Abstract
Objective
The aim of this study was to evaluate the effects of increased oxygen availability on gene expression and on collagen deposition/maturation in the periodontium following disease.
Methods
Male Wistar rats had ligatures placed around their molars to induce periodontal disease, and a subset of animals underwent hyperbaric oxygen (HBO) treatment for two hours twice per day. At 15 and 28 days, tissue gene expression of COL1A1, TGF-β1 and ALP was determined; other histological samples were stained with Picrosirius red to evaluate levels of collagen deposition, maturation and thickness.
Results
In animals that underwent HBO treatment, type I collagen expression was higher and collagen deposition, maturation and thickness were more robust. Reduced mRNA levels of TGF-β1 and ALP in HBO-treated rats on day 28 suggested that a quicker resolution in both soft tissue and bone remodeling occurred following oxygen treatment. No differences in inflammation were observed between groups.
Conclusions
In conclusion, the extra-cellular matrix (ECM) regenerated more quickly in the HBO-treated group as evidenced by higher collagen expression, deposition and maturation.
Keywords: hyperbaric oxygen, collagen type I, transforming growth factor beta1, alkaline phosphatase, cytokine, inflammation
Introduction
Oxygen is a key factor during wound healing as it is involved in many critical processes such as energy production, collagen synthesis, angiogenesis, epithelization, bactericidal reactions, and bone repair (1–3). In conditions where oxygen availability is increased there is generally a better outcome of wound healing and reduced rates of infection (4–6); in contrast, the opposite takes place during hypoxia (7,8). Hence, strategies that improve the delivery of oxygen to the injured site, e.g. hyperbaric oxygen (HBO) therapy, have been indicated as adjunct treatment for morbidities that require improved wound recovery such as diabetic foot ulcers, spinal lesions, and hidradenitis suppurativa (9–12).
Injured periodontal tissue is typically hypoxic due to an influx of cells with high oxygen demands and a disruption of blood supply (13–15). Under these conditions, the microbiological profile shifts from mainly aerobic bacteria in healthy periodontal tissues to an anaerobic population present deep inside the periodontal pockets (16,17). These environmental conditions during active disease create a hostile milieu in which rates of tissue damage are greatly increased. Several molecular components are present in mucosal and periodontal healing; these are involved in the processes of cell turnover and maturation, acting as markers for tissue destruction or repair. Collagen type 1 alpha 1 (COL1A1) is a gene that encodes the major component of collagen type 1, which is found in most connective tissues, and it is the most abundant type of collagen found overall and in the periodontal ligament (18). Its expression is linked to soft and hard periodontal tissue turnover. TGF-β1 is a multifunctional polypeptide and its expression has been associated with the healing and/or repair of periodontal tissues (19,20). TGF-β1 has been shown to interact with matrix metalloproteinases in the process of gingival tissue degradation (21,22). Thus, TGF-β1 can be regarded as an important regulator of the remodeling phase in periodontium. Alkaline phosphatase (ALP) is a marker for osteoblastic differentiation in bone remodeling and is important in the deposition of acellular cementum (23,24). This enzyme is sensitive to periodontal regeneration when released from periodontal ligament fibroblasts (24). Hence, TGF-β1 and ALP play a role in the remodeling of soft tissues and bone, respectively. In healthy periodontium, the underlying architecture of the extracellular matrix (ECM) follows a balance between degradation and synthesis; however, this balance can quickly shift towards a heavier load of tissue degradation when there is presence of disease (25,26). Collagenases and other proteins such as TGF- β1 have been shown to regulate this degradation (21,22,27,28). A quick regeneration process is important to reduce the destructive effects of periodontal disease.
HBO therapy, one of the vehicles to increase oxygen availability, has shown beneficial effects on periodontal healing (29–31); however, little is known about its effects on the regeneration of the ECM in the periodontal pocket. The aim of this study was to evaluate the effects of increased oxygen availability on gene expression and collagen deposition/maturation in the presence of ligature-induced periodontal disease in male Wistar rats. The hypothesis was that higher oxygen availability would improve collagen expression and deposition.
