Abstract
Mitochondrial dysfunction and associated oxidative stress is strongly linked to cardiovascular, neurodegenerative, and age associated disorders. More specifically cardiovascular diseases are common in patients with diabetes and significant contributor to the high mortality rates associated with diabetes. Studies have shown that the heart failure risk is increased in diabetic patients even after adjusting for coronary artery disease and hypertension. Although the actual basis of the increased heart failure risk is multifactorial, increasing evidences suggest that imbalances in mitochondrial function and associated oxidative stress play an important role in this process. This review summarizes these abnormalities in mitochondrial function and discusses potential underlying mechanisms.
1. Introduction
Over the last 20 years, our understanding of the pathophysiology of chronic heart failure has advanced substantially. However, heart disease still remains the number one cause of morbidity and mortality in the industrialized world, affecting over 27 million people in the United States alone[1]. Furthermore, the prevalence of Type 2 diabetes is reaching pandemic proportions, with estimates that by the year 2025 nearly 300 million adults will be affected by diabetes mellitus [2, 3]. Patients with diabetes are at increased risk of cardiovascular diseases associated mortality [4, 5]. Previously, it has been shown that patients with chronic heart failure and type 2 diabetes have almost two times higher risk of all cause of mortality that of similar patients without diabetes. To put this in context, if a patient with chronic heart failure (CHF) suffers from type 2 diabetes, their risk of cardiovascular-associated death is over two times higher [4, 6]. Interestingly, in many cases diabetic patients also develop heart failure even in the absence of cardiovascular risk factors such as hypertension and coronary artery disease [7, 8]. Recently the term “diabetic cardiomyopathy” is used to refer the cardiovascular dysfunction in diabetic patients that is out of proportion to their underlying vascular disease [9]. Mitochondria serve as the power houses of a cell and recent reports implicate mitochondrial injury to be a major player in the pathophysiology of diabetic heart disease [10, 11]. Therefore strategies to attenuate mitochondrial injury might be a potential therapeutic target for diabetic heart disease.
2. Mitochondrial dysfunction in diabetic heart
The mitochondrion serves a critical role as a platform for energy transduction, signaling, and cell death pathways related to common cardiovascular diseases such as heart failure [12]. Cardiac dysfunction in diabetic patients is caused by multiple pathologic mechanisms. Interestingly, all these mechanisms are associated with mitochondrial injury, which has been proposed to be an underlying cause, in the pathophysiology of diabetic heart disease [10, 11]. Indeed, numerous animal and human studies demonstrated the frequent appearance of damaged mitochondria in the diabetic hearts [13–15]. Dysfunctional mitochondria can cause more ROS production and release pro-death factors such as cytochrome C, apoptosis inducing factor, and Smac/DIABLO [15, 16]. Various ROS scavengers or antioxidants are able to reduce cardiomyocyte death and attenuate diabetic cardiac injury in experimental animal models [16, 17]. However, the antioxidant-based therapies have generally not been successful in diabetic patients [18, 19], suggesting that simply antagonizing existing ROS by antioxidants is not sufficient to abrogate diabetic cardiac injury. A potentially more effective treatment strategy may be to enhance the overall capacity of mitochondrial quality control to maintain a pool of healthy mitochondria that are needed for supporting cardiac contractile function in diabetic patients.
Recent evidences suggest that cardiac dysfunction in diabetic patients is linked to metabolic abnormalities and more often associated with mitochondrial dysfunction [20]. Diabetes and obesity, the major metabolic disorders, are characterized by high levels of circulating free fatty acids, which results in increased cardiac fatty acid uptake, storage and metabolism [21–23]. In heart, free fatty acids have taken up by cardiac cells such as cardiomyocytes, which are normally catabolized in mitochondrial and in some circumstances, peroxisomal fatty acid β-oxidation (FAO) pathways. Fatty acids are also incorporated into triglycerides (TAG) pools and are ultimately oxidized through β-oxidation flux [24, 25]. Peroxisome proliferator-activated receptor alpha (PPARα), which is upregulated in diabetic hearts, plays significant role in modulating TAG flux [21, 24, 25]. In general, heart does not store significant amounts of lipid, however, it can accumulate triglycerides when fatty acid supply is high. Both in diabetic patients and animal models, the myocardial triglyceride content is notably increased compared to healthy controls [26–28].
Myocardial energy substrate preference (glucose versus fatty acid) normally varies in a dynamic manner to meet the tremendous energy needs of the mammalian heart. In healthy heart vast majority of ATP is generated by oxidation of fatty acids (FAs) and glucose in mitochondria [29] [30–32]. During normal circumstances, nonesterified or free fatty acids (FAs) are the preferred substrate in the adult myocardium, supplying 60–90% of total ATP [29, 33–36]. FAs derived from circulating triglyceride-rich lipoproteins and albumin bound nonesterified FAs are oxidized in the mitochondrial matrix by FA β-oxidation (FAO) process, while pyruvate derived from glucose and lactate is oxidized by the pyruvate-dehydrogenase (PDH) complex, present within the inner mitochondrial membrane. The final product, acetyl-CoA, derived from both pathways, ultimately enters the tricarboxylic acid (TCA) cycle to generate ATP [12, 37]. In heart failure with reduced ejection fraction, both animal models and human studies demonstrate alterations in the otherwise versatile capacity of the myocardium to use alternative substrates. Emerging data demonstrated a reduced cardiac fatty acid use during heart failure. Previous studies in different heart failure models showed a reduced mRNA and protein expression of FA transporters [38–41]. FA uptake has been reported to reduce both in high-salt-diet-induced heart failure and by rapid pacing [39, 42]. Finally both in animal models and human subjects strongly advocate that FA oxidation is significantly reduced during cardiovascular abnormalities [38–41]. In contrast, the data on cardiac glucose use are less consistent [43–45]. In the presence of systolic dysfunction, cardiac glucose uptake was decreased in mice after aortic constriction [43], while unchanged in rats with myocardial infarction [46], and increased in Dahl salt-sensitive rats [39]. The impaired glucose oxidation that parallels systolic dysfunction might be attributable in part to mitochondrial dysfunction, reduced expression of genes involved in glycolysis and glucose oxidation, or decreased abundance of the PDH complex [39, 47]. Osorio et al showed increased glucose oxidation rates in failing dog hearts induced by rapid pacing [46], and Dávila-Román et al demonstrated higher total rates of glucose use in patients with idiopathic dilated cardiomyopathy [48]. Thus the changes in glucose oxidation in cardiac myocytes may depend on both the stage and the pathogenesis of heart failure.
