Abstract
Introduction
Spinal cord injury (SCI) results in skeletal muscle atrophy, increases in intramuscular fat, and reductions in skeletal muscle oxidative capacity. Endurance training elicited with neuromuscular electrical stimulation (NMES) may reverse these changes and lead to improvement in muscle metabolic health.
Methods
Fourteen participants with complete SCI performed 16 weeks of home-based endurance NMES training of knee extensors muscles. Skeletal muscle oxidative capacity, muscle composition, and blood metabolic and lipid profiles were assessed pre- and post-training.
Results
There was an increase in number of contractions performed throughout the duration of training. The average improvement in skeletal muscle oxidative capacity was 119%, ranging from −14% to 387% (P = 0.019). There were no changes in muscle composition or blood metabolic and lipid profiles.
Discussion
Endurance training improved skeletal muscle oxidative capacity, however endurance NMES of knee extensor muscles did not change blood metabolic and lipid profiles.
Keywords: spinal cord injury, neuromuscular electrical stimulation, near infrared spectroscopy, skeletal muscle oxidative capacity, mitochondrial capacity, endurance training
Introduction
Spinal cord injury (SCI) can result in long-term impairment or complete loss of function of skeletal muscles in affected areas. Improved treatments have extended life expectancy after SCI 1 to the extent that people with SCI may be more susceptible to chronic health problems compared to able-bodied individuals. Cardiovascular disease is more prevalent in people with SCI 2 and is also a leading cause of death in people with paraplegia 2–4. Similarly, dyslipidemia, metabolic syndrome, and Type II diabetes mellitus are also more prevalent in the SCI population when compared to the able-bodied population 5.
Following paralysis, profound changes in skeletal muscle size and strength occur rapidly 6,7. Decreases in muscle size occur concomitantly with increases in intramuscular fat (IMF) 8,9. Previous studies have suggested that decreases in muscle size and increases in IMF are related to a number of chronic health conditions including glucose intolerance 9,10, diabetes mellitus/metabolic syndrome 11, dyslipidemia 10, cardiovascular disease 12, and osteoporosis 13. Paralysis is also associated with increased muscle fatigue in response to electrical stimulation 14–16. Skeletal muscle oxidative capacity is a term that reflects oxygen uptake and utilization of skeletal muscle tissue, and several studies have used different measurement approaches to show that muscles in people with SCI have reduced skeletal muscle oxidative capacity 17–19. Reduced skeletal muscle oxidative capacity may be associated with development of chronic diseases.
Exercise is a potent stimulus that beneficially affects numerous tissue/organ systems to improve health and reduce the risk of chronic diseases. The paralysis that ensues following SCI provides a significant hurdle for patients to perform whole-body exercise training. Neuromuscular electrical stimulation (NMES) has been used extensively in rehabilitation settings, and numerous studies have demonstrated its efficacy in promoting adaptive responses in SCI patients. For example, NMES resistance exercise training (RET) induces skeletal muscle hypertrophy and may reduce IMF 20–23. A previous study reported that resistance NMES training also resulted in significant improvements in in vivo skeletal muscle oxidative capacity 24. These improvements averaged 25%, and while significant, were not enough to bring skeletal muscle oxidative capacity to near able-bodied levels. Twitch contractions have been shown to induce metabolic changes in skeletal muscle25. A previous study in people with SCI showed that low frequency stimulation is capable of increasing the expression of PGC-1α and other metabolic transcription factors26. The purpose of this study was to test whether an NMES endurance protocol using twitch contractions could enhance in vivo skeletal muscle oxidative capacity and improve blood metabolic and lipid profiles. This study adds to a previous case report that showed endurance NMES training led to increased skeletal muscle oxidative capacity in an individual with a motor-complete spinal cord injury 24.
Materials and Methods
This study used a pre- vs. post-test study design to evaluate the effects of endurance NMES training. Participants classified as AIS (ASIA Impairment Scale) level A or B (motor-complete or sensory-incomplete) were recruited for this study. Outcome variables assessed pre- vs. post-endurance NMES training included skeletal muscle oxidative capacity, muscle composition (muscle area, fat area, and % fat), and also blood metabolic and lipid profiles. All participants provided written informed consent prior to study participation. Institutional Review Board approval for this study was obtained from the Research Review Committee at the Shepherd Center (Atlanta, GA) as the primary review site, and the University of Georgia as the secondary review site.