Materials and methods
Animals
Immunocompetent adult male Wistar rats (Charles River Laboratories Inc, Wilmington, MA), about 18 weeks of age, were housed 2 per cage (n=62) in a temperature and humidity controlled facility, under a 12-hour light-dark cycle (lights on at 06:00 h). They were allowed one week to acclimatize to their new environment. All rats were fed a standardized diet of finely milled chow (Research Diets Inc, New Brunswick, NJ) and water ad libitum. Experiments were carried out in a facility approved by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and were approved by The University of Illinois at Chicago (UIC) Institutional Animal Care and Use Committee.
Placement of ligature
Examination of the dentition and the periodontium was done at baseline under anesthesia by probing sulcus depth with a UNC 15 periodontal probe, to ensure that no rats had periodontitis. Anesthesia was provided by an intraperitoneal injection of Xylazine 0.05ml/100g, and Ketamine 0.15 ml/100g. Then 4-0 silk ligatures were placed subgingivally, bilaterally, around the maxillary second molars of all animals, using high magnification loupes to visualize the gingival sulcus. The experimental periodontitis model followed has been described previously (32). Once a week, the rats were anesthetized and sutures were checked to ensure they remained in place.
Hyperbaric Oxygen Therapy
A subset of animals (n=17) was subjected to 100% oxygen, at 2.5 ATM pressure, for a 2-hour period twice a day starting on the day of ligature placement and continuing throughout the experiment (2 weeks or 4 weeks) (33). A specialized HBO chamber designed for rodents was used for the study. Food and water were supplied in the chamber. Slow compression and decompression cycles ensured that the animals were not subjected to sudden changes in pressure or temperature during HBO treatments. Hot water bottles below the chamber prevented temperature changes during the oxygenation process. The control group (n=45) were left in their home cages at this time.
Real-Time PCR
At days 15 and 28, different subsets of rats were sacrificed. For analyses of gene expression, periodontal tissues were harvested and flash-frozen in 1 ml of TRIzol (Life Technologies, Rockville, MA) and stored at −80°C. mRNA levels were determined for COL1A1, TGF-β1, ALP, IL-1α, IL-1β, IL-6, and IL-10. Frozen tissue was homogenized using a tissue-tearor, and total RNA was extracted following the manufacturer’s protocol(Life Technologies, Rockville, MA). From the total RNA, poly (A) tailed RNA were selected using magnetic oligo (dT) beads and a magnetic particle concentrator (Dynal A.S., Oslo, Norway) in accordance to the manufacturer’s protocol. The mRNA was processed to obtain cDNA and stored at −80°C until analysis. Primers and probes for real-time PCR were designed using Primer Express software (Applied Biosystems, Foster City, CA). Real-time (RT-PCR) was performed using PE Biosystems ABI PRISM 7000 sequence detection system. All gene expression was standardized to G3PDH (Rodent GAPDH Control Reagents, Applied Biosystems, Foster City, CA) gene expression in the respective samples.
Histological analyses
On days 15 and 28, a maxillary molar segment of each of the rats was harvested and placed in 10% buffered formalin. The harvested tissue sections were stained with Picrosirius red for analysis of collagen deposition and maturation. Sections of 5μm were then deparaffinized in xylene for 10 min and progressively rehydrated with ethanol/double-distilled water. Rehydrated sections were treated with 0.2% aqueous phosphomolybdic acid, rinsed and stained with 0.1% Sirius red in saturated picric acid (Electron Microscopy Sciences, Hatfield, PA) for 90 minutes. After staining, slides were washed in hydrochloric acid, washed in 70% ethanol, dehydrated, cleared and cover slips were mounted in Permount (Fisher, Fairlawn, NJ). Sections were then examined at 200x magnification using a Polarizing microscope (Zeiss AxioVert 200, Oberkochen, Germany).
Statistical analyses
Analyses of variance (ANOVA) were used to determine the effects of time and HBO-treatment on gene expression. Day was treated as a within-subjects variable and HBO-treatment as a between-subjects variable. α=.05 was used to determine significance. Data were analyzed with IBM SPSS 22.0 (Armonk, NY). Error bars represent standard error of the mean (SEM).
