Abstract
Many psychological theories posit foundational links between two fundamental constructs: (1) our ability to produce, perceive, and represent action; and (2) our ability to understand the meaning and motivation behind the action (i.e. Theory of Mind; ToM). This position is contentious, however, and long-standing competing theories of social-cognitive development debate roles for basic action-processing in ToM. Developmental research is key to investigating these hypotheses, but whether individual differences in neural and behavioral measures of motor action relate to social-cognitive development is unknown. We examined 3- to 5-year-old children’s (N = 26) EEG mu-desynchronization during production of object-directed action, and explored associations between mu-desynchronization and children’s behavioral motor skills, behavioral action-representation abilities, and behavioral ToM. For children with high (but not low) mu-desynchronization, motor skill related to action-representation abilities, and action-representation mediated relations between motor skill and ToM. Results demonstrate novel foundational links between action-processing and ToM, suggesting that basic motor action may be a key mechanism for social-cognitive development, thus shedding light on the origins and emergence of higher social cognition.
Introduction
Theory of Mind (ToM) – understanding that internal mental states such as beliefs, desires, intentions, and knowledge motivate outward action and interaction – is a cornerstone of social cognition, and has been the focus of decades of research across a remarkable breadth of fields. Yet, core questions about factors and processes supporting ToM and its development are debated, and remain unclear. In a comprehensive examination of ToM development in early childhood, the present study brings together measures of executive functioning (EF) and verbal intelligence (long-established correlates of ToM; see Devine & Hughes, 2014; Milligan, Astington & Dack, 2007, for meta-analyses) with measures of basic motor action. We demonstrate novel foundational links between individual differences in ToM and both behavioral and neural measures of action – beyond influence of EF and verbal intelligence – to shed new light on potential origins and developmental mechanisms of higher social cognition.
Theoretical links between action-processing and ToM
Theories in both classic and current literature posit links between action and ToM. Despite some differences, they converge on the position that the neural and/or mental processes involved in planning, executing, and perceiving basic bodily movements – referred to broadly in this manuscript as ‘action processes’ or ‘action-processing’ – contribute to understanding mental states.
To illustrate, several positions emphasize the role of internal representation of action in developing mental-state understanding. We define action-representation as the internal mental process of recreating some semblance of a perceived or executed action, in the absence of any real-time perception or execution. Some researchers argue that mental-state understanding evolved from a neural system for detecting and representing actions (Blakemore & Decety, 2001; Frith & Frith, 1999). In addition, proponents of ‘simulation’ accounts of mental-state understanding posit that others’ actions are mapped onto internal representations – or simulations – of one’s own actions. It is hypothesized that these simulations, by way of connection to one’s own mental states, can then guide inference about the mental states motivating others’ actions (e.g. Goldman, 1992; Gordon, 1996; Decety & Grèzes, 2006; Gallese & Sinigaglia, 2011).
Other theoretical positions linking action-processes and ToM emphasize an additional component beyond action-perception and action-representation: action-production – defined here as the execution of one’s own actions. Hunnius and Bekkering (2014) argue that infants’ action-production experience provides multifaceted representations of actions and their consequences, which lead to associations between actions and outcomes that can then be used to understand and predict others’ actions. Woodward (2013; Woodward & Gerson, 2014) also argues that infants’ action-representations are derived from action-production, and that together action-production and representation support developing understanding of the intentions and goals motivating action, particularly when infants experience their own and others’ goal-directed actions in co-occurrence. These ideas are echoed by Meltzoff (e.g. 2002, 2007, 2013) who proposes that ToM is jump-started through (1) initial detection of equivalences between perceived and produced actions, (2) developments in producing one’s own actions as motivated by one’s own mental states, and (3) the ability to represent others’ actions similarly to one’s own. In brief, when infants see others acting ‘like me’ (i.e. like the infant themself), they project that others have mental experiences also like their own. Theories of ‘embodied social cognition’ highlight similar themes and posit that social understanding arises from the production and mutual perception of body actions and their outcomes (e.g. Daum, Sommerville & Prinz, 2009).
However, these theoretical positions require substantiation with empirical research, especially considering that not all theories of ToM development emphasize a foundational role of basic action-processing. Indeed, other theories emphasize, for example, innate cortical modules dedicated specifically to mental-state reasoning (e.g. Scholl & Leslie, 1999), or naïve conceptual theories of how and why humans generally behave the way they do (e.g. Gopnik & Wellman, 1992, 2012), or advancements in general EF and inhibitory control (e.g. Carlson & Moses, 2001; Devine & Hughes, 2014) as alternative mechanisms supporting ToM development. Empirical research is therefore critical to substantiate action-processing as a key developmental construct that contributes to ToM development.
Behavioral relations between action-processing and ToM
Behavioral research in infancy provides some support for theories linking the emergence of mental-state understanding to action-processing (see Hunnius & Bekkering, 2014; Woodward, 2013, for reviews). Indeed, infants’ action-production experience is associated with understanding the goals and intentions behind others’ actions (e.g. Ambrosini, Reddy, de Looper, Costantini, Lopez et al., 2013; Brune & Woodward, 2007; Cannon, Woodward, Gredebäck, von Hofsten & Turek, 2012; Woodward & Guajardo, 2002; Kanakogi & Itakura, 2011; Gerson & Woodward, 2014; Sommerville, Hildebrand & Crane, 2008; Sommerville, Woodward & Needham, 2005). However, relations between action-processing and detection of intentions and goals in infancy do not provide clear evidence that action-processing facilitates development of the complex mental-state understanding characteristic of ToM. Developments in children’s explicit understanding (i.e. as measured with pointing or verbal response) of beliefs, desires, knowledge, and emotions are manifest in the preschool years, over roughly 3 to 5 years old (Wellman, Cross & Watson, 2001; Wellman & Liu, 2004). Thus, investigating how behavioral developments in action-processing relate to preschoolers’ developments in these advanced, explicit mental-state understandings is needed to provide direct evidence that action-processing contributes to developing higher social cognition and a fully-fledged ToM.
Mu-rhythm as a neural mechanism for linking action-processing with ToM development
A specific neural mechanism facilitating links between action and ToM, present in early life, would further support the notion that action-processing is important for ToM development. One possible neural mechanism may be captured in the electroencephalogram (EEG) mu-rhythm. In both adults and infants/children, mu-rhythm reflects EEG oscillations in ‘alpha’ frequency bands (~8–13 Hz in adults, ~6–9 Hz in infants/children) that desynchronize (i.e. decrease in spectral power relative to a resting/baseline period) during an action-production event (e.g. voluntary hand movement) (Muthukumaraswamy & Johnson 2004; Pfurtscheller, Neuper, Andrew & Edlinger, 1997). The analyses used to identify mu-rhythm are often referred to as event-related desynchronization (ERD) analyses. For mu-rhythm, desynchronization often occurs maximally over central scalp locations overlying sensorimotor cortex (Kuhlman, 1978), suggesting that it may index sensorimotor cortical activation (e.g. Leocani, Toro, Manganotti, Zhuang & Hallett, 1997; Toro, Deuschl, Thatcher, Sato, Kufta et al., 1994). Indeed, source localization studies have identified source estimates of mu-rhythm concentrated in sensorimotor areas (Hari, Salmelin, Mäkelä, Salenius & Helle, 1997; Salmelin & Hari, 1994a, 1994b; Thorpe, Cannon & Fox, 2016).
Though most robustly defined during action-production, mu-desynchronization also occurs during action-perception (see Fox, Bakermans-Kranenburg, Yoo, Bowman, Cannon et al., 2016, for meta-analysis; Marshall & Meltzoff, 2011). Given these dual properties, some researchers reason that mu-rhythm reflects activity associated with not only producing action, but also internally representing action in the absence of production (Muthukumaraswamy & Johnson, 2004; Lepage & Théoret, 2006; Marshall & Meltzoff, 2011). Given that both action-production and action-representation have been posited to support development of mental-state understanding as outlined above (e.g. Meltzoff, 2002; Woodward, 2013; Decety & Grèzes, 2006), it is possible that a neural system supporting both of these functions could constitute a specific neural mechanism facilitating links between action-processing and ToM (see e.g. Marshall & Meltzoff, 2011, 2014).