Endurance Neuromuscular Electrical Stimulation Training
Participants completed 16 weeks of home-based endurance NMES training consisting of twitch contractions (pulse duration/interval = 200/50 μs). Two surface electrodes were placed on the knee extensor muscles of both legs. Participants were instructed carefully to place the distal electrode approximately 1 cm above the knee and the proximal electrode 15–20 degrees laterally, approximately one-half to two-thirds up the femur (Figure 1). This electrode placement targets the entire knee extensor muscle group, including the vastus lateralis muscle. This NMES electrode placement is consistent with our previous studies that utilized a resistance training protocol 20,24,27,28. Parameters of endurance NMES including electrical stimulation frequency, duration of session, and number of days per week progressively increased as follows: 2 Hz to 7 Hz, 10 minutes to 75 minutes, and 3 to 5 days per week. These parameters were increased over the course of 16 weeks based on the onset of muscle fatigue, which was defined as visual observation of reduced muscle performance. The intensity of electrical stimulation varied among participants and was selected based on visual observation of vigorous muscle contraction and remained constant throughout the training.
Figure 1.
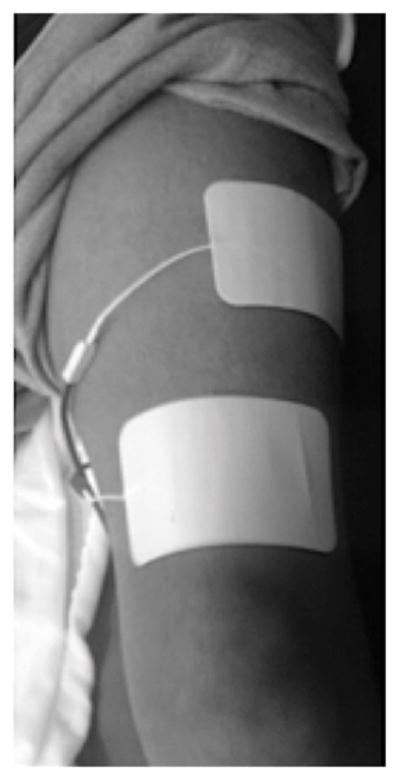
Illustration of surface electrode placement on distal and proximal ends of the vastus lateralis muscle during an endurance NMES training session.
The initial training session was performed in the Exercise Muscle Physiology Laboratory (Athens, GA) under the supervision of trained research personnel. Participants, or a caregiver, were taught proper electrode placement, as well as how to operate the portable electrical stimulation unit (Richmar Theramini 2). Participants completed the remainder of the training sessions at home using the portable electrical stimulation unit. After completion of each training session, participants reported to researchers through phone calls in order to monitor training progression. Electrical stimulation frequency, intensity, and session duration were recorded, and the number of contractions per session was calculated (frequency x session duration in seconds).
All participants initiated training at 2 Hz and trained at this frequency until their muscles were able to sustain strong contractions for 30 minutes. Once this was achieved, the twitch frequency was increased by 1 Hz each week, until 7 Hz was reached. Next, participants increased the training duration by approximately 2–5 minutes each training session, based on muscle performance. Finally, participants incorporated additional days into their training (progressing from 3 to 5 days per week). All participants followed these training guidelines based on feedback from researchers after each training session. Post-testing sessions occurred within 1 week after the last training session.
Skeletal Muscle Oxidative Capacity
Near infrared spectroscopy (NIRS) was used to measure skeletal muscle oxidative capacity of the vastus lateralis muscle pre- vs. post-16 weeks of endurance NMES training in 9 participants using methods described previously 18. For this protocol, participants lay supine on an exam table with an NIRS probe (Oxymon MK III, Artinis medical systems, the Netherlands) secured to the vastus lateralis muscle. Two surface electrodes were placed on the vastus lateralis muscle, adjacent to the NIRS probe. An arterial occlusion cuff was placed upstream of the NIRS probe around the upper thigh. A short bout of electrical stimulation (10 sec, 4 Hz) was applied to the muscle to increase skeletal muscle oxygen consumption (mVO2)18. This was followed by a series of brief (5–10 sec) arterial cuff occlusions that were performed to determine the recovery kinetics of mVO2. The slope of decline of the oxygenated-hemoglobin signal (O2HB) during each arterial occlusion was measured as a representation of the rate of skeletal muscle oxygen consumption. Next, the mVO2 measurements were fitted to a monoexponential curve, and a rate constant (min−1) was calculated. The rate constant is proportional to skeletal muscle oxidative capacity 29. All NIRS data were corrected for blood volume shifts 30. Ultrasound B-mode imaging (LOGIQ e; GE Healthcare, USA) was used to measure adipose tissue thickness on top of the muscle to ensure adequate penetration depth of NIRS signal.
