Abstract
A major challenge in anticancer treatment is the pre-existence or emergence of resistance to therapy. AXL and MER are two members of the TAM (TYRO3-AXL-MER) family of receptor tyrosine kinases, which, when activated, can regulate tumor cell survival, proliferation, migration and invasion, angiogenesis, and tumor-host interactions. An increasing body of evidence strongly suggests that these receptors play major roles in resistance to targeted therapies and conventional cytotoxic agents. Multiple resistance mechanisms exist, including the direct and indirect crosstalk of AXL and MER with other receptors and the activation of feedback loops regulating AXL and MER expression and activity. These mechanisms may be innate, adaptive, or acquired. A principal role of AXL appears to be in sustaining a mesenchymal phenotype, itself a major mechanism of resistance to diverse anticancer therapies. Both AXL and MER play a role in the repression of the innate immune response which may also limit response to treatment. Small molecule and antibody inhibitors of AXL and MER have recently been described, and some of these have already entered clinical trials. The optimal design of treatment strategies to maximize the clinical benefit of these AXL and MER targeting agents are discussed in relation to the different cancer types and the types of resistance encountered. One of the major challenges to successful development of these therapies will be the application of robust predictive biomarkers for clear-cut patient stratification.
Keywords: AXL, MER, Cancer, Drug resistance, Epithelial-to-mesenchymal transition, Immunomodulation
Introduction
Receptor tyrosine kinases (RTKs) are broadly involved in cellular signaling. Many RTKs are deregulated in cancer, many of them being oncogenic drivers. The TAM family of RTKs comprises three transmembrane receptors: TYRO-3, AXL, and MER. Their extracellular domain resembles to some extent that of cell adhesion molecules and contains two immunoglobulin-like and two fibronectin type III domains, while the intracellular kinase domain mediates activation of signaling pathways. Activation of the receptors is triggered by homodimerization following ligand binding, or ligand-independent mechanisms, such as heterodimerization with other TAM or non-TAM RTKs. Several ligands have been identified, with different affinities towards the three TAM receptors: GAS6, protein S, Tubby, Tubby-like protein 1 (TULP-1), and Galectin-3. More data are available on GAS6 and protein S since they were the first to be identified. GAS6 can bind all three receptors, whereas protein S is specific for MER and TYRO-3. The affinity of GAS6 is, however, 3- to 10-fold higher for AXL compared to MER and TYRO-3.
In normal adult tissues, TAM receptors have widespread expression patterns, being expressed in the brain (hippocampus, cerebellum), heart, and liver as well as in monocytes, platelets, and endothelial cells. Their physiological function resides mostly in the regulation of inflammation and elimination of debris via phagocytosis [1••, 2]. Overall, MER is more specifically expressed by cells from the hematopoietic lineage (monocytes, macrophages, dendritic cells, natural killer cells, platelets) while AXL expression pattern is more constrained to epithelial tissues.
TAM receptors are implicated in the regulation of the innate immune response as well as in several signaling cascades that are essential during cancer progression. Being initially discovered in cancers, their biological functions have mostly been studied in the oncology field [3, 4]. Overexpression of AXL and to a lesser extent of MER has been described in multiple malignancies from epithelial and hematological origins and is often associated with poor prognosis [1••, 5–9]. Several studies highlight the role of AXL activation in tumor progression and metastasis development. In most settings, AXL/MER expression is induced together with the appearance of drug resistance to conventional or targeted therapies (Table 1). Moreover, AXL expression is associated with epithelial to mesenchymal transition (EMT), a frequent feature of metastatic tumors often correlated to drug resistance. Both tumor and stromal cells from the microenvironment can produce GAS6, fostering a crosstalk between the two cell populations. Targeting AXL could thus be a good strategy to overcome drug resistance in multiple cancer types by targeting both tumor cells (mostly via AXL) and their microenvironment (via AXL and MER).
Table 1.
Resistance to conventional and targeted therapies involving AXL and MER
| Resistance setting | Therapy | Cancer type | References |
|---|---|---|---|
| AXL-mediated resistance to targeted therapies | HER2 inhibition | Breast | [35, 58••] |
| RAF inhibition | Melanoma | [84, 121–123, 124] | |
| MEK inhibition | Breast, melanoma | [63•] | |
| EGFR inhibition | NSCLC | [60, 82•, 115, 125] | |
| EGFR inhibition | HNSCC | [28] | |
| Sunitinib | Renal | [117] | |
| Imatinib | CML | [15] | |
| Imatinib | GIST | [39] | |
| ALK inhibition | Neuroblastoma | [126, 127] | |
| VEGFR inhibition | Diverse | [115, 128] | |
| PI3K/AKT inhibition | Diverse | [61••, 129] | |
| FTL-3 inhibition | AML | [130] | |
| AXL-mediated resistance to conventional therapies | AML | [36, 43] | |
| CML | [131] | ||
| Breast | [88, 131] | ||
| Esophageal | [132] | ||
| Lung | [133] | ||
| Ovarian | [134] | ||
| Colon | [135] | ||
| MER-mediated resistance to conventional therapies | B-ALL, T-ALL | [136, 137] | |
| Glioma | [68] | ||
| Lung | [133, 138] | ||
| AXL/MER-mediated resistance to immune checkpoint therapies | Breast | [97•, 98•] | |
| Colon | [99•] | ||
This review will focus on the role of AXL and MER in drug resistance and how TAM inhibitors could be best used to reverse innate or acquired resistance.
Regulation of AXL and MER Expression
Although the mechanisms involved are not fully understood, the roles of AXL and MER in cancer are governed essentially by deregulation of transcription leading, directly or indirectly, to increased levels of the activated proteins [1••, 2, 10]. In the few cases where chromosome amplifications, mutations or gene fusions have been described, functional evidence is lacking [11–14]. Mechanisms involved in AXL and MER transcriptional regulation are described below.
Transcription Factors and Regulators
Five transcription factor complexes have been shown to regulate AXL promoter activity: activator protein 1 (AP1), SP1/SP3, YAP/TAZ/TEAD, hypoxia inducible factor (HIF), and myeloid zinc finger 1 protein (MZF-1). Binding sites for the FOS and JUN components of AP1 are present in the AXL promoter and functional studies have confirmed their involvement in the regulation of AXL expression in chronic myeloid leukemia (CML) and bladder cancer, respectively [15, 16]. The transcription factors SP1 and SP3 bind to GC-rich regions on the AXL promoter to induce its expression. Methylation of CpG sites in these SP binding regions inhibits SP-driven transcription of AXL [17]. The YAP/TAZ/TEAD complex was shown to regulate AXL expression in gallbladder, hepatocellular carcinoma, and lung cancers [18–20]. In particular, one study shows that upregulation of the Hippo/YAP pathway in non-small cell lung cancer (NSCLC) cells induces AXL expression and leads to increased resistance to inhibition of the epidermal growth factor receptor (EGFR) [19]. Interestingly, an association between hypoxia and AXL has been suggested in clear cell renal carcinoma, where hypoxia-responsive elements are present in the proximal region of the AXL promoter and binding of HIF-1 and HIF-2 directly induces AXL expression [21]. Moreover, a correlation between HIF-1 and AXL expression has been shown in metastatic prostate cancer, where hypoxia stabilized the AXL protein [22]. Lastly, one study demonstrates the role of the myeloid zinc finger 1 protein (MZF-1) in colorectal and cervical cancer cell lines [23].
Some studies have highlighted the regulation of AXL transcription in cancer through feedback loops induced by other RTKs. In NSCLC and head and neck squamous cell carcinoma (HNSCC) for example, EGFR signaling and downstream MEK/ERK activation induces expression of AXL mRNA via the JUN transcription factor [24]. Similar findings have been described in bladder cancer where AXL mRNA is induced after MET activation and downstream MEK/ERK signaling [25].