Interestingly, during uncontrolled diabetes, cardiac energy substrate preference becomes constrained because of the need for insulin for myocardial glucose uptake. Glucose utilization in the diabetic heart is diminished at least in part because of insulin resistance, impaired pyruvate dehydrogenase activity, and reduced glucose transporter (e.g. Glut4). Thus, the diabetic heart relies almost exclusively on mitochondrial FAO for ATP synthesis. This reliance on FAO has potentially detrimental consequences, which includes impaired mitochondrial respiratory function. Mitochondria are the center of both fatty acid and glucose metabolism and thus are likely to be impacted by impaired metabolism associated with diabetes. Previously studies have demonstrated the mitochondrial abnormalities in skeletal muscle of insulin resistant and diabetic humans. Furthermore, reduced expression of targets genes associated with mitochondrial oxidative phosphorylation (OXPHOS) [49–51] peroxisome-proliferator-activated receptor (PPAR) gamma, co-activator-1α (PGC-1α) was observed during heart failure in diabetes [52]. PGC-1α is a master metabolic regulator that coordinates gene expression for pathways involved in mitochondrial biogenesis and respiratory function (Figure-1) [52]. Shulman and colleagues demonstrated a reduction in ATP synthesis and mitochondrial content in severely insulin-resistant offspring of Type 2 diabetes [53, 54]. Kelley et al., found impaired mitochondrial enzyme activities and reduced mitochondrial size and number in skeletal muscle from diabetic patients [55, 56]. In sum, these studies strongly implicate impaired mitochondrial function and biogenesis both in diabetic animals and human patients.
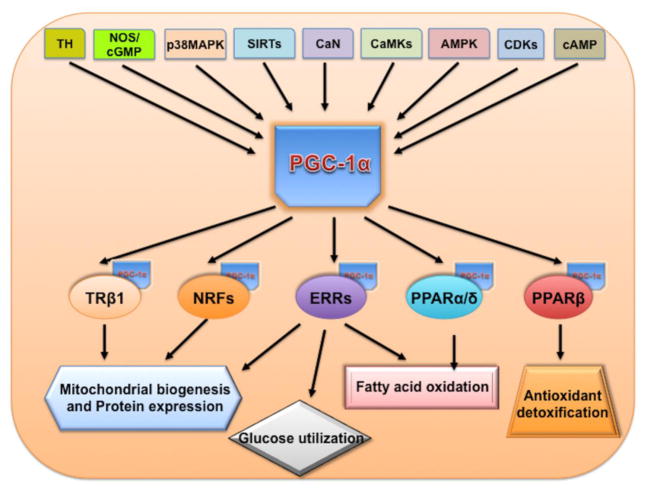
Figure 1.
PGC-1α regulatory cascade. Thyroid hormone, nitric oxide synthase, MAP kinase, sirtuins, calcinurin (CaN), CAMK, AMPK, CDK and β-adrenergic stimulus have been shown to influence the PGC1α activity. PGC1α then co-activate transcription factors such as PPARα/β, NRFs, TRb1 etc which is known to regulate different aspects of energy metabolism including mitochondrial biogenesis, fatty acid oxidation and antioxidant defense system.
The mitochondrial function has been directly studied in multiple animal models of diabetes. In chronic Type 1 diabetes (OVE26 mice) mice model, it was demonstrated that mice had evidence of mitochondrial biogenesis that was coupled to a reduction in mitochondrial function and mitochondrial ultrastructural defects [57]. Mitochondrial state III respiration is significantly reduced in type 2 diabetic animal models (db/db and ob/ob) [58–60]. Furthermore, there is evidence for increased cardiac mitochondrial biogenesis with ultrastructural defects in insulin resistance and diabetes animals [59, 61–64]. In summary, the animal model investigations provide precise and convincing evidence that mitochondrial function is impaired in the hearts of animals with insulin resistance and diabetes. In contrast, due to limited availability of human heart samples and multiple variant, cardiac mitochondrial function has been under studied in human subjects. Nonetheless, a number of studies provide indirect evidence for altered cardiac mitochondrial function in diabetic patients. Diamant et al. studied high-energy phosphate metabolism and cardiac function in asymptomatic well-controlled diabetic men and controls using MRI and 31P nuclear magnetic resonance spectroscopy (NMRS). They demonstrated a reduction in multiple indexes of diastolic function by MRI in the diabetic patients; these functional changes were associated with a reduction in the cardiac phosphocreatine/ATP ratios [65]. A reduction in cardiac phosphocreatine/ATP ratios has also been demonstrated in hearts of diabetic patients with normal cardiac function by echocardiography [66, 67], suggesting that changes in mitochondrial function may precede the reduction in contractility. In another study, Anderson et al. demonstrated in the left atrial appendage tissue from Type 2 diabetic patients undergoing coronary bypass surgery that mitochondrial respiratory function was impaired and hydrogen peroxide emission was increased, suggesting an increase in oxidative stress [68, 69]. Together with human data demonstrating altered lipid metabolism, these studies strongly implicate mitochondrial dysfunction in the human diabetic heart. In following sections, the potential mechanisms that contribute to mitochondrial dysfunction and leading to cardiovascular abnormalities in diabetes will be discussed.
3. Mechanism of mitochondrial dysfunction in diabetic heart
3.1. Altered energy metabolism
Heart is maximum energy consuming organ of the body and thus a subtle energy deficits can rapidly induced contractile dysfunction. The uninterrupted ATP generation is dependent on the continuous supply of oxygen and fuel substrates and on the integrity of oxidative phosphorylation (OxPhos), which produces virtually all the hearts’ ATP. [70–72] While the heart can switch its substrate [fatty acids (FAs), glucose, ketones, lactate, amino acids] preference depending on workload, oxygen supply and hormones, its main energetic substrate is FAs (60–70%) as discussed in preceding section. Due to high-energy demand, cardiomyocytes has a relatively higher number of mitochondria compared to other cells. Due to insulin resistance in type 2 diabetes, diabetic heart has increased rate of fatty acid oxidation. Previous studies have shown an increased expression of nuclear receptor transcription factor, PPARα/δ/β. PPARs is an important transcriptional regulator of fatty acid uptake and oxidation. In fact PPARα regulates most of the enzymes involved in fatty acid oxidation. PPARα knock out mice showed reduced fatty acid oxidation rate. Interestingly, cardiac specific PPARα overexpression significantly reduced enzymes involved in mitochondrial oxidative phosphorylation. Sack et al. [73] reported the down-regulation of genes required for myocardial FAs use in human hearts and rats with progressive heart failure. Davila-Roman et al. used in vivo imaging with positron emission tomography (PET) to confirm reduced FAs oxidation with increased dependence on glucose metabolism in patients with compensated dilated cardiomyopathy [48]. As heart failure progresses, myocardial insulin resistance develops [74], further compromising the versatility of substrate use and increasing the metabolic stress on the heart. Importantly, chronic heart failure patients with decreased systemic insulin sensitivity have a worse prognosis [75]. Notably, increase in FAO may be detrimental, as it requires more oxygen and generate plenty of ROS (Figure 2). Intriguingly, diabetic animals showed reduced cardiac efficiency, with increased myocardial oxygen consumption (VO2) associated with increased FAO [59, 76–78]. The increased demand for oxidizing fatty acids and the reduction in cardiac efficiency may contribute to contractile dysfunction in the diabetic heart. Furthermore, the altered substrate flexibility and the change in oxygen consumption potentially may contribute to increased mortality following ischemic damage in diabetic patients.
