Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. Elevated intraocular pressure (IOP) is considered to be a predominant risk factor for primary open angle glaucoma, the most prevalent form of glaucoma. Although the etiological mechanisms responsible for increased IOP are not completely clear, impairment in aqueous humor (AH) drainage through the conventional or trabecular pathway is recognized to be a primary cause in glaucoma patients. Importantly, lowering of IOP has been demonstrated to reduce progression of vision loss and is a mainstay of treatment for all types of glaucoma. Currently however, there are limited therapeutic options available for lowering IOP especially as it relates to enhancement of AH outflow through the trabecular pathway. Towards addressing this challenge, bench and bedside research conducted over the course of the last decade and a half has identified the significance of inhibiting Rho kinase for lowering IOP. Rho kinase is a downstream effector of Rho GTPase signaling that regulates actomyosin dynamics in numerous cell types. Studies from several laboratories have demonstrated that inhibition of Rho kinase lowers IOP via relaxation of the trabecular meshwork which enhances AH outflow. By contrast, activation of Rho GTPase/Rho kinase signaling in the trabecular outflow pathway increases IOP by altering the contractile, cell adhesive and permeability barrier characteristics of the trabecular meshwork and Schlemm’s canal tissues, and by influencing extracellular matrix production and fibrotic activity. This article, written in honor of the late David Epstein, MD, summarizes findings from both basic and clinical studies that have been instrumental for recognition of the importance of the Rho/Rho kinase signaling pathway in regulation of AH outflow, and in the development of Rho kinase inhibitors as promising IOP- lowering agents for glaucoma treatment.
Keywords: Glaucoma, Trabecular meshwork, Intraocular pressure, Rho kinase, Cytoskeleton, Aqueous humor outflow
1. Introduction
Glaucoma is a chronic optic neuropathy which represents a leading cause of irreversible blindness worldwide (Quigley and Broman, 2006). Globally there are nearly 60.5 million people affected by glaucoma and this number is expected to increase to 112 million by year 2040 (Tham et al., 2014). Primary open angle glaucoma (POAG) is considered to be the most prevalent among several different forms of glaucoma, (Kwon et al., 2009; Weinreb and Khaw, 2004). Although POAG is a multifactorial disease, elevated intraocular pressure (IOP) caused by impaired aqueous humor (AH) drainage from the eye is recognized as a primary risk factor (Kwon et al., 2009; Weinreb and Khaw, 2004). Elevated IOP in the anterior chamber of the eye damages optic nerve axons and leads to retinal ganglion cell (RGC) death which eventually impairs vision in glaucoma patients (Kwon et al., 2009; Quigley, 2011; Tian et al., 2015). Although the relationship between elevated IOP, optic nerve axonal damage and loss of RGCs is not completely clear at the mechanistic level, lowering IOP has been proven to delay further loss of RGCs in glaucoma patients (Higginbotham et al., 2004; Kass et al., 2005; Kwon et al., 2009; Tian et al., 2015). Moreover, since there are no proven neuroprotective therapeutic agents available to directly prevent optic nerve axonal damage and RGC loss in humans, lowering IOP remains the mainstay of glaucoma treatment (Kwon et al., 2009; Lee and Goldberg, 2011; Weinreb and Khaw, 2004).
Intraocular pressure is determined by the balance between production of AH by the ciliary epithelium and drainage of AH through the conventional and non-conventional outflow pathways (Gabelt and Kaufman, 2005; Weinreb and Khaw, 2004). In humans, most of the AH is drained via the conventional or trabecular pathway consisting of the trabecular meshwork (TM), juxtacanalicular tissue (JCT) and Schlemm’s canal (SC) (Gabelt and Kaufman, 2005; Lutjen-Drecoll, 1999). Importantly, blockage or increased resistance to AH outflow in the trabecular pathway is recognized as the main cause for elevated IOP in glaucoma patients (Gabelt and Kaufman, 2005; Lutjen-Drecoll, 1999; Stamer and Acott, 2012).
Cellular responses to physiological cues including cytokines, growth factors, steroids, miRNAs, ECM, mechanical stretch and reactive oxidants, have been demonstrated to influence AH outflow through the conventional pathway (Clark and Wordinger, 2009; Gabelt and Kaufman, 2005; Gagen et al., 2014; Gonzalez et al., 2014; Keller et al., 2009; Rao and Epstein, 2007; Sacca et al., 2016; Wiederholt et al., 2000). At the physiological level, cellular contraction/relaxation, permeability, cell stiffness, phagocytosis, ECM remodeling, cell survival and anti-oxidative activities are some of the cellular activities recognized to be important for maintaining homeostasis of AH outflow through the conventional pathway (Alvarado et al., 1981; Gabelt and Kaufman, 2005; Rao and Epstein, 2007; Sacca et al., 2016; Stamer and Acott, 2012; Wiederholt et al., 2000). Despite continued efforts however, we have yet to identify the definitive molecular pathways that serve as key determinants of trabecular AH outflow homeostasis, the disruption or impairment of which underlies increased resistance to AH outflow and eventually leads to elevated IOP in glaucoma patients (Stamer and Acott, 2012). Encouragingly, recent efforts using various animal and perfusion models in conjunction with molecular and pharmacological approaches have begun to not only identify certain major cellular pathways and molecular mechanisms regulating AH outflow and IOP, but also drive exploration of novel therapeutic avenues for targeted drug development to lower IOP and treat glaucoma (Agarwal and Agarwal, 2014; Gabelt and Kaufman, 2005; Inoue and Tanihara, 2013; Rao and Epstein, 2007; Stamer and Acott, 2012).
In this review, we have focused on describing (I) the Rho GTPase/Rho kinase signaling pathway and its role in TM and SC cell physiology, AH outflow and IOP in both normal and glaucoma subjects, and (II) the development of Rho kinase inhibitors as glaucoma therapeutics in humans.
2. The Rho GTPase and Rho kinase signaling pathway
Rho GTPases are a family of the Ras superfamily of monomeric small GTP-binding proteins, with the roles of RhoA, Rac1 and CDC42 being best characterized in the context of regulation of actin dynamics and various actin-associated cellular activities (Burridge and Wennerberg, 2004; Etienne-Manneville and Hall, 2002; Hall, 2012; Ridley, 2001). Briefly, these intracellular GTPases act as molecular switches that cycle between a GTP-bound, active conformation and a GDP-bound, inactive conformation. This cycling between bound GDP and GTP is regulated by guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) and guanine nucleotide dissociation inhibitors(GDIs) (Burridge and Wennerberg, 2004). The activities of GEFs and GAPs are regulated via engagement of various receptors at the plasma membrane (Cherfils and Zeghouf, 2013; Garcia-Mata and Burridge, 2007).
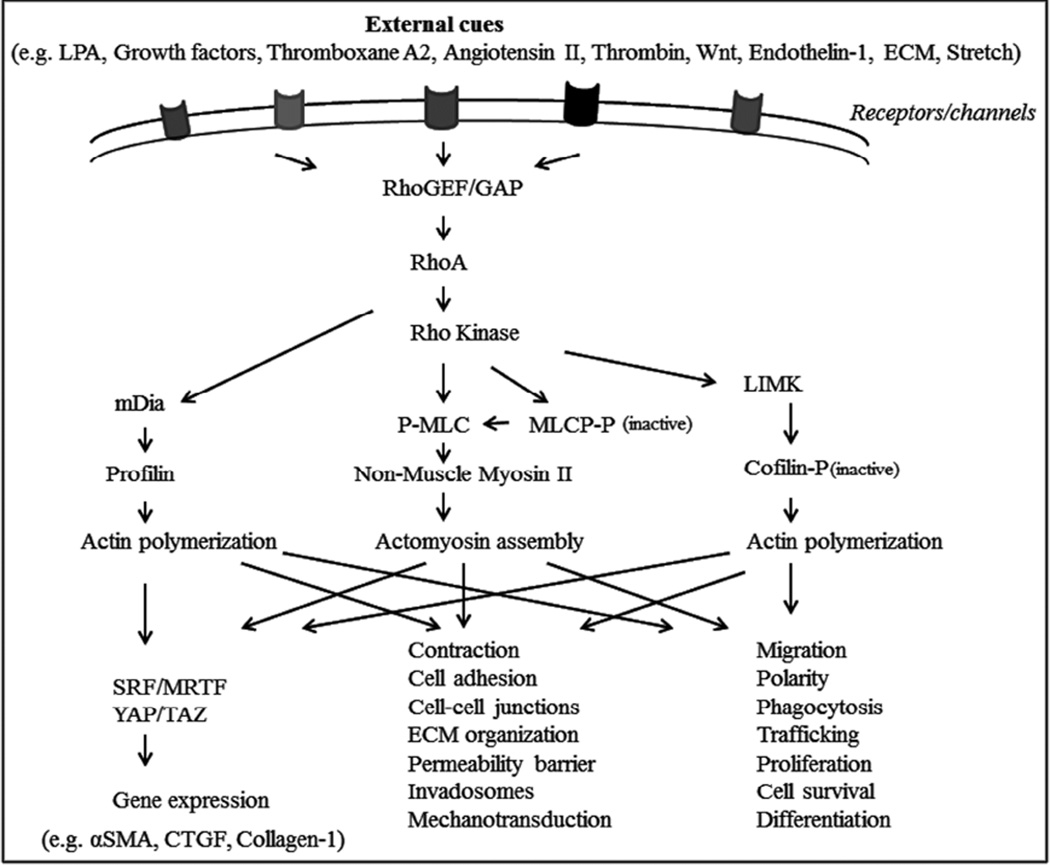
Among several downstream effectors of RhoA, the role of the Rho-associated, coiled-coil serine/threonine kinase, or Rho kinase, is well understood (Amin et al., 2013; Fukata et al., 2001; Hartmann et al., 2015; Julian and Olson, 2014; Knipe et al., 2015; Loirand, 2015; Thumkeo et al., 2013). There are two isoforms of Rho kinase, ROCK1 (ROKβ/P160) and ROCK2 (ROKα), which exhibit nearly 65% homology and are encoded by two different genes localized on human chromosomes 18 and 2, respectively (Fukata et al., 2001; Julian and Olson, 2014; Thumkeo et al., 2013). Both Rho kinase isoforms share many cellular effects, but also exhibit some isoform-specific activities (Thumkeo et al., 2005). ROCK1 and ROCK2 both contain an N-terminal serine/threonine kinase domain followed by a coiled-coil region containing the Rho-binding domain (RBD) and a pleckstrin homology domain with a cysteine-rich domain at the C terminus (Julian and Olson, 2014). Once activated by Rho GTPase, Rho kinase phosphorylates various intracellular substrates. The best characterized substrates include myosin light chain (MLC), myosin phosphatase substrate 1 (MYPT1, the regulatory subunit of myosin phosphatase), LIM kinase, CP1-17, calponin and the ERM (ezrin, radixin and moesin) proteins (Fukata et al., 2001; Somlyo and Somlyo, 2003; Thumkeo et al., 2013). Through these substrate interactions, Rho kinase regulates actin cytoskeletal dynamics, actomyosin contraction, cell adhesion, cell stiffness, cell morphology and ECM reorganization (Fig.1). While cellular contraction can be regulated via both calcium-dependent and independent means involving myosin light chain kinase and myosin phosphatase, respectively, Rho kinase has been demonstrated to regulate cellular contraction in smooth muscle tissues mainly through modulating myosin II activity in a calcium-independent manner (Fukata et al., 2001; Somlyo and Somlyo, 2003; Uehata et al., 1997).
