signaling within the OX system (Olney et al., 2015). Moreover, multiple reports have shown that blocking this increase in signaling via peripheral administration of an OX1R or OX2R antagonist disrupts binge-like consumption of ethanol, sucrose, or saccharin (Alcaraz-Iborra et al., 2014; Anderson et al., 2014; Olney et al., 2015). Together, these data indicate that (1) orexin-A is released from hypothalamic orexigenic neurons during binge-like ethanol drinking and (2) suppressing signaling onto OXRs attenuates this behavior. These data, however, were unable to identify the locus of this effect.
Although neurons that synthesize OX peptide are restricted to the hypothalamus, orexinergic fibers project extensively throughout the brain. Among this litany of brain regions that receive hypothalamic OX input are the ventral tegmental area (VTA) and central nucleus of the amygdala (CeA; Ch’ng and Lawrence, 2015; Nambu et al, 1999; Peyron et al, 1998; Schmitt et al, 2012). Accordingly, these structures also express both OXR subtypes (Cluderay et al., 2002; Marcus et al., 2001; Narita, 2006) and application of OX causes robust depolarization of neurons in these regions (Bisetti et al., 2006; Korotkova et al., 2003). Importantly, both the VTA and CeA are involved in ethanol drinking behavior as ethanol has been demonstrated to engage both regions (Bachtell and Ryabinin, 2001; Gessa et al., 1985; McBride et al., 2010; Pandey et al., 2008). Moreover, pharmacological manipulations of the VTA (Melón and Boehm, 2011; Moore and Boehm, 2009) or CeA (Lowery-Gionta et al, 2012; Rinker et al, In Press) disrupts binge-like ethanol drinking. As a whole, these findings reveal the VTA and CeA as probable sites where OX acts to modulate binge-like ethanol drinking behavior.
The current set of experiments were designed to further elucidate the role of the OX system in binge-like ethanol drinking. Using polymerase chain reaction (PCR), we examined the effect of repeated cycles of binge-like ethanol drinking on hypothalamic prepro-orexin mRNA expression; results, coupled with previous immunohistochemistry data, are consistent with increased OX signaling stemming from binge-like ethanol consumption. Furthermore, using site-directed pharmacological techniques, in tandem with OX compounds selective for either the OX1R or OX2R, we sought to characterize the contribution of each receptor subtype within the VTA and CeA. Our results indicate the OX1R is the principal OXR subtype that selectively modulates binge-like ethanol consumption in these regions.
2. Materials and Methods
Animals
Each of the following experiments used male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), aged 6–7 weeks and weighing 20–25 g upon arrival. Mice were individually housed in cages located in a vivarium with an ambient temperature of approximately 22°C and a reverse light/dark cycle (lights off at 8:30 am). Animals in the current study were used in only one experiment and were not reused between experiments- that is, mice from experiment 2a were not later reused in experiment 1 or 2b and vice-versa. Refer to Table S1 for a comprehensive list of the number of animals used in each experiment. All procedures used were in accordance with the National Institute of Health guidelines and were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Experiment 1
To determine whether multiple cycles of binge-like ethanol drinking causes alterations in the OX system, mice experienced one or three cycles of drinking-in-the-dark (DID) consuming either ethanol, sucrose, or water. The DID procedure is a preclinical model of binge-like ethanol drinking we have used previously to assess changes in the OX system following excessive ethanol consumption (Olney et al., 2015). In order to maintain consistency with these previous experiments, which assessed OX protein levels, our DID procedures used in Experiment 1 were identical to those we used previously. Briefly, animals were given 2-hr access to test bottles containing either ethanol (20% v/v) or sucrose (3% w/v) 3-hr into the dark cycle for three consecutive days. Binge-like consumption is assessed on the fourth day when access to test bottles is extended to 4-hr. Tail-blood samples are collected after test bottles are removed on the fourth day to assess blood ethanol concentration (BEC). Each 4-day test period constitutes a binge cycle and animals are allowed 3-days rest between cycles.
To assess changes in prepro-orexin expression while ethanol was still present in the brain, all animals were sacrificed immediately following the final binge session so that brains could be extracted and processed for PCR. Multiple cycles of DID (0, 1, or 3 cycles) were assessed in order to examine prepro-orexin mRNA changes over time in response to repeated binge-like ethanol drinking. Bilateral brain punches (1.0 mm x 1.0 mm) containing the hypothalamus- including all subregions (i.e. LH, perifornical area of the hypothalamus, and dorsomedial hypothalamus)- were subsequently collected from each animal.
Experiment 2a
Mice were cannulated targeting the VTA to assess the contribution of OXRs within this region. To assess regional specificity of these effects, a separate cohort of mice were cannulated dorsal to the VTA. Thirty minutes prior to testing, animals were bilaterally infused with either the selective OX1R antagonist, SB-334867 (SB; 0.0 or 6.0 μg; Tocris Bioscience, Bristol, UK), or the selective OX2R antagonist, TCS-OX2-29 (TCS; 0.0, 5.0, or 7.5 μg; Tocris Bioscience, Bristol, UK). These doses of SB and TCS were determined based on what has been demonstrated to be effective previously (Barson et al., 2015; Borgland et al., 2006). Both compounds were dissolved in 100% dimethyl sulfoxide (DMSO). High concentrations of DMSO are commonly used to dissolve compounds for intracranial infusions (James et al., 2011; Li et al., 2011; Srinivasan et al., 2012) with no observable consequences on behavior (Akbari et al., 2008; James et al., 2011; Naghdi and Asadollahi, 2004). All infusions were administered in a volume of 0.3 μl/side. In order to increase power during statistical analysis, a Latin-square design was used such that each animal received all doses of the drug over repeated trials in a randomized, counterbalanced order. Mice were given three days of rest between subsequent 4-day DID sessions in order to avoid carryover effects of the drug. The DID procedures were identical to those used in Experiment 1 except animals had access to test bottles for only 2-hr on the fourth day due to the short half-life (Mould et al., 2014; Porter et al., 2001) and transient effects on binge-like drinking behavior of the compounds used (Olney et al., 2015). It is worth noting that limiting the access to ethanol during DID from 4-hr to only 2-hr has the clear disadvantage of reducing the total amount of ethanol consumed across the entire test period. This is particularly problematic considering the fact that binge-like ethanol drinking is specifically defined by the amount of ethanol consumed (i.e. BEC ≥ 80 mg/dl; NIAAA, 2004). However, we observed that animals in the current study were nonetheless able to achieve binge levels of ethanol consumption despite the fact that access to ethanol was limited to only 2-hr.
