Abstract
Retention in HIV care is vital to the HIV care continuum. The current review aimed to synthesize qualitative research to identify facilitators and barriers to HIV retention in care interventions. A qualitative evidence meta-synthesis utilizing thematic analysis. Prospective review registration was made in PROSPERO and review procedures adhered to PRISMA guidelines. Nineteen databases were searched to identify qualitative research conducted with individuals living with HIV and their caregivers. Quality assessment was conducted using CASP and the certainty of the evidence was evaluated using CERQual. A total of 4419 citations were evaluated and 11 were included in the final meta-synthesis. Two studies were from high-income countries, 3 from middle-income countries, and 6 from low-income countries. A total of eight themes were identified as facilitators or barriers for retention in HIV care intervention: (1) Stigma and discrimination, (2) Fear of HIV status disclosure, (3) task shifting to lay health workers, (4) Human resource and institutional challenges, (5) Mobile Health (mHealth), (6) Family and friend support, (7) Intensive case management, and, (8) Relationships with caregivers. The current review suggests that task shifting interventions with lay health workers were feasible and acceptable. mHealth interventions and stigma reduction interventions appear to be promising interventions aimed at improving retention in HIV care. Future studies should focus on improving the evidence base for these interventions. Additional research is needed among women and adolescents who were under-represented in retention interventions.
Keywords: HIV, Retention, Care continuum, Meta-synthesis, ARV
Implementation of universal testing and treatment depends on individuals with HIV successfully navigating the care continuum. The World Health Organization 2013 [1] antiretroviral (ARV) guidelines are now being revised, necessitating systematic reviews of evidence to improve HIV care. Advances in HIV care informed by the HIV Prevention Trials Network (HPTN) 052 study [2] and other research [3–5] demonstrated the clinical and public health benefits of early antiretroviral therapy initiation. There is now urgency in expanding HIV testing and treatment across the world. However, universal test and treat strategies are dependent on successful retention in HIV care.
Retention in HIV care is defined as the continued engagement in health services, from enrollment in care to discharge or death of an individuals living with HIV [6]. Individuals retained in care have lower mortality [7, 8] and higher likelihood of viral suppression [9]. Previous evidence reviews on retention in HIV care interventions have focused on high-income country contexts [10–12] and on quantitative studies [12].
Implementation science within the HIV care context seeks to identify the social and behavioral factors known to influence the effectiveness of HIV interventions [10, 11, 13]. Studies are beginning to integrate qualitative evaluations to assess the mechanisms of treatment effectiveness and to identify barriers and facilitators of implementation, uptake, scalability, and usefulness for particular subpopulations within general healthcare [14–16], and studies are needed that refer to HIV treatment in particular. Given examples from these qualitative studies, integration and synthesis across settings and interventions can provide information to inform the WHO ARV guidelines revision and provide policy relevant information for clinicians and other policy stakeholders. The purpose of this review was to synthesize the qualitative literature evaluating HIV retention in care interventions in order to summarize facilitators and barriers and inform policy.
Methods
Search Strategy
We used a comprehensive search strategy to identify all relevant studies regardless of language or publication status, or year of publication through Feb 17th 2015 (see Supplementary Table 1). Data collection and analysis proceeded in accordance with the PRISMA review guidelines [17], utilized the ENTREQ checklist [18], and followed meta-synthesis guidance from the Cochrane group [15]. We registered our review in PROSPERO (CRD42015017328).
We searched the following nineteen electronic journal and dissertation/thesis databases: CENTRAL (Cochrane Central Register of Controlled Trials), EMBASE, LILACS, PsycINFO, PubMed (MEDLINE), Web of Science/Web of Social Science, CINAHL, British Nursing Index and Archive, Social Science Citation Index, AMED (Allied and Complementary Medicine Database), DAI (Dissertation Abstracts International), EPPI-Centre (Evidence for Policy and Practice Information and Coordinating Centre), ESRC (Economic and Social Research Council), Global Health (EBSCO), Anthrosource, and JSTOR. Conference proceedings from the Conferences on Retroviruses and Opportunistic Infections (CROI), International AIDS Conference (IAC), and International AIDS Society (IAS) clinical meetings were searched from their inception dates (1993, 1985 and 2001, respectively). We contacted researchers and relevant organizations and checked reference lists of all included studies. After identifying and deleting the duplicates, citations and abstracts were imported into Endnote X4.
