Abstract
Background
Pertuzumab (Perjeta®), a HER2/neu receptor antagonist, was approved by the US Food and Drug Administration in June 2012 for use in the first-line setting for patients with HER2-positive metastatic breast cancer (mBC).
Objective
This retrospective study investigated the clinical and demographic characteristics, treatment patterns, safety, and clinical outcomes for patients with HER2-positive mBC who received pertuzumab in the first-line setting in US community oncology practices.
Methods
Patients with HER2-positive mBC, who initiated pertuzumab within 60 days of mBC diagnosis between June 2012 and June 2014, followed through December 2014, had ≥2 visits within the McKesson Specialty Health/US Oncology Network, and were not on clinical trials during the study period, were eligible. This study utilized iKnowMed electronic health records, Claims Data Warehouse, and Social Security Death Index. Progression-free survival (PFS) was assessed by Kaplan–Meier methods.
Results
A total of 266 patients met the selection criteria. A vast majority of the patients (249/266, 93.6%) received a trastuzumab + pertuzumab + taxane (H + P + T) regimen. The number of patients with prior adjuvant/neoadjuvant therapy was higher than the CLEOPATRA trial, but age (median 57 years) and percentage of visceral disease (74.8%) were similar. The most common adverse events were fatigue (50.8%), diarrhea (44.7%), nausea (35.3%), peripheral neuropathy (33.5%), neutropenia (24.9%), and rash (24.4%). The median PFS was 16.9 months (95% CI 14.2–19.7).
Conclusions
In this retrospective study of patients with HER2-positive mBC receiving pertuzumab in the first-line setting, most patients were treated with H + P + T. The safety and PFS of H + P + T were consistent with those observed in the pivotal trial.
Key Points
| The purpose of this retrospective, observational study was to investigate the clinical use of pertuzumab in a population of patients with HER2-positive mBC treated in a real-world community oncology practice setting. |
| We found that most patients were treated with pertuzumab, trastuzumab, and a taxane in the first-line setting. |
| Our results confirmed the clinical safety and PFS of pertuzumab as first-line therapy for patients with HER2-positive mBC in the community treatment setting. |
| The use of pertuzumab in this setting was consistent with the NCCN guidelines (2012) and, for the most part, with the FDA-approved Perjeta® (pertuzumab) prescribing information. |
Introduction
Breast cancer is one of the most frequently diagnosed cancer types, and the second leading cause of death in women with cancer. It is estimated that in 2016, 246,660 women will have been diagnosed with breast cancer, and 40,450 will have died from the disease [1]. HER2-positive breast cancer is a subtype that overexpresses the human epidermal growth factor receptor 2 (HER2) protein that promotes the growth of cancer cells. Approximately 20% of breast cancers are HER2-positive (also known as HER2/neu positive), and overexpression of HER2 is associated with a poor prognosis [2, 3].
Pertuzumab is a novel HER2/neu receptor antagonist. It is a humanized monoclonal antibody that binds to HER2 [4]. Preclinical studies have shown that it acts as an antitumor agent by binding to the HER2 receptor and blocking its pairing with other HER receptors, and is an important targeted therapy for patients with HER2-positive breast cancer [5–11]. Pertuzumab was approved by the US Food and Drug Administration (FDA) in June 2012 to be used in the first-line setting in combination with trastuzumab and docetaxel for patients with HER2-positive metastatic breast cancer (mBC) [12]. The approved pertuzumab dosing schedule is an initial dose of 840 mg followed every 3 weeks thereafter by 420 mg. The CLEOPATRA Phase 3 trial demonstrated that the addition of pertuzumab to trastuzumab and docetaxel therapy for HER2-positive mBC significantly increased the median progression-free survival (PFS) and overall survival (OS) when compared to trastuzumab + docetaxel + placebo [13, 14]. However, few reports exist regarding the clinical use, safety, and effectiveness of pertuzumab for patients with mBC in the real-world setting.
The purpose of this retrospective, observational study was to investigate the clinical use of pertuzumab in a population of patients with HER2-positive mBC treated in a real-world community oncology practice setting. More specifically, this study included a description of the demographics, clinical characteristics, treatment patterns, and analysis of PFS and adverse events (AEs) for patients with HER2-positive mBC who received pertuzumab as first-line therapy.