Alternative Transcriptional Control
Two microRNAs (miRNAs) have been described as repressors of AXL expression: miR-34a and miR-199a/b. These miRNAs bind to the 3′-UTR of the AXL gene to negatively regulate its expression in breast, colorectal, head and neck, hepatocellular carcinoma, and lung cancer cell lines [26–31]. Recently, one elegant study showed that the miRNA-processing enzyme Dicer suppresses AXL expression in breast cancer cells by inducing expression of miR-494. As a consequence, cells lose their stem cell-like properties and have increased sensitivity to paclitaxel [32•].
AXL gene expression is also governed by epigenetic changes in histone acetylation and histone/DNA methylation. Histone demethylation by EZH2 increases AXL mRNA expression in glioma [33]. DNA methylation of AXL was detected in NSCLC cell lines and was associated with EMT features and resistance to EGFR inhibition [34]. Promoter hypomethylation is associated with increased expression of AXL in HER2 inhibitor-resistant breast cancers [35], acute myeloid leukemia (AML) [36], and some colorectal models [17]. Histone deacetylase (HDAC) inhibition has been shown to reduce AXL expression in AML, suggesting a link between histone acetylation and AXL expression [37]. One study performed in lung cancer cells suggests that mutant p53 could mediate histone acetylation on the AXL promoter, increasing AXL expression and triggering cell growth and motility [38]. A more detailed epigenetic map across tumor types and characterization of the methylation/acetylation status of the AXL gene is required to confirm these findings.
AXL and MER in Resistance Mediated by Feedback Loops and Receptor Crosstalk
Regulation of AXL and MER Activity
Both paracrine and autocrine loops can activate AXL/MER signaling cascades (Fig. 1). Multiple studies have shown that GAS6 is secreted by diverse cell types, from the tumor and/or stromal cells. To cite a few examples, autocrine activation and production of GAS6 by tumor cells have been described for melanoma, GIST, and breast cancers [39–42]. Secretion of GAS6 from the tumor microenvironment has been shown in colon, breast, and prostate cancers as well as in AML. In glioblastoma, breast cancer, and AML, both autocrine and paracrine secretion of ligands have been detected [6, 43]. The production of GAS6 by stromal cells can create a specific niche in which AXL signaling cascades are activated and favor metastasis development [44••]. Apart from ligand binding, little is known as to the regulation of AXL/MER activation. A soluble form of AXL/MER has been described to negatively regulate AXL/MER signaling by acting as an antagonist to GAS6 [45, 46]. The C1 domain-containing phosphatase and tensin homolog protein (C1-TEN) can dephosphorylate AXL and block downstream AKT activation [47]. AXL protein can be stabilized by binding to heat-shock protein 90 (HSP90) [48] or destabilized by ubiquitination by the casitas B-lineage lymphoma (CBL) E3 ligases [49, 50]. Interestingly, a downregulation of CBL has been described as playing a pivotal role in the resistance of CML to BCR-ABL inhibition [51].
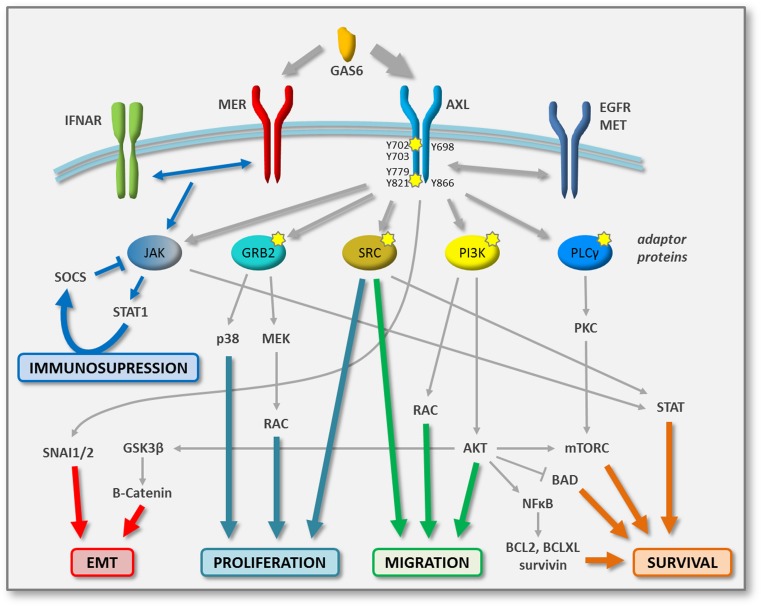
Fig. 1.
AXL and MER signaling networks in tumor cells. Schematic representing the major signaling networks activated upon binding of GAS6 with its TAM receptor in tumor cells. Affinity of GAS6 for AXL is higher than that for MER. Tyrosine docking sites in AXL are represented. Diverse adaptor proteins mediate activation of specific signaling pathways, involved in proliferation, migration, and survival. Potential direct and indirect phosphorylation biomarkers of AXL activity (pharmacodynamic markers) are indicated by yellow stars. Crosstalk between AXL and other RTKs is exemplified by the dimerization with EGFR and MET. Signaling implicated in the regulation of the immune response is depicted by blue arrows
AXL and MER Downstream Signaling Pathways
Like most RTKs, AXL and MER transmit signals into the cell via adaptor proteins. Importantly, the binding of adaptor proteins to phosphorylated AXL/MER appears to be context- and tissue-specific. It is thus important to understand the disease-specific phenotype in order to decipher in which signaling networks AXL and MER play a role. Activation of AXL/MER by ligand binding triggers receptor dimerization and subsequent autophosphorylation of their cytoplasmic domain [1••]. Each phosphorylated tyrosine residue (Tyr) serves as a docking site for specific adaptor proteins. Five phosphorylation sites have been described for MER: Tyr749, Tyr753, Tyr754, Tyr872, and Tyr929. The last two mediate binding to GRB2 and the p85 regulatory subunit of PI3K, which activate MEK/ERK and PI3K/AKT signaling pathways, respectively. Six phosphorylation sites have been identified in AXL: Tyr698, Tyr702, Tyr703, Tyr779, Tyr821, and Tyr866. While the three more N-terminal residues are putative autophosphorylation sites reflecting AXL activation, the three most C-terminal tyrosines are docking sites for downstream effector proteins [52]. Phospho-Tyr779 and phospho-Tyr821 bind to p85 and activate PI3K/AKT signaling; binding of GRB2 to phospho-Tyr821 transduces the MEK/ERK cascade; phospho-Tyr821 and phospho-Tyr866 bind to SRC, LYK, and PLCγ which can switch on additional signaling networks such as PKC or STAT [53–56] (Fig. 1).
Direct Roles of AXL and MER Signaling in Resistance
AXL expression can circumvent resistance to targeted agents and specifically to inhibitors of other RTKs by either maintaining activity of the same pathway via alternative effectors or by inducing activation of distinct signaling networks. For instance, in NSCLC and HNSCC models, AXL expression sustains PI3K/AKT and MEK/ERK signaling and thus mediates resistance to the EGFR inhibition [24, 57]. A positive feedback loop further reinforces this bypass mechanism in which the MEK/ERK pathway induces transcription of AXL by JUN [24]. Moreover, AXL can dimerize with non-TAM receptors, such as EGFR, MET, and PDGFR [25, 58••, 59, 60, 61••]. This crosstalk triggers a signaling switch, leading to the bypass of the RTK inhibitor effect. An elegant study in triple-negative breast cancers demonstrated that AXL binds to EGFR, other ErbB receptors, MET, and PDGFR depending on their expression levels and membrane localization [58••]. Similarly, dimerization of AXL and HER3 has been shown to bypass HER2 signaling inhibition by lapatinib in breast cancer cells [35]. In squamous cell carcinoma resistant to PI3K inhibitors, dimerization of AXL with EGFR activates the PLCγ/PKC/mTOR pathway to sustain tumor progression [61••], and in mesenchymal ovarian tumors and cell lines, AXL dimerized with MET, EGFR, and HER2, leading to sustained ERK activation [62•]. Switching between receptors is another escape mechanism that has been described in gastro-intestinal stromal tumors (GIST). Cells resistant to the KIT/PDGFR inhibitor imatinib express lower levels of KIT while upregulating AXL and GAS6 [39]. Finally, a recent study has very elegantly shown that inhibition of the MEK/ERK pathway decreases the proteolytic shedding of AXL from the surface of melanoma and breast cancer cells, thus removing negative feedback on its signaling activity [63•].
