High-dose BEAM (carmustine, etoposide, cytarabine, and melphalan) chemotherapy is the most common conditioning regimen for autologous hematopoietic cell transplantation (AHCT) recipients with lymphoma.1 Despite wide utilization of BEAM, there are limited data on the need for dose modifications in obese patients, resulting in center-specific and empiric adjustments.2,3,4 A guideline to dose BEAM in obese patients has become necessary since the obesity rate has doubled to >30% in the past few decades.5 Obese patients have relatively more lean body weight, bone mass, and blood volume than non-obese patients, and obesity may affect renal and hepatic function and increase the volume of distribution for lipophilic drugs all of which may lead to altered drug pharmacokinetics.6
A variety of dosing strategies have been employed in obese patients including using total body weight (TBW), adjusted body weight, or ideal body weight (IBW)7,8; however, arbitrary dose reductions may lead to under-dosing and result in treatment failure. In this study, we report a cohort of patients prospectively treated with BEAM using total body weight without weight-adjustment and compare short-term transplant outcomes focusing on toxicity and early disease control among non-obese versus obese patients.
We studied 91 consecutive patients prospectively enrolled on Institutional Research Board of University of Minnesota approved protocol using BEAM and AHCT between July 2013 and December 2015. All patients signed informed consent. The eligibility criteria included relapsed non-Hodgkin (NHL) or Hodgkin lymphoma with chemo-sensitive disease or high-risk NHL in 1st remission, and no extreme organ dysfunction. Detail on diagnosis of mucositis, gastrointestinal (GI) toxicity, infection rates, number of readmissions, number of inpatient days, and use of total parenteral nutrition (TPN) was completed using retrospective chart review. The BEAM conditioning regimen consisted of carmustine 300 mg/m2 IV once on day -6, etoposide 100 mg/m2 IV twice daily on days -5 to -2, cytarabine 100 mg/m2 IV twice daily on days -5 to -2, and melphalan 140 mg/m2 IV once on day -1. Doses were calculated using total body weight and were not adjusted for overweight or obese patients. Ten non-obese patients had melphalan dosed at 3.6 mg/kg and none in the obese group.2 Autologous hematopoietic cell graft was infused on day 0 and G-CSF started on day +5. Obesity was defined as ≥ 125% of IBW using standard Devine formulas (male IBW = 50 kg + 2.3[height (in) − 60(in)]; female IBW = 45.5 kg + 2.3[height (in) − 60(in)]). Hematologic recovery was defined as absolute neutrophil count (ANC) ≥ 0.5 × 109/L on the first of three consecutive days and platelet count ≥ 20 × 109/L.
Patient, disease, and treatment characteristics of 46 non-obese and 45 obese patients are compared in Table 1. The median weight and BMI for non-obese patients was 70 kg and 24.5 and for obese patients, 95 kg and 31.9 respectively. Two patients were below their IBW (both BMI’s were 18.3). The median age was similar for both groups. DLBCL and mantle cell lymphoma were the most common histologies. The non-obese group had more males, more patients in CR, a shorter median time form diagnosis to transplant and higher proportion of patients with KPS below 90% compared to the obese group. Mean total nucleated cell (TNC) and CD34+ counts per kg did not differ between the two cohorts. Median follow-up was 12 months (range 3–25 months).
Table 1.
Patient, disease and transplant characteristics by weight groups
| < 125% of IBW (n=46) | ≥ 125% of IBW (n=45) | |
|---|---|---|
| Male | 30 (65%) | 24 (53%) |
| Age, median (range) | 58 (22–74) | 59 (34–75) |
| BMI, median (range) | 24.5 (18–29) | 31.9 (26–49) |
| Weight (kg), median (range) | 70 (41–105) | 95 (66–139) |
| Lymphoma Diagnosis | ||
| Diffuse large B cell NHL | 21 (46%) | 30 (67%) |
| Mantle cell lymphoma | 11 (24%) | 8 (18%) |
| Follicular NHL | 5 (11%) | 4 (9%) |
| T cell NHL | 7 (15%) | 1 (2%) |
| Other* | 2 (4%) | 2 (4%) |
| Disease status | ||
| CR | 33 (72%) | 30 (67%) |
| PIF/REL sensitive | 12 (26%) | 14 (31%) |
| Stable disease | 1 (2%) | 1 (2%) |
| Months from dx to HCT, median (range) | 10 (3–113) | 19 (3–250) |
| Karnofsky score | ||
| 100 | 21 (46%) | 22 (49%) |
| 90 | 17 (37%) | 20 (44%) |
| 80 | 8 (17%) | 3 (7%) |
| HCT-CI | ||
| 0 | 18 (39%) | 18 (40%) |
| 1–2 | 17 (37%) | 19 (42%) |
| > 2 | 10 (22%) | 8 (18%) |
| Unknown | 1 (2%) | 0 (0%) |
| Year of HCT | ||
| 2013 | 6 (13%) | 4 (9%) |
| 2014 | 16 (35%) | 16 (36%) |
| 2015 | 24 (52%) | 25 (56%) |
| TNC infused × 108/kg, mean (SD) | 15.0 (8.4) | 14.8 (9.5) |
| CD34+ cells infused × 106/kg, mean (SD) | 5.2 (3.5) | 4.9 (2.2) |
IBW, ideal body weight; BMI, body mass index; HCT-CI, Hematopoietic Cell Transplantation-Comorbidity Index. TNC, total nucleated cell. N (% of column total) reported for categorical variables; median (range) or mean (SD) for continuous variables.
