Abstract
Sleep disorders are among the most common non-motor manifestations in Parkinson’s disease (PD) and have a significant negative impact on quality of life. While sleep disorders in PD share most characteristics with those that occur in the general population, there are several considerations specific to this patient population regarding diagnosis, management, and implications. The available research on these disorders is expanding rapidly, but many questions remain unanswered. We thus conducted a systematic review of the literature published from 2005-2015 on the following disorders of sleep and wakefulness in PD: REM sleep behavior disorder, insomnia, nocturia, restless legs syndrome and periodic limb movements, sleep disordered breathing, excessive daytime sleepiness, and circadian rhythm disorders. We discuss the epidemiology, etiology, clinical implications, associated features, evaluation measures, and management of these disorders. The influence on sleep of medications used in the treatment of motor and non-motor symptoms of PD is detailed. Additionally, we suggest areas in need of further research.
Keywords: Parkinson’s disease, sleep and neurodegeneration, REM sleep behavior disorder, insomnia, restless legs syndrome, periodic limb movements, sleep disordered breathing, excessive daytime sleepiness, circadian rhythm disorders
Introduction
While the non-motor manifestations of Parkinson’s disease (PD) were noted when it was first described in 1817, early research focused on motor symptoms. Decades of research have improved management of motor manifestations in PD, while also shedding light on the protean non-motor manifestations. Among these, sleep disorders stand out given their high prevalence and their severe impact on quality of life (QOL)[1-3]. Additionally, sleep dysfunction can be associated with and influence other motor and non-motor symptoms in this patient population. While the past decade has seen major advances in our understanding of sleep disorders in PD, much remains to be learned. Applying rigorous and comprehensive literature search/identification criteria, we review the state of our knowledge from the past decade on some of the most common disorders of sleep and wakefulness in PD, namely, Rapid eye movement (REM) sleep behavior disorder (RBD), insomnia, nocturia, Restless legs syndrome (RLS)/Periodic limb movement of disorder (PLMD), sleep disordered breathing (SDB), Excessive daytime sleepiness (EDS), and circadian rhythm disorders. We provide an overview of findings from the past decade of research, and propose key priorities for research in this area for the coming decade.
Methods
This was a qualitative systematic review conducted according to PRISMA guidelines[4]. This methodology was chosen as it allows for systematic vetting of all references on a given topic that meet pre-specified inclusion and exclusion criteria, and provides a comprehensive account of all references meeting those criteria (in contrast to narrative reviews)[4]. Two electronic databases, Pubmed and Embase, were searched for articles published between January 1, 2005 and January 1, 2015. The supplement to this manuscript details the search terms and methodology used for queries pertaining to each sleep disorder, including article inclusion and exclusion criteria.
REM Sleep Behavior Disorder
RBD is a parasomnia characterized by loss of the atonia that normally occurs during REM sleep, associated with dream enactment behavior. RBD has been an intensive area of investigation over the past decade, both as a characteristic of the “premotor” PD state[5], and as a potential marker of more severe disease manifestations in PD.
Epidemiology
The prevalence of RBD among individuals without PD or other neurodegenerative parkinsonian syndromes has not been well studied. Studies on the prevalence of RBD among community-dwelling older adults have not been conducted in over a decade.
Among patients with established PD seen at tertiary care centers, the prevalence of polysomnographically-defined RBD is 39-46%[6,7]. In early and de novo PD, the prevalence may be lower, at 30%[8]. There are no apparent sex differences in prevalence in this population[7,8]. REM sleep behavioral events, i.e. purposeful motor behaviors and/or vocalizations in REM sleep (irrespective of REM sleep without atonia (RSWA)), were identified in half of early PD patients[9].
RBD accounts for the majority of complex motor behaviors during sleep in PD, though apnea-related arousals are on the differential diagnosis[10], as are Periodic limb movements of sleep (PLMS). In studies of probable RBD (pRBD) in PD, violent dream content is often reported, especially in males[11], and there is a significantly increased risk of injury[12]. Normal language and non-violent, culturally-specific movements also occur[13]. Of note, seemingly normal motor function and vocalizations occur during dream enactment in PD patients with RBD[14], often in stark contrast to the bradykinesia and hypophonia seen during wakefulness.
As mentioned, RBD may precede PD motor manifestations or develop after PD onset[15]. In studies of pRBD in PD, RBD preceding PD was associated with younger age of PD onset and more severe disease manifestations[16]. Shorter duration between RBD symptom onset and development of motor manifestations has also been associated with greater risk of cognitive impairment[17]; possibly indicating greater neurodegeneration in these cases[17]. RBD persists in some patients throughout the course of their disease but resolves in others; in one study, approximately 30% of PD patients had resolution of pRBD within 4 years[18].
Etiology
Progress to determine the etiology of RBD has been slow, but several theories have been put forth, based largely on animal data and supported by imaging and pathological data in humans. It has been proposed that RBD results from dysfunction in brainstem nuclei including the glutamatergic peri-locus coeruleus, combined with abnormalities in brainstem locomotor centers[19-21].
Evaluation
RBD diagnosis requires polysomnographic demonstration of RSWA combined with either a history of dream enactment or demonstration of dream enactment during polysomnogram[22]. The definition of what constitutes RSWA is an area of active ongoing research[23-26]. Several screening questionnaires have been applied for the diagnosis of RBD in PD[27-31] (table 1), but demonstrate low specificity[27,32]. Questionnaires also poorly predict RSWA[32]. On the other hand, actigraphy may have high specificity but low sensitivity for the diagnosis of RBD in PD[67]. Four main conclusions that may be drawn from the literature over the past decade on diagnosis of RBD in PD include (i) both patient and bed-partner input is essential in optimizing sensitivity of any questionnaire used to diagnose RBD (ii) questionnaire-based diagnosis has low specificity (iii) RSWA is necessary for definitive diagnosis (vi) polysomnogram is required to detect RSWA.
Table 1.
Methods of evaluation of disorders of sleep and wakefulness in Parkinson’s disease (ICSD II: International classification of sleep disorders II; ESS: Epworth sleepiness scale; PD: Parkinson’s disease; REM: REM sleep behavior disorder; RLS: Restless legs syndrome; PLMD: Periodic limb movement disorder)
| Objective Measures | Questionnaires | |
|---|---|---|
| REM sleep behavior disorder |
Polysomnography with assessment of
atonia during REM sleep |
REM Behavior disorder screening questionnaire
(RBDSQ)[27]: 10-item questionnaire with scores ranging from 0 to 13. Sensitivity of English version in PD for ≥6 cutoff 68-90%, specificity 63-82.8%[30,33] |
| REM Sleep Behavior Disorder questionnaire Hong Kong (RBDQ-
HK)[34]: 13 item questionnaire. Sensitivity 82.2%, specificity 86.9% mixed (idiopathic and secondary) RBD population | ||
| Mayo Sleep Questionnaire (MSQ)[35] item 1: Single question. Sensitivity in PD 90.3%, specificity 87.9% among PD patients meeting ICSDII criteria[30] | ||
| RBD1Q[36]: Single
question. Sensitivity 93.8%, specificity 87.2% in idiopathic RBD population (not validated in PD). | ||
| Innsbruck REM Sleep Behavior Disorder Inventory[37]: 5 item questionnaire. In mixed (idiopathic and secondary) RBD population, sensitivity 91.4%, specificity 85.7%. For single RBD summary question, sensitivity 74.3%. specificity 92.9%. | ||
| Insomnia[38] | Polysomnography (in sleep maintenance insomnia, to exclude OSA or PLMD as a cause) |
Parkinson’s disease sleep scale version-2[39]: Developed specifically for PD, version 1 recommended[38]. 15 item questionnaire |
| Actigraphy[40,41] | Scales for Outcomes in Parkinson’s Disease
Sleep[42]: Developed specifically for PD and recommended[38]. 6 item night-time questionnaire[42]. Includes nocturia item, which is an important component of any PD sleep questionnaire[43] |
|
| Pittsburgh Sleep Quality Index[44] (not developed specifically for PD but among recommended scales[38]) | ||
| Nocturia | New-onset nocturia and/or acute worsening warrants a urinanalysis[45]. Post-void residual volume should also be checked[45]. Assessment for benign prostatic hypertrophy, bladder calculi, and/or other urologic disorders that may be contributing to nocturia is essential. In select cases, and in consultation with urology, urodynamic and cystoscopy may be appropriate for some patients[45]. |
Parkinson’s disease sleep scale version-2[39] and Scales for Outcomes in Parkinson’s Disease Sleep (SCOPA)[42] both include a nocturia item. Other possible tools not yet validated in PD include bladder diaries and symptom questionnaires such as the Nocturia,Nocturnal Enuresis and Sleep-Interruptions Questionnaire (NNES-Q)[46,47] and the overactive bladder symptom score[48]. |
| Restless legs syndrome |
Suggested immobilization test (SIT). Mean leg discomfort cutoff of 11, yields a sensitivity of 91% and specificity of 72% for RLS diagnosis in PD [49]. |
|
| Periodic limb movement disorder |
Polysomnography (>5/hr in children and >15/hr in adults consistent with PLMD in appropriate clinical context) |
|
| Sleep disordered breathing |
Diagnostic polysomnography (with additional in-lab monitoring as needed for positive airway pressure titration[50]) |
Berlin Questionnaire: 10 items; 3 categories: snoring
behavior, daytime sleepiness, hypertension/BMI; High risk if positive in at least 2 categories Predicts respiratory disturbance index >5 with 86% sensitivity and 77% specificity in general population[51] |
| Out of center sleep testing (OCST):
effective in diagnosis, but may underestimate respiratory events per hour if EEG is not recorded[22] |
STOP questionnaire: 4 questions: Snoring,
Tiredness/Sleepiness, Observed cessation of breathing, blood Pressure. Considered high risk if answer positively for at least 2 questions Predicts AHI >5 with sensitivity of 65.6% in general population[52] |
|
| STOP-BANG: STOP questionnaire plus BMI, Age, Neck
circumference, Gender Predicts AHI >5 with sensitivity of 83.6% and AHI >30 with sensitivity of 100% in general population[52] | ||
| Circadian rhythm disorders |
Actigraphy | |
| Dim Light Melatonin Onset: onset of melatonin secretion under dim light conditions | ||
| Excessive daytime sleepiness |
Multiple sleep latency test (MSLT) (measures tendency to fall asleep over 5 nap opportunities[22]) |
Epworth Sleepiness Scale (ESS): subject rates tendency to
doze off in 8 different situations over the past month; validated in PD[53,54] |
| Maintenance of wakefulness test (MWT) (measures ability to stay awake over 4 trials[55]) |
Stanford Sleepiness Scale (SSS): 1 question item, subject
rates level of alertness at time of assessment[56] |
|
| Actigraphy | Scales for Outcomes in Parkinson’s
disease-SLEEP-daytime sleepiness (SCOPA-SLEEP-DS): 6-question evaluation of daytime sleepiness designed for use in PD[42] Also validated in Thai[57] |
|
| Parkinson’s Disease Sleep Scale (PDSS): items 14:
“Do you feel tired and sleepy after waking in the morning?” and 15: “Have you unexpectedly fallen asleep during the day?”[58] Item 15 is an independent predictor of sleepiness in one study[59] but not another[60] Also validated in Italian, Chinese, Japanese, and Portugese[61- 64] | ||
| Movement Disorders Society-Unified Parkinson’s
Disease Rating Scale (MDS-UPDRS): item 1.8: “Daytime Sleepiness”[65] Shown to be effective screening tool for EDS, having good correlation with ESS[66] |
Clinical Implications
There are three broad implications of RBD. First, it is one of the strongest clinical predictors of future PD risk, and is thus seen as a prodromal state to PD[68] (as discussed further below, excessive daytime sleepiness[69] may be a manifestation of prodromal to PD as well). As will be discussed, significant advancements have been made in identifying characteristics in RBD that are strongly associated with this risk of conversion to PD[70]. The second important implication is that among PD patients, several reports in recent years have suggested that RBD is associated with more severe motor and non-motor manifestations (table 2). Finally, dream enactment behavior imposes significant risk of injury to the patient and bed-partner.
Table 2.
Clinical Implications (including associated motor and non-motor manifestations) identified in PD patients with specified sleep disorders compared to those without (EDS: Excessive daytime sleepiness; ICDs: Impulse control disorders; pRBD: Probable REM sleep behavior disorder; PLMS: Periodic limb movements of sleep; RBD: REM sleep behavior disorder; RLS: restless legs syndrome; SCOPA-AUT: Scales for outcomes in Parkinson’s–Autonomic; UPDRS: Unified Parkinson’s disease rating scale)
| Motor manifestations |
Neuropsychiatric
manifestations |
Co-morbid sleep
problems |
Autonomic
manifestations |
|---|---|---|---|
| RBD | |||
|
|||
| Insomnia | |||
| Greater prevalence of RBD (mainly in studies of pRBD[87,88] but also in studies with polysomnographic confirmation)[6] |
|||
| Associated with restless legs syndrome (RLS)[125] and leg motor restlessness (leg restlessness not meeting criteria for RLS)[127] |
|||
|
|||
|
|
||
| RLS/PLMD | |||
|
|
|
|
| |||
| Excessive Daytime Sleepiness | |||
|
|
||
In a subset of patients with RBD, an identifiable non-neurodegenerative cause is present at the time of presentation (including focal brainstem lesions or narcolepsy[20,68]). However, as mentioned, in the vast majority of so-called idiopathic RBD cases, a neurodegenerative disorder emerges years after onset of the dream enactment. Use of the term “idiopathic” RBD is thus likely a misnomer in most cases, and other terminology may be more appropriate (such as “cryptogenic” and “symptomatic” RBD in those with versus without an identifiable underlying etiology, respectively).
Several reports over the past decade have provided ample evidence that the majority of patients with RBD will go on to develop a neurodegenerative disorder[68,160], with synucleinopathies (PD, Dementia with Lewy Bodies, or Multiple System Atrophy) being the most common[161]. Clinical progression from idiopathic RBD to a clinically-diagnosable synucleinopathy, often with associated cognitive impairment, is largely consistent with Braak’s proposed PD neuropathological staging system[162], though not all patients follow this progression. In the largest prospective series (174 RBD patients) published at the time of our literature query, the 5-year and 14-year cumulative risk of developing a diagnosable neurodegenerative syndrome was 33.1% and 90.4%, respectively[68]. As discussed further below, like RBD, excessive daytime sleepiness[69] may be a manifestation prodromal to PD as well.
Polysomnographically-confirmed RBD has only rarely been reported in neurodegenerative tauopathies (including progressive supranuclear palsy[163] and Guadeloupian parkinsonism[164]). In fact, a history suggesting RBD in a patient otherwise presumed to have a tauopathy (based on clinical grounds) should prompt evaluation for RBD mimickers such as obstructive sleep apnea[165,166].
An exciting area of development has been delineation, in longitudinal studies, of clinical, imaging, and biochemical biomarkers that may help predict if and when so-called idiopathic RBD will progress to a diagnosable neurodegenerative disorder (table 3).
Table 3.
Clinical, imaging, and biochemical biomarkers that may predict emergence of a neurodegenerative disorder in patients with "idiopathic" RBD
|
Sensory and Motor Clinical Assessments
Greater olfactory loss[167] Impaired color vision[70] Abnormalities on objective motor testing[168] |
|
Autonomic
Orthostatic hypotension[91] Urinary dysfunction[91] Constipation[91] Reduced cardiac MIBG uptake[169] (only reported cross-sectionally) |
|
Imaging markers
Striatal dopaminergic deficit[170] PD-related covariance pattern on PET and SPECT[171] Substantia nigra hyperechogenicity on transcranial dopplersonography[172], (though not in isolation[173]) Increased hippocampal mean regional cerebral blood flow[174] |
|
Electrophysiologic markers
Tonic surface EMG activity on submental EMG[175] EEG slowing[176,177] (which predicts MCI but not parkinsonism). |
|
Biochemical biomarkers
Uric acid levels (associated with duration of RBD prior to manifestation of PD)[178] |
All potential biomarkers require validation in prospective cohorts and inclusion into multimodal models that minimize lead time, e.g. models that reliably predict future risk of neurodegeneration before disease onset (and long enough before disease onset to allow for preventative interventions (if/where available) to take effect).
In regards to RBD in already-manifest PD, QOL is reduced in PD patients with RBD compared to those without[17,97]. Whether this results from the RBD itself and/or the co-morbid signs and symptoms (table 2) requires further study. Individuals with RBD are at a significantly increased risk of sleep-related injury, ranging from superficial wounds to serious injuries that require intensive care and can be fatal[90]. Importantly, bed-partners of individuals with RBD are at a significantly increased risk of injury as well.
In line with the hypothesis that RBD in PD reflects more advanced neurodegeneration, smaller thalamic volumes on MRI [179] have been found in PD patients with pRBD compared to those without (but without significant structural cortical or white matter differences [180]). Furthermore, PD patients with RBD have greater EEG slowing during wakefulness[181], possibly suggesting more severe neurodegeneration.
Management
Adequately powered, randomized studies for treatment of RBD in PD have not been conducted. Clonazepam and melatonin are commonly used based on guidelines established for idiopathic RBD[90], but there are limited data on the efficacy of these drugs in individuals with RBD and a diagnosed neurodegenerative disorder. While data are limited, it is probable that this group of patients are more susceptible to side effects of benzodiazepines. In regards to other drug classes examined in studies including more than 30 patients, open-label data suggest that memantine may reduce the frequency of dream enactment[182].
An integral part of the management of RBD is intervention aimed at reducing injury to both the patient and the bed-partner[90]. This includes not only pharmacologic management but also institution of environmental modifications, such as removing sharp objects on which the patient can injure themselves, placing the mattress on the floor, and securing windows [90].
Nocturnal hallucinations are common in PD, particularly advanced PD, and are more common in PD patients with pRBD compared to those without[102]. Anecdotally, individuals with PD, particularly those with vivid dreams as occurs in RBD, often have difficulty distinguishing nocturnal hallucinations from dreams. In such individuals, particularly when hallucinations are frightening and contribute to insomnia, consideration for treatment with anti-psychotics such quetiapine is warranted. Of course, the risks of worsening parkinsonism and other side effects need to be weighed against potential benefits in such cases.
Insomnia
Epidemiology
While both sleep onset and sleep maintenance insomnia are common in PD, sleep fragmentation, a key indicator of sleep maintenance insomnia, has been the most commonly reported sleep complaint[111]. Poor nighttime sleep (questionnaire-assessed) is more common in PD than the general population[123].
There is a 20-80% prevalence of poor sleep reported in PD based on cross-sectional subjective assessments[59,121,122]. The prevalence based on physician interview is 30-59%[59,183] while it is 32% based on International Classification of Sleep Disorders (ICSD)-II criteria[105,184]. Risk factors for poor sleep include more advanced disease[119,185], female sex[121-123], and sporadic (as opposed to familial) PD[186]. In a longitudinal study of 89 PD patients over 8 years, 83% experienced insomnia at one or more study visits while 33% reported insomnia at all 3 visits and 20% who were free from insomnia at baseline developed it in follow-up[122].
Regarding objective measures of poor sleep in PD, polysomnography is useful in identifying potential causes of insomnia, such as OSA. One study found that PD patients have significantly lower total sleep time (TST), sleep efficiency (SE), and increased REM sleep latency compared to age-matched healthy controls. They spent more time in stage 1 and less in REM, but without difference in number of arousals[124]. Sleep efficiency and total sleep time were reduced in those with subjective insomnia but this was not statistically significant (p=0.07)[124].