Methods
Data Source
In this retrospective study, demographic, clinical, and treatment data were primarily abstracted from the McKesson Specialty Health/US Oncology Network (MSH/USON) iKnowMed (iKM) electronic health record (EHR) database. iKM is an oncology-specific, integrated, web-based system that captures outpatient practice encounter history for patients treated across 19 US states and at over 400 sites of care. Overall, the iKM EHR database captures data from approximately 10% of newly-diagnosed cancer patients in the USA (~750,000) annually and includes 1000 affiliated physicians. MSH/USON’s Claims Data Warehouse (CDW), which records all health insurance claims-related information, was used to obtain payer data. Documented vital status (death) in iKM was supplemented with data from the Social Security Death Index (SSDI). In addition, chart reviews were conducted by clinical staff to supplement and validate data captured by programmatic queries for prespecified endpoints from the iKM EHR.
Patient Selection Criteria
Patients with HER2-positive mBC who initiated treatment with pertuzumab within the study period of 1 June 2012, and 30 June 2014 were eligible for inclusion in the study. All patients were followed through 31 December 2014 (end of study follow-up). The primary analysis population met the following criteria: (1) female patients with HER2-positive mBC; (2) had at least two visits within USON during the study period; (3) received care at a practice utilizing the full EHR capabilities of iKM; (4) ≥18 years old at diagnosis; and (5) received pertuzumab within 60 days of mBC diagnosis during the study period. Patients were excluded if they were diagnosed with or treated for another primary cancer during the study period, or were concurrently enrolled in a clinical trial.
Measures
The date of initiation of pertuzumab was defined as the index date. PFS was defined as the time from the index date until the first disease progression, or death from any cause, whichever occurred earlier. Evidence of progression was captured from chart review. Patients without a progression or death event were censored at the last pertuzumab administration date within the study period, last visit date, or the end of study-follow up, whichever occurred first.
Adverse events were captured from the index date until 30 days after the pertuzumab discontinuation date, or the end of study follow-up, whichever occurred first. Pertuzumab treatment duration was defined as the time from the index date to the last pertuzumab administration date, last visit date, death date, or the end of study-follow up, whichever occurred first.
The Eastern Cooperative Oncology Group (ECOG) performance status at index was recorded for each patient. Comorbidities noted within 6 months prior to the index date were included, and assigned a weight based on the Charlson Comorbidity Index (CCI). Disease type at diagnosis was classified as non-visceral if the metastatic sites were breast, bone/bone marrow, lymph node, skin, or chest wall, or visceral if the metastatic sites were adrenal gland, ascites, brain, cervix, esophagus, gall bladder/bile duct, head and neck localized lesion, kidney, liver, lung, mediastinum, ovary, pancreas, pelvis (non-bone), pericardial effusion, rectum, trachea/bronchi, uterus, or other. Patients with both visceral and non-visceral metastatic sites were considered to be a part of the visceral subset. Among patients with recurrent disease, disease-free interval (DFI) was calculated as the duration from date of primary breast cancer surgery to mBC diagnosis.
Statistical Analysis
All statistical analyses were conducted on the primary analysis population. Descriptive statistics were utilized to summarize demographics, clinical characteristics, treatment patterns, and AEs. Categorical variables were reported as frequency and percentage, and continuous variables were reported as mean, standard deviation, median, and range. Since docetaxel was the taxane used in the CLEOPATRA pivotal trial [13, 14], and as trastuzumab + pertuzumab + docetaxel is also the FDA-approved indicated regimen in the mBC setting [12], a sensitivity analysis was performed on a subset of the primary analysis population who initiated treatment with trastuzumab + pertuzumab + docetaxel. Kaplan–Meier (KM) survival analyses were conducted to estimate median PFS, and PFS rates with their corresponding 95% confidence intervals (CIs) at 6, 9, 12, and 18 months, for the primary and sensitivity analysis populations. All statistical analyses were performed using SAS® 9.2 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
The primary analysis population, i.e., patients with HER2-positive mBC who initiated pertuzumab within 60 days of mBC diagnosis, included 266 patients. Mean (SD) age was 57 (13.7) years (Table 1). The majority of the population was Caucasian (70.7%), and had private insurance (63.1%). ECOG performance status was 0 or 1 for 83.9% of patients, and CCI was 0 for 78.2% of the patients. Visceral disease was noted for 74.8% of patients. About one-half of the patients (49.2%) had de novo mBC. Among patients with recurrent mBC, the median DFI was 33.7 months (range 3.8–471.8) and 61.5% (83/135) of these patients previously received trastuzumab in the adjuvant/neoadjuvant setting. The most common sites of metastases were lymph node (63.5%), bone/bone marrow (52.6%), liver (43.6%), and lung (32.3%). Around 60% of patients had tumors that were ER-positive and/or PgR-positive.