Experiment 2b
Mice were bilaterally cannulated targeting the CeA to assess the contribution of OXRs within this region while a separate cohort was cannulated targeting the BLA as a region-specific control. All other experimental procedures were identical to those described for Experiment 2a except only one dose of TCS were used (0.0 versus 7.5 μg) as the lower dose (5.0 μg) was found to be ineffective at modulating binge-like ethanol drinking during Experiment 2a; thus, only the effect of the higher dose (7.5 μg) was examined in Experiment 2b.
Data Analysis
For experiment 1, a univariate ANOVA was used to measure prepro-orexin mRNA expression in the hypothalamus using group (water, one-cycle ethanol, one-cycle sucrose, three-cycle ethanol, or three-cycle sucrose) as the independent variable. Fisher’s LSD post-hoc tests and Bonferroni corrections were used when applicable. To ensure that hypothalamic precursor expression was not affected by differential drinking levels, separate t-tests were used to assess consumption of each test solution (i.e. ethanol or sucrose) on the final day of testing between animals in the one or three DID cycle groups. Similarly, a t-test was also performed on the BEC data to measure differences in ethanol metabolism as a function of DID cycles (one or three). Finally, separate univariate ANOVAs were used to analyze changes in body mass (g) as a function of group. For all analyses, p < 0.05 (two-tailed) was used to determine statistical significance.
For experiments 2a & b, separate repeated-measures ANOVAs were used for either the VTA or CeA to assess hourly binge consumption with both time (hour 1 and hour 2) and dose (0.0 or 6.0 μg for SB; 0.0, 5.0, or 7.5 μg for TCS) being within-subject variables. Additionally, BEC and total binge consumption across the total two-hour test period was assessed using separate repeated-measures ANOVAs with dose (0.0 or 6.0 μg for SB; 0.0, 5.0, or 7.5 μg for TCS) as the within-subject variable. We also included drug order as a between-subjects variable in these analyses to ensure the order in which the animals were presented with the drug did not have any confounding effects on drinking behavior. Fisher’s LSD post-hoc tests and Bonferroni corrections were used when applicable. Importantly, due to the relatively short half-life and transient activity of the compounds (Mould et al., 2014; Porter et al., 2001), planned-comparisons were used to assess binge-like consumption of each drug group relative to vehicle during the first hour of testing. In fact, doses that were observed to be ineffective at reducing binge-like ethanol drinking across a four-hour DID test period (Anderson et al., 2014) were found to significantly reduce ethanol consumption, but only during the first hour of DID testing (Olney et al., 2015). Thus, planned-comparisons are necessary to detect the short-lived effects of these compounds on binge-like consumption. Notably, consumption of each test solution was adjusted to account for differences in body weight between animals. Specifically, binge-like consumption of ethanol was recorded as g/kg while sucrose consumption was in ml/kg form. Values of binge-like ethanol consumption for each of the studies in experiments 2a & 2b have been converted to ml/kg form and are provided in the supplemental section (Figure S2).
3. Results
Experiment 1: Assessing changes in orexin mRNA following DID
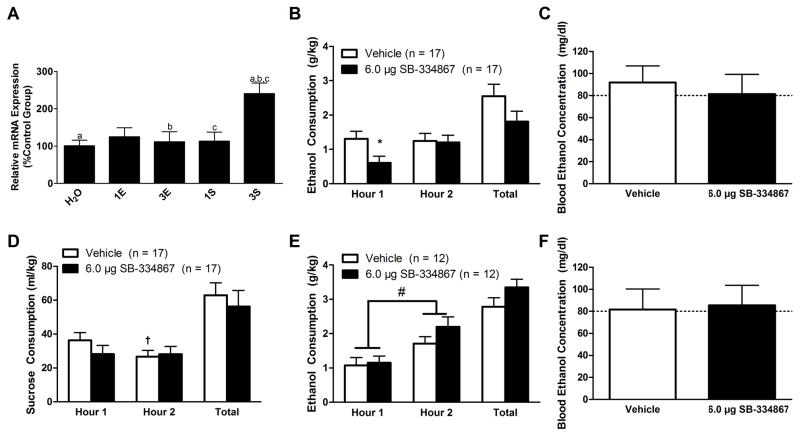
PCR analysis detected a significant difference in prepro-orexin expression within the hypothalamus as a function of group (Fig. 1A; F(4, 45) = 5.558, p = 0.001). Here, the three-cycle sucrose group was found to have significantly more precursor mRNA relative to all of the other groups except the one-cycle ethanol group (p’s ≤ 0.002). Importantly, all animals in our PCR study displayed comparable consumption, BECs, and final body mass regardless of DID history (Figure S1), which indicates that differences in precursor mRNA expression were not confounded by group differences in these measures.
Figure 1.
