Abstract
Although the phenotypic correlation between language and nonverbal cognitive ability is well-documented, studies examining the etiology of the covariance between these abilities are scant, particularly in very young children. The goal of the current study was to address this gap in the literature by examining the genetic and environmental links between language use, assessed through conversational language samples, and nonverbal cognition in a sample of 3-year-old twins (N = 281 pairs). Significant genetic and nonshared environmental influences were found for nonverbal cognitive ability and language measures, including mean length of utterance (MLU), and number of different words (NDW), as well as significant genetic covariance between cognitive ability and both language measures.
Language is a remarkable human skill, and the ability to understand and use language forms the building blocks for the development of several capacities, including social skills and general cognitive functioning, throughout life. Individual variation in language abilities is informative for both researchers and clinicians, as it can help us to identify the underlying etiology and mechanisms of language development. Several studies have examined individual differences in language skills, and have found that such variation is related to variation in other important areas, such as general cognitive ability.
Wide variation in language ability has been reported from infancy through adulthood. In early language development, receptive vocabulary (i.e., words that are understood) varies in both rate of acquisition and types of words acquired, and this variation predicts later language comprehension, even when controlling for important factors like gender and SES (Bates, Dale, & Thal, 1995). Such individual differences extend to related skills in both spoken and written language. For instance, in children with a history of language difficulties, conversational language abilities predict reading level, even beyond what can be predicted by formal language tests (DeThorne et al., 2010).
There is a well-documented relation between individual differences in language ability and environmental factors. One such factor is parental input, which has also been the subject of much empirical research. Studies have indicated that both the quantity and quality of maternal speech play a role in children’s expressive vocabulary (Hart & Risley, 1995; Hart, 2004; Hoff, 2003), and maternal responsiveness predicts achievement of language milestones in early childhood (Tamis-LeMonda, Bornstein, & Baumwell, 2001). Media exposure may also play a role in early language skills. In one study examining exposure to television media in infancy, duration of exposure was negatively related to later language and cognitive development (Tomopoulos et al., 2010). Behavioral genetic studies have also found that early vocabulary development is influenced by shared environmental factors—aspects of the environment that are common to family members and make siblings similar to one another (Dale et al., 2000).
These studies, which examine the etiology of language ability, have also indicated that a significant proportion of the variance between individuals is due to genetic factors. This is true for people of all ages, and for both typical populations as well as for individuals with language disorders (see Stromswold, 2001, for a review). For instance, moderate heritabilities have been found for both vocabulary (25%) and grammar (39%) in two-year-old twins, and in fact, there is little evidence that the two skills are dissociable, as substantial phenotypic and genetic associations have been found at that age (Dale et al., 2000). Similarly, verbal delay in young toddlers and the continuity of delay from age two to three are both substantially heritable (62% and 48%, respectively) (Eley et al., 2001). Longitudinal studies of twins have indicated that language skills are relatively stable across age, and that genetic influences become increasingly important for variation in language abilities as children get older (Hayiou-Thomas, Dale, & Plomin, 2012). Interestingly, though, although some of the genes that influence early language skills also affect language in middle childhood, new genes come online at this time, as genetic influences gain prominence in language capacities (Hayiou-Thomas, Dale, & Plomin, 2012; Hoekstra et al., 2007). Further, a study of school-aged twins found significant genetic influences for both conversational language and standardized tests of language ability (DeThorne, 2008). The genetic correlation between the two language measures was r = .37, indicating that there was substantial overlap in the genetic effects influencing both. Nonetheless, there were also genetic effects that were specific to conversational language. Thus it seems that individual variation in language ability largely stems from genetic factors, but that different genetic factors may affect different aspects of language, depending on the measures used or the age assessed (see also Stromswold, 2001).
Genetic influences on general cognitive ability have been similarly well-documented. For instance, across multiple behavioral genetic studies, including thousands of monozygotic and dizygotic twins, the heritability of intelligence in children has been found to be approximately 50 percent (Plomin & Spinath, 2004). Moreover, this heritability increases with age, with genetic factors accounting for close to 70 percent of the variance in measures of intelligence among adult twins (Bouchard, Lykken, McGue, Segal, & Tellegen, 1990; McGue, Bouchard, Iacono, & Lykken, 1993; Read et al., 2006).
Individual differences in language ability are related to general cognitive ability, as indicated by moderate to strong phenotypic associations between IQ measures and standardized measures of both semantic and morphosyntactic verbal abilities in middle childhood (DeThorne et al., 2006). Studies of children with specific language impairment (SLI), though, reveal the complexity of this relation. One of the diagnostic criteria for SLI is the presence of a language delay with no corresponding delay in general cognitive functioning. However, in longitudinal studies involving children with language disorders, there is an observable decline in cognitive abilities, with IQ scores dropping 20 points on average, across ages 7 to 14 years in children with continuing language delays (Botting, 2005). Although this may indicate that, in children with SLI, language is affected by a cognitive delay that is otherwise unnoticed, the results could also indicate that more stable IQ scores, such as those in typically-developing children, are linked to better language outcomes, or that language deficits create delays in learning such that standardized test performance lags behind that of children with no language disorder. Thus, it may be that language and general cognitive ability are part of an interactive process (Botting, 2005).