Results and discussion
In this study, Type I collagen expression in periodontal tissue was higher in animals that underwent HBO treatment. Histologically, fibrils were more mature and more numerous when compared to controls. Gene expression of TGF-β1 was lower at 28 versus 15 days in the HBO-treated group, indicating that the tissue remodeling process resolved itself more quickly in HBO-treated rats than in controls. Likewise, ALP expression was reduced at 28 versus 15 days in the HBO-treated group, suggesting that the activation, and subsequent de-activation, of the ALP mechanism important for bone repair occurred more quickly in HBO-treated rats. In the controls, ALP and TGF- β1 levels remained similar at both time points. These findings strongly suggest that oxygen availability regulates soft tissue and bone remodeling in periodontal disease.
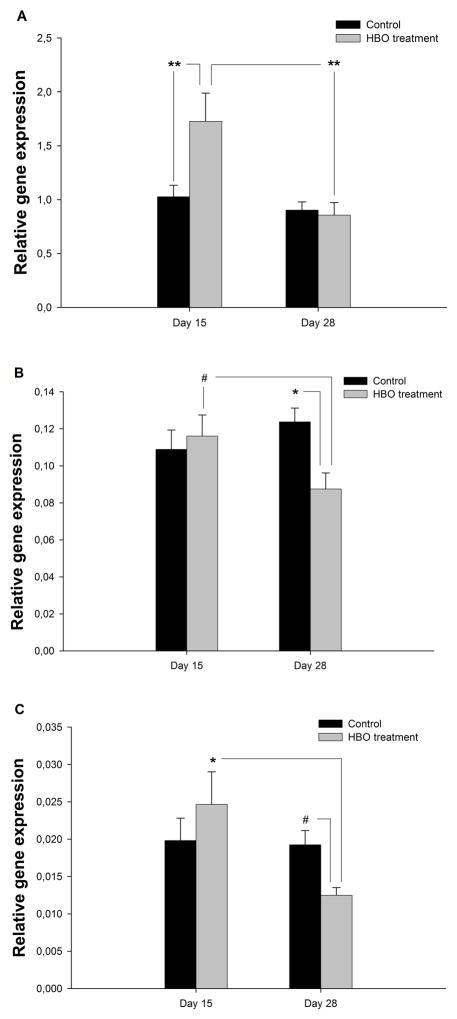
We observed an increase in collagen-related gene expression in periodontal tissues as a result of increased oxygen availability (Fig. 1A). In addition, for COL1A1, there was an interaction of Day x Treatment (F (1,57) =7.58, p=.008). In the HBO-group, COL1A1 expression (Fig. 1A) was higher on day 15 compared to controls (p=.007), and by day 28 had returned to that of controls (p=.009 vs. HBO day 15). Collagen deposition and polymerization requires molecular oxygen to be present, which will, in turn, be important for new capillary development during inflammation (7). In conditions of hypoxia, collagen is deposited poorly and angiogenesis is negatively affected, thus increasing risk for impaired healing and infection (2,7,34). In past studies, hyperbaric oxygen therapy has been shown to increase mRNA levels and collagen synthesis in the lacerated tendons of rats (35,36). In HBO-treated animals, the present result of elevated COL1A1 expression on day 15 suggests an enhanced immune response to periodontal disease. On day 28, the expression was lower and similar to control levels. By this time point, HBO-treated animals showed greater collagen deposition and maturation than non-treated controls. These results confirmed our initial hypothesis that higher oxygen availability would improve collagen expression and deposition.
Fig. 1.
Gene expression in tissue for HBO-treated (n=17) and control groups (n=45). ANOVA was used to determine the statistical significance of the effects of time and HBO-treatment on gene expression. Bars represent the standard error of the mean (SEM). (A) Collagen type 1; (B) TGF-β1; (C) ALP. #p<.10, *p<.05, **p<.01.