Etiology
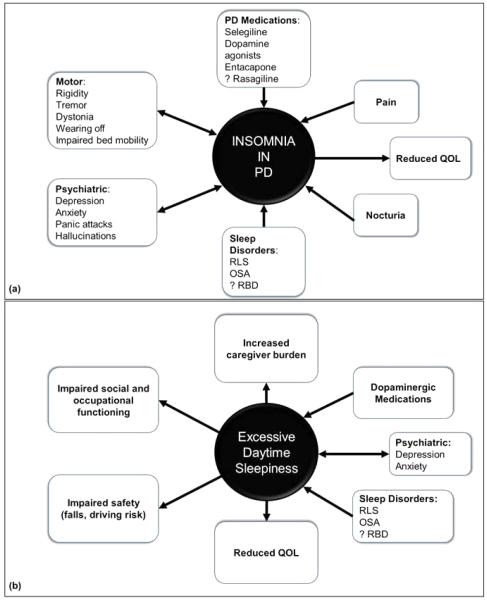
There are several potential causes of and/or contributors to insomnia in PD (figure 1a). Primary sleep disorders such as psychophysiologic insomnia (independent of the disease), RLS/PLMD, vivid dreams/nightmares, and OSA all may occur in the PD population, as discussed in this review. PD-related motor symptoms such as tremor[187], rigidity, leg cramps[187], and dystonia [111] are common. All of the latter may contribute to sleep-onset insomnia, and wearing off of dopaminergic medications overnight[111] may lead to or exacerbate sleep maintenance insomnia as well. Rigidity, bradykinesia, and other less well-defined causes also manifest with impaired bed mobility, which commonly presents subjectively with sleep-maintenance insomnia[188,189], and is associated with reduced sleep efficiency as assessed by polysomnography[188]. Given the significant contribution motor symptoms have on insomnia in PD, bedtime and overnight dopaminergic medication therapy may be required by many patients, which, while helpful for sleep in some patients, can have detrimental effects on sleep others (table 4).
Fig. 1.
Schematic of etiologies contributing to (a) insomnia and (b) excessive daytime sleepiness in Parkinson's disease.
Table 4.
Considerations in the Treatment of Insomnia in Parkinson’s disease (DA: dopamine agonist; LED: levodopa equivalent dose; LED-DA: levodopa equivalent dose–dopamine agonist amount; PD: Parkinson’s disease; RCT: randomized controlled trial; UPDRS: Unified Parkinson’s Disease Rating Scale)
| Potential Etiologic Factors | Treatment Considerations |
|---|---|
| Motor symptoms and complications[187,190]
Rigidity [187] Tremor [187,190] Motor fluctuations/nocturnal wearing off Dystonia |
Limited evidence suggest long-acting dopamine agonists may
be of benefit for subjective sleep disturbance and objective early morning motor symptoms[191-194]. Benefits on motor symptoms have to be weighed against risks of worsening insomnia due to dopaminergic therapy (see below) |
| Poor bed mobility [188,189] | Limited evidence support referral to physical and
occupational therapy for interventions to improve bed mobility[195]. During an open-label, one-night, in-lab polysomnogram study, the group treated with a dose of controlled-release carbidopa/levodopa 50/200 mg had 15 minutes less mean wake time after sleep onset compared to those who remained off dopaminergic medications that night, a significant difference[120]. |
| Medications (increased risk of insomnia compared
to placebo in RCTs): Selegiline[196] Dopamine agonists[197,198] and dopamine agonist withdrawal[199] Rasagiline[200] (though some data suggest otherwise[201]) Entacapone[198] |
Limited data guides timing of dopaminergic medication
intake in relation to sleep. Anecdotally, dosing of PD medications earlier in the day may be necessary for patients whose sleep is disrupted from dopaminergic meds. Higher total LED[202] and LED- DA[116] have been associated with worse measures of subjective and objective sleep in PD. In more advanced PD, treatment of motor symptoms disruptive to sleep may outweigh any negative effects on sleep physiology of nocturnal dopaminergic stimulation[191] |
| Psychiatric disorders[123,126,128][123,126,128]:
Depression Anxiety Panic attacks |
Treatment of underlying psychiatric symptoms such as
depression may improve insomnia in PD[203-205]. Low-level evidence and anecdotal experience suggest consideration for use of medications that treat psychiatric symptoms while capitalizing on sedating properties (such as trazodone and mirtazapine). Caution in worsening RBD and RLS is an important consideration[90] |
| Nocturia[111] | Limited evidence is available to guide treatment of
nocturia in PD. In the non-PD population, behavioral interventions (bladder training, bladder control strategies, pelvic floor muscle training) are recommended as first-line therapy[45]. Anti-muscarinics can be effective but in the PD population are associated with increased risk of cognitive dysfunction. Intradetrusor onabotulinumtoxin A may be of benefit in select cases[45]. Preliminary evidence suggests that posterior tibial nerve stimulation may be of benefit for nocturia in PD[206] and deserves further study. When possible, avoiding evening/nighttime administration of diuretics and other medications that may increase urine output may help. Potential benefit of dopamine agonists[111,207] and subthalamic nucleus deep brain stimulation[208,209] on nocturia warrant further study. |
Several PD-related non-motor symptoms may also play a prominent role, including psychiatric symptoms (anxiety, depression, panic attacks, nocturnal hallucinations and other psychotic symptoms)[126,128], pain (including nocturnal leg cramps)[111], and nocturia (see below).
The multifactorial nature of insomnia in PD is exemplified by the findings of one study that female gender, total LED, LED-DA, more depressive symptoms, better cognition, and more motor fluctuations accounted for 30% of variance in reported nighttime sleep problems, with depressive symptoms accounting for 21%[123]. Delving into any and all potential contributors to insomnia in PD is key to formulating a treatment plan (table 4).
Studies examining objective correlates of poor subjective sleep have reported conflicting results. Some studies have reported greater amounts of polysomnographic evidence of sleep fragmentation in patients with worse subjective sleep[112], but others have not found associations between subjective and objective sleep in PD. The explanation for this discrepancy may be due to sleep-state misperception and/or the different temporal measures of polysomnogram (1-3 nights of recording) and questionnaires (ask subjects to estimate symptoms over one or more weeks).
Evaluation: see Table 1
Clinical Implications
Several reports in recent years have suggested that insomnia is associated with more severe PD motor and non-motor manifestations (table 2). Insomnia has a significant negative impact on QOL in PD [3,210-213,213,214]; at least 10% of patients with advanced PD rank sleep problems as their most bothersome symptom[185].
Management
The majority of RCTs investigating treatment of insomnia in PD in the past decade have included <30 patients and were excluded from this review. The available evidence to support treatment of insomnia in PD was considered insufficient in the Movement Disorders Society Taskforce Guidelines of 2011[215]. Controlled release carbidopa-levodopa, eszopiclone, and melatonin 3-5 mg were considered to have an acceptable risk without need for specialized monitoring. Since that time, some additional randomized trial data has become available for eszopicilone[216] and melatonin[217], though the quality of evidence for these and other agents continues to be suboptimal. Sodium oxybate[218] and rotigotine[192] show promise in open-label trials.
While insomnia can be a side effect of anti-depressants, placebo-controlled studies of nortriptyline, paroxetine, and venlafaxine provide evidence that treating depression in PD improves symptoms of insomnia[203-205]. Though evidence supporting treatment of other psychiatric disorders that may be contributing to insomnia are limited, targeting specific psychiatric symptoms such as anxiety, nocturnal panic attacks, and nocturnal hallucinations in the management of insomnia are worthy of consideration and investigation in clinical trials (table 4). This applies also to treatment of other co-morbid signs/symptoms that may be contributing to insomnia in PD, such as nocturia and nocturnal PD motor symptoms, as detailed in table 4.
Nocturia
Epidemiology
Definitions of what constitutes clinically significant nocturia, or nighttime urination, vary but two or more episodes of nighttime urination lead to significant subjective sleep disruption and have detrimental effects on sleep in PD[219]. PD is a risk factor for nocturia among older adult men[220], and excessive nighttime urination is more common in PD than in age-matched controls[141], even early in the disease course[187,221]. It is one of the most commonly reported non-motor symptoms in PD, occurring in over two-thirds patients[47,121,208,210,222-225], and is among the most common urologic symptoms in PD patients presenting to movement disorders[226,227] and urology clinics[228]. Additionally, It is among the most common sleep complaints in this patient population[102,111,229], and is a key factor in sleep maintenance insomnia. In some studies, nocturia is more common in men[222,224,230,231], in older patients[231,232], in those with older age of PD onset[191,233] and increases with disease duration and severity[231].
Etiology
Nocturia in PD is likely multifactorial. Detrusor hyperactivity is present in most, but not all patients, with lower urinary tract symptoms such as nocturia[234,235]. Polyuria (production of greater-than-normal amounts of urine overnight) is present in some but not all patients. Other contributing factors may include factors unrelated to PD such as nocturnal diuresis and sleep apnea. Dopaminergic therapy[230] and higher LED[236] have been associated with greater report of nocturia, but whether this is a consequence of dopaminergic medications or a reflection of greater disease severity is not clear. In other studies, dopamine agonist use was associated with lower prevalence of nocturia[111].
Evaluation: see Table 1
Clinical Implications
Nocturia in PD has a significant negative impact on quality of life[227]. As mentioned, it is cited as one of the most frequent nocturnal symptoms and contributes to sleep maintenance insomnia. The consequences of nocturia on daytime symptoms and function in PD are not well studied, but likely include excessive daytime sleepiness and possibly other non-motor and even motor problems associated with insomnia as detailed above. Objectively, nocturia in PD is associated with increased nocturnal activity as measured by actigraphy[133] and lower total sleep time and sleep efficiency on polysomnography[219].
Management
Little evidence guides management of nocturia in the PD population. Until such evidence becomes available, principles of nocturia management developed for the general older population may be of utility, though there are factors unique to the PD population that must be considered (table 4).
Restless Legs Syndrome and Periodic Limb Movement Disorder
Restless legs syndrome (RLS) or Willis Ekbom disease belongs to the group of Sleep-Related Movement Disorders. According to ICSD III criteria[22], RLS diagnosis requires “an urge to move the legs, usually accompanied by uncomfortable and unpleasant sensations in the legs”. These symptoms must begin or worsen during periods of relative inactivity and must be partially or totally relieved by movement. The symptoms must cause significant distress or impairment in function. The interface of RLS and PD has been of increasing interest over the past decade.
Compared to RLS, PLMD has drawn less attention in PD research. PLMD is a sleep-related movement disorder characterized by periodic limb movements during sleep (PLMS) (>5/hr in children and >15/hr in adults) that result in clinically significant sleep disturbance or impairment of functioning[22].
Epidemiology
The temporal relationship between RLS and PD requires further study, but data to date suggest that RLS is not a risk factor for PD per se but rather that PD is a risk factor for a diagnosis of RLS[237] and a diagnosis of RLS may be an early manifestation of PD[238].
Several recent reports focused on the prevalence of RLS in PD and its associations with PD symptoms. In a cohort of several parkinsonian disorders (PD=134, PSP=27, MSA=21, DLB=5), RLS was the most common in PD (11.9%)[239]. The prevalence of RLS in recent PD cohorts ranges from 3%[131] (compared to 0.5% of healthy controls) to 21.3%[129,240,241]. Late-onset patients develop RLS sooner after PD diagnosis compared to those with young-onset [242].
Primary RLS is frequently associated with PLMS. The relationship between PLMS and RLS in PD is less well understood, with research yielding conflicting results. Some studies report an association between presence of PLMS and RLS in these patients[131] whereas others do not[243].
Etiology
The question of possible pathophysiological overlap between PD and RLS has been a subject of debate. Brain imaging and genetic clinical research approaches may be useful in examining the biological links between these disorders. A recent imaging study demonstrates evidence for different pathophysiological pathways between RLS and PD at the level of nigrostriatal presynaptic function, finding that striatal dopamine transporter binding measured by SPECT is reduced in PD but not in primary RLS[244].
In regards to the genetics of RLS in PD, alpha-synuclein promoter Rep1 allele 2 is known to confer a PD risk. In a genotyping study of 258 patients with RLS the Rep1 allele 2 showed significantly decreased frequency compared with 235 healthy controls. These data suggest that low alpha-synuclein function may contribute to RLS pathogenesis[245].
Transcranial sonography has been applied to both PD and RLS populations and can be used to improve understanding of pathophysiological mechanisms of these disorders. While it is clear that PD is associated with substantia nigra (SN) hyperechogenicity, and RLS is associated with SN hypoechogenicity, studies that employed this technique in PD with co-existent RLS revealed contradictory findings[246,247].
Evaluation
There exists a clear need for more accurate assessment and diagnosis of RLS in PD. The suggested immobilization test may be useful for this (table 1). Diagnosis of co-existent RLS in PD may be challenging due to several potential clinical confounders. For example, nocturnal leg restlessness is an important mimicker of RLS in PD, making the accurate diagnosis of RLS in PD difficult. Several studies have addressed nocturnal restlessness in the PD population[248-250]. In a cohort of 100 un-medicated PD patients, 40% had leg restlessness compared with 18% of age- and gender-matched controls[250]. However, only 15% of these PD patients met criteria for the diagnosis of RLS. Among PD patients with RLS, the urge to move legs and unpleasant sensations in the legs can be associated with wearing off[251]. These results emphasize the importance of differentiating between true RLS and RLS mimics. This has significant implications for patient care and clinical research.
Clinical Implications
RLS in PD negatively impacts quality of life[130]. While one study found associations between RLS and younger age of PD onset, male gender, higher Mini-Mental State Examination score and less advanced Hoehn and Yahr stage[240], another did not[239]. RLS has also been associated with non-motor PD symptoms (table 2), which suggests the role of a non-dopaminergic system in the link between RLS and PD. However, domperidone treatment has been linked with higher rates of RLS in PD, suggesting a role for dopaminergic neurons outside of the blood-brain barrier in the pathophysiology of RLS[252]. The main obstacle in interpreting these studies is that most enrolled PD patients are already treated with dopaminergic agents, which may mask co-existent RLS. Associations between RLS and neuropathy have been well recognized, but not systematically studied. In one study, no correlations were found between RLS and neuropathy, levodopa exposure, and vitamin B12 levels in patients with PD[248]. Although not systematically studied in the PD population, it is likely that RLS contributes not only to sleep onset insomnia but also sleep maintenance insomnia in PD. This aspect is even more significant considering that RLS frequently mimics many symptoms intrinsic to PD, making timely diagnosis challenging.
Management
While many of the dopaminergic agents used to treat PD (levodopa, ropinirole, pramipexole, and rotigotine) also have been independently demonstrated in randomized trials to be effective in treating RLS, there are no randomized trials examining the treatment of RLS specifically in the PD population. In addition, the occurrence and management of augmentation of RLS symptoms in PD patients being treated with dopaminergic medications for their motor symptoms is poorly described. Two mainstays of evidence-based treatment of RLS in the non-PD literature are applicable to PD as well: (i) assessment for and correction of any iron deficiency, and (ii) consideration of reduction of and/or discontinuation of contributing agents such as anti-depressants (table 4).
Deep brain stimulation (DBS) is an important treatment modality for patients with PD. Several groups reported positive postoperative effects of subthalamic nucleus DBS on RLS[253,254]. However, emergence of RLS subsequent to STN DBS may occur as well[255], emphasizing the need to screen for RLS postoperatively as the reduction in anti-parkinsonian medications may lead to unmasking of RLS.
Sleep Related Breathing Disorders
Epidemiology
In studies that prospectively evaluated PD participants without selection for sleep complaint, the prevalence of sleep disordered breathing (SDB) ranges from 15-76%[6,10,74,112,117,124,163,243,256-262]. Because patients with PD have been reported to have both obstructive and restrictive pulmonary dysfunction[263,264] as well as abnormal response to hypercapnia[265], researchers have hypothesized that sleep apnea risk is increased in PD relative to the general population. However, controlled studies on sleep apnea in PD over the last 10 years consistently failed to demonstrate any increased risk for sleep apnea. Regardless, the negative health implications of sleep apnea in older adults have been extensively documented. For example, obstructive sleep apnea (OSA) has been associated with significant cardiovascular, psychiatric, and cognitive comorbidities as well as increased healthcare utilization[266,267]. Therefore, it is important to fully explore this sleep disorder in PD.
Regarding controlled studies of prevalence of SDB in PD, one study demonstrated no significant difference in apnea hypopnea index (AHI) among Asian PD patients and controls, with neither group having significant central sleep apnea. Surprisingly, OSA was present in 49.1% PD patients compared to 65.7% of controls[124]. Another study showed sleep apnea in 40% controls and 27% of PD subjects and patients with apnea had more severe motor disability than those without[256]. In early PD, there was no difference in AHI between PD and control groups[257]. Further, in a study designed to compare the frequency of SDB in PD and controls, Trotti and colleagues evaluated PD patients with polysomnography (PSG) and compared outcomes to an historical control[268,269] and found no difference in frequency or severity of OSA. Interestingly, neither subjective sleepiness nor snoring predicted sleep apnea in PD patients[262].
There are also uncontrolled studies reporting SDB prevalence in PD. One found a prevalence of 22.4% for moderate to severe sleep apnea (AHI >15) among unselected PD participants[260]. In another cohort of PD subjects, sleep apnea was present in 54.6%[112]. Among subjects with mild-moderate PD, SDB (AHI ≥10) was present in 55%[6]. Other studies find at least mild apnea (AHI ≥5) in 74% of subjects[117] and severe apnea (AHI >30) in 17.9%[270]. In early PD, 33.7% of subjects had an AHI ≥5[243]. Similarly, among treatment naïve PD, 43.3% had an AHI ≥5 and the mean AHI was 8.3[271].
There have been a few studies of sleep disordered breathing in patients with other Parkinsonian syndromes such as Progressive Supranuclear Palsy (PSP), Dementia with Lewy Bodies (DLB), and Multiple Systems Atrophy (MSA). One such polysomnographic study found that OSA was present in 30.7% of PD and 34.8% of DLB subjects[74]. In another study, 55% of PD and PSP patients had OSA, which increased with age in both groups, but PSP patients had worse sleep efficiency[163]. A questionnaire study comparing MSA, PD, and control subjects found sleep-related breathing complaints in 30% PD, 19.8% control, and 54.7% MSA subjects[272]. Among MSA, PSP, and PD patients, 38.5%, 14.3%, and 18.8%, respectively, screened high risk for sleep apnea[273].
Etiology
As discussed previously, pulmonary dysfunction has been proposed as a potential risk for SDB in PD patients[263,264]. In individual patients, SDB could be related to rigidity of muscles of the chest wall, restricted lung volumes secondary to changes in posture/kyphoscoliosis, or altered flow volume loop due to 4-8Hz oscillations in upper airway muscles[274,275]. Interestingly, there was no increase in sleep apnea in PD patients with camptocormia[276]. Neurodegeneration was also proposed as a cause of SDB in PD, but studies show no correlation between SDB and caudal brainstem serotonergic innervation or striatal dopaminergic innervation[258]. For many PD patients, the etiology for sleep apnea is likely the same as for the general population.
Clinical Implications
Extensive research has documented the negative health effects of untreated sleep apnea in the general population but this is less well explored in PD patients. Some studies show an association between subjective sleepiness and sleep apnea: PD patients with AHI ≥5 had more EDS than those with AHI <5[112] and AHI correlates with subjective sleepiness in PD[152]. Other studies found no correlation between AHI and subjective sleepiness[6,243,260,270]. PD patients with higher AHI have shorter mean sleep latency in several studies[117,124,261,270], but one study found no difference[259]. PD subjects with sleep apnea have worse scores on cognitive testing and sleep disordered breathing independently predicts cognitive dysfunction[6]. Arousal related paroxysmal nocturnal behaviors are more frequent in PD patients with sleep disordered breathing, though most complex paroxysmal nocturnal behaviors were related to RBD[10].
Due to differences in SDB characteristics between PD patients and controls, PD patients may have less severe SDB-associated health consequences[256]. For example, for the same AHI, PD patients maintain higher oxygen saturation than controls and control subjects have more of their AHI accounted for by apnea, whereas PD patients have more hypopneas[260,277] indicating less severe consequences despite similar AHI. One study also suggested more central sleep apnea among PD patients compared to controls[278], but other studies don’t support this[124,256,277]. Central sleep apnea in PD patients has been associated with higher doses of dopamine agonists[278]. Interestingly, control subjects with OSA have higher levels of sympathetic activity during sleep than PD patients with OSA, suggesting that sympathetic dysfunction in PD leads to a blunted response to apneas[279]. These findings support the argument that sleep apnea in PD may have fewer cardiovascular consequences. In addressing cardiovascular health among PD patients with and without SDB, one study showed a trend toward more history of cardiovascular events in PD patients with sleep apnea, but this did not reach significance (33% versus 13%)[256]. Another study found no difference in presence of cardiovascular disease between PD participants with and without SDB or between controls and PD subjects with OSA[260].