Given these previous findings of phenotypic correlations between language ability and general cognitive ability, and their similar patterns of strong genetic influence and increasing heritability with age, it may be that common genetic factors affect both language and nonverbal cognition. However, only a small number of studies have looked at this possibility. More commonly, studies have looked at the overlap between nonverbal and verbal cognition, the latter of which is the ability to use language-based reasoning when considering new information. In school-aged children there is evidence of substantial genetic overlap between verbal and nonverbal cognitive abilities (e.g., Petrill et al., 2001). Interestingly, the genetic covariance between these abilities in younger children is much more modest, suggesting that these abilities are largely independent in early childhood (Hoekstra et al., 2007; Price et al., 2000). Research in populations with language or cognitive impairment, however, indicate strong genetic covariance between the two capacities, even in early childhood. Almost all of the overlap between verbal and nonverbal cognitive abilities in children with either verbal or cognitive delays is mediated by genetic factors (Purcell et al., 2001). Taken together, this research suggests that many of the same genetic influences are involved in both verbal and nonverbal cognitive ability, both in typical development and in children with delays in these abilities, although these capacities may be more independent in early childhood than in later development.
Studies of verbal and nonverbal cognition, however, still leave open the question of common genetic influences on language capacities and nonverbal cognitive abilities, as verbal reasoning is not interchangeable with language ability. Previous work has suggested that a majority of the genetic influences on individual differences in language ability, as assessed on standardized tests, overlap with those that influence individual differences in nonverbal cognition (Colledge et al., 2002). In addition, genetic factors explain a substantial proportion of the variation in nonverbal performance between children with language impairments and the typical population (Viding et al., 2003). However, research focused specifically on natural language abilities and nonverbal cognition is limited. The current study utilizes the twin method to expand on previous work on the etiology of language and nonverbal cognitive skills in toddlers, using conversational language measures to assess children’s language abilities.
To our knowledge, there have been no twin studies examining genetic and environmental influences on individual differences in preschoolers' conversational language. As indicated above, most previous studies have focused on verbal cognition, rather than conversational language use, and thus have left the relation between nonverbal cognitive ability and natural language skills unexamined. The few studies that have examined language abilities and their relation to nonverbal cognition, may have failed to capture the full picture of language ability in young children because they used either parent-report or standardized tests of language. Parent reports may artificially inflate familial similarity, especially for twins, because parents may confuse the twins’ vocabulary and skills (Bishop, Laws, Adams, & Norbury, 2006). Formal language tests also have limitations, as they may also tap into factors that influence test-taking ability such as attention, motivation, compliance, anxiety, coping ability, and frustration tolerance (e.g., Pena, Iglesias, & Lidz, 2001; Speltz, DeKlyen, Calderon, Greenberg, & Fisher, 1999), rather than language alone. On the other hand, conversational language measures may exploit pragmatic abilities, linguistic skills specific to context and conversation, such as syntactic and semantic bootstrapping, and temperament dimensions like extraversion (DeThorne et al., 2008). Thus, conversational language measures are more likely to assess language use, rather than language knowledge. These differences may lead to differences in motivation—children may have a performance motivation when completing a formal language test, but a communicative motivation in conversation. Consequently, the genetic and environmental influences on individual differences in conversational language skills may be different from those that underlie formal assessments, and the etiology of conversational language, and its relation to general cognitive ability, may provide unique information about the overlap of nonverbal skills with verbal abilities specific to natural language use.
We predicted that there would be genetic effects on both nonverbal cognitive ability and on language ability assessed within a conversational context. We also predicted that there would be shared environmental influences on children’s language, as the impact of parental and other input on early vocabulary has been supported by several studies, as mentioned above (Hart & Risley, 1995; Hoff, 2003; Tomopoulos et al., 2010). Finally, based on studies using formal language measures, as well as studies of verbal and nonverbal cognition, we expected moderate genetic covariance between non-verbal cognitive ability and children’s conversational language ability. By assessing the genetic and environmental influences on conversational language and nonverbal cognition in young children, this study can examine the etiology of language use and knowledge in an ecologically valid context. The language skills involved specifically in this conversational context may be informative in creating interventions for children with language delay, and are likely particularly important for social success. Thus, the potential genetic overlap between these skills and general cognitive ability could have important implications for language development in both typical and clinical populations.
Method
Participants
Families were recruited for the Boston University Twin Project through the Massachusetts Registry of Vital Records. Only twins who were considered full-term (i.e., born at 34 weeks gestation or later) and not very low-birth weight (i.e., more than 1750 g) were included in the study. Three-hundred-four twin pairs were assessed within one month of their third birthday (M = 2.99 years, SD = 0.96 years). Assessments took place between July 2004 and November 2007. The current study includes 281 (131 monozygotic [MZ], 150 dizygotic [DZ]) English-speaking twin pairs for which conversational language data was available. All twin pairs were same-sex, with 136 female pairs and 145 male pairs. Males and females were approximately equally distributed across zygosity. Zygosity was assessed through DNA cheek swab samples using 10 multiplex markers. Ethnicity of the full sample was generally representative of the state of Massachusetts. Most of the sample was White (85.4%), with smaller proportions of Black (3.2%), Asian (7%), mixed race (7.3%), and Other (2.2%). Socioeconomic status of the families ranged from low to upper middle class, based on the Hollingshead (1975) Four Factor Index.
Procedure
Twins visited the lab on two days, 48 hours apart. On each day, each twin completed one hour of testing, separate from his co-twin, participating in tasks assessing their temperament and cognitive ability. Each twin interacted with a different experimenter, who worked with that twin on both days of testing. On the first day, one twin participated in a standardized test of cognitive development, while the other twin participated in a laboratory play situation. This included several social imitation tasks, from which language samples were taken. On the second day of testing, the twins switched test situations.