Fig. 1.
Schematic illustration of the role of Rho GTPase/Rho kinase signaling pathway in regulation of actin cytoskeletal organization and cellular processes in smooth muscle and non-smooth muscle cells.
Based on data obtained from gene targeted mouse models, it is now well recognized that ROCK1 and ROCK2 play a vital role in several facets of cellular physiology (Thumkeo et al., 2003; Thumkeo et al., 2005). Importantly, there is also growing evidence that Rho kinase dysregulation is associated with various diseases (Amin et al., 2013; Hartmann et al., 2015; Knipe et al., 2015; Loirand, 2015; Mueller et al., 2005; Shimokawa et al., 2016; Thumkeo et al., 2013; Tsou et al., 2014). Therefore, Rho kinase has been considered a promising therapeutic target to explore the utility of inhibitors of the enzyme for treatment of a broad range of human diseases, with several clinical trials at various stages of completion (Feng and LoGrasso, 2014; Knipe et al., 2015; Loirand, 2015).
3. Role of Rho/Rho kinase signaling in the conventional AH outflow pathway and regulation of IOP
Well before several of us initiated research focused on the role of Rho GTPase and Rho kinase in TM tissue and the AH outflow pathway (Inoue and Tanihara, 2013; Rao and Epstein, 2007; Thieme et al., 2000), studies carried out in the laboratories of Paul Kaufman, Michael Wiederholt and David Epstein had demonstrated that integrity of the actin cytoskeleton and contractile properties of the TM influence AH outflow and IOP (Epstein et al., 1999; Erickson-Lamy et al., 1992; Kaufman and Barany, 1977; Peterson et al., 1999; Tian et al., 2000; Wiederholt et al., 2000). Moreover, certain broad specificity kinase inhibitors that influence cytoskeletal integrity and contraction of the TM had also been reported to alter AH outflow (Epstein et al., 1999; Tian et al., 1999; Tian et al., 1998). These significant and important initial studies spurred our interest in identifying specific and druggable signaling pathways that play a dominant role in the regulation of actin cytoskeletal organization and contractile properties in TM tissue and the AH outflow pathway. In this regard, the pioneering studies of Alan Hall (Hall, 1998), Anne Ridley (Ridley, 2001), Keith Burridge (Chrzanowska-Wodnicka and Burridge, 1996), Kozo Kaibuchi (Kimura et al., 1996) and Shuh Narumiya (Uehata et al., 1997) and others, demonstrating the role of Rho GTPases and Rho kinases in the regulation of actin dynamics, cell adhesion, cell shape and contraction, led us to postulate that Rho GTPase/Rho kinase signaling would play a critical role in TM function, AH outflow and IOP.
Since a great deal of work involving several laboratories has gone into exploring the role of Rho/Rho kinase signaling in TM cells, AH outflow and IOP, this review will separately and briefly discuss the effects of inhibition and activation of Rho/Rho kinase on TM cell biology and the aforementioned functional parameters.
3.1. Bench side research: The effects of inhibition of Rho GTPase and Rho kinase on TM, AH outflow and IOP
TM and SC cell studies
Early research into Rho GTPase and Rho kinase signaling demonstrated a major role for these proteins in regulating smooth muscle contractile activity and actomyosin organization in a calcium independent manner (Fukata et al., 2001; Somlyo and Somlyo, 2003; Uehata et al., 1997). Since it was recognized that contractile properties of the TM influenced AH outflow (Wiederholt et al., 2000), and that the TM possessed smooth muscle-like properties and expressed α-smooth muscle actin (αSMA) (de Kater et al., 1992; Wiederholt et al., 2000), we and others undertook an evaluation of the effects of Rho kinase inhibitor Y-27632 on TM cells and tissues of the AH outflow pathway. Inhibition of Rho kinase in TM cells was demonstrated to induce dose- and time-dependent reversible changes in cell shape in association with decreased actin stress fibers, focal adhesions and cell-cell interactions (Honjo et al., 2001; Inoue and Tanihara, 2013; Rao et al., 2005b; Rao et al., 2001). These cellular changes were closely regulated by nonmuscle myosin II activity via myosin light chain (MLC) phosphorylation (Rao et al., 2005b; Rao et al., 2001). Rho kinase inhibitors caused a decrease of MLC phosphorylation in TM cells and TM tissue via inhibition of phosphorylation of MLC and myosin phosphatase (Ramachandran et al., 2011a; Rao and Epstein, 2007).
Rho kinase inhibitors suppress the contractile response of TM and ciliary muscle to various extracellular factors, and this has been confirmed using several structural analogues of Y-27632 and fasudil (Cellini et al., 2005; Fukiage et al., 2001; Honjo et al., 2001; Rao and Epstein, 2007; Thieme et al., 2000). TM cells and the tissues of the AH outflow pathway (TM, JCT and SC) express both ROCK1 and ROCK 2 isoforms along with RhoA (Goldhagen et al., 2012; Nakajima et al., 2005; Rao et al., 2001). SC cells derived from human and monkey eyes exhibit very similar responses to Rho kinase inhibitors as those observed in TM cells in terms of changes in cell morphology, actin cytoskeleton and cell adhesive interactions (Kameda et al., 2012; Rao et al., 2001; Stamer et al., 2015). Inhibition of Rho GTPase and Rho kinase can also affect the integrity of inter-cellular junctions including the adherens junctions and tight junctions, thereby influencing the permeability barrier of the inner wall of SC (Kameda et al., 2012; Kaneko et al., 2016; Kumar and Epstein, 2011; Rao et al., 2001). It also is now well recognized that inhibition of Rho kinase reduces cell mechanical tension and stiffness, and decreases extracellular matrix synthesis and rigidity in various cell types including TM and SC cells through different molecular mechanisms (Pattabiraman et al., 2014; Zhang et al., 2008; Zhou et al., 2012).
Additionally, treatment of TM cells with Rho kinase inhibitors impairs Wnt planar cell polarity (PCP) signaling which regulates cytoskeletal organization (Yuan et al., 2013). Similarly, Rho kinase inhibitors suppress dexamethasone-induced cytoskeletal changes and enhance SC cell permeability revealing the possible involvement of Rho GTPase signaling in glucocorticoid-induced ocular hypertension (Fujimoto et al., 2012). Consistent with the effects of Rho kinase inhibitors, expression of dominant negative Rho kinase in TM cells decreases actin stress fibers, MLC phosphorylation and focal adhesions, confirming a definitive and specific role for Rho kinase in regulation of TM cell contractile activity (Rao et al., 2005a).
Moreover, Rho kinase inhibitors have been found to suppress the TGF-β2, lysophosphatidic acid (LPA), CTGF and RhoA-induced increase in TM cell transdifferentiation into myofibroblast-like cells, confirming the anti-fibrotic potential of Rho kinase inhibitors (Pattabiraman et al., 2014). It is also important to recognize that Rho kinase inhibitors influence nitric oxide (NO) mediated endothelial cell relaxation by increasing the expression and activation of endothelial NO synthase (Amin et al., 2013; Huveneers et al., 2015; Kolluru et al., 2014; Sawada and Liao, 2014). Although NO has been reported to modulate AH outflow and IOP, the interactions between the NO and Rho/Rho kinase pathways in TM or SC cells needs exploration in future studies (Cavet et al., 2015; Stamer and Acott, 2012).
