Abstract
Background
Non-human primates, particularly rhesus macaques, are ideal pre-clinical large animal models to investigate organ tolerance induction protocols using donor hematopoietic stem cells (HSCs) to induce microchimerism. Their relatively small size poses some challenges for the safe and effective collection of peripheral blood HSCs through apheresis procedures. We describe our experiences using the Spectra Optia apheresis unit to successfully obtain HSCs from mobilized peripheral blood of rhesus macaques.
Method
Mobilization of peripheral blood HSCs was induced using granulocyte stimulating factor (G-CSF) and Mozobil. The Spectra Optia unit was used in 18 apheresis procedures in 13 animals (4.9–10 kg). Animal health was carefully monitored during and after the procedure. Changes in peripheral blood cells before, during and after procedure were determined by complete blood count and flow cytometry.
Results
The automatic settings of the Spectra Optia unit were applied successfully to the procedures on the rhesus macaque. All animals tolerated the procedure well with no mortality. Mobilization of HSCs were most consistently achieved using 50μg/kg of G-CSF for 5 days and a single dose of Mozobil on the 5th day, followed by collection of cells 3 hours after Mozobil injection. The final apheresis product contained an average of 23 billion total nucleated cells with 47% granulocytes, 3,871 million total CD3 cells and 77 million CD34 cells which resulted in an average of 10 million CD34+ cells/kg of donor weight.
Conclusion
Apheresis of peripheral blood mobilized HSCs in rhesus macaques using Spectra Optia is a safe and effective procedure.
Keywords: apheresis, primate, chimerism
Introduction
Nonhuman primates, and specifically old world rhesus macaques, have proven to be a highly relevant pre-clinical large animal model for hematopoietic stem cell (HSC) transplantation [1–3]. In clinical practice apheresis has surpassed bone marrow harvest as the procedure of choice for collection of HSCs [4,5] These large animals are particularly relevant translational models because their hematopoietic cells have a high degree of cross reactivity with human reagents and responsiveness to human cytokines [6,7]. The use of rhesus macaques in HSC transplant models, however, is challenging due to their relative small size (typically 4–10 kg), which makes apheresis a demanding procedure with safety implications. Similar to use of apheresis in small pediatric patients the large extracorporeal blood volume mandates the priming of the apheresis machine with allogeneic blood [8]. The Spectra Optia Apheresis System, a newer generation of the COBE Spectra system, requires a lower extracorporeal volume compared to previous models, and was of interest to test for use in the small rhesus macaques. We have established a non-human primate HSC transplantation model for investigation of HSC-induced kidney transplant tolerance. We describe our experience with apheresis and its optimization for collection of G-CSF mobilized peripheral blood HSC in small sized rhesus macaques using the Spectra Optia Apheresis System.
Materials and Methods
Animals
Thirteen rhesus macaques (Macaca mulatta) were used for 18 apheresis procedures. Animals were 4.9–10 kg and 3.3–12.1 years old at the time of the procedure. All animals were negative for Mycobacterium tuberculosis, Macacine herpesvirus 1 (herpes B virus), simian T-lymphotropic virus, simian retrovirus type D, and simian immunodeficiency virus. All animals were treated in accordance with the 8th edition of the Guide for the Care and Use of Laboratory Animals published by National Research Council and the procedures and protocol were approved by the University of Wisconsin-Madison institutional animal care and use committee (IACUC).
Peripheral HSC Mobilization
Animals were treated for five days with granulocyte-colony stimulating factor (G-CSF) given subcutaneously (SQ); 50 μg/kg/day was administered prior to 12 procedures, 10 μg/kg/day for two procedures, and 100 μg/kg/day for a single procedure. In 16 procedures the animals were given 1 mg/kg Mozobil SQ (Plerixafor®), and in 2 procedures the animals were given MD3100 [Sigma Chemical Co, St. Louis, MO], all 3–6 hours prior to the apheresis procedure on the 5th day [9]. Two animals were treated with 50 μg/kg/day G-CSF for 6 days and received 1 mg/kg Mozobil on both days 5 and 6.
