Abstract
Combined hepatocellular-cholangiocarcinoma (CHC) is a rare tumor with poor prognosis, with incidence ranging from 1.0%-4.7% of all primary hepatic tumors. This entity will be soon renamed as hepato-cholangiocarcinoma. The known risk factors for hepatocellular carcinoma (HCC) have been implicated for CHC including viral hepatitis and cirrhosis. It is difficult to diagnose this tumor pre-operatively. The predominant histologic component within the tumor largely determines the predominant radiographic features making it a difficult distinction. Heterogeneous and overlapping imaging features of HCC and cholangiocarcinoma should raise the suspicion for CHC and multiple core biopsies (from different areas of tumor) are recommended before administering treatment. Serum tumor markers CA19-9 and alpha-fetoprotein can aid in the diagnosis, but it remains a challenging diagnosis prior to resection. There is sufficient data to support bipotent hepatic progenitor cells as the cell of origin for CHC. The current World Health Organization classification categorizes two main types of CHC based on histo-morphological features: Classical type and CHC with stem cell features. Liver transplant is one of the available treatment modalities with other management options including transarterial chemoembolization, radiofrequency ablation, and percutaneous ethanol injection. We present a review paper on CHC highlighting the risk factors, origin, histological classification and therapeutic modalities.
Keywords: Combined hepatocellular-cholangiocellular carcinoma, Hepatocellular carcinoma, Cholangiocellular carcinoma, Hepatic progenitor cell(s), Histogenesis, Classification
Core tip: Combined hepatocellular-cholangiocarcinoma is a rare tumor with ambiguous data in literature in relation to its clinical features, histogenesis, pathological classification and prognosis. The goal of our study was to review the literature and highlight the new updates on this entity.
INTRODUCTION
Combined hepatocellular-cholangiocarcinoma (CHC) is a rare tumor, with variation reported from 1.0%-4.7% of all primary hepatic tumors in series of patients undergoing hepatic resection[1-6], although accurate incidence is not known. CHC has been known with several nomenclatures in the literature including mixed hepatocellular carcinoma-cholangiocarcinoma (HCC-CC), hybrid HCC-CC or combined liver and bile duct carcinoma[7]. Some of the more common risk factors for CHC mentioned in the literature are hepatitis B virus (HBV) infection, male predominance, cirrhosis and hepatitis C virus (HCV) infection[8-12]. Molecular evidence supports that the hepatic progenitor cell (HPC) is the cell of origin for CHC. On histology, it is divided into two main subtypes-classical type and subtypes with stem cell features (further discussed in histology section)[13]. Separate HCC and CC in the same liver does not classify as CHC. We present a review of current understanding of clinical features, histogenesis and histology of the combined hepatocellular-cholangiocarcinoma that will be soon renamed as hepato-cholangiocarcinoma.
CLINICAL FEATURES AND RISK FACTORS
Owing to the rarity of CHC, the majority of the series describing clinical features and prognostic factors consist of retrospective studies from single institutions encompassing small patient populations with little statistical power. This is further compounded by inconsistent histologic inclusion criteria and definition of CHC across the studies, with many series including collision tumors and separate nodules of HCC and CC as CHC. Thus, it is not surprising that identification of clinical features and prognostic factors are not consistent or reproducible among the different studies. A clear profile of the patient demographic afflicted by this rare primary hepatic malignancy has remained vague and is highly dependent on geographic region.
Etiology and risk factors for these tumors may be common or differ in different regions in eastern and western series. This reflects variability in the prevalence of infectious agents such as hepatitis viruses and liver flukes, as well as the lifestyle and nutritional differences. Multiple studies have highlighted risk factors such as male gender, cirrhosis, hepatitis infection, family history of liver cancer, heavy alcohol consumption and diabetes mellitus[4,8-11,14-16]. The high male: Female ratio and prevalence of HBV in CHC patients in Asian countries are generally more similar to HCC compared to CC[15,16]. On the other hand, western studies have shown a less pronounced male predominance of CHC, paralleling with relatively low prevalence of HBV (15%-16.6%) and high prevalence of HCV[1,4,12,17]. Taken together, these findings suggest that geographical characteristics heavily influence clinical profiles of patients with CHC. This demonstrates that CHC is associated with overlapping clinical features of both HCC and CC. Some studies have reported that CHC has poor prognosis and more aggressive behavior in comparison to HCC and CC[18-20], which some authors attribute to increased lymph node involvement[8].
IMAGING CHARACTERISTICS AND PRE-OPERATIVE DIAGNOSIS
Historically, CHC has been an elusive and difficult pre-operative diagnosis. This is due to its heterogeneous imaging characteristics with overlapping features of both HCC and CC. The predominant histologic component within the tumor largely determines the predominant radiographic features. This is also true in tissue specimens, as sampling error (e.g., sampling only the area of HCC or CC within a CHC) may also lead to an erroneous pre-operative diagnosis. Thus, the majority of CHC cases in the literature were initially misdiagnosed as either HCC or CC and the proper diagnosis was only reached in the surgical resection specimens. Correct pre-operative diagnosis is important, especially distinction from HCC, as it may determine different management strategy. In the United States the vast majority of HCCs are diagnosed based on characteristic radiological features alone without pathologic confirmation. HCC in selected patients is an indication for liver transplant, with excellent outcomes equivalent to non-neoplastic entities and 5-year survival > 70%[21]. Taking into account the scarcity of grafts available for transplantation and the poor prognosis associated with CHC, differentiation from HCC becomes paramount.