Expression of prepro-orexin mRNA within the hypothalamus (N = 50; n = 10/group) following binge-like ethanol drinking as well as the effect of inhibiting signaling onto OX1Rs in the VTA on binge-like drinking behavior. PCR analysis revealed that the three cycles of binge-like sucrose consumption produced a marked increase in prepro-orexin mRNA expression within the hypothalamus relative to all other groups except for the one-cycle ethanol group (A). Inhibition of OX1Rs in the VTA selectively reduces binge-like ethanol consumption. Animals infused with SB directly into the VTA exhibited blunted binge-like ethanol consumption relative to vehicle-treated controls but only during the first hour of testing (B). Despite this reduction in consumption, no significant effect was observed in BECs as measured at the end of the two hour test period (C). Interestingly, intra-VTA infusion of SB did not significantly affect binge-like sucrose consumption at any point during the test (D), though a significant Time×Dose interaction revealed that vehicle-treated animals drank significantly more sucrose during the first hour of testing relative to the second. Importantly, this phenomenon was specific to the VTA treatment as SB infused directly dorsal to the VTA did not significantly impact binge-like ethanol drinking (E) or subsequent BECs (F) at the end of the two hour test period. We did, however, observe that animals drank significantly more ethanol during the second hour of testing relative to the first hour regardless of treatment group. H2O, water group; 1E, one-cycle ethanol group; 3E, three-cycle ethanol group; 1S, one-cycle sucrose group; 3S three-cycle sucrose group. Bars that share the same letter in (A) are significantly different from one another (p < 0.05). * denotes that p < 0.05 relative to vehicle-treated animals during the first hour of testing. † signifies that p < 0.05 relative to vehicle-treated animals during the first hour of testing. # denotes that animals drank significantly more during the second hour of testing relative to the first hour regardless of treatment group. Dotted line in (C, F) delineates 80 mg/dl, the minimum BEC to constitute a binge episode. Data presented as Mean ± SEM.
Experiment 2a: Characterizing orexin circuitry in the ventral tegmental area
Repeated-measures ANOVA revealed that binge-like ethanol consumption did not change over time (Fig. 1B; Main effect of Time: F(1,15) = 2.701, p = 0.121) nor did inhibition of OX1Rs in the VTA significantly alter binge-like ethanol drinking (Main effect of Dose: F(1,15) = 2.651, p = 0.096; Time×Dose Interaction: F(1,15) = 2.189, p = 0.160). However, planned-comparisons revealed that, relative to vehicle-treated controls, SB significantly blunted binge-like ethanol intake during the first hour of testing (t(16) = 2.208, p = 0.042), confirming our previous observation that the short half-life of SB (Porter et al., 2001) limits the window in which this compound is effective (Olney et al., 2015). Moreover, no significant effect was observed on total binge-like ethanol drinking across the 2-hr test period (F(1,15) = 3.161, p = 0.096) or on BECs (Fig. 1C; F(1,15) = 0.419, p = 0.527) between drug conditions. Importantly, the order in which animals received drug treatment in our Latin-square design did not confound binge-like ethanol drinking (F(1,15) = 3.489, p = 0.081) or BECs (F(1,15) = 0.134, p = 0.720).
Intra-VTA infusion of SB did not affect binge-like sucrose consumption across time (Fig. 1D; Main effect of Time: F(1,15) = 4.253, p = 0.064). Binge-like sucrose drinking was not impacted by treatment condition (Main effect of Dose: F(1,15) = 0.711, p = 0.412). There was, however, a significant interaction effect (Time×Dose Interaction: F(1,15) = 4.876, p = 0.043). Further probing revealed vehicle-treated animals consumed significantly greater volumes of sucrose during the first hour of testing relative to the second (t(16) = 2.473, p = 0.025). Notably, planned-comparisons revealed no significant difference in sucrose consumption during the first hour of testing between treatment groups (t(16) = 1.463, p = 0.163). Moreover, sucrose consumption across the entire two hour test period did not vary between treatment conditions (F(1, 15) = 0.711, p = 0.412). Additionally, the order in which the animal received the compound did not impact sucrose intake (F(1, 15) = 0.024, p = 0.878).
Importantly, effects were specific to the VTA as similar treatment dorsal to the VTA did not produce significant alterations in binge-like ethanol drinking as a function of treatment (Fig. 1E; Main effect of Dose: F(1,10) = 4.188, p = 0.068; Time×Dose Interaction: F(1,10) = 3.989, p = 0.074; Total ethanol consumption: F(1,10) = 4.188, p = 0.068; SB versus vehicle planned-comparisons at the first hour: t(11) = −0.278, p = 0.786) although we did observe that the animals drank significantly more ethanol during the second hour of testing relative to the first regardless of treatment condition (Main effect of Time: F(1,10) = 7.037, p = 0.024). Moreover, we did not observe an effect on BECs between groups (Fig. 1F; F(1,9) = 0.028, p = 0.870). Additionally, whether the animal received treatment with vehicle or SB first did not affect binge-like ethanol consumption (F(1,10) = 0.268, p = 0.616) or BECs (F(1,9) = 0.108, p = 0.750).
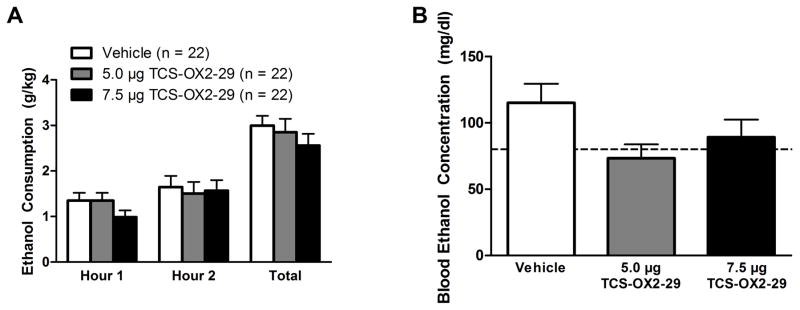
Intra-VTA infusion of TCS did not significantly alter binge-like ethanol drinking over time (Fig. 2A; Main effect of Time: F(1,19) = 3.175, p = 0.091). Similarly, none of the doses of TCS affected drinking behavior (Main effect of Dose: F(2,38) = 0.669, p = 0.518; Time×Dose Interaction: F(2,38) = 0.425, p = 0.657). Using planned-comparisons, we did not observe a significant effect of the low dose of TCS (5.0 μg) relative to vehicle-treated controls during the first hour of testing (t(21) = −0.005, p = 0.996) but there was a nonsignificant trend for the high dose of TCS (7.5 μg) to reduce ethanol consumption relative to vehicle (t(21) = 1.790, p = 0.088). Additionally, we did not observe an effect across the total 2-hr test period (F(2,38) = 0.669, p = 0.518). There was a marginal, albeit nonsignificant, effect for BEC levels to vary as a function of TCS dose (Fig. 2B; F(2,34) = 3.181, p = 0.054). Moreover, the order in which the animals received the drug treatment did not impact binge-like ethanol drinking (F(1, 19) = 0.194, p = 0.825) or BECs (F(1, 19) = 1.323, p = 0.292).
