Abstract
Objectives
Stable closure of full-thickness burn wounds remains a limitation to recovery from burns of greater than 50% of the total body surface area (TBSA). Hypothetically, engineered skin substitutes (ESS) consisting of autologous keratinocytes and fibroblasts attached to collagen-based scaffolds may reduce requirements for donor skin, and decrease mortality.
Methods
ESS were prepared from split-thickness skin biopsies collected after enrollment of 16 pediatric burn patients into an approved study protocol. ESS and split-thickness autograft (AG) were applied to 15 subjects with full-thickness burns involving a mean of 76.9% TBSA. Data consisted of photographs, tracings of donor skin and healed wounds, comparison of mortality with the National Burn Repository (NBR), correlation of TBSA closed wounds with TBSA full-thickness burn, frequencies of regrafting, and immunoreactivity to the biopolymer scaffold.
Results
One subject expired before ESS application, and 15 subjects received 2056 ESS grafts. The ratio of closed wound to donor areas was 108.7±9.7 for ESS compared with a maximum of 4.0±0.0 for AG. Mortality for enrolled subjects was 6.25%, and 30.3% for a comparable population from the NBR (p<0.05). Engraftment was 83.5±2.0% for ESS and 96.5±0.9 for AG. Percentage TBSA closed was 29.9±3.3% for ESS, and 47.0±2.0 for AG. These values were significantly different between the graft types. Correlation of % TBSA closed with ESS with % TBSA full-thickness burn generated an R2 value of 0.65 (p<0.001).
Conclusions
These results indicate that autologous ESS reduce mortality and requirements for donor skin harvesting, for grafting of full-thickness burns of greater than 50% TBSA.
Keywords: Burns, Wound healing, Engineered skin substitutes, Skin grafts, Regenerative medicine
Introduction
Permanent wound closure remains a limiting factor in recovery from extensive, full-thickness burn injuries. Recovery from massive burns requires complex critical care that includes, but is not limited to: fluid resuscitation, cardiovascular and respiratory support, nutritional support of hyper-metabolism and immune function, management of microbial contamination and infection, physical therapy and psycho-social adaptation. However, recovery depends ultimately on closure of the wounds with autologous epidermis and connective tissue to provide stable healing with minimal scar1. Furthermore, while wound closure is usually a requirement for discharge from the hospital, skin pliability and stability are essential for the recovery of range of motion2,3, and contribute importantly to long-term quality of life.
Several alternatives have been studied to accomplish more rapid wound closure4,5. Temporary wound coverage before autografting has been reported with a bi-layered, allogeneic skin substitute6. Cultured epithelial autografts applied as partially-stratified, keratinocyte sheets have been studied extensively7,8, but are reported to blister, ulcerate, and remain mechanically fragile due to deficient formation of basement membrane9. Cultured keratinocytes have also been sprayed as cell suspensions10 over partial-thickness burns11, or a dermal substitute12,13, but the time to healing may be lengthy due to the slow organization of the cultured cell suspensions into stratified, keratinized epidermis. Replacement of dermal tissue has also been shown to reduce long-term morbidity from scarring. Dermal substitutes from natural or engineered sources8,14–16 have been reported to provide connective tissue beneath either epidermal autograft, or cultured keratinocytes. More recently, favorable results have been reported using a bilayered, autologous skin substitute in an initial clinical trial17. However, none of these alternatives has displaced unmeshed, split-thickness skin autograft, which has been reported to provide superior results in pediatric burns, and grafting to the face, hands or genitalia18–20.
Previous reports from this laboratory have described the design and testing of engineered skin substitutes (ESS) prepared from epidermal keratinocytes and dermal fibroblasts attached to collagen-glycosaminoglycan scaffolds21–23. The epidermal substitute stratifies and keratinizes in vitro to initiate formation of an epidermal barrier24,25. Proliferating keratinocytes attach directly to dermal fibroblasts on the surface of the biopolymer scaffold, and initiate development of a basement membrane which inhibits blistering after healing23,26. Clinical experience with this model has shown healing of burns, surgical wounds or chronic wounds27–30. The present study is a pre-pivotal investigation of autologous ESS (previously called “cultured skin substitutes”29,31) to evaluate whether this device provides new medical benefits for treatment of burns of greater than 50% of the total body surface area (TBSA). Subjects were enrolled from 2007–2010, and were followed for one year for data collection. Reduced mortality for these subjects in comparison to data from the National Burn Repository (NBR)32 was presented previously33.
Methods
Subjects and experimental design
This study was performed from 2007–2010 with a protocol approved by the Institutional Review Board of the University of Cincinnati, and under an Investigational Device Exemption (IDE) application regulated by the US Food and Drug Administration (FDA). Under this IDE, autologous ESS were considered as medical devices. All subjects were enrolled into the study with Informed Consent Forms, and acknowledged the protection of healthcare information according to the Health Insurance Portability and Accountability Act. During an inspection by FDA in 2006, deficiencies in protocol performance were cited, including lack of data monitoring. In 2007, FDA issued a Data Integrity Hold on the IDE, but the investigators were permitted to continue subject recruitment by Compassionate Use Enrollments34. This report summarizes results from those Enrollments. In addition, an audit of retrospective data, and monitoring of prospective data were required. All data collection was completed for all subjects reported here, data were either audited or monitored, and the Hold was lifted. This study was registered on ClinicalTrials.gov titled, “Autologous Engineered Skin Substitutes for Closure of Skin Wounds”: https://clinicaltrials.gov/ct2/show/NCT00591513?term=Cultured+skin+substitutes&rank=2
The study design consisted of a prospective, randomized, open-label, paired-site comparison of excised, full-thickness burns grafted with ESS and split-thickness skin autograft (AG). Of the 16 subjects, one male subject expired before the ESS grafts were prepared. The remaining 15 subjects survived, completed the study and were included in the data analysis.
ESS were meshed at a ratio of 1 to 1.5 and not expanded, and AG was meshed and expanded between 1 to 1.5 and 1 to 4. Application sites were paired by selecting adjacent, contra-lateral or anterior-posterior areas that required skin grafting. Two sites (~150 cm2 each) were randomized as “A” or “B” prior to the beginning of the study31. Comparative grafting was performed in one procedure for each subject. If additional applications of ESS were performed, they were measured only for closed areas of wounds. If additional applications of AG were performed, they were not evaluated29,31. The main hypothesis of the study was that ESS close greater areas of wounds than AG per unit of skin autograft harvested.