Indirect Roles of AXL and MER in Resistance via Tumor Cell Proliferation, Survival, Migration, and Invasion
As described above, activation of AXL or MER initiates signaling cascades that are essential for tumor progression. While regulation of cell proliferation is mostly mediated by activation of the p38- and MEK-driven MAPK signaling cascades, most of the published work suggests a more prominent role of AXL in the PI3K/AKT/mTOR and JAK/STAT pathways in tumor cell survival [1••, 2, 10, 64] (Fig. 1). In particular, activation of AKT leads to the nuclear translocation of NF-kB which induces expression of anti-apoptotic proteins, such as survivin, BCL2, BCL-XL, and cyclinD1, as well as phosphorylation and inhibition of the pro-apoptotic protein BAD [10, 65–67]. As a consequence, inhibition of AXL/MER can induce apoptosis in tumor cells via blocking both MEK/ERK and PI3K/AKT pathways [68]. One study in chronic lymphocytic leukemia (CLL) also suggested that AXL inhibition mediates apoptosis by reducing the expression of the anti-apoptotic protein MCL1 [54]. Induction of the PLCγ-PKC signaling cascade by AXL leads to mTORC1 activation, which also promotes cell survival [61••] (Fig. 1). In addition, interplay between receptors of the TAM family can modify the signaling outcome induced by GAS6 [69]. Interestingly, one study suggests that the balance between AXL and TYRO-3 expression is important in the dormancy of prostate cancer cells, with high TYRO-3 levels promoting proliferation and high AXL expression leading to a quiescent phenotype [70].
Besides regulating proliferation and survival, AXL promotes cell migration, cell invasion, and metastasis development in several cancer types [7, 8, 71–73]. The induction of such a migratory phenotype is mediated by AKT and SRC pathways as well as RAC-induced cytoskeleton changes [74, 75]. It was also proposed that the kinase domain of AXL can bind to the actin cytoskeleton while the extracellular domain of the receptor modulates cell adhesion by regulating the expression of tight- and adherent-junction proteins [76]. Together, these observations strongly suggest that the increase of AXL/MER activity typically observed following conventional or targeted therapy will lead indirectly to drug resistance via marked promotion of tumor progression and aggressiveness (Table 1).
AXL and MER in Resistance Mediated by Epithelial-to-Mesenchymal Transition
The Importance of EMT in Resistance
Epithelial-to-mesenchymal transition, or EMT, corresponds to the reversible conversion of epithelial cells to mesenchymal cells, and plays an important role during embryonic development and wound healing. EMT involves profound phenotypic changes that include loss of epithelial characteristics with concomitant acquisition of mesenchymal traits, the latter being more appropriate for migration and invasion. In the cells that undergo EMT, typical epithelial markers, such as E-cadherin and cytokeratins, are repressed while mesenchymal markers, such as N-cadherin, vimentin, or fibronectin, are induced. A number of transcription factors are well described as EMT inducers: SNAI1/2, TWIST1/2, and ZEB1/2. EMT is also associated with modifications in matrix composition and matrix adhesion proteins that will contribute to enhance cell migration in the stromal compartment [77]. Due the plasticity of cells required during the invasion cascade, multiple studies have shown an association between EMT and metastasis development [78].
The loss of epithelial features by mesenchymal cells is very often correlated with induction of stem cell-like properties, such as decreased proliferation and as a consequence, increased resistance to anti-proliferative agents [79]. In this way, cells that are intrinsically mesenchymal or that have undergone an EMT show higher degree of resistance to chemotherapeutic agents as well as to targeted therapies [80•]. Interestingly, a recent study in mesenchymal NSCLC, TNBC, and HNSCC cell lines very elegantly demonstrated that the reversal of EMT by AXL inhibition was accompanied by decreased expression of DNA repair genes, diminished efficiency of homologous recombination and sensitivity to poly (ADP-ribose) polymerase (PARP) inhibition, leading to apoptotic cell death [81••].Together, these studies show that EMT can be considered a major, albeit indirect, mechanism of drug resistance [82•].
AXL as a Sustainer and Effector of EMT
The implication of AXL in EMT is supported by a multitude of studies, which frequently highlighted a correlation between AXL expression and features of EMT [77, 80•, 83]. Expression of AXL is upregulated in mesenchymal EMT-like cells, suggesting a role of AXL in this phenotypic transition. In addition, AXL scores as one of the top genes in EMT-specific signatures [44••, 82•, 84•]. AXL expression enhances migratory capabilities of cancer cells and is often associated with increased metastasis development and poor prognosis. However, whether AXL induces EMT or whether EMT induces AXL expression remains an open debate. More mechanistic data on their causal relationship are warranted to address this point.
Inhibition of AXL by small molecule inhibitors or depletion of AXL by siRNA has been shown to reverse resistance of mesenchymal cancer cells, without necessarily switching them back to an epithelial state. These findings support the fact that AXL is required to maintain EMT-driven drug resistance, but is not necessarily the cause of the mesenchymal state itself [10, 44••,85]. Indeed, several reports pinpoint AXL as a downstream effector of EMT. Most available data are focused on breast cancer, where SNAI1/2 transcription factors induce AXL expression together with regulation of the expression of key EMT genes [42, 86]. An EMT gene signature, which includes high levels of AXL, has been described as a predictive biomarker of resistance to EGFR or PI3K inhibitors in several solid tumors. However, in this context, inhibition of AXL by a small molecule inhibitor was sufficient to reverse EMT-associated resistance [82•]. Supporting this finding, a kinome-wide shRNA screen also identified AXL as a key regulator of the mesenchymal state and stem cell properties in glioblastoma [87]. A similar study in breast cancer models demonstrated a role of AXL in the maintenance of stemness and further showed that AXL downregulation could reverse the EMT phenotype of the cancer stem cell population [88]. A number of mechanistic studies also support the hypothesis that AXL is indeed an EMT inducer. AXL was shown to control the expression of the transcription factors SNAI1/2 and TWIST1/2 in pancreatic cancer [89] and, in breast cancer, to activate the AKT/GSK3β/β-Catenin cascade that induces expression of ZEB1 and other EMT-related genes [90]. A similar study performed in head and neck cancer suggests that resistance to the EGFR inhibitor erlotinib is associated with low miR34a and high AXL levels, the latter inducing EMT via the AKT pathway [28].
In conclusion, the causal connection between AXL and EMT is likely to be context- and tissue-specific. Since several reports have clearly highlighted the therapeutic value of inhibiting AXL/MER signaling cascades, AXL/MER receptors are likely not solely biomarkers of the mesenchymal phenotype and drug resistance but rather have a functional role in maintaining this drug tolerant state.
AXL and MER in Resistance Mediated by the Tumor Microenvironment
Role of AXL and MER in the Innate Immune Response
Besides the oncogenic signaling networks described in the previous sections, AXL and MER play important roles in the innate immune system by promoting phagocytosis of apoptotic cells and debris, supporting the maturation of natural killer cells (NK cells) and inhibiting inflammation driven by dendritic cells (DCs) and macrophages [1, 91, 92•]. Here, the functions of AXL and MER as regulators of inflammation are discussed in the context of their role in the immune response to cancer and resistance to anticancer treatment.
In inflammatory conditions, type I interferon (IFN) binds the IFNα receptors (IFNAR) on DCs to amplify the inflammatory response through activation of the JAK/STAT1 pathway, leading to the transcription of pro-inflammatory cytokines. AXL and MER have been shown to bind the IFNAR and redirect downstream signaling via STAT1 to activate transcription of the suppressor of cytokine signaling (SOCS) proteins which inhibit JAK [92•]. Thus, AXL/MER activation mediates a negative feedback loop in order to dampen the inflammation process and avoid tissue damage. Furthermore, activation of the TAM receptors in macrophages leads to a switch from a M1 to a M2 phenotype, the latter being unable to activate CD8 positive T cells [93]. As a consequence, activated TAM receptors in tumors induce immunosuppression which blocks the antitumor activity of cytotoxic T cells. In such an environment, the efficacy of anticancer treatment is decreased and resistance can develop. Supporting this hypothesis, a high level of M2 macrophages in tumors often correlates with poor prognosis. On contrary, inhibition of TAM receptors maintains macrophages in a M1 state, in which they secrete pro-inflammatory cytokines and can activate T cells [1••, 94, 95•].