Figure 2.
Inhibition of OX2Rs in the VTA does not significantly alter binge-like consumption. Infusion of TCS into the VTA does not significantly impact binge-like ethanol consumption at any point throughout the test period (A) nor does it significantly alter observed BEC levels (B). Dotted line in (B) delineates 80 mg/dl. Data presented as Mean ± SEM.
Experiment 2b: Characterizing orexin circuitry in the central nucleus of the amygdala
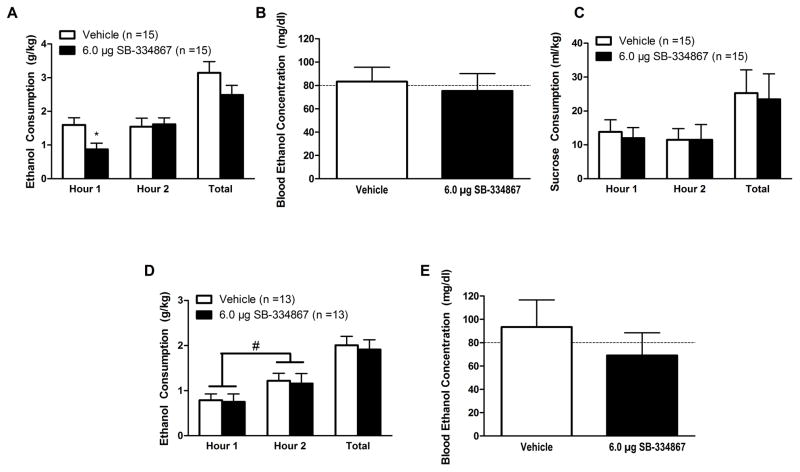
Repeated-measures ANOVA revealed that binge-like ethanol drinking did not change across the 2-hr test period (Fig. 3A; Main effect of Time: F(1,13) = 2.077, p = 0.173). Although there was no main effect of dose (F(1,13) = 2.242, p = 0.158), there was a nonsignificant trend for drinking levels to change over time as a function of treatment (Time×Dose Interaction: F(1,13) = 2.334, p = 0.056); however, planned-comparisons at the first hour of testing revealed that intra-CeA treatment with SB significantly reduced drinking levels relative to vehicle-treated controls (planned-comparisons: (t(14) = 2.961, p = 0.010). Similarly, our analyses did not reveal an effect of treatment on total drinking across the 2-hr test period (F(1,13) = 2.242, p = 0.158) nor did it impact BECs (Fig. 3B; F(1,11) = 0.149, p = 0.707). Importantly, drug order did not affect binge-like ethanol drinking (F(1,13) = 0.135, p = 0.719) or BECs (F(1,11) = 0.001, p = 0.982).
Figure 3.
Inhibition of OX1Rs in the CeA selectively reduces binge-like ethanol consumption. Animals infused with SB directly into the CeA exhibited blunted binge-like ethanol consumption relative to vehicle-treated controls but only during the first hour of testing (A). Despite this reduction in consumption, no significant effect was observed in BECs as measured at the end of the two hour test period (B). Interestingly, intra-CeA infusion of SB did not significantly affect binge-like sucrose consumption at any point during the test (C). Importantly, this phenomenon was specific to the CeA treatment as local infusion of SB into the BLA did not significantly impact binge-like ethanol drinking (D)- although animals did drink significantly more ethanol during the second hour of testing relative to the first, regardless of treatment condition. Moreover, intra-BLA SB did not affect subsequent BECs (E) at the end of the two hour test period. Data presented as mean ± SEM. * denotes that p < 0.05 relative to vehicle-treated animals during the first hour of testing. # denotes that animals drank significantly more during the second hour of testing relative to the first hour regardless of treatment group. Dotted line in (B) delineates 80 mg/dl, the minimum BEC to constitute a binge episode. Data presented as Mean ± SEM.
Similar treatment with SB did not alter hourly sucrose consumption (Main effect of time: F(1,13) = 1.707, p = 0.214; Main effect of Dose: F(1,13) = 1.957, p = 0.185; Time×Dose Interaction: F(1,13) = 0.547, p = 0.473; planned-comparisons at the first hour: t(14) = 2.035, p = 0.061) or total consumption across the 2-hr test period (F(1,13) = 1.957, p = 0.185; Fig. 3C). Moreover, drug order did not impact binge-like sucrose consumption (F(1,13) = 0.503, p = 0.491).
Intra-BLA infusion of SB did not affect binge-like ethanol drinking (Fig. 3D; Main effect of Dose: F(1,11) = 0.088, p = 0.772; Time×Dose Interaction: F(1,11) = 0.002, p = 0.962; planned-comparison at the first hour: t(12) = 0.134, p = 0.896)- although there was a significant effect that indicated animals drank significantly more ethanol during the second hour of testing relative to the first regardless of drug treatment (Main effect of Time: F(1,1) = 7.522, p = 0.019). Moreover, there was no significant effect of SB across the total 2-hr test period (F(1,11) = 0.088, p = 0.772) nor was there an effect on BECs (Fig. 3E; F(1,11) = 0.795, p = 0.392). The order in which the animals received the drug did not alter ethanol drinking (F(1,11) = 0.045, p = 0.836) or BECs (F(1,11) = 0.503, p = 0.493).