In addition to these studies of Rho kinase inhibition, inhibition of Rho GTPase activity in TM cells has been studied using dominant negative forms of RhoA (Borras et al., 2015; Vittitow et al., 2002), direct inhibitors of RhoA including C3 exoenzyme (Liu et al., 2005; Pattabiraman and Rao, 2010; Slauson et al., 2015), and inhibitors of protein isoprenylation, e.g. statins and geranylgeranyl transferase inhibitors-GGTIs (Pervan et al., 2015; Rao et al., 2008; Song et al., 2005; Von Zee et al., 2009). As expected, each of these agents reduces actin stress fibers, focal adhesions and MLC phosphorylation, mimicking the changes induced by Rho kinase inhibitors in TM cells (Rao et al., 2008; Song et al., 2005; Vittitow et al., 2002). Inhibition of Rho GTPase activity in TM cells using isoprenylation inhibitors and C3 exoenzyme decreases TGF-β2 expression and secretion (Pervan et al., 2015). Interestingly, myocilin, a mutation in which is linked to juvenile-and adult-onset primary open angle glaucoma (Stone et al., 1997), diminishes cell:matrix interactions in TM cells by inhibiting Rho GTPase activity, indicating a potential functional link between myocilin and Rho GTPase in regulation of cell adhesive interactions (Shen et al., 2008). Furthermore, forskolin and cAMP-induced TM cell relaxation is linked to inactivation of Rho GTPase signaling via phosphorylation of RhoA (Ramachandran et al., 2011b). These observations suggest that Rho GTPase plays a central role in TM biology by way of interacting with key regulatory molecular and cellular pathways.
AH outflow studies
Following recognition of the dramatic effects induced by inhibitors of Rho kinase and Rho GTPase on morphological, contractile and cell adhesive interactions in TM and SC cells, the effects of these inhibitors on AH outflow were evaluated in both enucleated eyes and in live animals (Inoue and Tanihara, 2013; Rao and Epstein, 2007; Tian and Kaufman, 2005). Since AH outflow occurs through the trabecular pathway and the uveoscleral pathway (Gabelt and Kaufman, 2005; Weinreb and Khaw, 2004), it is necessary to know whether a given agent lowers IOP through changes to one- or both pathways. For instance, the prostaglandin F2α analogs, which are most commonly used first line treatment for lowering IOP, are known to achieve their ocular hypotensive effect mainly by increasing AH outflow through the uveoscleral pathway (Alm, 1998; Gabelt and Kaufman, 1989; Weinreb and Khaw, 2004).
Therefore, we and others assessed the effects of Rho kinase inhibition on AH outflow and demonstrated that different Rho kinase inhibitors all mediate a dose-dependent increase in AH outflow primarily through the trabecular pathway (Gong and Yang, 2014; Inoue and Tanihara, 2013; Li et al., 2016; Rao et al., 2005a; Rao et al., 2005b; Rao et al., 2001; Tian and Kaufman, 2005). Importantly, this increase in AH outflow through the trabecular pathway was associated with TM tissue relaxation (mediated by decreased MLC phosphorylation), increases in giant vacuoles in the SC inner wall, expansion of JCT, widening of SC and washout of extracellular material in the trabecular pathway (Gong and Yang, 2014; Li et al., 2016; Rao et al., 2005b; Rao et al., 2001). In addition to pharmacological inhibitors of Rho kinase, expression of dominant negative Rho kinase in organ cultured human eyes also led to increase in AH outflow facility through the trabecular pathway, confirming a definitive role for Rho kinase activity in regulation of the conventional AH outflow (Rao et al., 2005a).
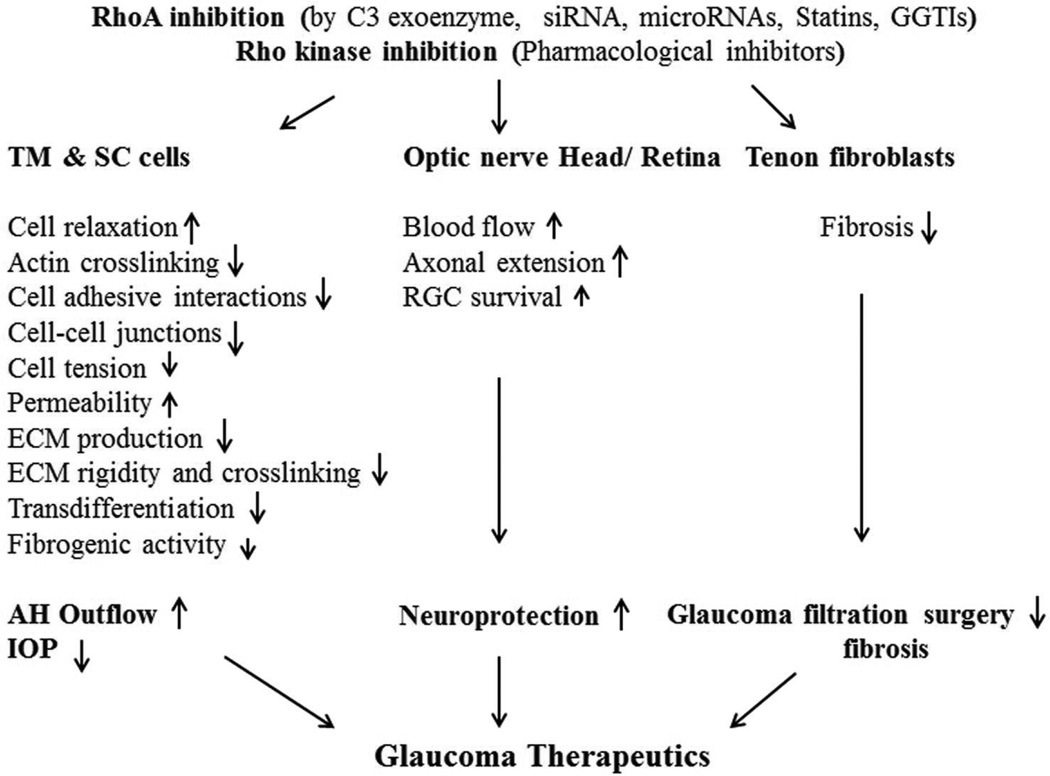
In analogous fashion, inhibition of Rho GTPase using C3 exoenzyme, statins and GGTIs has been shown to increase AH outflow facility through the trabecular pathway in porcine and monkey eyes (Liu et al., 2005; Rao et al., 2008; Song et al., 2005). Additionally, expression of dominant negative RhoA in the anterior chamber of human donor eyes has been demonstrated to increase AH outflow through the trabecular meshwork (Vittitow et al., 2002). Moreover, overexpression of miR-200c was reported to inhibit the contractile activity of TM cells and increase AH outflow through the TM by suppressing the expression of RhoA and activators of RhoA, including LPAR1 receptor and endothelin-1 (Luna et al., 2012). The studies using eyes from several different species consistently demonstrated that inhibitors of both Rho kinase and RhoA GTPase increase AH outflow through the trabecular pathway (Figs. 2), establishing the importance of this signaling pathway in homeostasis of AH outflow (Inoue and Tanihara, 2013; Rao and Epstein, 2007).
Fig. 2.
Inhibitors of Rho GTPase and Rho kinase lower IOP by increasing aqueous humor outflow through the conventional pathway and exhibit neuroprotective effects by stimulating axonal outgrowth and retinal ganglion cell survival.
IOP studies
Honjo et. al (Honjo et al., 2001) and Waki et. al (Waki et al., 2001) first reported that Rho kinase inhibition could lower IOP in rabbits via topical application and intracameral injections of Y-27632. Following this initial observation, several laboratories have confirmed that Y-27632 and other Rho kinase inhibitors consistently lower IOP in various animal models including rabbits, rodents, cats and monkeys, under both normal and ocular hypertensive conditions (Donegan and Lieberman, 2016; Feng et al., 2015; Fukunaga et al., 2009; Inoue and Tanihara, 2013; Isobe et al., 2014; Junglas et al., 2012; Li et al., 2016; Nishio et al., 2009; Pattabiraman et al., 2015b; Sumi et al., 2014; Van de Velde et al., 2014). Importantly, both ROCK1 and ROCK2 null mice exhibit lower basal IOP, confirming a definitive role for Rho kinase in IOP homeostasis (Whitlock et al., 2009).
Consistent with the IOP lowering effect of Rho kinase inhibitors, lowering of IOP in rodent eyes has been achieved by inhibition of Rho GTPase activity, either via expression of recombinant C3 exoenzyme which inhibits RhoA through ADP-ribosylation, or expression of dominant negative RhoA in the anterior chamber of rodent eyes (Borras et al., 2015; Liu et al., 2005; Slauson et al., 2015). Similarly, reduction of RhoA activity in the AH outflow pathway using shRNA lowers IOP in rodents (Liu et al., 2012).