Taking this role of AXL/MER into account, blocking their activity could improve antitumor response by (i) increasing pro-inflammatory cytokines and antitumor activity of cytotoxic T cells in tumors with an immunosuppressive environment (high M2 macrophages, low levels of activated CD8 T cells), (ii) simultaneously targeting tumor cells and macrophages in resistant AXL/MER-positive tumors, and (iii) combining TAM inhibitors with immunotherapies to improve antitumor T cell activity by blocking inhibitory checkpoints (e.g., anti-PD1 or anti-CTL4A). In support of this approach, a recent study by Hugo et al. in metastatic melanoma demonstrated that innate resistance to anti-PD1 therapies was associated with overexpression of AXL and an increased number of infiltrated macrophages [96••]. Of note, three recent studies in mouse syngenic models of colon and breast cancer showed marked synergy for tumor growth inhibition with dual inhibition of AXL and PD1 or CTLA4 [97•, 98•, 99•]. Incidentally, a combination of AXL/MER inhibition with immunotherapies may be of particular importance in tissues where inflammation could have a tumor-promoting function, as is the case of colon cancer [100].
In conclusion, these findings suggest that AXL/MER inhibitors could have an important tumor immunomodulatory role, causing a switch from an anti-inflammatory and immunosuppressive context (M2 macrophages) to a pro-inflammatory and immuno-active milieu (M1 macrophages, activated T cells). Immunoprofiling of patient tumors pre- and post-treatment and its correlation with TAM expression as well as more detailed preclinical studies in immunocompetent models are required to validate this hypothesis.
Role of AXL and MER in Angiogenesis
Another function of TAM receptors resides in vascular integrity and pro-angiogenic properties. During wound healing or vasculature damage, TAM receptor signaling promotes stabilization of platelet aggregation, survival of endothelial cells, and restitution of the endothelial barrier function [92•, 101–103]. Being expressed by endothelial cells, TAM receptors participate in the formation of new vessels and contribute to their stabilization via signaling in AXL-positive vascular smooth muscle cells. In particular, AXL has been described as a key modulator of endothelial cell functions that are required for angiogenesis and tumor growth [104]. Both autocrine and paracrine loops between AXL and its ligands GAS6 promote motility and proliferation of endothelial cells, modulate integrin function to facilitate migration and survival of endothelial and tumor cells, and facilitate cell motility via regulation of RAC and AKT pathways. In this context, activation of AXL and MER on endothelial cells has been proposed as a means of resistance to antiangiogenic therapies targeting the vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) receptors [102, 103, 105]. A recent phase III clinical study of cabozantinib—which inhibits AXL in addition to MET and VEGFR—in renal cell carcinoma (RCC) patients progressing after VEGFR inhibitor treatment, supports the hypothesis that AXL inhibition could target resistance to VEGFR inhibition [106].
Targeting AXL and MER in the Clinic
Optimal Therapeutic Strategies as a Function of Cancer Type and Resistance Mode
Overall, based on the current evidence, it seems that AXL and MER have a limited role to play in cancer initiation and progression per se. AXL/MER overexpression seems to be largely restricted to cells that are or have become refractory to anticancer treatments, where their roles in cancer cell proliferation, survival, migration, invasion, and EMT provide a strong rationale to block their activation in order to reverse the drug tolerant state and overcome resistance. AXL/MER inhibitors could thus be used in two distinct scenarios, as modulators of either innate or acquired resistance.
Some cancers have intrinsically high AXL or MER expression. Studies suggesting a role of AXL and MER in leukemia cells led to a phase I clinical trial of the specific AXL inhibitor BGB324 as a monotherapy in AML in which signs of clinical benefit were seen [107••]. Studies on treatment-naive colon and breast cancers have described mesenchymal subgroups with high AXL expression, suggesting that targeting of AXL in these specific patient populations could be beneficial, possibly as a monotherapy or, more likely, to reverse innate resistance to conventional and targeted therapies [108•, 109, 110]. However, a wider benefit of AXL/MER inhibitors is likely to be for patients with acquired resistance to conventional or targeted therapies, where AXL/MER inhibitors would be used in combination with the agent to which resistance was developed. Preclinical and clinical studies that form the basis of the different rationales for these combinations are summarized in Table 1; some are described in more detail in the preceding sections. Several clinical trials are currently evaluating the potential of AXL/MER inhibitors in these settings [10, 111•, 112]. Finally, the immunosuppressive and pro-angiogenic functions of TAM receptors place them as attractive targets for the tumor stroma. Indeed, inhibition of TAM could create an immune proficient niche and favor activation of cytotoxic T cells. Blocking AXL signaling could also reduce angiogenesis and tumor growth [105]. Hence, AXL/MER inhibitors could have a dual function by targeting both tumor cells and their stroma. However, careful toxicity evaluation should be done as chronic exposure to TAM inhibitors, particularly MER, may cause autoimmune disorders. There is, nonetheless, a certain degree of redundancy in the roles of the TAM receptors in immune suppression. The development of specific AXL inhibitors with lower affinity for either MER or TYRO-3 could thus be useful to avoid these potential side effects [1••, 92•].
Small Molecule AXL and MER Inhibitors in Preclinical and Clinical Development
Three strategies have been developed to inhibit TAM activity by (i) blocking ligand/receptor binding with antibodies, (ii) inhibiting kinase activity with ATP competitors, and (iii) reducing TAM expression [111•]. Anti-AXL monoclonal antibodies and an aptamer approach have been described but these two strategies have not been well documented so far [105, 113–116]. The second strategy, which is commonly used for RTK inhibition, has led to several ATP-competitive compounds [10, 111•, 112].
Many of these molecules are multi-kinase inhibitors, for which AXL is not the main target and that were not initially developed to block its activity (Table 2). So far, only three molecules are described as specific AXL inhibitors: the first-in-class compound BGB324 which has entered clinical trials and TP-0903 and SLC-0211 still at preclinical stage. The lack of specificity of some of the other molecules may nevertheless be used as an advantage for anticancer treatment as multiple RTKs are involved in tumor progression and disease recurrence. Due to the similarity of the ATP binding site between MET and AXL, many of the compounds target these two receptors. As MET and AXL are involved in resistance mechanisms, especially in NSCLC, inhibiting both simultaneously may target distinct resistant populations within the same tumor, or prevent the emergence of secondary mechanisms of resistance, and thus be highly beneficial for patients. In this context, monotherapy trials of the multi-kinase inhibitors Cabozantinib, Sitravatinib, and Glesatinib are specifically including NSCLC patients with high expression or genetic aberrations of AXL. However, based on the preclinical hypotheses outlined in this review, it may be necessary to combine with the agent to which resistance has developed to observe clinical benefit. Moreover, the fact that these multi-kinase inhibitors also target angiogenesis via inhibition of VEGFR2 will further confound the issue. The interpretation of the results of these trials will thus have to be made with caution in terms of any possible link between antitumor efficacy and high AXL expression. Results of the phase I/II NSCLC trials of the more selective Gilteritinib and BGB324 (no specific patient selection) and S49076 (including patient selection based on high AXL expression) in combination with EGFR inhibition are eagerly awaited. BGB324 is also being investigated in combination with docetaxel in NSCLC.
Table 2.