The characteristic features of HCC on contrast enhanced CT and MRI are arterial phase diffuse enhancement, portal venous washout, and an enhanced pseudocapsule on delayed imaging. The hallmark radiological findings of CC are arterial peripheral rim enhancement with progressive fibrous stroma central enhancement, dilation of the biliary system, and retraction of the capsule[22]. CHC may show all of these radiographic characteristics to varying degrees, making distinction from HCC and particularly from CC very challenging. Some authors have suggested that the presence of heterogeneous or overlapping imaging features should prompt an extended tissue biopsy from different appearing tumor areas to aid in this diagnostic conundrum and mitigate sampling bias[22]. Another clue that should raise suspicion for CHC pre-operatively is discordant tumor markers. Generally, elevated alpha-fetoprotein (AFP) levels are associated with HCC, while elevated CA 19-9 levels are associated with CC. If a tumor shows characteristic imaging features of HCC, but is associated with elevated CA 19-9 levels, or if a tumor has characteristic CC imaging features and is associated with elevated AFP levels, or if both serum markers are elevated, biopsy for pathologic confirmation should be strongly considered.
HISTOGENESIS
The concept of cancer stem cells may explain the origin and progression of different kinds of cancers, and CHC is no exception. Although histogenesis of the CHC has been a topic of debate, three types of tumor origins have been hypothesized: (1) collision tumors; (2) de-differentiation or re-differentiation of a primary HCC into a biliary phenotype or vice versa; (3) derivation from bipotent HPC[5,23]. The first theory, collision tumor consisting of separate populations of HCC and CC occurring in the same liver without intimate relationship, does not qualify as CHC. De-differentiation or redifferentiation of HCC or CC into the other component is controversial, as some studies have shown differences in the clinical features, histology and molecular genetics of CHC and HCC/CC while others have supported this theory owing to existing similarities between CHC and HCC as well as CHC and CC.
The bipotent HPC is a stem cell that differentiates into both hepatocytes and bile duct epithelial cells, and is suspected to be the cancer stem cell responsible for CHC growth[5,23-26]. Theise et al[23] described four primary liver cancers with three different components including hepatocellular, cholangiocellular and a third component of small or undifferentiated cells (oval-like cells) with high N/C ratio, scant basophilic cytoplasm and nuclear pleomorphism. Oval cells (described in animal models) or intermediate cells (in humans) or stem cells can be found in regenerative nodules. These cells are located in canals of Hering[23,27] and can differentiate bidirectionally into hepatocytes and cholangiocytes, also called as bipotent progenitor cells[5,23,24]. There is morphological and immunohistochemical similarity between these oval-cell like progenitors and hepatoblasts (both positive for CK19 and Hep-Par1).
HPC and stem cell markers which have been studied in relation to CHC include CD133, CD90, CD44, epithelial cell adhesion molecule (EpCAM), nuclear cell adhesion molecule (NCAM/CD56), OV6, CD13, c-kit, YAP1, SALL4 and Delta-like 1 homolog (DLK1)[5,28-33]. Kim et al[24] demonstrated that c-kit-positive HPCs have a potential to differentiate into both hepatocytes and cholangiocytes and are neoplastic counterparts of HPCs.
Evidence including identification of HPC-like cells merging with HCC and CC components, as well as shared expression of HPC markers in the different components, supports these cells as the origin for CHC[23,25]. Furthermore, inoculation of cells from a CHC cell line positive for the HPC marker EpCAM has been associated with development of CHC in mice[26]. HPC activation in non-tumor liver in CHC cases has been linked with recurrence and poor prognosis in CHC[34]. Microdissection has shown that both components share a single clonal background, which is consistent with the shared origin of both components deriving from HPCs[35].
The role of the HPC is reflected in the current World Health Organization (WHO) classification, which is subdivided into CHC, classical type and three subtypes of CHC with stem cell features[13]. In making this classification, the authors noted that it was uncertain whether biological differences existed between these subtypes, and determination of subtype relied mainly on histological and immunohistochemical features. HPC marker expression has been shown to varying degree in all stem cell subtypes and to a lesser degree in the classical subtype, while prominent in transitional areas of CHC[24,29-31]. Ikeda et al[30] showed that the stem cell markers DLK-1 and NCAM/CD56 were expressed most frequently in CHC with stem cell features, and were most frequently expressed in typical and cholangiocellular subtypes. Akiba et al[29] showed that stem cell markers CD133 and EpCAM were more often expressed in CHC with stem cell features compared to those with classical features. They further showed that among CHC subtypes with stem cell features, cholangiocellular subtype more often expressed CD133 and EpCAM in comparison to intermediate subtype (their study did not include sufficient cases of typical subtype for statistical analysis)[29]. Komuta et al[36] supported origin of cholangiolocellular carcinoma from HPCs that was initially a subtype of cholangiocarcinoma.
Molecular studies have shown that CHC shares some traits with HCC and others with CC, confirming its status as a distinct entity. Gene profiling of CC, HCC and CHC by microarray shows increased differential expression in CC vs HCC as compared to CC vs CHC, reflecting this concept[37]. Analysis of copy number changes in CC and HCC components of CHC showed concordance in the overall trend of gain or loss for several target genes although magnitude of copy number change differed. The copy number gains in the CC component were likely to be paired with a similar but not identical copy number gain in the HCC component of the tumor, with the same holding true for copy number losses. The specific genes most often amplified in this study were MYC, ADAMTSL4, TM4SF1 and CUL4A, which are each associated with HCC although CUL4A has also been associated with CC[38]. Similarly, comparative genomic hybridization showed specific chromosomal gains and losses similar to those of HCC[39]. This study also showed high prevalence of chromosomal imbalances similar to those seen in CC. Similarly, a high level of chromosomal instability, in addition to recurrent loss of heterozygosity at 3p and 14q is also noted[40].