Two sets of evidence are needed to support this possibility: (1) evidence that mu-desynchronization is associated with both action-production and action-representation in early life, and (2) evidence that mu-desynchronization is associated with ToM. There is some initial indirect support for this first set of evidence (see Cuevas, Cannon, Yoo & Fox, 2014; Marshall & Meltzoff, 2011, 2014, for reviews). Specifically, infants’ mu-desynchronization is clearly associated with action-production. It occurs when infants produce object-directed grasps (e.g. Southgate, Johnson, Osborne & Csibra, 2009; Marshall, Young & Meltzoff, 2011), and the magnitude of mu-desynchronization during infants’ action-perception relates to their experience or proficiency in action-production (Cannon, Simpson, Fox, Vanderwert, Woodward et al., 2016; Gerson, Bekkering & Hunnius, 2015; Upshaw, Bernier & Sommerville, 2016; Van Elk, van Schie, Hunnius, Vesper & Bekkering, 2008; Virji-Babul, Rose, Moiseeva & Makan, 2012; Warreyn, Ruysschaert, Wiesema, Handl, Pattyn et al., 2013). Some evidence suggests that mu-rhythm may also be somatatopically organized by 14 months of age (Marshall, Saby & Meltzoff, 2013; Saby, Meltzoff & Marshall 2013).
Support for associations between mu-desynchronization and action-representation comes from findings that, similar to action-production, infants’ mu-rhythm desynchronizes during mere perception of action, both in visual and auditory domains (e.g. Southgate et al., 2009; Marshall et al., 2011, during visual action perception; Gerson et al., 2015; Paulus, Hunnius, van Elk & Bekkering, 2012, during auditory perception of action-related sounds). This similar desynchronization across production and perception conditions has led researchers to argue that mu-desynchronization during action-perception reflects activation of the same sensorimotor neural system supporting action-production, and thus mu-rhythm indexes cortical representation of sensorimotor action even in the absence of actual production (Muthukumaraswamy & Johnson, 2004; Lepage & Théoret, 2006). In brief, a common conceptualization of mu-rhythm is that it reflects activity of a single sensorimotor neural system that is activated during both action-production and action-representation.
However, the idea that mu-desynchronization during action-perception reflects internal representation of sensorimotor action is difficult to verify because it rests on the assumption that the similarity in mu-desynchronization across production and perception conditions indicates activation of precisely the same sensorimotor neural system. This assumption is potentially problematic given that signals emanating from their source neural populations are volume conducted to the scalp surface, and thus spatial resolution of the EEG is low. Moreover, other conceptualizations of mu-rhythm posit that it reflects a dynamic, integrated system in which multiple neural networks of different functions are coupled and entrained (Pineda, 2005; Thorpe et al., 2016), and researchers caution against an exclusive emphasis on sensorimotor functions (Marshall & Meltzoff, 2014). Thus, global similarities in mu-rhythm desynchronization across action-production and action-perception conditions may not necessarily reflect activation of precisely the same parts of a potentially more complex underlying neural network. Further clarity on mu-rhythm function is needed.
More generally, investigations of infant mu-rhythm do not necessarily translate to an understanding of mu-rhythm function in early childhood, when key advancements in explicit ToM are manifest (Wellman et al., 2001; Wellman & Liu, 2004). To date there is no direct evidence examining relations between mu-rhythm and explicit ToM in preschool children. These examinations are critical for investigating whether mu-rhythm and action-processing (i.e. production and/or representation processes) may serve as a neural mechanism facilitating ToM development.
The present study
The present study aims to shed light on links between ToM and action-processing, and the possibility that mu-rhythm reflects a neural mechanism supporting this link. We examined relations between individual differences in ToM, and neural and behavioral measures of action-processing, in 3- to 5-year-old typically developing children. Guided by existing theories that posit both action-production and action-representation as core features supporting social-cognitive development (e.g. Meltzoff, 2002; 2013; Woodward, 2013; Decety & Grèzes, 2006), we assessed children’s behavioral skills in producing simple motor acts (using the motor skill test from the Movement Assessment Battery for Children; MABC; Henderson & Sugden, 1992), as well as their action-representation skills (using a novel task requiring mental representation of object-directed hand actions in the absence of either direct perception or production of action). We also assessed explicit ToM reasoning (using the Wellman & Liu, 2004, ToM scale and standard location-change false-belief tasks; Wimmer & Perner, 1983). In addition to these behavioral measures, we recorded children’s EEG activity during perception and production of simple object-directed grasping actions, to examine mu-desynchronization. This battery of assessments was designed to address several outstanding issues that currently create difficulty in evaluating links between ToM and action-processing.
First, behavioral measures of action-processes can help clarify mu-rhythm function, and in particular its hypothesized association with action-representation per se. Clarity on mu-rhythm function has important implications for the field more broadly, and is central to the present study given the hypothesized role of action-representation in ToM development (e.g. Woodward, 2013; Meltzoff, 2002; Decety & Grèzes, 2006) and the possibility that mu-rhythm reflects a neural mechanism facilitating links between action-processes and ToM. The addition of behavioral measures side-steps the issue of interpreting similarities in the EEG signal across action-production and action-perception conditions (which can be difficult to verify given volume conduction and low spatial resolution of ERD analyses). Specifically, mu-desynchronization in a single condition can be correlated with behavioral measures of both action-production and action-representation, as well as with other cognitive-behavioral measures (e.g. ToM, EF), to reveal potentially complex relations among neural and behavioral action constructs that may not be detectable in the EEG alone.
To guide our hypotheses for how mu-desynchronization might relate to behavioral measures of action-production and action-representation, we used a more complex conceptualization of mu-rhythm function (given recent caution against the conceptualization of mu-rhythm as reflecting activity in a single sensorimotor system; e.g. Marshall & Meltzoff, 2014). This more complex conceptualization posits that mu-rhythm reflects a dynamic and integrative network in which multiple neural systems of different specialized functions are interconnected (Pineda, 2005; Thorpe et al., 2016). We hypothesized that individual differences in mu-desynchronization may therefore reflect the extent to which systems supporting action-production and action-representation are interconnected into a common integrated network. Support for this ‘integrated network’ conceptualization would come from finding that mu-desynchronization moderates the relation between action-production and action-representation, rather than correlating directly with either construct (as would be predicted by the simplified ‘single sensorimotor system’ conceptualization).
Second, we examined relations between individual differences in ToM, and behavioral measures of action-production and action-representation. This examination provides the first direct assessment of links between action-processing and the explicit mental-state understanding evident in preschool years. We hypothesized that ToM performance would correlate with action-representation, but not necessarily with action-production. In infancy, intention-understanding is associated with acquisition of entirely new motor production skills (e.g. Sommerville et al., 2005), whereas individual differences in the fine-tuning of existing motor skills measured in early childhood (Manoel & Conolly, 1998) may not directly relate to ToM performance.
Third, to shed light on the possibility that mu-rhythm reflects a neural mechanism supporting links between ToM and action-processing, we explored relations between individual differences in mu-desynchronization and children’s ToM performance to gain insight into how such a neural mechanism may facilitate links between action and mental-state understanding.
Finally, to assess unique relations between behavioral and neural action constructs and ToM specifically, we also assessed children’s EF and verbal intelligence to use as covariates in all analyses given robust associations between ToM and each of these additional constructs (see Devine & Hughes, 2014; Milligan et al., 2007, for meta-analyses).