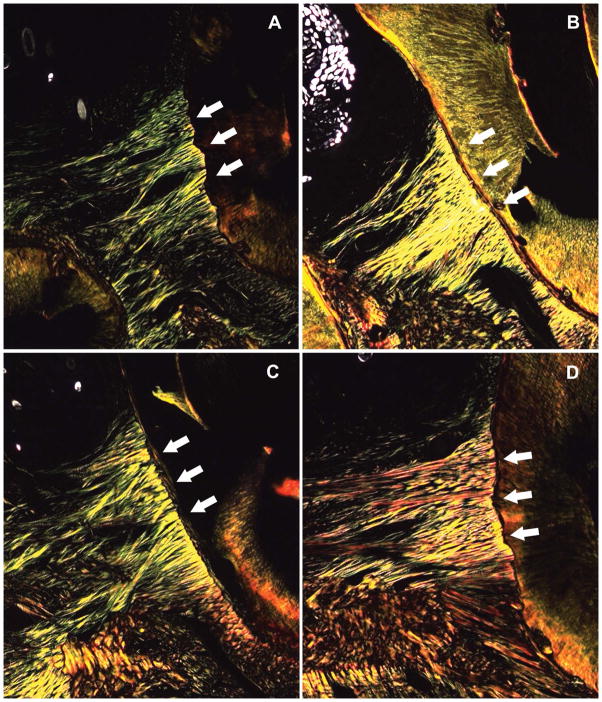
Collagen content and maturation was qualitatively assessed using Picrosirius red staining. Given issues faced in the standardization of the cuts, the quantification of color intensity was not attainable. Higher collagen deposition and improved fibril maturation were observed in the periodontal tissues of the experimental group, as compared to control animals, at both 15 days (denser with more yellow fibrils) and 28 days (denser with more red fibrils) after ligature placement (Fig. 2). Responsiveness to bacterial challenge is an important attribute for periodontal tissues, which are known to be constantly exposed to pathogenic microbiota. The ligature-induced periodontal disease led to tissue remodeling and collagen deposition, both of which appeared strengthened in the HBO-treated group. Greater collagen maturation and thickness in the treatment group indicates that higher oxygen availability early in periodontitis may improve collagen fiber development.
Fig. 2.
Collagen deposition and maturation for different days and treatment groups (Picrosirius red staining, x200 magnification). (A) Control group at 2 weeks; (B) Control group at 4 weeks; (C) HBO treatment group at 2 weeks; (D) HBO treatment group at 4 weeks. Fibril maturation was assessed through changes in the color spectrum. Images are representative of each respective group. Red, orange, yellow, and green coloring represent fibrils in order of decreasing maturation and thickness. White arrows indicate the location of periodontal fibrils.
These results should be considered with some caution, given that periodontal disease was not quantitatively assessed; abundant collagen expression and deposition may not be indicative of improved clinical outcomes. Such collagen deposition and maturation may be delayed in the face of other parameters such as inflammation and infection. While excessive inflammation was not noted in this study based on IL-1 levels, the process of collagen deposition and maturation was enhanced by higher oxygenation. Overall, the described changes have been related to a reduced risk of infection and a quicker healing process (11,37,38). Importantly, both the periodontitis model in Wistar rats (32) and the HBO treatment model in rodents (33) have been used individually in several studies. However, no studies have assessed these two factors in combination.
In HBO-treated rats, TGF-β1 gene expression (Fig. 1B) was lower on day 28 compared to day 15 (p=.066), and also compared to controls on day 28 (p=.021). Similarly, in HBO-treated animals ALP gene expression (Fig. 1C) was lower on day 28 compared to day 15 (p=.018), and also compared to controls on day 28 (p=.078). Importantly, gene expression for TGF-β1 and ALP did not change across time in controls; however, expression levels were reduced in HBO-treated animals on day 28. This reduced gene expression of TGF-β1 and ALP on day 28 suggests that the underlying tissue remodeling which occurs in response to periodontal injury resolved itself more quickly in HBO-treated rats than in controls. Our histological findings support this contention in soft tissue, and the ALP findings suggest that the process of bone remodeling may have also been enhanced following HBO-treatment.