Magnetic Resonance Imaging
T1-weighted magnetic resonance imaging (MRI) was performed on the quadriceps and hamstring muscles pre- vs. post-16 weeks of endurance NMES in 11 participants. Testing occurred in a 1.5-T whole-body magnet (Signa Excited) at the Shepherd Center (Atlanta, Georgia), using methods previously described (parameters: repetition time/echo time = 400/13.2 ms, flip angle = 90, echo-train length = 2, FOV = 40 x 40 cm, slice thickness = 1 cm, acquired matrix = 256 x 256) 31.
MRI image analysis was performed using Image J software, as previously described 31,32. Briefly, the area of the quadriceps muscles was circled manually to obtain a histogram of voxel intensity. Next, reference voxel intensity of each tissue category (muscle, fat, connective tissue) was determined by averaging the intensity of 3 areas per tissue category on each MRI slice. These reference voxel intensity values were used to differentiate each tissue category within the quadriceps area. Percent intramuscular fat was calculated as a weighted percentage of voxel intensity from reference fat values. The hamstring muscle groups were also analyzed in this manner to represent an untrained control muscle.
Metabolic and Lipid Profile
Blood metabolic and lipid profiles were obtained pre- vs. post-endurance NMES training in all participants to evaluate the systemic effects of the NMES training protocol. Briefly, after an overnight fast, participants reported to the Shepherd Center (Atlanta, GA) for a series of venous blood draws taken before and after ingestion of a 75-gram glucose drink. Blood was sampled at 0, 30, 60, 90, and 120 minutes post-glucose ingestion. Metabolic indices were calculated from fasting glucose and insulin values, including HOMA-IR, HOMA-β, %S, and IR, using the downloadable calculator: https://www.dtu.ox.ac.uk/homacalculator/index.php. The lipid panel consisted of total cholesterol, HDL cholesterol, triglycerides, and LDL cholesterol.
Statistical Analysis
Data are presented as means ± SD unless otherwise noted. Student two-tailed, paired t-tests were used to determine changes pre- vs. post-endurance NMES training. Significance was accepted at P < 0.05.
Results
Endurance Neuromuscular Electrical Stimulation Training
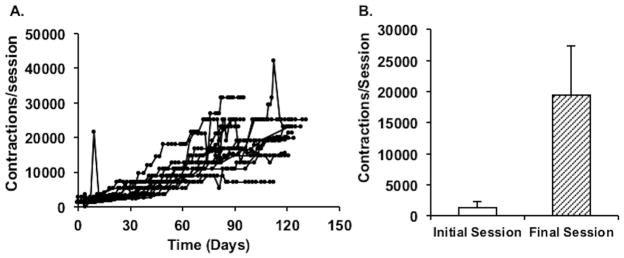
Participant characteristics are listed in Table 1. Fourteen of 15 participants completed the training. One participant was not trainable due to inadequate muscle activation during electrical stimulation, likely due to excessive adipose tissue. All participants who trained completed home-based endurance NMES training without adverse events. One participant suffered a leg injury unrelated to the study after completing 9 weeks of electrical stimulation training, resulting in a 20-day interruption period. Training resumed without incident following this period. All participants who completed the training experienced an increase in training volume, shown in Figure 2. The average electrical stimulation intensity used during training was 112 ± 45 mA (range: 100 – 200 mA).
Table 1.