Table 1.
Patient baseline demographics and disease characteristics
| Characteristics |
N = 266 N (%) |
|---|---|
| Age at metastatic breast cancer diagnosis (years) | |
| Mean (SD) | 57.0 (13.7) |
| Median | 57.3 |
| Min–max | 22.2–92.1 |
| Age category | |
| <65 years old | 188 (70.7) |
| ≥65 years old | 78 (29.3) |
| Geographic region | |
| Northeast | 11 (4.1) |
| Midwest | 29 (10.9) |
| South | 160 (60.2) |
| West | 66 (24.8) |
| Payer type | |
| Medicare | 72 (27.1) |
| Medicaid | 5 (1.9) |
| Private | 168 (63.1) |
| Others | 20 (7.5) |
| Unknown | 1 (0.4) |
| Race | |
| White/Caucasian | 188 (70.7) |
| Black/African American | 33 (12.4) |
| Asian | 14 (5.3) |
| Native American | 1 (0.4) |
| Hispanic | 4 (1.5) |
| Other | 2 (0.7) |
| No information | 24 (9.0) |
| Metastatic breast cancer diagnosis | |
| Recurrent mBC | 135 (50.8) |
| de novo mBC | 131 (49.2) |
| Disease-free interval (months) | |
| N | 135 (50.8) |
| Mean (SD) | 51.4 (57.6) |
| Median | 33.7 |
| Min–max | 3.8–471.8 |
| Inflammatory breast cancer (IBC) | |
| IBC disease | 27 (10.2) |
| Non-IBC disease | 239 (89.8) |
| Skin/chest wall involvement | |
| Skin | 39 (14.7) |
| Chest wall | 5 (1.9) |
| Both | 10 (3.8) |
| None | 212 (79.6) |
| Tumor (histological) grade | |
| G1 | 8 (3.0) |
| G2 | 63 (23.7) |
| G3 | 175 (65.8) |
| GX | 14 (5.3) |
| No information | 6 (2.2) |
| Primary tumor site | |
| Left | 129 (48.5) |
| Right | 136 (51.1) |
| No information | 1 (0.4) |
| Hormone receptor status | |
| ER and PgR negative | 111 (41.7) |
| ER and/or PgR positive | 155 (58.3) |
| ECOG performance status (PS) | |
| PS 0 | 64 (24.1) |
| PS 1 | 159 (59.8) |
| PS 2 | 30 (11.3) |
| PS 3 | 4 (1.5) |
| No information | 9 (3.4) |
| Number of sites of metastases | |
| 1 | 62 (23.3) |
| 2 | 76 (28.6) |
| 3 | 52 (19.5) |
| 4 and above | 76 (28.6) |
| Charlson comorbidity index | |
| 0 | 208 (78.2) |
| 1–2 | 52 (19.5) |
| 3–4 | 6 (2.3) |
| ≥5 | 0 |
SD standard deviation, DFI disease-free interval, IBC inflammatory breast cancer, ER estrogen receptor, PgR progesterone receptor, ECOG Eastern Cooperative Oncology Group, PS performance status
A subgroup of 221 patients (83.1% of 266 patients) constituted the sensitivity analysis population, i.e., initiated trastuzumab + pertuzumab + docetaxel within 60 days of mBC diagnosis. The remaining 45 patients were excluded from the sensitivity analysis population for the following reasons: five patients received docetaxel after 60 days of mBC diagnosis, 39 did not receive docetaxel, and one patient received ado-trastuzumab emtansine instead of trastuzumab.
Treatment Patterns
The median duration between the date of mBC diagnosis and that of pertuzumab initiation was 26 days (range 0–60 days). Of the 266 patients, 265 received pertuzumab in combination with trastuzumab, with one patient having received ado-trastuzumab emtansine instead of trastuzumab. The majority of the patients (N = 249/265, 94.0%) received trastuzumab with pertuzumab plus a taxane. Of the remaining patients, 14 (5.3%) patients did not receive any chemotherapy while 2 (0.7%) received vinorelbine. All patients received the indicated pertuzumab loading dose of 840 mg, with the exception of one who received 420 mg instead, and all received the indicated maintenance dose of 420 mg. The median number of cycles of pertuzumab administered was 11 (range 1–41), and the median pertuzumab treatment duration was 7.3 months (range 0.7–29). Among the patients receiving a taxane as their initial chemotherapy (N = 249), 226 (90.8%) received docetaxel, 21 (8.4%) received paclitaxel, and two (0.8%) received nab-paclitaxel. The median number of cycles of docetaxel administered was 6 (range 1–27), and the median duration of docetaxel was 4.1 months (range 0.7–21.9). At the time of this analysis, 38 (14.3%) patients were noted to have received endocrine therapy in the first-line setting. Thus, up to the point of this data cut-off, 24.5% of the patients with hormone receptor-positive disease had received endocrine therapy.