AXL and MER inhibitors in preclinical and clinical development
| Drug other names; developer |
Targets | Indications | Phase |
|---|---|---|---|
| Cabozantinib XL184, Cometriq®; Exelixis/Ipsen |
VEGFR2, MET, RET, AXL | Thyroid NSCLCa, RCC, other solid tumors |
MA II/III |
| Bosutinib SKI-606; Bosulif®; Pfizer |
BCR-ABL, SRC, AXL | CML breast, GBM, other solid tumors |
MA II |
| Glesatinib MGCD265; Mirati |
MET, VEGFR2, RON, AXL | NSCLCa, bladder, breast | II |
| Merestinib LY2801653; Eli Lilly |
RON, MET, AXL, FTL-3 | NSCLC, biliary tract | II |
| Gilteritinib ASP2215; Astellas Pharma |
AXL, FLT3 | NSCLCa (+EGFRi), AML | II |
| BMS-777607 ASLAN002; BMS/Aslan |
AXL, RON, MET, TYRO3, FLT3 | Solid tumors | I/II |
|
S49076 Servier |
MET, MER, AXL, FGFR1–3 | GBM (+VEGFi) NSCLCa (+EGFRi) |
I/II |
| BGB324 R428; BerGenBio |
AXL | AML, NSCLC (+EGFRi) | I/II |
| Sitravatinib MGCD516, Mirati |
VEGFR2, PDGFRA, AXL, MER, RET, MET, | NSCLCa, other solid tumors | I |
| DDR2, TRKA | |||
| Ningetinib HEC Pharm |
VEGFR2, MET, AXL, MER, FLT3, RON | GBM, other solid tumors | I |
| BPI-9016 M Betta Pharmaceuticals |
MET, AXL | Solid tumors | I |
| TP-0903 Tolero Pharmaceuticals |
AXL | – | Preclinical |
| ONO-9330547 Ono Pharmaceutical |
AXL, MER | – | Preclinical |
| SLC-0211 SignalChem Lifesciences |
AXL | – | Preclinical |
| NPS-1034 NeoPharm |
AXL, FLT3, KIT, MET, ROS1, TIE1 | – | Preclinical |
| LDC1267 Lead Discovery Center |
MER, TYRO3, AXL | – | Preclinical |
| UNC2250 University of N. Carolina |
MER | – | Preclinical |
| UNC2025 University of N. Carolina |
MER, FLT3, AXL, TYRO3 | – | Preclinical |
| RXDX106 Ignyta |
AXL, MER, TYRO3, MET | – | Preclinical |
Sources of information on clinical trials include ClinicalTrials.Gov (https://clinicaltrials.gov/) and TrialTrove (https://citeline.com/). Targets are listed in order of potency (most potently hit first) based on available information
MA market authorisation
aPatients selected based on high expression or genetic aberrations of AXL
Challenges in Identifying Pharmacodynamic and Predictive Biomarkers
The signaling cascades activated downstream of AXL/MER are dependent on the tissue context. These complex signaling networks have been a challenge for the identification of robust biomarkers of (i) AXL/MER activity and (ii) response to AXL/MER inhibition. Moreover, the discrepancy between the possible readouts proposed in different in vitro studies is, in part, due to the lack of robust relevant in vitro models to study AXL/MER cellular functions. Indeed, their activity is tightly regulated by interaction with their ligands, which may require the presence of stromal cells and/or a particular organization of tumor cells. More complex 3D co-culture systems may resolve this issue. So far, molecular data are mostly available on AXL rather than MER as it is the most commonly studied and targeted TAM receptor.
Pharmacodynamic Biomarkers
Levels of expression and activation of AXL in patient tumors are currently assessed by immunohistochemistry analysis of total protein and phosphorylation status of the receptors as well as expression of GAS6. The phosphorylation of the Tyr702 site, for which good antibodies are available, is commonly used as a marker of AXL activation.
The identification of a robust pharmacodynamic (PD) marker for direct target hitting has been challenging, largely due to the fact that there are no good commercially available antibodies to the autophosphorylation sites (Tyr779, Tyr821, and Tyr866). Interestingly, while some of the less selective AXL inhibitors may also indirectly lead to a marked reduction in phosphorylation of the Tyr702 site [117], more selective inhibitors such as BGB324 and S49076 do not [118, 119]. The implications of these different patterns of inhibition of the phosphosites in terms of downstream signaling remain to be determined. Good and specific antibodies for each docking site are also lacking, which precludes a precise molecular analysis of AXL phosphorylation status.
The activation of multiple signaling cascades, together with the fact that many if not all of these pathways are also downstream of other RTKs, excludes the use of phosphorylation of downstream molecules such as AKT. It is possible, however, that further insight into the impact of AXL-specific compounds on signaling via in depth phospho-proteomic studies would provide useful, specific, PD biomarkers.
Predictive Biomarkers of Response
The expression levels of AXL, and to a lesser extent GAS6, are described as broad markers of poor prognosis [6, 120]. In AXL-driven clinical trials, AXL and GAS6 expression levels are used to select AXL-positive populations. What threshold of AXL/GAS6 expression or whether a specific molecular context is associated with a better clinical response is still an open question. The comparison of responders and non-responders from the ongoing BGB324 and S49076 clinical trials will likely be very informative.
Conclusion
Resistance to conventional and targeted therapy is a major cause of failure of anticancer treatment. Further understanding of resistance mechanisms and identification of specific targets driving this resistance will allow development of compounds able to selectively kill the drug tolerant population and avoid disease recurrence. TAM receptors, in particular AXL, have emerged as key mediators of innate and acquired drug resistance in multiple cancer types, from both hematological and epithelial origins. As a consequence, several multi-kinase inhibitors have been repurposed to target AXL in the clinic to reverse resistance. Importantly, the role of AXL in dampening the immune response has led to promising novel therapeutic strategies combining AXL targeting compounds with immune checkpoint inhibitors. In conclusion, AXL has become an attractive target for anticancer treatment, most specifically in combination. More AXL-specific inhibitors are now being developed and will hopefully provide novel strategies to overcome drug resistance using well-tolerated drugs capable of being used in combination. The identification of robust biomarkers of activity and response for these novel molecules will be required for appropriate patient stratification and optimal clinical benefit.