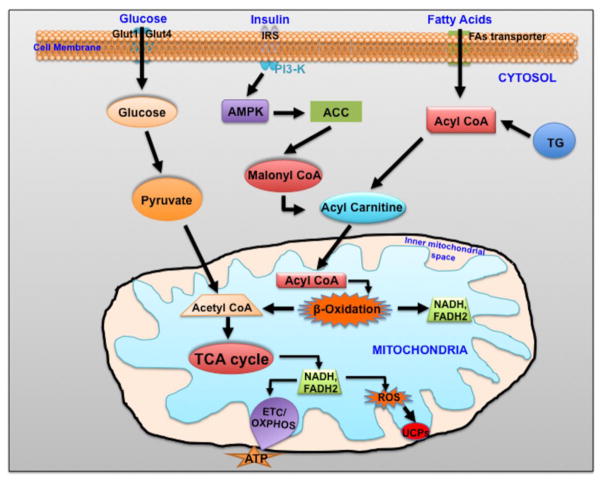
Figure 2.
Schematic diagram showing cardiomyocyte energetics. In the diabetic heart, high free fatty acids and insulin resistance alleviated FAs oxidation. ACC: Acetyl-Coenzyme A carboxylase, AMPK: 5AMP-activated protein kinase, CoA: Coenzyme A, FAs: Fatty acids, GLUT: glucose transporter, PI3K: Phosphatidylinositol 3-Kinase, ROS: Reactive oxygen species, TCA: Tricarboxylic acid cycle, TG: triglycerides: UPCs: uncoupling proteins.
Mitochondrial oxygen consumption is normally tightly coupled to ATP synthesis (via electron transport chain). The energy that is produced during electron transfer is used to create an electrochemical gradient by pumping protons from the mitochondrial matrix to the inter membrane space. These protons generally reenter the matrix via the ATP synthase/complex V (proton pump) and generate ATP from ADP (Figure 2). However, sometimes it is possible that protons bypass the ATPase system and reenter into matrix using uncoupling proteins (such as UCP-1, 2, 3, 4 and 5) [76]. This bypass system results in oxygen consumption that is not coupled to ATP production [79]. Recently, Boudina et al. demonstrated mitochondrial uncoupling in db/db mouse hearts [80]. This group has noted an increase in respiration in the setting of oligomycin, an inhibitor of the ATP synthase and an increase in proton leak from cardiac mitochondria isolated from db/db mice. Adding guanosine diphosphate (GDP), an inhibitor of UCPs, resulted in restoration of proton leak to wild-type levels, strongly suggesting that the increased uncoupling was mediated by UCPs. A second potential mediator of mitochondrial uncoupling is the adenine nucleotide translocator (ANT). Boudina et al. also found that atractyloside, an inhibitor of ANT-mediated uncoupling, altered proton leak in db/db mitochondria, suggesting that ANT may also contribute to uncoupling in diabetic hearts [80].
3.2. Oxidative stress and derangement of oxidative phosphorylation
Both preclinical and clinical studies suggest that ROS production is significantly enhanced in the failing myocardium [81, 82]. The majority of ROS in the heart appear to come from uncoupling of mitochondrial electron transport chain at the level of complexes I and III [81, 83, 84]. The activities of mitochondrial electron transport chain complexes are suppressed in HF, and disruption of mitochondrial bioenergetics function was found to increase ROS levels and oxidative DNA damage [85, 86], providing a possible pathophysiological link between mitochondrial dysfunction and ROS (Figure 2) [82, 87]. Thus, ROS generated by the mitochondria have the ability to modify multiple additional physiologic pathways. ROS can directly damage proteins by oxidation, or they can oxidize lipids to form lipid peroxidation products, which can induce protein or phospholipid damage. Therefore deficiencies in antioxidant system during diabetes may enhance the oxidative damage in cardiac cells. Previous studies have shown that mitochondrial ROS activates multiple pathways related to cellular damage in the setting of hyperglycemia [88–91]. An increase in 3-nitrotyrosine in association with increased cell death has been noted in human myocardial samples [15, 92], as well as in a streptozotocin (STZ)-induced model of Type 1 diabetes [93]. Indeed, Cai et al. demonstrated a reduction in nitrosative damage with overexpression of metallothionein, an antioxidant protein, in STZ-treated mice [94]. Furthermore, Boudina et al. have reported an increase in mitochondrial H2O2 in db/db mice, in association with increased levels of MnSOD, suggesting that there is an increase in ROS production [80]. In addition, catalase overexpression protected cardiomyocyte contractility in the agouti model of type 2 diabetes. [95]. Taken together, studies discussed above suggest the potential role of oxidative stress in the myocardial dysfunction in diabetics.
3.3. MicroRNAs in regulation of mitochondrial dysfunction in diabetic heart
Recent studies have highlighted the therapeutic potential of microRNAs (miRNA) in diabetic cardiomyopathy [96], [97]. We and others have previously shown that microRNAs (miR) regulates multiple cellular processes such as proliferation, differentiation, cell metabolism, apoptosis and angiogenesis [98–104]. This review also summarizes the knowledge on the effects of miRNAs in diabetic cardiomyopathy.
miRNAs’ biogenesis is characterized by a cleavage process catalyzed by Drosha, an RNase III, and its essential cofactor known as DGCR8 (DiGeorge syndrome critical region) [105–107]. After the cleavage process, an intermediate stem loop is released, known as the miRNA precursor or pre-miRNA. Pre miRNA hairpins are then recognized by Exportin-5 for nuclear export. At the cytoplasm, the pre-miRNA is cleaved by Dicer, an RNase III, into the mature form of 20–22 nucleotides[108]. This RNA is subsequently unwound by a helicase activity, binds to an Argonaute protein and gets incorporated as single-stranded RNA into the RNA-induced silencing complex (RISC) [108] which directs the miRNA to complementary sites within the 3′ UTRs (untranslated region) of target mRNAs leading to translational repression or degradation of the target mRNA [102, 109]. Interestingly, miRNAs can act as translational activators as well [26]. Based on computational algorithms, around 60% of human transcripts contain potential miRNA-binding sites within their 3′ UTRs. A “seed sequence” in the 5′ end of the mature miRNA pairs to nucleotides 2 through 8 at the 3′ UTR of target mRNAs [108]. However, miRNAs can interact with 5′ UTRs, protein-coding sequences and introns. Furthermore, miRNAs can also localize to the nucleus, where they may regulate transcription/splicing of transcripts, or serve as signaling molecules between two cells through exosome transfer[110]. Although a single miRNA can target many genes, it is possible that multiple miRNAs can regulate a single gene. These studies suggest that miRNA transcription to maturation involves several coordinated steps and that deregulation in miRNA biogenesis and function might contribute to the development of cardiovascular diseases [111, 112].