Apheresis Procedure
For the apheresis procedure the animals were sedated ketamine (15 mg/kg) and atropine (0.04mg/kg) administered intramuscularly (IM), then intubated and maintained on isoflurane (1.5%, range of 1–2%). Thermal support was provided using a heated table and forced warm air blanket (3M, Bair Hugger). The skin overlying the femoral triangle was aseptically prepared and local analgesic was administered (Lidocaine 2%, 2–4 mg SQ). Based on pilot procedures, the catheter placement was modified from using a 9 French dual lumen catheter to a 5 French central venous catheter (Jorgenson Laboratories, Loveland, CO) inserted into the femoral vein for blood outflow. For the majority of procedures, the catheter was placed percutaneously, but occasionally a cut down procedure was needed to directly visualize the femoral vein. A second intravenous catheter (20–22-gauge) was placed into the saphenous vein, in the opposite leg when possible, for return flow.
For each procedure, approximately 150 ml of whole blood was collected from a single 3rd party (unrelated) donor rhesus macaque negative for pathogens as described above, mixed in a 14:1 ratio of ACD-A, leukofiltered (Haemonetics, Braintree, MA) and stored for 1–3 days at 4°C. The morning of the procedure, the blood was irradiated (Gammacell 1000 Elite, Ottawa, Ontario) prior to being used for priming the apheresis machine (Spectra Optia, Terumo BCT, Lakewood, CO). The apheresis machine was prepared using a Spectra Optia Collection Set (Terumo BCT), the tubing first primed with saline, then primed with the allogeneic blood until blood appeared in the waste bag or the prime was manually ended. ACD-A was used at a rate of 2–2.5 ml/min/L of total blood volume (TBV) with a minimum inlet:AC ratio of 12:1 for most cases. To prevent citrate toxicity, calcium gluconate (230 mg/mL) diluted in crystalloid fluids (0.9% saline used for pilot studies, switched to plasmalyte due to concerns for hypokalemia) resulting in 4 mg/ml Ca++, was administered IV beginning typically at 35 ml/hr and adjusted as needed based on ionized calcium values obtained during procedure.
Adjustments were made in collection settings as needed. After the final chamber collection, the rinse back of the blood and fluid contained in the channel of the tubing set was performed. Following the procedure, the femoral catheter was removed and pressure applied for 20–40 minutes. If a cut down procedure was used, the vein was ligated and the skin was closed in 2 layers using absorbable suture. In cases where the full volume of allogeneic blood was not used to prime the instrument, the residual 40–60 mls was given IV over 10 minutes at the end of the procedure. Remaining catheters were removed and pressure applied for 5–10 minutes.
Clinical monitoring
During the procedure heart rate, respiratory rate, end tidal CO2, oxygen saturation, and blood pressure (HDO Vet, DVM Solutions, San Antonio, TX) were continuously monitored and recorded every 15 minutes. In addition to monitoring ionized calcium, animals were monitored for clinical signs of citrate toxicity (e.g. seizures, arrhythmias, or vomiting). Animals were given an analgesic (buprenorphine, 0.01 mg/kg IM) if catheter placement was difficult or if a femoral cut down procedure was used. If an animal showed signs of pain (such as favoring leg, change in posture or locomotion) after the procedure, buprenorphine (0.01 mg/kg IM) or meloxicam (0.2 mg/kg SQ) were given as needed. Animals were monitored closely for evidence of hemorrhage, especially from the femoral catheter site.
Laboratory Evaluation
Complete blood counts (CBCs) were performed before administration of Mozobil and immediately prior to start of apheresis (3–6 hours after Mozobil administration). Baseline white blood cell count, platelet, and hematocrit data entered into the Optia software were updated when results became available (typically 30–45 minutes after initiation of procedures). During the procedure, laboratory parameters were monitored using a portable point of care unit (i-STAT, Chem8+ cartridge, Abbott, Abbott Park, IL) to monitor changes in electrolytes, hematocrit, and ionized calcium. i-STATs were performed every 15–45 minutes during the procedure, with more frequent checks at the beginning. A complete chemistry panel was performed at the end of the procedure. Frequency of CD34 and CD3 was determined by flow cytometry captured using a Becton Dickinson LSRII flow cytometer (BD Biosciences, San Jose, CA) with CD34 (clone 563), CD45 (clone D058-1283) and CD3 (clone SP34-2, all from BD Biosciences). In some animals, a CBC and chemistry panel were also performed 1 and 3 days later for further monitoring.