Participant Characteristics
| Participant | Age years | Gender M/W | Height cm | Weight kg | BMI kg/m2 | Injury Level vertebrae region | Injury Duration years |
|---|---|---|---|---|---|---|---|
| 1 | 44 | M | 190.5 | 88.6 | 24 | C6–C7 | 7.2 |
| 2 | 39 | M | 195 | 108.0 | 28 | C5–C6 | 20 |
| 3 | 41 | M | 185.4 | 113.6 | 33 | C4–C5 | 24.8 |
| 4 | 47 | M | 182.8 | 88.6 | 27 | C7–C8 | 13.5 |
| 5 | 49 | M | 170.2 | 100.5 | 35 | T1–T2 | 15.9 |
| 6 | 63 | M | 167.6 | 78.2 | 28 | T11 | 7.4 |
| 7 | 53 | M | 185.5 | 118.2 | 34 | C5–C6 | 28.1 |
| 8 | 62 | W | 167.6 | 81.8 | 29 | C4 | 15.3 |
| 9 | 36 | M | 175.3 | 87.3 | 28 | T9 | 7.8 |
| 10 | 30 | M | 177.8 | 59.1 | 19 | T5 | 11.0 |
| 11 | 42 | W | 154.9 | 62.7 | 26 | C5–C6 | 8.2 |
| 12 | 43 | M | 180.3 | 95.5 | 29 | C4–C5 | 26 |
| 13 | 33 | W | 177.8 | 98.2 | 31 | C7–C8 | 5.6 |
| 14* | 33 | W | 157.5 | 62.3 | 25 | T4 | 6.6 |
| 15 | 61 | W | 157.5 | 56.8 | 23 | C6 | 3.7 |
| Summary | 45.1 ± 10.7 | 10/5 | 175 ± 12 | 86.6 ± 19.8 | 28 ± 4.3 | C4–T9 | 13.4 ± 8.0 |
Data are listed as Mean ± SD.
Participant was unable to train with NMES due to inadequate muscle activation. Participant 6 was classified as AIS B. All other participants were classified as AIS A.
Figure 2.
Panel A: Individual NMES training progression (AVG: 110 ± 20 days). Each line represents a participant, and each data point represents a training session. Contractions per session are calculated as: frequency of stimulation x session duration in seconds. Panel B: Average number of contractions performed per leg in initial vs. final session of training (initial AVG: 1366 ± 907 vs. final AVG: 19414 ± 8014).
Skeletal Muscle Oxidative Capacity
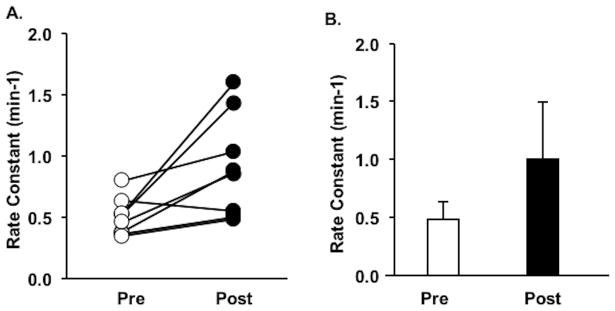
On average, skeletal muscle oxidative capacity significantly increased in response to endurance NMES training (pre: 0.49 ± 0.15 min−1 vs. post: 1.01 ± 0.49 min−1; n = 9, P = 0.019), shown in Figure 3. There was a wide range (−14% to 387%) of responses to endurance NMES training. During NIRS pre- or post-testing, 4 participants experienced muscle spasms that caused motion artifact in NIRS data resulting in unusable data, and 1 participant was not tested due to scheduling difficulties. No relationships were identified between the magnitude of change in skeletal muscle oxidative capacity and duration of injury or level of injury.
Figure 3.
Panel A: Individual rate constants of skeletal muscle oxidative capacity before and after endurance NMES training in 9 participants with SCI. Panel B: Average rate constants of skeletal muscle capacity before and after endurance NMES training (pre AVG: 0.49 ± 0.15 min−1 vs. post AVG: 1.01 ± 0.49 min−1, P = 0.019).
Magnetic Resonance Imaging (MRI)
Overall, muscle composition of the left and right quadriceps muscle did not change in response to NMES training (Table 2). Muscle area (left: P = 0.9; right: P = 0.48), fat area (left: P = 0.63; right: P = 0.73), and intramuscular fat (left: P = 0.62; right: P = 0.81) were not different pre- vs. post-NMES training. No changes were observed in the hamstring muscle group (untrained control). One participant was unable to fit in the MRI bore and could not be tested, and 2 participants were not tested due to scheduling difficulties.
Table 2.