Role of miRs in regulation of multiple cardiac remodeling genes has been well studied, however, a functional link between miRNA and diabetes-induced cardiac dysfunction is not well established. Recently altered miRs expression has been reported in isolated cardiac cells from diabetic rat heart [96]. Furthermore, antioxidant therapy (NAC treatment) significantly restored miRs (MiR-1, -133a, -133b, -499) expression and thus protects against diabetes-induced injury [96]. miRNA-141 plays important role by targeting inner mitochondrial membrane phosphate transporter Slc25a3 (solute carrier family-25 member 3) gene, which provide inorganic phosphate to mitochondrial matrix and thus essential for mitochondrial ATP production. Further, Baseler et. al have demonstrated an elevated miR-141 levels in diabetic mouse heart [97]. Thus further concluded that, miRNA-141 can regulate Slc25a3 protein expression in diabetic heart and could be involved in the pathogenesis of diabetic cardiomyopathy [97]. Therefore inhibition of miR-141 could be a potential target to improve mitochondrial function in diabetic heart. A recent study has demonstrated an increased expression of miR-223 in left ventricular biopsies from diabetic patients. Further miR-223 directly targets Glut-4 mediated glucose metabolism in the heart [113]. Shan et al. (2010) demonstrated that the high glucose induced expression of miR-1 and miR-206 in cardiomyocytes. Further induction of the miR’s resulted in increased cardiomyocyte cell death via directly targeting Hsp60 expression, contributing to hyperglycemia-induced cell death in cardiomyocytes [114]. Ingenuity miRNA pathway analysis in diabetic mice revealed that dysregulated miRNAs were implicated in myocardial signaling networks which can trigger apoptosis (miR-320b, miR-378, miR-34a), fibrosis (miR-125b, miR-150, miR-199a, miR-29b, miR30a), hypertrophic growth (miR-1, miR-150, miR-199a, miR-133a, miR-214, miR-29a, miR-125b, miR-221, miR-212), autophagy (miR-133a, miR-221, miR-212, miR30a), oxidative stress (miR-221, miR-146a, miR-34a, miR-210, miR-19b, miR-125b, miR27a, miR-155), and heart failure (miR-423, miR-499, miR-199a) [115]. These findings further signify the importance of miRNA in the diabetic heart disease.
4. Clinical relevance and future prospective
As unexplained cardiomyopathy is possibly driven by T2DM itself, good diabetic control seems naturally important for preventing dilated cardiomyopathy. However, only animal studies have shown that achieving early normal glucose levels reduces the progress of diabetes associated heart failure, and that certain diabetic drugs can have specific anti-cardiac remodeling effects.[116, 117]. For example, in ex vivo mice hearts, glucose–insulin infusions improved glucose oxidation and contractile efficiency, while incretin based therapies (Liraglutide and Exendin-4) reversed oxidative stress and SERCA down-regulation [118, 119]. Very limited data is available in humans, and some of them are not reproduced with animal’s findings. In a retrospective analysis, metformin use was associated with reduced natriuretic peptide (NPs) levels and lower cardiovascular morbidity and mortality [120, 121] while glucose–insulin–potassium trials yielded inconsistent results. [122] In contrast, increased hospitalizations due to heart failure were documented with thiazolidinediones (e.g. rosiglitazone) and sulfonyureas (e.g. glicazide). [122, 123] Thus, glycemic control alone might not be sufficient for preventing or managing heart failure. Because systolic dysfunction develops as diastolic cardiomyopathy progresses, and can itself accelerate DiCM independently by inducing mitochondrial dysfunction and oxidative stress, the mandated use of HF specific therapies is critical. They not only disrupt the vicious cycle between systolic failure and diastolic cardiomyopathy but certain agents such as β-blockers directly suppress oxidative stress and FAs metabolism [124]. However, because these agents are not suggested in the majority of patients (as their LV ejection fractions are adequate), alternative strategies are needed.
As discussed above, due to disrupted metabolism, increased FAs utilization occurs during Type 2 diabetes. Therefore, in cardiac cells, FAs metabolism can be regulated by drugs, which alter plasma-free FAs levels (lipoprotein lipase inhibitors), mitochondrial FAs uptake (CPT1 inhibitors) or FAs oxidation (β-oxidation inhibitors). In rats with Type 2 diabetes, acipimox and etomoxir reduced serum lipid levels and improved SERCA expression [125]. In diabetic heart failure patients, trimetazidine reduced natriuretic peptides and improved exercise capacity and LV function despite no change in cardiac perfusion [126, 127]. In a large clinical trial, ranolazine relieved angina more in diabetic than in non-diabetic patients [128]. Ranolazine also recovered LV function quicker in diabetic than in non-diabetic rats after myocardial infarction, with the benefits related to activation of the energy sensor, adenosine monophosphate kinase. [129] Besides inhibiting FAs utilization, directly stimulating glucose oxidation with dichloroacetate, a pyruvate dehydronase (PDH) activator, could also rebalance cardiac substrate uses in T2DM [130, 131].
Alternatively, inhibition of mitochondrial oxidative could be a potential therapeutic target to inhibit diabetic cardiomyopathy. In cell culture models of glucotoxicity and gluco-lipotoxicity, MitoQ (a mitochondrial targeted antioxidant) reduces oxidative stress, enhances oxidative phosphorylation (OxPhos) and stimulates cells survival. [132, 133] Alternatively, oxidative stress could be attenuated by boosting antioxidant defense system with agents such as resveratrol or N-acetyl cysteine (NAC), which improved cardiac oxidative phosphorylation in diabetic animals [134, 135]. Furthermore, direct ETC stimulation could also increase oxidative phosphorylation and improve heart function in diabetes.