Genome-wide transcriptional analysis of 20 CHC cases showed that CHC clustered with CC and separately from HCC, with upregulated signaling pathways of TGFβ and Wnt similar to those seen in CC. The TGFβ pathway upregulated in CHC recalled the key role of fibrosis and extracellular matrix remodeling in CC, and the Wnt pathway signature was similar to that seen in biliary ductal morphogenesis. However, CHC also clustered with a subset of poorly differentiated HCC with progenitor cell features, as would be expected given the postulated HPC origin of CHC, while CHC showed repression of the transcription factor HNF4A associated with mature hepatocyte differentiation[41]. Likewise it is also shown that CHCs were clustered with CC by gene expression profiling[42]. A recent whole genome sequencing analysis showed that genome-wide substitution patterns in liver cancers of biliary phenotype (both CC and CHC) overlapped with those of HCC in cases associated with chronic viral hepatitis, while biliary cancers (mostly CC in this study) unrelated to chronic hepatitis differed from HCC[43]. TERT promoter mutations, for example, were common in CHC and in other hepatitis-related cancers. Carcinogenesis arising from HBV acts largely through the HBV X protein that promotes HPC tumorigenesis so it is possible that these tumors may share a similar pathogenesis[44].
HISTOLOGY
Classification
There are multiple classifications for CHC in the literature. Allen and Lisa[7] made the first histological classification for CHC in 1949. They described three subtypes. Type 1 consisted of discrete foci of HCC and CC. Type 2 had contiguous masses with features of both HCC and CC. Type 3 was described as a solitary mass comprising of both components. Goodman et al[45] in 1985 proposed another classification, also encompassing three subtypes: Type 1 or collision tumor with separate and colliding areas of HCC and CC in the same liver; type 2 or transitional tumor with transitional areas with intimate intermingling of two components with actual transition of HCC elements to CC elements in the same tumor; and type 3 or mucin producing fibrolamellar tumor. Allen and Lisa[7]’s type 3 and Goodman et al[45]’s type 2 has similar features to the current WHO criteria for CHC. The current edition of the WHO classification describes two main types of CHC: Classical type and CHC with stem cell features. The stem-cell features type is further divided into 3 subtypes: Typical subtype, intermediate cell subtype and cholangiolocellular subtype[13]. Representative images of each subtype highlighting histological features and immunohistochemical profile are shown in Figures 1, 2, 3 and 4.
Figure 1.
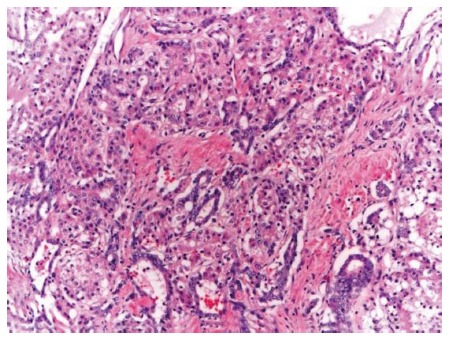
Representative picture classic type combined hepatocellular-cholangiocarcinoma, hematoxylin and eosin, 10 × - intermediate areas with both hepatocytic and chloangiocytic components.
Figure 2.
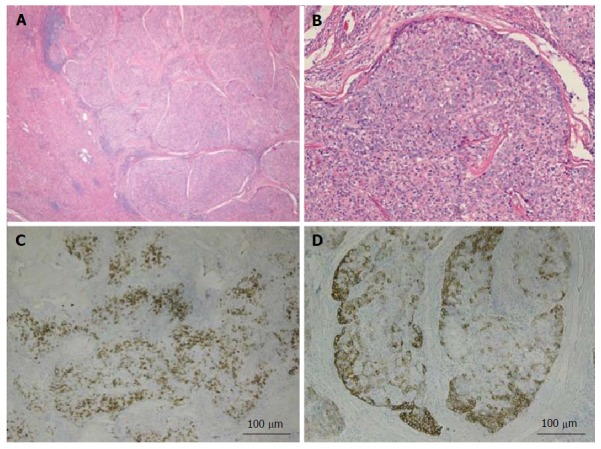
Combined hepatocellular-cholangiocarcinoma with stem cell features, typical subtype. A: H and E, 4 × - tumor nests present on the right side with non-neoplastic liver on the left side; B: H and E, 10 × - peripheral small cells with hyperchromatic nuclei with mature appearing hepatocytes in the center; C: CK7, 4 × - scattered expression of CK7 by tumor cells; D: CK19, 4 × - patchy staining of the tumor and highlighting small tumor cells located at the periphery. Tumor was also positive for Hep-Par1 (not shown). H and E: Hematoxylin and eosin.
Figure 3.