Methods
Participants
Forty-four children 3 to 5 years old (22 males, Mage = 55.75 months, SD = 6.93, range = 45–68 months) underwent EEG recording and behavioral testing. One child did not complete all behavioral tasks and was excluded from behavioral analyses. Seventeen additional children were excluded from EEG analyses: Three were left-handed (our EEG task required grasping toys with right hand only), and 14 did not provide more than 50% (i.e. ≥7/12 trials) useable (free of motion artifact) EEG data (in line with commonly reported attrition rates of 20–45% in preschool data due to excessive motion artifact; Bell & Cuevas, 2012) (see Supplemental Material for details on artifact detection and rejection processes). Our final sample consisted of 26 children (14 males, Mage = 55.98 months, SD = 7.42, range = 45–68 months; see Supplemental Material for detailed sample demographics). All included children had normal or corrected-to-normal vision, no history of neuropsychological disorders or trauma, and were born within 3 weeks of their original due-date, by parental report. Excluded children did not differ from included children on any demographic or behavioral measures (all ps > .14).
Measures
Behavioral assessments
Behavioral tasks were administered after EEG recording (see Table S1 for order). Descriptions of individual tasks for the ToM and EF batteries can be found in supporting information available online.
ToM battery
Diverse-desires, diverse-beliefs, knowledge-access, contents false-belief and explicit false-belief tasks from the Wellman and Liu (2004) scale were used, supplemented by two changed-location false-belief tasks (Wimmer & Perner, 1983). All tasks included warm-up and/or control questions to ensure that children correctly understood the task. Children received one point for every task they passed, thus higher scores indicate greater ToM understanding.
EF battery
Three EF tasks assessed cognitive flexibility and conflict-inhibition. Children completed grass-snow stroop (Carlson & Moses, 2001), dimensional-change card-sort (DCCS) (Zelazo, 2006), and less-is-more (Carlson, Davis & Leach, 2005). All tasks included warm-up and/or control questions to ensure that children correctly understood the task. Scores were either proportion/percent correct or sum correct on target, thus higher scores indicate greater EF skills.
Kaufman Brief Intelligence Test II (KBIT-2): Verbal intelligence (Kaufman & Kaufman, 1990)
The verbal intelligence test from the KBIT-2 (Kaufman & Kaufman, 1990) required selecting the picture correctly demonstrating a general fact or meaning of a target word. Higher scores indicate greater verbal intelligence.
Motor skill task
The posting coins test of motor skill (Henderson & Sugden, 1992) assessed children’s behavioral action-execution ability. Children were timed as they placed 12 coins as fast as they could into a slotted box. Shorter times indicate greater motor coordination and manual dexterity.
Action-representation task
This task assessed children’s ability to mentally represent different hand positions and object-directed hand actions. Correct responses on each trial required understanding how both body orientation (orientation of the hand in relation to the object) and grasping position (relative positions of fingers, palm, and wrist) should be optimized to grasp differently shaped and oriented handles (see Figure 1 for example stimulus). Critically, hand positions/orientations and handles were shown as static two-dimensional images. Thus, the task required representation of action in the absence of either observation or execution of action itself. For each item (eight total), children were told to pick the hand that was best shaped to grab the handle (only one option was correct). Higher scores indicate greater ability to mentally represent hand actions in the absence of action-execution or action-observation (see supporting information for scoring details). All trials were validated on a sample of 10 adults (sample achieved 100% accuracy).
Figure 1.
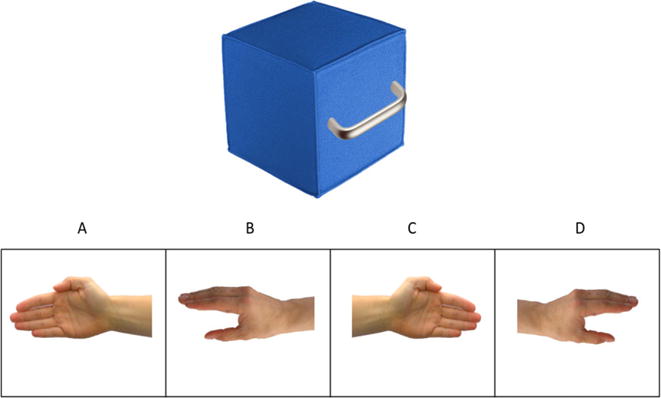
Example trial stimulus for action-representation task.
EEG grasp task
Children’s EEG was recorded while they grasped a small toy. Details of this task are also described in Thorpe et al. (2016) in which EEG data from a subset of the current sample were analyzed in a larger study, without connection to behavioral data. Children sat in front of a stage with an opaque curtain. The curtain was raised/lowered at the onset/offset of each trial (~1.5 second duration). Video-recording captured children’s grasping behavior, coded to identify EEG associated with each child’s exact point of grasping contact with the toy. Trials consisted of a static baseline interval (3 seconds; participants viewed black two-dimensional shapes on a white background), followed by an execution interval (ranged from 5 to 15 seconds depending on how quickly the child reached; a hidden experimenter pushed the top of the mobile stage towards the participant, and the participant picked up the toy). Participants were instructed to pick up the toy with their right (dominant) hand only (occasional left-hand execution trials were excluded from analyses).
Across trials, ten unique baseline shapes and toys were presented in unique orders, randomized across subjects. On 50% of trials (24 trials total), baseline was followed by an observation interval (~ 4 seconds; participants viewed a live female experimenter grasp a toy with her right hand); observation trials were interspersed throughout the task, randomly across participants, with the stipulation that the same interval type (i.e. execution or observation) occurred no more than twice consecutively. After every two to three trials, children played with stickers to prevent fatigue (average duration 1–2 minutes; five breaks total). The EEG net was not removed during breaks though no EEG was recorded. Total duration of the task including breaks and transitions was approximately 20–30 minutes.
Electrophysiological recording and analyses
EEG was recorded continuously at 500 Hz using a 64-channel Hydrocel Geodesic Sensor Net (Electrical Geodesic Inc., Eugene, OR) – a network of 64 Ag⁄AgCl electrodes embedded in an elastic geodesic tension structure. Impedance for all electrodes was kept below 100 KΩ. Signals were referenced to the vertex (Cz) during recording.
Details of EEG processing and analytic procedures can be found in Thorpe et al. (2016) (see also Supplemental Material). Continuous EEG data from each participant were first baseline-corrected, linear-detrended, and re-referenced to the average reference. Artifacts associated with body movement were edited using a thresholding procedure to remove high amplitude waveforms. Blinks/eye movements and net displacement over the front of the head were also identified and rejected using independent components analysis (Hyvärinen, 1999). EEG data were then reconstructed in channel space from the remaining set of clean components (components removed for each subject: M = 8.14, SD = 1.67). After pre-processing and artifact rejection, children on average contributed 10 trials (SD = 1.09) of artifact-free EEG data for analyses. This number is in line with current developmental studies on mu-rhythm desynchronization (e.g. Cannon et al., 2016; Yoo, Cannon, Thorpe & Fox, 2015), and with pediatric event-related desynchronization studies more generally (e.g. Cuevas, Raj & Bell, 2012).
Segmentation and parameters for ERD analysis
Resultant data were segmented into epochs containing a baseline interval (baseline/reference for ERD analyses) and its corresponding (subsequent) grasping interval (event of interest for ERD analyses) for both ‘observe trials’ (experimenter grasps object) and ‘execute trials’ (child grasps object). The grasping interval was identified for each trial from video recorded during task (coded frame-by-frame by two independent coders who achieved agreement within ~100 ms on 90% of trials) and consisted of the 2-second interval centered on the grasp-completion event (i.e. point at which child’s/ experimenter’s hand first touched the toy in an act resulting in a completed grasp), capturing 1 second pre and post grasp. This interval reasonably captured both the initiation of the voluntary grasping action from the reach towards the object (approx. mean duration of children’s initiation of reach to point of grasp = 1.33 seconds, SD = 0.92) as well as the completion of the voluntary grasping action ending with picking the object up off the table and retracting it towards the body (approx. mean duration of children’s point of grasp to completed retraction towards body = 1.34 seconds, SD = 0.83). The baseline interval consisted of the 2-second interval beginning 0.5 s after the static baseline image was shown. Only segments in which the baseline interval was free of any participant hand/arm motion were included in ERD analyses to ensure that all epochs consisted of clear grasping action referenced to clean, non-grasping baseline.