Intriguingly, therapies designed to increase glucose uptake by overcoming insulin resistance or other mechanisms hold promise for improving myocardial energetics and preventing the progression of contractile abnormalities in heart. In this context, glucagon-like peptide (GLP)-1, a naturally occurring incretin peptide, enhances glucose uptake by stimulating insulin secretion and by enhancing insulin sensitivity in target tissues [136–138]. Administration of exogenous GLP-1 by continuous infusion in patients with type 2 diabetes causes an impressive increase in insulin sensitivity in both skeletal muscle and adipose tissue, with substantial improvements in both insulin-mediated glucose uptake [138] and insulin-independent glucose uptake [139]. Receptors for GLP-1 have also been identified in human myocardium [140], thereby identifying the heart as a potential target for GLP-1 action. Although native GLP-1 is very unstable, degradation resistant GLP-1 analogues are now widely used in clinic for the treatment of type 2 diabetes. Recently, LePore et al. [141] had shown that GPL-10 agonist (albiglutide) ameliorates myocardial metabolic abnormalities in chronic heart failure.
Conclusion
Mitochondria are taking the center stage in cardiovascular research for novel therapeutics, as their dysfunction appears early and invariably in the development of hypertrophy and HF (Figure 3). Maintenance of mitochondrial integrity and biogenesis against cardiac insults and reduction in mitochondrial ROS production during diabetic cardiomyopathy are the promising directions to take in account for therapy. Although, in past decades, much advancement has been made in biomedical and pharmaceutical research, still several questions remained unanswered and suggest us for more systematic preclinical and clinical investigations to develop the better therapeutics to control the cardiovascular complications in diabetic patients.
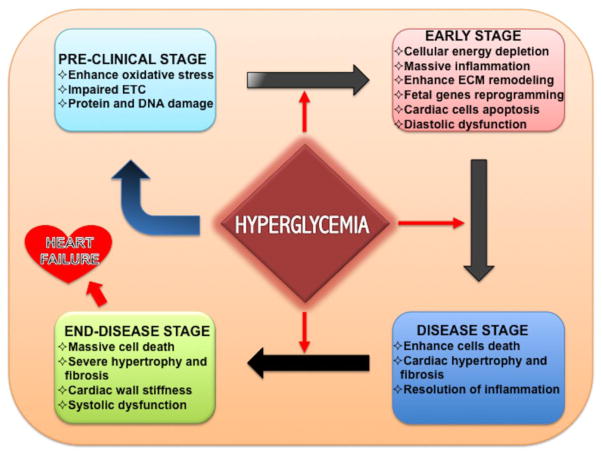
Figure 3.
Stages of heart failure in diabetics. Early stress stimulus enhances inflammatory milieu in myocardium, which triggers the mitochondrial dysfunction, oxidative stress and ultimately heart failure.
Highlights.
Mitochondrial function is impaired in diabetic cardiomyopathy.
Dysfunctional mitochondria triggers cardiac cells death in diabetic heart disease.
Fatty acid and glucose metabolism are impaired in diabetic heart disease.
PGC-1α a master metabolic regulator of mitochondrial biogenesis and respiratory function is dysregulated in diabetic cardiomyopathy.
Dysregulated microRNAs contribute to impaired mitochondrial function diabetic heart.
Acknowledgments
Work described in this manuscript was in part supported by National Institute of Health grants HL091983, HL126186, HL053354 and HL108795 (RK), American Heart Association- Scientist Development Grant 14SDG20480104 (SKV) and American Heart Association postdoctoral grant 15POST22720022 (VNSG).
Abbreviations
- CHF
Chronic heart failure
- ROS
Reactive oxygen species
- TGA
Triglycerides
- FAO
Fatty acid β oxidation
- PDH
Pyruvate dehydrogenase
- TCA
Tricarboxylic acid
- ATP
Adenosine triphosphate
- ADP
Adenosine diphosphate
- GDP
Guanosine diphosphate
- OXPHOS
Oxidative phosphorylation
- PPARα
Peroxisome proliferator-activated receptor alpha
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- ANT
Adenine nucleotide translocator
- miR
micoRNA
- NAC
N-acetyl cysteine
- Slc25a3
solute carrier family-25 member 3
- T2DM
type-2 diabetes
- GLP-1
Glucagon-like peptide
- ACC
Acetyl-Coenzyme A carboxylase
- AMPK
5AMP-activated protein kinase
- CoA
Coenzyme A
- FAs
Fatty acids
- GLUT
glucose transporter
- PI3K
Phosphatidylinositol 3-Kinase
- ROS
Reactive oxygen species
- UPCs
Uncoupling proteins
Footnotes
Disclosure: Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB C. American Heart Association Statistics, S. Stroke Statistics. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Annals of internal medicine. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 4.Kearney MT. Chronic heart failure and type 2 diabetes mellitus: The last battle? Diabetes & vascular disease research. 2015;12:226–227. doi: 10.1177/1479164115590324. [DOI] [PubMed] [Google Scholar]
- 5.Liang Q, Kobayashi S. Mitochondrial quality control in the diabetic heart. Journal of molecular and cellular cardiology. 2016;95:57–69. doi: 10.1016/j.yjmcc.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubbon RM, Woolston A, Adams B, Gale CP, Gilthorpe MS, Baxter PD, Kearney LC, Mercer B, Rajwani A, Batin PD, Kahn M, Sapsford RJ, Witte KK, Kearney MT. Prospective development and validation of a model to predict heart failure hospitalisation. Heart. 2014;100:923–929. doi: 10.1136/heartjnl-2013-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. Journal of cardiac failure. 2008;14:615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. Journal of the American College of Cardiology. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 9.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 10.Schilling JD. The mitochondria in diabetic heart failure: from pathogenesis to therapeutic promise. Antioxidants & redox signaling. 2015;22:1515–1526. doi: 10.1089/ars.2015.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants & redox signaling. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. The Journal of clinical investigation. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita M, Mukae S, Geshi E, Umetsu K, Nakatani M, Katagiri T. Mitochondrial respiratory impairment in streptozotocin-induced diabetic rat heart. Japanese circulation journal. 1996;60:673–682. doi: 10.1253/jcj.60.673. [DOI] [PubMed] [Google Scholar]
- 14.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clinical science. 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 15.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circulation research. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 16.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 17.Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, Salio M, Savino C, Melucci S, Santangelo F, Scanziani E, Masson S, Ghezzi P, Latini R. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. Journal of molecular and cellular cardiology. 2004;37:959–968. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR Hope H-TT Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. The New England journal of medicine. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 20.Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends in cardiovascular medicine. 2016;26:165–179. doi: 10.1016/j.tcm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Hafstad AD, Khalid AM, Hagve M, Lund T, Larsen TS, Severson DL, Clarke K, Berge RK, Aasum E. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovascular research. 2009;83:519–526. doi: 10.1093/cvr/cvp132. [DOI] [PubMed] [Google Scholar]
- 22.Malfitano C, de Souza AL, Junior, Carbonaro M, Bolsoni-Lopes A, Figueroa D, de Souza LE, Silva KA, Consolim-Colombo F, Curi R, Irigoyen MC. Glucose and fatty acid metabolism in infarcted heart from streptozotocin-induced diabetic rats after 2 weeks of tissue remodeling. Cardiovascular diabetology. 2015;14:149. doi: 10.1186/s12933-015-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal BR, Mehta AA. Diabetic cardiomyopathy: pathophysiological mechanisms and cardiac dysfuntion. Human & experimental toxicology. 2013;32:571–590. doi: 10.1177/0960327112450885. [DOI] [PubMed] [Google Scholar]