Data collection and calculations
The mortality rate in this study population was compared by a one sample z-test to values from the 2012 NBR32 for patients between 0–19.9 years of age, and burns of 50% TBSA and greater. Quantitative measurements consisted of tracings and planimetry of skin biopsies used to generate ESS, and tracings of treated areas on post-operative days (POD) 14 and 28. TBSA was calculated according to Mosteller35, and % burn by using the Lund-Browder formula36. From the area tracings and planimetry, calculations were made of:
-
(1)
% Area Closed at POD 14 and 28 = (closed area / total treated area) × 100
-
(2)
Ratio of Closed:Donor Areas at POD 28 = area closed with ESS / donor area
-
(3)
% TBSA closed at POD 28 = (area closed with ESS / TBSA) × 100
Engraftment was defined as the percentage of treated areas closed with dry epithelium at POD 14. For AG, the ratio of closed-to-donor areas was assigned as single value of 4 per harvest, and the % TBSA closed was calculated as the % TBSA full-thickness burn minus the % TBSA closed with ESS. Regrafting of comparative sites was recorded through POD 28, was scored as “None”, “Partial” or “Total”, and expressed as percentages of subjects treated.
Formation of antibodies to the biopolymer scaffold was assessed by collection of serum prior to the first application of ESS, and 28 days or later after exposure. Post-exposure sera were tested by Enzyme-Linked ImmunoSorbant Assay (ELISA) compared to pre-treatment sera, and positive control sera from rabbits immunized with a homogenate of the collagen-GAG scaffold together with Freund’s adjuvant.
Wound biopsies were collected before surgical application of ESS and AG, and as possible up to 1 year after grafting. Histological assessments were informational only.
Preparation, quality assurance and delivery of autologous ESS
Biopsy samples of split-thickness skin (0.010–0.012 inches thick) were collected as early as possible after injury, usually during the first operative procedure. The absolute areas (cm2) to be treated with ESS, and for the ESS biopsy for each patient were estimated with the following formulae31.
-
(4a)
% TBSA eligible for ESS = (% TBSA of full-thickness burn) − (40% TBSA estimated to be treated with AG)
-
(4b)
Absolute area (cm2) to be treated with ESS = (% TBSA eligible for ESS) × (TBSA (cm2))
-
(5)
Absolute area (cm2) of ESS biopsy = Absolute area (cm2) to be treated with ESS × 0.01
Formula 4a assumed that about 40% TBSA would be treated with AG during the time of ESS preparation, based on two skin grafting operations during about 4 weeks covering about 20% TBSA per operation. In cases of very extensive burns (e.g., >80% TBSA), the value of 40% TBSA coverage with AG was revised downward upon the advice of the medical staff, with a consequent increase in biopsy area (Formula 5). Epidermal keratinocytes and dermal fibroblasts were isolated and propagated as previously described37,38. Fibroblasts were inoculated at 3.75–5.0 × 105 cells/cm2 onto collagen-glycosaminoglycan scaffolds39, followed one day or later by keratinocytes at 0.75–1.0 × 106 cells/cm2, and incubation at the air-liquid interface was performed to promote attachment and keratinization23,40,41. ESS (approximately 25–30 cm2 each) were usually meshed as described above, and applied on incubation day 10–14. Each dose of ESS consisted typically of 32 ESS devices with a total area of 750–1000 cm2.
Wound preparation, grafting and post-operative care
Burn eschar was excised as early as possible after completion of resuscitation, and sites planned for treatment with ESS were covered with cadaveric allograft, or the dermal replacement, Integra® Dermal Regeneration Template (Integra LifeSciences Corp; Plainsboro, NJ)28,42. Grafting and post-operative care were performed as described previously31,43,44.
Statistical analyses
Student’s t-test was used for comparisons between graft types. Spearman’s rank order was applied to correlations between factors. Fisher’s exact test was used to distinguish differences in frequencies of events. Analysis of variance detected differences among multiple groups. Data were independently audited or monitored, and reviewed by an independent Data Safety Monitoring Board. Primary analyses of data for quantitative end points were performed on POD 28. Data from positive/negative scoring of site regrafting was subjected to Fischer’s exact test. For the end point, ratio of closed-to-donor areas, a single-value t-test was applied to compare ESS to a maximum value of 4 per harvest of AG. Actual values for expansion of AG were most often less than 3, but were not recorded for all AG applied to all patients in this study, and none was greater than 4. This statistical approach minimizes the benefit of ESS for this end point, and therefore was considered the most conservative statistical approach. Statistical significance was accepted at the 95% confidence level.
Results
Sixteen subjects were enrolled between February 2007 and July 2010. Subjects were consecutive hospital admissions who met enrollment inclusion/exclusion criteria. Mean age (±SEM) was 6.3 ± 1.1 years, range of 1.4 – 17.5. Fourteen subjects were male and 2 female. Mean TBSA burn was 79.1 ± 2.2%, range of 59.5–95.0%. Mean FT TBSA burn was 77.9 ± 2.4%, range 58.8–95.0%. Mean TBSA ESS per subject was 33.4 ± 3.5%, range 9.7–71.6%. Mean number of days from skin harvest to first application was 32.1 ± 1.1, range 24–42.
Figure 1 shows microscopic anatomy of AG (Figure 1A) and ESS (Figure 1B) prior to grafting. Both have dermal and epidermal components with a total thickness of less than 400 µm. The dermal component of ESS consisted of reticulations of collagen-glycosaminoglycan (GAG) biopolymer populated with cultured fibroblasts to which the epidermal component is attached biologically. The epidermal component consisted of cultured keratinocytes that stratified, and formed an analog of stratum corneum, which is the primary component of the epidermal barrier. The ESS lacked blood vessels, so were perfused entirely by angiogenesis, rather than by inosculation of blood vessels in the wound to those in the graft as occurred in AG.
Figure 1.