ERD scores were calculated across participants for each segment in 50 0.5 Hz bins from 0 to 25 Hz, at all channels (10(log10(Event EEG Power/Baseline EEG Power). The ‘observe trials’ did not yield desynchronization that was reliably significantly different from zero in clear and recognizable topographic patterns indicative of mu-rhythm. Given that a central aim of the study was to examine the possibility that mu-rhythm desynchronization reflects a neural mechanism supporting links between action and ToM, it was critical that we had confidence that EEG desynchronization reliably constituted the mu-rhythm. Thus, we did not continue analyses with ‘observe trials’. ‘Execute trials’ did yield clear and reliable mu-rhythm desynchronization (in line with a recent meta-analysis demonstrating stronger and topographically specific mu-desynchronization during action-execution versus action-observation conditions; Fox et al., 2016). Thus, analyses were concentrated on these ‘execute trials’.
For ‘execute trials’, the mu frequency band of interest was identified as 8–10 Hz: this band emerged from empirical bootstrap distributions as that which contained peak desynchronization (maximal negative values) across all channels (see Thorpe et al., 2016, for details). The ERD scores within this mu band were then averaged across all right-hand grasp trials to obtain an average ERD score for each participant, in each channel, that reflected desynchronization in the 8–10 Hz mu band during right-hand, object-directed grasp-execution.
Results
Preliminary analyses
Behavioral data
Reliable EF and ToM composites were created from individual tasks in the batteries, and acceptable reliability and validity was found for five items of the novel action-representation task (see supporting information for reliability and validity details). Table 1 shows correlations among all behavioral tasks. Interrelations among age, EF, verbal intelligence, and the action and ToM variables (all rs > .41, ps < .007) confirm the need to control for age, EF, and verbal intelligence in focal analyses.
Table 1.
Correlations among cognitive/behavioral variables and age
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Action-Representation | – | ||||
| 2. Motor Skill | −.33* | – | |||
| 3. ToM | .47** | −.41* | – | ||
| 4. Verbal Intelligence | .26 | −.41** | .48** | – | |
| 5. EF | .29 | −.51** | .53* | .41** | – |
| 6. Age | .10 | −.49** | .47** | .63** | .40* |
| Partial correlations among cognitive/behavioral variables controlling for age | |||||
| 1. Action-Representation | – | ||||
| 2. Motor Skill | −.33* | – | |||
| 3. ToM | .48** | −.24 | – | ||
| 4. Verbal Intelligence | .25 | −.15 | .27* | – | |
| 5. EF | .28 | −.40** | .42** | .23 | |
Notes:
Correlation is significant at p < .05 (two-tailed).
Correlation is significant at p < .01 (two-tailed). N = 43.
EEG data
As shown in the 64-channel topomap in Figure 2A and 2B, two clear, topographically distinct peaks were evident over bilateral central-parietal regions that represent maximal mu-desynchronization during children’s object-directed grasp-execution. From this whole-scalp map, electrode clusters were created corresponding to focal left and right central-parietal regions (Figure 2C). We also created contrast/control clusters in occipital regions, and left and right frontal regions. Repeated-measures ANOVAs revealed no effect of laterality: F(1, 26) = 1.575, p = .221. Figure 3 confirms maximal mu-desynchronization in bilateral central-parietal regions that was significantly greater than desynchronization in both occipital regions (t(26) = 2.184, p = .038) and bilateral frontal regions (t(26) = 2.254, p = .033). Occipital and frontal EEG desynchronization did not differ (t(26) = .482, p = .634). These results demonstrate a clear neural correlate (mu-desynchronization in central-parietal regions) of action-production (object-directed grasping) that was subsequently used in analyses with behavioral data.
Figure 2.
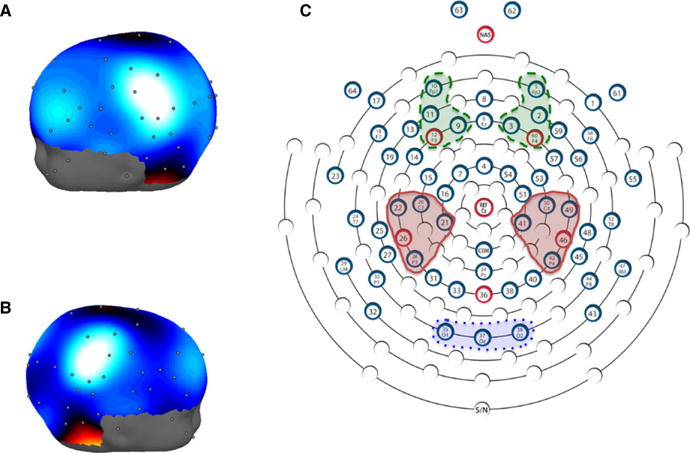
Three-dimensional topomaps overlayed on a 4-year-old child head model (NIH Pediatric MRI Database; University of South Carolina McCausland Brain Imaging Center Neurodevelopmental MRI Database) showing scalp regions of peak EEG desynchronization in the 8 to 10 Hz band (peak central-parietal desynchronization show in light blue/white regions), for left (A) and right (B) side views. Part C shows the clusters of electrodes corresponding to central-parietal peaks (center, solid red), frontal peaks (dashed green, top), and occipital channels (dotted blue, bottom) that were created for analyses.
Figure 3.
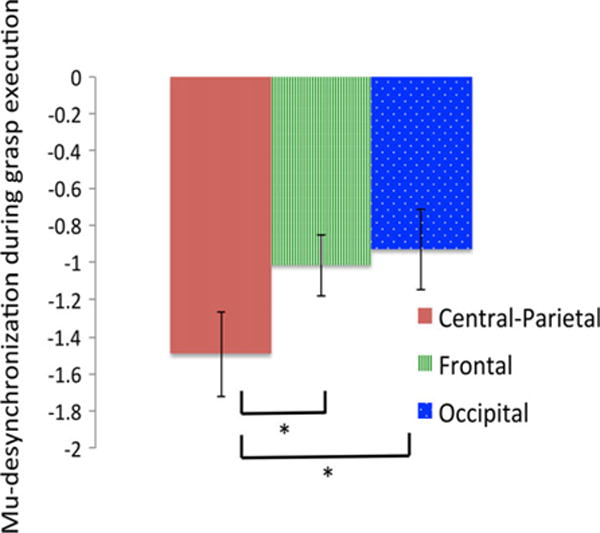
Grand average EEG desynchronization in 8 to 10 Hz band (N = 26) for bilateral central-parietal cluster (solid red, left), bilateral frontal cluster (striped green, middle) and occipital cluster (dotted blue, right). Error bars represent standard errors of the mean.
Relations among behavioral and neural measures of action
Our first focal analysis examined relations among behavioral measures of action-production (i.e. motor skill) and action-representation, and central-parietal mu-desynchronization, as a targeted investigation of proposed action-representation properties of mu. All analyses were repeated with contrast frontal and occipital clusters. To correct for these multiple comparisons, significance criterion was α < .017 (Bonferroni correction α = .05/3).