Inclusion Criteria and Study Selection
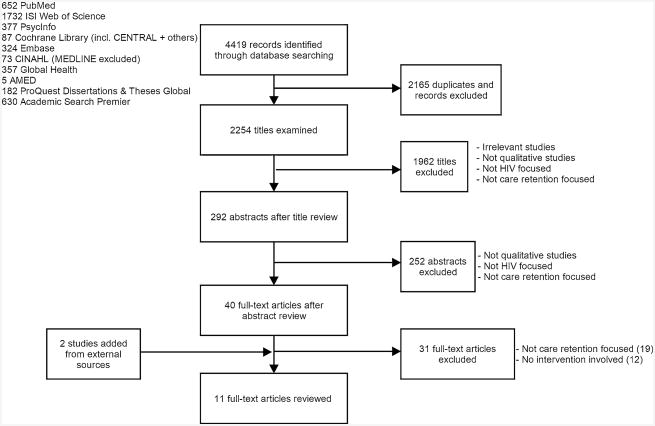
Two co-authors (BH and KS) independently assessed each title and abstract to identify relevant records and evaluated their eligibility according to the following inclusion criteria: (1) employed qualitative methods and results derived from qualitative analysis; (2) related to HIV care; (3) related to care retention; (4) intervention aimed at enhancing retention in HIV care. Qualitative methods included but were not limited to ethnographic research, case studies, process evaluations, and mixed methods research. Qualitative research findings (but not quantitative data) from mixed methods studies were included. Studies only reporting quantitative data were excluded. All full text manuscripts identified as potentially relevant by one or both reviewers were then retrieved and examined. The two reviewers further assessed final inclusion of the full text manuscripts independently. Disagreements were brought to a third independent reviewer and discussed until consensus was achieved among the three reviewers (See Fig. 1).
Fig. 1. Flow of reviewed literature on HIV retention in care interventions.
Data Extraction and Study Characteristics
After identifying studies for inclusion, one reviewer (BH) extracted the relevant data from the original selected reports and the second reviewer (KS) reviewed the manuscripts for a second time, extracted relevant data to an excel spreadsheet, and the two data extraction tables were compared and results were integrated. A set of data extraction categories was developed including the following: (1) primary source data (quotes from stakeholders in HIV retention interventions); (2) secondary source data (interpretation from qualitative research studies); (3) study characteristics such as location of trial (country, city), intervention type, mean age and sex distribution of participants, theoretical framework, analytical methodology, qualitative data type, subpopulation or key population included.
Assessment of Individual Study Quality
The Critical Appraisal Skills Program [CASP; 19] quality assessment tool for qualitative studies [20, 21] was adapted to assess individual study quality. The tool consisted of seven questions including (1) a screening question to ensure the manuscript was qualitative research, (2) whether the study context was clearly described, (3) if there was evidence of researcher reflexivity, (4) if the sampling method was clearly described and appropriate for the research question, (5) if the data collection was clearly described and appropriate, (6) if the analysis methods were clearly described and appropriate, and (7) whether the claims made supported by sufficient evidence (i.e., did the data provide sufficient depth and detail). Each article was independently assessed by both reviewers and a third reviewer provided their assessment in the case of disagreement. We followed the SPICE model to review the Setting, Perspective, Intervention, Comparator, and Evaluation (Table 1). The SPICE model is similar to the PICO model and has been used in several qualitative evidence reviews [22, 23].
Table 1. Summary of SPICE-model of our review.
| Setting | HIV care service |
|---|---|
| Perspective | Individuals living with HIV, caregivers, health care providers |
| Intervention | Interventions aimed to improve retention in HIV care |
| Comparison | Not applicable |
| Evaluation | Barriers and facilitators |
We further evaluated the certainty of the qualitative evidence for each review finding using the certainty of the qualitative evidence [24] approach. Using the CERQual approach, the certainty of each finding was graded as “Very low”, “Low”, “Moderate” and “High”, based on four criteria that included methodological limitations, coherence, relevance and adequacy of data. These four criteria respectively reflect methodological weaknesses, variation in the review findings across studies, relevance of the primary studies to the review question, and overall evaluation of the richness or scope of the data supporting a review finding.
Analysis
We (BH, KS) analyzed the data using framework thematic synthesis [25], which is particularly relevant for policymakers and those designing interventions [26], and one of the methods recommended by the Cochrane Qualitative Review Methods Group [27–30]. Similar to other primary analysis of qualitative data, framework thematic synthesis utilizes techniques to identify and develop themes related to the topic [31]. We identified emergent themes by reviewing primary findings reported in the results sections, including informant quotations, and secondary outcome data found in discussion sections that provided authors' interpretations of the qualitative data.
Results
A total of eleven studies were identified, including nine qualitative only studies [32–40] and two mixed method studies [41, 42] (Fig. 1).
Among the eleven qualitative studies, ten had full texts available [32–35,37–42] and one was available as a conference poster [36] (Table 2). Based on the CASP quality assessment tool for qualitative studies, seven studies [32–35, 37, 39, 40] were evaluated as having “minor” methodological limitations while the other four [36, 38, 41, 42] were evaluated as having “moderate” limitations (Table 3).
Table 2. Characteristics of the included studies in review.