Measures
Cognitive ability
The mental scale of the Bayley Scales of Infant Development-II (BSID-II; Bayley, 1993) was used to assess cognitive development. This measure involves a series of interactive tasks designed to measure children’s development in three domains: mental, motor, and behavioral. The BSID-II was standardized on a sample of 1700 children ranging in age from 1 to 42 months (Bayley, 1993). The mental scale provides a scaled Mental Development Index score reflecting general mental ability, but can be broken down into cognitive, social, and verbal facets (Bayley, 1993). Because we wanted to assess non-verbal cognitive ability, we limited our analyses to include only the cognitive facet. One item (154, “Identifies gender”) was included on both the verbal and cognitive facets and was removed from the cognitive facet score.
Language ability
Natural language samples were taken from 20-minute video-recorded interactions with an experimenter during a series of social imitation tasks, which included interacting with a puppet, using tools, and building objects. The experimenter provided scripted instructions for each task, but responses to children’s comments and questions were unscripted. This task encouraged interaction between the experimenter and the child, and was embedded late in the overall assessment, allowing the experimenter to build rapport with the child. Further, because the experimenter prompts were scripted, as noted above, variation between sessions was limited, and was due in large part to child-initiated factors.
Transcripts used the Systematic Analysis for Language Transcripts (SALT) format (Miller & Chapman, 2000), and were completed by trained research assistants. One transcriber prepared each transcript, and a second transcriber checked 20% of transcripts against the video recordings. The samples ranged from one to 411 complete, intelligible utterances (M = 95.43, SD = 66.65). Although previous researchers have questioned the reliability and validity of short language samples (Cole, Mills, & Dale, 1989; Gavin & Giles, 1996; Miller, 1981), recent research has indicated that short samples, as short as one minute and including as few as 12 total utterances, may provide the same information when compared to longer samples (Heilmann, Nockerts, & Miller, 2010). Further, all 281 twin pairs were included in the current analysis because we were specifically interested in variation in children’s conversational language, including limited conversational skills. Two language measures were used in the analyses: number of different words (NDW) and mean length of utterance in words (MLU). NDW was used as an indicator of lexical diversity, and its reliability and validity have been well-established (e.g., Klee, 1992). MLU was used as a measure of overall expressive language ability, involving both semantic and syntactic skills, as suggested by DeThorne and colleagues (2005). The clinical validity of MLU has been measured extensively, and it has been used to distinguish children with language disabilities from typically developing children (Klee, Schaffer, May, Membrino, & Mougey, 1989; Scarborough, Rescorla, Tager-Flusberg, Fowler, 1991).
Each child’s transcript was compared to a SALT reference database matched for age and transcript length. On average, the current twin sample was approximately one standard deviation below the norms provided by the SALT reference database. This difference corresponds to previous research indicating that twins lag behind singleton children in early vocabulary acquisition and grammatical development (see Stromswold, 2001).
Design
The twin design decomposes the observed (i.e., phenotypic) variance of a variable into additive genetic (A), shared (C) and nonshared (E) environmental variance components. Heritability, the genetic effect size, is the proportion of phenotypic variance that can be attributed to genetic factors. If genetic influences are important to a trait or behavior, then behavioral similarity should covary with genetic relatedness (i.e., genetically identical monozygotic twins who share 100% of their genes should be more similar in behavior than dizygotic twins who share on average, only 50% of their segregating genes). Shared environmental variance is familial resemblance that is not explained by genetic variance and comprises environmental influences that are shared by family members such as family demographics, one’s rearing neighborhood, shared friends, or even such things as the number of TVs or books in the house. If shared environments are important to individual differences in the behavior under study, they should enhance the similarity of family members. Nonshared environmental variance is a residual variance that includes environmental influences that are unique to each individual. These unique environmental influences operate to make members of the same family different from one another. Possible sources of nonshared environmental variance include differential parental treatment; relationships with friends, peers and teachers; and nonsystematic factors such as accidents, illness and measurement error (Plomin, Chipuer, & Neiderhiser, 1994).
Analyses
Correlational analyses
Descriptive statistics, as well as phenotypic and twin intraclass correlations were calculated for both cognitive and language abilities. In order to assess the multivariate component of the current analyses, cross-twin/cross-trait correlations were computed. In these correlations, one twin’s score on the first variable (e.g., cognitive ability) is correlated with his co-twin’s score on the second variable (e.g., NDW), and vice versa. For both the intraclass and cross-twin/cross-trait correlations, if the correlation is greater for MZ twins than it is for DZ twins, it suggests a genetic influence on the trait variance or covariance between the two traits, respectively.