For pro-inflammatory cytokines, a main effect of Day was observed for IL-1α (F (1,58) =12.32, p<.001), IL-1β (F (1,58) =8.16, p=.006) and IL-6 (F (1,58) =9.93, p=.003) gene expression. Higher inflammation was observed in all groups at day 15. HBO treatment did not alter these effects (data not shown). In general, animals in this study showed greater inflammation (via greater expression for IL-1) on day 15 rather than day 28. Previous reports indicate that less oxygen contributes to higher inflammation and impaired healing in periodontal tissues (39,40). Moreover, hyperbaric oxygen therapy has been shown to enhance neutrophil apoptosis and clearance by macrophages, thus, contributing to the resolution of inflammation (41). Our results found no significant differences in inflammation between HBO-treated rats and controls. Of note, bacterial levels and reaction to oxidative stress were not assessed in this study, and inflammation was not monitored until its full resolution. Such factors could play a role in obscuring the relationship between hyper-oxygenation and inflammatory levels.
Using a model of ligature-induced periodontitis, this study found that the extracellular matrix regenerated more quickly in HBO-treated rats compared to non-treated controls. This was primarily evidenced by higher gene expression for type I collagen, greater collagen deposition, and faster collagen maturation in the healing tissue. Gene expression for TGF-β1 and ALP further suggests that the degradation and restructuring of underlying tissues occurred earlier following HBO treatment. Future research is needed to elucidate how higher oxygen availability interplays with other factors such as the microbiota during periodontal disease. Increasing oxygenation in the periodontal tissues may be useful as adjunctive therapy in complex cases of periodontal disease. However, the investigation of alternative methods for local oxygen delivery is warranted due to potential complications related to HBO therapy such as aggravation of congestive heart failure, middle ear barotrauma, and oxygen toxicity to the central nervous system (CNS) and to the lungs (42,43).
Acknowledgments
This study was financially supported by the National Institute of Dental and Craniofacial Research (NIH) grants: NIDCR R03DE018052, 3R03DE018052-02S1.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Yip WL. Influence of oxygen on wound healing. Int Wound J. 2015;12:620–624. doi: 10.1111/iwj.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopf HW, Rollins MD. Wounds: an overview of the role of oxygen. Antioxid Redox Signal. 2007;9:1183–1192. doi: 10.1089/ars.2007.1641. [DOI] [PubMed] [Google Scholar]
- 3.Sammarco MC, Simkin J, Cammack AJ, Fassler D, Gossmann A, Marrero L, Lacey M, Van Meter K, Muneoka K. Hyperbaric Oxygen Promotes Proximal Bone Regeneration and Organized Collagen Composition during Digit Regeneration. PLoS One. 2015;10:e0140156. doi: 10.1371/journal.pone.0140156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao LS, Millas SG, Pedroza C, Tyson JE, Lally KP. Should perioperative supplemental oxygen be routinely recommended for surgery patients? A Bayesian meta-analysis. Ann Surg. 2012;256:894–901. doi: 10.1097/SLA.0b013e31826cc8da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalliainen LK, Gordillo GM, Schlanger R, Sen CK. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology. 2003;9:81–87. doi: 10.1016/s0928-4680(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 6.Chandra PK, Ross CL, Smith LC, et al. Peroxide-based oxygen generating topical wound dressing for enhancing healing of dermal wounds. Wound Repair Regen. 2015;23:830–841. doi: 10.1111/wrr.12324. [DOI] [PubMed] [Google Scholar]
- 7.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17:1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tandara AA, Mustoe TA. Oxygen in wound healing--more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz H, Senol L, Ercan E, Bilgili ME, Karabudak Abuaf O. A prospective randomized controlled trial assessing the efficacy of adjunctive hyperbaric oxygen therapy in the treatment of hidradenitis suppurativa. Int J Dermatol. 2015;55:232–237. doi: 10.1111/ijd.12936. [DOI] [PubMed] [Google Scholar]