| Author | Study design |
Methods | Location of study (City, Country) |
Income settingsa |
N | Subpops | % Female | Mean age | Intervention type |
Theoretical framework |
Retention definition |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agbonyitor et al. [32] | Qual | FGD | Amper, Fobur, Barkin Ladi, and Langtang, Nigeria | Middle | 53 | 30 PLWH; 22 volunteer | PLWH: 80 %, volunteer: 82% | PLWH: 40.7, volunteer: 35.8 | Home-based care | Thematic analysis | NR | |
| Alamo et al. [41] | Mixed | In-depth interviews | Uganda | Low | 394 | 347 patients; 47 CATTS | Patients: 71 %, CATTS: 63 % | Patients: 37(mdn), CATTS: 39 (mdn) | Task shifting to community health workers (CHW) & (CATTS) | Thematic analysis | Lost to follow-up | |
| Assefa et al. [42] | Mixed | KII + FGD | Ethiopia | Low | 84 | 72 KI;12 ART mentors | NR | NR | Complex | Self-generated framework | Continuous engagement of patients in care | |
| Bezabhe et al. [33] | Qual | Semi-structured interview + FGD | Ethiopia | Low | 58 | 24 PLWH, 15 nurses, 19 case managers | PLWH: 50 %, healthcare providers: 65 % | PLWH: 36, healthcare providers: 32 | Case management | Grounded theory | Loss to follow-up | |
| Busza et al. [34] | Qual | In-depth interview | Dar es Salaam, and Tanga region, Tanzania | Low | 36 | 14 adolescents living with HIV, 10 caregivers, 12 home based care providers | Adolescents: 64%, caregivers: 90 %, HBC providers: 83 % | NR | Home-based care | NR | NR | |
| Busza et al. [35] | Qual | Semi-structured | Harare, Zimbabwe | Low | 20 | 15 primary caregivers,5 CBO | Primary caregiver: 80 %, CBO: 78 % | NR | Community-based intervention (home visits) | Skovdal et al. Conceptual framework | NR | |
| Mallinson et al. [37] | Qual | In-depth interview | United States | High | 76 | None | 46% | 38.9 | Outreach initiative program -focus on physician relationship | Grounded theory | NR | |
| Miller et al. [38] | Qual | Face-to-face or telephone interview | South Africa | Middle | 30 | 14 treatment defaulters;16 transfer patients | 75 %; 42 % | 31; 34 | ARV treatment | NR | 1 month late for the next scheduled consultation or meds pick up | |
| Rajabiun et al. [39] | Qual | Semi-structured | United States | High | 76 | None | 46% | 38.9 | Outreach intervention | Grounded theory | NR | |
| Smillie et al. [40] | Qual | Semi-structured | Nairobi, Kenya | Middle | 20 | 5 health care providers;15 patients living with HIV | 73 % | 29 | mHealth | Theory of reasoned action and the technology acceptance model | NR | |
| Decroo et al. [36] | Qual | FGD | Lesotho | Low | NR | NR | NR | NR | Community antiretroviral therapy groups (CAGs) | NR | NR | |
Country income information is based on the World Bank category.
Qual qualitative study. Mixed mixed methods study including qualitative and quantitative methods. FGD focus group discussion. KII Key informant Interview. CATTS community antiretroviral therapy and tuberculosis treatment supporters. PLWH people living with HIV. NR none reported
Table 3. Summary of CASP scores of individual studies.
| First author | Year published | Qualitative research? | Clear context? | Reflexivity? | Clear and appropriate sampling method? | Clear and appropriate method of data collection? | Clear and appropriate method of analysis? | Sufficient evidence? | Total score | Methodological limitation (i.e., 1–2 = major; 3–4 = moderate; 5–6 = minor; 7 = none) |
|---|---|---|---|---|---|---|---|---|---|---|
| Agbonyitor, M. | 2009 | Yes | Yes | No | Yes | Yes | No | Yes | 5 | Minor |
| Alamo, S. | 2012 | Yes | Yes | No | No | Yes | Yes | No | 4 | Moderate |
| Assefa, Y. | 2014 | Yes | Yes | No | No | Yes | No | Yes | 4 | Moderate |
| Bezabhe, W. M. | 2014 | Yes | Yes | No | Yes | Yes | Yes | Yes | 6 | Minor |
| Busza, J. | 2014a | Yes | Yes | No | Yes | Yes | Yes | Yes | 6 | Minor |
| Busza, J. | 2014b | Yes | Yes | No | No | Yes | Yes | Yes | 5 | Minor |
| Mallinson, R. K. | 2007 | Yes | Yes | No | Yes | Yes | Yes | Yes | 6 | Minor |
| Miller, C. M. | 2010 | Yes | Yes | No | No | No | Yes | Yes | 4 | Moderate |
| Rajabiun, S. | 2007 | Yes | Yes | No | Yes | Yes | Yes | Yes | 6 | Minor |
| Smillie, K. | 2014 | Yes | Yes | No | No | Yes | Yes | Yes | 5 | Minor |
| Decroo, T. | 2015 | Yes | Yes | No | No | No | No | No | 2 | Major |
Settings and Population
The eleven studies were conducted between 2006 and 2013 and covered high-income countries [37, 39], middle-income countries [32, 38, 40], and low-income countries [33–36, 41, 42]. Study locations included Nigeria: Amper [32], Fobur [32], Barkin Ladi [32]; Langtang [32]; Uganda [41]; Ethiopia [33, 42]; Tanzania [34]; Zimbabwe [35]; South Africa [38]; Kenya [40]; Lesotho [36]; and the United States [37, 39].
Information was collected from the individuals living with HIV [32–34, 37–41], community caregiver volunteers [32] and healthcare providers [33–35, 38, 40–42]. All studies included adults, two also included adolescents [34, 41], one involved children [35] but none involved pregnant women.