Interestingly, inhibition of LIM kinase, a well characterized downstream target of Rho kinase also lowers IOP in ocular hypertensive mice (Harrison et al., 2009). Recently, LX7101, a dual inhibitor of both LIM kinase and Rho kinase, was reported to lower IOP in mice exhibiting dexamethasone-induced ocular hypertension (Harrison et al., 2015). Steroid-induced ocular hypertension is associated with changes in the TM that include stiffening of the tissue, actin cytoskeletal crosslinking and overproduction of ECM (Clark and Wordinger, 2009; Raghunathan et al., 2015). Therefore, it is reasonable to believe that the LIM kinase and Rho kinase inhibitors decrease IOP in steroid-induced ocular hypertensive mice through TM relaxation, actin depolymerization and decreased ECM production. Additionally, the ability of a Rho kinase inhibitor to lower IOP in rodents with ocular hypertension induced by the overexpression of CTGF, a pro-fibrotic cytokine (Junglas et al., 2012) suggests that the anti-fibrotic activity of Rho kinase inhibitors might account for their IOP lowering effects.
The mechanisms and tissues involved in the increase in trabecular outflow that occurs upon treatment with inhibitors of Rho GTPase and Rho kinase are still being actively investigated. A relatively new Rho kinase inhibitor currently in clinical development, netarsudil (previously AR-13324), has been shown to decrease IOP in monkeys by increasing trabecular AH outflow facility (Wang et al., 2015), and in rabbits it has also been shown to decrease episcleral venous pressure (Kiel and Kopczynski, 2015). Netarsudil, in addition to inhibiting Rho kinase, also exhibits a norepinephrine transport inhibitory activity (Sturdivant et al., 2016; Wang et al., 2015), so it is unclear whether its ability to reduce episcleral venous pressure is due to inhibition of Rho kinase, norepinephrine transporter, or both.
3.2.Bench side research: The effects of Rho GTPase activation on TM, AH outflow and IOP
TM and SC cell studies
Rho kinase is activated downstream of Rho GTPase in response to engagement of receptors for various extracellular factors and ligands that activate the Rho GTPase signaling pathway (Somlyo and Somlyo, 2003). In cultured TM cells derived from different species, RhoA GTPase activity has been demonstrated to be stimulated by LPA, S1P (sphingosine 1-phosphate), thromboxane A2, thrombin, Angiotensin II, endothelin-1, ECM proteins, dexamethasone, TGF-beta, CTGF, Wnt, mechanical stretch and galactin-8 (Diskin et al., 2012; Fujimoto et al., 2012; Iyer et al., 2012; Kumar and Epstein, 2011; Mettu et al., 2004; Pattabiraman et al., 2015a; Rao et al., 2005b; Sumida and Stamer, 2010; Yu et al., 2010; Yuan et al., 2013; Zhou et al., 2012). Some of these agents are known to stimulate Rho GTPase activity in SC cells as well, with many of them influencing the permeability barrier function of TM and SC and increasing resistance to AH outflow (Kumar and Epstein, 2011; Mettu et al., 2004; Stamer et al., 2009). Moreover, the levels of TGF-β2, endothelin-1 and CTGF are known to be elevated in AH derived from human glaucoma patients (Choritz et al., 2012; Noske et al., 1997; Tripathi et al., 1994). Importantly, mechanical stretch due to elevated IOP activates Rho GTPase signaling in the TM tissue (Pattabiraman et al., 2015a). Interestingly, dexamethasone treatment not only results in increased levels of Rho GTPase, but also decreases the levels of Rho GDI, and activates Rho GTPase activity in TM cells (Yu et al., 2010). Wnt-mediated nonconventional signaling in TM cells also activates Rho GTPase signaling and induces cross-linking of actin filaments (Yuan et al., 2013). RhoA activation was found to mediate TGF-β induced αSMA expression and contractile activity in TM cells (Nakamura et al., 2002). Collectively, these different observations reveal that Rho GTPase activity in AH outflow pathway cells, is responsive to, and can be dynamically regulated by various external cues.
Since dysregulated Rho GTPase function, especially chronic activation, has been implicated in various age related diseases (Amin et al., 2013; Hartmann et al., 2015; Loirand, 2015), studies from our group and others have explored the effects of sustained activation of Rho GTPase signaling in TM cells. Several laboratories have reported that Rho GTPase activation in TM and SC cells induces cellular contraction, stiffness and permeability barrier alterations in association with increased formation of actin stress fibers, focal adhesions, cell-cell interactions and increased myosin light chain phosphorylation (Kumar and Epstein, 2011; Mettu et al., 2004; Rao et al., 2005b; Stamer et al., 2015; Sumida and Stamer, 2010). Expression of constitutively active RhoA in TM cells increases the expression and levels of several ECM proteins and cytokines including the pro-fibrotic cytokines TGF-β and CTGF (Zhang et al., 2008). Moreover, activation of RhoA influences ECM rigidity and induces expression of αSMA in TM cells (Pattabiraman and Rao, 2010).
At a mechanistic level, in addition to its well understood role in actin cytoskeletal dynamics, Rho GTPase has also been demonstrated to directly control the transcriptional activity of SRF (serum response factor) and its co-activator MRTF (myocardin-related transcription factor), by regulating the ratio of G-actin to F-actin (Small, 2012; Treisman et al., 1998). MRTF binds to G-actin and accumulates in the cytosol. However, under conditions involving Rho GTPase activation, which drives increased F-actin generation via G-actin polymerization, MRTF is freed from G-actin and translocates to the nucleus to interact with SRF and initiate transcription of SRF/MRTF responsive genes including αSMA, CTGF and collagen-1(Knipe et al., 2015).
In our studies, Rho GTPase activation induced the expression of αSMA and various ECM proteins along with an enhanced fibrogenic response in TM cells, in an SRF/MRTF-dependent manner (Pattabiraman et al., 2014; Pattabiraman and Rao, 2010). Activation of SRF/MRTF transcription initiates transdifferentiation of fibroblasts into myofibroblasts and leads to fibrosis in various tissues (Knipe et al., 2015). Importantly, LPA and TGF-β-mediated activation of Rho GTPase leads to an increase in levels of αSMA, promoting transdifferentiation of TM cells and causing them to acquire a myofibroblast-like phenotype. These changes occur in association with the expression of Slug, Twist and snail1 fibrotic transcription factors, leading to mechanical changes due to hypercontraction of TM cells (Pattabiraman et al., 2014). Additionally, activation of Rho GTPase/Rho kinase signaling by external cues (e.g., dexamethasone) was documented to regulate the activity of transcriptional factors YAP/TAZ in different cell types, which control the expression of fibrotic genes including CTGF and collagen-1(Liu et al., 2015), and influence biomechanical characteristics of cells via the mechanosensitive Hippo signaling pathway (Mo et al., 2012; Sorrentino et al., 2014) (Fig.1).
AH outflow and IOP studies
Expression of constitutively active RhoA GTPase (RhoAV14) in organ cultured porcine eyes inducing an increase in TM contractile activity has been shown to decrease AH outflow facility, confirming the direct influence of Rho GTPase activity on AH outflow (Zhang et al., 2008). Additionally, lentiviral-mediated expression of RhoAV14 in the anterior chamber of an in vivo rat model was found to increase IOP in a sustained manner (Pattabiraman et al., 2015b). An important finding is that the ocular hypertension noted in these animals was associated with increased fibrotic activity in tissues of the AH outflow pathway (Pattabiraman et al., 2015b). Significantly, topical application of Rho kinase inhibitor Y-27632 was found to lower IOP in rats exhibiting RhoAV14 induced ocular hypertension (Pattabiraman et al., 2015b). The effects of Y-27632 on elevated IOP in this animal model were suggested to be mediated partly through the known anti-fibrotic activity of this Rho-kinase inhibitor (Knipe et al., 2015; Moriyama and Nagatoya, 2004; Pattabiraman et al., 2014).
The potential importance of dysregulated Rho GTPase /Rho kinase signaling in the pathogenesis of human glaucoma with ocular hypertension gains support from human genetic studies. Recent genome-wide association studies conducted in human glaucoma patients identified an association between increased IOP and a well characterized nucleotide exchange factor (ARHGEF12) of Rho GTPase (Springelkamp et al., 2015). However, human polymorphism studies have not revealed an association between the Rho kinases and propensity for glaucoma (Demiryurek et al., 2016).
4. Bedside research: Rho kinase inhibitors as glaucoma therapeutics
Based on consistent and promising results demonstrating the IOP lowering effects of Rho kinase inhibitors in both live animals and eye perfusion models (Inoue and Tanihara, 2013; Rao and Epstein, 2007; Tian and Kaufman, 2005), several pharmaceutical companies initiated exploration of the ocular hypotensive effects of Rho kinase inhibitors in humans. To date, the Rho kinase inhibitors that have been evaluated for clinical safety and efficacy in human subjects, including ripasudil (K-115), fasudil, SNJ-1656, AMA0076, AR-12286 and netarsudil (AR-13324), have all demonstrated ocular hypotensive effects (Inoue and Tanihara, 2013; Pakravan et al., 2016). Two of these compounds, ripasudil (Kowa) and netarsudil (Aerie), were successfully advanced to phase 3 clinical studies in Japan and the US, respectively. Twice-daily topical administration of 0.4% ripasudil in phase 3 trials reduced mean diurnal IOP by 2.9 mmHg in glaucoma patients. Ripasudil was recently approved in Japan for the treatment of ocular hypertension and glaucoma (Garnock-Jones, 2014; Inoue and Tanihara, 2013). This compound also demonstrated additive efficacy when used in combination with the beta blocker timolol, which reduces IOP by reducing AH production, and the prostaglandin F2α analog latanoprost, which reduces IOP by increasing uveoscleral outflow (Tanihara et al., 2015). The demonstration of additive efficacy is significant, since it indicates that the Rho kinase mechanism of lowering IOP by increasing trabecular outflow is compatible with other currently approved classes of glaucoma drugs.