Evaluation methods: See Table 1
Management
Treatment for SDB in Parkinson’s disease is the same as for the general population. Only one study in the past ten years has addressed the impact of sleep apnea treatment in PD patients. This randomized, controlled study demonstrated that, compared to sham CPAP, therapeutic CPAP reduced AHI and improved the mean sleep latency[50].
Excessive Daytime Sleepiness
Epidemiology
Excessive daytime somnolence is common among patients with Parkinson’s disease, affecting 20-60% of patients[57,89,102,123,124,137,144,145,151,182,183,243,259,261,270,272,273,280-296]. Multiple factors influence the prevalence of EDS, including duration and severity of disease, age, gender, cognition, presence of nocturnal sleep disorders, and mood disorders. Some controversy exists as to whether excessive somnolence is a prodromal feature of PD or a symptom only in advanced disease. One study showed that EDS was significantly worse in drug-naïve PD patients compared to control subjects and even worse in advanced PD[187]. In contrast, another study showed no significant difference in EDS between newly diagnosed PD and controls, although PD subjects took more naps[243]. Two small studies found no difference in subjective or objective sleepiness between drug-naïve PD and controls[297,298]. Similarly de novo PD patients and SWEDD (Scans Without Evidence of Dopaminergic Deficits) subjects had no significant difference in ESS and EDS was uncommon in both groups[299]. Interestingly, a large population-based study showed EDS to be a possible risk factor for future development of PD[69]. Larger, controlled studies in de novo PD patients are needed to further evaluate the true prevalence of daytime sleepiness in this population.
That subjective sleepiness is more common among PD patients than the general population is well established. Supportive controlled studies show subjective sleepiness in 33.5-54% of PD patients, compared to 16-19% controls[40,151,284,291,296]. Similarly, a large study demonstrated EDS in 43% of PD patients compared to 10% of controls, with sleepiness being more common in PD patients with higher age, higher dopamine agonist dose, more severe disease, autonomic dysfunction, and psychiatric symptoms[123]. In longitudinal studies, the 8-year prevalence of EDS was 54.2% for all PD subjects evaluated and 46.5% in those never treated with dopamine agonists[137]. Similarly, a longitudinal evaluation at 3.5, 5, and 7-year time-points showed 49%, 53%, and 44%, respectively, had daytime somnolence[283].
Several studies have suggested that sleepiness is more likely to affect patients with advanced PD[102,144,146,282,298,300]. An objective study demonstrated that advanced PD patients had significantly shorter mean sleep latency (MSL) compared to early PD and controls. The MSL did not correlate with subjective sleepiness, but was related to disease duration and motor impairment[298]. Another study demonstrated that PD patients with Hoehn &Yahr stage 4 had significantly more daytime sleepiness than stages 1, 2, or 3, without group differences for dopaminergic therapy[301]. In a study of non-motor symptoms in nursing home residents with PD, 68% reported daytime sleepiness[295], supporting the idea that EDS is related to disease duration and severity.
Sex also influences EDS prevalence in PD, with men being more likely to be sleepy[287]. In fact, EDS is best predicted by male sex, duration of PD, and presence of anxiety[282], and more men than women have subjective sleepiness, even after controlling for levodopa equivalent dose[41].
The prevalence of EDS has also been compared between PD and other movement disorders. One study found no difference in EDS between essential tremor (ET) and PD subjects[286], while another found EDS to be more common in PD than ET[280]. In a study comparing daytime sleepiness in MSA, PD, and controls, EDS was found in 28%, 29%, and 2% respectively[272]. EDS appears to be more common among subjects with Parkinson’s disease dementia (PDD) or DLB compared to PD patients without dementia[182,281].
Etiology
The etiology of EDS in Parkinson’s disease is multifactorial (figure 1b). Some studies report no significant correlation between nighttime sleep and subjective sleepiness[134]. However, nocturnal sleep disorders can certainly contribute to daytime somnolence. For example, two studies found more subjective sleepiness in PD patients with RBD compared to those without RBD, although in one of the studies, the difference became not significant after controlling for other predictors[86,97]. Interestingly, treatment-naïve PD patients without RBD had significantly shorter MSL than subjects with RBD, though neither group had abnormal MSL, and the Epworth Sleepiness Scale (ESS) was not different between the groups[8]. Regarding restless legs syndrome (RLS), one study showed no difference in subjective sleepiness between PD patients with and without RLS[239]. However, other studies show that RLS severity correlates with sleepiness[129] and RLS is an independent predictor of EDS in PD patients[272].
The association between daytime sleepiness and sleep apnea has not been definitively established in PD. Among PD patients not selected for any sleep complaint, patients with objective sleepiness by MSLT had higher apnea hypopnea index (AHI), with no correlation between subjective sleepiness and AHI[258,270]. Similar findings of absence of correlation between ESS and AHI have been reported in other studies as well[256,260,262,293], and one study found no correlation between self-reported snoring and subjective sleepiness[302]. In contrast, other studies have demonstrated a relationship between subjective sleepiness and sleep apnea[112,303], including one showing significant correlation between both subjective and objective sleepiness and AHI[261]. Available data indicate that objective sleepiness is consistently influenced by the presence of sleep-disordered breathing, but its effect on subjective sleepiness is less well established. This suggests that some PD patients may underestimate their degree of sleepiness.
Dopaminergic medications may also affect levels of somnolence, and studies of dopamine agonists frequently note somnolence as an adverse effect. However, some investigations have shown no correlation between medications and subjective sleepiness[134,243]. Studies investigating the impact of medications on somnolence are outlined in Table 5. To summarize, dopaminergic medications, particularly dopamine agonists, influence subjective sleepiness in some patients, but do not appear to cause changes in objective measures of sleepiness. This is another example of the disconnect between patient perception and objective outcomes.
Table 5.
Effects of medications on excessive daytime sleepiness in PD (ESS: Epworth sleepiness scale: EDS: excessive daytime sleepiness; MSLT: multiple sleep latency test)
| Dopamine Agonists |
|---|
| Pramipexole |
| Somnolence is often reported by PD patients on immediate
or extended release formulations in both placebo controlled and open label extension studies[134,243] |
| In early PD, patients randomized to pramipexole had slight
worsening of subjective sleepiness compared to a slight improvement in those randomized to rasagiline[304] |
| Among patients randomized to initial therapy with
pramipexole or levodopa, EDS was present at 6 years in 57% of those on initial pramipexole compared to 35% of those initially on levodopa[305] |
| EDS may be exacerbated in patients with kidney disease
since pramipexole is renally excreted-- One study showed correlation between EDS and renal function in patients on pramipexole but not ropinirole[306] |
| Transdermal Rotigotine |
| EDS affects 33% of PD patients on rotigotine compared to 20% on placebo[307,308] |
| EDS occurs at a rate of 23% per patient year on rotigotine[309] |
| In open label extension studies, ESS scores increased over
time and EDS was reported as an adverse event in 13-24.9% of subjects on rotigotine[193,310,311] |
| Ropinirole |
| Studies of ropinirole do not show increased somnolence for
immediate or extended release formulations in placebo-controlled or open label extension studies[194,312,313] |
| A within-subject study showed improvement in ESS following
change from immediate to prolonged- release formulation of ropinirole[314] |
| Apomorphine |
| Somnolence was reported in 7.8-14.3% of PD patients on
apomorphine in a placebo controlled trial[315] |
| EDS was reported as an adverse event by 21% PD patients on
apomorphine in an open label extension study[316] |
| Rasagiline |
| No increased EDS compared to placebo[316] |
| Entacapone |
| Somnolence was reported by 6.5% of PD subjects who were
switched from immediate release carbidopa/levodopa to carbidopa/levodopa/entacapone[317] |
| Selegiline |
| In an open label study, selegiline in combination with
reduction or discontinuation of dopamine agonists led to reduction or resolution of somnolence in 94% subjects with EDS[196] |
| Piribedil |
| PD patients with EDS on pramipexole or ropinirole were
randomized to either change to the non- ergot dopamine agonist piribedil or to remain on current therapy. Those changed to piribedil had improvement in sleepiness[318] |
| Studies supporting relationship between dopaminergic therapy and EDS |
| Levodopa equivalent dose independently predicts subjective sleepiness[117] |
| Sudden onset sleep (SOS) episodes are more likely among patients on dopamine agonists[319] |
| Subjective sleepiness correlates with use of dopamine agonists[261,293,320] |
| A longitudinal study showed that dopamine agonist use was
an independent predictor of EDS at 7 years[283] |
| Micro-sleep episodes (by actigraphy) are more frequent in
the 30 minutes following levodopa dose and PD patients report more somnolence within 1 hour of taking dopamine agonists[259,321] |
|
Studies not supporting a relationship between
dopaminergic therapy and
EDS[57,285,290,322] |
| A longitudinal study showed no change in EDS one year after initiating dopaminergic therapy[322] |
| Dopamine agonist use correlated with EDS but was not an
independent predictor of subjective sleepiness in regression analyses[282] |
| Objective evaluations of sleepiness (MSLT) show no effect
of dopamine agonists on MSL[303] and
no difference in MSL between those on levodopa alone versus dopamine agonists alone[124] |
Imaging has been explored in an effort to identify neurodegenerative changes that could explain the etiology of somnolence in Parkinson’s disease. In studies comparing PD patients with and without EDS, SPECT imaging showed that left parietal hypoperfusion and right thalamus hyperperfusion were significant predictors of EDS[323] and diffusion tensor imaging showed reduced fractional anisotrophy in the fornix in PD subjects with EDS[324]. Additionally, PD patients with EDS have regional brain atrophy in the frontal lobes, temporal lobes, occipital lobes, and in the area of the nucleus basalis of Meynert[325] as well as in the middle cerebellar peduncle compared to PD patients without sleepiness[273]. Neurodegeneration could also impact circadian rhythm regulation. In fact, PD patients with EDS have lower amplitude of the melatonin rhythm[89]. In a search for genetic causes of EDS in PD, no relationship was found between sleepiness and the catechol-O-methyltransferase val158met polymorphism[326]. Additional imaging and post mortem studies in larger groups of patients with and without EDS are needed to determine the true impact of neurodegenerative brain changes on somnolence in PD.
Clinical Implications
Excessive daytime sleepiness has been associated with many motor and non-motor symptoms, as outlined in Table 2. EDS negatively correlates with and is an independent predictor of QoL in PD [1,289], although another study showed that nocturnal sleep quality was a better predictor of QoL[2]. EDS is also an independent predictor of Health related quality of life (HRQoL) through its impact on activities of daily living[327]. In addition, EDS in PD negatively impacts caregiver burden[328].
Studies investigating the safety implications of EDS in PD have shown that subjective sleepiness predicts worse driving performance during distraction[329] and sleepiness predicts poorer processing speed/reaction time even after controlling for levodopa equivalent dose[330]. Among 5,210 PD patients with a driver’s license, 8% reported sudden onset sleep (SOS) while driving; 27% and 28% of those, respectively, had near or actual accidents associated with SOS[85]. In a French PD cohort, EDS and male sex were predictors of drowsy driving and SOS while driving[287]. SOS was more frequent and more likely to occur during active behaviors in PD compared to control subjects, but subjective sleepiness was not a good predictor of SOS[296]. PD subjects may be less likely to adjust their behavior based on sleepiness (don’t adjust driving speed based on sleepiness)[331]. In addition to safety concerns among PD patients who continue to drive, there is also the potential for loss of independence, increased caregiver burden, and further impairment of quality of life in those who no longer drive due to sleepiness.
While some PD patients reported improved motor symptoms on awakening from nighttime or daytime sleep, there was no difference between EDS in PD patients with or without sleep benefit (improvement in motor symptoms following sleep)[332]
Evaluation methods
Measures for evaluation of EDS in PD are listed in Table 5. Comparison between studies can be challenging due to use of different measures and different definitions of EDS, such as defining sleepiness as Epworth Sleepiness Scale (ESS) >10 versus ≥10. There is also disparity between subjective and objective sleepiness in many studies--some showing no correlation between mean sleep latency (MSL) and ESS[117,135,333] and others demonstrating a significant relationship[259,261]. Similarly, actigraphy measures of napping correlate with subjective sleepiness in some studies but not others[321,334]. These findings again raise concern that PD patients may have poor insight about their degree of sleepiness.
Because the profound hypersomnolence in PD patients is reminiscent of narcolepsy, hypocretin levels in CSF have been examined in PD. In one study, levels were normal in PD but did correlate with mean sleep latency[298]. In another study, there was no correlation between hypocretin levels and subjective or objective measures of sleepiness in PD patients with or without dementia[135]
Management
Several therapies have been evaluated for management of excessive sleepiness in PD. Treatments found to be no more effective than placebo for EDS include: modafinil up to 400mg/day for 4 weeks[335], memantine[182,336], and bright light therapy[337]. Additionally, following STN DBS, PD patients report improvement in sleep quality but not subjective sleepiness[338]. Melatonin at 5mg improved subjective sleepiness on the General Sleep Disturbance Scale, but not on the ESS[339]. Some potentially promising therapies for EDS include: sodium oxybate, which improved ESS, but was associated with a small but significant increase in apneic events[218]; atomoxetine[340]; and caffeine, which showed a trend toward improvement in sleepiness compared to placebo[341]. These studies indicate that some treatment options may improve somnolence in PD, but the promising studies need to be replicated in larger groups.
Circadian Rhythms Disruption
Circadian rhythms are biological rhythms with a periodicity of approximately 24 hours in humans. These rhythms influence many physiological and behavioral functions. The sleep-wake cycle is one of the most robust outputs of circadian timekeeping. Compared to other sleep disorders in PD, systematic clinical investigations of circadian rhythm disruption are just recently emerging.
Endogenous circadian rhythms can be characterized by analyzing circadian markers (Table 1). Prolongation of the phase angle of melatonin rhythm was recently reported in medicated compared to un-medicated PD patients and controls[342]. Although two other studies did not show alterations in the circadian phase of melatonin secretion, both reported decreased amplitudes of melatonin secretion, which was significantly lower in patients with EDS[343,344]. PD patients are also found to have elevated cortisol levels, and flattened expression rhythm of a major core clock gene, Bmal1[344]. Additionally, the mesor and nocturnal fall in core-body temperature were lower in PD compared to controls[345]. Somatotrophic, thyrotrophic and lactotrophic axes appear to be intact in early-stage PD[346]. Overall, these investigations strongly suggest alterations of the endogenous circadian rhythmicity in PD. This will need to be better delineated in longitudinal studies employing larger cohorts of PD patients.
Blood pressure (BP) and heart rate (HR) have a distinct diurnal rhythm, and are important outcomes for investigations of the interface of circadian biology and autonomic function in PD. 24-hour ambulatory BP monitoring reveals significant differences in the rhythm of non-dipping, the percent of nocturnal BP decrease, nighttime BP levels, and nocturnal decrease of HR between PD patients and controls[347]. These changes do not appear related to disease severity and phenotype, supporting the hypothesis that these alterations may stem from intrinsic circadian dysregulation. Reversal of circadian BP rhythm, postprandial hypotension and nocturnal hypertension in PD has also been reported[348]. Circadian profile of BP and HR may be good metrics to differentiate between Multiple System Atrophy (MSA) and PD as patients with MSA have higher nocturnal HR and lower nocturnal decline in HR compared with PD[349]. Low frequency components of heart rate variability and the low/high frequency ratio tend to be more reduced in patients with MSA compared to PD patients[350,351]. Further studies will be needed to better delineate these findings.
Circadian disruption has been associated with neuropsychiatric disturbances in PD. PD patients with hallucinations have diminished inter-daily stability of rest-activity cycle, reduced amplitude of activity and increased nighttime activity compared to non-hallucinators[352]. Similarly, PD patients with depression have lower amplitudes of core body temperature and higher minimum rectal temperature relative to PD patients without depression[301].
Systematic study of circadian function in the PD population is in early stages. While clinical significance of circadian disruption may prove to be very relevant to both motor and non-motor aspects of the disease, published literature to date provides the rationale for exploring circadian based interventions in the management of disrupted sleep-wake cycle, especially excessive daytime somnolence.
Influence of Dopaminergic Therapy on Sleep in Parkinson’s disease
The complexity of sleep dysfunction in Parkinson’s disease is further compounded by the influence of dopaminergic and other PD medications on sleep. The majority of studies investigating sleep in PD have included patients already on therapy, with only a few exploring sleep in unmedicated patients [9, 199, 250, 342]. For each sleep disorder discussed in this review, anti-Parkinsonian medications can have potential positive or negative effects. For example, in RBD, anecdotally, many patients report improvement in dream-enactment behavior with dopaminergic therapy. As discussed in the section on insomnia and in Table 4, insomnia can be worsened both by wearing off of medications [111,199] as well as by use of certain medications such as selegiline[196] and others. Excessive daytime sleepiness in the context of dopamine agonist use has been extensively evaluated, as outlined in Table 5. The influence of PD therapeutic medications on sleep disorders is further explored throughout this review within each specific section. The influence of these treatments on sleep dysfunction in combination with the heterogeneity of the PD phenotype further emphasizes the need for additional studies to address this complex non-motor symptom.
Conclusions and Future Directions
This review of the literature of sleep-related disorders in PD research over the past decade demonstrates that sleep dysfunction is common in these patients and has a significant impact on quality of life, motor symptom severity, and other non-motor symptoms. As is evidenced from our findings, significant advancements have been made in our understanding of disorders of sleep and wakefulness in PD. Despite the growing body of knowledge, much remains to be understood in terms of diagnostic assessments, epidemiology, pathophysiology, clinical impact and implications on the underlying disease and its manifestations, and evidence-guided management. In table 6, gaps in knowledge and priorities for future research are proposed.
Table 6.
Priorities for research, areas of knowledge deficits, and potential future directions in PD Research. (EDS: Excessive daytime sleepiness; PLMS: periodic limb movements of sleep; RLS: restless legs syndrome; RBD: REM sleep behavior disorder; SDB: sleep disordered breathing)
| RBD | Insomnia | Nocturia | RLS/PLMS | EDS | SDB |
Circadian
Rhythm Disruption |
|
|---|---|---|---|---|---|---|---|
| Epidemiology |
|
|
|
|
|
|
|
| Etiology |
|
|
|
|
|
|
|
| Evaluation |
|
|
|
|
|
|
|
| Clinical Implications |
|
|
|
|
What is the long-term impact of EDS on safety, motor outcomes, and healthcare utilization? |
|
|
| Management |
|
|
|
|
|
|
|
Practice Points. Present the important points for readers to remember in clearly indicated box(es), e.g.:
Practice Points.
REM sleep behavior disorder is a clinical biomarker of increased risk of synuclein-related neurodegeneration in individuals without evidence of a neurologic disorder. It is associated with increased risk of injury to the patient and bed-partner, and institution of safety measures is a key component of its management.
Insomnia in PD is multifactorial, resulting from a combination of nocturnal motor symptoms, nocturia, impaired bed mobility, medications, depression, and co-morbid primary sleep disorders. Etiologies contributing to insomnia may need to be treated separately. Reduced sleep time related to insomnia has the potential to worsen motor symptoms.
RLS is common in patients with PD, and is sometimes challenging to diagnose and distinguish from motor restlessness of PD and other RLS mimics. A detailed history and evaluation is essential in a PD patient with possible RLS, as its presence may influence timing and nature of therapy.
Circadian dysfunction is under-recognized in PD and likely influences not only sleep-wake cycles, but also may affect mood, cognition, autonomic and motor functions. Circadian based therapies, such as timed light exposure and melatonin, should be considered in PD patients with evidence of circadian dysregulation.