Compliance with Ethical Standards
Conflict of Interest
Marie Schoumacher is an employee of Servier Laboratories. Mike Burbridge is an employee of Servier Laboratories.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Evolving Therapies
References
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
- 1.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14(12):769–785. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 2.Scaltriti M, Elkabets M, Baselga J. Molecular pathways: AXL, a membrane receptor mediator of resistance to therapy. Clin Cancer Res. 2016;22(6):1313–1317. doi: 10.1158/1078-0432.CCR-15-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neubauer A, Fiebeler A, Graham DK, O'Bryan JP, Schmidt CA, Barckow P, et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84(6):1931–1941. [PubMed] [Google Scholar]
- 4.O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11(10):5016–5031. doi: 10.1128/MCB.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rochlitz C, Lohri A, Bacchi M, Schmidt M, Nagel S, Fopp M, et al. Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK) Leukemia. 1999;13(9):1352–1358. doi: 10.1038/sj.leu.2401484. [DOI] [PubMed] [Google Scholar]
- 6.Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(1):130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 7.Song X, Wang H, Logsdon CD, Rashid A, Fleming JB, Abbruzzese JL, et al. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer. 2011;117(4):734–743. doi: 10.1002/cncr.25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vajkoczy P, Knyazev P, Kunkel A, Capelle HH, Behrndt S, von Tengg-Kobligk H, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103(15):5799–5804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10(10):1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 10.Corno C, Gatti L, Lanzi C, Zaffaroni N, Colombo D, Perego P. Role of the receptor tyrosine kinase Axl and its targeting in cancer cells. Curr Med Chem. 2016;23(15):1496–1512. doi: 10.2174/0929867323666160405112954. [DOI] [PubMed] [Google Scholar]
- 11.Seo JS, Ju YS, Lee WC, Shin JY, Lee JK, Bleazard T, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012;22(11):2109–2119. doi: 10.1101/gr.145144.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hucthagowder V, Meyer R, Mullins C, Nagarajan R, DiPersio JF, Vij R, et al. Resequencing analysis of the candidate tyrosine kinase and RAS pathway gene families in multiple myeloma. Cancer Genet. 2012;205(9):474–478. doi: 10.1016/j.cancergen.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaver TM, Lehmann BD, Beeler JS, Li CI, Li Z, Jin H, et al. Diverse, biologically relevant, and targetable gene rearrangements in triple-negative breast cancer and other malignancies. Cancer Res. 2016;76(16):4850–4860. doi: 10.1158/0008-5472.CAN-16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, et al. Mechanisms of AXL overexpression and function in imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2(11):874–885. doi: 10.18632/oncotarget.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayan AE, Stanford R, Vickery R, Grigorenko E, Diesch J, Kulbicki K, et al. Fra-1 controls motility of bladder cancer cells via transcriptional upregulation of the receptor tyrosine kinase AXL. Oncogene. 2012;31(12):1493–1503. doi: 10.1038/onc.2011.336. [DOI] [PubMed] [Google Scholar]
- 17.Mudduluru G, Allgayer H. The human receptor tyrosine kinase Axl gene—promoter characterization and regulation of constitutive expression by Sp1, Sp3 and CpG methylation. Biosci Rep. 2008;28(3):161–176. doi: 10.1042/BSR20080046. [DOI] [PubMed] [Google Scholar]
- 18.Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30(10):1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Wei Y, Wu S, Wang Y, Wang Z, Sun Y, et al. Up-regulation of the Hippo pathway effector TAZ renders lung adenocarcinoma cells harboring EGFR-T790 M mutation resistant to gefitinib. Cell Biosci. 2015;5:7. doi: 10.1186/2045-3701-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Lu J, Zhang F, Li H, Zhang B, Wu X, et al. Yes-associated protein 1 (YAP1) promotes human gallbladder tumor growth via activation of the AXL/MAPK pathway. Cancer Lett. 2014;355(2):201–209. doi: 10.1016/j.canlet.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111(37):13373–13378. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra A, Wang J, Shiozawa Y, McGee S, Kim J, Jung Y, et al. Hypoxia stabilizes GAS6/Axl signaling in metastatic prostate cancer. Mol Cancer Res. 2012;10(6):703–712. doi: 10.1158/1541-7786.MCR-11-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudduluru G, Vajkoczy P, Allgayer H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol Cancer Res. 2010;8(2):159–169. doi: 10.1158/1541-7786.MCR-09-0326. [DOI] [PubMed] [Google Scholar]
- 24.Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, et al. AXL mediates resistance to cetuximab therapy. Cancer Res. 2014;74(18):5152–5164. doi: 10.1158/0008-5472.CAN-14-0294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 25.Yeh CY, Shin SM, Yeh HH, Wu TJ, Shin JW, Chang TY, et al. Transcriptional activation of the Axl and PDGFR-alpha by c-Met through a ras- and Src-independent mechanism in human bladder cancer. BMC Cancer. 2011;11:139. doi: 10.1186/1471-2407-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaller M, Liffers ST, Oeljeklaus S, Kuhlmann K, Roh S, Hoffmann R, et al. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10(8):M111 010462. doi: 10.1074/mcp.M111.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH, Ambs S, Caplen NJ. Identification of the receptor tyrosine kinase AXL in breast cancer as a target for the human miR-34a microRNA. Breast Cancer Res Treat. 2011;130(2):663–679. doi: 10.1007/s10549-011-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giles KM, Kalinowski FC, Candy PA, Epis MR, Zhang PM, Redfern AD, et al. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol Cancer Ther. 2013;12(11):2541–2558. doi: 10.1158/1535-7163.MCT-13-0170. [DOI] [PubMed] [Google Scholar]
- 29.Li XY, Wen JY, Jia CC, Wang TT, Li X, Dong M, et al. MicroRNA-34a-5p enhances sensitivity to chemotherapy by targeting AXL in hepatocellular carcinoma MHCC-97 L cells. Oncol Lett. 2015;10(5):2691–2698. doi: 10.3892/ol.2015.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mudduluru G, Ceppi P, Kumarswamy R, Scagliotti GV, Papotti M, Allgayer H. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene. 2011;30(25):2888–2899. doi: 10.1038/onc.2011.13. [DOI] [PubMed] [Google Scholar]
- 31.Cho CY, Huang JS, Shiah SG, Chung SY, Lay JD, Yang YY, et al. Negative feedback regulation of AXL by miR-34a modulates apoptosis in lung cancer cells. RNA. 2016;22(2):303–315. doi: 10.1261/rna.052571.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang TY, Chen HA, Chiu CF, Chang YW, Kuo TC, Tseng PC, et al. Dicer elicits paclitaxel chemosensitization and suppresses cancer stemness in breast cancer by repressing AXL. Cancer Res. 2016;76(13):3916–3928. doi: 10.1158/0008-5472.CAN-15-2555. [DOI] [PubMed] [Google Scholar]
- 33.Ott M, Litzenburger UM, Sahm F, Rauschenbach KJ, Tudoran R, Hartmann C, et al. Promotion of glioblastoma cell motility by enhancer of zeste homolog 2 (EZH2) is mediated by AXL receptor kinase. PLoS One. 2012;7(10):e47663. doi: 10.1371/journal.pone.0047663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin SH, Wang J, Saintigny P, Wu CC, Giri U, Zhang J, et al. Genes suppressed by DNA methylation in non-small cell lung cancer reveal the epigenetics of epithelial-mesenchymal transition. BMC Genomics. 2014;15:1079. doi: 10.1186/1471-2164-15-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69(17):6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 36.Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008;268(2):314–324. doi: 10.1016/j.canlet.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Silva G, Cardoso BA, Belo H, Almeida AM. Vorinostat induces apoptosis and differentiation in myeloid malignancies: genetic and molecular mechanisms. PLoS One. 2013;8(1):e53766. doi: 10.1371/journal.pone.0053766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaughan CA, Singh S, Windle B, Yeudall WA, Frum R, Grossman SR, et al. Gain-of-function activity of mutant p53 in lung cancer through up-regulation of receptor protein tyrosine kinase Axl. Genes Cancer. 2012;3(7–8):491–502. doi: 10.1177/1947601912462719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26(27):3909–3919. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 40.Sensi M, Catani M, Castellano G, Nicolini G, Alciato F, Tragni G, et al. Human cutaneous melanomas lacking MITF and melanocyte differentiation antigens express a functional Axl receptor kinase. J Invest Dermatol. 2011;131(12):2448–2457. doi: 10.1038/jid.2011.218. [DOI] [PubMed] [Google Scholar]
- 41.Schlegel J, Sambade MJ, Sather S, Moschos SJ, Tan AC, Winges A, et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J Clin Invest. 2013;123(5):2257–2267. doi: 10.1172/JCI67816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107(3):1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS, et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013;122(14):2443–2452. doi: 10.1182/blood-2013-03-491431. [DOI] [PubMed] [Google Scholar]
- 44.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, 2nd, Treacy D, Trombetta JJ, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352(6282):189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109(3):1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinger JG, Omari KM, Marsden K, Raine CS, Shafit-Zagardo B. Up-regulation of soluble Axl and Mer receptor tyrosine kinases negatively correlates with Gas6 in established multiple sclerosis lesions. Am J Pathol. 2009;175(1):283–293. doi: 10.2353/ajpath.2009.080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hafizi S, Ibraimi F, Dahlback B. C1-TEN is a negative regulator of the Akt/PKB signal transduction pathway and inhibits cell survival, proliferation, and migration. FASEB J. 2005;19(8):971–973. doi: 10.1096/fj.04-2532fje. [DOI] [PubMed] [Google Scholar]