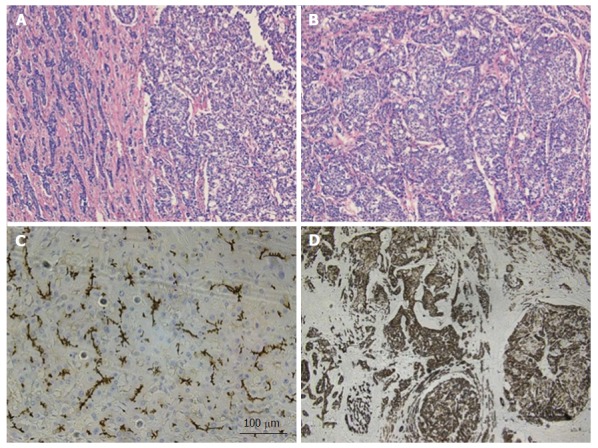
Combined hepatocellular-cholangiocarcinoma with stem cell features, intermediate subtype. A: H and E, 4 × - tumor is present in trabecular/nested pattern on the right side with ill-formed gland like structures seen on the left side; B: H and E, 10 × - tumor cells with intermediate features between hepatocytes and cholangiocytes; C: CD10, 10 × - tumor showing canalicular staining pattern for CD10 (hepatocytic marker); D: CK19, 4 × - tumor cells strongly and diffusely expressing CK19 (chlolangiocytic marker). Focal tumor cells were positive for Hep-Par1 and CD56 (not shown). H and E: Hematoxylin and eosin.
Figure 4.
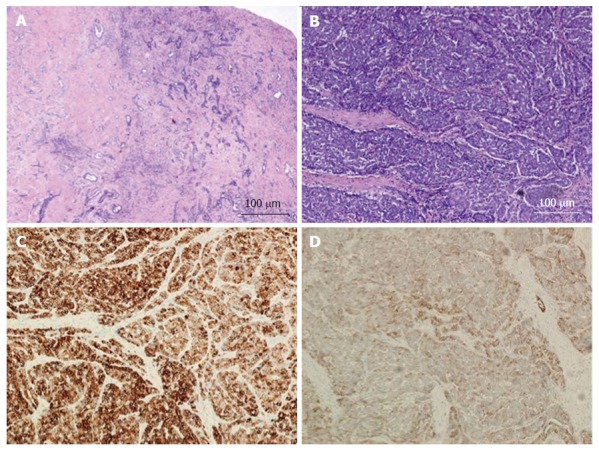
Combined hepatocellular-cholangiocarcinoma with stem cell features, cholangiocellular subtype. A: H and E, 4 × - tumor cells present in tubular, anastomosing (antler-like) pattern; B: H and E, 10 × - small hyperchromatic tumor cells with high nuclear to cytoplasmic ratio present within dense fibrous stroma; C: CK7, 10 × - tumor is diffusely positive for CK7; D: CD56, 10 × - CD56 staining the cholangiolocellular component as well as the tumor cells at the periphery of the trabeculae. The tumor was diffusely positive for CK19 while negative for HepPar-1 and AFP (not shown). H and E: Hematoxylin and eosin.
Histopathology
WHO 2010 classification defines these tumors as histological demonstration of unequivocally differentiated hepatocellular and biliary components in the tumor with intermingling of the two components[13]. Collision tumor, which is a separate entity, consists of HCC and CC occurring in the same liver without intimate relationship and is not categorized as CHC. The definite diagnosis of CHC can be made by histology only along with use of IHC and special stains[5]. The diagnosis of CHC is a challenging diagnosis on core biopsy as it depends on the area sampled[46]. Hepatocytic differentiation is defined by bile production, Mallory-Denk bodies, alpha-1 antitrypsin globules and trabecular arrangement of tumor cells. The cholangiocarcinoma component is appreciated by mucin production, prominent desmoplastic stroma and glandular structures. Combined fibrolamellar HCC in combination with cholangiocellular component has also been described in the literature[45,47].
In the CHC classic type (Figure 1), both the hepatocytic and cholangiocarcinoma components are present and can vary from well to poorly differentiated[13] with intermediate areas demonstrating features of both the components. Hepatocytic component is usually represented by thickened trabeculae composed of polygonal cells with abundant granular eosinophilic cytoplasm and scant stroma while cholangiocarcinoma component has gland formation with low cuboidal/columnar cells and dense fibrotic stroma. HCC with pseudoglandular pattern and expression of CK7/CK19 is not classified as CHC[5].
The first subtype of CHC with stem cell features, the typical subtype (Figure 2A and B), is characterized by peripheral small cells with hyperchromatic nuclei and a high nuclear to cytoplasmic ratio with nests of mature appearing hepatocytes in the center. The intermediate cell subtype (Figure 3A and B) consists of tumor cells with intermediate features between hepatocytes and cholangiocytes. Kim et al[24] described these tumor cells as small and oval shaped with hyperchromatic nuclei and scant cytoplasm arranged in either trabeculae, solid nests or strands, present within a desmoplastic stroma. Well-formed glands are not seen but ill-formed gland like structures may be present. These tumors were initially termed as intermediate carcinomas (hepatocyte-cholangiocyte) as these had features that were intermediate between HCC and CC[13,24]. The cholangiolocellular subtype (Figure 4A and B) is characterized by small cells with a high nuclear to cytoplasmic ratio and hyperchromatic oval shaped nuclei arranged in a tubular, cord like, anastomosing pattern (also referred to as an “antler-like” pattern) within a dense fibrous stroma. No significant cellular atypia or evidence of mucin production is seen in both intermediate and cholangiolocellular subtypes[13]. Although there are distinct histological features described by the WHO to classify all these types/subtypes, there is no mention of percentage of stem cell area required to categorize CHC with stem cell features. If the stem cells predominate, the tumor is classified as one of the subtype of CHC with stem cell features depending on the histologic appearance. It appears that CHCs with less than 5% stem cell area have better prognosis than those with stem cell areas greater than 5%[30]. Sasaki et al[48] proposed certain clinico-pathological findings for CHC with stem cell features. The intermediate subtype was more commonly associated with female patients, larger tumor size, higher histological grade of HCC component, and less fibrosis while cholangiocellular subtype had smaller tumor size and lower histological grade of HCC. The typical subtype has less inflammation in comparison with the cholangiocellular subtype[48].