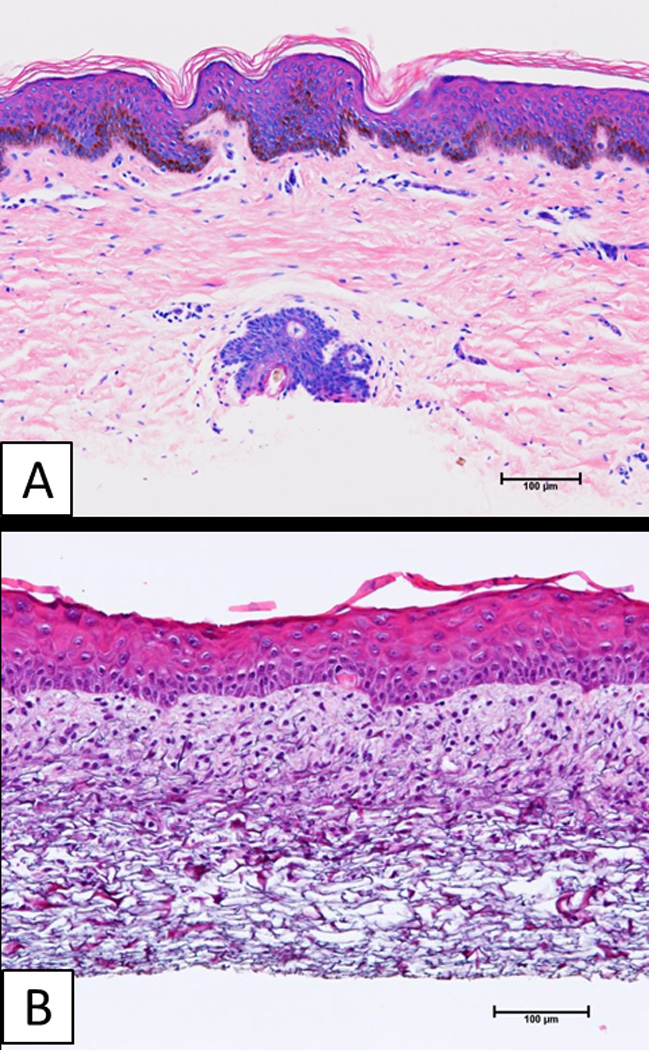
Histologic anatomy of split-thickness skin autograft (AG), and engineered skin substitutes (ESS) prior to surgery. A) Split-thickness skin has a fully keratinized epidermis, and vascularized dermis with epidermal appendages. B) Engineered skin substitutes have partially keratinized epidermis, and a dermal substitute without a vascular network. Scale bar = 0.1 mm.
In this study, 2056 ESS grafts totaling an area of 4.89 m2 were applied in 59 operative procedures. An informational example of surgical application of ESS, and healing during the first two months after surgery is shown in Figure 2. In this subject, ESS were applied (Figures 2A, 2B), and accomplished more than 90% wound closure at POD 14. At POD 28 (Figure 2C), the closed wounds were stable and use of pressure garments was begun. The healed ESS remained stable, pliable and hypopigmented at POD 62 (Figure 2D). Histological anatomy of wound beds had reticulations of Integra with fibro-vascular tissue, and were observed at the pre-graft sites of both ESS and AG (Figures 2E, 2F). At POD 105, the epidermis had matured, and remained stable and tightly-adhered to connective tissue in both closed wounds (Figures 2G, 2H). Neither healed ESS nor AG developed glands or follicles. The dermal-epidermal junction remained relatively linear indicating the absence of rete peg formation.
Figure 2.
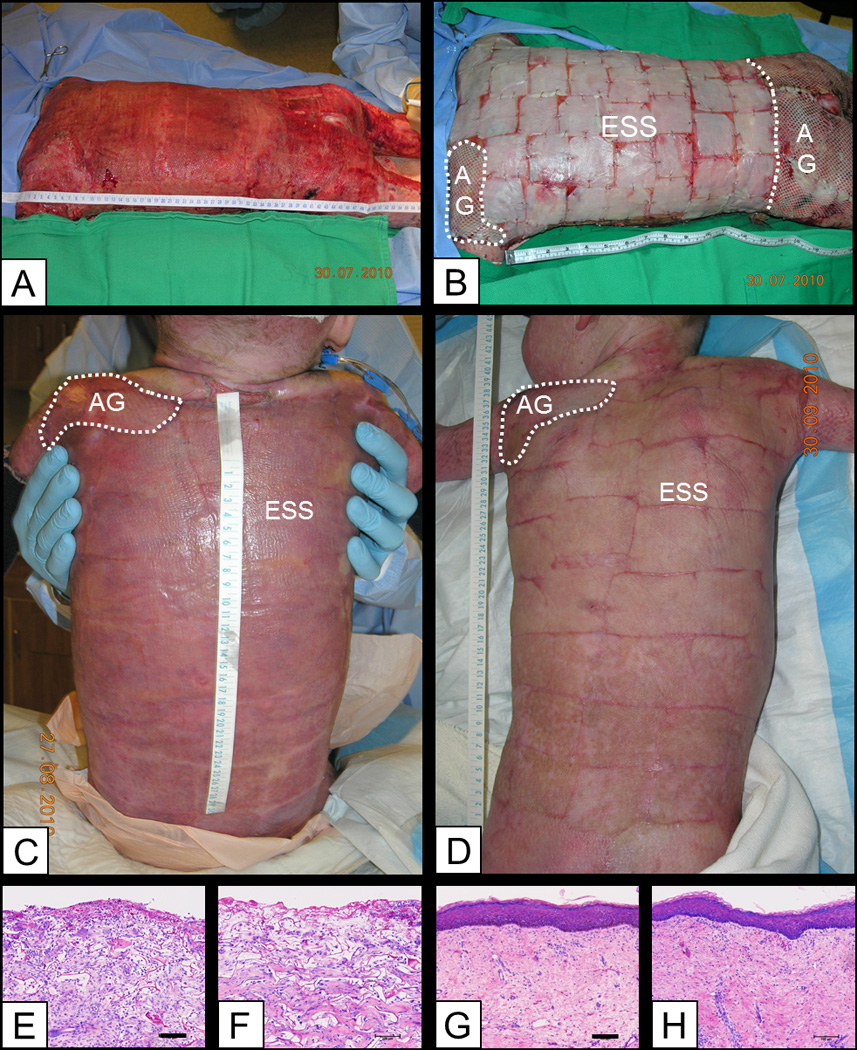
Clinical observations from surgical application of ESS and AG to a single subject through post-operative day (POD) 62. A) Prepared wound bed consisting of Integra Dermal Regeneration Template. B) Surgical application of engineered skin substitutes (ESS) and autograft (AG) comparative site. C) POD 28. D) POD 62 shows areas treated with split-thickness autograft (left shoulder), or ESS. Scales in cm. E, F) Wound beds for ESS (E) and AG (F) showed development of fibro-vascular tissue into reticulations of Integra Dermal Regeneration Template. G, H) At POD 105, ESS (G) showed a vascularized connective tissue component with orthogonal collagen fibers, and a stratified epidermis with a relatively flat dermal-epidermal junction. Similarly, the connective tissue component of AG (H) was well vascularized with a fully-stratified epidermis and a relatively flat dermal-epidermal junction. Scale bar = 0.1 mm.