- 48.Wu Z, Gholami AM, Kuster B. Systematic identification of the HSP90 candidate regulated proteome. Mol Cell Proteomics. 2012;11(6):M111 016675. doi: 10.1074/mcp.M111.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valverde P. Effects of Gas6 and hydrogen peroxide in Axl ubiquitination and downregulation. Biochem Biophys Res Commun. 2005;333(1):180–185. doi: 10.1016/j.bbrc.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 50.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gioia R, Tregoat C, Dumas PY, Lagarde V, Prouzet-Mauleon V, Desplat V, et al. CBL controls a tyrosine kinase network involving AXL, SYK and LYN in nilotinib-resistant chronic myeloid leukaemia. J Pathol. 2015;237(1):14–24. doi: 10.1002/path.4561. [DOI] [PubMed] [Google Scholar]
- 52.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinger JG, Gohari P, Yan Y, Backer JM, Varnum B, Shafit-Zagardo B. In brain, Axl recruits Grb2 and the p85 regulatory subunit of PI3 kinase; in vitro mutagenesis defines the requisite binding sites for downstream Akt activation. J Neurochem. 2008;106(1):134–146. doi: 10.1111/j.1471-4159.2008.05343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, et al. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood. 2011;117(6):1928–1937. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, et al. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14(22):2619–2631. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 56.Brown M, Black JR, Sharma R, Stebbing J, Pinato DJ. Gene of the month: Axl. J Clin Pathol. 2016;69(5):391–397. doi: 10.1136/jclinpath-2016-203629. [DOI] [PubMed] [Google Scholar]
- 57.Tian Y, Zhang Z, Miao L, Yang Z, Yang J, Wang Y, et al. Anexelekto (AXL) increases resistance to EGFR-TKI and activation of AKT and ERK1/2 in non-small cell lung cancer cells. Oncol Res. 2016;24(5):295–303. doi: 10.3727/096504016X14648701447814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal. 2013;6(287):ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gujral TS, Karp RL, Finski A, Chan M, Schwartz PE, MacBeath G, et al. Profiling phospho-signaling networks in breast cancer using reverse-phase protein arrays. Oncogene. 2013;32(29):3470–3476. doi: 10.1038/onc.2012.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gusenbauer S, Vlaicu P, Ullrich A. HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors. Oncogene. 2013;32(33):3846–3856. doi: 10.1038/onc.2012.396. [DOI] [PubMed] [Google Scholar]
- 61.Elkabets M, Pazarentzos E, Juric D, Sheng Q, Pelossof RA, Brook S, et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27(4):533–546. doi: 10.1016/j.ccell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antony J, Tan TZ, Kelly Z, Low J, Choolani M, Recchi C, et al. The GAS6-AXL signaling network is a mesenchymal (Mes) molecular subtype-specific therapeutic target for ovarian cancer. Sci Signal. 2016;9(448):ra97. doi: 10.1126/scisignal.aaf8175. [DOI] [PubMed] [Google Scholar]
- 63.Miller MA, Oudin MJ, Sullivan RJ, Wang SJ, Meyer AS, Im H, et al. Reduced proteolytic shedding of receptor tyrosine kinases is a post-translational mechanism of kinase inhibitor resistance. Cancer Discov. 2016;6(4):382–399. doi: 10.1158/2159-8290.CD-15-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204(1):36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- 65.Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, et al. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene. 2013;32(6):689–698. doi: 10.1038/onc.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demarchi F, Verardo R, Varnum B, Brancolini C, Schneider C. Gas6 anti-apoptotic signaling requires NF-kappa B activation. J Biol Chem. 2001;276(34):31738–31744. doi: 10.1074/jbc.M104457200. [DOI] [PubMed] [Google Scholar]
- 67.Lee WP, Wen Y, Varnum B, Hung MC. Akt is required for Axl-Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene. 2002;21(3):329–336. doi: 10.1038/sj.onc.1205066. [DOI] [PubMed] [Google Scholar]
- 68.Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–1307. doi: 10.1158/1535-7163.MCT-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown JE, Krodel M, Pazos M, Lai C, Prieto AL. Cross-phosphorylation, signaling and proliferative functions of the Tyro3 and Axl receptors in Rat2 cells. PLoS One. 2012;7(5):e36800. doi: 10.1371/journal.pone.0036800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8(4):e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7(12):1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rettew AN, Young ED, Lev DC, Kleinerman ES, Abdul-Karim FW, Getty PJ, et al. Multiple receptor tyrosine kinases promote the in vitro phenotype of metastatic human osteosarcoma cell lines. Oncogenesis. 2012;1:e34. doi: 10.1038/oncsis.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He L, Zhang J, Jiang L, Jin C, Zhao Y, Yang G, et al. Differential expression of Axl in hepatocellular carcinoma and correlation with tumor lymphatic metastasis. Mol Carcinog. 2010;49(10):882–891. doi: 10.1002/mc.20664. [DOI] [PubMed] [Google Scholar]
- 74.Mahajan NP, Earp HS. An SH2 domain-dependent, phosphotyrosine-independent interaction between Vav1 and the Mer receptor tyrosine kinase: a mechanism for localizing guanine nucleotide-exchange factor action. J Biol Chem. 2003;278(43):42596–42603. doi: 10.1074/jbc.M305817200. [DOI] [PubMed] [Google Scholar]
- 75.Abu-Thuraia A, Gauthier R, Chidiac R, Fukui Y, Screaton RA, Gratton JP, et al. Axl phosphorylates Elmo scaffold proteins to promote Rac activation and cell invasion. Mol Cell Biol. 2015;35(1):76–87. doi: 10.1128/MCB.00764-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cichon MA, Szentpetery Z, Caley MP, Papadakis ES, Mackenzie IC, Brennan CH, et al. The receptor tyrosine kinase Axl regulates cell-cell adhesion and stemness in cutaneous squamous cell carcinoma. Oncogene. 2014;33(32):4185–4192. doi: 10.1038/onc.2013.388. [DOI] [PubMed] [Google Scholar]
- 77.Thomson S, Petti F, Sujka-Kwok I, Mercado P, Bean J, Monaghan M, et al. A systems view of epithelial-mesenchymal transition signaling states. Clin Exp Metastasis. 2011;28(2):137–155. doi: 10.1007/s10585-010-9367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 79.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15(5):311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 81.Balaji K, Vijayaraghavan S, Diao L, Tong P, Fan Y, Carey JP, et al. AXL inhibition suppresses the DNA damage response and sensitizes cells to PARP inhibition in multiple cancers. Mol Cancer Res. 2016 doi: 10.1158/1541-7786.MCR-16-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19(1):279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kohn KW, Zeeberg BR, Reinhold WC, Sunshine M, Luna A, Pommier Y. Gene expression profiles of the NCI-60 human tumor cell lines define molecular interaction networks governing cell migration processes. PLoS One. 2012;7(5):e35716. doi: 10.1371/journal.pone.0035716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller J, Krijgsman O, Tsoi J, Robert L, Hugo W, Song C, et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun. 2014;5:5712. doi: 10.1038/ncomms6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson C, Ye X, Pham T, Lin E, Chan S, McNamara E, et al. AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer Res. 2014;74(20):5878–5890. doi: 10.1158/0008-5472.CAN-14-1009. [DOI] [PubMed] [Google Scholar]
- 86.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30(12):1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 87.Cheng P, Phillips E, Kim SH, Taylor D, Hielscher T, Puccio L, et al. Kinome-wide shRNA screen identifies the receptor tyrosine kinase AXL as a key regulator for mesenchymal glioblastoma stem-like cells. Stem Cell Reports. 2015;4(5):899–913. doi: 10.1016/j.stemcr.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asiedu MK, Beauchamp-Perez FD, Ingle JN, Behrens MD, Radisky DC, Knutson KL. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene. 2014;33(10):1316–1324. doi: 10.1038/onc.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8(7):618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C, Jin H, Wang N, Fan S, Wang Y, Zhang Y, et al. Gas6/Axl axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3beta/beta-catenin signaling. Theranostics. 2016;6(8):1205–1219. doi: 10.7150/thno.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest. 2013;123(8):3231–3242. doi: 10.1172/JCI67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loges S, Schmidt T, Tjwa M, van Geyte K, Lievens D, Lutgens E, et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115(11):2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 95.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gausdal G, Davidsen K, Wnuk-Lipinska K, Wiertel K, Kang J, Engelsen A, et al. Abstract 566: BGB324, a selective small molecule inhibitor of the receptor tyrosine kinase AXL, enhances immune checkpoint inhibitor efficacy. Cancer Res. 2016 [Google Scholar]
- 98.Soh KK, Kim W, Lee YS, Peterson P, Siddiqui-Jain A, Warner SL, et al. Abstract 235: AXL inhibition leads to a reversal of a mesenchymal phenotype sensitizing cancer cells to targeted agents and immuno-oncology therapies. Cancer Res. 2016 [Google Scholar]
- 99.Yoshizawa T, Tanaka K, Yasuhiro T, Fujikawa R, Ri S, Kawabata K. Abstract LB-218: development of Axl/Mer inhibitor, ONO-9330547: preclinical evidence supporting the combination with immunotherapeutics. Cancer Res. 2016 [Google Scholar]
- 100.Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci U S A. 2013;110(32):13091–13096. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cosemans JM, Van Kruchten R, Olieslagers S, Schurgers LJ, Verheyen FK, Munnix IC, et al. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J Thromb Haemost. 2010;8(8):1797–1808. doi: 10.1111/j.1538-7836.2010.03935.x. [DOI] [PubMed] [Google Scholar]
- 102.Ruan GX, Kazlauskas A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012;31(7):1692–1703. doi: 10.1038/emboj.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruan GX, Kazlauskas A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/Akt and promote angiogenesis. J Biol Chem. 2013;288(29):21161–21172. doi: 10.1074/jbc.M113.474619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE, et al. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res. 2005;65(20):9294–9303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28(39):3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 106.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 107.•• Loges S, Gjertsen BT, Heuser M, Ben-Batalla I, Micklem DR, Jorg C et al. A first-in-patient phase I study of BGB324, a selective Axl kinase inhibitor in patients with refractory/relapsed AML and high-risk MDS. J Clin Oncol. 2016;34. First report of a clinical study with a selective AXL inhibitor, and showing good responses in monotherap.