Correlation analyses and regressions controlling for age, EF, and verbal intelligence indicated that neither motor skill nor action-representation directly related to mu-desynchronization in any of central-parietal, frontal, or occipital regions1 (rs < .23, ps > .13; bs < .050, ts < .85, ps > .41). There was, however, a significant correlation between behavioral motor skill and action-representation that withstood correction for age (Table 1). Given that central-parietal mu-desynchronization exhibited notable variance (range = 4.74, SD = 1.18, variance = 1.402), and given the clear hypotheses that central-parietal mu-desynchronization supports both action-production and action-representation, we tested a more complex relation between central-parietal mu and the behavioral action measures. This model served as direct test of our hypothesis that mu-desynchronization may moderate the relation between motor skill and action-representation, in support of a more complex conceptualization of mu-rhythm as an integrated network supporting multiple interconnected neural systems (e.g. action-production and action-representation) (e.g. Pineda, 2005; Thorpe et al., 2016).
For this moderation analysis, we conducted a hierarchical regression with action-representation as the dependent variable. Age, executive functioning, and verbal intelligence were entered first as covariates to control for domain-general developments, motor skill was entered next as the predictor of interest, and then the moderator of central-parietal mu-desynchronization was entered as a dichotomous variable with high mu-desynchronization (top half of median split) coded as 0 and low mu coded as 1. All of these variables combined accounted for 21.2% of the variance in action-representation, and no entry was associated with any significant change in the model (all ΔR2 < .11, ps > .11).
Critically, when the interaction term between motor skill and mu-desynchronization was entered in the final block, it significantly predicted action-representation (b = .383, SE = .108, p = .002; CI95% [.157, .610]). The interaction term alone accounted for an additional 31.4% of the variance in action-representation (greater than all other variables combined) and yielded a significant change in the model (F(1, 19) = 12.58, p = .002). These results demonstrate a moderating effect of central-parietal mu, in line with our hypothesis. For this final model that examined children at high levels of mu-desynchronization, advances in motor skill significantly predicted advances in action-representation (b = −.413, SE = .101, p < .001; CI95% [−.201, −.624]). In contrast, in a parallel regression examining children at low central-parietal mu (bottom half of median split), motor skill was not associated with action-representation (b = −.029, SE = .049, p = .559; CI95% [.0733, −.132]). Figure 4 shows the overall moderating effect of central-parietal mu on the relation between action-representation and motor skill. When moderation analyses were repeated with central-parietal mu-desynchronization as a continuous moderator, results revealed an identical pattern: The addition of the interaction term between motor skill and continuous mu-desynchronization yielded a significant change in the model (F(1, 19) = 4.94, p = .039), and only children with mu-desynchronization that was at least one standard deviation greater than the mean showed a relation between motor skill and action-representation (b = −.257, SE = .090, p = .010; CI95% [−.444, −.069]); the relation between the two behavioral action constructs was diminished for children at mean mu-desynchronization (b = −.096, SE = .052, p = .082), and absent for children below the mean (b = .065, SE = .089, p = .472). EEG desynchronization in neither frontal nor occipital regions moderated relations between action-representation and motor skill (all ps > .50).
Figure 4.
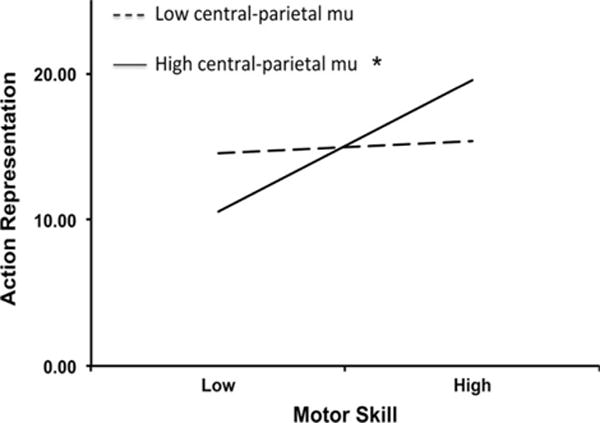
Moderation effect of central-parietal mu-desynchronization (8–10 Hz) on relation between behavioral developments in action-representation ability and motor skill. There is a significant positive relation between action-representation and motor skill performance for children with high central-parietal mu-desynchronization during object-directed grasp (solid line) but not for children with low central-parietal mu desynchronization (dashed line).
Relations among action measures and ToM
Our second set of focal analyses examined relations among behavioral and neural measures of action, and ToM performance. We first targeted relations between ToM and the behavioral measures of motor skill and action-representation in order to take advantage of the larger sample size (N = 43) associated with behavioral measures alone. We conducted a hierarchical regression with ToM as the dependent variable, and age, EF, and verbal intelligence entered first as controls. Motor skill and action-representation were then entered separately as potential predictors of ToM, to directly test our hypothesis that action-production likely would not directly predict individual differences in ToM but that there may be an association between ToM performance and action-representation. Control variables accounted for a significant proportion of the variance in ToM (Table 2). In line with our predictions, the addition of motor skill did not significantly change the model, and did not predict ToM beyond the controls, whereas action-representation accounted for an additional 9.1% variance in ToM and yielded a significant change in the model: (F(1, 37) = 6.457, p = .015). Considering all variables together, action-representation was the only significant predictor of ToM. These results evince behavioral links between ToM-reasoning and action-representation: advances in ToM were associated with advances in action-representation, even beyond variance accounted for by age, and by EF, and verbal intelligence – two core predictors of preschoolers’ explicit ToM (e.g. Devine & Hughes, 2014; Milligan et al., 2007).
Table 2.
Summary of Hierarchical Regression Analysis for Variables Predicting Theory of Mind
| Variable | b (SE) | CI95% | t | R | R2 | ΔR2 |
|---|---|---|---|---|---|---|
| Stage 1 | .618 | .382 | .382* | |||
| Age | .047 (.042) | [−.038, .131] | 1.119 | |||
| EF | .239 (.091) | [.055, .423] | 2.626* | |||
| Verbal Intelligence | .061 (049) | [−.037, .159] | 1.256 | |||
| Stage 2 | .621 | .385 | .003 | |||
| Age | .041 (.044) | [−.047, .130] | .944 | |||
| EF | .222 (.099) | [.021, .423] | 2.236* | |||
| Verbal Intelligence | .059 (.049) | [−.040, .159] | 1.209 | |||
| Motor Skill | −.011 (.024) | [.038, −.060] | −.444 | |||
| Stage 3 | .690 | .477 | .091* | |||
| Age | .063 (.042) | [−.022, .146] | 1.487 | |||
| EF | .190 (.094) | [.000, .379] | 2.022 | |||
| Verbal Intelligence | .037 (.047) | [−.058, .132] | .783 | |||
| Motor Skill | .003 (.023) | [.051, −.044] | .146 | |||
| Action-Representation | .291 (.086) | [.044, .394] | 2.541* |
Notes: N = 43,
p < .05.
Next, we conducted a more exploratory analysis to examine relations between ToM and mu-desynchronization. There were no direct relations between ToM and any of central-parietal, frontal, or occipital mu for either correlations (rs < .11, ps > .29) or regressions controlling for age, EF, and verbal intelligence (bs < .076, ts < .38, ps > .71). However, guided by the above sets of results, we explored a more complex model, this time testing a moderated mediation model linking mu-desynchronization, motor skill, action-representation, and ToM.
Specifically, given the association between motor skill and action-representation found in the previous moderation model, and the relation between action-representation and ToM in the behavioral data above, we reasoned that it was possible that action-representation could be a mediator (or mechanism) through which developments in motor skill relate to developments in ToM. Given that central-parietal mu moderated the relation between motor skill and action-representation (i.e. the relation was only present in children with high central-parietal mu), we examined whether central-parietal mu also moderated this possible mediation (i.e. tested whether the mediation exists in children with high but not low central-parietal mu) (see Figure 5).
Figure 5.