Healed wounds remained pliable and hypopigmented at POD 360 (Figure 3A, 3B). By 1 year after grafting in this subject, the autograft comparative site had developed hair growth by transplantation from the donor site (Figure 3C), and the ESS site was smooth and without hair or glands (Figure 3D).
Figure 3.
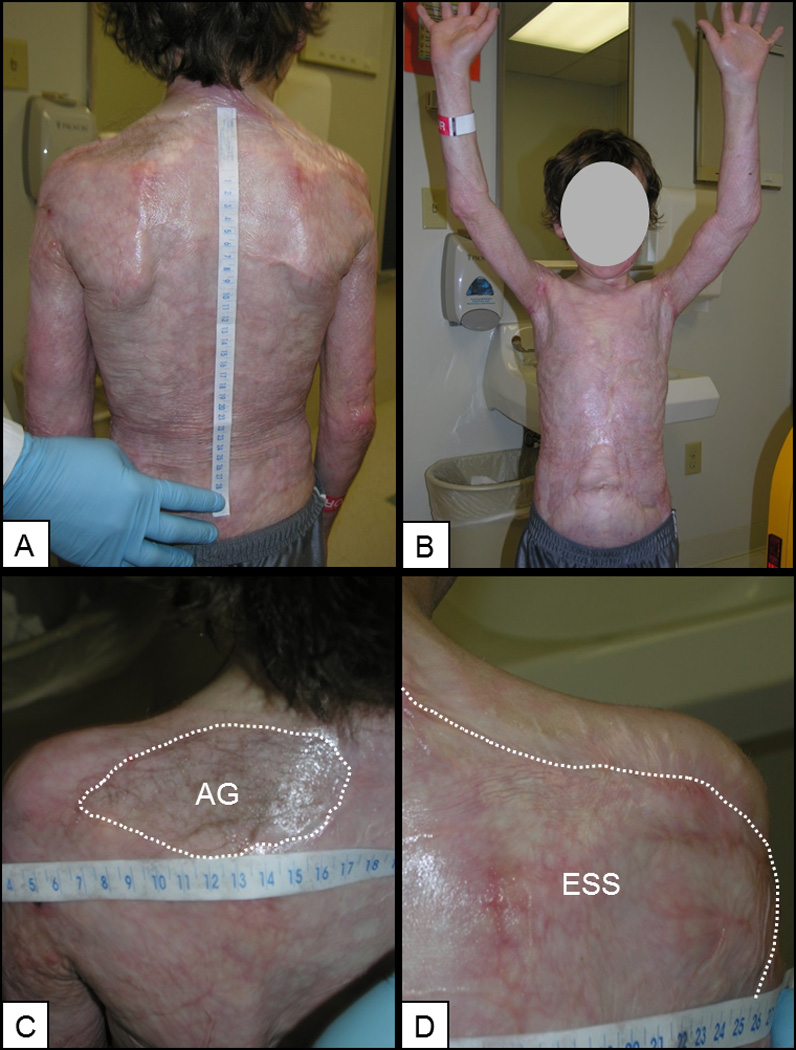
Clinical examination of a single subject at POD 360. A,B) Posterior and anterior torso, and demonstration of range of motion of the upper extremities. C) Autograft (AG) has developed hair transferred from the donor site. D) ESS has no hair or glands. Scales in cm.
The ratio of closed wound area to donor skin area for ESS was 108.7, versus a maximum of 4 per harvest of AG (Figure 4A). This significant difference (p<0.01) represents a reduction of donor skin harvesting of more than an order of magnitude by use of ESS, and demonstrates the primary medical benefit of this alternative therapy. Mortality in this study (Figure 4B) was 6.25% (1/16) which was significantly lower (p=0.037) than a rate of 30.3% (305/1008) for a population of similar age (0–19.9 years) and burn magnitude (50% TBSA or greater) reported in the NBR. Epithelial engraftment and wound closure at POD 14 (Figure 4C) was 83.5% for ESS compared to 96.5% for AG which was a significant difference (p<0.05). Percentage TBSA closed with ESS was 29.9% and 47.0% for AG at POD 28 (Figure 4D). This difference was significant (p<0.05).
Figure 4.