- 24.Banke NH, Wende AR, Leone TC, O’Donnell JM, Abel ED, Kelly DP, Lewandowski ED. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circulation research. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolwicz SC, Jr, Liu L, Goldberg IJ, Tian R. Enhancing Cardiac Triacylglycerol Metabolism Improves Recovery From Ischemic Stress. Diabetes. 2015;64:2817–2827. doi: 10.2337/db14-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 27.Dewald O, Sharma S, Adrogue J, Salazar R, Duerr GD, Crapo JD, Entman ML, Taegtmeyer H. Downregulation of peroxisome proliferator-activated receptor-alpha gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation. 2005;112:407–415. doi: 10.1161/CIRCULATIONAHA.105.536318. [DOI] [PubMed] [Google Scholar]
- 28.Niu YG, Evans RD. Myocardial metabolism of triacylglycerol-rich lipoproteins in type 2 diabetes. The Journal of physiology. 2009;587:3301–3315. doi: 10.1113/jphysiol.2009.173542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart failure reviews. 2002;7:115–130. doi: 10.1023/a:1015320423577. [DOI] [PubMed] [Google Scholar]
- 30.Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circulation Heart failure. 2012;5:493–503. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 31.Pellieux C, Montessuit C, Papageorgiou I, Pedrazzini T, Lerch R. Differential effects of high-fat diet on myocardial lipid metabolism in failing and nonfailing hearts with angiotensin II-mediated cardiac remodeling in mice. American journal of physiology Heart and circulatory physiology. 2012;302:H1795–1805. doi: 10.1152/ajpheart.01023.2011. [DOI] [PubMed] [Google Scholar]
- 32.Ardehali H, Sabbah HN, Burke MA, Sarma S, Liu PP, Cleland JG, Maggioni A, Fonarow GC, Abel ED, Campia U, Gheorghiade M. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. European journal of heart failure. 2012;14:120–129. doi: 10.1093/eurjhf/hfr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdurrachim D, Luiken JJ, Nicolay K, Glatz JF, Prompers JJ, Nabben M. Good and bad consequences of altered fatty acid metabolism in heart failure: evidence from mouse models. Cardiovascular research. 2015;106:194–205. doi: 10.1093/cvr/cvv105. [DOI] [PubMed] [Google Scholar]
- 34.Nickel A, Loffler J, Maack C. Myocardial energetics in heart failure. Basic research in cardiology. 2013;108:358. doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- 35.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. The Journal of clinical investigation. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. The American journal of medicine. 1954;16:504–515. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 37.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circulation research. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovascular research. 2010;86:461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 39.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circulation Heart failure. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 40.Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovascular research. 2006;72:430–437. doi: 10.1016/j.cardiores.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqi N, Singh S, Beadle R, Dawson D, Frenneaux M. Cardiac metabolism in hypertrophy and heart failure: implications for therapy. Heart failure reviews. 2013;18:595–606. doi: 10.1007/s10741-012-9359-2. [DOI] [PubMed] [Google Scholar]
- 42.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 43.Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovascular research. 2013;97:676–685. doi: 10.1093/cvr/cvs424. [DOI] [PubMed] [Google Scholar]
- 44.Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C, Abel ED. GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. Journal of molecular and cellular cardiology. 2014;72:95–103. doi: 10.1016/j.yjmcc.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sankaralingam S, Lopaschuk GD. Cardiac energy metabolic alterations in pressure overload-induced left and right heart failure (2013 Grover Conference Series) Pulmonary circulation. 2015;5:15–28. doi: 10.1086/679608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amorim PA, Nguyen TD, Shingu Y, Schwarzer M, Mohr FW, Schrepper A, Doenst T. Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. The Journal of thoracic and cardiovascular surgery. 2010;140:1160–1167. doi: 10.1016/j.jtcvs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT, MacCoss MJ, Rabinovitch PS. Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovascular research. 2012;93:79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 49.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kristensen JM, Skov V, Petersson SJ, Ortenblad N, Wojtaszewski JF, Beck-Nielsen H, Hojlund K. A PGC-1alpha- and muscle fibre type-related decrease in markers of mitochondrial oxidative metabolism in skeletal muscle of humans with inherited insulin resistance. Diabetologia. 2014;57:1006–1015. doi: 10.1007/s00125-014-3187-y. [DOI] [PubMed] [Google Scholar]
- 51.Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. International journal of molecular sciences. 2013;14:21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. The Journal of clinical investigation. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. The Journal of clinical investigation. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. The New England journal of medicine. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 56.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 57.Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr, Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. American journal of physiology Endocrinology and metabolism. 2004;287:E896–905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- 58.Pham T, Loiselle D, Power A, Hickey AJ. Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. American journal of physiology Cell physiology. 2014;307:C499–507. doi: 10.1152/ajpcell.00006.2014. [DOI] [PubMed] [Google Scholar]
- 59.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 60.Kuo TH, Moore KH, Giacomelli F, Wiener J. Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes. 1983;32:781–787. doi: 10.2337/diab.32.9.781. [DOI] [PubMed] [Google Scholar]
- 61.Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1alpha and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:3225–3237. doi: 10.1096/fj.13-245241. [DOI] [PubMed] [Google Scholar]
- 62.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circulation research. 2014;114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitra R, Nogee DP, Zechner JF, Yea K, Gierasch CM, Kovacs A, Medeiros DM, Kelly DP, Duncan JG. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. Journal of molecular and cellular cardiology. 2012;52:701–710. doi: 10.1016/j.yjmcc.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. Journal of the American College of Cardiology. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 66.Banks L, Wells GD, McCrindle BW. Cardiac energy metabolism is positively associated with skeletal muscle energy metabolism in physically active adolescents and young adults. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39:363–368. doi: 10.1139/apnm-2013-0312. [DOI] [PubMed] [Google Scholar]