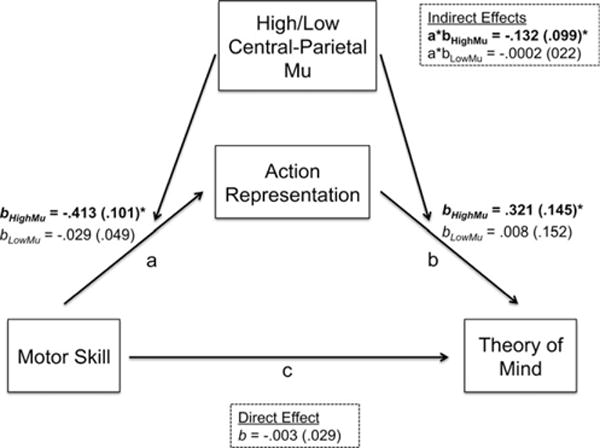
Moderated mediation model (N = 26) depicting action-representation mediating the relation between motor skill and ToM only when children exhibit high mu-desynchronization during grasp execution (bolded values). Figure shows unstandardized betas, with standard error in parentheses. *p ≤ .05.
To run this model, we used the PROCESS tool for SPSS (Hayes, 2013), which computes a bias-corrected bootstrap distribution (1000 iterations) for each direct and indirect effect estimate. Results replicated the initial moderation effect of central-parietal mu-desynchronization on the relation between motor skill and action-representation, and further showed that action-representation predicted ToM at high levels of central-parietal mu (b = .321, SE = .145, p = .039; CI95% [.017, .625]) but not at low mu (b = .008, SE = .152, p = .961; CI95% [−.312, .327]). As shown in Figure 5, results suggested a moderated mediation effect. Action-representation mediated the relation between motor skill and ToM at high levels of central-parietal mu-desynchronization (conditional indirect effect for high mu: a*b = −.132, SE = .099; CI95% [−.033, −.442]; Sobel test = −.195, SE = .068, p = .05). But there was no mediation effect at low mu (conditional indirect effect for low central-parietal mu: a*b = −.0002, SE = .022; CI95% [.013, −.069]; Sobel test = −.049, SE = .004, p = .96). In addition, the index of moderated mediation showed that the mediation effects at high and low central-parietal mu were indeed different (index = .132, SE = .098, CI95% [.036, .466]).
Discussion
We investigated relations among individual differences in the neural correlates of action-production (mu-desynchronization during execution of object-directed grasping), and performance on behavioral measures of action-production (i.e. motor skill), action-representation, and explicit ToM. We found evidence for novel foundational links between individual differences in ToM and both behavioral and neural measures of action – beyond the influence of executive functioning and verbal intelligence. These findings shed new light on the potential origins and developmental mechanisms of higher social cognition.
Several researchers posit that action-production and action-representation are important for mental-state understanding (e.g. Woodward, 2013; Meltzoff, 2002; Hunnius & Bekkering, 2014). Neuroscientific research has identified a neural system – indexed with the EEG mu-rhythm – that is associated with action-processing, and is hypothesized to reflect action-representation (e.g. Muthukumaraswamy & Johnson, 2004). It is therefore possible that the mu-rhythm represents a neural mechanism facilitating links between action and developing ToM (e.g. see Marshall & Meltzoff, 2011, 2014). Our data help inform these hypotheses.
Functions of mu-rhythm: new insights
Central-parietal mu-desynchronization moderated relations between behavioral motor skill and action-representation, demonstrating that mu-desynchronization is associated with action-production – as previously shown (e.g. Muthukumaraswamy & Johnson, 2004; Pfurtscheller et al., 1997) – and with action-representation – as many have hypothesized. However, the associations appear to be more complex than previously thought.
To review, researchers have interpreted the similar EEG signal during action-perception as evidence of an internal representation of the action that critically is supported by the same neural system as that which supports action-production. In brief, it is commonly hypothesized that mu-desynchronization reflects activity in a single sensorimotor neural system that is activated during both action-production and action-representation. Under this hypothesis, one might expect direct relations between mu-desynchronization and the ability to both produce and represent actions. In contrast, the present study did not reveal direct correlations with mu-desynchronization, but rather, as mu-desynchronization increased, the strength of relation between behavioral action-production and action-representation performance also increased, as indicated by the moderation model. This pattern suggests a slightly altered hypothesis for the function of mu-rhythm, at least as it operates in early childhood, in line with more complex conceptualizations of mu-rhythm as reflective of an integrated network of multiple neural systems with specialized functions (Pineda, 2005; Thorpe et al., 2016).
Indeed, our data suggest that individual differences in mu-desynchronization reflect the extent to which action-production and action-representation are integrated. At high mu-desynchronization (conceptualized here as reflecting stronger interconnections between network populations supporting action-production and action-representation), behavioral performance on action-representation and action-production was positively correlated. Conversely, at low mu-desynchronization, performance on the two behavioral action constructs was unrelated (e.g. possibly due to a lack of integration in their underlying neural network).
This ‘integrated network’ conceptualization of mu-rhythm can also be used to explain existing phenomena now clearly uncovered in the infant mu-rhythm literature – namely, that infants who gain more action-production experience also show greater mu-desynchronization during action-perception (e.g. Cannon et al., 2016; Marshall et al., 2013; Saby, Marshall & Meltzoff, 2012; van Elk et al., 2008; Paulus et al., 2012). To illustrate, mu-rhythm may reflect integration of systems specialized for action-production and action-representation, but each system could be differentially activated across perception and production modalities. For instance, action-perception may most strongly activate the system for action-representation. Thus, when an infant activates their action-representation system during action-perception, this system could be more or less integrated with a system supporting action-production. When that infant then gains experience in production of a specific motor act, this experience could serve to slightly enhance the integration between the infant’s own motor act and a stored representation of it. Critically, when the infant then perceives that motor act again, it would result in greater mu-desynchronization because the neural populations underlying action-representation (activated most directly by action-perception) have more connections to the neural populations underlying action-production, to result in an overall greater neural response as reflected in higher mu-desynchronization.
It is important to note that though this integrated network model for mu-rhythm fits with existing infant findings as outlined in the paragraph above, it does not preclude other possible conceptualizations of mu-rhythm development. Indeed, two differences exist between our study and the approaches most commonly taken in the infant mu literature, which point to other possible conceptualizations. First, we examined mu-desynchronization in children 3 to 5 years old, whereas much existing research on associations between behavior and mu-rhythm examines infants less than 2 years old. It is therefore possible that mu-rhythm reflects different neural processes in infancy compared to early childhood. The infant mu-rhythm may reflect activity in a single sensorimotor neural system, but then, as production and representational abilities mature, there could be accompanied neural reorganization and specialization into separate systems that then integrate through further reorganization and development. Second, we examined mu-desynchronization during action-production, whereas infant studies more commonly examine mu-desynchronization during action-perception. It is therefore also possible that the desynchronization in production and perception conditions reflects activity in different underlying neural systems. To shed light on each of these possibilities, future research should look for direct relations between mu-desynchronization and behavioral measures of action-representation and action-production in infants and children prior to age 3 years, as well as track these relations longitudinally over infancy to early childhood. The present study lays a foundation for these future directions.
Finally, the moderation model evident in our data provides only indirect support for the notion of mu-rhythm as an integrated network of neural systems. More direct support would come from network connectivity analyses of mu-desynchronization, which could represent an important replication of our analyses should mu-rhythm network interconnectivity predict the strength of correlation between behavioral measures of action-production and action-representation and/or should functional connections increase in strength as infants and children mature and advance in their action-production and representation abilities.
Even in advance of future research, the conceptualization of mu-rhythm as an ‘integrated network’ (versus more common conceptualizations that emphasize specifically sensorimotor activity) has implications for theorizing about neural mechanisms supporting cognitive development. For example, an action-representation system, which may be separate from but networked to the motor system, could plausibly store representations of actions to then be evaluated at higher levels. Such a network would have clear implications for how developments in action experience (which may be supported by the motor system more directly) could shape a deeper understanding and evaluation of actions in terms of the cognitive constructs that motivate them, as we discuss next.