Phase 3 clinical trials of Aerie Pharmaceuticals’ netarsudil, a Rho kinase/norepinephrine transporter inhibitor, are expected to be completed by mid-2016. In Phase 2 clinical trials in patients with elevated IOP, once-daily dosing of 0.02% netarsudil produced reductions in mean diurnal IOP ranging from 5.7 to 6.8 mmHg (Bacharach et al., 2015; Levy et al., 2015; Lewis et al., 2016). Interestingly, in contrast to latanoprost which was most effective in subjects with higher baseline IOPs and less effective in subjects with lower baseline IOPs, 0.02% netarsudil maintained the same IOP lowering effect at both lower and higher baseline IOPs (Bacharach et al., 2015). In a separate Phase 1 study of healthy subjects with low baseline IOPs (14–20 mmHg), 0.02% netarsudil again produced significant IOP reductions, reducing mean diurnal IOP from 16.2 mmHg to 11.3 mmHg after 8 days of treatment (Levy et al., 2015). The substantial IOP reductions observed in subjects with lower baseline IOPs could be related to netarsudil’s reported ability to lower episcleral venous pressure in rabbits (Kiel and Kopczynski, 2015; Sturdivant et al., 2016), given that episcleral venous pressure can be responsible for more than half of measured IOP in normotensive patients (Selbach et al., 2005).
Based on demonstrated additive efficacy between Rho kinase inhibitors and prostaglandin F2α analogs, Aerie Pharmaceuticals developed a fixed combination of 0.02%netarsudil plus 0.005% latanoprost, referred to as PG324. In a Phase 2 study, once-daily dosing of 0.02% PG324 provided IOP reductions that were 1.6 to 3.2 mmHg greater than latanoprost alone (Lewis et al., 2016). Phase 3 clinical development of 0.02% PG324 was initiated in 2015.
The most common adverse event associated with different Rho kinase inhibitors was conjunctival hyperemia, which was typically transient and asymptomatic. Conjunctival hyperemia is an expected pharmacological effect of Rho kinase inhibitors due to their ability to relax smooth muscle cells and dilate blood vessels (Somlyo and Somlyo, 2003; Uehata et al., 1997).
5. Effects of Rho GTPase and Rho kinase signaling on optic nerve axons and retinal ganglion cell survival
Optic nerve degeneration and retinal ganglion cell (RGC) loss are the main cause for the loss of vision in glaucoma (Kwon et al., 2009; Van de Velde et al., 2015). Although lowering IOP slows the neuronal degeneration of axons and loss of RGCs, in a large population of normotensive glaucoma patients, optic nerve atrophy and loss of RGC occur with no obvious elevation in IOP (Kwon et al., 2009; Quigley, 2011; Van de Velde et al., 2015). Interestingly, Rho/Rho kinase signaling is recognized to be involved in the pathobiology of various central nervous system (CNS) disorders (Chrissobolis and Sobey, 2006; Forgione and Fehlings, 2014; Fujita and Yamashita, 2014). The Rho GTPase signaling pathway has been demonstrated to be especially involved in the inhibition of axonal outgrowth by Nogo, myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), and other repulsive guidance molecules (Fujita and Yamashita, 2014; Hall and Lalli, 2010). Moreover, Rho GTPase and Rho kinase influence neuronal cell death as well via regulating the activities of several molecules involved in cell survival and death including PTEN (Phosphatase and Tensin homolog) and microtubule-associated proteins (Boomkamp et al., 2012; Mimura et al., 2006; Varma et al., 2010). Importantly, optic nerve head and optic nerve head astrocytes derived from glaucoma patients show elevated levels of RhoA and Rho kinase, indicating a possible involvement for Rho GTPase signaling in glaucoma pathobiology at the optic nerve (Goldhagen et al., 2012; Lukas et al., 2008). In the rat optic nerve crush model, RGC cell apoptosis and axonal degeneration were associated with elevated levels of RhoA, RhoA activation, caspase-3 and ROCK2 in the retinal ganglion cell layer (Tan et al., 2011; Xu et al., 2014).
It also is becoming increasingly evident from various experimental studies that inhibition of the Rho/Rho kinase signaling pathway suppresses neuronal damage in different CNS disease models and promoting both increased axonal extension and increased neuronal survival (DeGeer and Lamarche-Vane, 2013; Fujita and Yamashita, 2014). RhoA inhibition using C3 exoenzyme and suppression of RhoA expression by shRNA increases axonal outgrowth and RGC survival in various animal models of glaucomatous damage (Bertrand et al., 2007; Bertrand et al., 2005; Drummond et al., 2014; Koch et al., 2014). Similarly, different Rho kinase inhibitors including HA1007 (fasudil), Y-39983 (SJN-1656), ripasudil (K-115) and Y-27632 were found to exhibit neuroprotection by promoting axonal outgrowth and RGC survival in different animal models (Bermel et al., 2009; Hirata et al., 2008; Kitaoka et al., 2004; Sagawa et al., 2007; Sugiyama et al., 2011; Van de Velde et al., 2015; Yamamoto et al., 2014; Yu et al., 2015; Yu et al., 2016). Fasudil and Y-39983 have also been shown to increase blood flow to the optic nerve head in rabbits (Sugiyama et al., 2011; Tokushige et al., 2011).
Although there are a vast number of preclinical studies that support the importance of Rho GTPase and Rho kinase inhibitors as potential neuroprotective agents (Van de Velde et al., 2015), the efficacy of these drugs as direct neuroprotective agents has yet to be tested in human patients.
6. Concluding remarks
The current time is undoubtedly an exciting period in which remarkable progress is being made toward understanding the physiology of the conventional AH outflow pathway and the mechanisms of IOP homeostasis. Through this understanding, the ultimate goal of developing targeted therapies to lower IOP in glaucoma patients via increasing trabecular outflow appears to be coming to fruition. The Rho GTPase/Rho kinase signaling pathway has been identified as one of the central mechanisms that integrates inputs from various external factors and generates outputs and cellular effects that regulate AH outflow homeostasis through the trabecular pathway. The first Rho kinase inhibitor has been approved for the treatment of patients with glaucoma, and a Rho kinase/norepinephrine transporter inhibitor will soon complete Phase 3 clinical development. Additionally, combinatorial therapy using Rho kinase inhibitors in combination with timolol or latanoprost is being exploited to harness additive efficacy to lower IOP.
Importantly, as this work continues, it is providing important and novel insights into other related molecular pathways and targets for pharmacological manipulation of IOP. Most significantly, additional studies are required to understand how Rho/Rho kinase signaling is regulated by external factors in the healthy AH outflow pathway, as well as to identify the basis for dysregulation of this pathway with aging and ultimately in glaucomatous eyes.
Beyond lowering of IOP, Rho kinase inhibitors may provide additional benefits for the treatment of glaucoma. The anti-fibrotic activity of Rho kinase inhibitors may be able to slow or stop the functional deterioration of trabecular outflow tissues that is the underlying cause of elevated IOP in glaucoma patients, and to prevent tissue scarring following glaucoma filtration surgery. Furthermore, it is imperative that we continue to explore this class of drugs for their potential to provide neuroprotective activity that could directly rescue or mitigate optic nerve axonal damage and enhance RGC survival in patients with glaucoma.
Highlights.
Rho/Rho kinase signaling plays a crucial role in homeostasis of AH outflow and IOP
Dysregulation of Rho/Rho kinase signaling impairs AH outflow and leads to increased IOP
Inhibition of Rho/Rho kinase signaling results in an ocular hypotensive response
Inhibition of Rho/Rho kinase has neuroprotective effects
Rho kinase inhibition exhibits anti-fibrotic effects in TM and Tenon fibroblasts
Acknowledgments
We are greatly indebted to the late Dr. David Epstein for the constant encouragement he provided during our studies on the Rho/Rho kinase signaling pathway in the AH outflow pathway and his central role in advancing basic science towards clinical practice. The studies performed in the Rao laboratory were supported by the National Institutes of Health grants (R01 EY018590 and EY25096).