Results
Collection parameters
The mean (± S.D.) time for apheresis was 229 (± 69) minutes, with a mean (± S.D.) of 5.1 (± 1.2) total blood volumes collected. The run speed was 10–10.5 ml/min. Because of the very high platelet count in some animals at the time of collection, the ideal collection preference [CP, =60−(0.2*white blood cell count)+(0.8* platelet count)] was occasionally <20 (the lowest setting possible for the Optia). In those cases, the collection preference was set for 20. In most other cases, the preference was set slightly higher than the ideal collection preference automatically calculated by the instrument. There was no correlation between collection preference settings and total CD34 collected, or efficiency of collection.
Unlike the Cobe Spectra system, which continuously collects the buffy coat into the collection bag, the Spectra Optia collects product into a 20 ml chamber and then releases the contents into the collection bag. For maximum efficiency of collection, the system is designed to trigger a collection based on a calculation of the amount of blood that would have to be processed to fill the chamber with white blood cells, including the CD34 cells of interest. Because of the small total blood volume in the rhesus macaque, it was not clear if the automatic processes would work appropriately. We therefore modified the collection parameters to trigger a collection when a set volume of blood had been processed; however, we found that this did not yield as high a quality product (frequency of CD34) as when the automatic triggering mechanism was utilized. Therefore, the majority of procedures were performed using the automatic collection parameters.
Clinical Monitoring
Animals occasionally showed mild facial muscle fasciculation or became less deep under anesthesia when ionized calcium dropped below 1.0 mmol/L; therefore, the goal was to maintain ionized calcium above 1.0 mmol/L by adjusting the calcium infusion. We found a concentration of calcium gluconate for infusion of 4 mg/ml Ca++ allowed adequate ionized calcium and electrolytes to be maintained in a safe range without fluid overload. The total fluids received (plasmalyte, ACD-A, and whole blood) ranged from 55–95 ml/kg for each procedure.
Following apheresis, appetite for chow was mild to moderately reduced and gradually improved over 2 weeks. Weight loss variably occurred following the procedure (7 animals had 6–8% weight loss and 2 animals had 13–20% weight loss).
All animals tolerated the procedure well. One animal had mild azotemia (BUN 61 mg/dL, creatinine 2.35 mg/dL) and a marked elevation in CPK (111,260 U/L), LDH (10,760 IU/L), and AST (1,115 IU/L) the day following apheresis. Review of the anesthesia record indicated no evidence of low blood pressure or seizure activity and the ionized calcium was appropriately maintained. There were no mortalities.
Laboratory parameters
The mean serum ionized calcium concentration was 1.17 mmol/L (normal range 1.01–1.15 mmol/L) at the beginning of the procedure, 1.12 mmol/L at the end of the procedure, and with a range of 0.87–1.52 mmol/L during the procedure. With calcium supplementation required to maintain ionized calcium during the procedure the mean serum calcium at the end of the procedure was 23.5 mg/dL for 17 procedures (range of 18.7–33.7 mg/dL). Serum calcium values returned to normal limits (9.0–11.1 mg/dL) the following morning (average 9.4 mg/dL, range 8.9–10.3 mg/dL for 9 animals).