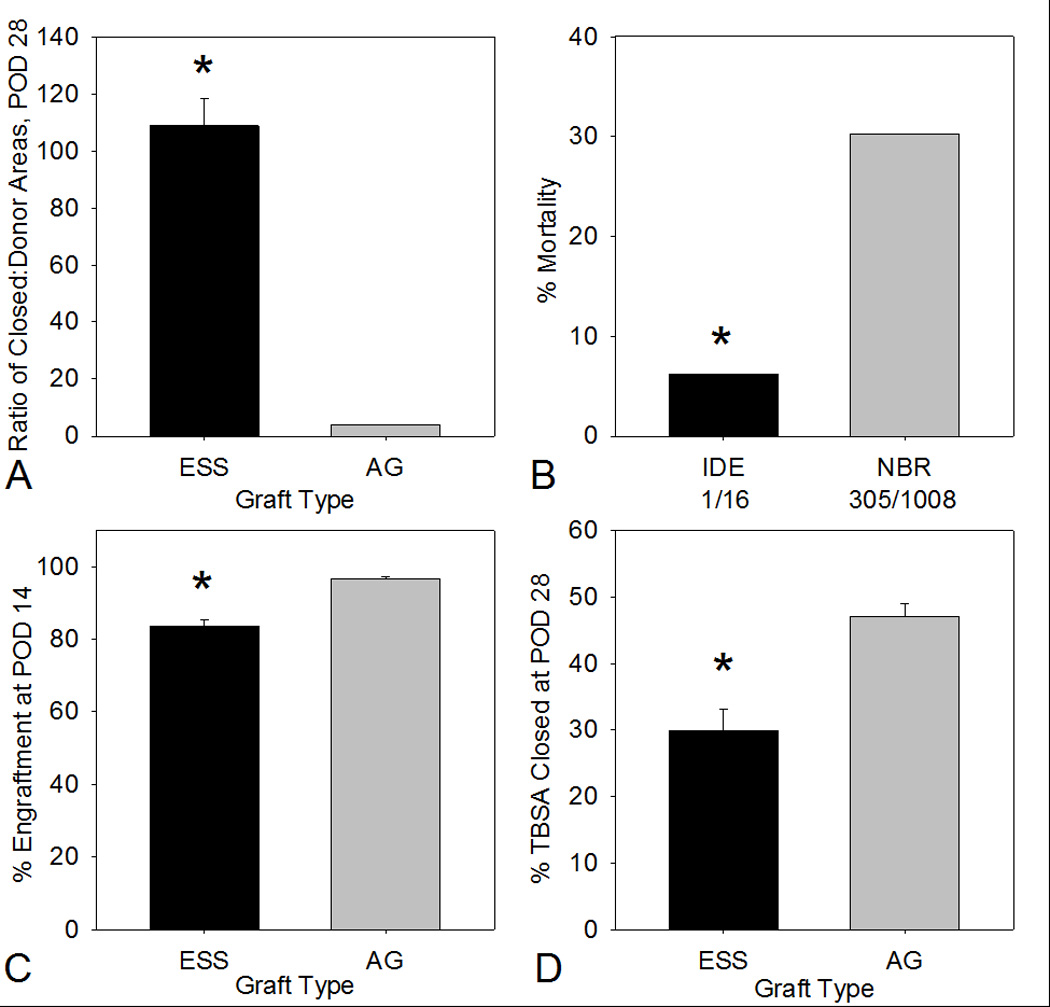
Plots of ratio of areas of closed wounds to donor biopsies, percentage mortality, percentage engraftment, and percentage total body surface area (TBSA) at POD 28. A) Ratios of closed-to-donor areas at POD 28 were 108.8 ± 9.7 for ESS and 4.0 ± 0.0 for AG. B) Percentages mortality were 6.25% (1/16) for subjects enrolled and treated under the IDE protocol which was significantly lower than 30.3% (305/1008) as reported in the 2012 National Burn Repository. C) Percentages of engraftment (mean ± SEM) at POD 14 were 83.5 ± 2.0 for ESS and 96.5 ± 0.9 for AG. D) Percentages of TBSA closed at POD 28 were 29.9 ± 3.3 % for ESS and 47.0 ± 2.0 for AG. Asterisks: p<0.05 between groups.
A strong positive correlation (R2 = 0.65) was found between the % TBSA of wound closure with ESS at POD 28, and the % TBSA full-thickness burn (Figure 5A). Importantly, the range of %TBSA closed extended to 60% or greater in selected cases, emphasizing the therapeutic impact of this device in life-threatening burns. Regrafting of comparative graft sites before POD 28 was not significantly different between groups (Figure 5B), occurred at a rate of 26.7% (4/15) for ESS, and each of these regrafting events was partial. There was no regrafting of comparative sites treated with AG.
Figure 5.
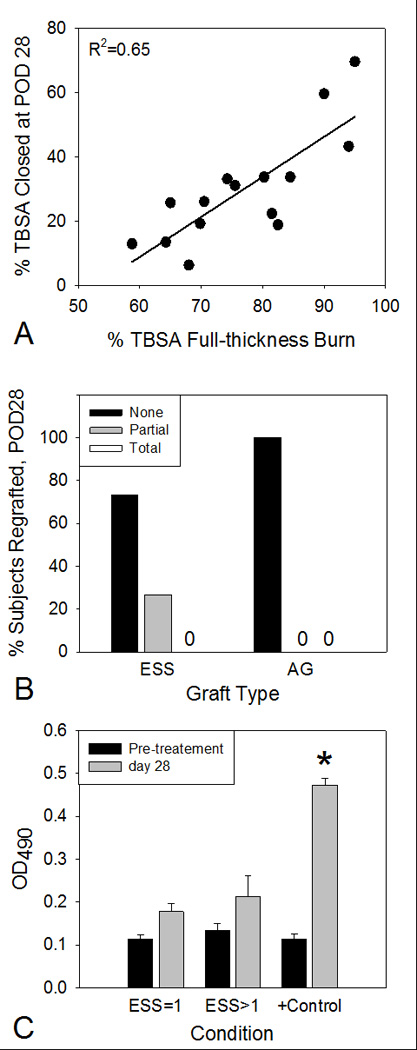
Correlation of % Total Body Surface Area (TBSA) closed with % TBSA full-thickness burn, frequencies of regrafting prior to POD 28, and antibodies to the biopolymer scaffold. A) A strong positive correlation (R2 = 0.65, p<0.001) was detected between % TBSA closed with ESS at POD 28, and % TBSA full-thickness burn. B) Neither condition required total regrafting of comparative sites. ESS comparative sites required partial regrafting in 26.7% of the ESS procedures (4/15), and AG required no regrafting. C) Enzyme-Linked ImmunoSorbant Assay (ELISA) of pre-immune and post-immune sera. ELISA values (OD490) for subject sera to homogenized collagen-GAG matrices were not statistically different before or 28 days after one or more graftings of ESS. Control sera from rabbits immunized with homogenized matrices showed statistically significant increases of ELISA values.
Antibody production specific to the collagen-GAG biopolymer scaffold is shown in Figure 5C. Single or multiple graftings of ESS did not stimulate significant increases in specific antibodies to the scaffold. By comparison, immunization of rabbits with homogenized scaffolds stimulated a significant increase in specific antibody binding.