- 10.Onen MR, Yuvruk E, Karagoz G, Naderi S. Efficiency of hyperbaric oxygen therapy in iatrogenic spinal infections. Spine (Phila Pa 1976) 2015;40:1743–1748. doi: 10.1097/BRS.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 11.Huang ET, Mansouri J, Murad MH, Joseph WS, Strauss MB, Tettelbach W, Worth ER, Committee UCO. A clinical practice guideline for the use of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers. Undersea Hyperb Med. 2015;42:205–247. [PubMed] [Google Scholar]
- 12.Dauwe PB, Pulikkottil BJ, Lavery L, Stuzin JM, Rohrich RJ. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast Reconstr Surg. 2014;133:208e–215e. doi: 10.1097/01.prs.0000436849.79161.a4. [DOI] [PubMed] [Google Scholar]
- 13.Ng KT, Li JP, Ng KM, Tipoe GL, Leung WK, Fung ML. Expression of hypoxia-inducible factor-1alpha in human periodontal tissue. J Periodontol. 2011;82:136–141. doi: 10.1902/jop.2010.100100. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Xiang X, Xu M, Fan C, Sowa MG, Liu KZ. Assessment of tissue oxygenation of periodontal inflammation in patients with coronary artery diseases using optical spectroscopy. BMC Oral Health. 2014;14:25. doi: 10.1186/1472-6831-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mettraux GR, Gusberti FA, Graf H. Oxygen tension (pO2) in untreated human periodontal pockets. J Periodontol. 1984;55:516–521. doi: 10.1902/jop.1984.55.9.516. [DOI] [PubMed] [Google Scholar]
- 16.Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junemann S, Prior K, Szczepanowski R, Harks I, Ehmke B, Goesmann A, Stoye J, Harmsen D. Bacterial community shift in treated periodontitis patients revealed by ion torrent 16S rRNA gene amplicon sequencing. PLoS One. 2012;7:e41606. doi: 10.1371/journal.pone.0041606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaku M, Yamauchi M. Mechano-regulation of collagen biosynthesis in periodontal ligament. J Prosthodont Res. 2014;58:193–207. doi: 10.1016/j.jpor.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck LS, DeGuzman L, Lee WP, Xu Y, Siegel MW, Amento EP. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993;92:2841–2849. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skaleric U, Kramar B, Petelin M, Pavlica Z, Wahl SM. Changes in TGF-beta 1 levels in gingiva, crevicular fluid and serum associated with periodontal inflammation in humans and dogs. Eur J Oral Sci. 1997;105:136–142. doi: 10.1111/j.1600-0722.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 21.Mercado AM, Padgett DA, Sheridan JF, Marucha PT. Altered kinetics of IL-1 alpha, IL-1 beta, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav Immun. 2002;16:150–162. doi: 10.1006/brbi.2001.0623. [DOI] [PubMed] [Google Scholar]
- 22.Martelli-Junior H, Cotrim P, Graner E, Sauk JJ, Coletta RD. Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. J Periodontol. 2003;74:296–306. doi: 10.1902/jop.2003.74.3.296. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Calle J, Sanudo C, Sanchez-Verde L, Garcia-Renedo RJ, Arozamena J, Riancho JA. Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone. 2011;49:830–838. doi: 10.1016/j.bone.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Groeneveld MC, Van den Bos T, Everts V, Beertsen W. Cell-bound and extracellular matrix-associated alkaline phosphatase activity in rat periodontal ligament. Experimental Oral Biology Group. J Periodontal Res. 1996;31:73–79. doi: 10.1111/j.1600-0765.1996.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 25.Tjaderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–1629. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 26.Bartold PM, Narayanan AS. Molecular and cell biology of healthy and diseased periodontal tissues. Periodontol 2000. 2006;40:29–49. doi: 10.1111/j.1600-0757.