EDS is common among PD patients and negatively impacts quality of life and impairs safety. This symptom can be severe enough to cause sudden onset sleep episodes and significantly impair driving safety. Further, the propensity for sleep during the daytime can interrupt work and social activities, increase caregiver burden, and has the potential to further disrupt nighttime sleep.
While sleep disordered breathing is not more common among PD patients compared to the general population, patients may not present with the typical symptoms of snoring and daytime sleepiness, so a low threshold for polysomnogram should be considered. Though some data suggest that PD patients may have fewer cardiovascular consequences of apnea than the general population, CPAP therapy is effective and should be recommended.
Research Agenda. Please indicate points which you feel would repay further research in box(es), e.g.:
Research Agenda.
Future research on REM sleep behavior disorder will be facilitated by the development of improved diagnostic criteria, specifying practical and yet sensitive and specific definitions of REM sleep without atonia, as well as questionnaires with improved specificity.
In regards to knowledge gaps pertaining to insomnia in PD, treatment options are markedly under-studied and randomized clinical trials of pharmacologic and non-pharmacologic approaches to treatment of insomnia in PD are much needed.
Further research is needed to understand a potential overlap in pathophysiological mechanisms that underlie PD and restless legs syndrome. Future investigations will need to define criteria specific to restless legs syndrome in the PD population and established guidance for the optimal treatment approaches for restless legs syndrome in co-existent PD.
Future research on sleep disordered breathing in PD should include development of screening tools more sensitive to detection of sleep apnea in this population and execution of randomized controlled trials to determine the impact of treatment of sleep disordered breathing on motor and non-motor as well as cardiovascular outcomes in PD.
Important areas of future research on daytime sleepiness in PD will include understanding the discrepancy between objective and subjective measures of sleepiness in PD and conducting placebo-controlled trials of both pharmacologic and non-pharmacologic treatments with the potential to improve sleepiness in these patients.
One of the main questions in the field of circadian rhythms and PD is whether circadian disruption represents a consequence of PD-specific neurodegeneration, or whether it may lead to and/or promote the neurodegenerative process of PD. Longitudinal studies centered on circadian function in PD will be needed to answer this and similar questions that may position circadian system as a novel diagnostic and therapeutic target in PD
Supplementary Material
Acknowledgements
The authors do not have any direct conflicts of interest with this work. Drs. Amara and Videnovic both receive grant funding from the National Institutes of Health: Dr. Videnovic: K23 NS072283; Dr. Amara: K23 NS080912. Dr. Chahine receives support from (i) the NIH (P50 NS053488) (ii) receives support as site Principal Investigator of the Parkinson’s Progression Marker’s Initiative and (iii) receives royalties from Wolters Kluwel (for book authorship)
Abbreviation List
- AHI
Apnea hypopnea index
- DBS
Deep brain stimulation
- EDS
Excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- LED
Levodopa equivalent dose
- LED-DA
Levodopa equivalent dose contributed by dopamine agonists
- PD
Parkinson’s disease
- OSA
Obstructive sleep apnea
- PLMS
Periodic limb movements of sleep
- PLMD
Periodic limb movement disorder
- pRBD
Probable REM sleep behavior disorder (not polysomnographically confirmed)
- RBD
REM sleep behavior disorder
- RSWA
REM sleep without atonia
- RLS
Restless legs syndrome
- QOL
Quality of life
- SIT
Suggested immobility test
- STN
Subthalamic nucleus
- TST
Total sleep time
- WASO
Wake time after sleep onset
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Glossary of terms: n/a
References
- *[1].Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord. 2010 Nov 15;25(15):2493–2500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- [2].Gomez-Esteban JC, Tijero B, Somme J, Ciordia R, Berganzo K, Rouco I, et al. Impact of psychiatric symptoms and sleep disorders on the quality of life of patients with Parkinson's disease. J Neurol. 2011 Mar;258(3):494–499. doi: 10.1007/s00415-010-5786-y. [DOI] [PubMed] [Google Scholar]
- *[3].Avidan A, Hays RD, Diaz N, Bordelon Y, Thompson AW, Vassar SD, et al. Associations of sleep disturbance symptoms with health-related quality of life in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2013;25(4):319–326. doi: 10.1176/appi.neuropsych.12070175. Fall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009 Jul 21;339(7):b2700. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berg D, Lang AE, Postuma RB, Maetzler W, Deuschl G, Gasser T, et al. Changing the research criteria for the diagnosis of Parkinson's disease: obstacles and opportunities. Lancet Neurol. 2013 May;12(5):514–524. doi: 10.1016/S1474-4422(13)70047-4. [DOI] [PubMed] [Google Scholar]
- [6].Neikrug AB, Maglione JE, Liu L, Natarajan L, Avanzino JA, Corey-Bloom J, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med. 2013 Nov 15;9(11):1119–1129. doi: 10.5664/jcsm.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011 Sep 13;77(11):1048–1054. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- [8].Plomhause L, Dujardin K, Duhamel A, Delliaux M, Derambure P, Defebvre L, et al. Rapid eye movement sleep behavior disorder in treatment-naive Parkinson disease patients. Sleep Med. 2013 Jul 25; doi: 10.1016/j.sleep.2013.04.018. [DOI] [PubMed] [Google Scholar]
- [9].Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep. 2014 Mar 1;37(3):431–438. doi: 10.5665/sleep.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Manni R, Terzaghi M, Repetto A, Zangaglia R, Pacchetti C. Complex paroxysmal nocturnal behaviors in Parkinson's disease. Mov Disord. 2010 Jun 15;25(8):985–990. doi: 10.1002/mds.22990. [DOI] [PubMed] [Google Scholar]
- [11].Bjornara KA, Dietrichs E, Toft M. REM sleep behavior disorder in Parkinson's disease--is there a gender difference? Parkinsonism Relat Disord. 2013 Jan;19(1):120–122. doi: 10.1016/j.parkreldis.2012.05.027. [DOI] [PubMed] [Google Scholar]
- [12].Scaglione C, Vignatelli L, Plazzi G, Marchese R, Negrotti A, Rizzo G, et al. REM sleep behaviour disorder in Parkinson's disease: a questionnaire-based study. Neurol Sci. 2005 Feb;25(6):316–321. doi: 10.1007/s10072-004-0364-7. [DOI] [PubMed] [Google Scholar]
- [13].Oudiette D, De Cock VC, Lavault S, Leu S, Vidailhet M, Arnulf I. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology. 2009 Feb 10;72(6):551–557. doi: 10.1212/01.wnl.0000341936.78678.3a. [DOI] [PubMed] [Google Scholar]
- [14].De Cock VC, Vidailhet M, Leu S, Texeira A, Apartis E, Elbaz A, et al. Restoration of normal motor control in Parkinson's disease during REM sleep. Brain. 2007 Feb;130:450–456. doi: 10.1093/brain/awl363. Pt 2. [DOI] [PubMed] [Google Scholar]
- [15].Kumru H, Santamaria J, Tolosa E, Iranzo A. Relation between subtype of Parkinson's disease and REM sleep behavior disorder. Sleep Med. 2007 Nov;8(7-8):779–783. doi: 10.1016/j.sleep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [16].Nihei Y, Takahashi K, Koto A, Mihara B, Morita Y, Isozumi K, et al. REM sleep behavior disorder in Japanese patients with Parkinson's disease: a multicenter study using the REM sleep behavior disorder screening questionnaire. J Neurol. 2012 Aug;259(8):1606–1612. doi: 10.1007/s00415-011-6386-1. [DOI] [PubMed] [Google Scholar]
- [17].Gong Y, Xiong KP, Mao CJ, Shen Y, Hu WD, Huang JY, et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. 2014 Jun;15(6):647–653. doi: 10.1016/j.sleep.2013.12.021. [DOI] [PubMed] [Google Scholar]
- [18].Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008 Apr;79(4):387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- [19].Kim YE, Yang HJ, Yun JY, Kim HJ, Lee JY, Jeon BS. REM sleep behavior disorder in Parkinson disease: association with abnormal ocular motor findings. Parkinsonism Relat Disord. 2014 Apr;20(4):444–446. doi: 10.1016/j.parkreldis.2013.12.003. [DOI] [PubMed] [Google Scholar]
- [20].Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007 Nov;130:2770–2788. doi: 10.1093/brain/awm056. Pt 11. [DOI] [PubMed] [Google Scholar]
- [21].Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson's disease. Brain. 2013 Jul;136:2120–2129. doi: 10.1093/brain/awt152. Pt 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].American Academy of Sleep Medicine . In: International Classification of Sleep Medicine. 3rd Darien IL, editor. American Academy of Sleep Medicine; 2014. [Google Scholar]
- [23].McCarter SJ, Louis EK, Duwell EJ, Timm PC, Sandness DJ, Boeve BF, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep. 2014 Oct 1;37(10):1649–1662. doi: 10.5665/sleep.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kempfner J, Sorensen G, Zoetmulder M, Jennum P, Sorensen HB. REM behaviour disorder detection associated with neurodegenerative diseases. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:5093–5096. doi: 10.1109/IEMBS.2010.5626212. [DOI] [PubMed] [Google Scholar]
- [25].Ferri R, Fulda S, Cosentino FI, Pizza F, Plazzi G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson's disease with or without REM sleep behavior disorder. Sleep Med. 2012 Jun;13(6):707–713. doi: 10.1016/j.sleep.2012.01.003. [DOI] [PubMed] [Google Scholar]
- [26].Sixel-Doring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Intraindividual Variability of REM Sleep Behavior Disorder in Parkinson's Disease: A Comparative Assessment Using a New REM Sleep Behavior Disorder Severity Scale (RBDSS) for Clinical Routine. J Clin Sleep Med. 2011 Feb 15;7(1):75–80. [PMC free article] [PubMed] [Google Scholar]
- [27].Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007 Dec;22(16):2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- [28].Frauscher B, Jennum P, Ju YE, Postuma RB, Arnulf I, Cochen De Cock V, et al. Comorbidity and medication in REM sleep behavior disorder: A multicenter case-control study. Neurology. 2014 Mar 25;82(12):1076–1079. doi: 10.1212/WNL.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nomura T, Inoue Y, Kagimura T, Uemura Y, Nakashima K. Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson's disease patients. Sleep Med. 2011 Aug;12(7):711–713. doi: 10.1016/j.sleep.2011.01.015. [DOI] [PubMed] [Google Scholar]
- [30].Chahine LM, Daley J, Horn S, Colcher A, Hurtig H, Cantor C, et al. Questionnaire-based diagnosis of REM sleep behavior disorder in Parkinson's disease. Mov Disord. 2013 Mar 20;28(8):1146–1149. doi: 10.1002/mds.25438. [DOI] [PubMed] [Google Scholar]
- [31].Shen SS, Shen Y, Xiong KP, Chen J, Mao CJ, Huang JY, et al. Validation study of REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK) in east China. Sleep Med. 2014 Aug;15(8):952–958. doi: 10.1016/j.sleep.2014.03.020. [DOI] [PubMed] [Google Scholar]
- [32].Bolitho SJ, Naismith SL, Terpening Z, Grunstein RR, Melehan K, Yee BJ, et al. Investigating rapid eye movement sleep without atonia in Parkinson's disease using the rapid eye movement sleep behavior disorder screening questionnaire. Mov Disord. 2014 May;29(6):736–742. doi: 10.1002/mds.25832. [DOI] [PubMed] [Google Scholar]
- [33].Stiasny-Kolster K, Sixel-Doring F, Trenkwalder C, Heinzel-Gutenbrunner M, Seppi K, Poewe W, et al. Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson's disease. Sleep Med. 2015 Jan;16(1):186–189. doi: 10.1016/j.sleep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- [34].Li SX, Wing YK, Lam SP, Zhang J, Yu MW, Ho CK, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Med. 2010 Jan;11(1):43–48. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- [35].Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin SC, Bieniek K, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011 May;12(5):445–453. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, et al. A single-question screen for rapid eye movement sleep behavior disorder: A multicenter validation study. Mov Disord. 2012 Jun;27(7):913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Frauscher B, Ehrmann L, Zamarian L, Auer F, Mitterling T, Gabelia D, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012 Nov;27(13):1673–1678. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- [38].Hogl B, Arnulf I, Comella C, Ferreira J, Iranzo A, Tilley B, et al. Scales to assess sleep impairment in Parkinson's disease: critique and recommendations. Mov Disord. 2010 Dec 15;25(16):2704–2716. doi: 10.1002/mds.23190. [DOI] [PubMed] [Google Scholar]
- [39].Trenkwalder C, Kohnen R, Hogl B, Metta V, Sixel-Doring F, Frauscher B, et al. Parkinson's disease sleep scale-validation of the revised version PDSS-2. Mov Disord. 2011 Mar;26(4):644–652. doi: 10.1002/mds.23476. [DOI] [PubMed] [Google Scholar]
- [40].Stavitsky K, Saurman JL, McNamara P, Cronin-Golomb A. Sleep in Parkinson's disease: a comparison of actigraphy and subjective measures. Parkinsonism Relat Disord. 2010 May;16(4):280–283. doi: 10.1016/j.parkreldis.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stavitsky K, Cronin-Golomb A. Sleep quality in Parkinson disease: an examination of clinical variables. Cogn Behav Neurol. 2011 Jun;24(2):43–49. doi: 10.1097/WNN.0b013e31821a4a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marinus J, Visser M, van Hilten JJ, Lammers GJ, Stiggelbout AM. Assessment of sleep and sleepiness in Parkinson disease. Sleep. 2003 Dec 15;26(8):1049–1054. doi: 10.1093/sleep/26.8.1049. [DOI] [PubMed] [Google Scholar]
- [43].Scullin MK, Harrison TL, Factor SA, Bliwise DL. A Neurodegenerative Disease Sleep Questionnaire: principal component analysis in Parkinson's disease. J Neurol Sci. 2014 Jan 15;336(1-2):243–246. doi: 10.1016/j.jns.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [45].Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012 Dec;188(6 Suppl):2455–2463. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- [46].Bing MH, Moller LA, Jennum P, Mortensen S, Lose G. Validity and reliability of a questionnaire for evaluating nocturia, nocturnal enuresis and sleep-interruptions in an elderly population. Eur Urol. 2006 Apr;49(4):710–719. doi: 10.1016/j.eururo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- [47].Romenets SR, Wolfson C, Galatas C, Pelletier A, Altman R, Wadup L, et al. Validation of the non-motor symptoms questionnaire (NMS-Quest) Parkinsonism Relat Disord. 2012 Jan;18(1):54–58. doi: 10.1016/j.parkreldis.2011.08.013. [DOI] [PubMed] [Google Scholar]
- [48].Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, et al. Symptom assessment tool for overactive bladder syndrome--overactive bladder symptom score. Urology. 2006 Aug;68(2):318–323. doi: 10.1016/j.urology.2006.02.042. [DOI] [PubMed] [Google Scholar]
- [49].De Cock VC, Bayard S, Yu H, Grini M, Carlander B, Postuma R, et al. Suggested immobilization test for diagnosis of restless legs syndrome in Parkinson's disease. Mov Disord. 2012 May;27(6):743–749. doi: 10.1002/mds.24969. [DOI] [PubMed] [Google Scholar]
- *[50].Neikrug AB, Liu L, Avanzino JA, Maglione JE, Natarajan L, Bradley L, et al. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 2014 Jan 1;37(1):177–185. doi: 10.5665/sleep.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999 Oct 5;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- [52].Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008 May;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- [53].Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991 Dec;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- [54].Hagell P, Broman JE. Measurement properties and hierarchical item structure of the Epworth Sleepiness Scale in Parkinson's disease. J Sleep Res. 2007 Mar;16(1):102–109. doi: 10.1111/j.1365-2869.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- [55].Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005 Jan;28(1):113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- [56].MacLean AW, Fekken GC, Saskin P, Knowles JB. Psychometric evaluation of the Stanford Sleepiness Scale. J Sleep Res. 1992 Mar;1(1):35–39. doi: 10.1111/j.1365-2869.1992.tb00006.x. [DOI] [PubMed] [Google Scholar]
- [57].Setthawatcharawanich S, Limapichat K, Sathirapanya P, Phabphal K. Validation of the Thai SCOPA-sleep scale for assessment of sleep and sleepiness in patients with Parkinson's disease. J Med Assoc Thai. 2011 Feb;94(2):179–184. [PubMed] [Google Scholar]
- [58].Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002 Dec;73(6):629–635. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tse W, Liu Y, Barthlen GM, Halbig TD, Tolgyesi SV, Gracies JM, et al. Clinical usefulness of the Parkinson's disease sleep scale. Parkinsonism Relat Disord. 2005 Aug;11(5):317–321. doi: 10.1016/j.parkreldis.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [60].Martinez-Martin P, Visser M, Rodriguez-Blazquez C, Marinus J, Chaudhuri KR, van Hilten JJ, et al. SCOPA-sleep and PDSS: two scales for assessment of sleep disorder in Parkinson's disease. Mov Disord. 2008 Sep 15;23(12):1681–1688. doi: 10.1002/mds.22110. [DOI] [PubMed] [Google Scholar]
- [61].Pellecchia MT, Antonini A, Bonuccelli U, Fabbrini G, Ferini Strambi L, Stocchi F, et al. Observational study of sleep-related disorders in Italian patients with Parkinson's disease: usefulness of the Italian version of Parkinson's disease sleep scale. Neurol Sci. 2012 Jun;33(3):689–694. doi: 10.1007/s10072-011-0826-7. [DOI] [PubMed] [Google Scholar]
- [62].Uemura Y, Nomura T, Inoue Y, Yamawaki M, Yasui K, Nakashima K. Validation of the Parkinson's disease sleep scale in Japanese patients: a comparison study using the Pittsburgh Sleep Quality Index, the Epworth Sleepiness Scale and Polysomnography. J Neurol Sci. 2009 Dec 15;287(1-2):36–40. doi: 10.1016/j.jns.2009.09.015. [DOI] [PubMed] [Google Scholar]