Multivariate model fitting analyses
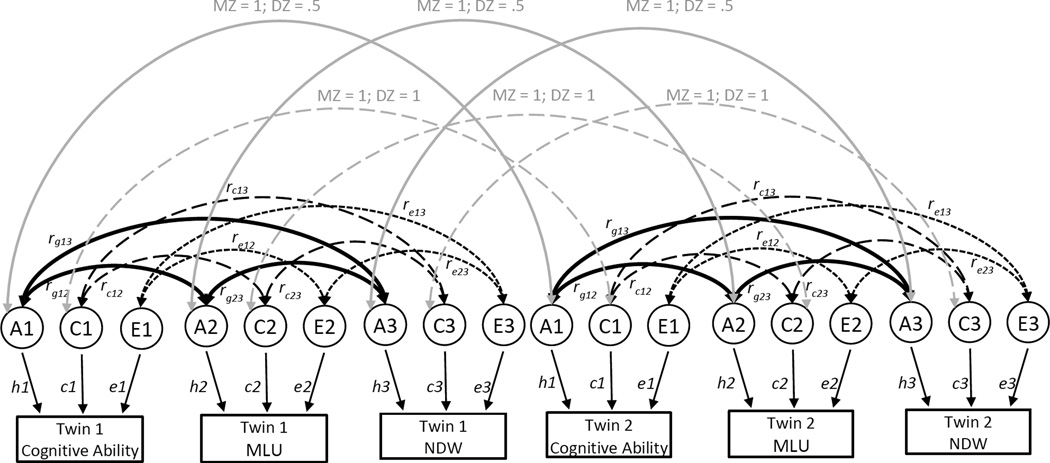
To examine the relative contributions of common genetic and environmental influences on cognitive ability, as measured by the BSID-II cognitive scale, NDW, and MLU, trivariate Correlated Factors models were fitted to the raw data in Mx (Neale, 1994). This model is illustrated in Figure 1. Factors A1, C1, and E1 represent the additive genetic, shared environmental, and nonshared environmental effects, respectively, on nonverbal cognitive ability; A2, C2, and E2 represent genetic and environmental effects on MLU; and A3, C3, and E3 represent effects on NDW. The gray double-headed paths denote correlations between co-twins. Following the logic of the twin design described earlier, each latent genetic variable A1, A2, and A3 for Twin 1 is correlated with its’ corresponding variable for Twin 2 (i.e., A1 Twin 1 with A1 Twin 2, etc.) based on the degree of genetic relatedness (i.e., 1 for MZ twins and .5 for DZ twins). Similarly, because the twins are reared together, each latent shared environmental variable for Twin 1 (i.e., C1, C2, and C3) is correlated 1 with the corresponding variable for Twin 2.
Figure 1.
Trivariate Correlated Factors Model. The circles represent the latent additive genetic (A), shared environmental (C), and nonshared environmental (E) factors that influence each phenotype. The path coefficients, h, c and e, are standardized partial regression coefficients indicating the relative influence of the latent factors on each phenotype. The black double-headed paths are the estimated genetic and environmental correlations between phenotypes. rg (shown in bold) shows the genetic correlations between each pairs of variables; rc (dashed) shows the shared-environmental correlations, and re (dotted) the nonshared environmental correlations. The grey double headed paths reflect genetic (solid) and shared environmental (dashed) correlations between co-twins (values are provided in the model based on the assumptions of the twin design). MLU = mean length of utterance. NDW = number of different words.
The path coefficients, h, c and e, for each variable are standardized partial regression coefficients indicating the relative influence of the latent factors on the phenotypes. The square of these path coefficients represent the genetic, shared environmental and nonshared environmental variances for each phenotype. For example, the square of path estimate for h1 yields the heritability of nonverbal cognitive ability. Similarly, the square of c2 estimates the shared environmental variance for MLU. This model allows us to directly estimate the genetic, shared environmental, and nonshared environmental correlations between phenotypes (i.e., rg, rc and re, depicted as black double-headed paths in Figure 1). For example in Figure 1, rg13 estimates the genetic correlation between nonverbal cognitive ability and NDW. The genetic correlation indicates the extent to which genetic effects on one phenotype correlates with genetic effects on another, independent of the heritability of each phenotype. The genetic factors that influence two phenotypes can covary perfectly even though the genetic effects on each phenotype contribute only slightly to the phenotypic variance. Thus, rg can be 1.0 even though the genetic contribution to the phenotypic correlation is only modest if the heritability of each phenotype is modest and the same genetic effects operate on each phenotype. Conversely, two phenotypes may be substantially heritable, but the genetic correlation would be zero if the genetic effects on the two phenotypes do not overlap. Similar logic applies to rc and re.
The overall fit of the model can be assessed by calculating twice the difference between the negative log-likelihood (−2LL) of the model and that of a saturated model (i.e., a model in which the variance/covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated). The difference in −2LL is asymptotically distributed as chi square (χ2) with degrees of freedom equal to the difference in the number of parameters in the full ACE model and that in the saturated model.
Results
Means and standard deviations for all measures are listed in Table 1. There was a strong, positive association between NDW and MLU (r = .70, p < .001), controlling for the effect of cognitive ability, as well as a moderate association between MLU and cognitive ability (r = .26, p < .001), and a small but significant relation between NDW and cognitive ability (r = .20, p < .001). Table 2 shows the twin intraclass correlations and cross-twin/cross-trait correlations for cognitive ability, NDW, and MLU. For all three variables, intraclass correlations between DZ twins were lower than those of MZ twins, suggesting genetic influences on all three traits. This difference did not reach significance for NDW, though. A similar pattern was seen in the MZ and DZ cross-twin/cross-trait correlations, indicating possible genetic influences on the correlations between cognitive ability, NDW, and MLU. However, model-fitting analyses allow a more powerful test of genetic and environmental sources of variance and covariance.
Table 1.