Discussion
Data from this study support the hypothesis that autologous ESS reduce harvesting of donor skin in pediatric patients for closure of burn injuries involving greater than 50% TBSA. The reduction in donor skin requirements implies reductions in donor site morbidity, numbers of skin-grafting operations, and intensive care days, but those data were not collected in this study. The reduction in donor site harvesting is interpreted to result from quantitative advantages provided by ESS.
The epithelium of ESS forms a partial barrier and basement membrane in vitro24,29 which promote epithelial closure after grafting. Engraftment of ESS occurs between connective tissue in the wound and the dermal substitute of ESS in analogy to AG28. Upon vascularization of the dermal component of ESS, which occurs during the first week after grafting, the ESS begins to stabilize as barrier function, basement membrane, and nutrient supply are restored27. By POD 7, engrafted ESS have closed the wounds with functional epidermal barrier45. By POD 14, healed ESS has sufficient mechanical strength to allow physical therapy to begin29. By POD 28 (Figure 2B), pressure garments, which help to control scar, can be worn without loss of ESS46. Unmeshed AG applied as sheet grafts on the hands and face has been reported to reduce scar formation, and improve functional and cosmetic outcomes18,19. Application of ESS sheets without expansion of the mesh may provide similar advantages. In this subject population, engraftment (Figure 4C) was greater than 80%, but remained significantly lower than AG. This difference introduced a requirement for minor regrafting of ESS sites at a higher, but not significantly different, frequency (4/15) than AG (0/15) (Figure 5B), despite a reduction in donor site harvesting.
The primary medical benefit of ESS is defined by a ratio of closed areas to donor areas of greater than 100 (Figure 4A). This value was compared statistically to a maximum expansion of 1:4 for AG, but the actual expansion of AG was not measured in this study. In most cases, the usual expansion of AG was 1:2. Therefore, the conservation of donor skin with ESS compared to AG may actually have been as much as 50-fold. The factor of donor skin expansion of greater than 100-fold by ESS suggests hypothetically that less than 1% TBSA of split-thickness donor skin is sufficient to resurface the body completely with ESS. This benefit has been realized in selected cases of greater than 90% TBSA full-thickness burns, in which a donor biopsy of less than 1% TBSA was required. Based on these selected cases, it may be possible that common use of ESS could increase the LD50 for burns which is estimated between 70–80% TBSA in healthy adults, but is much lower in the elderly, and the very young32. The positive correlation of % TBSA closed with ESS to % TBSA full-thickness burn (Figure 5A) demonstrates that ESS remain effective even as the magnitude and complexity of the burn injury are at their greatest. This was shown not to be true for cultured epithelial autografts in which effectiveness correlated inversely with burn magnitude47.
Despite conservation of donor skin, ESS average area (29.9% TBSA) covered was less than AG (47.0% TBSA). This apparent anomaly was attributed to greater frequencies of burns between 50–80% TBSA in which lower %TBSA was treated with ESS, and to limited laboratory capacity to generate the engineered grafts. Nonetheless, the range of areas closed with ESS extended to about 70% TBSA. It was also observed that because of the sparing of donor skin by ESS, the mesh ratio for AG for most cases could be reduced to 1:2 or less, compared to as much as 1:4. This reduction of mesh ratio likely promoted faster healing, and less scarring of wounds closed with AG18,19. This indirect benefit may also contribute to improved functional outcome and long-term recovery.
Among the limitations and qualifications of this study were the small sample size, the paired-site comparison format rather than randomized subject populations, and performance at a single clinical site. Regarding samples size and mortality, the mortality rate of 6.25% (1/16) was significantly different from data in the NBR, but subjects were not matched for demographic or medical parameters, so the observed mortality rate will require a larger sample size, or subject matching, before it may be considered conclusive.
Certain end points, such as length of hospital stay, and numbers of operations to complete skin grafting, were not assessed due to the limited capacity of the investigators’ laboratories to produce the ESS grafts (~900 cm2/week). However, in young subjects with small absolute TBSA, but large TBSA full-thickness burns (e.g., >80%), production capacity was sufficient to complete skin grafting in less than eight weeks from subject enrollment. Therefore, the technical capability to complete closure of large TBSA, full-thickness burns should be possible within two months for most patients who have few co-morbid conditions, if production capacity of ESS is not limiting. In addition, the follow-up period for this study was limited to one year, and numbers of reconstructive surgeries needed during several years of pediatric growth was not included in the data collection. Anecdotal observations from a previous study31, and from this study, suggest that the ESS grows proportionately with the pediatric subjects. These observations require careful examination clinically and biologically, and will be considered for quantification as end points in future studies.
This study was intended to serve as a Phase I/II trial to demonstrate safety and efficacy of autologous ESS for closure of extensive full-thickness burns. Because subjects were enrolled at only one burn center, there was no ‘standard of care’ population without use of ESS as an investigative therapy. There were also no exclusion criteria for known factors that increase mortality such as inhalation injury, sepsis, or comorbid conditions such as obesity, diabetes, cardiovascular disease, substance abuse, or alcohol or tobacco use. However, in this pediatric population, these co-morbid conditions occurred less frequently than in adults.
Remaining anatomic deficiencies of ESS compared to AG include, but are not limited to: hypopigmentation, and absence of blood vessels, nerve, sweat and sebaceous glands, and hair follicles. Hypopigmentation and lack of a vascular plexus have been addressed in pre-clinical studies from this laboratory48,49. Hypothetically, hair follicles50,51, and sweat and sebaceous glands52, may be regenerated in vitro, but accomplishment of these goals will require regulation of developmental signals in vitro which is beyond the scope of the present studies. However, it is important to recognize that split-thickness skin AG also does not regenerate glands or follicles. Therefore, regeneration of hair and/or glands in ESS would offer anatomic structures found only in full-thickness skin.
Conclusions
These results show that the ESS model offers new alternatives for: increased availability of autologous skin substitutes for grafting and closure of extensive, full-thickness burns; reduced morbidity from harvesting of donor skin to patients with needs for closure of extensive full-thickness burns; and, reduced mortality in pediatric burn patients.