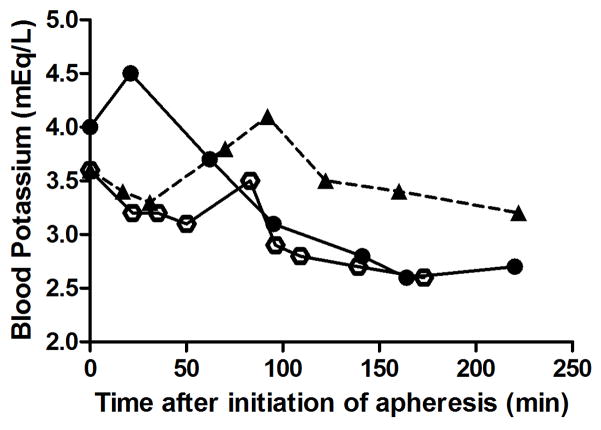
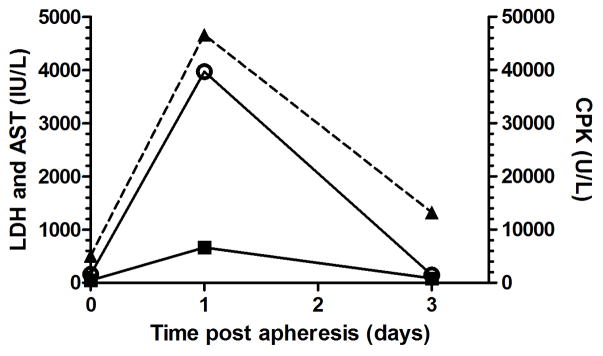
Hypokalemia was common during initial pilot studies when 0.9% NaCl was used for fluid support; however, this was corrected when plasmalyte was used (Figure 1). Serum CPK, LDH, and AST levels were moderate to markedly increased the morning following apheresis and normalized within 3 days after the procedure (Figure 2). Other changes on chemistry panel included mild increase in iron (normal range 48–209 μg/dL), with a mean of 110 μg/dL at the end of the procedure and increased to a mean of 269 μg/dL 3 days later.
Figure 1.
Change in blood potassium levels during apheresis. A single rhesus macaque was supplemented with 0.9% NaCl with 2 mg/ml Ca++ (open circles), 0.9% NaCl with 4 mg/ml Ca++ (closed circles), or plasmalyte with 4mg/ml Ca++ (triangles, dashed line) for three separate apheresis procedures.
Figure 2.
Circulating mean levels of creatinine phosphokinase (CPK, open circle, normal range 0–1565 U/L), lactate dehydrogenase (LDH, filled triangle, dashed line, normal range 45–718 IU/L), and aspartate aminotransferase (AST, filled squares, normal range 15–69 IU/L) were measured in the blood day 0 immediately after procedure (n=18), day 1 (n=7), and day 3 (n=6).
For most of the procedures, the entire reserved allogeneic (non-donor 3rd party) blood (~150mls) was used to prime the machine, but for the last 6 procedures, 40–60ml was reserved and transfused into the animal at the end of the procedure. There was no difference in hematocrit or platelet counts at the end of the procedure when the blood product was used only to prime the instrument or was divided and used both to prime the instrument and transfused into the animal at the end of the procedure (data not shown). This may be due to the small total volume of the transfusion or to the fact that at the end of the procedure, the rinse back procedure returns residual cells in the tubing system to the animal.
Mobilization
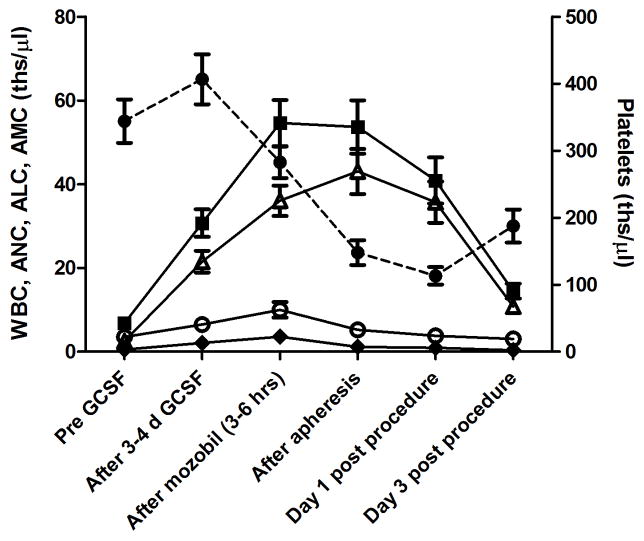
Figure 3 shows the kinetics of white blood cell (WBC) counts after administration of G-CSF. The absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), and absolute monocyte counts (AMC) increased ~9-, 2- and 4-fold, respectively. The counts further increased with addition of Mozobil to 17-, 3.2-, and 8.6-fold total increase in ANC, ALC, and AMC over pre-treatment, respectively.
Figure 3.
Changes in peripheral plateletes (circles, dashed line), total white blood cells (WBC, filled squares), neutrophils (ANC, open triangles), lymphocytes (ALC, open circles), and monocytes (AMC, filled diamonds) before treatment, after 3–4 days of G-CSF (50 μg/ml), after mobilization (3–6 hours), at the end of the procedure (4–6 hours), the day after the procedure (day 1, n=8 animals) and the 3rd day after the procedure (day 3, n=7 animals). Data represents averages (+/− SEM) at each time point from 11 animals (13 procedures) that were treated with 50 μg/kg G-CSF.