Intervention
Task shifting to non-specialist community caregivers [35, 36, 39, 41, 42], was the most common intervention identified in this review. Other interventions included home-based care [32, 34], case management [33, 42], primary HIV medical care [37], counseling [42], and mHealth [33, 40].
Themes
Of the evidence available, the following eight overarching themes emerged: stigma and discrimination, fear of HIV status disclosure, lay worker support, human resource and institutional challenges, mHealth, family and friend support, intensive case management challenges, and relationships with those implementing interventions (see Table 4).
Table 4. Summary of qualitative review findings.
| Review finding | Contributing studies | Confidence in the evidencea | Explanation of confidence in the evidence assessment |
|---|---|---|---|
| Stigma and discrimination influenced treatment retention. Some people living with HIV perceived long wait times and lack of individual care by clinic treatment providers as examples of discrimination. Fear of negative reactions by others prevented people living with HIV from seeking assistance from family members or friends. People living with HIV reported community level discrimination | [32–36, 39–11] | High | 6 studies with minor methodological limitations and 2 with moderate limitations. Reasonably thick data from 8 countries, mostly from low-and middle-income contexts. Interventions in middle-income countries were less intensive. High coherence, relevance, and adequacy |
| Fear of disclosure Lack of disclosure of HIV serostatus was associated with clinic avoidance. Fear of disclosure prevented home health workers from making home visits and limited their effectiveness to provide care. Individuals living with HIV skipped doses of medication to conceal status. Conversely, patients who openly shared their HIV status mobilized social supports and had fewer difficulties accessing medication | [33, 35, 40–42] | High | 3 studies with minor methodological limitation and 2 with moderate limitations. Reasonably thick data from 4 countries (all low-income). High coherence, relevance, and adequacy |
| Task shifting to lay health workers was generally acceptable to individuals living with HIV. Lay counselors were able to spend more time with individuals living with HIV, provided more social and nonmedical instrumental support (arrangement of rides to clinic). Lay health worker counseling and peer health education nurtured hope and a positive attitude, which increased retention in care | [34, 39, 41, 42] | Moderate | 2 studies with minor and 2 studies with moderate methodological limitations. Reasonably thick data from 4 countries (3 low income, 1 high income). High coherence and relevance, medium adequacy |
| Human Resource and institutional challenges Clinician shortage and high clinician workload led to poor care provision for individuals infected with HIV. Lack of available pharmacists, limited drug availability and stock outs, and long clinic waiting times led to sub-optimal dosing and skipped doses of medication for individuals living with HIV | [33, 35, 36, 38, 39, 41] | Moderate | 3 studies with minor, 2 with moderate and 1 with major methodological limitations. Reasonably thick data from 6 countries (1 high income; 1 middle income, and 4 low income). High coherence and relevance, medium adequacy |
| mHealth Passive and active methods for reaching individuals living with HIV increased retention in care. Cell phone based interventions are promising for retention in care | [33, 40] | Moderate | 2 studies with minor methodological limitations. Thick data available from 2 countries (1 low income and 1 middle income). High coherence and relevance, medium adequacy |
| Family and friend support Some individuals living with HIV reported that receiving support from family and friends increased retention. Others reported that family was even more important than friendships, citing higher levels of familial trust | [33, 36, 39, 40] | Moderate | 3 studies with minor and 1 with major methodological limitations. Reasonably thick data available from 3 countries (1 low income and 1 middle income, 1 high income). Thin data available from 1 country (low income). High coherence and relevance, medium adequacy |
| Intensive case management Intensive follow-up is useful to promote retention in care interventions. Using a stepped care approach of following and only individuals living with HIV who previously defaulted from treatment can minimized resource burdens. Working in interdisciplinary treatment teams facilitated retention in care through coordination of documentation and sharing patient information. However, some individuals living with HIV reported wanting less involvement with home-health care providers due to competing work demands. Some treatment providers reported that intensive follow-up was burdensome | [33, 42] | Moderate | 1 study with moderate and 1 study with minor methodological limitations. Thick data available from 1 country (low income). High coherence and relevance, medium adequacy |
| Relationship with caregivers Positive and supportive relationships with caregivers was associated with retention outcomes. Collaborative healthcare decision-making, patience to answer questions, and being treated with respect enhanced retention. Conversely, patronizing behaviors and not physically touching individuals living with HIV was reported as a barrier to retention | [32, 33, 37–39] | Moderate | 4 studies with minor and 1 with major methodological limitations. Reasonably thick data available from 4 countries (1 low income and 2 middle income, 1 high income). High coherence and relevance, medium adequacy |
The CER Qual confidence refers to the overall confidence in the review finding based on assessing components related to relevance, adequacy, coherence, and methodological rigor as described in detail in the methods section
Stigma and Discrimination (High CERQual Confidence)
Eight studies [32–36, 39–41] suggested that stigma had a major influence on treatment retention intervention effectiveness.
Community Stigma
Actual and perceived discrimination from family and community members disrupted treatment retention by discouraging individuals living with HIV from seeking emotional or instrumental (e.g., transportation to clinic appointment) assistance from significant others [33]. The implementation of retention interventions sometimes inadvertently led to disclosure of the participant's HIV serostatus, decreasing intervention engagement [34]. Community health workers making home visits normalized having relationships with individuals living with HIV and thereby decreased community stigma [32].