Through the end-of-study follow-up, pertuzumab was discontinued in 170 of the 266 patients (63.9%). The most common reasons for pertuzumab discontinuation were disease progression (N = 76, 28.6%), loss to follow-up (N = 28, 10.5%), toxicity (N = 19, 7.1%), and death (N = 12, 4.5%).
Clinical Outcomes
The most common AEs were fatigue (50.8%), followed by diarrhea (44.7%), nausea (35.3%), and peripheral neuropathy (33.5%) (Table 2). Some patients (N = 17, 6.4%) were noted to have been hospitalized due to AEs. Prophylactic G-CSF use (defined as use during the first cycle of chemotherapy) was noted in 70 (26.3%) patients.
Table 2.
Incidence of select adverse eventsa
| Adverse event |
N = 266 N (%) |
|---|---|
| Fatigue | 135 (50.8) |
| Diarrhea | 119 (44.7) |
| Nausea | 94 (35.3) |
| Peripheral neuropathy | 89 (33.5) |
| Neutropenia | 66 (24.9) |
| Rash | 65 (24.4) |
| Vomiting | 44 (16.5) |
| Decreased appetite | 37 (13.9) |
| Constipation | 35 (13.2) |
| Mucosal inflammation | 33 (12.4) |
| Peripheral edema | 28 (10.5) |
| Alopecia | 19 (7.1) |
| Pruritus | 13 (4.9) |
| Asthenia | 12 (4.5) |
| Dysgeusia | 9 (3.4) |
| Febrile neutropenia | 6 (2.3) |
| Dry skin | 4 (1.5) |
| Hypersensitivity | 4 (1.5) |
| Left ventricular dysfunction | 2 (0.8) |
| Cardiovascular event | 0 |
aAny adverse events regardless of grade
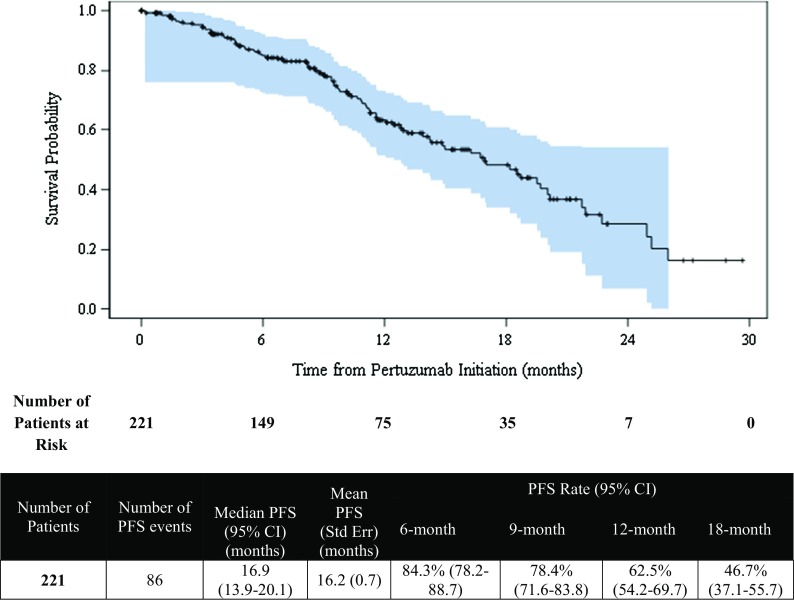
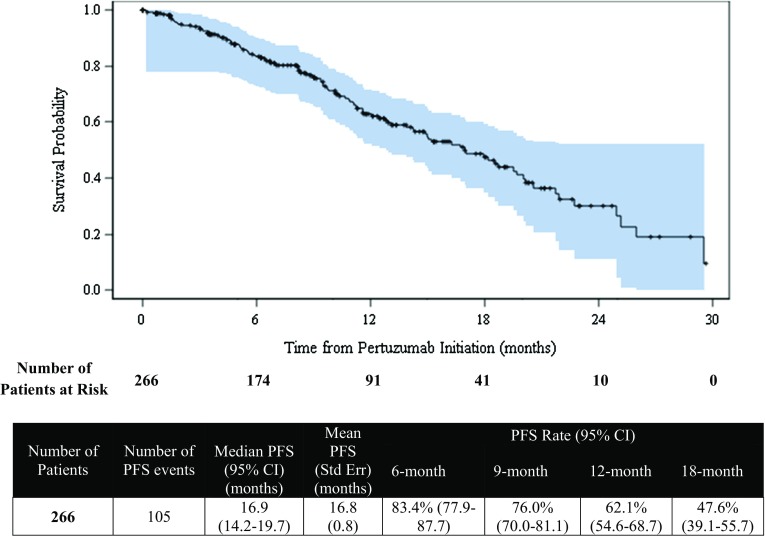
The median duration from pertuzumab initiation to end-of-study follow-up was 16.4 months (range 6.2–30.2). The median PFS was 16.9 months (95% CI 14.2–19.7) for the primary analysis population, as demonstrated in Fig. 1, and the estimated PFS rate at 1 year was 62.1% (95% CI 54.6–68.7). For the sensitivity analysis population, the median PFS was 16.9 months (95% CI 13.9–20.1), and the estimated PFS rate at 1 year was 62.5% (95% CI 54.2–69.7), as shown in Fig. 2.
Fig. 1.
Progression-free survival (PFS) from initiation of pertuzumab (Kaplan–Meier analysis)—primary analysis population
Fig. 2.
Progression-free survival from initiation of pertuzumab (Kaplan–Meier analysis)—sensitivity analysis population
Discussion