Descriptive Statistics for Cognitive and Language Ability, by Zygosity
| MZ Twins | DZ Twins | |||||
|---|---|---|---|---|---|---|
| n | M (SD) | Range | n | M (SD) | Range | |
| Cognitive Ability |
277 | 108.94 (4.08) | 97.00 – 121.00 | 312 | 109.46 (4.75) | 89.00 – 124.00 |
| MLU | 261 | 2.42 (0.74) | 1.00 – 5.00 | 289 | 2.49 (0.75) | 1.00 – 4.64 |
| NDW | 262 | 68.53 (44.18) | 0.00 – 193.00 | 290 | 76.34 (44.02) | 0.00 – 183.00 |
Note. MLU = mean length of utterance. NDW = number of different words. MZ = monozygotic. DZ = dizygotic.
Table 2.
Intraclass and Cross-twin/cross-trait Correlations for Cognitive Ability, MLU, and NDW.
| Intraclass Correlations | |||
| MZ | DZ | p* | |
| Cognitive Ability | .68 | .43 | < .01 |
| MLU | .51 | .30 | .04 |
| NDW | .50 | .37 | .18 |
| Cross-twin/Cross-trait Correlations | |||
| MZ | DZ | ||
| Cognitive Ability-MLU | .24 | .19 | .67 |
| Cognitive Ability-NDW | .22 | .08 | .23 |
| MLU-NDW | .39 | .28 | .30 |
p = significance of the differences between correlations.
MLU = mean length of utterance. NDW = number of different words. MZ = monozygotic. DZ = dizygotic.
Multivariate model-fitting
Table 3 presents the fit statistics for the correlated factors model of cognitive ability, NDW, and utterance length (MLU). Reduced models that set either the genetic (Model 3) or the nonshared environmental (Model 5) correlations between variables to zero resulted in significant decrements of fit, demonstrating significant genetic and nonshared environmental covariance between cognitive ability and the language measures. On the other hand, a model dropping all shared environmental correlations between variables (Model 4) showed no significant decrement of fit, indicating that the shared environment did not contribute to the covariance between traits. Finally, a model in which all shared environmental paths were dropped (Model 6) also showed no significant change in fit; suggesting that in addition to no significant shared environmental covariance between the variables, there was no significant shared environmental variance for any of the measures. Thus, both genetic and nonshared environmental factors, but not shared environmental factors, contributed to cognitive ability, NDW and MLU, and to the covariance between measures.
Table 3.
Model Fit Statistics for Trivariate Models Including Cognitive Ability, MLU, and NDW.
| Overall fit of Model | Relative fit of Model | |||||||
|---|---|---|---|---|---|---|---|---|
| −2LL | df | Δdf | p | AIC | Δχ2 | df | p | |
| 1. Saturated Model | 9908.225 | 1668 | ||||||
| 2. Full ACE Model | 9938.923 | 1692 | 24 | 0.16 | −17.302 | |||
| 3. Drop rg; no genetic covariance |
9947.188 | 1695 | 27 | 0.06 | −2.265 | 8.265 | 3 | 0.04 |
| 4. Drop rc; no shared environment covariance |
9942.384 | 1695 | 27 | 0.16 | 2.539 | 3.461 | 3 | 0.33 |
| 5. Drop re; no nonshared environment covariance |
10040.769 | 1695 | 27 | <0.01 | −95.846 | 101.846 | 3 | <0.01 |
| 6. AE only | 9942.979 | 1698 | 30 | 0.25 | 7.945 | 4.055 | 6 | 0.67 |
Note. MLU = mean length of utterance. NDW = number of different words.
Variance components and genetic and environmental sources of covariance between cognitive ability and language from the best-fitting model are presented in Figure 2. Genetic effects explained approximately three-quarters of the variance in nonverbal cognitive ability and fifty percent of variance in both language measures. The remaining variance for all three phenotypes was due to nonshared environmental influences. The genetic covariances between nonverbal cognitive ability and language abilities were moderate, ranging from .32 for NDW to .48 for MLU. Although moderate, it was these overlapping genetic influences that fully explained the phenotypic correlation between nonverbal cognitive ability and both language measures (i.e., there were no environmental sources of covariance between cognitive ability and language). In contrast, the covariance between NDW and MLU was due to both genetic and environmental factors. Approximately 78% of the genetic effects and 64% of the nonshared environmental effects overlapped across language measures.
Figure 2.
Estimates of genetic and nonshared environmental variances and covariances (and 95% CI) for cognitive ability, MLU (mean length of utterance) and NDW (number of different words) from the best-fitting model.
Discussion
The present study found significant genetic influences on two measures of conversational language ability, NDW and MLU, as well as significant genetic covariance between these measures in a preschool sample. In addition, we found moderate genetic overlap between conversational language ability and nonverbal cognitive ability. These results replicate previous findings by Dale (2000) and Price (2000) and their colleagues, and extend these prior findings by using conversational language measures rather than formal language tests or parent reports of language ability. In this way, we were able to assess language use, rather than decontextualized language knowledge, and our measures were likely less influenced by other cognitive functions, such as anxiety or frustration tolerance, which may be especially relevant in this age group. This enabled us to more directly assess our findings in the context of studies with school-aged children that more often use natural language samples in their analysis (e.g., De Thorne et al., 2010).