On cytology specimens, diagnosis of CHCs can be challenging[49,50]. Cell blocks and immunohistochemical stains can prove helpful in reaching a correct diagnosis of CHC. With CHC being an uncommon tumor, arriving at a diagnosis and classification of CHC can be difficult with histology alone.
Special and immunohistochemical stains
Immunohistochemical stains are required for demonstrating hepatic and biliary phenotypes. The hepatocellular component is positive for HepPar1, pCEA, CD10 and glypican[5]. The CC component shows expression of CK7, CK19 and mucin/mucicarmine although HCC components can also express CK7 and CK19[5]. Mucin is essential to demonstrate the biliary component[51]. CAM 5.2 and AE1 can also be useful to differentiate between HCC and CC component; HCC will be positive for CAM5.2 while AE1 will be positive in CC component[49]. CHC with stem cell features expresses stem cell markers including c-kit, NCAM, and EpCAM. Kim et al[32] demonstrated similar expression of these stem cell markers in CHC and HCC. Oval cell-like progenitors or small cells (or HPCs) described originally by Theise et al[23] are focally positive for alpha-fetoprotein and alpha-antitrypsin while negative for Hep-Par1, c-Kit, vimentin and CHR-A. CD44, which is one of the cancer stem cell markers, is associated with poor prognosis and early recurrence in patients with CHC[32]. DLK1 is another marker of HPCs in adult liver[30]. Survival of patients with high expression of DLK1 is worse[30] suggesting that patients with CHC with stem cell features do worse in comparison to classical type CHC.
CHC with stem cell features, typical subytype stains positively with CK7, CK19 (Figure 2C and D), NCAM1/CD56, cKIT and/or EpCAM.
The intermediate subtype that is characterized by intermediate cells (between hepatocytes and cholangiocytes), shows simultaneous expression of hepatocyte and biliary markers (Figure 3C and D). Akiba et al[52] demonstrated that intermediate cells stain better with Arginase-1 and CK8. Biliary phenotypes (CK7 and CK19) are more commonly positive in intermediate subtype than Hep-Par1[29].
The cholangiolocellular subtype is positive for CK19, and stem cell markers- cKIT, NCAM1/CD56 and EpCAM (Figure 4C and D).
TREATMENT OPTIONS
Currently, minor or major hepatic resection, with or without lymph node dissection, is the consensus recommended treatment for CHC. However, the absence of randomized prospective studies precludes the determination of optimal management strategies in these patients.
The role of liver transplant in the treatment of CHC remains to be defined. Most of the data on transplanted CHC patients comes from patients that were initially misdiagnosed with HCC or from incidentally discovered tumors in the explanted livers. The outcome data of this cohort is mixed, although outcomes are consistently worse when compared with HCC due to associated higher recurrence rates after transplant in CHC[53,54]. A recent study found no survival benefit of transplant over resection in CHC, with 3-year overall survival of 48% and 46% (P = 0.56), respectively[55]. Interestingly, a few studies have reported favorable outcomes in CHC patients undergoing transplantation. Chan et al[56] reported three cases, with two of them alive without recurrence 25 and 35 mo post-transplant. The other patient died of metastatic disease 16.5 mo after transplant. Another more recent publication found that transplanted CHC patients have better 5-year overall survival than those treated with major resection (41.1% vs 28.1%, P = 0.039)[57]. However, the 5-year overall survival rate of transplanted CHC patients was still much worse than transplanted HCC patients in both studies (41.1% vs 67%, P < 0.001 and 48% vs 78%, P = 0.01)[55,57]. These findings were further supported by Vilchez et al[12] in their UNOS database analysis. They found an overall 5-year survival of 40% in transplanted CHC patients, which was similar to CC (47%) but much worse than HCC (62%, P = 0.002). The authors concluded that currently, liver transplant is not a viable option for these patients. It should be emphasized that these results may be influenced by the fact that majority of CHC patients are misdiagnosed pre-operatively and managed as HCC. Improved initial diagnostic accuracy may allow for optimization and more aggressive neoadjuvant therapies for CHC patients. This may in turn improve outcomes in this group and make transplant a viable option in the future.
Other treatment modalities that have been reported in CHC include transarterial chemoembolization, radiofrequency ablation, and percutaneous ethanol injection, but the data regarding the benefits of these interventions are inconclusive. The role of chemotherapy and radiotherapy remains to be defined.
PROGNOSIS
Despite the ambiguity and discordance of CHC clinical features reported in the literature, the studies consistently supported that CHC is associated with a more aggressive course and a worse prognosis than HCC. With regards to CC, the studies are varied with some showing CHC to have a worse[9] or similar prognosis while others show an improved outcome. Overall most studies showed that the prognosis of CHC is grim. Reported 3-year and 5-year overall survivals range from (37.3%, 47%, 46%, 12.4%, 10.5%, 34.6%) and (9.2%, 40%, 32%, 23.1%, 33%), respectively[8-12,55,57,58].