- [63].Margis R, Donis K, Schonwald SV, Fagondes SC, Monte T, Martin-Martinez P, et al. Psychometric properties of the Parkinson's Disease Sleep Scale--Brazilian version. Parkinsonism Relat Disord. 2009 Aug;15(7):495–499. doi: 10.1016/j.parkreldis.2008.12.008. [DOI] [PubMed] [Google Scholar]
- [64].Wang G, Cheng Q, Zeng J, Bai L, Liu GD, Zhang Y, et al. Sleep disorders in Chinese patients with Parkinson's disease: validation study of a Chinese version of Parkinson's disease sleep scale. J Neurol Sci. 2008 Aug 15;271(1-2):153–157. doi: 10.1016/j.jns.2008.04.008. [DOI] [PubMed] [Google Scholar]
- [65].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008 Nov 15;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- [66].Horvath K, Aschermann Z, Acs P, Bosnyak E, Deli G, Pal E, et al. Is the MDS-UPDRS a Good Screening Tool for Detecting Sleep Problems and Daytime Sleepiness in Parkinson's Disease? Parkinsons Dis. 2014;2014:806169. doi: 10.1155/2014/806169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Louter M, Arends JB, Bloem BR, Overeem S. Actigraphy as a diagnostic aid for REM sleep behavior disorder in Parkinson's disease. BMC Neurol. 2014 Apr 6;14 doi: 10.1186/1471-2377-14-76. 76-2377-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[68].Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative Disorder Risk in Idiopathic REM Sleep Behavior Disorder: Study in 174 Patients. PLoS One. 2014 Feb 26;9(2):e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[69].Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005 Nov 8;65(9):1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- *[70].Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011 May;69(5):811–818. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- [71].Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008 Oct;79(10):1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- [72].Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008 Sep 15;23(12):1665–1672. doi: 10.1002/mds.22099. [DOI] [PubMed] [Google Scholar]
- [73].Romenets SR, Gagnon JF, Latreille V, Panniset M, Chouinard S, Montplaisir J, et al. Rapid eye movement sleep behavior disorder and subtypes of Parkinson's disease. Mov Disord. 2012 Jun 25; doi: 10.1002/mds.25086. [DOI] [PubMed] [Google Scholar]
- [74].Terzaghi M, Arnaldi D, Rizzetti MC, Minafra B, Cremascoli R, Rustioni V, et al. Analysis of video-polysomnographic sleep findings in dementia with Lewy bodies. Mov Disord. 2013 Sep;28(10):1416–1423. doi: 10.1002/mds.25523. [DOI] [PubMed] [Google Scholar]
- [75].Vendette M, Gagnon JF, Decary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology. 2007 Nov 6;69(19):1843–1849. doi: 10.1212/01.wnl.0000278114.14096.74. [DOI] [PubMed] [Google Scholar]
- [76].Sinforiani E, Zangaglia R, Manni R, Cristina S, Marchioni E, Nappi G, et al. REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson's disease. Mov Disord. 2006 Apr;21(4):462–466. doi: 10.1002/mds.20719. [DOI] [PubMed] [Google Scholar]
- [77].Gagnon JF, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009 Jul;66(1):39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- [78].Marques A, Dujardin K, Boucart M, Pins D, Delliaux M, Defebvre L, et al. REM sleep behaviour disorder and visuoperceptive dysfunction: a disorder of the ventral visual stream? J Neurol. 2010 Mar;257(3):383–391. doi: 10.1007/s00415-009-5328-7. [DOI] [PubMed] [Google Scholar]
- [79].Plomhause L, Dujardin K, Boucart M, Herlin V, Defebvre L, Derambure P, et al. Impaired visual perception in rapid eye movement sleep behavior disorder. Neuropsychology. 2014 May;28(3):388–393. doi: 10.1037/neu0000006. [DOI] [PubMed] [Google Scholar]
- [80].Gaudreault PO, Gagnon JF, Montplaisir J, Vendette M, Postuma RB, Gagnon K, et al. Abnormal occipital event-related potentials in Parkinson's disease with concomitant REM sleep behavior disorder. Parkinsonism Relat Disord. 2013 Feb;19(2):212–217. doi: 10.1016/j.parkreldis.2012.10.006. [DOI] [PubMed] [Google Scholar]
- *[81].Gunn DG, Naismith SL, Bolitho SJ, Lewis SJ. Actigraphically-defined sleep disturbance in Parkinson's disease is associated with differential aspects of cognitive functioning. J Clin Neurosci. 2014 Jul;21(7):1112–1115. doi: 10.1016/j.jocn.2013.09.017. [DOI] [PubMed] [Google Scholar]
- *[82].Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: A prospective study. Mov Disord. 2012 May;27(6):720–726. doi: 10.1002/mds.24939. [DOI] [PubMed] [Google Scholar]
- [83].Sinforiani E, Pacchetti C, Zangaglia R, Pasotti C, Manni R, Nappi G. REM behavior disorder, hallucinations and cognitive impairment in Parkinson's disease: a two-year follow up. Mov Disord. 2008 Jul 30;23(10):1441–1445. doi: 10.1002/mds.22126. [DOI] [PubMed] [Google Scholar]
- [84].Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014 Sep 30;83(14):1253–1260. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[85].Meindorfner C, Korner Y, Moller JC, Stiasny-Kolster K, Oertel WH, Kruger HP. Driving in Parkinson's disease: mobility, accidents, and sudden onset of sleep at the wheel. Mov Disord. 2005 Jul;20(7):832–842. doi: 10.1002/mds.20412. [DOI] [PubMed] [Google Scholar]
- [86].Poryazova R, Oberholzer M, Baumann CR, Bassetti CL. REM Sleep Behavior Disorder in Parkinson's Disease: A Questionnaire-Based Survey. J Clin Sleep Med. 2013 Jan 15;9(1):55–59. doi: 10.5664/jcsm.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Suzuki K, Miyamoto T, Miyamoto M, Watanabe Y, Suzuki S, Tatsumoto M, et al. Probable rapid eye movement sleep behavior disorder, nocturnal disturbances and quality of life in patients with Parkinson's disease: a case-controlled study using the rapid eye movement sleep behavior disorder screening questionnaire. BMC Neurol. 2013 Feb 9;13 doi: 10.1186/1471-2377-13-18. 18-2377-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vibha D, Shukla G, Goyal V, Singh S, Srivastava AK, Behari M. RBD in Parkinson's disease: a clinical case control study from North India. Clin Neurol Neurosurg. 2011 Jul;113(6):472–476. doi: 10.1016/j.clineuro.2011.02.007. [DOI] [PubMed] [Google Scholar]
- *[89].Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014 Apr;71(4):463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010 Feb 15;6(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- [91].Postuma RB, Gagnon JF, Pelletier A, Montplaisir J. Prodromal autonomic symptoms and signs in Parkinson's disease and dementia with Lewy bodies. Mov Disord. 2013 May;28(5):597–604. doi: 10.1002/mds.25445. [DOI] [PubMed] [Google Scholar]
- [92].Postuma RB, Lanfranchi PA, Blais H, Gagnon JF, Montplaisir JY. Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord. 2010 Oct 30;25(14):2304–2310. doi: 10.1002/mds.23347. [DOI] [PubMed] [Google Scholar]
- [93].Postuma RB, Montplaisir J, Lanfranchi P, Blais H, Rompre S, Colombo R, et al. Cardiac autonomic denervation in Parkinson's disease is linked to REM sleep behavior disorder. Mov Disord. 2011 Jul;26(8):1529–1533. doi: 10.1002/mds.23677. [DOI] [PubMed] [Google Scholar]
- [94].Miyamoto T, Miyamoto M, Iwanami M, Hirata K. Cardiac (123)I-MIBG accumulation in Parkinson's disease differs in association with REM sleep behavior disorder. Parkinsonism Relat Disord. 2011 Mar;17(3):219–220. doi: 10.1016/j.parkreldis.2010.11.020. [DOI] [PubMed] [Google Scholar]
- [95].Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Age, drugs, or disease: what alters the macrostructure of sleep in Parkinson's disease? Sleep Med. 2012 Oct;13(9):1178–1183. doi: 10.1016/j.sleep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- [96].Benninger DH, Michel J, Waldvogel D, Candia V, Poryazova R, van Hedel HJ, et al. REM sleep behavior disorder is not linked to postural instability and gait dysfunction in Parkinson. Mov Disord. 2010 Aug 15;25(11):1597–1604. doi: 10.1002/mds.23121. [DOI] [PubMed] [Google Scholar]
- [97].Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, Nithi K, Talbot K, Ben-Shlomo Y, et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson's disease. J Neurol Neurosurg Psychiatry. 2014 May;85(5):560–566. doi: 10.1136/jnnp-2013-306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Aygun D, Turkel Y, Onar MK, Sunter T. Clinical REM sleep behavior disorder and motor subtypes in Parkinson's disease: a questionnaire-based study. Clin Neurol Neurosurg. 2014 Apr;119:54–58. doi: 10.1016/j.clineuro.2014.01.011. [DOI] [PubMed] [Google Scholar]
- [99].Zibetti M, Rizzi L, Colloca L, Cinquepalmi A, Angrisano S, Castelli L, et al. Probable REM sleep behaviour disorder and STN-DBS outcome in Parkinson's Disease. Parkinsonism Relat Disord. 2010 May;16(4):265–269. doi: 10.1016/j.parkreldis.2010.01.001. [DOI] [PubMed] [Google Scholar]
- [100].Pacchetti C, Manni R, Zangaglia R, Mancini F, Marchioni E, Tassorelli C, et al. Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson's disease. Mov Disord. 2005 Nov;20(11):1439–1448. doi: 10.1002/mds.20582. [DOI] [PubMed] [Google Scholar]
- [101].Forsaa EB, Larsen JP, Wentzel-Larsen T, Goetz CG, Stebbins GT, Aarsland D, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010 Aug;67(8):996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- [102].Bjornara KA, Dietrichs E, Toft M. Clinical features associated with sleep disturbances in Parkinson's disease. Clin Neurol Neurosurg. 2014 Sep;124:37–43. doi: 10.1016/j.clineuro.2014.06.027. [DOI] [PubMed] [Google Scholar]
- [103].Fantini ML, Macedo L, Zibetti M, Sarchioto M, Vidal T, Pereira B, et al. Increased risk of impulse control symptoms in Parkinson's disease with REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2014 Jul 8; doi: 10.1136/jnnp-2014-307904. [DOI] [PubMed] [Google Scholar]
- [104].Bayard S, Dauvilliers Y, Yu H, Croisier-Langenier M, Rossignol A, Charif M, et al. Impulse control disorder and rapid eye movement sleep behavior disorder in Parkinson's disease. Parkinsonism Relat Disord. 2014 Dec;20(12):1411–1414. doi: 10.1016/j.parkreldis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- [105].Ylikoski A, Martikainen K, Partinen M. Parasomnias and isolated sleep symptoms in Parkinson's disease: A questionnaire study on 661 patients. J Neurol Sci. 2014 Nov 15;346(1-2):204–208. doi: 10.1016/j.jns.2014.08.025. [DOI] [PubMed] [Google Scholar]
- [106].Munhoz RP, Teive HA, Eleftherohorinou H, Coin LJ, Lees AJ, Silveira-Moriyama L. Demographic and motor features associated with the occurrence of neuropsychiatric and sleep complications of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013 Aug;84(8):883–887. doi: 10.1136/jnnp-2012-304440. [DOI] [PubMed] [Google Scholar]
- [107].Di Fabio N, Poryazova R, Oberholzer M, Baumann CR, Bassetti CL. Sleepwalking, REM sleep behaviour disorder and overlap parasomnia in patients with Parkinson's disease. Eur Neurol. 2013;70(5-6):297–303. doi: 10.1159/000353378. [DOI] [PubMed] [Google Scholar]
- [108].Sommerauer M, Valko PO, Werth E, Poryazova R, Hauser S, Baumann CR. Revisiting the impact of REM sleep behavior disorder on motor progression in Parkinson's disease. Parkinsonism Relat Disord. 2014;20(4):460–464. doi: 10.1016/j.parkreldis.2014.01.005. [DOI] [PubMed] [Google Scholar]
- [109].Bugalho P, Viana-Baptista M. REM sleep behavior disorder and motor dysfunction in Parkinson's disease - A longitudinal study. Parkinsonism Relat Disord. 2013 Aug 5; doi: 10.1016/j.parkreldis.2013.07.017. [DOI] [PubMed] [Google Scholar]
- [110].Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson's disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol. 2009;61(3):164–170. doi: 10.1159/000189269. [DOI] [PubMed] [Google Scholar]
- [111].Gomez-Esteban JC, Zarranz JJ, Lezcano E, Velasco F, Ciordia R, Rouco I, et al. Sleep complaints and their relation with drug treatment in patients suffering from Parkinson's disease. Mov Disord. 2006 Jul;21(7):983–988. doi: 10.1002/mds.20874. [DOI] [PubMed] [Google Scholar]
- [112].Norlinah MI, Afidah KN, Noradina AT, Shamsul AS, Hamidon BB, Sahathevan R, et al. Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism Relat Disord. 2009 Nov;15(9):670–674. doi: 10.1016/j.parkreldis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- [113].Riedel O, Klotsche J, Spottke A, Deuschl G, Forstl H, Henn F, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson's disease. J Neurol. 2010 Jul;257(7):1073–1082. doi: 10.1007/s00415-010-5465-z. [DOI] [PubMed] [Google Scholar]
- [114].Erro R, Santangelo G, Picillo M, Vitale C, Amboni M, Longo K, et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J Neurol. 2012 Sep;259(9):1808–1813. doi: 10.1007/s00415-011-6407-0. [DOI] [PubMed] [Google Scholar]
- [115].Stavitsky K, Neargarder S, Bogdanova Y, McNamara P, Cronin-Golomb A. The impact of sleep quality on cognitive functioning in Parkinson's disease. J Int Neuropsychol Soc. 2012 Jan;18(1):108–117. doi: 10.1017/S1355617711001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P, ELEP Group Relationship between sleep disorders and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2013 Dec;19(12):1152–1155. doi: 10.1016/j.parkreldis.2013.07.026. [DOI] [PubMed] [Google Scholar]
- [117].Chung S, Bohnen NI, Albin RL, Frey KA, Muller ML, Chervin RD. Insomnia and sleepiness in Parkinson disease: associations with symptoms and comorbidities. J Clin Sleep Med. 2013 Nov 15;9(11):1131–1137. doi: 10.5664/jcsm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tijero B, Somme J, Gomez-Esteban JC, Berganzo K, Adhikari I, Lezcano E, et al. Relationship between sleep and dysautonomic symptoms assessed by self-report scales. Mov Disord. 2011 Aug 15;26(10):1967–1968. doi: 10.1002/mds.23761. [DOI] [PubMed] [Google Scholar]
- [119].Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010 Jun;9(6):573–580. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- [120].Wailke S, Herzog J, Witt K, Deuschl G, Volkmann J. Effect of controlled-release levodopa on the microstructure of sleep in Parkinson's disease. Eur J Neurol. 2011 Apr;18(4):590–596. doi: 10.1111/j.1468-1331.2010.03213.x. [DOI] [PubMed] [Google Scholar]
- [121].Porter B, Macfarlane R, Walker R. The frequency and nature of sleep disorders in a community-based population of patients with Parkinson's disease. Eur J Neurol. 2008 Jan;15(1):50–54. doi: 10.1111/j.1468-1331.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- [122].Gjerstad MD, Wentzel-Larsen T, Aarsland D, Larsen JP. Insomnia in Parkinson's disease: frequency and progression over time. J Neurol Neurosurg Psychiatry. 2007 May;78(5):476–479. doi: 10.1136/jnnp.2006.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Verbaan D, van Rooden SM, Visser M, Marinus J, van Hilten JJ. Nighttime sleep problems and daytime sleepiness in Parkinson's disease. Mov Disord. 2008 Jan;23(1):35–41. doi: 10.1002/mds.21727. [DOI] [PubMed] [Google Scholar]
- [124].Yong MH, Fook-Chong S, Pavanni R, Lim LL, Tan EK. Case control polysomnographic studies of sleep disorders in Parkinson's disease. PLoS One. 2011;6(7):e22511. doi: 10.1371/journal.pone.0022511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yu SY, Sun L, Liu Z, Huang XY, Zuo LJ, Cao CJ, et al. Sleep disorders in Parkinson's disease: clinical features, iron metabolism and related mechanism. PLoS One. 2013 Dec 23;8(12):e82924. doi: 10.1371/journal.pone.0082924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Suzuki K, Miyamoto M, Miyamoto T, Okuma Y, Hattori N, Kamei S, et al. Correlation between depressive symptoms and nocturnal disturbances in Japanese patients with Parkinson's disease. Parkinsonism Relat Disord. 2009 Jan;15(1):15–19. doi: 10.1016/j.parkreldis.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [127].Shimohata T, Nishizawa M. Sleep disturbance in patients with Parkinson's disease presenting with leg motor restlessness. Parkinsonism Relat Disord. 2013 May;19(5):571–572. doi: 10.1016/j.parkreldis.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [128].Morgante L, Colosimo C, Antonini A, Marconi R, Meco G, Pederzoli M, et al. Psychosis associated to Parkinson's disease in the early stages: relevance of cognitive decline and depression. J Neurol Neurosurg Psychiatry. 2012 Jan;83(1):76–82. doi: 10.1136/jnnp-2011-300043. [DOI] [PubMed] [Google Scholar]
- [129].Verbaan D, van Rooden SM, van Hilten JJ, Rijsman RM. Prevalence and clinical profile of restless legs syndrome in Parkinson's disease. Mov Disord. 2010 Oct 15;25(13):2142–2147. doi: 10.1002/mds.23241. [DOI] [PubMed] [Google Scholar]
- [130].Covassin N, Neikrug AB, Liu L, Corey-Bloom J, Loredo JS, Palmer BW, et al. Clinical correlates of periodic limb movements in sleep in Parkinson's disease. J Neurol Sci. 2012 May 15;316(1-2):131–136. doi: 10.1016/j.jns.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Loo HV, Tan EK. Case-control study of restless legs syndrome and quality of sleep in Parkinson's disease. J Neurol Sci. 2008 Mar 15;266(1-2):145–149. doi: 10.1016/j.jns.2007.09.033. [DOI] [PubMed] [Google Scholar]
- [132].van Rooden SM, Visser M, Verbaan D, Marinus J, van Hilten JJ. Patterns of motor and non-motor features in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2009 Aug;80(8):846–850. doi: 10.1136/jnnp.2008.166629. [DOI] [PubMed] [Google Scholar]
- [133].Perez Lloret S, Rossi M, Merello M, Rascol O, Cardinali DP. Nonmotor symptoms groups in Parkinson's disease patients: results of a pilot, exploratory study. Parkinsons Dis. 2011;2011:473579. doi: 10.4061/2011/473579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Goldman JG, Ghode RA, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Dissociations among daytime sleepiness, nighttime sleep, and cognitive status in Parkinson's disease. Parkinsonism Relat Disord. 2013 Sep;19(9):806–811. doi: 10.1016/j.parkreldis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Compta Y, Santamaria J, Ratti L, Tolosa E, Iranzo A, Munoz E, et al. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson's disease dementia. Brain. 2009 Dec;132:3308–3317. doi: 10.1093/brain/awp263. Pt 12. [DOI] [PubMed] [Google Scholar]
- [136].Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Visual symptoms in Parkinson's disease and Parkinson's disease dementia. Mov Disord. 2011 Nov;26(13):2387–2395. doi: 10.1002/mds.23891. [DOI] [PubMed] [Google Scholar]
- [137].Gjerstad MD, Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology. 2006 Sep 12;67(5):853–858. doi: 10.1212/01.wnl.0000233980.25978.9d. [DOI] [PubMed] [Google Scholar]