Several previous studies have suggested that language and nonverbal cognition are more independent in infancy and toddlerhood, and become more entwined with age, as indicated by their increasing genetic correlations across development (Colledge et al., 2002; Hoekstra et al., 2007). Our findings indicate that this may be only partially true. The moderate genetic correlations between conversational language ability and nonverbal cognitive ability in this study were comparable to those seen in a previous study of four-year-olds using standardized measures of language (Colledge et al., 2002), indicating some independence of these abilities in toddlerhood. However, studies using conversational language measures in school-aged children have found the same moderate overlap (DeThorne et al., 2006). Thus, it may be that the genetic covariance between language and nonverbal cognitive abilities increase only for formal language measures. This could mean that language itself remains relatively independent of cognitive ability, but that the other skills involved in formal language assessment, such as executive functioning capacities, test-taking skills, and temperamental traits, account for the greater overlap seen between language and general cognitive ability in later childhood and adulthood. This has important implications for the study and treatment of SLI in young children. These results lend support to the defining characteristic of language delay without a corresponding cognitive delay in this disorder, as it indicates that these two areas may indeed be dissociable, at least when measuring conversational language use. Further, the current results emphasize the importance of assessing the method of measurement in both language development research and child development research in general, as associations between abilities and the underlying genetic and environmental factors may differ depending on the methodology. Thus, more research using conversational language measures in toddlers and young children is needed to further elucidate the varying connections between nonverbal cognitive ability and language ability.
Unlike previous studies, we did not find significant shared environmental effects on cognitive ability, language, or the relation between them. This could be explained by the fact that the present research used only experimenter-administered measures, and that a separate experimenter interacted with each twin, whereas most previous studies with toddlers have used parent-report measures of language. The validity of parent reports of children's language ability has been well-documented with singleton children, but may be more complex in twin samples (Bishop, Laws, Adams, Norbury, 2006; Feldman et al., 2006). When the same rater assesses both twins in a pair, rater bias could be shared across twins, increasing the similarity in their scores and increasing estimates of shared environmental influence. Our use of only experimenter-administered tasks could also explain, in part, our finding of substantial nonshared environmental influences on language abilities—a unique finding in the literature. Possible sources of nonshared environmental influences include the context of the conversational language sample, and differential parental treatment and responsiveness, as well as other child-specific experiences and measurement error. In addition, because a different experimenter worked with each twin, differences between those experimenters are a possible source of nonshared environmental influence. Although most of the experimenters’ linguistic input was scripted and only those aspects of conversation that were child-driven, such as responses to questions, were improvised, it is possible that even in this constrained situation, different testers might differentially elicit verbal responses from children either through their verbal responses or their demeanor while testing. This does not seem to be the case in the current sample, however; the effect of rater was very small, accounting for less than 1% of the variance in MLU and in NDW.
A possible alternative source of the nonshared environmental effects could be parental input and responsiveness, which are important factors in language development (Hart & Risley, 1995; Tamis-Lemonda et al., 2001). Parental influence is often thought of as a shared environmental influence, but if parents provide different amounts or types of input to each twin, or if their positivity and responsiveness differs between twins, this could, instead, be a nonshared factor. Such differential parental treatment may stem from differences in children’s language and cognitive ability (O’Connor, 2002), and parents may respond to such differences subconsciously, while still conflating their twins' knowledge when responding to parent-report questionnaires. Children with varying linguistic and nonverbal skills may also elicit different types of input from others' in their environment. For example, children with higher IQs or more advanced conversational abilities may be involved in more in-depth discussions than children who are less skilled. Thus, rather than differences in input from parents and others causing later vocabulary differences, variation in children’s language and cognitive abilities may evoke different linguistic input. This potential gene-environment correlation could have important implications for studies of parental input in language development, as genetic influences may play an important role in children’s early language environment. Future longitudinal research examining the direction of the relation between linguistic input and children’s conversational language skill can provide a greater understanding of this possible source of nonshared environmental influence.
Research should also examine the links between language ability and nonverbal cognitive ability in other populations. Although the current sample was recruited from birth records in order to recruit the most representative sample possible, the results are limited in that, as with many twin studies, participants were predominantly white and middle- to upper-middle-class. Future studies using weighted sampling methods in order to gain a more diverse sample will provide more generalizable results.
When studying language in a twin sample, it is important to keep in mind that expressive language abilities in twins generally lag behind those of singleton children. These delays may be associated with the higher rates of premature birth and perinatal complications associated with twinning. However, a great deal of evidence indicates that twins may show delays in their language development because of differences in the social environment (see Thorpe, 2006, for a review). For instance, classic research has indicated that, because of the constant presence of a sibling, twins engage in fewer individual interactions than singleton children, and experience more interruptions when they do (Lytton, Watts, & Dunn, 1987; Tomasello, Mannie, & Kruger, 1986). Although delays are consistently found in twins, they are most likely temporary and mild. When twins with overt neurological impairment or who were born very premature (< 33 weeks) are excluded from analyses, twins’ language abilities are only modestly delayed (i.e., approximately 3 months behind singletons) (Rutter, Thorpe, Greenwood, North, & Golding, 2003). Thus, it is likely that the findings of twin studies are applicable to both twins and single children.
The present findings indicate the importance of genetic influences on both cognitive ability and conversational language capacities, and suggest that both measurement-specific and general contexts are significant in measuring language capacity. Further, conversational language abilities, which are particularly important for social success, appear relatively genetically independent of nonverbal cognitive ability, at least in toddlers. These results could have meaningful implications for intervention, as they suggest that differential experiences may play a role in language outcomes, and for developmental theory, as they speak to the etiology and trajectories of nonverbal cognitive ability and language skills, and thereby, of both cognitive and social development.