Peripharal platelet counts decreased after Mozobil administration (range 112–488 ths/μl) and further decreased during the apheresis procedure (range 55–249 ths/μl, Figure 3). Total WBCs and the ANC component were higher at the end of the procedure. The ALCs decreased as expected since this population was collected during the procedure within the mononuclear cell fraction. All peripheral major hematologic subsets returned to near normal levels by day 3 after apheresis. One animal each received 10 μg/kg and 100 μg/kg G-CSF, respectively for mobilization. As expected, lower doses of G-CSF mobilized fewer cells than higher doses, with ANC mobilization the highest after 100 μg/kg G-CSF (data not shown).
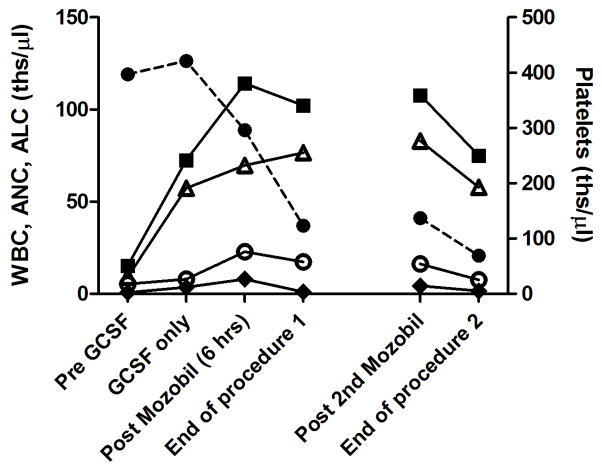
Six animals underwent repeat apheresis procedures with timing between the procedures ranging from 24 hours to 5 months. All 6 animals tolerated multiple procedures well, but apheresis procedures on the rhesus macaque on two sequential days resulted in a significant decrease in peripheral platelet counts (to 69 ths/μl) and mild decrease in hematocrit (32%) at the end of the procedure on the second day (Figure 4).
Figure 4.
Changes in cell subsets for a single animal that underwent apheresis procedures on two sequential days. Peripheral plateletes (circles, dashed line), total white blood cells (WBC, filled squares), neutrophils (ANC, open triangles), lymphocytes (ALC, open circles), and monocytes (AMC, filled diamonds) before treatment, after 4 days of G-CSF only (50 μg/ml), 6 hours after mobilization with Mozobil, at the end of the first procedure, 6 hours after administration of 2nd dose of Mozobil, and at the end of the second procedure.
Mobilization of CD34+ hematopoetic stem cells
Flow cytometry to measure CD34 cells in peripheral blood, were performed before, during and after the mobilization and apheresis. As shown in Figure 5, mobilization of CD34 cells was higher after administration of 50 μg/ml G-CSF than with 10 μg/ml G-CSF. Mobilization using 100 μg/ml in the one animal tested was actually compared to animals in which 50 μg/ml was used. Administration of Mozobil further enhanced the mobilization of CD34 cells into the peripheral blood.
Figure 5.
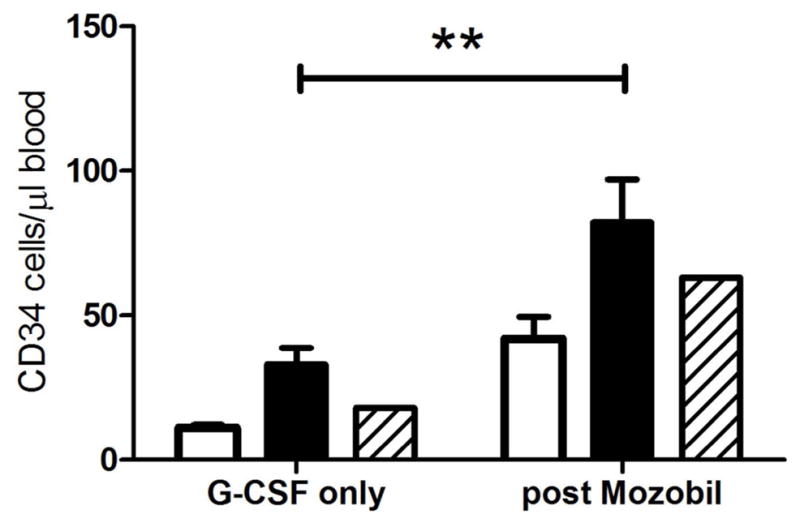
Mean ± SEM of CD34 cells mobilized to the peripheral blood with G-CSF [10 μg/ml (open bars), 50 μg/ml (filled bars) or 100 μg/ml (stripped bars)] before and after (3–6 hours) Mozobil administration.