Other diagnoses consisted of HIV-associated primary effusion lymphoma and lymphomatoid granulomatosis in the non-obese group and Hodgkin’s and Burkitt’s lymphoma in the obese group.
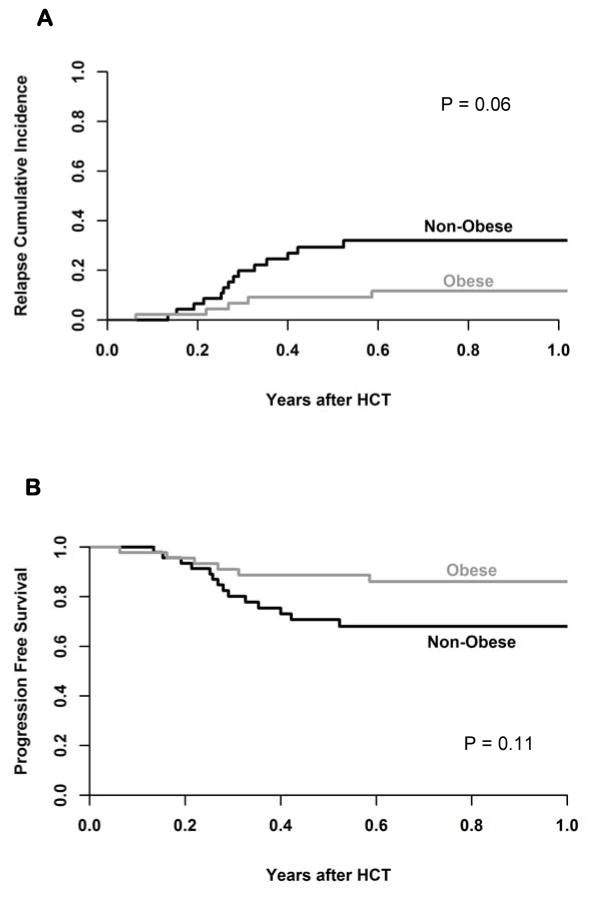
We observed no transplant related mortality (TRM) using the BEAM regimen in this cohort. The cumulative incidence of relapse at 1 year was 32% (95% CI 20–48%) for non-obese and 12% (95% CI 3–25%, P=0.06) for obese patients (Figure 1A); this relapse rate resulted in similar PFS of 68% (95% CI 52–80%) for non-obese and 86% (95% CI 71–94%; P=0.11) for obese patients. OS at one year was 79% (95% CI 62–89%) for non-obese and 91% (95% CI 78–97%, P=0.2) for obese patients (Figure 1B). In univariate analysis, obesity was not predictive of OS or PFS. The eight patients with T-cell NHL had 3.3-fold higher risk of treatment failure as compared to DLBCL, and all 9 patients with follicular lymphoma survived free from progression at 1-year. Patients without CR prior to HCT had a tendency to have lower PFS (Hazard ratio 1.9, 95% CI 0.8–4.8). Gender, age, KPS, HCT-comorbidity index, and graft cell dose were not predictors of PFS.
Figure 1.
(A) cumulative incidence of relapse by groups and (B) Progression free survival.
Median day of absolute neutrophil count (ANC) recovery occurred on days 10 and 11 (P=0.59), and median day of platelet recovery occurred on day 19 (P=0.52) in the non-obese and obese cohorts respectively. Two thirds of patients (65% and 71% among the non-obese and obese) were re-admitted with post-transplant complications and were hospitalized for a median 12 and 14 days. Complications requiring admission for IV pain medication and IV hydration such as moderate to severe oro-pharyngeal mucositis (30% non-obese vs 36% obese; P=0.66) and non-infectious diarrhea (41% non-obese vs 58% obese; P=0.14) occurred frequently and contributed to use of total parenteral nutrition (TPN) support (22% of non-obese and 24% of obese patients; P=0.81). The cumulative density of bacteremia through day 100 was 0.55 (95% CI 0.37–0.81) and 0.45 (95% CI 0.29–0.70; P=0.55) for non-obese and obese cohorts, respectively.