Adverse clinicopathologic prognostic factors associated with increased tumor recurrence and worse survival in various studies include large tumor size (> 5 cm), presence of satellite nodules, lymph node involvement, multifocality, vascular invasion, portal vein invasion, high tumor stage, high levels of CA 19-9, decreased capsule formation, free surgical resection margins < 2 cm, and GGT levels > 60 U/L[11,58,59]. However, many of these factors did not reach statistical significance on multivariate analysis. This may be due to the retrospective nature and low number of patients within each study due to the low incidence of CHC.
A recent population level study analyzed the SEER database for patients diagnosed with CHC between 1988-2009 in the United States. Of the 465 cases studied, they founded that a majority of CHC patients were male (66%) and Caucasian (74.9%), and a plurality were 66 years or older (44.3%)[57]. Clinical features of CHC patients fell in between those for HCC and CC, suggestive of the mixed characteristics associated with this tumor and in concordance with its bi-phenotypic differentiation. The authors found that CHC had a worse overall survival when compared to HCC, but better when compared with CC. The reported 5-year overall survival and disease specific survival for HCC, CHC, and CC were 11.7%, 10.5%, 5.7% and 21%, 17.8% and 11.9%, respectively (P < 0.001). The authors of the study concluded that CHC patients have intermediate clinical characteristics, demographics, and prognosis when compared with HCC and CC patients. Another study compared the post-resection outcomes of CHC, HCC and CC and found no significant differences in tumor recurrence rates, but did find worse survival rates when compared to HCC. However, results of this study are not representative of true CHCs as the authors used the Allen and Lisa[7] classification. The majority of patients in this study were classified as “combined type” or type 2 of the Allen and Lisa classification, which is not currently considered a true CHC according to the WHO classification[6].
The intermediate biological behavior of CHC has been further supported by other studies. Multiple series have found clinico-pathologic features in CHC that are commonly associated with either HCC or CC. These include a high rate of lymph node metastasis, commonly associated with CC, and vascular invasion and portal vein invasion, commonly associated with HCC. Reported rates of lymph node metastasis in CHC cases are as high as 42%[8]. This may be explained by the biphenotypic nature of these malignancies[9].
In summary, CHC is a rare tumor with bad prognosis and overlapping clinical and radiological features with HCC and CC. More studies are required to adequately define its histogenesis including molecular genetics of this tumor.
ACKNOWLEDGMENTS
The authors wish to thank Hongfa Zhu, MD, PhD, at Mount Sinai Hospital, New York, United States, for providing the microphotographs of classic type combined hepatocellular-cholangiocarcinoma.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All the authors of this study have nothing to declare.
Peer-review started: September 14, 2016
First decision: October 20, 2016
Article in press: January 3, 2017
P- Reviewer: Tomizawa M, Yoshida K S- Editor: Gong XM L- Editor: A E- Editor: Li D
References
- 1.Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040–2046. doi: 10.1002/cncr.10392. [DOI] [PubMed] [Google Scholar]
- 2.Liu CL, Fan ST, Lo CM, Ng IO, Lam CM, Poon RT, Wong J. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg. 2003;138:86–90. [PubMed] [Google Scholar]
- 3.Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, Joh JW. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892–897. doi: 10.1007/s00595-006-3276-8. [DOI] [PubMed] [Google Scholar]
- 4.Portolani N, Baiocchi GL, Coniglio A, Piardi T, Grazioli L, Benetti A, Ferrari Bravo A, Giulini SM. Intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma: a Western experience. Ann Surg Oncol. 2008;15:1880–1890. doi: 10.1245/s10434-008-9933-y. [DOI] [PubMed] [Google Scholar]
- 5.Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485–1492. doi: 10.1111/j.1440-1746.2010.06430.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoon YI, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Lee JW, et al. Postresection Outcomes of Combined Hepatocellular Carcinoma-Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2016;20:411–420. doi: 10.1007/s11605-015-3045-3. [DOI] [PubMed] [Google Scholar]
- 7.Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647–655. [PMC free article] [PubMed] [Google Scholar]
- 8.Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol. 2002;17:401–405. doi: 10.1046/j.1440-1746.2002.02734.x. [DOI] [PubMed] [Google Scholar]
- 9.Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T, Makuuchi M. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283–287. doi: 10.1093/jjco/hyg056. [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, Hsieh SY, Chang CJ, Lin YJ. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol. 2013;28:122–127. doi: 10.1111/j.1440-1746.2012.07289.x. [DOI] [PubMed] [Google Scholar]
- 11.Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol. 2014;26:192–199. doi: 10.1097/MEG.0b013e3283625df9. [DOI] [PubMed] [Google Scholar]