Acknowledgments
The Boston University Twin Project (BUTP) is supported by grants R01 MH062375 and R01 HD068435 from the National Institutes of Health to Dr. Saudino. The authors gratefully acknowledge the families in the BUTP, the BUTP staff and volunteers. Thank you also to the BU Center for Autism Research Excellence for assistance with the language analysis.
References
- Bates E, Dale P, Thal D. Individual differences and their implications for theories of language development. In: Fletcher P, MacWhinney B, editors. The Handbook of Child Language. Oxford: Blackwell; 1995. pp. 96–151. [Google Scholar]
- Bayley N. Bayley Scales of Infant Development: Manual. Psychological Corporation; 1993. [Google Scholar]
- Bishop D, Laws G, Adams C, Norbury C. High heritability of speech and language impairments in 6-year-old twins demonstrated using parent and teacher report. Behavior Genetics. 2006;36:173–184. doi: 10.1007/s10519-005-9020-0. [DOI] [PubMed] [Google Scholar]
- Botting N. Non-verbal cognitive development and language impairment. Journal of Child Psychology and Psychiatry. 2005;46:317–326. doi: 10.1111/j.1469-7610.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- Bouchard T, Lykken D, McGue M, Segal N, Tellegen A. Sources of human psychological differences: The Minnesota study of twins reared apart. Science. 1990;250:223–228. doi: 10.1126/science.2218526. [DOI] [PubMed] [Google Scholar]
- Cole K, Mills P, Dale P. Examination of test-retest and split-half reliability for measures derived from language samples of young handicapped children. Language, Speech, and Hearing Services in Schools. 1989;20:259–268. [Google Scholar]
- Colledge E, Bishop D, Dale P, Koeppen-Schomerus G, Price T, Happe F, Plomin R. The structure of language abilities at 4 years: A twin study. Developmental Psychology. 2002;38:749. doi: 10.1037//0012-1649.38.5.749. [DOI] [PubMed] [Google Scholar]
- Dale P, Dionne G, Eley T, Plomin R. Lexical and grammatical development: A behavioral genetic perspective. Journal of Child Language. 2000;27:619–642. doi: 10.1017/s0305000900004281. [DOI] [PubMed] [Google Scholar]
- DeThorne L, Hart S, Petrill S, Deater-Deckard K, Thompson L, Schatschneider C, Davison M. Children’s history of speech-language difficulties: Genetic influences and associations with reading. Journal of Speech, Language, and Hearing Research. 2006;49:1280–1293. doi: 10.1044/1092-4388(2006/092). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeThorne L, Johnson B, Loeb J. A closer look at MLU: What does it really measure? Clinical Linguistics and Phonetics. 2005;19:635–648. doi: 10.1080/02699200410001716165. [DOI] [PubMed] [Google Scholar]
- DeThorne L, Petrill S, Hart S, Channell R, Campbell R, Deater-Deckard K, Thompson L, Vandenbergh D. Genetic effects on children’s conversational language use. Journal of Speech, Language, and Hearing Research. 2008;51:423–435. doi: 10.1044/1092-4388(2008/031). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeThorne L, Petrill S, Schatschneider C, Cutting L. Conversational language use as a predictor of early reading development: Language history as a moderating variable. Journal of Speech, Language, and Hearing Research. 2010;53:209–223. doi: 10.1044/1092-4388(2009/08-0060). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley T, Dale P, Bishop D, Price T, Plomin R. Longitudinal analysis of components of cognitive delay: Examining aetiology of verbal and performance aspects of cognitive delay. Journal of Educational Psychology. 2001;93:698–707. [Google Scholar]
- Feldman H, Campbell T, Kurs-Lasky M, Rockette H, Dale P, Paradise J. Concurrent and predictive validity of parent reports of child language at ages 2 and 3 years. Child Development. 2005;76:856–868. doi: 10.1111/j.1467-8624.2005.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin W, Giles L. Sample size effects on temporal reliability of language sample measures of preschool children. Journal of Speech, Language, and Hearing Research. 1996;39:1258–1262. doi: 10.1044/jshr.3906.1258. [DOI] [PubMed] [Google Scholar]
- Hart B. What toddlers talk about. First Language. 2004;24:91–106. [Google Scholar]
- Hart B, Risley T. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Brookes Publishing; 1995. [Google Scholar]
- Hayiou-Thomas M, Dale P, Plomin R. The etiology of variation in language skills changes with development: A longitudinal twin study of language from 2 to 12 years. Developmental Science. 2012;15:233–249. doi: 10.1111/j.1467-7687.2011.01119.x. [DOI] [PubMed] [Google Scholar]
- Heilmann J, Nockerts A, Miller J. Language sampling: does the length of the transcript matter? Language, Speech, and Hearing Services in Schools. 2010;41:393–404. doi: 10.1044/0161-1461(2009/09-0023). [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Bartels M, Boomsma D. Longitudinal genetic study of verbal and nonverbal IQ from early childhood to young adulthood. Learning and Individual Differences. 2007;17:97–114. [Google Scholar]
- Hoff E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development. 2003;74:1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Klee T. Developmental and diagnostic characteristics of quantitative measures of children's language production. Topics in Language Disorders. 1992;12:28–41. [Google Scholar]