Graft composition
Apheresis procedures were only performed on animals that received both G-CSF and Mozobil, a protocol that has been shown to mobilize CD34+ cells into the periphery that are capable of engraftment in the rhesus macaque [9,10] and other large animal models [11]. The final apheresis product contained a variable number of cells with an average of 23 × 109 (+/− 11 × 109) total nucleated cells with 47% granulocytes, 3.8 × 109 (+/− 3.7 × 109) total CD3 cells, and 77 × 106 (+/− 56 × 106) CD34 cells which resulted in a mean of 10 million CD34+ cells/kg of donor weight. The collection efficiency [(% CD34 in product x TNC x volume)/(% CD34 in blood at time of collection x WBC x total volume of blood processed)] was 37% +/− 17% and ranged from 13% to 67%. The collection efficiency was most strongly correlated with the time of apheresis (r2 = 0.244, p=0.04). As expected there was a clear correlation between the concentration of CD34+ cells in the blood at the time of collection and both the total number of cells collected (r2= 0.48, p=0.002) as well as the number of CD34+ cells collected/liter of blood processed (r2= 0.39, p=0.007).
Discussion
Collection of peripheral blood stem cells via apheresis in rhesus macaques provides the opportunity to utilize this large animal model in translationally-relevant hematopoietic cell transplant experiments. We have established a rhesus macaque combined hematopoietic and solid organ transplantation model to investigate novel strategies for induction of tolerance to kidney allografts. In this model hematopoietic stem cells are mobilized with G-CSF and Mozobil and then collected via apheresis using the Spectra Optia machine. We used the Spectra Optia Apheresis System, a newer generation of the COBE Spectra system, because this machine requires a lower extracorporeal volume compared to previous models [12,13], and thus was of interest to test for use in the small size rhesus macaques. Although in this study we only tested the intermittent chamber collection, a recent clinical study has shown that the continuous collection of MNCs using a redesigned disposable kit and software program for the Spectra Optia does not compromise collection efficiency [14].
The increase in CPK, LDH, AST, and iron were likely secondary to G-CSF administration and has been reported in humans [15]; however, muscle trauma associated with catheter placement and hemolysis from the procedure could have also contributed to the rise in LDH. All values were significantly improved at 3 days following the procedure. Because of the potential for azotemia and significant muscle enzyme elevations following the apheresis procedure, we administered crystalloid fluids (plasmalyte 20 ml/kg SQ) the morning after apheresis procedures.
Our stem cell mobilization efficiency using G-CSF and Mozobil was in line with previous reports [9,16]. In two animals that were mobilized with 10 μg/kg of G-CSF and Mozobil the CD34 yield was comparatively low. However, when the G-CSF dose was increased to 50 μg/kg it resulted in an improved CD34 cell yield. These doses of both G-CSF and Mozobil (1 mg/kg) are higher than typically administered to human subjects, but are typical of what has been used in nonhuman primate studies in the last two decades [9,17–20]. There was an animal that was mobilized twice but had poor mobilization on both occasions, consistent with individual variability, a phenomenon that is also seen in normal healthy human volunteer donors [21]. Like humans we also observed the common side effect of thrombocytopenia in the non-human primate model.
In humans Mozobil is recommended to be given 10–11 hours prior to the apheresis [22] but most studies using the rhesus macaque model report initiation of apheresis earlier after drug administration [9]. Early in the study, apheresis procedures were initiated 6 hours after administration of Mozobil, but in order to determine the best time after administration of Mozobil to initiate the apheresis process, we sampled blood at 1–2 hour intervals after administration in 2 animals. We found that in the animals serially monitored, the highest number of CD34 cells were found in the peripheral blood at about 3–4 hours post-Mozobil administration (data not shown). Based on these observations, all subsequent procedures were started 3 hours after Mozobil administration.