The results of CLEOPATRA demonstrated improved outcomes in trastuzumab + pertuzumab + docetaxel compared to trastuzumab + docetaxel + placebo, which has led to trastuzumab + pertuzumab + docetaxel becoming the new standard treatment regimen for HER2-positive mBC in the first-line setting [13–16]. These study findings led to the US FDA approval of the use of pertuzumab in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease. In 2014, the American Society of Clinical Oncology (ASCO) Clinical Practice guidelines recommended pertuzumab and trastuzumab in combination with a taxane chemotherapy as the preferred option for first-line therapy of HER2-positive mBC [17]. The findings of our study are consistent with the CLEOPATRA trial results—the PFS of the primary and sensitivity analysis populations are both quite similar to that observed in the randomized clinical trial.
In this retrospective study, we looked at the clinical and demographic characteristics, treatment patterns, safety, and PFS in a population of patients with HER2-positive mBC who received pertuzumab in the first-line setting in community oncology practices, the US Oncology Network. Between June 2012 and June 2014, a total of 266 patients with HER2-positive mBC initiated pertuzumab based therapy with 221 patients who started treatment with trastuzumab + pertuzumab + docetaxel. The other chemotherapy drugs given were paclitaxel, nab-paclitaxel, vinorelbine, carboplatin, capecitabine, or gemcitabine. The median duration of treatment with pertuzumab was 7.3 months (range 0.7–29). The pertuzumab fixed dose was as in the prescribing information. At 1 year, the estimated PFS rate for patients who initiated pertuzumab was 62.1%, and for those who started trastuzumab + pertuzumab + docetaxel was 62.5%. We found that similar to the pivotal clinical trial, the chemotherapy component was administered for a limited period, with patients then continuing with pertuzumab and trastuzumab. Unlike the CLEOPATRA trial in which endocrine therapy was not permitted, endocrine therapy was used in at least 24.5% of patients with hormone receptor-positive tumors.
There are some differences with our population compared to the CLEOPATRA trial. There was a greater use of adjuvant/neoadjuvant trastuzumab (61.5 vs. 38.6%). The age and percentage of visceral disease were similar in both groups. Regarding AEs, there were fewer side effects reported, but this is probably due to the retrospective nature of this analysis, which is unlike the emphasis of reporting of side effects, including low-grade ones, in a randomized clinical trial. Cardiac toxicities were minimal, which is in accordance with previous studies [18, 19], and there were no CNS toxicities noted.