Quadriceps Muscle Composition
| Pre | Post | % Change | P-value | ||
|---|---|---|---|---|---|
| Left Quad (cm2) | Muscle | 28.2 ± 10 | 28.5 ± 8 | 7.3 ± 32 | 0.90 |
| Fat | 8.4 ± 4 | 9.3 ± 5 | 12.1 ± 34 | 0.63 | |
| % Fat | 23.5 ± 4 | 24.2 ± 2 | 4.7 ± 18 | 0.62 | |
|
| |||||
| Right Quad (cm2) | Muscle | 28.2 ± 10 | 29.9 ± 7 | 10.1 ± 31 | 0.48 |
| Fat | 9.0 ± 5 | 9.4 ± 4 | 14.6 ± 35 | 0.73 | |
| % Fat | 23.8 ± 4 | 23.6 ± 2 | 0.9 ± 12 | 0.81 | |
Data are listed as AVG ± SD. Changes in muscle composition after 16 weeks of electrical stimulation training measured as the amount of fat, muscle, and % fat of quadriceps muscle in T1 weighted MRI images (5–7 slices per leg) in 11 participants. % change represents the mean % change of participants.
Metabolic and Lipid Panel
Blood metabolic and lipid profiles are shown in Table 3. A small, but statistically significant reduction in HbA1C was seen after NMES training (3% average reduction; P = 0.03). No changes were observed in other blood or metabolic lipid indices.
Table 3.
Blood Metabolic and Lipid Panel
| Pre | Post | % change | P value | ||
|---|---|---|---|---|---|
| HOMA-IR | 4.3 ± 2.6 | 4.0 ± 2.8 | 8.6 | 0.83 | |
| HOMA-β | 142.4 ± 57.8 | 136.7 ± 48 | 4.7 | 0.63 | |
| %S | 72.5 ± 68.1 | 66 ± 44.7 | 6.3 | 0.47 | |
| IR | 2.3 ± 1.2 | 2.4 ± 1.7 | 17.4 | 0.64 | |
| HbA1C | 5.6 ± 0.4 | 5.4 ± 0.5 | −2.9 | 0.03* | |
| Fasting Glucose | 95.6 ± 10.1 | 95.3 ± 10.6 | 1.7 | 0.66 | |
| 120 min Glucose | 123.1 ± 36.1 | 123.8 ± 37.2 | 8.2 | 0.94 | |
| 120 min Insulin | 228.1 ± 211.6 | 190.4 ± 273.3 | −0.7 | 0.46 | |
|
| |||||
| Total Cholesterol | 175.3 ± 50 | 167.5 ± 47 | −3.9 | 0.21 | |
| HDL Cholesterol | 39.6 ± 11.1 | 37.9 ± 10.1 | −3.1 | 0.31 | |
| Triglycerides | 139.4 ± 117 | 121.8 ± 88.1 | −4.9 | 0.15 | |
| LDL Cholesterol | 112.5 ± 46.1 | 105.2 ± 42.4 | −4.0 | 0.34 | |
Data are listed as Mean ± SD. Units are glucose/lipids: mg/dL and insulin: IU/L. % change shows changes in metabolic and lipid profile after 16 weeks of endurance NMES training.
P < 0.05 using Student paired t-test.
Discussion
The primary finding of this study is that skeletal muscle oxidative capacity increased after 16 weeks of home-based endurance NMES training. With the exception of the 1 participant who was not trainable due to inadequate muscle activation with electrical stimulation, all participants were able to complete the study, albeit with some unrelated health issues. This was similar to the completion rate for previous resistance NMES training studies 31,33. Previous studies have reported improvements in skeletal muscle oxidative capacity after electrical stimulation. For example, 1 study showed a 49–340% increase in succinate dehydrogenase activity after 6 months of chronic electrical stimulation using repeated tetanic contractions 2–8 hrs/day in the tibialis anterior muscle in people with SCI 34. A second study reported an 102% increase in citrate synthase activity after 3 months of 30 minute sessions of tetanic contractions using FES cycling 35, which is similar in magnitude to our study, where there was a 119% improvement in skeletal muscle oxidative capacity. This improvement in skeletal muscle oxidative capacity induced by endurance training is larger than a previous investigation of resistance training, which found a 25% improvement of 31P recovery rates 31.