- 67.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 68.Elezaby A, Sverdlov AL, Tu VH, Soni K, Luptak I, Qin F, Liesa M, Shirihai OS, Rimer J, Schaffer JE, Colucci WS, Miller EJ. Mitochondrial remodeling in mice with cardiomyocyte-specific lipid overload. Journal of molecular and cellular cardiology. 2015;79:275–283. doi: 10.1016/j.yjmcc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. Journal of the American College of Cardiology. 2009;54:1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Margulies KB. Evolving Challenges for Targeting Metabolic Abnormalities in Heart Failure. JACC Heart failure. 2016;4:567–569. doi: 10.1016/j.jchf.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Annals of the New York Academy of Sciences. 2004;1015:202–213. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 72.Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrate and lipids. Progress in cardiovascular diseases. 1972;15:289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- 73.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 74.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovascular research. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 75.Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. Journal of the American College of Cardiology. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 76.Banke NH, Lewandowski ED. Impaired cytosolic NADH shuttling and elevated UCP3 contribute to inefficient citric acid cycle flux support of postischemic cardiac work in diabetic hearts. Journal of molecular and cellular cardiology. 2015;79:13–20. doi: 10.1016/j.yjmcc.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 78.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 79.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochimica et biophysica acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 80.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 81.Akhmedov AT, Rybin V, Marin-Garcia J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart failure reviews. 2015;20:227–249. doi: 10.1007/s10741-014-9457-4. [DOI] [PubMed] [Google Scholar]
- 82.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and mitochondrial DNA damage in heart failure. Circulation journal: official journal of the Japanese Circulation Society. 2008;72(Suppl A):A31–37. doi: 10.1253/circj.cj-08-0014. [DOI] [PubMed] [Google Scholar]
- 83.Siebels I, Drose S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochimica et biophysica acta. 2013;1827:1156–1164. doi: 10.1016/j.bbabio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circulation research. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 85.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. Journal of the American College of Cardiology. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung HJ, Ma W, Wang PY, Hynes J, O’Riordan TC, Combs CA, McCoy JP, Jr, Bunz F, Kang JG, Hwang PM. Mitochondrial respiration protects against oxygen-associated DNA damage. Nature communications. 2010;1:5. doi: 10.1038/ncomms1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circulation research. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 88.Dymkowska D, Drabarek B, Podszywalow-Bartnicka P, Szczepanowska J, Zablocki K. Hyperglycaemia modifies energy metabolism and reactive oxygen species formation in endothelial cells in vitro. Archives of biochemistry and biophysics. 2014;542:7–13. doi: 10.1016/j.abb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Glushakova LG, Judge S, Cruz A, Pourang D, Mathews CE, Stacpoole PW. Increased superoxide accumulation in pyruvate dehydrogenase complex deficient fibroblasts. Molecular genetics and metabolism. 2011;104:255–260. doi: 10.1016/j.ymgme.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Remor AP, de Matos FJ, Ghisoni K, da Silva TL, Eidt G, Burigo M, de Bem AF, Silveira PC, de Leon A, Sanchez MC, Hohl A, Glaser V, Goncalves CA, Quincozes-Santos A, Borba Rosa R, Latini A. Differential effects of insulin on peripheral diabetes-related changes in mitochondrial bioenergetics: involvement of advanced glycosylated end products. Biochimica et biophysica acta. 2011;1812:1460–1471. doi: 10.1016/j.bbadis.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 91.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 92.Munasinghe PE, Riu F, Dixit P, Edamatsu M, Saxena P, Hamer NS, Galvin IF, Bunton RW, Lequeux S, Jones G, Lamberts RR, Emanueli C, Madeddu P, Katare R. Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. International journal of cardiology. 2016;202:13–20. doi: 10.1016/j.ijcard.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 93.Han SS, Wang G, Jin Y, Ma ZL, Jia WJ, Wu X, Wang XY, He MY, Cheng X, Li WJ, Yang X, Liu GS. Investigating the Mechanism of Hyperglycemia-Induced Fetal Cardiac Hypertrophy. PloS one. 2015;10:e0139141. doi: 10.1371/journal.pone.0139141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 95.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type, 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 96.Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell biochemistry and biophysics. 2013;67:1397–1408. doi: 10.1007/s12013-013-9672-y. [DOI] [PubMed] [Google Scholar]
- 97.Baseler WA, Thapa D, Jagannathan R, Dabkowski ER, Croston TL, Hollander JM. miR-141 as a regulator of the mitochondrial phosphate carrier (Slc25a3) in the type 1 diabetic heart. American journal of physiology. Cell physiology. 2012;303:C1244–1251. doi: 10.1152/ajpcell.00137.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garikipati VN, Krishnamurthy P, Verma SK, Khan M, Abramova T, Mackie AR, Qin G, Benedict C, Nickoloff E, Johnson J, Gao E, Losordo DW, Houser SR, Koch WJ, Kishore R. Negative Regulation of miR-375 by Interleukin-10 Enhances Bone Marrow-Derived Progenitor Cell-Mediated Myocardial Repair and Function After Myocardial Infarction. Stem cells. 2015;33:3519–3529. doi: 10.1002/stem.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Joladarashi D, Srikanth Garikipati VN, Thandavarayan RA, Verma SK, Mackie AR, Khan M, Gumpert AM, Bhimaraj A, Youker KA, Uribe C, Suresh Babu S, Jeyabal P, Kishore R, Krishnamurthy P. Enhanced Cardiac Regenerative Ability of Stem Cells After Ischemia-Reperfusion Injury: Role of Human CD34+ Cells Deficient in MicroRNA-377. Journal of the American College of Cardiology. 2015;66:2214–2226. doi: 10.1016/j.jacc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulation research. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation research. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature reviews. Genetics. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 104.Almeida MI, Reis RM, Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mutation research. 2011;717:1–8. doi: 10.1016/j.mrfmmm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 105.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 106.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang XX, Su YF, Li XS, Zhang Y, Wu Y, Mao N. Human fetal heart-derived adherent cells with characteristics similar to mesenchymal progenitor cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14:1191–1194. [PubMed] [Google Scholar]
- 109.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 110.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hendrickson DG, Hogan DJ, McCullough HL, Myers JW, Herschlag D, Ferrell JE, Brown PO. Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 2009;7:e1000238. doi: 10.1371/journal.pbio.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovascular research. 2010;86:410–420. doi: 10.1093/cvr/cvq010. [DOI] [PubMed] [Google Scholar]