In this study we demonstrate the feasibility and safety of apheresis procedures in the rhesus macaque model in animals as small as 4.9 kg using Spectra Optia machine. The mobilization and collection procedures defined above demonstrated that consistent and acceptable yields of CD34 cells can be obtained. Side effects from the procedures were transient and resolved with minimal intervention and may happen in humans, but may be underestimated.
Acknowledgments
This work was supported by National Institutes of Health Grant U01AI102456 (to DBK) and P51 OD011106.
References
- 1.Hematti P. Modeling human hematopoietic stem cell gene therapy in nonhuman primates. Current hematology reports. 2004;3(4):282–289. [PubMed] [Google Scholar]
- 2.Donahue RE, Kuramoto K, Dunbar CE. Large animal models for stem and progenitor cell analysis. Curr Protoc Immunol. 2005;Chapter 22(Unit 22A):21. doi: 10.1002/0471142735.im22a01s69. [DOI] [PubMed] [Google Scholar]
- 3.Herodin F, Thullier P, Garin D, Drouet M. Nonhuman primates are relevant models for research in hematology, immunology and virology. European cytokine network. 2005;16(2):104–116. [PubMed] [Google Scholar]
- 4.Pulsipher MA, Chitphakdithai P, Logan BR, Shaw BE, Wingard JR, Lazarus HM, Waller EK, Seftel M, Stroncek DF, Lopez AM, Maharaj D, Hematti P, O’Donnell PV, Loren AW, Leitman SF, Anderlini P, Goldstein SC, Levine JE, Navarro WH, Miller JP, Confer DL. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121(1):197–206. doi: 10.1182/blood-2012-03-417667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, Urbano-Ispizua A, Cutler CS, Bacigalupo AA, Battiwalla M, Flowers ME, Juckett MB, Lee SJ, Loren AW, Klumpp TR, Prockup SE, Ringden OT, Savani BN, Socie G, Schultz KR, Spitzer T, Teshima T, Bredeson CN, Jacobsohn DA, Hayashi RJ, Drobyski WR, Frangoul HA, Akpek G, Ho VT, Lewis VA, Gale RP, Koreth J, Chao NJ, Aljurf MD, Cooper BW, Laughlin MJ, Hsu JW, Hematti P, Verdonck LF, Solh MM, Norkin M, Reddy V, Martino R, Gadalla S, Goldberg JD, McCarthy PL, Perez-Simon JA, Khera N, Lewis ID, Atsuta Y, Olsson RF, Saber W, Waller EK, Blaise D, Pidala JA, Martin PJ, Satwani P, Bornhauser M, Inamoto Y, Weisdorf DJ, Horowitz MM, Pavletic SZ Graft-vs-Host Disease Working Committee of the C. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266–274. doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larochelle A, Dunbar CE. Hematopoietic stem cell gene therapy:assessing the relevance of preclinical models. Seminars in hematology. 2013;50(2):101–130. doi: 10.1053/j.seminhematol.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hematti P, Tuchman S, Larochelle A, Metzger ME, Donahue RE, Tisdale JF. Comparison of retroviral transduction efficiency in CD34+ cells derived from bone marrow versus G-CSF-mobilized or G-CSF plus stem cell factor-mobilized peripheral blood in nonhuman primates. Stem Cells. 2004;22(6):1062–1069. doi: 10.1634/stemcells.22-6-1062. [DOI] [PubMed] [Google Scholar]
- 8.Pathiraja V, Matar AJ, Gusha A, Huang CA, Duran-Struuck R. Leukapheresis protocol for nonhuman primates weighing less than 10 kg. Journal of the American Association for Laboratory Animal Science: JAALAS. 2013;52(1):70–77. [PMC free article] [PubMed] [Google Scholar]