In our study, electrical stimulation training varied among participants. The progression of the number of contractions (exercise stimulus) was limited by the observed contraction strength in order to ensure that the contractions were safe. There was a wide range of improvement in skeletal muscle oxidative capacity, from −14% to 387%. This wide range in responses is likely due to differences in exercise stimulus applied to the muscle 36. The 3 greatest improvements in skeletal muscle oxidative capacity were 387%, 202%, and 175%. Interestingly, these 3 participants also performed the greatest number of muscle contractions during the final month of the NMES training program, further supporting the relationship between improvements in skeletal muscle oxidative capacity and number of muscle contractions performed. The average improvement in the remaining participants was 37%; this was not far from the expected improvements for endurance training in able-bodied individuals, which ranges between 50–100% 36–40.
Contrary to our hypothesis, we did not see changes in blood metabolic or lipid profiles. This lack of change may be due to the relatively healthy baseline metabolic and lipid profiles in this cohort. Previous resistance NMES training studies of the quadriceps muscles have demonstrated modest 28,41 or no 31 improvements in whole-body glucose tolerance. FES cycling has been shown to improve glucose tolerance 42–44. FES cycling differs from our endurance training protocol in several ways. FES cycling uses higher frequencies (50 Hz) to elicit tetanic contractions, while the endurance protocol in our study used low frequencies (2 Hz to 7 Hz) to elicit twitch contractions without summation. Also, FES cycling uses a hybrid of active and passive movement. In contrast, our endurance protocol did not result in limb movement. FES cycling also involves contracting muscles against a load (flywheel resistance); thus, FES cycling has endurance and resistance components, possibly contributing to some of the differences between the studies. In addition, the lack of change in glucose tolerance seen in our study may be due to the relatively small amount of muscle mass activated during the endurance NMES protocol. FES cycling activates considerably more muscle mass (gluteal, hamstring, and quadriceps muscles) compared to our endurance training that targeted the quadriceps muscles. Despite the positive health benefits of FES cycling, this exercise modality is limited due to high cost and low accessibility, so additional exercise approaches are desirable. To date, no investigations have examined a hybrid approach of resistance and endurance NMES training without the FES rowing and cycling devices. Another approach may be incorporation of additional muscle groups (i.e. upper body musculature) through voluntary exercise 45,46, which would subsequently increase the caloric expenditure during exercise and possibly lead to whole-body metabolic changes.
Participants showed a robust ability to increase the intensity and duration of NMES training, demonstrated by the increase in electrical stimulation frequency, duration of sessions, and frequency of training sessions. This could be considered a qualitative surrogate for improved muscle endurance. The improvement in muscle endurance is consistent with previous findings that showed reduced muscle fatigability after 18 weeks of NMES resistance training 47, as well as reduced muscle fatigability after 6 weeks of FES cycling 48. Previous resistance NMES training studies demonstrated skeletal muscle hypertrophy after long-term SCI. Similarly, FES cycling has been shown to lead to improvement in muscle cycling performance 35,43. Taken together, the results of these studies show that paralyzed skeletal muscle responds to electrical stimulation even after long-term SCI, and improvements in endurance, hypertrophy, and performance demonstrate remarkable skeletal muscle plasticity.
In this study, muscle size and intramuscular fat composition did not change. This is similar to results of a previous study that also reported lack of hypertrophy in response to chronic electrical stimulation of paralyzed muscle34, which suggests that duration and type of stimulation may determine whether or not hypertrophy occurs. Endurance training is not expected to cause hypertrophy in able-bodied individuals, although expected changes in people with SCI are not well established. The lack of change in IMF was somewhat surprising, as endurance training might be expected to reduce IMF in able-bodied individuals. It is likely that the changes in IMF are linked tightly to the caloric expense of the exercise training. Because our NMES training only involved contraction of the quadriceps muscles, it is unlikely that twitch contractions of only this small muscle mass would significantly impact energy expenditure. In fact, none of the participants in this study experienced a body weight change during the course of training. It is also possible that variability in participant characteristics including gender, injury level, duration of injury, age, and BMI influenced the changes seen in our study.
In conclusion, 16 weeks of endurance NMES training resulted in large improvements in skeletal muscle oxidative capacity, in some cases reaching levels seen in able-bodied controls. The magnitude of improvement may be related to the number of muscle contractions performed during the training program. Despite improvements in skeletal muscle oxidative capacity, metabolic and lipid profiles did not improve. Exercise training of additional muscle groups, whether through voluntary or NMES approaches, may be required to elicit whole-body changes. Endurance NMES training is a feasible approach to exercise training for the spinal cord injury population, as it can be performed easily at home with minimal risk.