Self-Stigma
Self-stigma negatively influenced individuals living with HIV from accessing services [32]. The influence of a home-based care program decreased self-stigma: “Before people are afraid to boldly come out and accept their HIV status, but with this programme people have started to come out and tell their HIV status.” (Woman living with HIV, age unknown, Nigeria) [32].
Fear of HIV Status Disclosure (High CERQual Confidence)
According to five studies [33, 35, 40–42] fear of serostatus disclosure increased clinic avoidance behavior among individuals living with HIV. Home–based care interventions were relocated away from the home to prevent neighbors and others from knowing about their participation in the intervention and by inference, their HIV serostatus. This increased the burden on home-based care providers [41]. “Some of my patients have not disclosed their HIV status and they prefer that I do not visit them at home but rather meet them anywhere else in the community for follow-up.” (Community health worker, age and sex unknown, Uganda) [41].
Their fear also contributed to poor ARV retention and reduced engagement in mHealth interventions [40]. “If someone else reads the [SMS] message and they find out about my status” (31 year old female living with HIV, Nigeria) [40].
Individuals living with HIV who openly shared their HIV status were more likely to benefit from interventions [33, 35]. “I have no problem of disclosing my (HIV) status to my family, friends, and others. When I usually introduce myself, I often tell them that I am living with HIV… this helped me to take the pills regularly, without any anxiety, and to recover from my illness as well. (35 year old woman living with HIV, Ethiopia) [33].
Task Shifting to Lay Health Workers (Moderate CERQual Confidence)
Results from four studies [34, 39, 41, 42] suggested that lay health workers provided excellent health education and counseling and outreach activities, and their involvement was acceptable to most patients. Several sub-themes were identified.
Interpersonal Relationships
Lay health workers living with HIV established strong personal relationships with other individuals living with HIV receiving retention interventions [41]. Local lay health workers increased the likelihood that individuals infected with HIV would access retention services [41]. Lay health workers who spent more time with individuals living with HIV provided greater education on HIV and medication use [34]. Coordination with lay health workers as case managers was effective in retention in care: “We are responsible to ensure that the patient is getting adequate and holistic care. Clinicians identify patients at risk of poor adherence and/or retention, and send those patients to us. We then assess the level of risk for poor adherence and/or retention of the patient and develop a plan to improve it. Potential reasons (causes) for poor adherence and/or retention are identified. We will accordingly devise appropriate solutions and develop a targeted action plan to reduce the risk of the patient for poor adherence and/or retention.” (Case manager, age and sex unknown, Ethiopia)” [42].
Social Support Provision
The provision of emotional support offered by lay health workers and peer counselors nurtured hope and a positive outlook, which increased retention in care [33, 39, 42]. Non-medical support such as making arrangements for rides to clinics and providing soap and other basic needs by lay health workers improved relationships with children in care [34].
Counseling and Education
Counseling and encouragement from lay health workers provided hope for good treatment outcomes. Education about proper medication usage, side effects, and HIV serostatus disclosure can facilitate interventions to improve retention in care [39]. “I get good counseling on adherence in a way that I don't lose hope. The [lay health worker] encourage me.” (Female living with HIV, age unknown, Uganda) [41].
Barriers to lay health worker retention intervention effectiveness included high patient caseloads and lack of preparedness in dealing with acute stressors (e.g., patient adverse events and patients moving), which affected maintaining follow-up appointments for other individuals with HIV [41].
Human Resources and Institutional Challenges (Moderate CERQual Confidence)
Six studies [33, 35, 36, 38, 39, 41] demonstrated that clinician and lay health worker shortage and high workload led to poor care provision and long wait times for individuals living with HIV, contributing to suboptimal retention. Limited trained pharmacy personnel and absenteeism also exacerbated poor retention in care [35, 39]. “Sometimes the service is poor. I come in the morning, like I came around 8 but I might still be here until 1 pm. The pharmacist leaves for tea break and he does not come back. You will wait.” (Female caregiver, age unknown, Zimbabwe) [35]. Moreover, drug unavailability and stock outs could lead to sub-optimal dosing, skipped doses of medication, and treatment dropout [35, 38, 39]. “Last week there was a child who came saying that she had been to [a Hospital] but there were no drugs. She could not get Cotrimoxazole because it was out of stock and she didn't have money. Therefore this is another challenge that we face. (Woman working for community based organization, age unknown, Zimbabwe)” [35].
Mobile Health (mHealth) (Moderate CERQual Confidence)
According to two studies [33, 40] mHealth (using mobile phone applications) were useful to increase retention in care. mHealth interventions included passive ARV medication reminders on cellphones [33] and active inquiring and addressing barriers to retention through text messaging [40]. “Texting the health concerns will help. It is good. I think when you are texting matters relating to health, that is when you can speak about everything that you are having. I don't have any concerns, you are free. You can be more free when you text” (35 year old male living with HIV, Kenya) [40].