Action-representation mechanisms for ToM development
Our data demonstrate links between children’s developing ToM and developments in the action domain, and thus they critically extend infant work that evinces ties between action and social understanding in early life (Woodward, 2013). Specifically, results suggest that action-representation is particularly important for explicit ToM development in early childhood: action-representation was the single best predictor of individual differences in 3- to 5-year-olds’ explicit ToM, even beyond verbal intelligence and EF (classic, robust predictors). Results therefore provide the first more direct support for theories that posit a foundational role for action-processing in more complex mental-state understanding and ToM (e.g. Meltzoff, 2002, 2013).
We also found evidence that behavioral action-representation mediated relations between children’s motor skill and ToM development, but only for children exhibiting high central-parietal mu-desynchronization during grasp-execution. This moderated mediation model was not directly hypothesized, but rather was inspired by our findings that mu-desynchronization moderated relations between action-production (motor skill) and action-representation performance, and that action-representation strongly and directly predicted children’s ToM. Replication of these results is important given the somewhat post-hoc nature of this model, the novelty of our action-representation task, and the very limited sample size.
Nonetheless, the moderated mediation model suggests that in addition to action-representation abilities, action-production abilities and the neural systems associated with action-processing may also be important for ToM development. Speculatively, the ability to hold an action in one’s mind, in the absence of direct perception or production (i.e. as a representation), is perhaps a critical step in facilitating the necessary computations to then reason about the motivating mental states behind that action (ToM). Indeed, several researchers have hypothesized that action-representations are derived from experience in action-production, and that together they facilitate developing understanding of the mental states motivating action (e.g. Hunnius & Bekkering, 2014; Woodward, 2013; Meltzoff, 2002, 2013).
Intriguingly, the moderation aspect of our model further suggests that this mediation pathway from action-production to action-representation to ToM is only viable once a neural network supporting action-production and action-representation is sufficiently integrated (high mu-desynchronization). When there is no neural system in place to link action-production and action-representation, then advancements in these action domains do not serve to advance ToM.
As noted above, an action-representation system that is separate from yet networked to the motor system is a plausible system for storing actions (in the absence of execution or perception) and thus subsequently facilitating evaluation of those actions at higher levels. Therefore, this system may be particularly important for supporting evaluations of the mental states that motivate action, thereby helping facilitate the development of ToM. That is, speculatively, an action-representation system – when sufficiently integrated with action-production via a common neural network – may be helpful for identifying connections between one’s own executed motor actions and the mental states that motivate them. These connections may in turn be helpful for identifying and reasoning about the mental states motivating the actions of others. In line with this reasoning, Meltzoff (2002, 2007, 2013) has argued that the development of executing one’s own actions as motivated by one’s own mental states, and the ability to identify similarities between one’s own and others’ actions, are two central components in jump-starting ToM. Longitudinal research that tracks associations between individual differences in mu-desynchronization, developing understanding of mental states, action-production, and action-representation from infancy to childhood will be critical in further illuminating the model and hypotheses outlined here.
Additional considerations for future research
Although our final moderated mediation model suggested a direction from action-production to action-representation to ToM (with models testing the same measures in other positions returning null results), longitudinal data are necessary to identify true direction and causality. Future research should also extend to younger as well as older age ranges: given dissociations between action and ToM systems in adults (Van Overwalle, 2009), it is possible that complex relations between mu-desynchronization, action-production, action-representation, and ToM might be particularly important (and/or evident) when children are newly assembling social-cognitive abilities, with diminished relations among variables later in development. An additional consideration for future research is the possibility that our action-representation task included a component of mental spatial rotation, and that developments in ToM may also be related to developments in some aspect of spatial cognition. Finally, although our data fit with theoretical models emphasizing action mechanisms for social-cognitive development, they do not preclude alternative theories (e.g. Theory Theory; Gopnik & Wellman, 1992, 2012). For example, developments in action-production and action-representation – in particular once integrated in a mature network – may allow children to identify important contingencies between their own actions and mental states, which, in line with ‘Theory Theory’, may then lead to the formation and revision of naïve theories that guide mental state inferences and interpretation of others’ behavior. The findings from the present study therefore open important avenues for future research and conceptualizations of the origins and course of ToM development.
Supplementary Material
Figure S1 Histogram of children’s action-representation task performance scores (N=43) showing the frequency distribution of scores across the sample. Bars represent the number of children who achieved a given score. The distribution of scores reasonably approaches normal, as can be seen by reference to the simulated normal curve superimposed on the data.
Research highlights.
We provide evidence that individual differences in children’s explicit ToM relate to individual differences in neural and behavioral measures of basic action, demonstrating novel links between action and social cognition. Specifically, when neural systems supporting action-processing are highly integrated, children’s action-representation abilities mediate relations between their basic action-production skills and ToM.
Whether/how neural systems underlying action support higher social cognition is controversial and debated across many fields. Results help clarify functions of the mu-rhythm – a measure commonly used to investigate neural systems supporting action – and shed light on the origins and course of social-cognitive development.
Acknowledgments
We thank the research assistants who helped with data collection and analyses, and the parents and children who participated in the study. This research was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P01-HD064653, awarded to A. L. Woodward and N. A. Fox.
Footnotes
Mu-desynchronization in central-parietal, frontal, and occipital regions also showed no relations with age, EF, or verbal intelligence (all rs < .20, ps > .16).