In univariate analysis, the most significant factor associated with increased readmission was low CD34+ count < 4.5 × 106/kg (Odds Ratio (OR) 2.6, 95% CI 1.1–6.6). Additional factors that non-significantly increased re-admission risk were age > 62 years (OR 1.9, 95% CI 0.6–6.0) and HCT-CI score >2 (OR 3.2, 95% CI 0.8–13). Obesity, gender, lymphoma diagnosis, disease status, KPS, and TNC count did not affect the readmission rate.
Considerable variation of preparative chemotherapy dosing strategies/modifications for obese and overweight patients exists among transplant centers. As a result, the Center of International Blood and Marrow Transplantation Registry (CIBMTR) initiated a task-force to develop recommendations on dose adjustments of preparative regimen for obese and overweight patients. In this single-center study utilizing the BEAM regimen followed by AHCT, we found that obesity does not impact short-term survival of lymphoma patients. Moreover, the use of BEAM using unadjusted full doses based on total body weight followed by AHCT yields similar OS and PFS in obese and non-obese patients. In addition, the rates of GI and infectious complications and re-admission were not increased in obese patients, suggesting that arbitrary dose reduction of the BEAM regimen is not needed. While the negative impact of obesity on long-term survival in the general population and cancer patients has been previously reported9, obesity did not increase the risk of treatment related morbidity and mortality in AHCT for lymphoma. Both carmustine and cytarabine have large volumes of distribution and etoposide is highly lipophilic. These properties could potentially alter the pharmacokinetic and pharmacodynamic properties in obese patients and may result in a depot effect and prolonged drug exposure, supporting the observed trend to lower relapse in obese patients, yet without increased toxicity.
A registry study by Navarro et al. compared the outcomes by weight cohorts in 4681 Hodgkin’s and NHL patients undergoing AHCT.10 The overweight and obese cohorts had a lower relative risk of overall mortality compared to the normal BMI cohort without significant differences for TRM or relapse. Interestingly, the authors found that underweight patients had significantly worse outcomes. Our study only had two patients below normal BMI; one had a prolonged hospital admission with GI toxicity and TPN use, and both are surviving long-term. Another study with 80 DLBCL patients dosed BEAM conditioning using an adjusted body weight (defined as IBW plus 25% of the difference between actual and IBW) and reported no significant differences in relapse or OS among the weight groups.2 Obese patients had shorter hospital stays and less occurrence and severity of mucositis, potentially suggesting less drug exposure or lower drug peaks.
In conclusion, our results support the use of total body weight to calculate the chemotherapy doses for obese patients treated with BEAM. While the size and follow-up of this cohort is limited, our research design focused on short-term outcomes and post-transplant morbidities, and all patients were followed for at least 100 days including details on hospitalizations and TPN use. InnovativeFuture investigations should focus on strategies to reduce morbidity and prevent GI and infectious complication in all patients treated with BEAM. Future research using larger registry data may elucidate subtler differences in major outcomes of interest.
Footnotes
Financial disclosures: None
Conflict of interest: Authors declare no conflicts of interest.
References
- 1.Puig N, de la Rubia J, Remigia MJ, Jarque I, Martín G, Cupelli L, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma. 2006;47(8):1488–94. doi: 10.1080/10428190500527769. [DOI] [PubMed] [Google Scholar]
- 2.Costa LJ, Micallef IN, Inwards DJ, Johnston PB, Porrata LF, Litzow MR, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol. 2008;143(2):268–73. doi: 10.1111/j.1365-2141.2008.07342.x. [DOI] [PubMed] [Google Scholar]
- 3.Blijlevens N, Schwenkglenks M, Bacon P, D’Addio A, Einsele H, Maertens J, et al. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy--European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol. 2008;26(9):1519–25. doi: 10.1200/JCO.2007.13.6028. [DOI] [PubMed] [Google Scholar]
- 4.Tarella C, Caracciolo D, Gavarotti P, Argentino C, Zallio F, Corradini P, et al. Overweight as an adverse prognostic factor for non-Hodgkin’s lymphoma patients receiving high-dose chemotherapy and autograft. Bone Marrow Transplant. 2000;26(11):1185–91. doi: 10.1038/sj.bmt.1702692. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bubalo J, Carpenter PA, Majhail N, Perales MA, Marks DI, Shaughnessy P, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biol Blood Marrow Transplant. 2014;20(5):600–16. doi: 10.1016/j.bbmt.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Navarro WH. Impact of obesity in the setting of high-dose chemotherapy. Bone Marrow Transplant. 2003;31(11):961–6. doi: 10.1038/sj.bmt.1704052. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Navarro WH, Loberiza FR, Bajorunaite R, van Besien K, Vose JM, Lazarus HM, et al. Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12(5):541–51. doi: 10.1016/j.bbmt.2005.12.033. [DOI] [PubMed] [Google Scholar]