- [138].Goldman JG, Stebbins GT, Leung V, Tilley BC, Goetz CG. Relationships among cognitive impairment, sleep, and fatigue in Parkinson's disease using the MDS-UPDRS. Parkinsonism Relat Disord. 2014 Aug 13; doi: 10.1016/j.parkreldis.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhu K, van Hilten JJ, Marinus J. Predictors of dementia in Parkinson's disease; findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism Relat Disord. 2014 Sep;20(9):980–985. doi: 10.1016/j.parkreldis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- [140].Jain S, Siegle GJ, Gu C, Moore CG, Ivanco LS, Studenski S, et al. Pupillary unrest correlates with arousal symptoms and motor signs in Parkinson disease. Mov Disord. 2011 Jun;26(7):1344–1347. doi: 10.1002/mds.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007 Jul 24;69(4):333–341. doi: 10.1212/01.wnl.0000266593.50534.e8. [DOI] [PubMed] [Google Scholar]
- [142].Spindler M, Gooneratne NS, Siderowf A, Duda JE, Cantor C, Dahodwala N. Daytime sleepiness is associated with falls in Parkinson's disease. J Parkinsons Dis. 2013 Jan 1;3(3):387–391. doi: 10.3233/JPD-130184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bryant MS, Rintala DH, Hou JG, Rivas SP, Fernandez AL, Lai EC, et al. The relation of falls to fatigue, depression and daytime sleepiness in Parkinson's disease. Eur Neurol. 2012;67(6):326–330. doi: 10.1159/000335877. [DOI] [PubMed] [Google Scholar]
- [144].Suzuki K, Miyamoto T, Miyamoto M, Okuma Y, Hattori N, Kamei S, et al. Excessive daytime sleepiness and sleep episodes in Japanese patients with Parkinson's disease. J Neurol Sci. 2008 Aug 15;271(1-2):47–52. doi: 10.1016/j.jns.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [145].Ataide M, Franco CM, Lins OG. Daytime sleepiness and Parkinson's disease: the contribution of the multiple sleep latency test. Sleep Disord. 2014;2014:767181. doi: 10.1155/2014/767181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Naismith SL, Hickie IB, Lewis SJ. The role of mild depression in sleep disturbance and quality of life in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2010 Fall;22(4):384–389. doi: 10.1176/jnp.2010.22.4.384. [DOI] [PubMed] [Google Scholar]
- [147].Kummer A, Scalzo P, Cardoso F, Teixeira AL. Evaluation of fatigue in Parkinson's disease using the Brazilian version of Parkinson's Fatigue Scale. Acta Neurol Scand. 2011 Feb;123(2):130–136. doi: 10.1111/j.1600-0404.2010.01364.x. [DOI] [PubMed] [Google Scholar]
- [148].Miwa H, Miwa T. Fatigue in patients with Parkinson's disease: impact on quality of life. Intern Med. 2011;50(15):1553–1558. doi: 10.2169/internalmedicine.50.4954. [DOI] [PubMed] [Google Scholar]
- [149].Hagell P, Brundin L. Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry. 2009 May;80(5):489–492. doi: 10.1136/jnnp.2008.159772. [DOI] [PubMed] [Google Scholar]
- [150].Havlikova E, van Dijk JP, Rosenberger J, Nagyova I, Middel B, Dubayova T, et al. Fatigue in Parkinson's disease is not related to excessive sleepiness or quality of sleep. J Neurol Sci. 2008 Jul 15;270(1-2):107–113. doi: 10.1016/j.jns.2008.02.013. [DOI] [PubMed] [Google Scholar]
- [151].Goulart FO, Godke BA, Borges V, Azevedo-Silva SM, Mendes MF, Cendoroglo MS, et al. Fatigue in a cohort of geriatric patients with and without Parkinson's disease. Braz J Med Biol Res. 2009 Aug;42(8):771–775. doi: 10.1590/s0100-879x2009000800014. [DOI] [PubMed] [Google Scholar]
- [152].Wegelin J, McNamara P, Durso R, Brown A, McLaren D. Correlates of excessive daytime sleepiness in Parkinson's disease. Parkinsonism Relat Disord. 2005 Nov;11(7):441–448. doi: 10.1016/j.parkreldis.2005.05.007. [DOI] [PubMed] [Google Scholar]
- [153].Scullin MK, Sollinger AB, Land J, Wood-Siverio C, Zanders L, Lee R, et al. Sleep and impulsivity in Parkinson's disease. Parkinsonism Relat Disord. 2013 Nov;19(11):991–994. doi: 10.1016/j.parkreldis.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Berendse HW, Roos DS, Raijmakers P, Doty RL. Motor and non-motor correlates of olfactory dysfunction in Parkinson's disease. J Neurol Sci. 2011 Nov 15;310(1-2):21–24. doi: 10.1016/j.jns.2011.06.020. [DOI] [PubMed] [Google Scholar]
- [155].Lee AH, Weintraub D. Psychosis in Parkinson's disease without dementia: common and comorbid with other non-motor symptoms. Mov Disord. 2012 Jun;27(7):858–863. doi: 10.1002/mds.25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Verbaan D, van Rooden SM, Visser M, Marinus J, Emre M, van Hilten JJ. Psychotic and compulsive symptoms in Parkinson's disease. Mov Disord. 2009 Apr 15;24(5):738–744. doi: 10.1002/mds.22453. [DOI] [PubMed] [Google Scholar]
- [157].Barnes J, Connelly V, Wiggs L, Boubert L, Maravic K. Sleep patterns in Parkinson's disease patients with visual hallucinations. Int J Neurosci. 2010 Aug;120(8):564–569. doi: 10.3109/00207454.2010.494790. [DOI] [PubMed] [Google Scholar]
- [158].Zhu K, van Hilten JJ, Putter H, Marinus J. Risk factors for hallucinations in Parkinson's disease: results from a large prospective cohort study. Mov Disord. 2013 Jun;28(6):755–762. doi: 10.1002/mds.25389. [DOI] [PubMed] [Google Scholar]
- [159].Gallagher DA, Parkkinen L, O'Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson's disease. Brain. 2011 Nov;134:3299–3309. doi: 10.1093/brain/awr225. Pt 11. [DOI] [PubMed] [Google Scholar]
- [160].Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009 Apr 14;72(15):1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013 Mar 6; doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003 Mar-Apr;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- [163].Sixel-Doring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Polysomnographic findings, video-based sleep analysis and sleep perception in progressive supranuclear palsy. Sleep Med. 2009 Apr;10(4):407–415. doi: 10.1016/j.sleep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [164].De Cock VC, Lannuzel A, Verhaeghe S, Roze E, Ruberg M, Derenne JP, et al. REM sleep behavior disorder in patients with guadeloupean parkinsonism, a tauopathy. Sleep. 2007 Aug;30(8):1026–1032. doi: 10.1093/sleep/30.8.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001 Jul;16(4):622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- [166].Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005 Feb;28(2):203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- [167].Mahlknecht P, Iranzo A, Hogl B, Frauscher B, Muller C, Santamaria J, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015 Jan 21; doi: 10.1212/WNL.0000000000001265. [DOI] [PubMed] [Google Scholar]
- [168].Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012 Jun;135:1860–1870. doi: 10.1093/brain/aws093. Pt 6. [DOI] [PubMed] [Google Scholar]
- [169].Nomura T, Inoue Y, Hogl B, Uemura Y, Kitayama M, Abe T, et al. Relationship between (123)I-MIBG scintigrams and REM sleep behavior disorder in Parkinson's disease. Parkinsonism Relat Disord. 2010 Dec;16(10):683–685. doi: 10.1016/j.parkreldis.2010.08.011. [DOI] [PubMed] [Google Scholar]
- [170].Iranzo A, Lomena F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] Lancet Neurol. 2010 Nov;9(11):1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- [171].Holtbernd F, Gagnon JF, Postuma RB, Ma Y, Tang CC, Feigin A, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014 Feb 18;82(7):620–627. doi: 10.1212/WNL.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Berg D, Seppi K, Behnke S, Liepelt I, Schweitzer K, Stockner H, et al. Enlarged substantia nigra hyperechogenicity and risk for Parkinson disease: a 37-month 3-center study of 1847 older persons. Arch Neurol. 2011 Jul;68(7):932–937. doi: 10.1001/archneurol.2011.141. [DOI] [PubMed] [Google Scholar]
- [173].Iranzo A, Stockner H, Serradell M, Seppi K, Valldeoriola F, Frauscher B, et al. Five-year follow-up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2014 Dec;29(14):1774–1780. doi: 10.1002/mds.26055. [DOI] [PubMed] [Google Scholar]
- [174].Dang-Vu TT, Gagnon JF, Vendette M, Soucy JP, Postuma RB, Montplaisir J. Hippocampal perfusion predicts impending neurodegeneration in REM sleep behavior disorder. Neurology. 2012 Dec 11;79(24):2302–2306. doi: 10.1212/WNL.0b013e318278b658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010 Jan 19;74(3):239–244. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Fantini ML, Gagnon JF, Petit D, Rompre S, Decary A, Carrier J, et al. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003 Jun;53(6):774–780. doi: 10.1002/ana.10547. [DOI] [PubMed] [Google Scholar]
- [177].Iranzo A, Isetta V, Molinuevo JL, Serradell M, Navajas D, Farre R, et al. Electroencephalographic slowing heralds mild cognitive impairment in idiopathic REM sleep behavior disorder. Sleep Med. 2010 Jun;11(6):534–539. doi: 10.1016/j.sleep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [178].Uribe-San Martin R, Venegas Francke P, Lopez Illanes F, Jones Gazmuri A, Salazar Rivera J, Godoy Fernndez J, et al. Plasma urate in REM sleep behavior disorder. Mov Disord. 2013 Jul;28(8):1150–1151. doi: 10.1002/mds.25441. [DOI] [PubMed] [Google Scholar]
- [179].Salsone M, Cerasa A, Arabia G, Morelli M, Gambardella A, Mumoli L, et al. Reduced thalamic volume in Parkinson disease with REM sleep behavior disorder: volumetric study. Parkinsonism Relat Disord. 2014 Sep;20(9):1004–1008. doi: 10.1016/j.parkreldis.2014.06.012. [DOI] [PubMed] [Google Scholar]
- [180].Ford AH, Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Burn DJ, et al. Rapid eye movement sleep behavior disorder in Parkinson's disease: magnetic resonance imaging study. Mov Disord. 2013 Jun;28(6):832–836. doi: 10.1002/mds.25367. [DOI] [PubMed] [Google Scholar]
- [181].Gagnon JF, Fantini ML, Bedard MA, Petit D, Carrier J, Rompre S, et al. Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology. 2004 Feb 10;62(3):401–406. doi: 10.1212/01.wnl.0000106460.34682.e9. [DOI] [PubMed] [Google Scholar]
- [182].Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson's disease dementia. Int J Geriatr Psychiatry. 2010 Oct;25(10):1030–1038. doi: 10.1002/gps.2506. [DOI] [PubMed] [Google Scholar]
- [183].Jongwanasiri S, Prayoonwiwat N, Pisarnpong A, Srivanitchapoom P, Chotinaiwattarakul W. Evaluation of sleep disorders in Parkinson's disease: a comparison between physician diagnosis and self-administered questionnaires. J Med Assoc Thai. 2014 Mar;97(Suppl 3):S68–77. [PubMed] [Google Scholar]
- [184].American Academy of Sleep Medicine . Diagnostic and coding manual, American Academy of Sleep Medicine. 2nd Westchester, IL: 2005. International classification of sleep disorders. [Google Scholar]
- [185].Politis M, Wu K, Molloy S, G Bain P, Chaudhuri KR, Piccini P. Parkinson's disease symptoms: the patient's perspective. Mov Disord. 2010 Aug 15;25(11):1646–1651. doi: 10.1002/mds.23135. [DOI] [PubMed] [Google Scholar]
- [186].Vibha D, Shukla G, Singh S, Goyal V, Srivastava AK, Behari M. Lower prevalence of sleep disturbances in familial versus sporadic Parkinson's disease: a questionnaire based study. J Neurol Sci. 2010 Aug 15;295(1-2):27–30. doi: 10.1016/j.jns.2010.05.024. [DOI] [PubMed] [Google Scholar]
- [187].Dhawan V, Dhoat S, Williams AJ, Dimarco A, Pal S, Forbes A, et al. The range and nature of sleep dysfunction in untreated Parkinson's disease (PD). A comparative controlled clinical study using the Parkinson's disease sleep scale and selective polysomnography. J Neurol Sci. 2006 Oct 25;248(1-2):158–162. doi: 10.1016/j.jns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [188].Louter M, van Sloun RJ, Pevernagie DA, Arends JB, Cluitmans PJ, Bloem BR, et al. Subjectively impaired bed mobility in Parkinson disease affects sleep efficiency. Sleep Med. 2013 Jul;14(7):668–674. doi: 10.1016/j.sleep.2013.03.010. [DOI] [PubMed] [Google Scholar]
- [189].Stack EL, Ashburn AM. Impaired bed mobility and disordered sleep in Parkinson's disease. Mov Disord. 2006 Sep;21(9):1340–1342. doi: 10.1002/mds.20944. [DOI] [PubMed] [Google Scholar]
- [190].Gomez-Esteban JC, Zarranz JJ, Tijero B, Velasco F, Barcena J, Rouco I, et al. Restless legs syndrome in Parkinson's disease. Mov Disord. 2007 Oct 15;22(13):1912–1916. doi: 10.1002/mds.21624. [DOI] [PubMed] [Google Scholar]
- [191].Zhou CQ, Zhang JW, Wang M, Peng GG. Meta-analysis of the efficacy and safety of long-acting non-ergot dopamine agonists in Parkinson's disease. J Clin Neurosci. 2014 Jul;21(7):1094–1101. doi: 10.1016/j.jocn.2013.10.041. [DOI] [PubMed] [Google Scholar]
- [192].Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, Honczarenko K, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER) Mov Disord. 2011 Jan;26(1):90–99. doi: 10.1002/mds.23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Trenkwaldera C, Kiesb B, Dioszeghyc P, Hilld D, Surmanne E, Boroojerdie B, et al. Rotigotine transdermal system for the management of motor function and sleep disturbances in Parkinson’s disease: Results from a 1-year, open-label extension of the RECOVER study. Basal Ganglia. 2012;2(2):79–85. [Google Scholar]
- [194].Ray Chaudhuri K, Martinez-Martin P, Rolfe KA, Cooper J, Rockett CB, Giorgi L, et al. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson's disease. Eur J Neurol. 2012 Jan;19(1):105–113. doi: 10.1111/j.1468-1331.2011.03442.x. [DOI] [PubMed] [Google Scholar]
- [195].Janssens J, Malfroid K, Nyffeler T, Bohlhalter S, Vanbellingen T. Application of LSVT BIG intervention to address gait, balance, bed mobility, and dexterity in people with Parkinson disease: a case series. Phys Ther. 2014 Jul;94(7):1014–1023. doi: 10.2522/ptj.20130232. [DOI] [PubMed] [Google Scholar]
- [196].Lyons KE, Friedman JH, Hermanowicz N, Isaacson SH, Hauser RA, Hersh BP, et al. Orally disintegrating selegiline in Parkinson patients with dopamine agonist-related adverse effects. Clin Neuropharmacol. 2010 Jan-Feb;33(1):5–10. doi: 10.1097/WNF.0b013e3181b7926f. [DOI] [PubMed] [Google Scholar]
- [197].Parkinson Study Group Pramipexole in levodopa-treated Parkinson disease patients of African, Asian, and Hispanic heritage. Clin Neuropharmacol. 2007 Mar-Apr;30(2):72–85. doi: 10.1097/01.wnf.0000240943.59617.4c. [DOI] [PubMed] [Google Scholar]
- [198].Stowe R, Ives N, Clarke CE, Deane K, van Hilten, Wheatley K, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev. 2010 Jul 7;(7):CD007166. doi: 10.1002/14651858.CD007166.pub2. doi(7):CD007166. [DOI] [PubMed] [Google Scholar]
- [199].Pondal M, Marras C, Miyasaki J, Moro E, Armstrong MJ, Strafella AP, et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J Neurol Neurosurg Psychiatry. 2013 Feb;84(2):130–135. doi: 10.1136/jnnp-2012-302684. [DOI] [PubMed] [Google Scholar]
- [200].Solis-Garcia del Pozo J, Minguez-Minguez S, de Groot PW, Jordan J. Rasagiline meta-analysis: a spotlight on clinical safety and adverse events when treating Parkinson's disease. Expert Opin Drug Saf. 2013 Jul;12(4):479–486. doi: 10.1517/14740338.2013.790956. [DOI] [PubMed] [Google Scholar]
- [201].Elmer L, Schwid S, Eberly S, Goetz C, Fahn S, Kieburtz K, et al. Rasagiline-associated motor improvement in PD occurs without worsening of cognitive and behavioral symptoms. J Neurol Sci. 2006 Oct 25;248(1-2):78–83. doi: 10.1016/j.jns.2006.05.014. [DOI] [PubMed] [Google Scholar]
- [202].Chahine LM, Daley J, Horn S, Duda JE, Colcher A, Hurtig H, et al. Association between dopaminergic medications and nocturnal sleep in early-stage Parkinson's disease. Parkinsonism Relat Disord. 2013 Oct;19(10):859–863. doi: 10.1016/j.parkreldis.2013.05.009. [DOI] [PubMed] [Google Scholar]
- [203].Dobkin RD, Menza M, Bienfait KL, Gara M, Marin H, Mark MH, et al. Depression in Parkinson's disease: symptom improvement and residual symptoms after acute pharmacologic management. Am J Geriatr Psychiatry. 2011 Mar;19(3):222–229. doi: 10.1097/JGP.0b013e3181e448f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Buyske S, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology. 2009 Mar 10;72(10):886–892. doi: 10.1212/01.wnl.0000336340.89821.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Richard IH, McDermott MP, Kurlan R, Lyness JM, Como PG, Pearson N, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology. 2012 Apr 17;78(16):1229–1236. doi: 10.1212/WNL.0b013e3182516244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].El-Senousy M, Mohammed ES, Fayed HA, El-Seidy EA, Khalil MK, Abo El-Enen M, et al. Efficacy of Posterior Tibial Nerve Stimulation on Detrusor Overactivity of Idiopathic Parkinson’s Disease Patients Clinical and Urodynamic Evaluation. Egypt J Neurol Psychiat Neurosurg. 2013;50(3):265–270. [Google Scholar]
- [207].Giladi N, Fichtner A, Poewe W, Boroojerdi B. Rotigotine transdermal system for control of early morning motor impairment and sleep disturbances in patients with Parkinson's disease. J Neural Transm. 2010 Dec;117(12):1395–1399. doi: 10.1007/s00702-010-0506-4. [DOI] [PubMed] [Google Scholar]
- [208].Winge K, Nielsen KK. Bladder dysfunction in advanced Parkinson's disease. Neurourol Urodyn. 2012 Nov;31(8):1279–1283. doi: 10.1002/nau.22237. [DOI] [PubMed] [Google Scholar]
- [209].Nazzaro JM, Pahwa R, Lyons KE. The impact of bilateral subthalamic stimulation on non-motor symptoms of Parkinson's disease. Parkinsonism Relat Disord. 2011 Sep;17(8):606–609. doi: 10.1016/j.parkreldis.2011.05.009. [DOI] [PubMed] [Google Scholar]
- [210].Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 2009 Aug 15;24(11):1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- [211].Shearer J, Green C, Counsell CE, Zajicek JP. The impact of motor and non motor symptoms on health state values in newly diagnosed idiopathic Parkinson's disease. J Neurol. 2012 Mar;259(3):462–468. doi: 10.1007/s00415-011-6202-y. [DOI] [PubMed] [Google Scholar]
- [212].Forsaa EB, Larsen JP, Wentzel-Larsen T, Herlofson K, Alves G. Predictors and course of health-related quality of life in Parkinson's disease. Mov Disord. 2008 Jul 30;23(10):1420–1427. doi: 10.1002/mds.22121. [DOI] [PubMed] [Google Scholar]
- [213].Duncan GW, Khoo TK, Yarnall AJ, O'Brien JT, Coleman SY, Brooks DJ, et al. Health-related quality of life in early Parkinson's disease: the impact of nonmotor symptoms. Mov Disord. 2014 Feb;29(2):195–202. doi: 10.1002/mds.25664. [DOI] [PubMed] [Google Scholar]