Supplementary Material
Acknowledgments
Acknowledgements and Author Contributions:
This study was supported by funding from the Shriners Hospitals for Children, and was performed in its hospital facilities. Regulatory oversight was provided by the University of Cincinnati Office of Research Integrity. STB and RJK participated in the design of the study. All authors were investigators in a human subjects protocol approved by the Institutional Review Board of the University of Cincinnati, and an Investigative Device Exemption application regulated by the US Food and Drug Administration. All authors were involved in clinical administration of the investigative therapy and data collection. STB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank our staff biostatistician, Laura James, for biostatistical analysis of the study data. The authors gratefully acknowledge the expert assistance of Christopher Lloyd, Rachel Zimmerman, John Besse and Mary Rolfes in the technical performance of this study.
Disclosure of Financial Interests:
All authors were paid members of the medical or scientific staff of the Shriners Hospitals for Children. STB has patents and intellectual property that are assigned to the University of Cincinnati and Shriners Hospitals for Children, and are licensed for commercial development. During the performance period of this study (2007–2011), STB had financial interests that were disclosed and managed under the policies and regulations of the University of Cincinnati for financial conflict of interest. Currently, RJK has retired from the medical staffs of the University of Cincinnati and the Shriners Hospitals for Children, and serves as a consultant for the licensee of the patents and intellectual property.
Acronyms
- AG
split-thickness skin autograft
- ESS
autologous engineered skin substitutes
- ELISA
Enzyme-Linked Immuno-Sorbent Assay
- FDA
US Food and Drug Administration
- IDE
Investigational Device Exemption
- IRB
Institutional Review Board
- NBR
National Burn Repository
- POD
post-operative day
- SEM
standard error of the mean
- TBSA
total body surface area
Footnotes
This report was presented as an abstract at the 47th meeting of the American Burn Association; Chicago, IL; 21–24 April 2015.
References
- 1.Herndon DN. Total Burn Care. Phildelphia, PA: W.B. Saunders; 2012. [Google Scholar]
- 2.Schwanholt CA, Ridgway CL, Greenhalgh DG, et al. A prospective study of burn scar maturation in pediatrics: does age matter? J Burn Care Rehabil. 1994;15:416–420. doi: 10.1097/00004630-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Covey MH, Dutcher K, Marvin JA, et al. Efficacy of continuous passive motion (CPM) devices with hand burns. J Burn Care Rehabil. 1988;9:397–400. [PubMed] [Google Scholar]
- 4.Brusselaers N, Pirayesh A, Hoeksema H, et al. Skin replacement in burn wounds. J Trauma. 2010;68:490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 5.Kamel RA, Ong JF, Eriksson E, et al. Tissue engineering of skin. J Am Coll Surg. 2013;217:533–555. doi: 10.1016/j.jamcollsurg.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Centanni JM, Straseski JA, Wicks A, et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg. 2011;253:672–683. doi: 10.1097/SLA.0b013e318210f3bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31:559–568. doi: 10.1097/BCR.0b013e3181e4ca29. [DOI] [PubMed] [Google Scholar]
- 8.Bottcher-Haberzeth S, Biedermann T, Schiestl C, et al. Matriderm® 1 mm versus Integra(R) Single Layer 1.3 mm for one-step closure of full thickness skin defects: a comparative experimental study in rats. Pediatr Surg Int. 2012;28:171–177. doi: 10.1007/s00383-011-2990-5. [DOI] [PubMed] [Google Scholar]
- 9.Woodley DT, Peterson HD, Herzog SR, et al. Burn wounds resurfaced by cultured epidermal autografts show abnormal reconstitution of anchoring fibrils. JAMA. 1988;259:2566–2671. [PubMed] [Google Scholar]
- 10.Wood FM, Giles N, Stevenson A, et al. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns. 2012;38:44–51. doi: 10.1016/j.burns.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Sood R, Roggy DE, Zieger MJ, et al. A comparative study of spray keratinocytes and autologous meshed split-thickness skin graft in the treatment of acute burn injuries. Wounds. 2015;27:31–40. [PubMed] [Google Scholar]
- 12.Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713–1726. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 13.Navarro FA, Stoner ML, Lee HB, et al. Melanocyte repopulation in full-thickness wounds using a cell spray apparatus. J Burn Care Rehabil. 2001;22:41–46. doi: 10.1097/00004630-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan RL, Morgan JR, Cusick JL, et al. Initial experience with a composite autologous skin substitute. Burns. 2001;27:421–424. doi: 10.1016/s0305-4179(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 15.Jansen LA, De CP, Guay NA, et al. The evidence base for the acellular dermal matrix AlloDerm: a systematic review. Ann Plast Surg. 2013;70:587–594. doi: 10.1097/SAP.0b013e31827a2d23. [DOI] [PubMed] [Google Scholar]
- 16.Chester DL, Balderson DS, Papini RP. A review of keratinocyte delivery to the wound bed. J Burn Care Rehabil. 2004;25:266–275. doi: 10.1097/01.bcr.0000124749.85552.cd. [DOI] [PubMed] [Google Scholar]
- 17.Duranceau L, Genest H, Bortoluzzi P, et al. Successful grafting of a novel autologous tissue-engineered skin substitutes (dermis and epidermis) on twelve burn patients. J Burn Care Res. 2014;35(3):S121. [Google Scholar]
- 18.Housinger TA, Hills J, Warden GD. Management of pediatric facial burns. J Burn Care Rehabil. 1994;15(5):408–411. [PubMed] [Google Scholar]
- 19.Archer SB, Henke A, Greenhalgh DG, et al. The use of sheet autografts to cover extensive burns in patients. J Burn Care Rehabil. 1998;19:33–38. doi: 10.1097/00004630-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Housinger TA, Keller B, Warden GD. Management of burns of the penis. J Burn Care Rehabil. 1993;14:525–527. doi: 10.1097/00004630-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Boyce ST, Hansbrough JF. Biologic attachment, growth, and differentiation of cultured human epidermal keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery. 1988;103:421–431. [PubMed] [Google Scholar]