- 12.Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford) 2016;18:29–34. doi: 10.1016/j.hpb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theise ND, Park YN, Nakanuma Y. Bosman FT, Carneiro, F, Hruban RH, Theise ND. Lyon, France: IARC; 2010. WHO Classification of Tumours of the Digestive System. Combined hepatocellular-cholangiocarcinoma; pp. 225–227. [Google Scholar]
- 14.Park SE, Lee SH, Yang JD, Hwang HP, Hwang SE, Yu HC, Moon WS, Cho BH. Clinicopathological characteristics and prognostic factors in combined hepatocellular carcinoma and cholangiocarcinoma. Korean J Hepatobiliary Pancreat Surg. 2013;17:152–156. doi: 10.14701/kjhbps.2013.17.4.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Park YN, Lim JH, Choi GH, Choi JS, Kim KS. Characteristics of combined hepatocelluar-cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2014;40:976–981. doi: 10.1016/j.ejso.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YM, Zhang XF, Wu LP, Sui CJ, Yang JM. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-control study. World J Gastroenterol. 2014;20:12615–12620. doi: 10.3748/wjg.v20.i35.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panjala C, Senecal DL, Bridges MD, Kim GP, Nakhleh RE, Nguyen JH, Harnois DM. The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am J Transplant. 2010;10:1263–1267. doi: 10.1111/j.1600-6143.2010.03062.x. [DOI] [PubMed] [Google Scholar]
- 18.Zuo HQ, Yan LN, Zeng Y, Yang JY, Luo HZ, Liu JW, Zhou LX. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:161–165. [PubMed] [Google Scholar]
- 19.Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, Yoon JH, Lee HS, Yi NJ, Suh KS, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69–75. doi: 10.1097/MCG.0b013e3181ce5dfa. [DOI] [PubMed] [Google Scholar]
- 20.Maximin S, Ganeshan DM, Shanbhogue AK, Dighe MK, Yeh MM, Kolokythas O, Bhargava P, Lalwani N. Current update on combined hepatocellular-cholangiocarcinoma. Eur J Radiol Open. 2014;1:40–48. doi: 10.1016/j.ejro.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle MB, Vachharajani N, Maynard E, Shenoy S, Anderson C, Wellen JR, Lowell JA, Chapman WC. Liver transplantation for hepatocellular carcinoma: long-term results suggest excellent outcomes. J Am Coll Surg. 2012;215:19–28; discussion 28-30. doi: 10.1016/j.jamcollsurg.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Shetty AS, Fowler KJ, Brunt EM, Agarwal S, Narra VR, Menias CO. Combined hepatocellular-cholangiocarcinoma: what the radiologist needs to know about biphenotypic liver carcinoma. Abdom Imaging. 2014;39:310–322. doi: 10.1007/s00261-013-0069-6. [DOI] [PubMed] [Google Scholar]
- 23.Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298–304. doi: 10.1016/j.jhep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Itoyama M, Hata M, Yamanegi K, Yamada N, Ohyama H, Hirano H, Terada N, Nakasho K. Expression of both hepatocellular carcinoma and cholangiocarcinoma phenotypes in hepatocellular carcinoma and cholangiocarcinoma components in combined hepatocellular and cholangiocarcinoma. Med Mol Morphol. 2012;45:7–13. doi: 10.1007/s00795-010-0534-z. [DOI] [PubMed] [Google Scholar]
- 26.Ogasawara S, Akiba J, Nakayama M, Nakashima O, Torimura T, Yano H. Epithelial cell adhesion molecule-positive human hepatic neoplastic cells: development of combined hepatocellular-cholangiocarcinoma in mice. J Gastroenterol Hepatol. 2015;30:413–420. doi: 10.1111/jgh.12692. [DOI] [PubMed] [Google Scholar]
- 27.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 28.Walther Z, Jain D. Molecular pathology of hepatic neoplasms: classification and clinical significance. Patholog Res Int. 2011;2011:403929. doi: 10.4061/2011/403929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, Kondo R, Nomura Y, Koura K, Ueda K, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496–505. doi: 10.1097/PAS.0b013e31827332b0. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda H, Harada K, Sato Y, Sasaki M, Yoneda N, Kitamura S, Sudo Y, Ooi A, Nakanuma Y. Clinicopathologic significance of combined hepatocellular-cholangiocarcinoma with stem cell subtype components with reference to the expression of putative stem cell markers. Am J Clin Pathol. 2013;140:329–340. doi: 10.1309/AJCP66AVBANVNTQJ. [DOI] [PubMed] [Google Scholar]
- 31.Kim GJ, Kim H, Park YN. Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS One. 2013;8:e75449. doi: 10.1371/journal.pone.0075449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim R, Kim SB, Cho EH, Park SH, Park SB, Hong SK, Chae G. CD44 expression in patients with combined hepatocellular cholangiocarcinoma. Ann Surg Treat Res. 2015;89:9–16. doi: 10.4174/astr.2015.89.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka Y, Aishima S, Kohashi K, Okumura Y, Wang H, Hida T, Kotoh K, Shirabe K, Maehara Y, Takayanagi R, et al. Spalt-like transcription factor 4 immunopositivity is associated with epithelial cell adhesion molecule expression in combined hepatocellular carcinoma and cholangiocarcinoma. Histopathology. 2016;68:693–701. doi: 10.1111/his.12806. [DOI] [PubMed] [Google Scholar]
- 34.Cai X, Zhai J, Kaplan DE, Zhang Y, Zhou L, Chen X, Qian G, Zhao Q, Li Y, Gao L, et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012;56:1804–1816. doi: 10.1002/hep.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii H, Zhu XG, Matsumoto T, Inagaki M, Tokusashi Y, Miyokawa N, Fukusato T, Uekusa T, Takagaki T, Kadowaki N, et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol. 2000;31:1011–1017. doi: 10.1053/hupa.2000.9782. [DOI] [PubMed] [Google Scholar]