- [214].Kasten M, Kertelge L, Tadic V, Bruggemann N, Schmidt A, van der Vegt J, et al. Depression and quality of life in monogenic compared to idiopathic, early-onset Parkinson's disease. Mov Disord. 2012 May;27(6):754–759. doi: 10.1002/mds.24999. [DOI] [PubMed] [Google Scholar]
- [215].Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011 Oct;26(Suppl 3):S42–80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Menza M, Dobkin RD, Marin H, Gara M, Bienfait K, Dicke A, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Mov Disord. 2010 Aug 15;25(11):1708–1714. doi: 10.1002/mds.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhaes MC, de Lourdes Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007 Apr;254(4):459–464. doi: 10.1007/s00415-006-0390-x. [DOI] [PubMed] [Google Scholar]
- [218].Ondo WG, Perkins T, Swick T, Hull KL, Jr, Jimenez JE, Garris TS, et al. Sodium oxybate for excessive daytime sleepiness in Parkinson disease: an open-label polysomnographic study. Arch Neurol. 2008 Oct;65(10):1337–1340. doi: 10.1001/archneur.65.10.1337. [DOI] [PubMed] [Google Scholar]
- [219].Vaughan CP, Juncos JL, Trotti LM, Johnson TM, 2nd, Bliwise DL. Nocturia and overnight polysomnography in Parkinson disease. Neurourol Urodyn. 2013 Nov;32(8):1080–1085. doi: 10.1002/nau.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Gourova LW, van de Beek C, Spigt MG, Nieman FH, van Kerrebroeck PE. Predictive factors for nocturia in elderly men: a cross-sectional study in 21 general practices. BJU Int. 2006 Mar;97(3):528–532. doi: 10.1111/j.1464-410X.2006.06029.x. [DOI] [PubMed] [Google Scholar]
- [221].Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O'Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013 Jan 15;80(3):276–281. doi: 10.1212/WNL.0b013e31827deb74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Cheon SM, Ha MS, Park MJ, Kim JW. Nonmotor symptoms of Parkinson's disease: prevalence and awareness of patients and families. Parkinsonism Relat Disord. 2008;14(4):286–290. doi: 10.1016/j.parkreldis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [223].Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord. 2011 Feb 15;26(3):399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- [224].Breen KC, Drutyte G. Non-motor symptoms of Parkinson's disease: the patient's perspective. J Neural Transm (Vienna) 2013 Apr;120(4):531–535. doi: 10.1007/s00702-012-0928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Lolekha P, Kulkantrakorn K. Non-motor symptoms in Thai Parkinson’s disease patients: Prevalence, manifestation and health related quality of life. Neurology Asia. 2014;19(2):163–170. [Google Scholar]
- [226].Robinson JP, Bradway CW, Bunting-Perry L, Avi-Itzhak T, Mangino M, Chittams J, et al. Lower urinary tract symptoms in men with Parkinson disease. J Neurosci Nurs. 2013 Dec;45(6):382–92. doi: 10.1097/JNN.0b013e3182a3cf67. quiz E1-2. [DOI] [PubMed] [Google Scholar]
- [227].Sammour ZM, Gomes CM, Barbosa ER, Lopes RI, Sallem FS, Trigo-Rocha FE, et al. Voiding dysfunction in patients with Parkinson's disease: impact of neurological impairment and clinical parameters. Neurourol Urodyn. 2009;28(6):510–515. doi: 10.1002/nau.20681. [DOI] [PubMed] [Google Scholar]
- [228].Ragab MM, Mohammed ES. Idiopathic Parkinson's disease patients at the urologic clinic. Neurourol Urodyn. 2011 Sep;30(7):1258–1261. doi: 10.1002/nau.20983. [DOI] [PubMed] [Google Scholar]
- [229].Bhidayasiri R, Mekawichai P, Jitkritsadakul O, Panyakaew P, Kaewwilai L, Boonrod N, et al. Nocturnal journey of body and mind in Parkinson's disease: the manifestations, risk factors and their relationship to daytime symptoms. Evidence from the NIGHT-PD study. J Neural Transm (Vienna) 2014 Aug;121(Suppl 1):S59–68. doi: 10.1007/s00702-014-1199-x. [DOI] [PubMed] [Google Scholar]
- [230].Picillo M, Erro R, Amboni M, Longo K, Vitale C, Moccia M, et al. Gender differences in non-motor symptoms in early Parkinson's disease: a 2-years follow-up study on previously untreated patients. Parkinsonism Relat Disord. 2014 Aug;20(8):850–854. doi: 10.1016/j.parkreldis.2014.04.023. [DOI] [PubMed] [Google Scholar]
- [231].Rana AQ, Vaid H, Akhter MR, Awan NY, Fattah A, Cader MH, et al. Prevalence of nocturia in Parkinson's disease patients from various ethnicities. Neurol Res. 2014 Mar;36(3):234–238. doi: 10.1179/1743132813Y.0000000264. [DOI] [PubMed] [Google Scholar]
- [232].Forjaz MJ, Ayala A, Rodriguez-Blazquez C, Frades-Payo B, Martinez-Martin P, Longitudinal Parkinson's Disease Patient Study. Estudio longitudinal de pacientes con enferedad de Parkinson-ELEP Group Assessing autonomic symptoms of Parkinson's disease with the SCOPA-AUT: a new perspective from Rasch analysis. Eur J Neurol. 2010 Feb;17(2):273–279. doi: 10.1111/j.1468-1331.2009.02835.x. [DOI] [PubMed] [Google Scholar]
- [233].Spica V, Pekmezovic T, Svetel M, Kostic VS. Prevalence of non-motor symptoms in young-onset versus late-onset Parkinson's disease. J Neurol. 2013 Jan;260(1):131–137. doi: 10.1007/s00415-012-6600-9. [DOI] [PubMed] [Google Scholar]
- [234].Mohammed ES, Ragab MM. Idiopathic Parkinson's Disease: Lower Urinary Tract Dysfunctions and Urodynamic Abnormalities. Egypt J Neurol Psychiat Neurosurg. 2010;47(1):381–386. [Google Scholar]
- [235].Xue P, Wang T, Zong H, Zhang Y. Urodynamic analysis and treatment of male Parkinson's disease patients with voiding dysfunction. Chin Med J (Engl) 2014;127(5):878–881. [PubMed] [Google Scholar]
- [236].Iacovelli E, Gilio F, Meco G, Fattapposta F, Vanacore N, Brusa L, et al. Bladder symptoms assessed with overactive bladder questionnaire in Parkinson's disease. Mov Disord. 2010 Jul 15;25(9):1203–1209. doi: 10.1002/mds.23093. [DOI] [PubMed] [Google Scholar]
- [237].Calzetti S, Angelini M, Negrotti A, Marchesi E, Goldoni M. A long-term prospective follow-up study of incident RLS in the course of chronic DAergic therapy in newly diagnosed untreated patients with Parkinson's disease. J Neural Transm. 2014 May;121(5):499–506. doi: 10.1007/s00702-013-1132-8. [DOI] [PubMed] [Google Scholar]
- [238].Wong JC, Li Y, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep. 2014 Feb 1;37(2):369–372. doi: 10.5665/sleep.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Bhalsing K, Suresh K, Muthane UB, Pal PK. Prevalence and profile of Restless Legs Syndrome in Parkinson's disease and other neurodegenerative disorders: a case-control study. Parkinsonism Relat Disord. 2013 Apr;19(4):426–430. doi: 10.1016/j.parkreldis.2012.12.005. [DOI] [PubMed] [Google Scholar]
- [240].Azmin S, Khairul Anuar AM, Nafisah WY, Tan HJ, Raymond AA, Hanita O, et al. Restless legs syndrome and its associated risk factors in Parkinson's disease. Parkinsons Dis. 2013;2013:535613. doi: 10.1155/2013/535613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Rana AQ, Siddiqui I, Mosabbir A, Athar A, Syed O, Jesudasan M, et al. Association of pain, Parkinson's disease, and restless legs syndrome. J Neurol Sci. 2013 Apr 15;327(1-2):32–34. doi: 10.1016/j.jns.2013.01.039. [DOI] [PubMed] [Google Scholar]
- [242].Nomura T, Inoue Y, Nakashima K. Pathogenetic heterogeneity ofrestless legs syndrome in Parkinson's disease. Sleep Biol Rhythms. 2009;7(1):31–33. [Google Scholar]
- [243].Prudon B, Duncan GW, Khoo TK, Yarnall AJ, Anderson KN. Primary sleep disorder prevalence in patients with newly diagnosed Parkinson's disease. Mov Disord. 2014 Feb;29(2):259–262. doi: 10.1002/mds.25730. [DOI] [PubMed] [Google Scholar]
- [244].Linke R, Eisensehr I, Wetter TC, Gildehaus FJ, Popperl G, Trenkwalder C, et al. Presynaptic dopaminergic function in patients with restless legs syndrome: are there common features with early Parkinson's disease? Mov Disord. 2004 Oct;19(10):1158–1162. doi: 10.1002/mds.20226. [DOI] [PubMed] [Google Scholar]
- [245].Lahut S, Vadasz D, Depboylu C, Ries V, Krenzer M, Stiasny-Kolster K, et al. The PD-associated alpha-synuclein promoter Rep1 allele 2 shows diminished frequency in restless legs syndrome. Neurogenetics. 2014 Aug;15(3):189–192. doi: 10.1007/s10048-014-0407-z. [DOI] [PubMed] [Google Scholar]
- [246].Kwon DY, Seo WK, Yoon HK, Park MH, Koh SB, Park KW. Transcranial brain sonography in Parkinson's disease with restless legs syndrome. Mov Disord. 2010 Jul 30;25(10):1373–1378. doi: 10.1002/mds.23066. [DOI] [PubMed] [Google Scholar]
- [247].Ryu JH, Lee MS, Baik JS. Sonographic abnormalities in idiopathic restless legs syndrome (RLS) and RLS in Parkinson's disease. Parkinsonism Relat Disord. 2011 Mar;17(3):201–203. doi: 10.1016/j.parkreldis.2010.11.014. [DOI] [PubMed] [Google Scholar]
- [248].Rajabally YA, Martey J. No association between neuropathy and restless legs in Parkinson's disease. Acta Neurol Scand. 2013 Mar;127(3):216–220. doi: 10.1111/ane.12011. [DOI] [PubMed] [Google Scholar]
- [249].Suzuki K, Miyamoto M, Miyamoto T, Tatsumoto M, Watanabe Y, Suzuki S, et al. Nocturnal disturbances and restlessness in Parkinson's disease: using the Japanese version of the Parkinson's disease sleep scale-2. J Neurol Sci. 2012 Jul 15;318(1-2):76–81. doi: 10.1016/j.jns.2012.03.022. [DOI] [PubMed] [Google Scholar]
- [250].Gjerstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology. 2011 Nov 29;77(22):1941–1946. doi: 10.1212/WNL.0b013e31823a0cc8. [DOI] [PubMed] [Google Scholar]
- [251].Peralta CM, Wolf E, Seppi K, Wenning GK, Hogl B, Poewe W. Restless Legs in Idiopathic Parkinson's Disease. Movement Disorders. 2005;20(Suppl. 10):S108. doi: 10.1002/mds.22694. [DOI] [PubMed] [Google Scholar]
- [252].Rios Romenets S, Dauvilliers Y, Cochen De Cock V, Carlander B, Bayard S, Galatas C, et al. Restless legs syndrome outside the blood-brain barrier--exacerbation by domperidone in Parkinson's disease. Parkinsonism Relat Disord. 2013 Jan;19(1):92–94. doi: 10.1016/j.parkreldis.2012.07.019. [DOI] [PubMed] [Google Scholar]
- [253].Driver-Dunckley E, Evidente VG, Adler CH, Hillman R, Hernandez J, Fletcher G, et al. Restless legs syndrome in Parkinson's disease patients may improve with subthalamic stimulation. Mov Disord. 2006 Aug;21(8):1287–1289. doi: 10.1002/mds.20911. [DOI] [PubMed] [Google Scholar]
- [254].Chahine LM, Ahmed A, Sun Z. Effects of STN DBS for Parkinson's disease on restless legs syndrome and other sleep-related measures. Parkinsonism Relat Disord. 2011 Mar;17(3):208–211. doi: 10.1016/j.parkreldis.2010.11.017. [DOI] [PubMed] [Google Scholar]
- [255].Kedia S, Moro E, Tagliati M, Lang AE, Kumar R. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology. 2004 Dec 28;63(12):2410–2412. doi: 10.1212/01.wnl.0000147288.26029.b8. [DOI] [PubMed] [Google Scholar]
- [256].Cochen De Cock V, Abouda M, Leu S, Oudiette D, Roze E, Vidailhet M, et al. Is obstructive sleep apnea a problem in Parkinson's disease? Sleep Med. 2010 Mar;11(3):247–252. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- [257].Diederich NJ, Rufra O, Pieri V, Hipp G, Vaillant M. Lack of polysomnographic Non-REM sleep changes in early Parkinson's disease. Mov Disord. 2013 Sep;28(10):1443–1446. doi: 10.1002/mds.25520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Lelieveld IM, Muller ML, Bohnen NI, Koeppe RA, Chervin RD, Frey KA, et al. The role of serotonin in sleep disordered breathing associated with Parkinson disease: a correlative [11C]DASB PET imaging study. PLoS One. 2012;7(7):e40166. doi: 10.1371/journal.pone.0040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Monaca C, Duhamel A, Jacquesson JM, Ozsancak C, Destee A, Guieu JD, et al. Vigilance troubles in Parkinson's disease: a subjective and objective polysomnographic study. Sleep Med. 2006 Aug;7(5):448–453. doi: 10.1016/j.sleep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- [260].Nomura T, Inoue Y, Kobayashi M, Namba K, Nakashima K. Characteristics of obstructive sleep apnea in patients with Parkinson's disease. J Neurol Sci. 2013 Apr 15;327(1-2):22–24. doi: 10.1016/j.jns.2013.01.036. [DOI] [PubMed] [Google Scholar]
- [261].Poryazova R, Benninger D, Waldvogel D, Bassetti CL. Excessive daytime sleepiness in Parkinson's disease: characteristics and determinants. Eur Neurol. 2010;63(3):129–135. doi: 10.1159/000276402. [DOI] [PubMed] [Google Scholar]
- [262].Trotti LM, Bliwise DL. No increased risk of obstructive sleep apnea in Parkinson's disease. Mov Disord. 2010 Oct 15;25(13):2246–2249. doi: 10.1002/mds.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Sabate M, Rodriguez M, Mendez E, Enriquez E, Gonzalez I. Obstructive and restrictive pulmonary dysfunction increases disability in Parkinson disease. Arch Phys Med Rehabil. 1996 Jan;77(1):29–34. doi: 10.1016/s0003-9993(96)90216-6. [DOI] [PubMed] [Google Scholar]
- [264].Monteiro L, Souza-Machado A, Valderramas S, Melo A. The effect of levodopa on pulmonary function in Parkinson's disease: a systematic review and meta-analysis. Clin Ther. 2012 May;34(5):1049–1055. doi: 10.1016/j.clinthera.2012.03.001. [DOI] [PubMed] [Google Scholar]
- [265].Seccombe LM, Giddings HL, Rogers PG, Corbett AJ, Hayes MW, Peters MJ, et al. Abnormal ventilatory control in Parkinson's disease--further evidence for non-motor dysfunction. Respir Physiol Neurobiol. 2011 Dec 15;179(2-3):300–304. doi: 10.1016/j.resp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- [266].Diaz K, Faverio P, Hospenthal A, Restrepo MI, Amuan ME, Pugh MJ. Obstructive sleep apnea is associated with higher healthcare utilization in elderly patients. Ann Thorac Med. 2014 Apr;9(2):92–98. doi: 10.4103/1817-1737.128854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002 Jan;121(1):164–172. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- [268].Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000 Apr 12;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- [269].Redline S, Min NI, Shahar E, Rapoport D, O'Connor G. Polysomnographic predictors of blood pressure and hypertension: is one index best? Sleep. 2005 Sep;28(9):1122–1130. doi: 10.1093/sleep/28.9.1122. [DOI] [PubMed] [Google Scholar]
- [270].Cochen De Cock V, Bayard S, Jaussent I, Charif M, Grini M, Langenier MC, et al. Daytime sleepiness in Parkinson's disease: a reappraisal. PLoS One. 2014 Sep 8;9(9):e107278. doi: 10.1371/journal.pone.0107278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Joy SP, Sinha S, Pal PK, Panda S, Philip M, Taly AB. Alterations in Polysomnographic (PSG) profile in drug-naive Parkinson's disease. Ann Indian Acad Neurol. 2014 Jul;17(3):287–291. doi: 10.4103/0972-2327.138501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Moreno-Lopez C, Santamaria J, Salamero M, Del Sorbo F, Albanese A, Pellecchia MT, et al. Excessive daytime sleepiness in multiple system atrophy (SLEEMSA study) Arch Neurol. 2011 Feb;68(2):223–230. doi: 10.1001/archneurol.2010.359. [DOI] [PubMed] [Google Scholar]
- [273].Gama RL, Tavora DG, Bomfim RC, Silva CE, de Bruin VM, de Bruin PF. Sleep disturbances and brain MRI morphometry in Parkinson's disease, multiple system atrophy and progressive supranuclear palsy - a comparative study. Parkinsonism Relat Disord. 2010 May;16(4):275–279. doi: 10.1016/j.parkreldis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [274].Schiffman PL. A "saw-tooth" pattern in Parkinson's disease. Chest. 1985 Jan;87(1):124–126. doi: 10.1378/chest.87.1.124. [DOI] [PubMed] [Google Scholar]
- [275].Vincken W, Cosio MG. "Saw-tooth" pattern in the flow-volume loop. Chest. 1985 Sep;88(3):480–481. doi: 10.1378/chest.88.3.480-a. [DOI] [PubMed] [Google Scholar]
- [276].Lavault S, Bloch F, Houeto JL, Konofal E, Welter ML, Agid Y, et al. Periodic leg movements and REM sleep without atonia in Parkinson's disease with camptocormia. Mov Disord. 2009 Dec 15;24(16):2419–2423. doi: 10.1002/mds.22854. [DOI] [PubMed] [Google Scholar]
- [277].Diederich NJ, Vaillant M, Leischen M, Mancuso G, Golinval S, Nati R, et al. Sleep apnea syndrome in Parkinson's disease. A case-control study in 49 patients. Mov Disord. 2005 Nov;20(11):1413–1418. doi: 10.1002/mds.20624. [DOI] [PubMed] [Google Scholar]
- [278].Valko PO, Hauser S, Sommerauer M, Werth E, Baumann CR. Observations on sleep-disordered breathing in idiopathic Parkinson's disease. PLoS One. 2014 Jun 26;9(6):e100828. doi: 10.1371/journal.pone.0100828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Valko PO, Hauser S, Werth E, Waldvogel D, Baumann CR. Heart rate variability in patients with idiopathic Parkinson's disease with and without obstructive sleep apnea syndrome. Parkinsonism Relat Disord. 2012 Jun;18(5):525–531. doi: 10.1016/j.parkreldis.2012.01.023. [DOI] [PubMed] [Google Scholar]
- [280].Adler CH, Hentz JG, Shill HA, Sabbagh MN, Driver-Dunckley E, Evidente VG, et al. Probable RBD is increased in Parkinson's disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord. 2011 Jul;17(6):456–458. doi: 10.1016/j.parkreldis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Boddy F, Rowan EN, Lett D, O'Brien JT, McKeith IG, Burn DJ. Subjectively reported sleep quality and excessive daytime somnolence in Parkinson's disease with and without dementia, dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry. 2007 Jun;22(6):529–535. doi: 10.1002/gps.1709. [DOI] [PubMed] [Google Scholar]
- [282].Borek LL, Kohn R, Friedman JH. Mood and sleep in Parkinson's disease. J Clin Psychiatry. 2006 Jun;67(6):958–963. doi: 10.4088/jcp.v67n0613. [DOI] [PubMed] [Google Scholar]
- [283].Breen DP, Williams-Gray CH, Mason SL, Foltynie T, Barker RA. Excessive daytime sleepiness and its risk factors in incident Parkinson's disease. J Neurol Neurosurg Psychiatry. 2013 Feb;84(2):233–234. doi: 10.1136/jnnp-2012-304097. [DOI] [PubMed] [Google Scholar]
- [284].Chotinaiwattarakul W, Dayalu P, Chervin RD, Albin RL. Risk of sleep-disordered breathing in Parkinson's disease. Sleep Breath. 2011 Sep;15(3):471–478. doi: 10.1007/s11325-010-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Ferreira T, Prabhakar S, Kharbanda PS. Sleep disturbances in drug naive Parkinson's disease (PD) patients and effect of levodopa on sleep. Ann Indian Acad Neurol. 2014 Oct;17(4):416–419. doi: 10.4103/0972-2327.144016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Gerbin M, Viner AS, Louis ED. Sleep in essential tremor: a comparison with normal controls and Parkinson's disease patients. Parkinsonism Relat Disord. 2012 Mar;18(3):279–284. doi: 10.1016/j.parkreldis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Ghorayeb I, Loundou A, Auquier P, Dauvilliers Y, Bioulac B, Tison F. A nationwide survey of excessive daytime sleepiness in Parkinson's disease in France. Mov Disord. 2007 Aug 15;22(11):1567–1572. doi: 10.1002/mds.21541. [DOI] [PubMed] [Google Scholar]