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article:
References
- Ambrosini E, Reddy V, de Looper A, Costantini M, Lopez B, et al. Looking ahead: anticipatory gaze and motor ability in infancy. PLoS ONE. 2013;8:e67916. doi: 10.1371/journal.pone.0067916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Cuevas K. Using EEG to study cognitive development: issues and practices. Journal of Cognition and Development. 2012;13:281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001;2:561–567. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Brune CW, Woodward AL. Social cognition and social responsiveness in 10-month-old infants. Journal of Cognition and Development. 2007;8:133–158. [Google Scholar]
- Cannon EN, Simpson EA, Fox NA, Vanderwert RE, Woodward AL, et al. Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Developmental Science. 2016;19(1):50–62. doi: 10.1111/desc.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Woodward AL, Gredebäck G, von Hofsten C, Turek C. Action production influences 12-month-old infants’ attention to others’ actions. Developmental Science. 2012;15:35–42. doi: 10.1111/j.1467-7687.2011.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Davis A, Leach JG. Less is more: executive function and symbolic representation in preschool children. Psychological Science. 2005;16:609–616. doi: 10.1111/j.1467-9280.2005.01583.x. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s Theory of Mind. Child Development. 2001;72:1032–1063. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Cannon EN, Yoo K, Fox NA. The infant EEG mu rhythm: methodological considerations and best practices. Developmental Review. 2014;34:26–43. doi: 10.1016/j.dr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Raj V, Bell MA. A frequency band analysis of two-year-olds’ memory processes. International Journal of Psychophysiology. 2012;83:315–322. doi: 10.1016/j.ijpsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum MM, Sommerville JA, Prinz W. How social experience is related to children’s intergroup attitudes. European Journal of Social Psychology. 2009;39:1196–1206. doi: 10.1002/ejsp. [DOI] [Google Scholar]
- Decety J, Grèzes J. The power of simulation: imagining one’s own and other’s behavior. Brain Research. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Devine RT, Hughes C. Relations between false belief understanding and executive function in early childhood: a meta-analysis. Child Development. 2014;85(5):1777–1794. doi: 10.1111/cdev.12237. [DOI] [PubMed] [Google Scholar]
- Fox NA, Bakermans-Kranenburg MJ, Yoo KH, Bowman LC, Cannon EN, et al. Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychological Bulletin. 2016;142(3):291–313. doi: 10.1037/bul0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds: a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallese V, Sinigaglia C. What is so special about embodied simulation? Trends in Cognitive Sciences. 2011;15:512–519. doi: 10.1016/j.tics.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gerson SA, Bekkering H, Hunnius S. Short-term motor training, but not observational training, alters neurocognitive mechanisms of action processing in infancy. Journal of Cognitive Neuroscience. 2015;27(6):1207–1214. doi: 10.1162/jocn_a_00774. [DOI] [PubMed] [Google Scholar]
- Gerson SA, Woodward AL. Learning from their own actions: the unique effect of producing actions on infants’ action understanding. Child Development. 2014;85:264–277. doi: 10.1111/cdev.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AI. In defense of simulation theory. Mind & Language. 1992;7:104–119. [Google Scholar]
- Gordon RM. ‘Radical’ simulationism. In: Carruthers P, Smith PK, editors. Theories of theories of mind. Cambridge: Cambridge University Press; 1996. pp. 11–21. [Google Scholar]
- Gopnik A, Wellman HM. Why the child’s Theory of Mind really is a theory. Mind & Language. 1992;7:145–171. [Google Scholar]
- Gopnik A, Wellman HM. Reconstructing constructivism: causal models, Bayesian learning mechanisms, and the theory theory. Psychological Bulletin. 2012;138(6):1085–1108. doi: 10.1037/a0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M. Magnetoencephalographic cortical rhythms. International Journal of Psychophysiology. 1997;26:51–62. doi: 10.1016/S0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Transactions on Neural Networks. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Hayes AG. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: The Guilford Press; 2013. [Google Scholar]
- Henderson SE, Sugden DA. Movement assessment battery for children manual. London: The Psychological Corporation; 1992. [Google Scholar]
- Hunnius S, Bekkering H. What are you doing? How active and observational experience shape infants’ action understanding. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1644):20130490. doi: 10.1098/rstb.2013.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakogi Y, Itakura S. Develomental correspondence between action prediction and motor ability in early infancy. Nature Communications. 2011;2:41. doi: 10.1038/ncomms1342. [DOI] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. Kaufman Brief Intelligence Test. Bloomington, MN: Pearson; 1990. [Google Scholar]
- Kuhlman W. Functional topography of the human mu rhythm. Electroencephalography and Clinical Neurophysiology. 1978;44:83–93. doi: 10.1016/0013-4694(78)90107-4. [DOI] [PubMed] [Google Scholar]
- Leocani L, Toro C, Manganotti P, Zhuang P, Hallett M. Event-related coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced move- ments. Electroencephalography and Clinical Neurophysiology. 1997;104:199–206. doi: 10.1016/S0168-5597(96)96051-7. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Théoret H. EEG evidence for the presence of an action observation–execution matching system in children. European Journal of Neuroscience. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- Manoel EJ, Connolly KJ. The development of manual dexterity in young children. In: Connolly KJ, editor. The psychobiology of the hand. London: MacKeith Press; 1998. pp. 177–198. (Clinics in developmental medicine, no. 147). [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring mechanisms and imitation in human infants. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2014;369:20130620. doi: 10.1098/rstb.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Saby JN, Meltzoff AN. Infant brain responses to object weight: exploring goal-directed actions and self-experience. Infancy. 2013;18:942–960. doi: 10.1111/infa.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Developmental Science. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Imitation as a mechanism of social cognition: origins of empathy, Theory of Mind, and the representation of action. In: Goswami U, editor. Blackwell handbook of childhood cognitive development. Oxford: Blackwell; 2002. pp. 6–25. [Google Scholar]
- Meltzoff AN. ‘Like me’: a foundation for social cognition. Developmental Science. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Origins of social cognition: bidirectional self–other mapping and the ‘like-me’ hypothesis. In: Banaji M, Gelman S, editors. Navigating the social world: What infants, children, and other species can teach us. New York: Oxford University Press; 2013. pp. 139–144. [Google Scholar]
- Milligan K, Astington JW, Dack LA. Language and Theory of Mind: meta-analysis of the relation between language ability and false-belief understanding. Child Development. 2007;78:622–646. doi: 10.1111/j.1467-8624.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology. 2004;41:152–156. doi: 10.1046/j.1469-8986.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Paulus M, Hunnius S, van Elk M, Bekkering H. How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: electrophysiological evidence for action-effect binding in infancy. Developmental Cognitive Neuroscience. 2012;2:90–96. doi: 10.1016/j.dcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. International Journal of Psychophysiology. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating ‘seeing’ and ‘hearing’ into ‘doing’. Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Saby JN, Marshall PJ, Meltzoff AN. Neural correlates of being imitated: an EEG study in preverbal infants. Social Neuroscience. 2012;7:650–661. doi: 10.1080/17470919.2012.691429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby JN, Meltzoff AN, Marshall PJ. Infants’ somatotopic neural responses to seeing human actions: I’ve got you under my skin. PLoS ONE. 2013;8:e77905. doi: 10.1371/journal.pone.0077905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994a;60:537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalography and Clinical Neurophysiology. 1994b;91:237–248. doi: 10.1016/0013-4694(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Scholl BJ, Leslie AM. Modularity, development and ‘Theory of Mind’. Mind & Language. 1999;14:131–153. [Google Scholar]
- Sommerville JA, Hildebrand EA, Crane CC. Experience matters: the impact of doing versus watching on infants’ subsequent perception of tool-use events. Developmental Psychology. 2008;44:1249–1256. doi: 10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S, Cannon E, Fox N. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Journal of Clinical Neurophysiology. 2016;127(1):254–269. doi: 10.1016/j.clinph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, et al. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalography and Clinical Neurophysiology. 1994;93:380–389. doi: 10.1016/0168-5597(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Upshaw MB, Bernier RA, Sommerville JA. Infants’ grip strength predicts mu rhythm attenuation during observation of lifting actions with weighted blocks. Developmental Science. 2016;19(2):195–207. doi: 10.1111/desc.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage. 2008;43:808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji-Babul N, Rose A, Moiseeva N, Makan N. Neural correlates of action understanding in infants: influence of motor experience. Brain and Behavior. 2012;2:237–242. doi: 10.1002/brb3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warreyn P, Ruysschaert L, Wiesema JR, Handl A, Pattyn G, et al. Infants’ mu suppression during the observation of real and mimicked goal-directed actions. Developmental Science. 2013;16(2):173–185. doi: 10.1111/desc.12014. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of Theory of Mind development: the truth about false belief. Child Development. 2001;72:655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Liu D. Scaling of Theory of Mind tasks. Child Development. 2004;75:523–541. doi: 10.1111/j.1467-8624.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infant foundations of intentional understanding. In: Banaji MR, Gelman SA, editors. Navigating the social world: A developmental perspective. Oxford: Oxford University Press; 2013. pp. 75–80. [Google Scholar]
- Woodward AL, Gerson SA. Mirroring and the development of action understanding. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2014;369:20130181. doi: 10.1098/rstb.2013.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AL, Guajardo JJ. Infants’ understanding of the point gesture as an object-directed action. Cognitive Development. 2002;17:1061–1084. doi: 10.1016/S0885-2014(02)00074-6. [DOI] [Google Scholar]
- Yoo KH, Cannon EN, Thorpe SG, Fox NA. Desynchronization in EEG during perception of means–end actions and relations with infants’ grasping skill. British Journal of Developmental Psychology. 2015;34:24–37. doi: 10.1111/bjdp.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD. The dimensional change card sort (DCCS): a method of assessing executive function in children. Nature Protocols. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Histogram of children’s action-representation task performance scores (N=43) showing the frequency distribution of scores across the sample. Bars represent the number of children who achieved a given score. The distribution of scores reasonably approaches normal, as can be seen by reference to the simulated normal curve superimposed on the data.


