Abstract
Mutations in the insulin receptor gene can render the cell resistant to the biological action of insulin. We have studied a patient with leprechaunism (leprechaun/Minn-1), a genetic syndrome associated with intrauterine growth retardation and extreme insulin resistance. Genomic DNA from the patient was amplified by the polymerase chain reaction catalyzed by Thermus aquaticus (Taq) DNA polymerase, and the amplified DNA was directly sequenced. A nonsense mutation was identified at codon 897 in exon 14 in the paternal allele of the patient's insulin receptor gene. Levels of insulin receptor mRNA are decreased to less than 10% of normal in Epstein-Barr virus-transformed lymphoblasts and cultured skin fibroblasts from this patient. Thus, this nonsense mutation appears to cause a decrease in the levels of insulin receptor mRNA. In addition, we have obtained indirect evidence that the patient's maternal allele of the insulin receptor gene contains a cis-acting dominant mutation that also decreases the level of mRNA, but by a different mechanism. The nucleotide sequence of the entire protein-coding domain and the sequences of the intron-exon boundaries for all 22 exons of the maternal allele were normal. Presumably, the mutation in the maternal allele maps elsewhere in the insulin receptor gene. Thus, we conclude that the patient is a compound heterozygote for two cis-acting dominant mutations in the insulin receptor gene: (i) a nonsense mutation in the paternal allele that reduces the level of insulin receptor mRNA and (ii) an as yet unidentified mutation in the maternal allele that either decreases the rate of transcription or decreases the stability of the mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Frapier C., Mosthaf L., McKeon C., Elbein S. C., Permutt M. A., Ramos E., Lander E., Ullrich A., Taylor S. I. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J. 1989 Sep;8(9):2509–2517. doi: 10.1002/j.1460-2075.1989.tb08388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Kazazian H. H., Jr The molecular basis of hemophilia A in man. Trends Genet. 1988 Aug;4(8):233–237. doi: 10.1016/0168-9525(88)90156-4. [DOI] [PubMed] [Google Scholar]
- Atweh G. F., Brickner H. E., Zhu X. X., Kazazian H. H., Jr, Forget B. G. New amber mutation in a beta-thalassemic gene with nonmeasurable levels of mutant messenger RNA in vivo. J Clin Invest. 1988 Aug;82(2):557–561. doi: 10.1172/JCI113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Endo F., Nagata N., Priest J. H., Longo N., Elsas L. J., 2nd Structural analysis of normal and mutant insulin receptors in fibroblasts cultured from families with leprechaunism. Am J Hum Genet. 1987 Sep;41(3):402–417. [PMC free article] [PubMed] [Google Scholar]
- Fojo S. S., Stalenhoef A. F., Marr K., Gregg R. E., Ross R. S., Brewer H. B., Jr A deletion mutation in the ApoC-II gene (ApoC-II Nijmegen) of a patient with a deficiency of apolipoprotein C-II. J Biol Chem. 1988 Dec 5;263(34):17913–17916. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Moncada V. Y., Taylor S. I. Insulin receptor biosynthesis in cultured lymphocytes from insulin-resistant patients. J Clin Invest. 1985 Dec;76(6):2355–2361. doi: 10.1172/JCI112247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Goodbourn S. E., Lamb J., Clegg J. B., Weatherall D. J., Proudfoot N. J. Alpha-thalassaemia caused by a polyadenylation signal mutation. Nature. 1983 Nov 24;306(5941):398–400. doi: 10.1038/306398a0. [DOI] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Goldstein J. L., Russell D. W. Deletion of exon encoding cysteine-rich repeat of low density lipoprotein receptor alters its binding specificity in a subject with familial hypercholesterolemia. J Biol Chem. 1986 Oct 5;261(28):13114–13120. [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Russell D. W., Davignon J., Goldstein J. L. Deletion in the gene for the low-density-lipoprotein receptor in a majority of French Canadians with familial hypercholesterolemia. N Engl J Med. 1987 Sep 17;317(12):734–737. doi: 10.1056/NEJM198709173171204. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Bevins C. L., Cama A., Ojamaa K., Marcus-Samuels B., Kadowaki H., Beitz L., McKeon C., Taylor S. I. Two mutant alleles of the insulin receptor gene in a patient with extreme insulin resistance. Science. 1988 May 6;240(4853):787–790. doi: 10.1126/science.2834824. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Sasaoka T., Takata Y., Hisatomi A., Shigeta Y. Insulin resistance by uncleaved insulin proreceptor. Emergence of binding site by trypsin. Diabetes. 1988 May;37(5):653–656. doi: 10.2337/diab.37.5.653. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance). N Engl J Med. 1988 Dec 8;319(23):1526–1529. doi: 10.1056/NEJM198812083192306. [DOI] [PubMed] [Google Scholar]
- Moncada V. Y., Hedo J. A., Serrano-Rios M., Taylor S. I. Insulin-receptor biosynthesis in cultured lymphocytes from an insulin-resistant patient (Rabson-Mendenhall syndrome). Evidence for defect before insertion of receptor into plasma membrane. Diabetes. 1986 Jul;35(7):802–807. doi: 10.2337/diab.35.7.802. [DOI] [PubMed] [Google Scholar]
- Muller-Wieland D., Taub R., Tewari D. S., Kriauciunas K. M., Sethu S., Reddy K., Kahn C. R. Insulin-receptor gene and its expression in patients with insulin resistance. Diabetes. 1989 Jan;38(1):31–38. doi: 10.2337/diab.38.1.31. [DOI] [PubMed] [Google Scholar]
- Myerowitz R. Splice junction mutation in some Ashkenazi Jews with Tay-Sachs disease: evidence against a single defect within this ethnic group. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3955–3959. doi: 10.1073/pnas.85.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
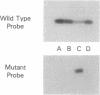
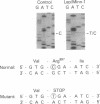
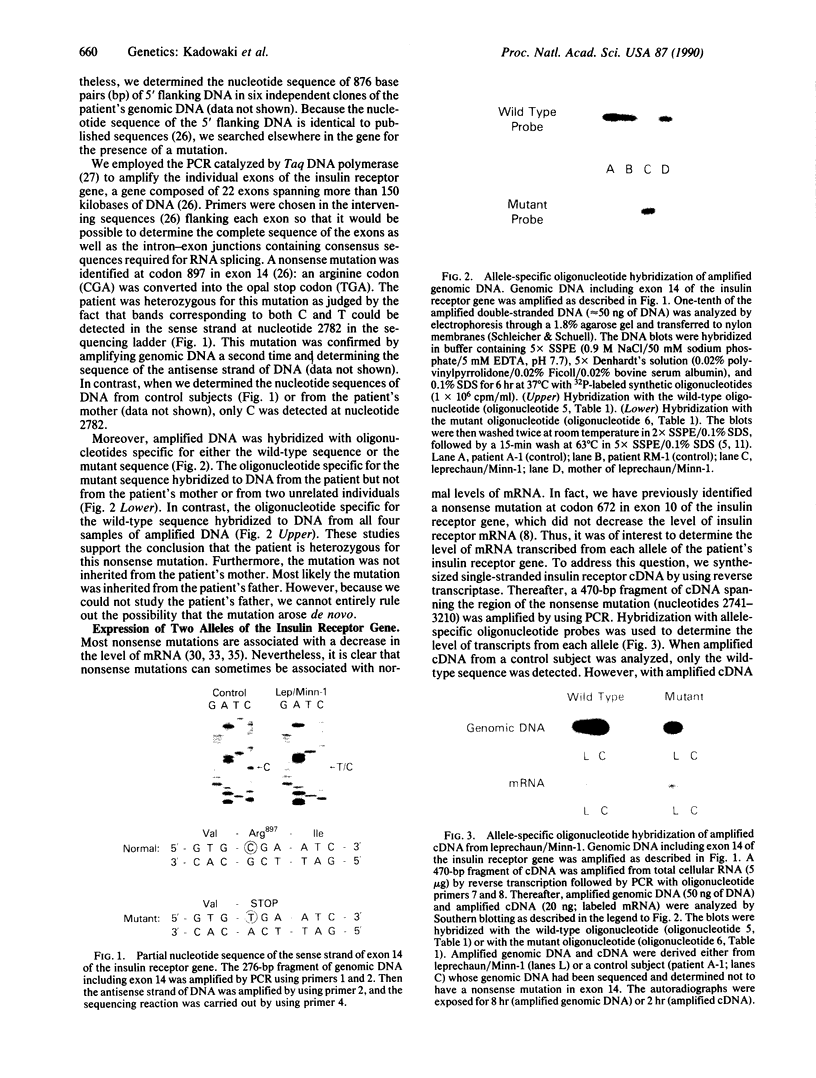
- Odawara M., Kadowaki T., Yamamoto R., Shibasaki Y., Tobe K., Accili D., Bevins C., Mikami Y., Matsuura N., Akanuma Y. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):66–68. doi: 10.1126/science.2544998. [DOI] [PubMed] [Google Scholar]
- Ojamaa K., Hedo J. A., Roberts C. T., Jr, Moncada V. Y., Gorden P., Ullrich A., Taylor S. I. Defects in human insulin receptor gene expression. Mol Endocrinol. 1988 Mar;2(3):242–247. doi: 10.1210/mend-2-3-242. [DOI] [PubMed] [Google Scholar]
- Podskalny J. M., Kahn C. R. Cell culture studies on patients with extreme insulin resistance. I. Receptor defects on cultured fibroblasts. J Clin Endocrinol Metab. 1982 Feb;54(2):261–268. doi: 10.1210/jcem-54-2-261. [DOI] [PubMed] [Google Scholar]
- Reddy S. S., Lauris V., Kahn C. R. Insulin receptor function in fibroblasts from patients with leprechaunism. Differential alterations in binding, autophosphorylation, kinase activity, and receptor-mediated internalization. J Clin Invest. 1988 Oct;82(4):1359–1365. doi: 10.1172/JCI113739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Seino S., Seino M., Nishi S., Bell G. I. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci U S A. 1989 Jan;86(1):114–118. doi: 10.1073/pnas.86.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Taira M., Hashimoto N., Shimada F., Suzuki Y., Kanatsuka A., Nakamura F., Ebina Y., Tatibana M., Makino H. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- Taylor S. I. Insulin action and inaction. Clin Res. 1987 Sep;35(5):459–472. [PubMed] [Google Scholar]
- Taylor S. I. Receptor defects in patients with extreme insulin resistance. Diabetes Metab Rev. 1985;1(1-2):171–202. doi: 10.1002/dmr.5610010109. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Samuels B., Roth J., Kasuga M., Hedo J. A., Gorden P., Brasel D. E., Pokora T., Engel R. R. Decreased insulin binding in cultured lymphocytes from two patients with extreme insulin resistance. J Clin Endocrinol Metab. 1982 May;54(5):919–930. doi: 10.1210/jcem-54-5-919. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasa Y., Seino S., Whittaker J., Kakehi T., Kosaki A., Kuzuya H., Imura H., Bell G. I., Steiner D. F. Insulin-resistant diabetes due to a point mutation that prevents insulin proreceptor processing. Science. 1988 May 6;240(4853):784–787. doi: 10.1126/science.3283938. [DOI] [PubMed] [Google Scholar]