Acknowledgments
The authors would like to thank all of the research subjects for their willingness to participate in this study and study personnel who contributed to participant recruitment and data collection. We also thank Rebecca Keipper for her assistance in MRI data analysis.
Funding Support: This research study was supported by NIH/NICHD R01HD039676.
Abbreviations
- AIS
ASIA Impairment Scale
- FES
Functional electrical stimulation
- FOV
Field of view
- HbA1C
Hemoglobin A1C
- HDL
High density lipoprotein
- HOMA-β
Homeostasis model assessment beta cell function
- HOMA-IR
Homeostasis model assessment insulin resistance
- Hz
Hertz
- IMF
Intramuscular fat
- IR
Insulin resistance
- LDL
Low density lipoprotein
- MRI
Magnetic resonance imaging
- NIRS
Near infrared spectroscopy
- NMES
Neuromuscular electrical stimulation
- %S
insulin sensitivity
- SCI
Spinal cord injury
- SD
Standard deviation
Footnotes
Conflict of Interest: The authors have not conflicts of interest to report.
References
- 1.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 2.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–152. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 3.Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord. 2000;38(10):604–610. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- 4.Flank P, Wahman K, Levi R, Fahlstrom M. Prevalence of risk factors for cardiovascular disease stratified by body mass index categories in patients with wheelchair-dependent paraplegia after spinal cord injury. J Rehabil Med. 2012;44(5):440–443. doi: 10.2340/16501977-0964. [DOI] [PubMed] [Google Scholar]
- 5.Lavela SL, Weaver FM, Goldstein B, et al. Diabetes mellitus in individuals with spinal cord injury or disorder. J Spinal Cord Med. 2006;29(4):387–395. doi: 10.1080/10790268.2006.11753887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 7.Gorgey ASDG. Spasticity may defend skeletal muscle mize in composition after incomplete spinal cord injury. Spinal Cord. 2007;46:96–102. doi: 10.1038/sj.sc.3102087. [DOI] [PubMed] [Google Scholar]
- 8.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 9.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42(12):711–716. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 10.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–277. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- 11.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord. 1999;37:765–771. doi: 10.1038/sj.sc.3100893. [DOI] [PubMed] [Google Scholar]
- 12.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466–476. doi: 10.1038/sj.sc.3102161. [DOI] [PubMed] [Google Scholar]
- 13.Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10(3):371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 14.Olive JL, Slade JM, Dudley GA, McCully KK. Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol. 2003;94(2):701–708. doi: 10.1152/japplphysiol.00736.2002. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney E, Puetz TW, Dudley GA, McCully KK. Low-frequency fatigue in individuals with spinal cord injury. J Spinal Cord Med. 2007;30(5):458–466. doi: 10.1080/10790268.2007.11753510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bickel CS, Slade JM, VanHiel LR, Warren GL, Dudley GA. Variable-frequency-train stimulation of skeletal muscle after spinal cord injury. J Rehabil Res Dev. 2004;41(1):33–40. doi: 10.1682/jrrd.2004.01.0033. [DOI] [PubMed] [Google Scholar]
- 17.McCully KK, Mulcahy TK, Ryan TE, Zhao Q. Skeletal muscle metabolism in individuals with spinal cord injury. Journal of Applied Physiology. 2011;111(1):143–148. doi: 10.1152/japplphysiol.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson ML, Ryan TE, Young HJ, McCully KK. Near-infrared assessments of skeletal muscle oxidative capacity in persons with spinal cord injury. Eur J Appl Physiol. 2013;113(9):2275–2283. doi: 10.1007/s00421-013-2657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992;72(4):1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- 20.Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80(4):394–396. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- 21.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol. 2003;94(6):2255–2262. doi: 10.1152/japplphysiol.00014.2003. [DOI] [PubMed] [Google Scholar]