- 36.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 37.Xue TC, Zhang BH, Ye SL, Ren ZG. Differentially expressed gene profiles of intrahepatic cholangiocarcinoma, hepatocellular carcinoma, and combined hepatocellular-cholangiocarcinoma by integrated microarray analysis. Tumour Biol. 2015;36:5891–5899. doi: 10.1007/s13277-015-3261-1. [DOI] [PubMed] [Google Scholar]
- 38.You HL, Weng SW, Li SH, Wei YC, Sheu JJ, Chen CM, Huang WT. Copy number aberrations in combined hepatocellular carcinoma and cholangiocarcinoma. Exp Mol Pathol. 2012;92:281–286. doi: 10.1016/j.yexmp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Homayounfar K, Gunawan B, Cameron S, Haller F, Baumhoer D, Uecker S, Sander B, Ramadori G, Lorf T, Füzesi L. Pattern of chromosomal aberrations in primary liver cancers identified by comparative genomic hybridization. Hum Pathol. 2009;40:834–842. doi: 10.1016/j.humpath.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanché H, Franco D, Monges G, Belghiti J, Sa Cunha A, Laurent-Puig P, et al. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41:292–298. doi: 10.1016/j.jhep.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Coulouarn C, Cavard C, Rubbia-Brandt L, Audebourg A, Dumont F, Jacques S, Just PA, Clément B, Gilgenkrantz H, Perret C, et al. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis. 2012;33:1791–1796. doi: 10.1093/carcin/bgs208. [DOI] [PubMed] [Google Scholar]
- 42.Woo HG, Lee JH, Yoon JH, Kim CY, Lee HS, Jang JJ, Yi NJ, Suh KS, Lee KU, Park ES, et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res. 2010;70:3034–3041. doi: 10.1158/0008-5472.CAN-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujimoto A, Furuta M, Shiraishi Y, Gotoh K, Kawakami Y, Arihiro K, Nakamura T, Ueno M, Ariizumi S, Nguyen HH, et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120. doi: 10.1038/ncomms7120. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Yang W, Yan HX, Luo T, Zhang J, Tang L, Wu FQ, Zhang HL, Yu LX, Zheng LY, et al. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated HBx transgenic mice. Hepatology. 2012;55:108–120. doi: 10.1002/hep.24675. [DOI] [PubMed] [Google Scholar]
- 45.Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.O’Connor K, Walsh JC, Schaeffer DF. Combined hepatocellular-cholangiocarcinoma (cHCC-CC): a distinct entity. Ann Hepatol. 2014;13:317–322. [PubMed] [Google Scholar]
- 47.Tanaka K, Honna T, Kitano Y, Kuroda T, Tanaka K, Morikawa N, Matsuda H, Kawashima N, Matsuoka K, Miyauchi J. Combined fibrolamellar carcinoma and cholangiocarcinoma exhibiting biphenotypic antigen expression: a case report. J Clin Pathol. 2005;58:884–887. doi: 10.1136/jcp.2004.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular-cholangiocarcinoma. Liver Int. 2015;35:1024–1035. doi: 10.1111/liv.12563. [DOI] [PubMed] [Google Scholar]
- 49.Dusenbery D. Combined hepatocellular-cholangiocarcinoma. Cytologic findings in four cases. Acta Cytol. 1997;41:903–909. doi: 10.1159/000332726. [DOI] [PubMed] [Google Scholar]
- 50.Wee A, Nilsson B. Combined hepatocellular-cholangiocarcinoma. Diagnostic challenge in hepatic fine needle aspiration biopsy. Acta Cytol. 1999;43:131–138. doi: 10.1159/000330966. [DOI] [PubMed] [Google Scholar]
- 51.Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34–40. doi: 10.1111/j.1440-1746.1998.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 52.Akiba J, Nakashima O, Hattori S, Naito Y, Kusano H, Kondo R, Nakayama M, Tanikawa K, Todoroki K, Umeno Y, et al. The expression of arginase-1, keratin (K) 8 and K18 in combined hepatocellular-cholangiocarcinoma, subtypes with stem-cell features, intermediate-cell type. J Clin Pathol. 2016;69:846–851. doi: 10.1136/jclinpath-2015-203491. [DOI] [PubMed] [Google Scholar]
- 53.Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934–942. doi: 10.1002/lt.22307. [DOI] [PubMed] [Google Scholar]
- 54.Park YH, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Namgoong JM, et al. Long-term outcome of liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma. Transplant Proc. 2013;45:3038–3040. doi: 10.1016/j.transproceed.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 55.Groeschl RT, Turaga KK, Gamblin TC. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J Surg Oncol. 2013;107:608–612. doi: 10.1002/jso.23289. [DOI] [PubMed] [Google Scholar]
- 56.Chan AC, Lo CM, Ng IO, Fan ST. Liver transplantation for combined hepatocellular cholangiocarcinoma. Asian J Surg. 2007;30:143–146. doi: 10.1016/S1015-9584(09)60149-4. [DOI] [PubMed] [Google Scholar]
- 57.Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952–959. doi: 10.1002/lt.23897. [DOI] [PubMed] [Google Scholar]
- 58.Kim KH, Lee SG, Park EH, Hwang S, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Kim KM, et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol. 2009;16:623–629. doi: 10.1245/s10434-008-0278-3. [DOI] [PubMed] [Google Scholar]
- 59.Song S, Moon HH, Lee S, Kim TS, Shin M, Kim JM, Park JB, Kwon CH, Kim SJ, Lee SK, et al. Comparison between resection and transplantation in combined hepatocellular and cholangiocarcinoma. Transplant Proc. 2013;45:3041–3046. doi: 10.1016/j.transproceed.2013.08.064. [DOI] [PubMed] [Google Scholar]