The strengths of this study include the inclusion of recent data, the ability to observe patients longitudinally over time, and the use of EHRs, which include much more detailed information on medication use than the medical claims often used in studies of this nature. The results provide insights into the real-world use of pertuzumab in the first-line setting among patients with HER2-positive mBC in a rapidly changing therapeutic environment characterized by the recent introduction of new treatment options and the nearly continuous updating of best practices based on emerging data from clinical trials. Our study is subject to inherent limitations of retrospective analyses based on electronic medical records, in that databases may contain inaccurate or missing data. This study utilized abstraction from unstructured clinical notes to assess for and confirm pre-specified endpoints which can be susceptible to error and bias. Finally, progression was evaluated by the treating physicians with the assessments done per the individual physician’s standard-of-care, which may be subjected to their own judgment. Also in this study, only univariate analyses were conducted; further investigation with a larger population, longer follow-up time, and multivariate analyses is warranted.
Conclusions
In this retrospective study of a real-world setting utilizing pertuzumab as part of the first-line therapy of HER2-positive mBC, we found that most patients were treated with pertuzumab, trastuzumab, and a taxane. The dose and frequency of pertuzumab administration were consistent with the prescribing information. Our results confirmed the clinical safety and PFS of pertuzumab as first-line therapy for patients with HER2-positive mBC in the community treatment setting. Moreover, the use of pertuzumab in this setting was consistent with the NCCN guidelines and, for the most part, with the FDA-approved Perjeta® (pertuzumab) prescribing information.
Acknowledgements
Portions of the research described in this article were presented at the 18th ECCO—40th ESMO European Congress, 25–29 September 2015, Vienna, Austria, and San Antonio Breast Cancer Symposium, 8–12 December 2015, San Antonio, TX, USA. We acknowledge Wan-Yu Tseng, Eduardo Santos, Lina Asmar, and Chia Portera for their valuable contributions in the early stages of this study, and Marley Boyd for assistance with editing.
Author contributions
Each author (N. Robert, H. P. Goertz, P. Chopra, X. Jiao, B. Yoo, D. Patt, V. Antao) made substantial contributions to all aspects of this manuscript including conception and planning of the work that led to the manuscript or acquisition, analysis, and interpretation of the data; drafting and/or critical revision of the manuscript for important intellectual content; and approval of the final submitted version of the manuscript for the entire content of this manuscript.
Compliance with Ethical Standards
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. This study was approved by the Institutional Review Board.
Conflict of interest
Hans-Peter Goertz, Bongin Yoo, and Vince Antao are employees of Genentech, Inc, and own stock/stock options in Roche. Pooja Chopra, Nicholas Robert, and Xiaolong Jiao are employees of McKesson Specialty Health, which has received consultancy fees from Genentech, Inc. Debra Patt has no conflicts of interest to declare.
Funding
This study was funded by Genentech, Inc.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 5.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/S1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 6.Sliwkowski MX, Schaefer G, Akita RW, et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- 7.Schaefer G, Fitzpatrick VD, Sliwkowski MX. Gamma-heregulin: a novel heregulin isoform that is an autocrine growth factor for the human breast tumor cell line, MDA-MB-175. Oncogene. 1997;15:1385–1394. doi: 10.1038/sj.onc.1201317. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick VD, Pisacane PI, Vandlen RL, et al. Formation of a high affinity heregulin binding site using the soluble extracellular domains of ErbB2 with ErbB3 or ErbB4. FEBS Lett. 1998;431:102–106. doi: 10.1016/S0014-5793(98)00737-6. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol. 2002;27:306–313. doi: 10.1165/rcmb.4850. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza N, Phillips GL, Silva J, et al. Inhibition of ligand-mediated HER2 activation in androgen-independent prostate cancer. Cancer Res. 2002;62:5485–5488. [PubMed] [Google Scholar]
- 11.Capelan M, Pugliano L, Azambuja ED, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2012;0:1–10. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 12.Perjeta Prescribing Information. Genentech, Inc., South San Francisco, CA. 2016. http://www.gene.com/download/pdf/perjeta_prescribing.pdf. Accessed March 2016.
- 13.Swain SM, Baselga J, Kim SB, CLEOPATRA Study Group et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack PL. Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer. Drugs. 2013;73:1491–1502. doi: 10.1007/s40265-013-0109-0. [DOI] [PubMed] [Google Scholar]
- 16.Chung C, Lam MS. Pertuzumab for the treatment of human epidermal growth factor receptor type 2-positive metastatic breast cancer. Am J Health Syst Pharm. 2013;70:1579–1587. doi: 10.2146/ajhp120735. [DOI] [PubMed] [Google Scholar]
- 17.Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2078–2099. doi: 10.1200/JCO.2013.54.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain SM, Ewer MS, Cortés J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]