- 108.Martinelli E, Martini G, Cardone C, Troiani T, Liguori G, Vitagliano D, et al. AXL is an oncotarget in human colorectal cancer. Oncotarget. 2015;6(27):23281–23296. doi: 10.18632/oncotarget.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feneyrolles C, Spenlinhauer A, Guiet L, Fauvel B, Dayde-Cazals B, Warnault P, et al. Axl kinase as a key target for oncology: focus on small molecule inhibitors. Mol Cancer Ther. 2014;13(9):2141–2148. doi: 10.1158/1535-7163.MCT-13-1083. [DOI] [PubMed] [Google Scholar]
- 112.Myers SH, Brunton VG, Unciti-Broceta A. AXL inhibitors in cancer: a medicinal chemistry perspective. J Med Chem. 2016;59(8):3593–3608. doi: 10.1021/acs.jmedchem.5b01273. [DOI] [PubMed] [Google Scholar]
- 113.Liu R, Gong M, Li X, Zhou Y, Gao W, Tulpule A, et al. Induction, regulation, and biologic function of Axl receptor tyrosine kinase in Kaposi sarcoma. Blood. 2010;116(2):297–305. doi: 10.1182/blood-2009-12-257154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leconet W, Larbouret C, Chardes T, Thomas G, Neiveyans M, Busson M, et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene. 2014;33(47):5405–5414. doi: 10.1038/onc.2013.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29(38):5254–5264. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 116.Cerchia L, Esposito CL, Camorani S, Rienzo A, Stasio L, Insabato L, et al. Targeting Axl with an high-affinity inhibitory aptamer. Mol Ther. 2012;20(12):2291–2303. doi: 10.1038/mt.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70(4):1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 119.Burbridge MF, Bossard CJ, Saunier C, Fejes I, Bruno A, Leonce S, et al. S49076 is a novel kinase inhibitor of MET, AXL, and FGFR with strong preclinical activity alone and in association with bevacizumab. Mol Cancer Ther. 2013;12(9):1749–1762. doi: 10.1158/1535-7163.MCT-13-0075. [DOI] [PubMed] [Google Scholar]
- 120.Ishikawa M, Sonobe M, Nakayama E, Kobayashi M, Kikuchi R, Kitamura J, et al. Higher expression of receptor tyrosine kinase Axl, and differential expression of its ligand, Gas6, predict poor survival in lung adenocarcinoma patients. Ann Surg Oncol. 2013;20(Suppl 3):S467–S476. doi: 10.1245/s10434-012-2795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tworkoski K, Singhal G, Szpakowski S, Zito CI, Bacchiocchi A, Muthusamy V, et al. Phosphoproteomic screen identifies potential therapeutic targets in melanoma. Mol Cancer Res. 2011;9(6):801–812. doi: 10.1158/1541-7786.MCR-10-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014;4(7):816–827. doi: 10.1158/2159-8290.CD-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dugo M, Nicolini G, Tragni G, Bersani I, Tomassetti A, Colonna V, et al. A melanoma subtype with intrinsic resistance to BRAF inhibition identified by receptor tyrosine kinases gene-driven classification. Oncotarget. 2015;6(7):5118–5133. doi: 10.18632/oncotarget.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu F, Li H, Sun Y. Inhibition of Axl improves the targeted therapy against ALK-mutated neuroblastoma. Biochem Biophys Res Commun. 2014;454(4):566–571. doi: 10.1016/j.bbrc.2014.10.126. [DOI] [PubMed] [Google Scholar]
- 127.Debruyne DN, Bhatnagar N, Sharma B, Luther W, Moore NF, Cheung NK, et al. ALK inhibitor resistance in ALK(F1174 L)-driven neuroblastoma is associated with AXL activation and induction of EMT. Oncogene. 2016;35(28):3681–3691. doi: 10.1038/onc.2015.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pinato DJ, Mauri FA, Lloyd T, Vaira V, Casadio C, Boldorini RL, et al. The expression of Axl receptor tyrosine kinase influences the tumour phenotype and clinical outcome of patients with malignant pleural mesothelioma. Br J Cancer. 2013;108(3):621–628. doi: 10.1038/bjc.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park IK, Mundy-Bosse B, Whitman SP, Zhang X, Warner SL, Bearss DJ, et al. Receptor tyrosine kinase Axl is required for resistance of leukemic cells to FLT3-targeted therapy in acute myeloid leukemia. Leukemia. 2015;29(12):2382–2389. doi: 10.1038/leu.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Y, Sun X, Jiang L, Yang F, Zhang Z, Jia L. Differential expression of Axl and correlation with invasion and multidrug resistance in cancer cells. Cancer Investig. 2012;30(4):287–294. doi: 10.3109/07357907.2012.657816. [DOI] [PubMed] [Google Scholar]
- 132.Hong J, Peng D, Chen Z, Sehdev V, Belkhiri A. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res. 2013;73(1):331–340. doi: 10.1158/0008-5472.CAN-12-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32(29):3420–3431. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Macleod K, Mullen P, Sewell J, Rabiasz G, Lawrie S, Miller E, et al. Altered ErbB receptor signaling and gene expression in cisplatin-resistant ovarian cancer. Cancer Res. 2005;65(15):6789–6800. doi: 10.1158/0008-5472.CAN-04-2684. [DOI] [PubMed] [Google Scholar]
- 135.Dunne PD, McArt DG, Blayney JK, Kalimutho M, Greer S, Wang T, et al. AXL is a key regulator of inherent and chemotherapy-induced invasion and predicts a poor clinical outcome in early-stage colon cancer. Clin Cancer Res. 2014;20(1):164–175. doi: 10.1158/1078-0432.CCR-13-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brandao LN, Winges A, Christoph S, Sather S, Migdall-Wilson J, Schlegel J, et al. Inhibition of MerTK increases chemosensitivity and decreases oncogenic potential in T-cell acute lymphoblastic leukemia. Blood Cancer J. 2013;3:e101. doi: 10.1038/bcj.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Linger RM, Lee-Sherick AB, DeRyckere D, Cohen RA, Jacobsen KM, McGranahan A, et al. Mer receptor tyrosine kinase is a therapeutic target in pre-B-cell acute lymphoblastic leukemia. Blood. 2013;122(9):1599–1609. doi: 10.1182/blood-2013-01-478156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kurokawa M, Ise N, Omi K, Goishi K, Higashiyama S. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci. 2013;104(7):904–911. doi: 10.1111/cas.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]