Dedication
The authors would like to dedicate this article to the memory of Dr. David Epstein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal R, Agarwal P. Newer targets for modulation of intraocular pressure: focus on adenosine receptor signaling pathways. Expert Opin Ther Targets. 2014;18:527–539. doi: 10.1517/14728222.2014.888416. [DOI] [PubMed] [Google Scholar]
- Alm A. Prostaglandin derivates as ocular hypotensive agents. Prog Retin Eye Res. 1998;17:291–312. doi: 10.1016/s1350-9462(97)00003-7. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Investigative ophthalmology & visual science. 1981;21:714–727. [PubMed] [Google Scholar]
- Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV, Ahmadian MR. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharach J, Dubiner HB, Levy B, Kopczynski CC, Novack GD Group, A.-C.S. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122:302–307. doi: 10.1016/j.ophtha.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Bermel C, Tonges L, Planchamp V, Gillardon F, Weishaupt JH, Dietz GP, Bahr M, Lingor P. Combined inhibition of Cdk5 and ROCK additively increase cell survival, but not the regenerative response in regenerating retinal ganglion cells. Mol Cell Neurosci. 2009;42:427–437. doi: 10.1016/j.mcn.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Di Polo A, McKerracher L. Enhanced survival and regeneration of axotomized retinal neurons by repeated delivery of cell-permeable C3-like Rho antagonists. Neurobiol Dis. 2007;25:65–72. doi: 10.1016/j.nbd.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Winton MJ, Rodriguez-Hernandez N, Campenot RB, McKerracher L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci. 2005;25:1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomkamp SD, Riehle MO, Wood J, Olson MF, Barnett SC. The development of a rat in vitro model of spinal cord injury demonstrating the additive effects of Rho and ROCK inhibitors on neurite outgrowth and myelination. Glia. 2012;60:441–456. doi: 10.1002/glia.22278. [DOI] [PubMed] [Google Scholar]
- Borras T, Buie LK, Spiga MG, Carabana J. Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats. JAMA Ophthalmol. 2015;133:182–190. doi: 10.1001/jamaophthalmol.2014.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Cavet ME, Vollmer TR, Harrington KL, VanDerMeid K, Richardson ME. Regulation of Endothelin-1-Induced Trabecular Meshwork Cell Contractility by Latanoprostene Bunod. Investigative ophthalmology & visual science. 2015;56:4108–4116. doi: 10.1167/iovs.14-16015. [DOI] [PubMed] [Google Scholar]
- Cellini M, Versura P, Trere D, Campos EC. Effects of endothelin-1 on human trabecular meshwork cell contraction. an in vitro cell culture model. Ophthalmic Res. 2005;37:43–49. doi: 10.1159/000083021. [DOI] [PubMed] [Google Scholar]
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- Choritz L, Machert M, Thieme H. Correlation of endothelin-1 concentration in aqueous humor with intraocular pressure in primary open angle and pseudoexfoliation glaucoma. Investigative ophthalmology & visual science. 2012;53:7336–7342. doi: 10.1167/iovs.12-10216. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke. 2006;37:2174–2180. doi: 10.1161/01.STR.0000231647.41578.df. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Experimental eye research. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- de Kater AW, Shahsafaei A, Epstein DL. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Investigative ophthalmology & visual science. 1992;33:424–429. [PubMed] [Google Scholar]
- DeGeer J, Lamarche-Vane N. Rho GTPases in neurodegeneration diseases. Exp Cell Res. 2013;319:2384–2394. doi: 10.1016/j.yexcr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Demiryurek S, Okumus S, Bozgeyik I, Oztuzcu S, Coskun E, Mat E, Durucu E, Tatar MG, Erbagci I, Gurler B, Demiryurek AT. Investigation of the Rho-kinase Gene Polymorphism in Primary Open-angle Glaucoma. Ophthalmic genetics. 2016;37:9–13. doi: 10.3109/13816810.2014.895016. [DOI] [PubMed] [Google Scholar]
- Diskin S, Chen WS, Cao Z, Gyawali S, Gong H, Soza A, Gonzalez A, Panjwani N. Galectin-8 promotes cytoskeletal rearrangement in trabecular meshwork cells through activation of Rho signaling. PloS one. 2012;7:e44400. doi: 10.1371/journal.pone.0044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan RK, Lieberman RL. Discovery of Molecular Therapeutics for Glaucoma: Challenges, Successes, and Promising Directions. J Med Chem. 2016;59:788–809. doi: 10.1021/acs.jmedchem.5b00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond ES, Rodger J, Penrose M, Robertson D, Hu Y, Harvey AR. Effects of intravitreal injection of a Rho-GTPase inhibitor (BA-210), or CNTF combined with an analogue of cAMP, on the dendritic morphology of regenerating retinal ganglion cells. Restor Neurol Neurosci. 2014;32:391–402. doi: 10.3233/RNN-130360. [DOI] [PubMed] [Google Scholar]
- Epstein DL. Soaring aspirations: lessons from my mentors and colleagues: the Weisenfeld award. Investigative ophthalmology & visual science. 2013;54:5219–5226. doi: 10.1167/iovs.13-11879. [DOI] [PubMed] [Google Scholar]
- Epstein DL, Roberts BC, Skinner LL. Nonsulfhydryl-reactive phenoxyacetic acids increase aqueous humor outflow facility. Investigative ophthalmology & visual science. 1997;38:1526–1534. [PubMed] [Google Scholar]
- Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Investigative ophthalmology & visual science. 1999;40:74–81. [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder A, Epstein DL. Ethacrynic acid induces reversible shape and cytoskeletal changes in cultured cells. Investigative ophthalmology & visual science. 1992;33:2631–2640. [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Feng Y, LoGrasso PV. Rho kinase inhibitors: a patent review (2012 – 2013) Expert Opin Ther Pat. 2014;24:295–307. doi: 10.1517/13543776.2014.863279. [DOI] [PubMed] [Google Scholar]
- Feng Y, LoGrasso PV, Defert O, Li R. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J Med Chem. 2015 doi: 10.1021/acs.jmedchem.5b00683. [DOI] [PubMed] [Google Scholar]
- Forgione N, Fehlings MG. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014;82:e535–e539. doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Inoue T, Kameda T, Kasaoka N, Inoue-Mochita M, Tsuboi N, Tanihara H. Involvement of RhoA/Rho-associated kinase signal transduction pathway in dexamethasone-induced alterations in aqueous outflow. Investigative ophthalmology & visual science. 2012;53:7097–7108. doi: 10.1167/iovs.12-9989. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338. doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Fukiage C, Mizutani K, Kawamoto Y, Azuma M, Shearer TR. Involvement of phosphorylation of myosin phosphatase by ROCK in trabecular meshwork and ciliary muscle contraction. Biochem Biophys Res Commun. 2001;288:296–300. doi: 10.1006/bbrc.2001.5751. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Ikesugi K, Nishio M, Sugimoto M, Sasoh M, Hidaka H, Uji Y. The effect of the Rho-associated protein kinase inhibitor, HA-1077, in the rabbit ocular hypertension model induced by water loading. Current eye research. 2009;34:42–47. doi: 10.1080/02713680802531353. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Prostaglandin F2 alpha increases uveoscleral outflow in the cynomolgus monkey. Experimental eye research. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gagen D, Faralli JA, Filla MS, Peters DM. The role of integrins in the trabecular meshwork. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:110–120. doi: 10.1089/jop.2013.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones KP. Ripasudil: first global approval. Drugs. 2014;74:2211–2215. doi: 10.1007/s40265-014-0333-2. [DOI] [PubMed] [Google Scholar]
- Goldhagen B, Proia AD, Epstein DL, Rao PV. Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. Journal of glaucoma. 2012;21:530–538. doi: 10.1097/IJG.0b013e318241b83c. [DOI] [PubMed] [Google Scholar]
- Gong H, Yang CY. Morphological and hydrodynamic correlations with increasing outflow facility by rho-kinase inhibitor Y-27632. J Ocul Pharmacol Ther. 2014;30:143–153. doi: 10.1089/jop.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Li G, Qiu J, Wu J, Luna C. Role of microRNAs in the trabecular meshwork. J Ocul Pharmacol Ther. 2014;30:128–137. doi: 10.1089/jop.2013.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BA, Almstead ZY, Burgoon H, Gardyan M, Goodwin NC, Healy J, Liu Y, Mabon R, Marinelli B, Samala L, Zhang Y, Stouch TR, Whitlock NA, Gopinathan S, McKnight B, Wang S, Patel N, Wilson AG, Hamman BD, Rice DS, Rawlins DB. Discovery and Development of LX7101, a Dual LIM-Kinase and ROCK Inhibitor for the Treatment of Glaucoma. ACS Med Chem Lett. 2015;6:84–88. doi: 10.1021/ml500367g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BA, Whitlock NA, Voronkov MV, Almstead ZY, Gu KJ, Mabon R, Gardyan M, Hamman BD, Allen J, Gopinathan S, McKnight B, Crist M, Zhang Y, Liu Y, Courtney LF, Key B, Zhou J, Patel N, Yates PW, Liu Q, Wilson AG, Kimball SD, Crosson CE, Rice DS, Rawlins DB. Novel class of LIM-kinase 2 inhibitors for the treatment of ocular hypertension and associated glaucoma. J Med Chem. 2009;52:6515–6518. doi: 10.1021/jm901226j. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Ridley AJ, Lutz S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front Pharmacol. 2015;6:276. doi: 10.3389/fphar.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham EJ, Gordon MO, Beiser JA, Drake MV, Bennett GR, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Archives of ophthalmology. 2004;122:813–820. doi: 10.1001/archopht.122.6.813. [DOI] [PubMed] [Google Scholar]
- Hirata A, Inatani M, Inomata Y, Yonemura N, Kawaji T, Honjo M, Tanihara H. Y-27632, a Rho-associated protein kinase inhibitor, attenuates neuronal cell death after transient retinal ischemia. Graefes Arch Clin Exp Ophthalmol. 2008;246:51–59. doi: 10.1007/s00417-007-0666-6. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Investigative ophthalmology & visual science. 2001;42:137–144. [PubMed] [Google Scholar]
- Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res. 2015;116:895–908. doi: 10.1161/CIRCRESAHA.116.305720. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Isobe T, Mizuno K, Kaneko Y, Ohta M, Koide T, Tanabe S. Effects of K-115, a rho-kinase inhibitor, on aqueous humor dynamics in rabbits. Current eye research. 2014;39:813–822. doi: 10.3109/02713683.2013.874444. [DOI] [PubMed] [Google Scholar]
- Iyer P, Maddala R, Pattabiraman PP, Rao PV. Connective tissue growth factor-mediated upregulation of neuromedin U expression in trabecular meshwork cells and its role in homeostasis of aqueous humor outflow. Investigative ophthalmology & visual science. 2012;53:4952–4962. doi: 10.1167/iovs.12-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Kameda T, Inoue T, Inatani M, Fujimoto T, Honjo M, Kasaoka N, Inoue-Mochita M, Yoshimura N, Tanihara H. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Investigative ophthalmology & visual science. 2012;53:3092–3103. doi: 10.1167/iovs.11-8018. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Ohta M, Inoue T, Mizuno K, Isobe T, Tanabe S, Tanihara H. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm's canal endothelial cells. Sci Rep. 2016;6:19640. doi: 10.1038/srep19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MA, Gordon MO, Kymes SM. Incorporating the results of the Ocular Hypertension Treatment Study into clinical practice. Archives of ophthalmology. 2005;123:1021–1022. doi: 10.1001/archopht.123.7.1021-b. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Barany EH. Cytochalasin B reversibly increases outflow facility in the eye of the cynomolgus monkey. Investigative ophthalmology & visual science. 1977;16:47–53. [PubMed] [Google Scholar]
- Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Experimental eye research. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel JW, Kopczynski CC. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015;31:146–151. doi: 10.1089/jop.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitaoka Y, Kitaoka Y, Kumai T, Lam TT, Kuribayashi K, Isenoumi K, Munemasa Y, Motoki M, Kobayashi S, Ueno S. Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res. 2004;1018:111–118. doi: 10.1016/j.brainres.2004.05.070. [DOI] [PubMed] [Google Scholar]
- Knipe RS, Tager AM, Liao JK. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev. 2015;67:103–117. doi: 10.1124/pr.114.009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JC, Tonges L, Michel U, Bahr M, Lingor P. Viral vector-mediated downregulation of RhoA increases survival and axonal regeneration of retinal ganglion cells. Front Cell Neurosci. 2014;8:273. doi: 10.3389/fncel.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluru GK, Majumder S, Chatterjee S. Rho-kinase as a therapeutic target in vascular diseases: striking nitric oxide signaling. Nitric Oxide. 2014;43:45–54. doi: 10.1016/j.niox.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Kumar J, Epstein DL. Rho GTPase-mediated cytoskeletal organization in Schlemm's canal cells play a critical role in the regulation of aqueous humor outflow facility. J Cell Biochem. 2011;112:600–606. doi: 10.1002/jcb.22950. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. The New England journal of medicine. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert Opin Emerg Drugs. 2011;16:137–161. doi: 10.1517/14728214.2011.521631. [DOI] [PubMed] [Google Scholar]
- Levy B, Ramirez N, Novack GD, Kopczynski C. Ocular hypotensive safety and systemic absorption of AR-13324 ophthalmic solution in normal volunteers. American journal of ophthalmology. 2015;159:980–985. e981. doi: 10.1016/j.ajo.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Levy B, Ramirez N, C CK, Usner DW, Novack GD Group, P.C.S. Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016;100:339–344. doi: 10.1136/bjophthalmol-2015-306778. [DOI] [PubMed] [Google Scholar]
- Li G, Mukherjee D, Navarro I, Ashpole NE, Sherwood JM, Chang J, Overby DR, Yuan F, Gonzalez P, Kopczynski CC, Farsiu S, Stamer WD. Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. European journal of pharmacology. 2016 doi: 10.1016/j.ejphar.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wu K, Qiu X, Yang Y, Lin X, Yu M. siRNA silencing of gene expression in trabecular meshwork: RhoA siRNA reduces IOP in mice. Curr Mol Med. 2012;12:1015–1027. doi: 10.2174/156652412802480907. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu Y, Filla MS, Gabelt BT, Peters DM, Brandt CR, Kaufman PL. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Molecular vision. 2005;11:1112–1121. [PubMed] [Google Scholar]
- Loirand G. Rho Kinases in Health and Disease: From Basic Science to Translational Research. Pharmacol Rev. 2015;67:1074–1095. doi: 10.1124/pr.115.010595. [DOI] [PubMed] [Google Scholar]
- Lukas TJ, Miao H, Chen L, Riordan SM, Li W, Crabb AM, Wise A, Du P, Lin SM, Hernandez MR. Susceptibility to glaucoma: differential comparison of the astrocyte transcriptome from glaucomatous African American and Caucasian American donors. Genome Biol. 2008;9:R111. doi: 10.1186/gb-2008-9-7-r111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Huang J, Qiu J, Wu J, Yuan F, Epstein DL, Gonzalez P. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PloS one. 2012;7:e51688. doi: 10.1371/journal.pone.0051688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Mettu PS, Deng PF, Misra UK, Gawdi G, Epstein DL, Rao PV. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Investigative ophthalmology & visual science. 2004;45:2263–2271. doi: 10.1167/iovs.03-0960. [DOI] [PubMed] [Google Scholar]
- Mimura F, Yamagishi S, Arimura N, Fujitani M, Kubo T, Kaibuchi K, Yamashita T. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J Biol Chem. 2006;281:15970–15979. doi: 10.1074/jbc.M510934200. [DOI] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Nagatoya K. The Rho-ROCK system as a new therapeutic target for preventing interstitial fibrosis. Drug news & perspectives. 2004;17:29–34. doi: 10.1358/dnp.2004.17.1.829023. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Nakajima E, Nakajima T, Minagawa Y, Shearer TR, Azuma M. Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes. J Pharm Sci. 2005;94:701–708. doi: 10.1002/jps.20285. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Hirano S, Suzuki K, Seki K, Sagara T, Nishida T. Signaling mechanism of TGF-beta1-induced collagen contraction mediated by bovine trabecular meshwork cells. Investigative ophthalmology & visual science. 2002;43:3465–3472. [PubMed] [Google Scholar]
- Nishio M, Fukunaga T, Sugimoto M, Ikesugi K, Sumi K, Hidaka H, Uji Y. The effect of the H-1152P, a potent Rho-associated coiled coil-formed protein kinase inhibitor, in rabbit normal and ocular hypertensive eyes. Current eye research. 2009;34:282–286. doi: 10.1080/02713680902783763. [DOI] [PubMed] [Google Scholar]
- Noske W, Hensen J, Wiederholt M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes Arch Clin Exp Ophthalmol. 1997;235:551–552. doi: 10.1007/BF00947082. [DOI] [PubMed] [Google Scholar]
- Pakravan M, Beni AN, Ghahari E, Varshochian R, Yazdani S, Esfandiari H, Ahmadieh H. The Ocular Hypotensive Efficacy of Topical Fasudil, a Rho-Associated Protein Kinase Inhibitor, in Patients With End-Stage Glaucoma. Am J Ther. 2016 doi: 10.1097/MJT.0000000000000362. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Inoue T, Rao PV. Elevated intraocular pressure induces Rho GTPase mediated contractile signaling in the trabecular meshwork. Experimental eye research. 2015a;136:29–33. doi: 10.1016/j.exer.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Maddala R, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J Cell Physiol. 2014;229:927–942. doi: 10.1002/jcp.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–C763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Rinkoski T, Poeschla E, Proia A, Challa P, Rao PV. RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork. Am J Pathol. 2015b;185:496–512. doi: 10.1016/j.ajpath.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervan CL, Lautz JD, Blitzer AL, Langert KA, Stubbs EB., Jr Rho GTPase signaling promotes constitutive expression and release of TGF-beta2 by human trabecular meshwork cells. Experimental eye research. 2015;146:95–102. doi: 10.1016/j.exer.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Tian B, Bershadsky AD, Volberg T, Gangnon RE, Spector I, Geiger B, Kaufman PL. Latrunculin-A increases outflow facility in the monkey. Investigative ophthalmology & visual science. 1999;40:931–941. [PubMed] [Google Scholar]
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investigative ophthalmology & visual science. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran C, Patil RV, Combrink K, Sharif NA, Srinivas SP. Rho-Rho kinase pathway in the actomyosin contraction and cell-matrix adhesion in immortalized human trabecular meshwork cells. Molecular vision. 2011a;17:1877–1890. [PMC free article] [PubMed] [Google Scholar]
- Ramachandran C, Patil RV, Sharif NA, Srinivas SP. Effect of elevated intracellular cAMP levels on actomyosin contraction in bovine trabecular meshwork cells. Investigative ophthalmology & visual science. 2011b;52:1474–1485. doi: 10.1167/iovs.10-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Molecular vision. 2005a;11:288–297. [PubMed] [Google Scholar]
- Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Experimental eye research. 2005b;80:197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Investigative ophthalmology & visual science. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Rao PV, Peterson YK, Inoue T, Casey PJ. Effects of pharmacologic inhibition of protein geranylgeranyltransferase type I on aqueous humor outflow through the trabecular meshwork. Investigative ophthalmology & visual science. 2008;49:2464–2471. doi: 10.1167/iovs.07-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007;21:167–177. doi: 10.2165/00063030-200721030-00004. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Sacca SC, Gandolfi S, Bagnis A, Manni G, Damonte G, Traverso CE, Izzotti A. The Outflow Pathway: A Tissue with Morphological and Functional Unity. J Cell Physiol. 2016 doi: 10.1002/jcp.25305. [DOI] [PubMed] [Google Scholar]