Family and Friend Support (Moderate CERQual Confidence)
Four studies [33, 36, 39, 40] addressed the importance of family and friend support for retention. Some individuals living with HIV reported that receiving support from family members increased retention [33, 40]. “Sometimes when I feel fatigued, am busy with work or sleep at dose time my children remind me to take the pills. They bring me a glass of water and the medication bottle.” (36 year old woman living with HIV, Ethiopia) [33]. Family support was mentioned as more important than community support for some individuals living with HIV since they felt greater trust of family members. Peer support also empowered individuals living with HIV to overcome stigma [36]. Retention was enhanced by the realization of parents living with HIV about how their retention in care affected their children's well-being [33]. “… I have a boy born free of the virus. As long as I am alive, I wish to see him achieving better opportunities. If I am not taking the medication properly or abandoned it altogether, I am ruining his chance.” (35 year old man living with HIV, Ethiopia) [33].
Intensive Case Management (Moderate CERQual Confidence)
Based on the findings from two studies [33, 42], intensive case management interventions were acceptable and useful according to individuals living with HIV. Intensive case management interventions involved frequent home visits, face-to-face encounters, and collaborative problem solving to decrease barriers to retention. Providing transportation to attend clinic appointments and interdisciplinary cooperation facilitated retention in care. However, intensive interventions required substantial staff time to implement and required multiple encounters with individuals living with HIV. This burdened health care workers and individuals living with HIV alike due to time and scheduling conflicts. Using a stepped care approach to only provide intensive case management to individuals living with HIV who were most at risk for treatment dropout minimized resource burden.
Relationships with Caregivers (Moderate CERQual Confidence)
Five studies [32, 33, 37–39] stated that better relationships between those implementing interventions (lay person or specialist) and individuals living with HIV enhanced retention outcomes. “When she visited me I was so happy, because at first I was feeling very rejected and when she came she was so friendly to me and people gathered and they were surprised that I had a visitor in the house. So she brought some few gifts (of bread, sugar, and milk) for me.” (Man living with HIV, age unknown, Nigeria) [32]. Patience and respect shown toward individuals living with HIV increased retention behavior. Conversely, patronizing behaviors, lacking empathy, and lack of physical touch with individuals living with HIV was reported as a barrier to retention [33, 37, 39]. Fear of punitive staff reactions after missed appointments or loss of medical card adversely affected retention [38].
Discussion
This qualitative evidence meta-synthesis identified multiple themes relevant for retention in care intervention and broader policy implications for HIV. Our review extends the literature on retention in HIV care [10, 12] by synthesizing evidence from studies conducted in geographically diverse locations and by formally assessing the quality of this data and the confidence in review findings. Qualitative meta-synthesis enables a more nuanced approach to understanding facilitators and barriers to retention in HIV care as it capitalizes on the strength of qualitative research to emphasize individuals' perspectives on retention in care.
Reducing stigma (both self-stigma and community stigma) against individuals living with HIV and their families may enhance retention in care. One study indicated that stigma prevented PLWH to disclose their status and receive emotional or instrumental support, which would enhance retention in HIV care [33]. A recent review documented the potential to reduce HIV-related stigma at the individual and community-level [44]. However, a critical gap in the literature remains regarding whether interventions to reduce HIV self- and community-stigma can increase retention in care. Increased development and evaluation of stigma reduction programs may enhance retention in care and improve quality of life for individuals living with HIV.
Fear of serostatus disclosure is implicated in poor retention in care [33, 35, 40–42]. Fear of disclosure placed greater demands on treatment providers [41]. It also undermined treatment retention through avoidance of treatment centers and reluctance to utilize mHealth [40]. Fear of disclosure is driven in part by stigma and perceived lack of acceptance by their families and communities, so increasing acceptance of PLWH may be a critical area for intervention development.
Our review identified broad categories of interventions focused on task shifting interventions to lay health workers and community volunteers, case management, clinic- and community-based health promotion, and mHealth. Our review highlighted that lay health workers are integral in delivering HIV interventions within locations where there are few healthcare providers [34, 39, 41, 42]. Studies demonstrated that lay health workers aid in delivering interventions that retain individuals living with HIV in care. Key features of the success of lay health workers included the importance of their relationship to individuals living with HIV, the support they provided, and their familiarity with the social context [33, 39, 42]. Enhanced coordination of care with community based agencies and lay health workers is likely to promote intervention effectiveness. Despite the promising evidence for the usefulness of task shifting interventions for retention in HIV care, difficulties were also identified. Issues involved in successful utilization of lay health workers includes training burden [41], issues of trust and concerns about home-based care inadvertently leading to HIV serostatus disclosures [41]. Continued evaluation of the integration of lay health workers in retention in care packages and evaluation of their effectiveness is needed.
Human resource and institutional challenges were highlighted in six studies [33, 35, 36, 38, 39, 41]. Skilled and lay health worker shortages were key issues that led to poor retention in care [35, 39]. Long waiting times and medication stock-outs were also critical concerns associated with poor retention [35, 38, 39]. These structural barriers are of critical concern and require greater comprehensive policy support, which may include government subsidies to increase training of local health workers and increase medication stocks. Relatedly, intensive case management interventions are challenging to administer due to structural and resource barriers [33, 42].