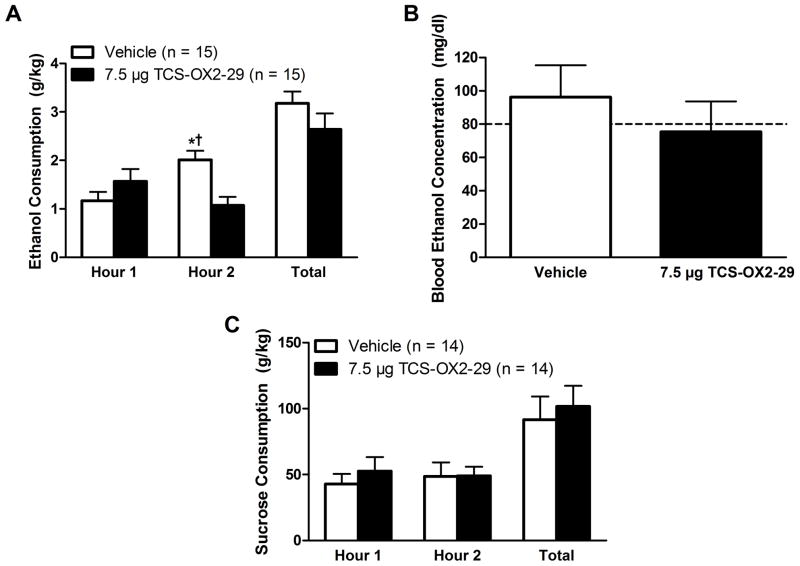
No significant main effects were observed following intra-CeA infusion of TCS (Fig. 4A; Main effect of Dose: F(1,13) = 2.344, p = 0.150; Main effect of Time: F(1,13) = 0.833, p = 0.378); however, we observed a significant interaction effect (Time×Dose Interaction: F(1,13) = 8.980, p = 0.010). Further probing revealed that this effect was driven by the fact that vehicle-treated animals drank significantly more ethanol during the second hour of testing relative to the first (t(14) = −3.076, p = 0.008). Additionally, vehicle-treated animals consumed significantly more ethanol relative to TCS-treated animals during the second hour of testing (t(14) = 3.190, p = 0.007). Notably, no difference was observed between the two treatment groups during the first hour of testing (t(14) = −1.284, p = 0.220). No effect was observed across the total 2-hr test period (F(1,13) = 2.330, p = 0.151) nor was there an effect on BECs (Fig. 4B; F(1,13) = 0.631, p = 0.445). Treatment order did not affect ethanol drinking (F(1,13) = 150, p = 0.705) or BECs (F(1,13) = 4.148, p = 0.069).
Figure 4.
Inhibition of CeA OX2Rs impacts binge-like consumption. Vehicle treated animals displayed an enhancement in binge-like ethanol drinking during the second hour of testing relative to the first (A) and infusion of TCS into the CeA blocked this behavior. However, no changes in BECs were observed as a function of treatment with TCS (B) nor did it impact binge-like sucrose intake (C). Data presented as mean ± SEM. * denotes that p < 0.05 relative to vehicle treated animals during the first hour of testing. † signifies that p < 0.05 relative to TCS treated animals at the same time point. Dotted line in (B) delineates 80 mg/dl. Data presented as Mean ± SEM.
No significant effects on any measure of binge-like sucrose consumption were observed following intra-CeA infusions of TCS (Fig. 4C; Main effect of Time: F(1,12) = 0.046, p = 0.834; Main effect of Dose: F(1,12) = 0.462, p = 0.510; Time×Dose Interaction: F(1,12) = 0.872, p = 0.369; planned-comparisons at the first hour: t(13) = −0.709, p = 0.491; total drinking across the full two hours: F(1,12) = 0.462, p = 0.510; drug order: F(1,12) = 0.277, p = 0.608).
4. Discussion
Findings from these experiments extend upon the existing OX literature in a number of ways. First, results from our PCR studies showed that- unlike other experimental models of ethanol administration- binge-like ethanol drinking did not produce compensatory changes in hypothalamic prepro-orexin expression; however, these findings are consistent with previous inferences made that binge-like ethanol drinking triggers increased signaling in hypothalamic OX neurons (Olney et al, 2015). What is more, we were able to identify and characterize OX circuitry within the VTA and CeA that modulates binge-like ethanol drinking behavior by demonstrating that the OX1R is the predominate receptor subtype that selectively regulates binge-like ethanol consumption in these regions. Notably, the data collected from these experiments- to our knowledge- provide the first evidence that OX signaling within the CeA contributes to ethanol drinking behavior.
It is worth noting that our intra-VTA infusion of SB may have partially diffused from its target location within the VTA into the surrounding brain tissue- including the substantia nigra (SN), which also robustly expresses both OXR subtypes (Ch’ng and Lawrence, 2015; Marcus et al., 2001). What is more, the SN has recently generated much interest due to its observed role in motivated behaviors (see Magnard et al., 2016 for review). Therefore, there remains the possibility that inadvertent inhibition of OX1Rs in the SN may- in part- have contributed to the observed decrease in binge-like ethanol drinking. However, the role of OXRs on ethanol intake within this specific brain region has previously been directly examined when Srinivasan and colleagues (2012) demonstrated that intra-SN infusion of a nonselective OX1R/OX2R antagonist failed to impact ethanol self-administration. Thus, the possibility that our observed reduction in binge-like ethanol drinking was due to nonspecific effects of SB in the SN is unlikely. Further reinforcing site-specificity we failed to find an effect of SB on ethanol intake when injected into the control site.
Additionally, it should be noted that, given the within-subjects nature of the experiments, mice in these studies received multiple infusions over the course of testing. Indeed, one may argue that multiple injections may lead to neurodegeneration of the tissue in the infusion zone. Had multiple infusions caused damage to the surrounding tissue, then one would expect that the OXR antagonist would be effective only during the first week of testing as the continued infusions would cause sufficient damage to limit the effects of the drug. However, we did not observe this to be the case as our statistical analyses did not reveal a main effect of drug order in any of our experiments. This indicates that the drug was equally effective across multiple weeks and suggests the absence of sufficient damage to the surrounding tissue that would limit the effects of the drug.