- 22.Smiley AK, Klingenberg JM, Aronow BJ, et al. Microarray analysis of gene expression in cultured skin substitutes compared with native human skin. J Invest Dermatol. 2005;125:1286–1301. doi: 10.1111/j.0022-202X.2005.23971.x. [DOI] [PubMed] [Google Scholar]
- 23.Boyce ST, Supp AP, Swope VB, et al. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol. 2002;118:565–572. doi: 10.1046/j.1523-1747.2002.01717.x. [DOI] [PubMed] [Google Scholar]
- 24.Boyce ST, Supp AP, Harriger MD, et al. Surface electrical capacitance as a noninvasive index of epidermal barrier in cultured skin substitutes in athymic mice. J Invest Dermatol. 1996;107(1):82–87. doi: 10.1111/1523-1747.ep12298286. [DOI] [PubMed] [Google Scholar]
- 25.Barai ND, Supp AP, Kasting GB, et al. Improvement of epidermal barrier properties in cultured skin substitutes after grafting onto athymic mice. Skin Pharmacol Physiol. 2006;20:21–28. doi: 10.1159/000096168. [DOI] [PubMed] [Google Scholar]
- 26.Woodley DT. Covering wounds with cultured keratinocytes. JAMA. 1989;262(15):2140–2141. [PubMed] [Google Scholar]
- 27.Hansbrough JF, Boyce ST, Cooper ML, et al. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substrate. JAMA. 1989;262:2125–2130. [PubMed] [Google Scholar]
- 28.Boyce ST, Kagan RJ, Meyer NA, et al. The 1999 Clinical Research Award. Cultured skin substitutes combined with Integra to replace native skin autograft and allograft for closure of full-thickness burns. J Burn Care Rehabil. 1999;20:453–461. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 29.Boyce ST, Kagan RJ, Yakuboff KP, et al. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Annals of Surgery. 2002;235:269–279. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harriger MD, Warden GD, Greenhalgh DG, et al. Pigmentation and microanatomy of skin regenerated from composite grafts of cultured cells and biopolymers applied to full-thickness burn wounds. Transplantation. 1995;59:702–707. doi: 10.1097/00007890-199503150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Boyce ST, Kagan RJ, Greenhalgh DG, et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006;60:821–829. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 32.American Burn Association. 2012 National Burn Repository. American Burn Association; 2012. [Google Scholar]
- 33.Boyce ST, Rieman MT, Kagan RJ. Reduced mortality in pediatric burn patients treated with autologous cultured skin substitutes during an FDA Integrity Hold. Proceedings of an FDA Public Workshop on Medical Countermeasures for a Burn Mass Casualty Incident. 2012 [Google Scholar]
- 34.US Food and Drug Administration. Early and expanded access: compassionate use. 2014 [Google Scholar]
- 35.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 36.Lund CC, Browder NC. The estimate of areas of burn. Surg Gynecol Obstet. 1944;79:352–358. [Google Scholar]
- 37.Boyce ST, Ham RG. Cultivation, frozen storage, and clonal growth of normal human epidermal keratinocytes in serum-free media. J Tiss Cult Meth. 1985;9:83–93. [Google Scholar]
- 38.Boyce ST. Methods for the serum-free culture of keratinocytes and transplantation of collagen-GAG-based skin substitutes. In: Morgan JR, Yarmush ML, editors. Methods in Molecular Medicine, Vol. 18: Tissue Engineering Methods and Protocols. Totowa, NJ: Humana Press Inc; 1999. pp. 365–389. [DOI] [PubMed] [Google Scholar]
- 39.Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res. 1988;22:939–957. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- 40.Boyce ST. Fabrication, quality assurance, and assessment of cultured skin substitutes for treatment of skin wounds. Biomed Eng J. 2004;20:107–112. [Google Scholar]
- 41.Swope VB, Supp AP, Schwemberger S, et al. Increased expression of integrins and decreased apoptosis correlate with increased melanocyte retention in cultured skin substitutes. Pigment Cell Res. 2006;19:424–433. doi: 10.1111/j.1600-0749.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 42.Heimbach D, Luterman A, Burke JF, et al. Artificial dermis for major burns; a multi-center randomized clinical trial. Ann Surg. 1988;208:313–320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyce ST, Warden GD, Holder IA. Non-cytotoxic combinations of topical antimicrobial agents for use with cultured skin. Antimicrob Agents Chemother. 1995;39(6):1324–1328. doi: 10.1128/aac.39.6.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warden GD, Saffle JR, Kravitz M. A two-stage technique for excision and grafting of burn wounds. J Trauma. 1982;22:98–103. doi: 10.1097/00005373-198202000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Goretsky MJ, Supp AP, Greenhalgh DG, et al. The 1995 Young Investigator Award: Surface electrical capacitance as an index of epidermal barrier properties of composite skin substitutes and skin autografts. Wound Rep Reg. 1995;3(4):419–425. doi: 10.1046/j.1524-475X.1995.30406.x. [DOI] [PubMed] [Google Scholar]
- 46.Boyce ST, Kagan RJ, Greenhalgh DG, et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. Journal of Trauma. 2006;60:821–829. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 47.Williamson J, Snelling C, Clugston P, et al. Cultured epithelial autograft: Five years of clinical experience with twenty-eight patients. J Trauma. 1995;39:309–319. doi: 10.1097/00005373-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 48.Swope VB, Supp AP, Boyce ST. Regulation of cutaneous pigmentation by titration of human melanocytes in cultured skin substitutes grafted to athymic mice. Wound Repair Regen. 2002;10:378–386. doi: 10.1046/j.1524-475x.2002.10607.x. [DOI] [PubMed] [Google Scholar]
- 49.Supp DM, Wilson-Landy K, Boyce ST. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002;16:797–804. doi: 10.1096/fj.01-0868com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sriwiriyanont P, Lynch KA, McFarland KL, et al. Characterization of hair follicle development in engineered skin substitutes. PLoS One. 2013;8:e65664. doi: 10.1371/journal.pone.0065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sriwiriyanont P, Lynch KA, Maier EA, et al. Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol. 2012;21:783–785. doi: 10.1111/exd.12003. [DOI] [PubMed] [Google Scholar]
- 52.McNairn AJ, Doucet Y, Demaude J, et al. TGFbeta signaling regulates lipogenesis in human sebaceous glands cells. BMC Dermatol. 2013;13:2. doi: 10.1186/1471-5945-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