Only two studies that utilized mHealth applications for HIV retention in care were reviewed. With the continued proliferation of mobile phones and mobile devices in low-and middle-income countries [43], mHealth provides an alternative platform to deliver HIV retention in care interventions. However, care should be taken to mitigate the risk associated with inadvertent serostatus disclosure [40] and the fears related to this disclosure risk, which may influence mHealth uptake.
Four studies [33, 36, 39, 40] provided evidence that family and friend support was critical in retention in care. Family level counseling or peer-based interventions may be important additional services to increase retention in care. This may especially be true in collectivistic cultures where community and family engagement may be critical mediators of optimal treatment outcomes. A related theme emerged that touched upon the importance of human connection. Five studies [32, 33, 37–39] provided support for patience, care, and support shown by caregivers is vital to increase retention in care. Given the importance of this theme, trainings of physicians or lay health workers to deliver compassionate and empathic care may be vital to enhance retention in care programming.
This review has several limitations. Although eight themes were identified, few studies were available to provide adequate richness to each of the themes. All of the qualitative data provided was integrated data based on single interviews or focus groups. Few studies focused on important sub populations including pregnant women, children and adolescents, and other key populations, like gay, bisexual and other men who have sex with men [45]. Additional qualitative studies are needed to contextualize retention in care interventions and provide enhanced viewpoints on the mechanisms underlying these interventions. Current qualitative evidence for retention in care interventions is lacking. Some quotations included in this paper may be also be relevant to adherence, but we believe they are important for retention in care. Future studies are needed that provide particular emphasis on the perspectives of individuals living with HIV and providers involved in intervention delivery. This would greatly enhance subsequent implementation and development of tailored retention interventions to retain individuals living with HIV in care.
Supplementary Material
Acknowledgments
We thank the WHO HIV/AIDS Department and the Guangdong Provincial Centers for Skin Diseases and STI Control for their contribution and support. We would like to thank Zhang Ye, Alice Armstrong, Nathan Ford of the WHO and Simon Lewin of the Norwegian Knowledge Centre for the Health Services for their support during concept development and manuscript review processes.
Financial Disclosure: This project was originally commissioned and supported by the World Health Organization. Additional funding and support was provided by the Macau SAR Government and the University of Macau (SRG-000001-2014-FSS & MYRG2015-00109-FSS, PI: Hall), and (NIAID 1R01AI114310-01 and FIC 1D43TW009532-01, PI: Tucker) from the National Institutes of Health.
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s10461-016-1537-0) contains supplementary material, which is available to authorized users.
Conflicts of interest All authors declare they have no conflict of interest.
Compliance with Ethical Standards: Ethical approval This article does not contain any studies with human participants performed by any of the authors.
References
- 1.World Health Organization (WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach June 2013. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, McCauley M, Sugarman J. Establishing HIV treatment as prevention in the HIV Prevention Trials Network 052 randomized trial: an ethical odyssey. Clin Trials. 2012;9:340–7. doi: 10.1177/1740774512443594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The TEMPRANO ANRS. 12136 Study Group. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373:808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 6.Stricker SM, Fox KA, Baggaley R, Negussie E, de Pee S, Grede N, et al. Retention in care and adherence to ART are critical elements of HIV care interventions. AIDS Behav. 2014;18(Suppl 5):S465–75. doi: 10.1007/s10461-013-0598-6. [DOI] [PubMed] [Google Scholar]
- 7.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 8.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed Visits and Mortality among Patients Establishing Initial Outpatient HIV Treatment. Clin Infect Dis. 2009;48:248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59:86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9:313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57:1164–71. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 12.Okeke NL, Ostermann J, Thielman NM. Enhancing linkage and retention in HIV care: a review of interventions for highly resourced and resource-poor settings. Curr HIV/AIDS Rep. 2014;11:376–92. doi: 10.1007/s11904-014-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 14.Brookes D. Understanding qualitative research and its value in healthcare. Nurs Timesnet. 2007;103:32–3. [Google Scholar]
- 15.Hannes K. Chapter 4: Critical appraisal of qualitative research. In: Noyes J, Booth A, Hannes K, et al., editors. Supplementary guidance for inclusion of qualitative research in Cochrane Systematic Reviews of Interventions edition: Version 1. Cochrane Qualitative Research Methods Group; 2011. [Google Scholar]
- 16.Holloway I, Wheeler S. Qualitative Research in Nursing and Healthcare. New York: Wiley; 2009. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181. doi: 10.1186/1471-2288-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Programme CAS. Qualitative appraisal checklist for qualitative research. 2006 [Google Scholar]