- 22.Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med. 2010;33(1):90–95. doi: 10.1080/10790268.2010.11689681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bickel CSY-FC, Mahoney ET, McCully KK. Neuromuscular Electrical Stimulation-Induced Resistance Training After SCI: A Review of the Dudly Protocol. Top Spinal Cord Inj Rehabil. 2015;21(4):294–302. doi: 10.1310/sci2104-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan TE, Erickson ML, Young HJ, McCully KK. Case report: endurance electrical stimulation training improves skeletal muscle oxidative capacity in chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94(12):2559–2561. doi: 10.1016/j.apmr.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Pette D, Tyler KR. Response of succinate dehydrogenase activity in fibres of rabbit tibialis anterior muscle to chronic nerve stimulation. J Physiol. 1983;338:1–9. doi: 10.1113/jphysiol.1983.sp014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie MA, Suneja M, Faidley E, Shields RK. A minimal dose of electrically induced muscle activity regulates distinct gene signaling pathways in humans with spinal cord injury. PLoS One. 2014;9(12):e115791. doi: 10.1371/journal.pone.0115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal Cord. 2007;45(1):49–56. doi: 10.1038/sj.sc.3101940. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney ET, Bickel CS, Elder C, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil. 2005;86(7):1502–1504. doi: 10.1016/j.apmr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. Journal of applied physiology (Bethesda, Md : 1985) 2013;115(12):1757–1766. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: Correcting for blood volume changes. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan TE, Brizendine JT, Backus D, McCully KK. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil. 2013;94(11):2166–2173. doi: 10.1016/j.apmr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Young HJ, Jenkins NT, Zhao Q, McCully KK. Measurement of Intramuscular Fat by Muscle Echo Intensity. Muscle Nerve. 2015 doi: 10.1002/mus.24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorgey AS, Dolbow DR, Cifu DX, Gater DR. Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J Electromyogr Kinesiol. 2013;23(4):977–984. doi: 10.1016/j.jelekin.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. Journal of applied physiology (Bethesda, Md : 1985) 1992;72(4):1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- 35.Mohr T, Andersen JL, Biering-Sorensen F, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(1):1–16. doi: 10.1038/sj.sc.3100343. [DOI] [PubMed] [Google Scholar]
- 36.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. Journal of applied physiology: respiratory, environmental and exercise physiology. 1982;53(4):844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- 37.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–2282. [PubMed] [Google Scholar]
- 38.Gollnick PD, Armstrong RB, Saltin B, Saubert CWt, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 39.Ryan TE, Southern WM, Brizendine JT, McCully KK. Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med Sci Sports Exerc. 2013;45(12):2346–2352. doi: 10.1249/MSS.0b013e31829a726a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Southern WM, Ryan TE, Kepple K, Murrow JR, Nilsson KR, McCully KK. Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiological reports. 2015;3(4) doi: 10.14814/phy2.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–174. doi: 10.1249/MSS.0b013e31822672aa. [DOI] [PubMed] [Google Scholar]
- 42.Griffin L, Decker MJ, Hwang JY, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol. 2009;19(4):614–622. doi: 10.1016/j.jelekin.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Jeon JY, Weiss CB, Steadward RD, et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord. 2002;40(3):110–117. doi: 10.1038/sj.sc.3101260. [DOI] [PubMed] [Google Scholar]
- 44.Jeon JY, Hettinga D, Steadward RD, Wheeler GD, Bell G, Harber V. Reduced plasma glucose and leptin after 12 weeks of functional electrical stimulation-rowing exercise training in spinal cord injury patients. Arch Phys Med Rehabil. 2010;91(12):1957–1959. doi: 10.1016/j.apmr.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Mutton DL, Scremin AM, Barstow TJ, Scott MD, Kunkel CF, Cagle TG. Physiologic responses during functional electrical stimulation leg cycling and hybrid exercise in spinal cord injured subjects. Arch Phys Med Rehabil. 1997;78(7):712–718. doi: 10.1016/s0003-9993(97)90078-2. [DOI] [PubMed] [Google Scholar]
- 46.Raymond J, Davis GM, Climstein M, Sutton JR. Cardiorespiratory responses to arm cranking and electrical stimulation leg cycling in people with paraplegia. Med Sci Sports Exerc. 1999;31(6):822–828. doi: 10.1097/00005768-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Sabatier MJ, Stoner L, Mahoney ET, et al. Electrically stimulated resistance training in SCI individuals increases muscle fatigue resistance but not femoral artery size or blood flow. Spinal Cord. 2006;44(4):227–233. doi: 10.1038/sj.sc.3101834. [DOI] [PubMed] [Google Scholar]
- 48.Gerrits HL, de Haan A, Sargeant AJ, Dallmeijer A, Hopman MT. Altered contractile properties of the quadriceps muscle in people with spinal cord injury following functional electrical stimulated cycle training. Spinal Cord. 2000;38(4):214–223. doi: 10.1038/sj.sc.3100974. [DOI] [PubMed] [Google Scholar]