Although we have identified the specific OX pathways that modulate binge-like ethanol drinking, these studies were unable to specifically characterize the nature of these circuits. That is, it remains to be determined which population of neurons within the VTA and CeA are expressing OXRs that drive binge drinking behavior. That said, it is well-established that OX acts directly on dopaminergic neurons within the VTA. Indeed, OX has been demonstrated to induce plasticity at postsynaptic sites on these dopaminergic neurons that is believed to contribute to the development of drug addiction (Borgland et al., 2006). The actions of OX in the CeA, however, are poorly understood. Schmeichel and colleagues (2016) recently reported that SB significantly blocked a cocaine-induced enhancement of GABAergic neurotransimission within the CeA. Taken together, these observations suggest that OX may regulate binge drinking- at least in part- by modulating the activity of dopaminergic and GABAergic neurons within the VTA and CeA, respectively. However, future studies will seek to more directly investigate these mechanisms in order to provide further insight into the specific OX circuitry involved in binge drinking behavior.
In accordance with the existing literature (Olszewski et al., 2009), our PCR study demonstrated multiple cycles of binge-like sucrose consumption led to elevated levels of hypothalamic prepro-orexin mRNA expression. Specifically, we observed a marked increase in prepro-orexin mRNA within the hypothalamus following three cycles of binge-like sucrose drinking. These data suggest that hypothalamic OX signaling is involved in sucrose consumption. In fact, schedule-induced feeding of a high-sugar diet causes a robust enhancement of hypothalamic prepro-orexin mRNA expression relative to a standard diet (Olszewski et al., 2009). Together with the data from the current study, this suggests that hypothalamic OX neurons are recruited following repeated access to highly palatable reinforcers. Importantly, although previous findings suggest that OX signaling in the parabrachial nucleus (Kay et al., 2014), ventral pallidum (Ho and Berridge, 2013), or posterior paraventricular nucleus of the thalamus (Barson et al., 2015) regulates sucrose consumption, data from our site-directed pharmacology studies (Experiments 2a & 2b) indicate that OX signaling in the VTA and CeA does not modulate binge-like sucrose consumption.
Contrary to the previous studies that found increases in prepro-orexin mRNA following ethanol exposure (Barson et al., 2015; Lawrence et al., 2006), we found that binge-like ethanol drinking did not impact hypothalamic precursor expression. Although inconsistent with much the current literature, other reports exist that deviate from this pattern of results as well. Indeed, chronic ethanol consumption tends to increase OX mRNA (Barson et al., 2015; Lawrence et al., 2006). However, Morganstern and colleagues (2010) reported that acute ethanol caused an increase in OX expression while chronic ethanol consumption resulted in a significant reduction in hypothalamic OX mRNA. Given that different models of ethanol exposure can lead to differential effects on the OX system, it may be the case that the DID procedure does not produce compensatory changes in the OX system. Alternatively, the timing of the tissue collection may have impacted the results as well. For example, we sacrificed animals immediately after the final binge session whereas others waited 30 min (Barson et al., 2015) or two hours (Morganstern et al., 2010) afterwards to assess OX mRNA. Thus, we may have collected tissue before the system responded to the depleted levels of OX peptide and mobilized prepro-orexin mRNA to replenish those levels. Indeed, waiting longer to assess brain tissue may reveal compensatory changes in levels of prepro-orexin mRNA that are consistent with our drinking data (i.e. that the OX system is recruited during binge-like ethanol consumption).
Although we did not observe significant changes in precursor mRNA as a function of binge-like ethanol drinking, these results do provide insight as to the nature of the changes we previously reported concerning orexin-A immunoreactivity (IR) in which we observed that binge-like ethanol drinking reduces orexin-A IR in the LH (Olney et al., 2015). Specifically, we proposed that this reduction in orexin-A IR reflected an increase in signaling stemming from reduced stores of OX peptide in neurons. Given this reduction in orexin-A IR, had expression of prepro-orexin mRNA similarly decreased following DID in our present study then a more parsimonious explanation would be that the OX system is downregulated following binge-like ethanol drinking. However, the fact that prepro-orexin mRNA did not change indicates the system is not downregulated following binge-like ethanol consumption and that reduced orexin-A IR was likely reflective of depleted cellular stores following increased release. Moreover, this interpretation is consistent with the notion that binge-like ethanol drinking parallels OX signaling as pharmacological blockade of OXRs disrupts this behavior (Anderson et al., 2014; Olney et al., 2015).
Our findings provide support that the OX1Rs within the VTA and CeA contribute to binge-like ethanol drinking while the contribution of the OX2R may be relatively more limited. Notably, inhibiting intra-VTA OX2Rs via TCS failed to significantly alter binge-like ethanol drinking in our experiments across a range of doses. Although one may surmise that- based on our findings- OX2Rs within the VTA do not contribute to binge drinking behavior, it may be more appropriate to conclude that, within the VTA, OX2Rs play a subsidiary role to OX1Rs in modulating ethanol drinking. Indeed, previous studies have demonstrated that the OX2R is capable of modulating ethanol drinking (Anderson et al., 2014; Barson et al., 2015; Shoblock et al., 2011). In fact, we did observe a slight, albeit nonsignificant, trend for the higher dose of TCS (7.5 μg) to disrupt ethanol drinking during the first hour of testing relative to vehicle-treated animals. Furthermore, intra-VTA TCS marginally reduced BECs relative to vehicle-treated controls. What is more, animals treated with the lower dose of TCS (5.0 μg) achieved an average BEC (73.32 ± 10.50 mg/dl) below the threshold to be considered a binge episode (80 mg/dl). Together, these findings suggest that OX2Rs in the VTA may have relatively more subtle effects on ethanol consumption. Indeed, it has previously been demonstrated that suppressing OX2R signaling within the anterior paraventricular nucleus of the thalamus (PVT) via local infusion of a dose of TCS comparable to our low dose (5.0 μg) effectively attenuated ethanol consumption (Barson et al., 2015). As a whole, the fact that the observed reductions in binge-like ethanol drinking following our VTA manipulation did not reach statistical significance across a range of doses (5.0 and 7.5 μg) suggests that that the OX2R is not the predominate OXR subtype that modulates binge-like ethanol drinking within the VTA but OX2R signaling in other brain regions (e.g. anterior PVT) makes significant contributions to ethanol drinking.