- 9.Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, Bridger G, Dunbar CE, Hematti P. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107(9):3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida N, Bonifacino A, Krouse AE, Metzger ME, Csako G, Lee-Stroka A, Fasano RM, Leitman SF, Mattapallil JJ, Hsieh MM, Tisdale JF, Donahue RE. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34+ cells mobilized by G-CSF and plerixafor. Exp Hematol. 2011;39(7):795–805. doi: 10.1016/j.exphem.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burroughs L, Mielcarek M, Little MT, Bridger G, Macfarland R, Fricker S, Labrecque J, Sandmaier BM, Storb R. Durable engraftment of AMD3100-mobilized autologous and allogeneic peripheral-blood mononuclear cells in a canine transplantation model. Blood. 2005;106(12):4002–4008. doi: 10.1182/blood-2005-05-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karafin MS, Graminske S, Erickson P, Walters MC, Scott EP, Carter S, Padmanabhan A. Evaluation of the Spectra Optia apheresis system for mononuclear cell (MNC) collection in G-CSF mobilized and nonmobilized healthy donors: results of a multicenter study. Journal of clinical apheresis. 2014;29(5):273–280. doi: 10.1002/jca.21319. [DOI] [PubMed] [Google Scholar]
- 13.Long G, Waller EK, Gregurek S, Tricot G, Marschner S, Bill J. Evaluation of the spectra Optia(R) mononuclear cell collection procedure in multiple myeloma patients. Journal of clinical apheresis. 2015;30(1):1–7. doi: 10.1002/jca.21341. [DOI] [PubMed] [Google Scholar]
- 14.Lisenko K, Pavel P, Bruckner T, Puthenparambil J, Hundemer M, Schmitt A, Witzens-Harig M, Ho AD, Wuchter P. Comparison between intermittent and continuous spectra optia leukapheresis systems for autologous peripheral blood stem cell collection. J Clin Apher. 2016 doi: 10.1002/jca.21463. [DOI] [PubMed] [Google Scholar]
- 15.Anderlini P, Przepiorka D, Champlin R, Korbling M. Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood. 1996;88(8):2819–2825. [PubMed] [Google Scholar]
- 16.Kean LS, Sen S, Onabajo O, Singh K, Robertson J, Stempora L, Bonifacino AC, Metzger ME, Promislow DE, Mattapallil JJ, Donahue RE. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood. 2011;118(25):6580–6590. doi: 10.1182/blood-2011-06-359331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, Kepes S, Gray J, Dunbar CE, Persons DA, Nienhuis AW. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103(11):4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- 18.Hematti P, Sellers SE, Agricola BA, Metzger ME, Donahue RE, Dunbar CE. Retroviral transduction efficiency of G-CSF+SCF-mobilized peripheral blood CD34+ cells is superior to G-CSF or G-CSF+Flt3-L-mobilized cells in nonhuman primates. Blood. 2003;101(6):2199–2205. doi: 10.1182/blood-2002-08-2663. [DOI] [PubMed] [Google Scholar]
- 19.Kiem HP, Sellers S, Thomasson B, Morris JC, Tisdale JF, Horn PA, Hematti P, Adler R, Kuramoto K, Calmels B, Bonifacino A, Hu J, von Kalle C, Schmidt M, Sorrentino B, Nienhuis A, Blau CA, Andrews RG, Donahue RE, Dunbar CE. Long-term clinical and molecular follow-up of large animals receiving retrovirally transduced stem and progenitor cells: no progression to clonal hematopoiesis or leukemia. Mol Ther. 2004;9(3):389–395. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Wu T, Kim HJ, Sellers SE, Meade KE, Agricola BA, Metzger ME, Kato I, Donahue RE, Dunbar CE, Tisdale JF. Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods. Mol Ther. 2000;1(3):285–293. doi: 10.1006/mthe.2000.0034. [DOI] [PubMed] [Google Scholar]
- 21.Gattillo S, Marktel S, Rizzo L, Malato S, Malabarba L, Coppola M, Assanelli A, Milani R, De Freitas T, Corti C, Bellio L, Ciceri F. Plerixafor on demand in ten healthy family donors as a rescue strategy to achieve an adequate graft for stem cell transplantation. Transfusion. 2015;55(8):1993–2000. doi: 10.1111/trf.13059. [DOI] [PubMed] [Google Scholar]
- 22.Stewart DA, Smith C, MacFarland R, Calandra G. Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant. 2009;15(1):39–46. doi: 10.1016/j.bbmt.2008.10.018. [DOI] [PubMed] [Google Scholar]