- Sagawa H, Terasaki H, Nakamura M, Ichikawa M, Yata T, Tokita Y, Watanabe M. A novel ROCK inhibitor, Y-39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats. Exp Neurol. 2007;205:230–240. doi: 10.1016/j.expneurol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Sawada N, Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid Redox Signal. 2014;20:1251–1267. doi: 10.1089/ars.2013.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach JM, Posielek K, Steuhl KP, Kremmer S. Episcleral venous pressure in untreated primary open-angle and normal-tension glaucoma. Ophthalmologica. 2005;219:357–361. doi: 10.1159/000088378. [DOI] [PubMed] [Google Scholar]
- Shen X, Koga T, Park BC, SundarRaj N, Yue BY. Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells. J Biol Chem. 2008;283:603–612. doi: 10.1074/jbc.M708250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Sunamura S, Satoh K. RhoA/Rho-Kinase in the Cardiovascular System. Circ Res. 2016;118:352–366. doi: 10.1161/CIRCRESAHA.115.306532. [DOI] [PubMed] [Google Scholar]
- Slauson SR, Peters DM, Schwinn MK, Kaufman PL, Gabelt BT, Brandt CR. Viral Vector Effects on Exoenzyme C3 Transferase-Mediated Actin Disruption and on Outflow Facility. Investigative ophthalmology & visual science. 2015;56:2431–2438. doi: 10.1167/iovs.14-15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM. The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J Cardiovasc Transl Res. 2012;5:794–804. doi: 10.1007/s12265-012-9397-0. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Investigative ophthalmology & visual science. 2005;46:2424–2432. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- Springelkamp H, Iglesias AI, Cuellar-Partida G, Amin N, Burdon KP, van Leeuwen EM, Gharahkhani P, Mishra A, van der Lee SJ, Hewitt AW, Rivadeneira F, Viswanathan AC, Wolfs RC, Martin NG, Ramdas WD, van Koolwijk LM, Pennell CE, Vingerling JR, Mountain JE, Uitterlinden AG, Hofman A, Mitchell P, Lemij HG, Wang JJ, Klaver CC, Mackey DA, Craig JE, van Duijn CM, MacGregor S. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Human molecular genetics. 2015;24:2689–2699. doi: 10.1093/hmg/ddv027. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Braakman ST, Zhou EH, Ethier CR, Fredberg JJ, Overby DR, Johnson M. Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction. Prog Retin Eye Res. 2015;44:86–98. doi: 10.1016/j.preteyeres.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Read AT, Sumida GM, Ethier CR. Sphingosine-1-phosphate effects on the inner wall of Schlemm's canal and outflow facility in perfused human eyes. Experimental eye research. 2009;89:980–988. doi: 10.1016/j.exer.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Sturdivant JM, Royalty SM, Lin CW, Moore LA, Yingling JD, Laethem CL, Sherman B, Heintzelman GR, Kopczynski CC, deLong MA. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorganic & medicinal chemistry letters. 2016;26:2475–2480. doi: 10.1016/j.bmcl.2016.03.104. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Shibata M, Kajiura S, Okuno T, Tonari M, Oku H, Ikeda T. Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Investigative ophthalmology & visual science. 2011;52:64–69. doi: 10.1167/iovs.10-5265. [DOI] [PubMed] [Google Scholar]
- Sumi K, Inoue Y, Nishio M, Naito Y, Hosoya T, Suzuki M, Hidaka H. IOP-lowering effect of isoquinoline-5-sulfonamide compounds in ocular normotensive monkeys. Bioorganic & medicinal chemistry letters. 2014;24:831–834. doi: 10.1016/j.bmcl.2013.12.085. [DOI] [PubMed] [Google Scholar]
- Sumida GM, Stamer WD. Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm's canal endothelial cell monolayers. Investigative ophthalmology & visual science. 2010;51:6633–6638. doi: 10.1167/iovs.10-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HB, Zhong YS, Cheng Y, Shen X. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. International journal of ophthalmology. 2011;4:652–657. doi: 10.3980/j.issn.2222-3959.2011.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Suganami H, Araie M Group, K.C.S. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined With Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015;133:755–761. doi: 10.1001/jamaophthalmol.2015.0525. [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Thieme H, Nuskovski M, Nass JU, Pleyer U, Strauss O, Wiederholt M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Investigative ophthalmology & visual science. 2000;41:4240–4246. [PubMed] [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92:303–315. doi: 10.1016/j.ejcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Kaufman PL. Effect of staurosporine on outflow facility in monkeys. Investigative ophthalmology & visual science. 1999;40:1009–1011. [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Investigative ophthalmology & visual science. 2000;41:619–623. [PubMed] [Google Scholar]
- Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Experimental eye research. 2005;80:215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Archives of ophthalmology. 1998;116:633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- Tian K, Shibata-Germanos S, Pahlitzsch M, Cordeiro MF. Current perspective of neuroprotection and glaucoma. Clin Ophthalmol. 2015;9:2109–2118. doi: 10.2147/OPTH.S80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokushige H, Waki M, Takayama Y, Tanihara H. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Current eye research. 2011;36:964–970. doi: 10.3109/02713683.2011.599106. [DOI] [PubMed] [Google Scholar]
- Treisman R, Alberts AS, Sahai E. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb Symp Quant Biol. 1998;63:643–651. doi: 10.1101/sqb.1998.63.643. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Experimental eye research. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Tsou PS, Haak AJ, Khanna D, Neubig RR. Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription. Am J Physiol Cell Physiol. 2014;307:C2–C13. doi: 10.1152/ajpcell.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Van de Velde S, De Groef L, Stalmans I, Moons L, Van Hove I. Towards axonal regeneration and neuroprotection in glaucoma: Rho kinase inhibitors as promising therapeutics. Prog Neurobiol. 2015;131:105–119. doi: 10.1016/j.pneurobio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Van de Velde S, Van Bergen T, Sijnave D, Hollanders K, Castermans K, Defert O, Leysen D, Vandewalle E, Moons L, Stalmans I. AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Investigative ophthalmology & visual science. 2014;55:1006–1016. doi: 10.1167/iovs.13-13157. [DOI] [PubMed] [Google Scholar]
- Varma H, Yamamoto A, Sarantos MR, Hughes RE, Stockwell BR. Mutant huntingtin alters cell fate in response to microtubule depolymerization via the GEF-H1-RhoA-ERK pathway. J Biol Chem. 2010;285:37445–37457. doi: 10.1074/jbc.M110.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittitow JL, Garg R, Rowlette LL, Epstein DL, O'Brien ET, Borras T. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Molecular vision. 2002;8:32–44. [PubMed] [Google Scholar]
- Von Zee CL, Richards MP, Bu P, Perlman JI, Stubbs EB., Jr Increased RhoA and RhoB protein accumulation in cultured human trabecular meshwork cells by lovastatin. Investigative ophthalmology & visual science. 2009;50:2816–2823. doi: 10.1167/iovs.08-2466. [DOI] [PubMed] [Google Scholar]
- Waki M, Yoshida Y, Oka T, Azuma M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Current eye research. 2001;22:470–474. doi: 10.1076/ceyr.22.6.470.5489. [DOI] [PubMed] [Google Scholar]
- Wang RF, Williamson JE, Kopczynski C, Serle JB. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. Journal of glaucoma. 2015;24:51–54. doi: 10.1097/IJG.0b013e3182952213. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Whitlock NA, Harrison B, Mixon T, Yu XQ, Wilson A, Gerhardt B, Eberhart DE, Abuin A, Rice DS. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J Ocul Pharmacol Ther. 2009;25:187–194. doi: 10.1089/jop.2008.0142. [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Xu F, Huang H, Wu Y, Lu L, Jiang L, Chen L, Zeng S, Li L, Li M. Upregulation of Gem relates to retinal ganglion cells apoptosis after optic nerve crush in adult rats. J Mol Histol. 2014;45:565–571. doi: 10.1007/s10735-014-9579-y. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Maruyama K, Himori N, Omodaka K, Yokoyama Y, Shiga Y, Morin R, Nakazawa T. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Investigative ophthalmology & visual science. 2014;55:7126–7136. doi: 10.1167/iovs.13-13842. [DOI] [PubMed] [Google Scholar]
- Yu J, Lan S, Wang R, Maier A, Luan X. Fasudil alleviates traumatic optic neuropathy by inhibiting Rho signaling pathway. Int J Clin Exp Med. 2015;8:13377–13382. [PMC free article] [PubMed] [Google Scholar]
- Yu J, Luan X, Lan S, Yan B, Maier A. Fasudil, a Rho-Associated Protein Kinase Inhibitor, Attenuates Traumatic Retinal Nerve Injury in Rabbits. J Mol Neurosci. 2016;58:74–82. doi: 10.1007/s12031-015-0691-6. [DOI] [PubMed] [Google Scholar]
- Yu M, Sun J, Peng W, Chen Z, Lin X, Liu X, Li M, Wu K. Protein expression in human trabecular meshwork: downregulation of RhoGDI by dexamethasone in vitro. Molecular vision. 2010;16:213–223. [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Call MK, Yuan Y, Zhang Y, Fischesser K, Liu CY, Kao WW. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Investigative ophthalmology & visual science. 2013;54:6502–6509. doi: 10.1167/iovs.13-12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008;295:C1057–C1070. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou EH, Krishnan R, Stamer WD, Perkumas KM, Rajendran K, Nabhan JF, Lu Q, Fredberg JJ, Johnson M. Mechanical responsiveness of the endothelial cell of Schlemm's canal: scope, variability and its potential role in controlling aqueous humour outflow. J R Soc Interface. 2012;9:1144–1155. doi: 10.1098/rsif.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]