- 20.Carlsen B, Glenton C, Pope C. Thou shalt versus thou shalt not: a meta-synthesis of GPs' attitudes to clinical practice guidelines. Br J Gen Pract. 2007;57:971–8. doi: 10.3399/096016407782604820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Vol-mink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. Plos Med. 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makanjuola T, Taddese HB, Booth A. Factors associated with adherence to treatment with isoniazid for the prevention of tuberculosis amongst people living with HIV/AIDS: a systematic review of qualitative data. PLoS ONE. 2014;9:e87166. doi: 10.1371/journal.pone.0087166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis SA, Noyes J, Hastings RP. Systematic review of epilepsy self-management interventions integrated with a synthesis of children and young people's views and experiences. J Adv Nurs. 2015;71:478–97. doi: 10.1111/jan.12511. [DOI] [PubMed] [Google Scholar]
- 24.Lewin S, Glenton C, Munthe-Kaas H, Carlsen B, Colvin CJ, Gülmezoglu M, et al. Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual) Plos Med. 2015;12:e1001895. doi: 10.1371/journal.pmed.1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth A. Formulating answerable questions. In: Booth A, Brice A, editors. Evidence Based Practice: An Information Professional's Handbook. London: Facet; 2004. pp. 61–70. [Google Scholar]
- 26.Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol. 2009;9:59. doi: 10.1186/1471-2288-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haley DF, Golin CE, Farel CE, Wohl DA, Scheyett AM, Garrett JJ, et al. Multilevel challenges to engagement in HIV care after prison release: a theory-informed qualitative study comparing prisoners' perspectives before and after community reentry. BMC Public Health. 2014;14:1253. doi: 10.1186/1471-2458-14-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunn A, Eng W, Cornwall A, Beckwith C, Dickman S, Flanigan T, et al. African American patient experiences with a rapid HIV testing program in an urban public clinic. J Natl Med Assoc. 2012;104:5–13. doi: 10.1016/s0027-9684(15)30125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinlivan EB, Messer LC, Adimora AA, Roytburd K, Bowditch N, Parnell H, et al. Experiences with HIV testing, entry, and engagement in care by HIV-infected women of color, and the need for autonomy, competency, and relatedness. AIDS Patient Care STDs. 2013;27:408–15. doi: 10.1089/apc.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systemic reviews. BMC Med Res Methodol. 2008;8:45. doi: 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll C, Booth A, Lloyd-Jones M. Should we exclude inadequately reported studies from qualitative systematic reviews? An evaluation of sensitivity analyses in two case study reviews. Qual Health Res. 2012;22:1425–34. doi: 10.1177/1049732312452937. [DOI] [PubMed] [Google Scholar]
- 32.Agbonyitor M. Home-based care for people living with HIV/AIDS in Plateau State, Nigeria: findings from a qualitative study. Global Public Health. 2009;4:303–12. doi: 10.1080/17441690902783165. [DOI] [PubMed] [Google Scholar]
- 33.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM, Bimirew MA, Kassie DM. Barriers and facilitators of adherence to antiretroviral drug therapy and retention in care among adult HIV-positive patients: a qualitative study from Ethiopia. PLoS ONE. 2014;9:e97353. doi: 10.1371/journal.pone.0097353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busza J, Besana GVR, Mapunda P, Oliveras E. Meeting the needs of adolescents living with HIV through home based care: lessons learned from Tanzania. Child Youth Serv Rev. 2014;45:137–42. [Google Scholar]
- 35.Busza J, Dauya E, Bandason T, Mujuru H, Ferrand RA. “I don't want financial support but verbal support” How do caregivers manage children's access to and retention in HIV care in urban Zimbabwe? J Int AIDS Soc. 2014;17:18839. doi: 10.7448/IAS.17.1.18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decroo T, Vandendyck M, Motsamai M, Mubanga M, Makhakhe S, Jonckheree S, et al. Community Antiretroviral Therapy Groups (CAGs) in Nazareth, Lesotho: the way forward for an effective community model for HIV care? J Acquir Immune Defic Syndr. 2011;56:e39–44. [Google Scholar]
- 37.Mallinson RK, Rajabiun S, Coleman S. The provider role in client engagement in HIV care. AIDS Patient Care STDs. 2007;21(Suppl 1):S77–84. doi: 10.1089/apc.2007.9984. [DOI] [PubMed] [Google Scholar]
- 38.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Health. 2010;15:48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajabiun S, Mallinson RK, McCoy K, Coleman S, Drainoni ML, Rebholz C, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDs. 2007;21(Suppl 1):S20–9. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 40.Smillie K, Van Borek N, van der Kop ML, Lukhwaro A, Li N, Karanja S, et al. Mobile health for early retention in HIV care: a qualitative study in Kenya (WelTel Retain) Ajar-African J AIDS Res. 2014;13:331–8. doi: 10.2989/16085906.2014.961939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alamo S, Wabwire-Mangen F, Kenneth E, Sunday P, Laga M, Colebunders RL. Task-shifting to community health workers: evaluation of the performance of a peer-led model in an antiretroviral program in Uganda. AIDS Patient Care STDs. 2012;26:101–7. doi: 10.1089/apc.2011.0279. [DOI] [PubMed] [Google Scholar]
- 42.Assefa Y, Lynen L, Wouters E, Rasschaert F, Peeters K, Van Damme W. How to improve patient retention in an antiretroviral treatment program in Ethiopia: a mixed-methods study. BMC Health Serv Res. 2014;14:45. doi: 10.1186/1472-6963-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Bank. Information and Communications for Development: Maximizing Mobile. Washington, DC: World Bank; 2012. [Google Scholar]
- 44.Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc. 2013;16(3Suppl 2):18734. doi: 10.7448/IAS.16.3.18734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO) Chapter 4: Organization of Guidelines Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva: World Health Organization; 2014. pp. 54–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.