Additionally, our data suggest that OX2Rs within the CeA may participate in binge-like ethanol drinking. Indeed, we observed that TCS-treated animals consumed less ethanol relative to vehicle treated animals during the second hour of our ethanol DID test. Specifically, this effect was driven by the fact that vehicle-treated animals displayed an increase in ethanol consumption during the second hour of testing relative to the first. Thus, treatment with TCS may not have reduced binge-like drinking behavior, per se, but rather blocked an increase in drinking behavior that occurred during the second hour of testing. Further investigations may seek to elucidate the particular role of the OX2R in the CeA.
Our data suggest that these compounds have a narrow window in which binge-like ethanol drinking is altered due to their relatively short half-life. Indeed, drinking was significantly blunted only during the first hour of testing following local infusions of SB into the VTA or CeA. Despite these changes in drinking behavior, we did not observe any significant differences in BECs at the end of the two-hour test period. Perhaps differences in BECs would have been observed had we taken tail-blood samples at a time that coincided with the observed differences in drinking behavior (i.e. one hour after ethanol access). However, collecting tail-blood samples requires the use of a restrainer and can be rather stressful for the animal (Harris et al., 2004; Tuli et al., 1995), which may ultimately confound subsequent drinking behavior (Nash and Maickel, 1985; Rockman et al., 1986). Thus, blood samples were collected at the end of the 2-hour test period, which may have provided sufficient time for the drug to clear the system and for the drug-treated animals to drink enough ethanol to achieve a BEC comparable to their vehicle-treated counterparts.
Perhaps our most exciting finding was the fact that OX signaling within the VTA and CeA was reinforcer-specific. Specifically, these OX circuits were demonstrated to modulate binge-like ethanol drinking, yet did not contribute to binge-like sucrose consumption. Previously, we and others have shown that a systemic injection of an OXR antagonist reduces binge-like consumption of ethanol as well as other natural reinforcers including sucrose and the non-nutritional sweetener, saccharin (Alcaraz-Iborra et al., 2014; Anderson et al., 2014; Olney et al., 2015). Taken alone, these previous findings indicate that the OX system regulates the overarching hedonic properties shared by salient reinforcers in general.
However, a growing number of studies have emerged reporting that pharmacological suppression of signaling onto OXRs disrupts ethanol self-administration yet leaves responding to sucrose intact. Indeed, early investigations reported that systemic (Jupp et al., 2011) or intra-VTA administration of an OXR antagonist reduces operant responding (Srinivasan et al., 2012) and seeking behavior (Brown et al., 2016) of ethanol, but not sucrose. More recently, it has also been demonstrated that infusion of OX into the anterior PVT augments ethanol drinking without altering sucrose drinking (Barson et al., 2015). A similar pattern has also been observed in other natural rewards as infusion of orexin-A into the paraventricular nucleus of the hypothalamus or LH enhances ethanol drinking but does not alter food or water intake (Schneider et al., 2007). To be clear, this collection of findings does not invalidate the conclusion that the OX system modulates responses to general, salient reinforcers. They do, however, indicate that specific OX pathways regulating binge-like ethanol drinking may be largely independent of those that modulate responding to natural rewards. Indeed, this ability to manipulate ethanol consumption without affecting responding to natural rewards makes the OX system an appealing target for novel pharmacotherapies used to treat alcohol use disorders (AUDs).
Although these data help elucidate the OX circuitry involved in binge-like ethanol drinking, the psychological mechanisms by which OX signaling in these regions modulates this behavior remains unknown. Two well-established psychological processes, positive- and negative-reinforcement, have been identified as principal motivators of ethanol consumption (Edwards, 2016; Gilpin and Koob, 2008). Indeed, both the VTA and CeA are involved in aspects of both positive- and negative-reinforcement (Gessa et al., 1985; Gilpin and Koob, 2008; Hata et al., 2011; Hodge et al., 1993; Hyytiä and Koob, 1995). Moreover, the OX system has been implicated in both of these behavioral principles (Anderson et al., 2014; Bayerlein et al., 2011; Kuru et al., 2000; Sakamoto et al., 2004; Taslimi et al., 2012; von der Goltz et al., 2011; Winsky-Sommerer et al., 2004). Therefore, inhibiting OXR signaling within these regions may diminish the reinforcing properties of ethanol consumption- thereby attenuating the motivation to consume ethanol. Further investigation is needed in order to identify the psychological process mediating the effect of OX signaling on ethanol consumption.
Through our current set of experiments, we have demonstrated that OX signaling within the VTA and CeA- particularly onto the OX1R- modulates binge-like ethanol drinking. Interestingly, OX1R signaling in these regions is selective for ethanol as pharmacological inhibition of the OX1R did not alter binge-like sucrose consumption. Though we observed no changes in prepro-orexin mRNA as a function of binge-like ethanol drinking, this observation provides further support to interpretations of previous experimental findings that binge-like ethanol drinking leads to increased signaling of hypothalamic OX neurons (Olney et al., 2015). Indeed, a substantial body of literature, which encompasses various behavioral paradigms that model ethanol consumption, clearly demonstrates that OX signaling modulates ethanol drinking behavior (Lawrence, 2010; Mahler et al., 2012). As a whole, these data provide converging evidence that OX inhibition blocks ethanol consumption and further reinforces the potential value of OX compounds in treating AUDs. What is more, the selectivity of our manipulations for ethanol over sucrose suggests that targeting specific OX circuits, such as those involving the VTA and CeA, may be beneficial in reducing binge-like ethanol consumption without impacting responding to natural rewards.
Supplementary Material
Acknowledgments
This work was funded by National Institute of Health grants AA022048, AA013573, and AA015148.
We would like to thank Dr. Hyung-Suk Kim of the UNC Animal Clinical Chemistry and Gene Expression Laboratories for his assistance during the PCR analysis.