- [288].Happe S, Baier PC, Bachmann CG, Helmschmied MD, Paulus W. Nocturnal restlessness and distressing dreams in patients with early Parkinson's disease: Role of the Parkinson's disease sleep scale (PDSS) Somnologie. 2008;12(1):50–55. [Google Scholar]
- [289].Havlikova E, van Dijk JP, Nagyova I, Rosenberger J, Middel B, Dubayova T, et al. The impact of sleep and mood disorders on quality of life in Parkinson's disease patients. J Neurol. 2011 Dec;258(12):2222–2229. doi: 10.1007/s00415-011-6098-6. [DOI] [PubMed] [Google Scholar]
- [290].Hu M, Cooper J, Beamish R, Jones E, Butterworth R, Catterall L, et al. How well do we recognise non-motor symptoms in a British Parkinson's disease population? J Neurol. 2011 Aug;258(8):1513–1517. doi: 10.1007/s00415-011-5972-6. [DOI] [PubMed] [Google Scholar]
- [291].Knipe MD, Wickremaratchi MM, Wyatt-Haines E, Morris HR, Ben-Shlomo Y. Quality of life in young-compared with late-onset Parkinson's disease. Mov Disord. 2011 Sep;26(11):2011–2018. doi: 10.1002/mds.23763. [DOI] [PubMed] [Google Scholar]
- [292].Louter M, van der Marck MA, Pevernagie DA, Munneke M, Bloem BR, Overeem S. Sleep matters in Parkinson's disease: use of a priority list to assess the presence of sleep disturbances. Eur J Neurol. 2013 Feb;20(2):259–265. doi: 10.1111/j.1468-1331.2012.03836.x. [DOI] [PubMed] [Google Scholar]
- [293].Valko PO, Waldvogel D, Weller M, Bassetti CL, Held U, Baumann CR. Fatigue and excessive daytime sleepiness in idiopathic Parkinson's disease differently correlate with motor symptoms, depression and dopaminergic treatment. Eur J Neurol. 2010 Dec;17(12):1428–1436. doi: 10.1111/j.1468-1331.2010.03063.x. [DOI] [PubMed] [Google Scholar]
- [294].Wang G, Wan Y, Cheng Q, Xiao Q, Wang Y, Zhang J, et al. Malnutrition and associated factors in Chinese patients with Parkinson's disease: Results from a pilot investigation. Parkinsonism Relat Disord. 2010 Feb;16(2):119–123. doi: 10.1016/j.parkreldis.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [295].Weerkamp NJ, Tissingh G, Poels PJ, Zuidema SU, Munneke M, Koopmans RT, et al. Nonmotor symptoms in nursing home residents with Parkinson's disease: prevalence and effect on quality of life. J Am Geriatr Soc. 2013 Oct;61(10):1714–1721. doi: 10.1111/jgs.12458. [DOI] [PubMed] [Google Scholar]
- [296].Ferreira JJ, Desboeuf K, Galitzky M, Thalamas C, Brefel-Courbon C, Fabre N, et al. Sleep disruption, daytime somnolence and 'sleep attacks' in Parkinson's disease: a clinical survey in PD patients and age-matched healthy volunteers. Eur J Neurol. 2006 Mar;13(3):209–214. doi: 10.1111/j.1468-1331.2006.01262.x. [DOI] [PubMed] [Google Scholar]
- [297].Buskova J, Klempir J, Majerova V, Picmausova J, Sonka K, Jech R, et al. Sleep disturbances in untreated Parkinson's disease. J Neurol. 2011 Dec;258(12):2254–2259. doi: 10.1007/s00415-011-6109-7. [DOI] [PubMed] [Google Scholar]
- [298].Wienecke M, Werth E, Poryazova R, Baumann-Vogel H, Bassetti CL, Weller M, et al. Progressive dopamine and hypocretin deficiencies in Parkinson's disease: is there an impact on sleep and wakefulness? J Sleep Res. 2012 Dec;21(6):710–717. doi: 10.1111/j.1365-2869.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- [299].Yang HJ, Kim YE, Yun JY, Ehm G, Kim HJ, Jeon BS. Comparison of sleep and other non-motor symptoms between SWEDDs patients and de novo Parkinson's disease patients. Parkinsonism Relat Disord. 2014 Dec;20(12):1419–1422. doi: 10.1016/j.parkreldis.2014.09.024. [DOI] [PubMed] [Google Scholar]
- [300].Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, Garcia-Sanchez C, Gironell A, Trapecio Group Study Prevalence and correlates of neuropsychiatric symptoms in Parkinson's disease without dementia. Mov Disord. 2008 Oct 15;23(13):1889–1896. doi: 10.1002/mds.22246. [DOI] [PubMed] [Google Scholar]
- [301].Suzuki K, Okuma Y, Hattori N, Kamei S, Yoshii F, Utsumi H, et al. Characteristics of sleep disturbances in Japanese patients with Parkinson's disease. A study using Parkinson's disease sleep scale. Mov Disord. 2007 Jul 15;22(9):1245–1251. doi: 10.1002/mds.21257. [DOI] [PubMed] [Google Scholar]
- [302].Suzuki K, Miyamoto M, Miyamoto T, Suzuki S, Watanabe Y, Numao A, et al. Snoring is associated with an impaired motor function, disease severity and the quality of life but not with excessive daytime sleepiness in patients with Parkinson's disease. Intern Med. 2013;52(8):863–869. doi: 10.2169/internalmedicine.52.9083. [DOI] [PubMed] [Google Scholar]
- [303].Shpirer I, Miniovitz A, Klein C, Goldstein R, Prokhorov T, Theitler J, et al. Excessive daytime sleepiness in patients with Parkinson's disease: a polysomnography study. Mov Disord. 2006 Sep;21(9):1432–1438. doi: 10.1002/mds.21002. [DOI] [PubMed] [Google Scholar]
- [304].Viallet F, Pitel S, Lancrenon S, Blin O. Evaluation of the safety and tolerability of rasagiline in the treatment of the early stages of Parkinson's disease. Curr Med Res Opin. 2013 Jan;29(1):23–31. doi: 10.1185/03007995.2012.752351. [DOI] [PubMed] [Google Scholar]
- [305].Parkinson Study Group CALM Cohort Investigators Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol. 2009 May;66(5):563–570. doi: 10.1001/archneur.66.1.nct90001. [DOI] [PubMed] [Google Scholar]
- [306].Baba Y, Higuchi MA, Fukuyama K, Abe H, Uehara Y, Inoue T, et al. Effect of chronic kidney disease on excessive daytime sleepiness in Parkinson disease. Eur J Neurol. 2011 Nov;18(11):1299–1303. doi: 10.1111/j.1468-1331.2011.03391.x. [DOI] [PubMed] [Google Scholar]
- [307].Jankovic J, Watts RL, Martin W, Boroojerdi B. Transdermal rotigotine: double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol. 2007 May;64(5):676–682. doi: 10.1001/archneur.64.5.676. [DOI] [PubMed] [Google Scholar]
- [308].Watts RL, Jankovic J, Waters C, Rajput A, Boroojerdi B, Rao J. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology. 2007 Jan 23;68(4):272–276. doi: 10.1212/01.wnl.0000252355.79284.22. [DOI] [PubMed] [Google Scholar]
- [309].Elmer LW, Surmann E, Boroojerdi B, Jankovic J. Long-term safety and tolerability of rotigotine transdermal system in patients with early-stage idiopathic Parkinson's disease: a prospective, open-label extension study. Parkinsonism Relat Disord. 2012 Jun;18(5):488–493. doi: 10.1016/j.parkreldis.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [310].LeWitt PA, Boroojerdi B, Surmann E, Poewe W, SP716 Study Group. SP715 Study Group Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson's disease: results of two open-label extension studies, CLEOPATRA-PD and PREFER. J Neural Transm. 2013 Jul;120(7):1069–1081. doi: 10.1007/s00702-012-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Giladi N, Boroojerdi B, Surmann E. The safety and tolerability of rotigotine transdermal system over a 6-year period in patients with early-stage Parkinson's disease. J Neural Transm. 2013 Sep;120(9):1321–1329. doi: 10.1007/s00702-013-1001-5. [DOI] [PubMed] [Google Scholar]
- [312].Pahwa R, Stacy MA, Factor SA, Lyons KE, Stocchi F, Hersh BP, et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology. 2007 Apr 3;68(14):1108–1115. doi: 10.1212/01.wnl.0000258660.74391.c1. [DOI] [PubMed] [Google Scholar]
- [313].Rektorova I, Balaz M, Svatova J, Zarubova K, Honig I, Dostal V, et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol. 2008 Sep-Oct;31(5):261–266. doi: 10.1097/WNF.0b013e31815d25ce. [DOI] [PubMed] [Google Scholar]
- [314].Dusek P, Buskova J, Ruzicka E, Majerova V, Srp A, Jech R, et al. Effects of ropinirole prolonged-release on sleep disturbances and daytime sleepiness in Parkinson disease. Clin Neuropharmacol. 2010 Jul;33(4):186–190. doi: 10.1097/WNF.0b013e3181e71166. [DOI] [PubMed] [Google Scholar]
- [315].Pahwa R, Koller WC, Trosch RM, Sherry JH, APO303 Study Investigators Subcutaneous apomorphine in patients with advanced Parkinson's disease: a dose-escalation study with randomized, double-blind, placebo-controlled crossover evaluation of a single dose. J Neurol Sci. 2007 Jul 15;258(1-2):137–143. doi: 10.1016/j.jns.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [316].Hauser RA, Silver D, Choudhry A, Eyal E, Isaacson S, ANDANTE study investigators Randomized, controlled trial of rasagiline as an add-on to dopamine agonists in Parkinson's disease. Mov Disord. 2014 Jul;29(8):1028–1034. doi: 10.1002/mds.25877. [DOI] [PubMed] [Google Scholar]
- [317].Koller W, Guarnieri M, Hubble J, Rabinowicz AL, Silver D. An open-label evaluation of the tolerability and safety of Stalevo (carbidopa, levodopa and entacapone) in Parkinson's disease patients experiencing wearing-off. J Neural Transm. 2005 Feb;112(2):221–230. doi: 10.1007/s00702-004-0184-1. [DOI] [PubMed] [Google Scholar]
- [318].Eggert K, Ohlwein C, Kassubek J, Wolz M, Kupsch A, Ceballos-Baumann A, et al. Influence of the nonergot dopamine agonist piribedil on vigilance in patients With Parkinson Disease and excessive daytime sleepiness (PiViCog-PD): an 11-week randomized comparison trial against pramipexole and ropinirole. Clin Neuropharmacol. 2014 Jul-Aug;37(4):116–122. doi: 10.1097/WNF.0000000000000041. [DOI] [PubMed] [Google Scholar]
- [319].Avorn J, Schneeweiss S, Sudarsky LR, Benner J, Kiyota Y, Levin R, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol. 2005 Aug;62(8):1242–1248. doi: 10.1001/archneur.62.8.1242. [DOI] [PubMed] [Google Scholar]
- [320].Oved D, Ziv I, Treves TA, Paleacu D, Melamed E, Djaldetti R. Effect of dopamine agonists on fatigue and somnolence in Parkinson's disease. Mov Disord. 2006 Aug;21(8):1257–1261. doi: 10.1002/mds.20929. [DOI] [PubMed] [Google Scholar]
- [321].Kotschet K, Johnson W, McGregor S, Kettlewell J, Kyoong A, O'Driscoll DM, et al. Daytime sleep in Parkinson's disease measured by episodes of immobility. Parkinsonism Relat Disord. 2014 Jun;20(6):578–583. doi: 10.1016/j.parkreldis.2014.02.011. [DOI] [PubMed] [Google Scholar]
- [322].Stefansdottir S, Gjerstad MD, Tysnes OB, Larsen JP. Subjective sleep problems in patients with early Parkinson's disease. Eur J Neurol. 2012 Dec;19(12):1575–1581. doi: 10.1111/j.1468-1331.2012.03791.x. [DOI] [PubMed] [Google Scholar]
- [323].Matsui H, Nishinaka K, Oda M, Hara N, Komatsu K, Kubori T, et al. Excessive daytime sleepiness in Parkinson disease: a SPECT study. Sleep. 2006 Jul 1;29(7):917–920. doi: 10.1093/sleep/29.7.917. [DOI] [PubMed] [Google Scholar]
- [324].Matsui H, Nishinaka K, Oda M, Niikawa H, Komatsu K, Kubori T, et al. Disruptions of the fornix fiber in Parkinsonian patients with excessive daytime sleepiness. Parkinsonism Relat Disord. 2006 Jun;12(5):319–322. doi: 10.1016/j.parkreldis.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [325].Kato S, Watanabe H, Senda J, Hirayama M, Ito M, Atsuta N, et al. Widespread cortical and subcortical brain atrophy in Parkinson's disease with excessive daytime sleepiness. J Neurol. 2012 Feb;259(2):318–326. doi: 10.1007/s00415-011-6187-6. [DOI] [PubMed] [Google Scholar]
- [326].Rissling I, Frauscher B, Kronenberg F, Tafti M, Stiasny-Kolster K, Robyr AC, et al. Daytime sleepiness and the COMT val158met polymorphism in patients with Parkinson disease. Sleep. 2006 Jan;29(1):108–111. [PubMed] [Google Scholar]
- [327].Visser M, van Rooden SM, Verbaan D, Marinus J, Stiggelbout AM, van Hilten JJ. A comprehensive model of health-related quality of life in Parkinson's disease. J Neurol. 2008 Oct;255(10):1580–1587. doi: 10.1007/s00415-008-0994-4. [DOI] [PubMed] [Google Scholar]
- [328].Ozdilek B, Gunal DI. Motor and non-motor symptoms in Turkish patients with Parkinson's disease affecting family caregiver burden and quality of life. J Neuropsychiatry Clin Neurosci. 2012 Fall;24(4):478–483. doi: 10.1176/appi.neuropsych.11100315. [DOI] [PubMed] [Google Scholar]
- [329].Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Driving with distraction in Parkinson disease. Neurology. 2006 Nov 28;67(10):1774–1780. doi: 10.1212/01.wnl.0000245086.32787.61. [DOI] [PubMed] [Google Scholar]
- [330].Naismith SL, Terpening Z, Shine JM, Lewis SJ. Neuropsychological functioning in Parkinson's disease: Differential relationships with self-reported sleep-wake disturbances. Mov Disord. 2011 Jul;26(8):1537–1541. doi: 10.1002/mds.23640. [DOI] [PubMed] [Google Scholar]
- [331].Crizzle AM, Myers AM, Roy EA, Almeida QJ. Drivers with Parkinson's disease: are the symptoms of PD associated with restricted driving practices? J Neurol. 2013 Oct;260(10):2562–2568. doi: 10.1007/s00415-013-7017-9. [DOI] [PubMed] [Google Scholar]
- [332].Sherif E, Valko PO, Overeem S, Baumann CR. Sleep benefit in Parkinson's disease is associated with short sleep times. Parkinsonism Relat Disord. 2014 Jan;20(1):116–118. doi: 10.1016/j.parkreldis.2013.09.005. [DOI] [PubMed] [Google Scholar]
- [333].Bliwise DL, Trotti LM, Juncos JJ, Factor SA, Freeman A, Rye DB. Daytime REM sleep in Parkinson's disease. Parkinsonism Relat Disord. 2013 Jan;19(1):101–103. doi: 10.1016/j.parkreldis.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Bolitho SJ, Naismith SL, Salahuddin P, Terpening Z, Grunstein RR, Lewis SJ. Objective measurement of daytime napping, cognitive dysfunction and subjective sleepiness in Parkinson's disease. PLoS One. 2013 Nov 21;8(11):e81233. doi: 10.1371/journal.pone.0081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry. 2005 Dec;76(12):1636–1639. doi: 10.1136/jnnp.2005.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Ondo WG, Shinawi L, Davidson A, Lai D. Memantine for non-motor features of Parkinson's disease: a double-blind placebo controlled exploratory pilot trial. Parkinsonism Relat Disord. 2011 Mar;17(3):156–159. doi: 10.1016/j.parkreldis.2010.12.003. [DOI] [PubMed] [Google Scholar]
- [337].Paus S, Schmitz-Hubsch T, Wullner U, Vogel A, Klockgether T, Abele M. Bright light therapy in Parkinson's disease: a pilot study. Mov Disord. 2007 Jul 30;22(10):1495–1498. doi: 10.1002/mds.21542. [DOI] [PubMed] [Google Scholar]
- [338].Lyons KE, Pahwa R. Effects of bilateral subthalamic nucleus stimulation on sleep, daytime sleepiness, and early morning dystonia in patients with Parkinson disease. J Neurosurg. 2006 Apr;104(4):502–505. doi: 10.3171/jns.2006.104.4.502. [DOI] [PubMed] [Google Scholar]
- [339].Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson's disease. Sleep Med. 2005 Sep;6(5):459–466. doi: 10.1016/j.sleep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- [340].Weintraub D, Mavandadi S, Mamikonyan E, Siderowf AD, Duda JE, Hurtig HI, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010 Aug 3;75(5):448–455. doi: 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012 Aug 14;79(7):651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014 Mar;15(3):342–347. doi: 10.1016/j.sleep.2013.10.016. [DOI] [PubMed] [Google Scholar]
- [343].Videnovic A, Lazar AS, Barker RA, Overeem S. 'The clocks that time us'--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014 Dec;10(12):683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014 May;71(5):589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Zhong G, Bolitho S, Grunstein R, Naismith SL, Lewis SJ. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson's disease. PLoS One. 2013 Aug 21;8(8):e72661. doi: 10.1371/journal.pone.0072661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA. Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson's disease. J Neuroendocrinol. 2011 Jun;23(6):519–524. doi: 10.1111/j.1365-2826.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- [347].Oh YS, Kim JS, Park IS, Song IU, Son YM, Park JW, et al. Association between nocturnal/supine hypertension and restless legs syndrome in patients with Parkinson's disease. J Neurol Sci. 2014 Sep 15;344(1-2):186–189. doi: 10.1016/j.jns.2014.06.056. [DOI] [PubMed] [Google Scholar]
- [348].Ejaz AA, Sekhon IS, Munjal S. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson's disease. Eur J Intern Med. 2006 Oct;17(6):417–420. doi: 10.1016/j.ejim.2006.02.020. [DOI] [PubMed] [Google Scholar]
- [349].Pilleri M, Levedianos G, Weis L, Gasparoli E, Facchini S, Biundo R, et al. Heart rate circadian profile in the differential diagnosis between Parkinson disease and multiple system atrophy. Parkinsonism Relat Disord. 2014 Feb;20(2):217–221. doi: 10.1016/j.parkreldis.2013.11.006. [DOI] [PubMed] [Google Scholar]
- [350].Harada T, Ishizaki F, Tachiki N, Tomoko M, Horie N, Noriko T, et al. Circadian rhythm of cardiovascular autonomic function in Parkinson's disease and multiple system atrophy. International Medical Journal. 2005;12(1):37–39. [Google Scholar]
- [351].Harada T, Ishizaki F, Nitta Y, Yamada T, Horie N, Hamada M, et al. Circadian rhythm of cardiovascular autonomic nervous function in Parkinson's disease, early-onset Parkinsonism and Multiple System Atrophy. International Medical Journal. 2006;13(2):131–133. [Google Scholar]
- [352].Whitehead DL, Davies AD, Playfer JR, Turnbull CJ. Circadian rest-activity rhythm is altered in Parkinson's disease patients with hallucinations. Mov Disord. 2008 Jun 15;23(8):1137–1145. doi: 10.1002/mds.22057. [DOI] [PubMed] [Google Scholar]
- [353].Kotagal V, Albin RL, Muller ML, Koeppe RA, Chervin RD, Frey KA, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012 Apr;71(4):560–568. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Nardone R, Bergmann J, Brigo F, Christova M, Kunz A, Seidl M, et al. Functional evaluation of central cholinergic circuits in patients with Parkinson's disease and REM sleep behavior disorder: a TMS study. J Neural Transm. 2013 Mar;120(3):413–422. doi: 10.1007/s00702-012-0888-6. [DOI] [PubMed] [Google Scholar]
- [355].Stavitsky K, McNamara P, Durso R, Harris E, Auerbach S, Cronin-Golomb A. Hallucinations, dreaming, and frequent dozing in Parkinson disease: impact of right-hemisphere neural networks. Cogn Behav Neurol. 2008 Sep;21(3):143–149. doi: 10.1097/WNN.0b013e318185e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.