Acute vasoreactivity testing is indicated in patients with idiopathic pulmonary arterial hypertension (IPAH) to identify subjects who will respond favorably to long-term treatment with calcium channel blockers (CCB) [1]. The result of the test determines the choice of treatment and predicts survival of the patient [2, 3]. It has been suggested that molecular etiology of IPAH is different in patients who respond or do not respond to CCB treatment [4]. In the current guidelines [1, 5] acute vasoreactivity testing is recommended in all patients with IPAH but treatment with CCB is advised only in those in World Health Organization functional class (WHO-FC) I–III. No guidelines are provided for patients with positive acute vasoreactivity testing who are in WHO-FC IV or who are hemodynamically unstable.
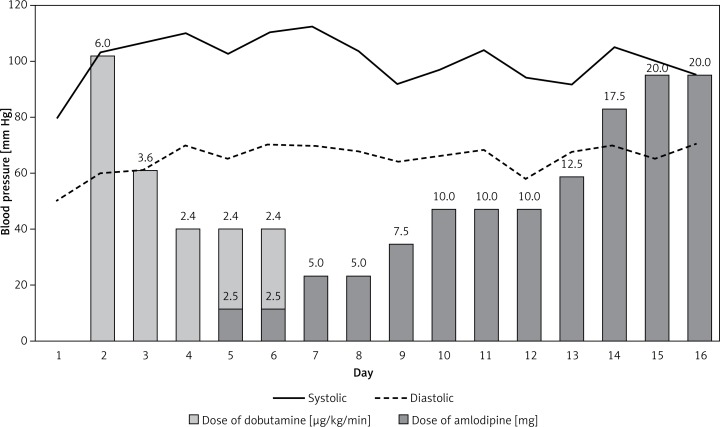
We present a case of a 29-year-old woman who was urgently admitted to the reference center for pulmonary hypertension from her local hospital due to rapidly progressing dyspnea and recurrent episodes of syncope. Her symptoms started 2 months before and progressed to WHO-FC IV at the time of admission. She presented with resting dyspnea, blood pressure of 90/60 mm Hg, a heart rate of 85 beats per minute and arterial blood saturation of 95%. The electrocardiogram showed sinus rhythm with an incomplete right bundle branch block. Echocardiography showed typical signs of precapillary pulmonary hypertension with right ventricular dysfunction (Table I). N-terminal pro b-type natriuretic peptide (NT-proBNP) was significantly elevated (Table I). On the first day of hospitalization the patient experienced syncope after minimal exertion. It was followed by prolonged hypotension which required management with intravenous fluid and continuous infusion of dobutamine. After hemodynamic stabilization, still on dobutamine, she underwent further diagnostic tests according to the current guidelines [1]. She was HIV negative; pulmonary function tests and lung perfusion scans were normal. Abdominal USG and laboratory tests excluded liver disease. Physical examination and laboratory tests did not suggest a diagnosis of connective tissue disease. Echocardiography was negative for congenital heart disease. These results together with right heart catheterization (day 4) confirmed the diagnosis of IPAH (Table I) [6]. Acute pulmonary vasoreactivity (20 ppm of nitric oxide over 5 min) was positive. Mean pulmonary artery pressure decreased from 49 mm Hg to 26 mm Hg without a change in the cardiac index (2.1 l/min/m2). We started treatment with amlodipine 2.5 mg while continuing inotropic support. After 2 days we were able to stop dobutamine infusion (Figure 1). At discharge the dose of amlodipine was 20 mg, and the patient was in WHO-FC II with 6 min walk distance of 370 m. The dose of amlodipine was increased in the outpatient clinic up to 40 mg daily. Three months follow-up showed marked hemodynamic and functional improvement, as shown in Table I. At 12 months follow-up the patient was at WHO-FC I, with 6 min walking distance of 490 m and no episodes of syncope.
Table I.
Functional and hemodynamic status of the patient at admission and at 3-month follow-up
| Parameter | Baseline | 3-month follow-up |
|---|---|---|
| Functional status: | ||
| WHO-FC | IV | II |
| 6MWD [m] | 0 at admission; 370 m at discharge | 450 |
| Echocardiography: | ||
| Proximal RVOT [mm] | 34 | 29 |
| TAPSE [mm] | 14 | 17 |
| RAA [cm2] | 24 | 17 |
| PAd [mm] | 42 | 28 |
| RVSP [mm Hg] | 88 | 55 |
| Pericardial fluid | Present | Absent |
| NT-proBNP [pg/ml] | 3141* | 132* |
| Right heart catheterization: | ||
| PAP s/m/d [mm Hg] | 72/49/38 | 53/29/18 |
| Mean RAP [mm Hg] | 1 | 1 |
| CI [l/min/m2] | 2.1 | 2.9 |
| Mean PAWP [mm Hg] | 10 | 8 |
| PVR [WU] | 10.2 | 3.9 |
| SVR [WU] | 25.9 | 16.7 |
| SpO2 in pulmonary artery [%] | 66 | 72 |
| Systemic pressure s/m/d [mm Hg] | 120/97/85 | 111/89/78 |
CI – cardiac index, PAd – pulmonary artery diameter, PAP – pulmonary artery pressure, PAWP – pulmonary artery wedge pressure, PVR – pulmonary vascular resistance, RAA – right atrium area, RAP – right atrial pressure, RVOT – right ventricular outflow tract, RVSP – right ventricular systolic pressure, SpO2 – oxygen saturation, s/m/d – systolic/mean/diastolic, SVR – systemic vascular resistance, TAPSE – tricuspid annular plane systolic excursion;
the upper reference limit is 125 pg/ml.
Figure 1.
Monitoring of systemic arterial pressure. The pressure was stabilized on dobutamine infusion and then maintained despite starting treatment with amlodipine
Observational studies showed a long-term benefit from CCB therapy in stable patients with vasoreactive IPAH at WHO-FC I–IV [3]. Our report is the first to present successful treatment with CCB of a hemodynamically compromised patient with vasoreactive IPAH. Based on this observation we suggest that in patients with vasoreactive IPAH an attempt of CCB therapy should be considered even when hemodynamic stabilization is dependent on inotropic support. The treatment should be closely monitored and prostacyclin therapy should be started in case of treatment failure with CCB [1, 7].
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 2.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 3.Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 4.Hemnes AR, Trammell AW, Archer SL, et al. A peripheral blood signature of vasodilator-responsive pulmonary arterial hypertension. Circulation. 2015;131:401–9. doi: 10.1161/CIRCULATIONAHA.114.013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D73–81. doi: 10.1016/j.jacc.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Sinha N, Devabhaktuni S, Kadambi A, McClung JA, Aronow WS, Lehrman SG. Can echocardiographically estimated pulmonary arterial elastance be a non-invasive predictor of pulmonary vascular resistance? Arch Med Sci. 2014;29:692–700. doi: 10.5114/aoms.2014.44860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergot E, Sitbon O, Cottin V, et al. Current epoprostenol use in patients with severe idiopathic, heritable or anorexigen-associated pulmonary arterial hypertension: data from the French pulmonary hypertension registry. Int J Cardiol. 2014;172:561–7. doi: 10.1016/j.ijcard.2013.12.313. [DOI] [PubMed] [Google Scholar]