- Klee T, Schaffer M, May S, Membrino I, Mougey K. A comparison of the age-MLU relation in normal and specifically language-impaired preschool children. Journal of Speech and Hearing Disorders. 1989;54:226–233. doi: 10.1044/jshd.5402.226. [DOI] [PubMed] [Google Scholar]
- Lytton H, Watts D, Dunn B. Twin-singleton differences in verbal ability: Where do they stem from? Intelligence. 1987;11:359–369. [Google Scholar]
- McGue M, Bouchard T, Iacono W, Lykken D. Behavioral genetics of cognitive ability: A life-span perspective. In: Plomin R, McClearn G, editors. Nature, Nurture, and Psychology. Washington, DC: American Psychological Association; 1993. pp. 59–76. [Google Scholar]
- Miller J. Assessing Language Production in Children: Experimental Procedures. Baltimore, MD: University Park Press; 1981. [Google Scholar]
- Miller J, Chapman R. Systematic Analysis of Language Transcripts (Vol. 6.01) Madison, WI: Language Analysis Lab, University of Wisconsin; 2000. [Google Scholar]
- Neale M. Mx: Statistical Modeling. 2nd. Richmond, VA: VCU, Department of Psychiatry; 1994. [Google Scholar]
- O’Connor T. The ‘effects’ of parenting reconsidered: Findings, challenges, and applications. Journal of Child Psychology and Psychiatry. 2002;43:555–572. doi: 10.1111/1469-7610.00046. [DOI] [PubMed] [Google Scholar]
- Pena E, Iglesias A, Lidz C. Reducing test bias through dynamic assessment of children’s word learning ability. American Journal of Speech-Language Pathology. 2001;10:138–154. [Google Scholar]
- Plomin R, Chipuer H, Neiderhiser J. Behavioral genetic evidence for the importance of nonshared environment. In: Hetherington E, Reiss D, Plomin R, editors. Separate social worlds of siblings: The impact of nonshared environment on development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994. pp. 1–31. [Google Scholar]
- Plomin R, Spinath F. Intelligence: Genetics, genes, and genomics. Journal of Personality and Social Psychology. 2004;86:112. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- Price T, Eley T, Dale P, Stevenson J, Plomin R. Genetic and environmental covariation between verbal and non-verbal cognitive development in infancy. Child Development. 2000;71:948–959. doi: 10.1111/1467-8624.00201. [DOI] [PubMed] [Google Scholar]
- Purcell S, Eley T, Dale P, Oliver B, Petrill S, Price T, Plomin R. Comorbidity between verbal and non-verbal cognitive delays in 2-year-olds: A bivariate twin analysis. Developmental Science. 2001;4:195–208. [Google Scholar]
- Read S, Pedersen NL, Gatz M, Berg S, Vuoksimaa E, Malmberg B, McClearn GE. Sex differences after all those years? heritability of cognitive abilities in old age. The Journals of Gerontology Series. B. Psychological Sciences and Social Sciences. 2006;61:P137–P143. doi: 10.1093/geronb/61.3.p137. [DOI] [PubMed] [Google Scholar]
- Rutter M, Thorpe K, Greenwood R, North K, Golding J. Twins as a natural experiment to study the causes of language delay: I. Examination of obstetric and perinatal environment. Journal of Child Psychology and Psychiatry. 2003;44:326–341. doi: 10.1111/1469-7610.00125. [DOI] [PubMed] [Google Scholar]
- Scarborough H, Rescorla L, Tager-Flusberg H, Fowler A. The relation of utterance length to grammatical complexity in normal and language-disordered groups. Applied Psycholinguistics. 1991;12:23–46. [Google Scholar]
- Speltz M, DeKlyen M, Calderon R, Greenberg M, Fisher P. Neuropsychological characteristics and test behaviors of boys with early onset conduct problems. Journal of Abnormal Psychology. 1999;108:315. doi: 10.1037/0021-843X.108.2.315. [DOI] [PubMed] [Google Scholar]
- Stromswold K. The heritability of language: A review and metaanalysis of twin, adoption, and linkage studies. Langauge. 2001;77:647–723. [Google Scholar]
- Tamis-LeMonda C, Bornstein M, Baumwell L. Maternal responsiveness and children’s achievement of language milestones. Child Development. 2001;72:748–767. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Thorpe K. Twin children's language development. Early human development. 2006;82:387–395. doi: 10.1016/j.earlhumdev.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Mannie S, Kruger A. Linguistic environment of 1- to 2-year-old twins. Developmental Psychology. 1986;22:169–176. [Google Scholar]
- Tomopoulos S, Dreyer B, Berkule S, Fierman A, Brockmeyer C, Mendelsohn A. Infant media exposure and toddler development. Archives of Pediatrics & Adolescent Medicine. 2010;164:1105–1111. doi: 10.1001/archpediatrics.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen I, Brouwer R, van Leeuwen M, Kahn R, Hulshoff Pol H, Boomsma D. Heritability of verbal and performance intelligence in a pediatric longitudinal sample. Twin Research and Human Genetics. 2011;14:119–128. doi: 10.1375/twin.14.2.119. [DOI] [PubMed] [Google Scholar]
- Viding E, Price T, Spinath F, Bishop D, Dale P, Plomin R. Genetic and environmental mediation of the relationship between language and nonverbal impairment in 4-year-old twins. Journal of Speech, Language, and Hearing Research. 2003;46:1271–1282. doi: 10.1044/1092-4388(2003/099). [DOI] [PubMed] [Google Scholar]