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- 27.Ding Y, Uitto VJ, Firth J, Salo T, Haapasalo M, Konttinen YT, Sorsa T. Modulation of host matrix metalloproteinases by bacterial virulence factors relevant in human periodontal diseases. Oral Dis. 1995;1:279–286. doi: 10.1111/j.1601-0825.1995.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 28.Kiili M, Cox SW, Chen HY, Wahlgren J, Maisi P, Eley BM, Salo T, Sorsa T. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J Clin Periodontol. 2002;29:224–232. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 29.Nogueira-Filho GR, Rosa BT, David-Neto JR. Effects of hyperbaric oxygen therapy on the treatment of severe cases of periodontitis. Undersea Hyperb Med. 2010;37:107–114. [PubMed] [Google Scholar]
- 30.Chen T, Zhou Y, Liu J, Xu B, Wu Z, Li D. Biological effects of hyperbaric oxygen on human severe periodontitis. Undersea Hyperb Med. 2002;29:159–166. [PubMed] [Google Scholar]
- 31.Oliveira PA, Oliveira AM, Pablos AB, Costa FO, Silva GA, Santos JN, Cury PR. Influence of hyperbaric oxygen therapy on peri-implant bone healing in rats with alloxan-induced diabetes. J Clin Periodontol. 2012;39:879–886. doi: 10.1111/j.1600-051X.2012.01922.x. [DOI] [PubMed] [Google Scholar]
- 32.Takada T, Yoshinari N, Sugiishi S, Kawase H, Yamane T, Noguchi T. Effect of restraint stress on the progression of experimental periodontitis in rats. J Periodontol. 2004;75:306–315. doi: 10.1902/jop.2004.75.2.306. [DOI] [PubMed] [Google Scholar]
- 33.Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun. 2005;19:217–222. doi: 10.1016/j.bbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson K, Jensen JA, Goodson WH, 3rd, Scheuenstuhl H, West J, Hopf HW, Hunt TK. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214:605–613. doi: 10.1097/00000658-199111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeyama N, Sakai H, Ohtake H, Mashitori H, Tamai K, Saotome K. Effects of hyperbaric oxygen on gene expressions of procollagen, matrix metalloproteinase and tissue inhibitor of metalloproteinase in injured medial collateral ligament and anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15:443–452. doi: 10.1007/s00167-006-0241-4. [DOI] [PubMed] [Google Scholar]
- 36.Ishii Y, Miyanaga Y, Shimojo H, Ushida T, Tateishi T. Effects of hyperbaric oxygen on procollagen messenger RNA levels and collagen synthesis in the healing of rat tendon laceration. Tissue Eng. 1999;5:279–286. doi: 10.1089/ten.1999.5.279. [DOI] [PubMed] [Google Scholar]
- 37.Widgerow AD. Cellular/extracellular matrix cross-talk in scar evolution and control. Wound Repair Regen. 2011;19:117–133. doi: 10.1111/j.1524-475X.2010.00662.x. [DOI] [PubMed] [Google Scholar]
- 38.Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;6:CD004123. doi: 10.1002/14651858.CD004123.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant MM, Kolamunne RT, Lock FE, Matthews JB, Chapple IL, Griffiths HR. Oxygen tension modulates the cytokine response of oral epithelium to periodontal bacteria. J Clin Periodontol. 2010;37:1039–1048. doi: 10.1111/j.1600-051X.2010.01622.x. [DOI] [PubMed] [Google Scholar]
- 40.Shannon MD, Hallmon WW, Mills MP, Lane JJ, Newell DH. Periodontal wound healing responses to varying oxygen concentrations and atmospheric pressures. J Clin Periodontol. 1988;15:222–226. doi: 10.1111/j.1600-051x.1988.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 41.Almzaiel AJ, Billington R, Smerdon G, Moody AJ. Hyperbaric oxygen enhances neutrophil apoptosis and their clearance by monocyte-derived macrophages. Biochem Cell Biol. 2015;93:405–416. doi: 10.1139/bcb-2014-0157. [DOI] [PubMed] [Google Scholar]
- 42.Heyboer M, 3rd, Wojcik SM, Grant WD, Chambers P, Jennings S, Adcock P. Middle ear barotrauma in hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:393–397. [PubMed] [Google Scholar]
- 43.Foster JH. Hyperbaric oxygen therapy: contraindications and complications. J Oral Maxillofac Surg. 1992;50:1081–1086. doi: 10.1016/0278-2391(92)90495-l. [DOI] [PubMed] [Google Scholar]