References
- Akbari E, Motamedi F, Naghdi N, Noorbakhshnia M. The effect of antagonization of orexin 1 receptors in CA1 and dentate gyrus regions on memory processing in passive avoidance task. Behavioural Brain Research. 2008;187:172–177. doi: 10.1016/j.bbr.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I. Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: Pharmacological and molecular evidence of orexin involvement. Behav Brain Res. 2014;272:93–99. doi: 10.1016/j.bbr.2014.06.049. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Frontiers in Neuroscience. 2014:8. doi: 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Ryabinin AE. Interactive effects of nicotine and alcohol co-administration on expression of inducible transcription factors in mouse brain. Neuroscience. 2001;103:941–954. doi: 10.1016/s0306-4522(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerlein K, Kraus T, Leinonen I, Pilniok D, Rotter A, Hofner B, Schwitulla J, Sperling W, Kornhuber J, Biermann T. Orexin A expression and promoter methylation in patients with alcohol dependence comparing acute and protracted withdrawal. Alcohol. 2011;45:541–547. doi: 10.1016/j.alcohol.2011.02.306. [DOI] [PubMed] [Google Scholar]
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Mühlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Borgland S, Taha S, Sarti F, Fields H, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brown RM, Kim AK, Khoo SYS, Kim JH, Jupp B, Lawrence AJ. Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol. 2016;21:603–612. doi: 10.1111/adb.12251. [DOI] [PubMed] [Google Scholar]
- Ch’ng SS, Lawrence AJ. Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: A characterisation of OX1R-eGFP mice. Journal of Chemical Neuroanatomy. 2015;66–67:1–9. doi: 10.1016/j.jchemneu.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regulatory Peptides, Special Issue: Orexin. 2002;104:131–144. doi: 10.1016/S0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FSN. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. Progress in Brain Research. Elsevier; 2016. Reinforcement principles for addiction medicine; from recreational drug use to psychiatric disorder; pp. 63–76. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Harris RBS, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav. 2004;81:557–568. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Hata T, Chen J, Ebihara K, Date Y, Ishida Y, Nakahara D. Intra-ventral tegmental area or intracerebroventricular orexin-A increases the intra-cranial self-stimulation threshold via activation of the corticotropin-releasing factor system in rats. Eur J Neurosci. 2011;34:816–826. doi: 10.1111/j.1460-9568.2011.07808.x. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic “liking” for sweetness. Neuropsychopharmacology. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. The International Journal of Neuropsychopharmacology. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin1 receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain research. 2011;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Kay K, Parise EM, Lilly N, Williams DL. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl) 2014;231:419–427. doi: 10.1007/s00213-013-3248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of Ventral Tegmental Area Dopaminergic and Nondopaminergic Neurons by Orexins/Hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ. Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain research. 2010;1314:124–129. doi: 10.1016/j.brainres.2009.07.072. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British journal of pharmacology. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, Kirouac GJ. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav. 2011;102:42–50. doi: 10.1016/j.physbeh.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnard R, Vachez Y, Carcenac C, Krack P, David O, Savasta M, Boulet S, Carnicella S. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl Psychiatry. 2016;6:e753. doi: 10.1038/tp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Progress in brain research. 2012;198:79. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, Schultz JA, McClintick JN, Edenberg HJ, Bell RL. Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol. 2010;44:171–183. doi: 10.1016/j.alcohol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, Boehm SL., 2nd GABAA receptors in the posterior, but not anterior, ventral tegmental area mediate Ro15-4513-induced attenuation of binge-like ethanol consumption in C57BL/6J female mice. Behav Brain Res. 2011;220:230–237. doi: 10.1016/j.bbr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Boehm SL. Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123:555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcoholism: Clinical and Experimental Research. 2010;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould R, Brown J, Marshall FH, Langmead CJ. Binding kinetics differentiates functional antagonism of orexin-2 receptor ligands. Br J Pharmacol. 2014;171:351–363. doi: 10.1111/bph.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi N, Asadollahi A. Genomic and nongenomic effects of intrahippocampal microinjection of testosterone on long-term memory in male adult rats. Behavioural Brain Research. 2004;153:1–6. doi: 10.1016/j.bbr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Narita M. Direct Involvement of Orexinergic Systems in the Activation of the Mesolimbic Dopamine Pathway and Related Behaviors Induced by Morphine. Journal of Neuroscience. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Jr, Maickel RP. Stress-induced consumption of ethanol by rats. Life Sci. 1985;37:757–765. doi: 10.1016/0024-3205(85)90546-6. [DOI] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE. Binge-Like Consumption of Ethanol and Other Salient Reinforcers is Blocked by Orexin-1 Receptor Inhibition and Leads to a Reduction of Hypothalamic Orexin Immunoreactivity. Alcohol Clin Exp Res. 2015;39:21–29. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Shaw TJ, Grace MK, Höglund CE, Fredriksson R, Schiöth HB, Levine AS. Complexity of neural mechanisms underlying overconsumption of sugar in scheduled feeding: involvement of opioids, orexin, oxytocin and NPY. Peptides. 2009;30:226–233. doi: 10.1016/j.peptides.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE. Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Hall A, Glavin GB. Effects of restraint stress on voluntary ethanol intake and ulcer proliferation in rats. Pharmacol Biochem Behav. 1986;25:1083–1087. doi: 10.1016/0091-3057(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF. Hypocretin Neurotransmission Within the Central Amygdala Mediates Escalated Cocaine Self-Administration and Stress-Induced Reinstatement in Rats. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, Wree A. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcoholism: Clinical and Experimental Research. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology. 2011;215:191–203. doi: 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE. The Dual Orexin/Hypocretin Receptor Antagonist, Almorexant, in the Ventral Tegmental Area Attenuates Ethanol Self-Administration. PLOS ONE. 2012;7:e44726. doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslimi Z, Arezoomandan R, Omranifard A, Ghalandari-Shamami M, Riahi E, Vafaei AA, Rashidy-Pour A, Haghparast A. Orexin A in the ventral tegmental area induces conditioned place preference in a dose-dependent manner: involvement of D1/D2 receptors in the nucleus accumbens. Peptides. 2012;37:225–232. doi: 10.1016/j.peptides.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Tuli JS, Smith JA, Morton DB. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim. 1995;29:90–95. doi: 10.1258/002367795780740339. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, Wiedemann K, Kiefer F. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm Behav. 2011;60:644–650. doi: 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.