- 114.Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP, Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, Lin SG, Yu XY. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS letters. 2010;584:3592–3600. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 115.Costantino S, Paneni F, Luscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. European heart journal. 2016;37:572–576. doi: 10.1093/eurheartj/ehv599. [DOI] [PubMed] [Google Scholar]
- 116.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. American journal of physiology. Endocrinology and metabolism. 2000;279:E1104–1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 117.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. American journal of physiology. Heart and circulatory physiology. 2013;305:H295–304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 119.Hafstad AD, Khalid AM, How OJ, Larsen TS, Aasum E. Glucose and insulin improve cardiac efficiency and postischemic functional recovery in perfused hearts from type 2 diabetic (db/db) mice. American journal of physiology. Endocrinology and metabolism. 2007;292:E1288–1294. doi: 10.1152/ajpendo.00504.2006. [DOI] [PubMed] [Google Scholar]
- 120.Riphagen IJ, Logtenberg SJ, Groenier KH, van Hateren KJ, Landman GW, Struck J, Navis G, Kootstra-Ros JE, Kema IP, Bilo HJ, Kleefstra N, Bakker SJ. Is the association of serum sodium with mortality in patients with type 2 diabetes explained by copeptin or NT-proBNP? (ZODIAC-46) Atherosclerosis. 2015;242:179–185. doi: 10.1016/j.atherosclerosis.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 121.Rosiak M, Postula M, Kaplon-Cieslicka A, Trzepla E, Czlonkowski A, Filipiak KJ, Opolski G. Metformin treatment may be associated with decreased levels of NT-proBNP in patients with type 2 diabetes. Advances in medical sciences. 2013;58:362–368. doi: 10.2478/ams-2013-0009. [DOI] [PubMed] [Google Scholar]
- 122.Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D’Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK, Richards ME, Aufderheide TP, Braude DA, Pirrallo RG, Doyle DD, Frascone RJ, Kosiak DJ, Leaming JM, Van Gelder CM, Walter GP, Wayne MA, Woolard RH, Opie LH, Rackley CE, Apstein CS, Udelson JE. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. Jama. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ, Team RS. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 124.Huang H, Shan J, Pan XH, Bao XF, Qian LB, Xia Q. Carvedilol Protects Early Diabetic Rat Hearts through Reducing Oxidative Stress. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2005. pp. 929–932. [DOI] [PubMed] [Google Scholar]
- 125.Rupp H, Elimban V, Dhalla NS. Modification of myosin isozymes and SR Ca(2+)-pump ATPase of the diabetic rat heart by lipid-lowering interventions. Molecular and cellular biochemistry. 1994;132:69–80. doi: 10.1007/BF00925676. [DOI] [PubMed] [Google Scholar]
- 126.Zhou X, Chen J. Is treatment with trimetazidine beneficial in patients with chronic heart failure? PloS one. 2014;9:e94660. doi: 10.1371/journal.pone.0094660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belardinelli R, Cianci G, Gigli M, Mazzanti M, Lacalaprice F. Effects of trimetazidine on myocardial perfusion and left ventricular systolic function in type 2 diabetic patients with ischemic cardiomyopathy. Journal of cardiovascular pharmacology. 2008;51:611–615. doi: 10.1097/FJC.0b013e31817bdd66. [DOI] [PubMed] [Google Scholar]
- 128.Kosiborod M, Arnold SV, Spertus JA, McGuire DK, Li Y, Yue P, Ben-Yehuda O, Katz A, Jones PG, Olmsted A, Belardinelli L, Chaitman BR. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) Journal of the American College of Cardiology. 2013;61:2038–2045. doi: 10.1016/j.jacc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 129.Mourouzis I, Mantzouratou P, Galanopoulos G, Kostakou E, Dhalla AK, Belardinelli L, Pantos C. The beneficial effects of ranolazine on cardiac function after myocardial infarction are greater in diabetic than in nondiabetic rats. Journal of cardiovascular pharmacology and therapeutics. 2014;19:457–469. doi: 10.1177/1074248414524481. [DOI] [PubMed] [Google Scholar]
- 130.Koshkarian GM. Congestive heart failure and sodium dichloroacetate. Journal of the American College of Cardiology. 1995;25:804–805. doi: 10.1016/s0735-1097(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 131.Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. Journal of the American College of Cardiology. 1994;23:1617–1624. doi: 10.1016/0735-1097(94)90665-3. [DOI] [PubMed] [Google Scholar]
- 132.Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free radical biology & medicine. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, Kim SS. Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28:873–886. doi: 10.1159/000335802. [DOI] [PubMed] [Google Scholar]
- 134.Bhatt NM, Aon MA, Tocchetti CG, Shen X, Dey S, Ramirez-Correa G, O’Rourke B, Gao WD, Cortassa S. Restoring redox balance enhances contractility in heart trabeculae from type 2 diabetic rats exposed to high glucose. American journal of physiology. Heart and circulatory physiology. 2015;308:H291–302. doi: 10.1152/ajpheart.00378.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beaudoin MS, Perry CG, Arkell AM, Chabowski A, Simpson JA, Wright DC, Holloway GP. Impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede development of diabetic cardiomyopathy are prevented by resveratrol in ZDF rats. The Journal of physiology. 2014;592:2519–2533. doi: 10.1113/jphysiol.2013.270538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCormick LM, Hoole SP, White PA, Read PA, Axell RG, Clarke SJ, O’Sullivan M, West NE, Dutka DP. Pre-treatment with glucagon-like Peptide-1 protects against ischemic left ventricular dysfunction and stunning without a detected difference in myocardial substrate utilization. JACC. Cardiovascular interventions. 2015;8:292–301. doi: 10.1016/j.jcin.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 137.Margulies KB, Anstrom KJ, Hernandez AF, Redfield MM, Shah MR, Braunwald E, Cappola TP N. Heart Failure Clinical Research. GLP-1 agonist therapy for advanced heart failure with reduced ejection fraction: design and rationale for the functional impact of GLP-1 for heart failure treatment study. Circulation. Heart failure. 2014;7:673–679. doi: 10.1161/CIRCHEARTFAILURE.114.000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fields AV, Patterson B, Karnik AA, Shannon RP. Glucagon-like peptide-1 and myocardial protection: more than glycemic control. Clinical cardiology. 2009;32:236–243. doi: 10.1002/clc.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circulation. Heart failure. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wei Y, Mojsov S. Distribution of GLP-1 and PACAP receptors in human tissues. Acta physiologica Scandinavica. 1996;157:355–357. doi: 10.1046/j.1365-201X.1996.42256000.x. [DOI] [PubMed] [Google Scholar]
- 141.Lepore JJ, Olson E, Demopoulos L, Haws T, Fang Z, Barbour AM, Fossler M, Davila-Roman VG, Russell SD, Gropler RJ. Effects of the Novel Long-Acting GLP-1 Agonist, Albiglutide, on Cardiac Function, Cardiac Metabolism, and Exercise Capacity in Patients With Chronic Heart Failure and Reduced Ejection Fraction. JACC Heart failure. 2016;4:559–566. doi: 10.1016/j.jchf.2016.01.008. [DOI] [PubMed] [Google Scholar]