Abstract
Children with an anxious temperament (AT) are prone to heightened shyness and behavioral inhibition (BI). When chronic and extreme, this anxious, inhibited phenotype is an important early-life risk factor for the development of anxiety disorders, depression, and co-morbid substance abuse. Individuals with extreme AT often show persistent distress in the absence of immediate threat and this contextually inappropriate anxiety predicts future symptom development. Despite its clear clinical relevance, the neural circuitry governing the maladaptive persistence of anxiety remains unknown. Here, we used a well-established nonhuman primate model of childhood temperament and high-resolution 18fluorodeoxyglucose positron emission tomography (FDG-PET) imaging to understand the neural systems governing persistent anxiety and clarify their relevance to early-life phenotypic risk. We focused on BI, a core component of anxious temperament, because it affords the moment-by-moment temporal resolution needed to assess contextually appropriate and inappropriate anxiety. From a pool of 109 peri-adolescent rhesus monkeys, we formed groups characterized by high or low levels of BI, as indexed by freezing in response to an unfamiliar human intruder’s profile. The High-BI group showed consistently elevated signs of anxiety and wariness across more than 2 years of assessments. At the time of brain imaging, 1.5 years after initial phenotyping, the High-BI group showed persistently elevated freezing during a 30-min ‘recovery’ period following an encounter with the intruder — more than an order of magnitude greater than the Low-BI group — and this was associated with increased metabolism in the bed nucleus of the stria terminalis, a key component of the central extended amygdala. These observations provide a neurobiological framework for understanding the early phenotypic risk to develop anxiety-related psychopathology, for accelerating the development of improved interventions, and for understanding the origins of childhood temperament.
Keywords: affective neuroscience, bed nucleus of the stria terminalis (BST), behavioral inhibition (BI), developmental psychopathology, extended amygdala, fear and anxiety, neuroimaging, temperament
When stable and extreme, anxious temperament is a prominent childhood risk factor for the development of anxiety disorders, depression, and co-morbid substance abuse1, 2. These disorders are common, debilitating, and challenging to treat3, highlighting the need to develop a deeper understanding of the neural systems that support elevated levels of dispositional anxiety and confer increased risk for the development of anxiety-related psychopathology.
Children with an anxious temperament often show sustained levels of heightened shyness, neuroendocrine activity, and behavioral inhibition (BI; e.g. freezing) in response to novelty and potential threat4, 5. Anxious temperament is a trait-like phenotype that is determined by a combination of heritable and non-heritable factors, evident early in life, decreased by the administration of anxiolytic agents, and expressed similarly in children and young monkeys4–8.
Persistent distress in the absence of immediate danger is a key feature of temperamental anxiety9, 10. Like adults11–13, children with extreme anxiety show potentiated defensive responses (e.g. startle) and report increased distress during periods of explicit safety before and after the presentation of threat14–18. Contextually inappropriate anxiety in the laboratory prospectively predicts heightened anxiety and exaggerated avoidance in the real world19, 20. Among adults with anxiety and depressive disorders, heightened ‘spillover’ and inertia of negative mood are common and predict the severity of clinical symptoms21, 22, the onset of future episodes of psychopathology23, and treatment response22.
Despite its clear clinical relevance, the neural circuitry governing the maladaptive persistence of anxiety in the minutes or hours following encounters with threat or other stressors remains poorly understood24. Recent work highlights the potential importance of the central extended amygdala, an anatomical concept encompassing the central (Ce) nucleus of the amygdala and the neighboring bed nucleus of the stria terminalis (BST). Mechanistic work in rodents indicates that the extended amygdala coordinates persistent defensive responses elicited by prolonged exposure to uncertain threat cues (e.g. cues paired with temporally unpredictable shock delivery) and diffusely threatening contexts (e.g. elevated-plus maze, brightly lit open field)11, 25–27. Imaging studies in monkeys demonstrate that activity in the extended amygdala predicts individual differences in freezing and other components of anxious temperament28, 29. Imaging studies in humans reveal increased activation in the dorsal amygdala in response to novel and potentially threatening faces among individuals with a childhood history of extreme BI5.
Here, we used a well-established nonhuman primate model of childhood anxiety and high-resolution 18fluorodeoxyglucose (18FDG) positron emission tomography (PET) imaging to establish the contribution of the primate extended amygdala to persistently enhanced, context-inappropriate freezing during the ‘recovery’ period following an encounter with potential threat. We focused on freezing, a core component of the BI phenotype in children4, 30 and the broader anxious temperament phenotype in monkeys5–7, because it affords the moment-by-moment temporal resolution needed to assess contextually appropriate and inappropriate anxiety. Young rhesus monkeys are ideal for understanding the neurobiology of extreme early-life anxiety. Reflecting the two species comparatively recent evolutionary divergence, the brains of rhesus monkeys and human children are genetically, anatomically, and functionally similar31, 32. This is important given known anatomical differences in the extended amygdala between rodents and primates33, 34. Homologous neurobiological substrates endow monkeys and humans with a shared repertoire of complex cognitive and socio-emotional behaviors and a common set of defensive responses to potential danger5, 7.
Adopting the longitudinal, extreme-groups strategy widely used to identify children at greatest risk1, 4, we phenotyped 109 peri-adolescent rhesus monkeys (M (SD)=2.19 years (.50); range=1.45–3.42 years) on two occasions one week apart and formed groups with stable-high (n=11; top quartile) or stable-low levels of BI (n=12; bottom quartile) (Figures 1a, b). Here, the BI phenotype was quantified by measuring freezing in response to an unfamiliar human intruder’s profile (i.e. the ‘No Eye Contact’ condition of the Human Intruder Paradigm35), paralleling methods used in children7. To further assess the long-term stability and generality of the monkey BI phenotype, snake anxiety was assessed approximately 2 years after the initial phenotyping (Figure 1c).
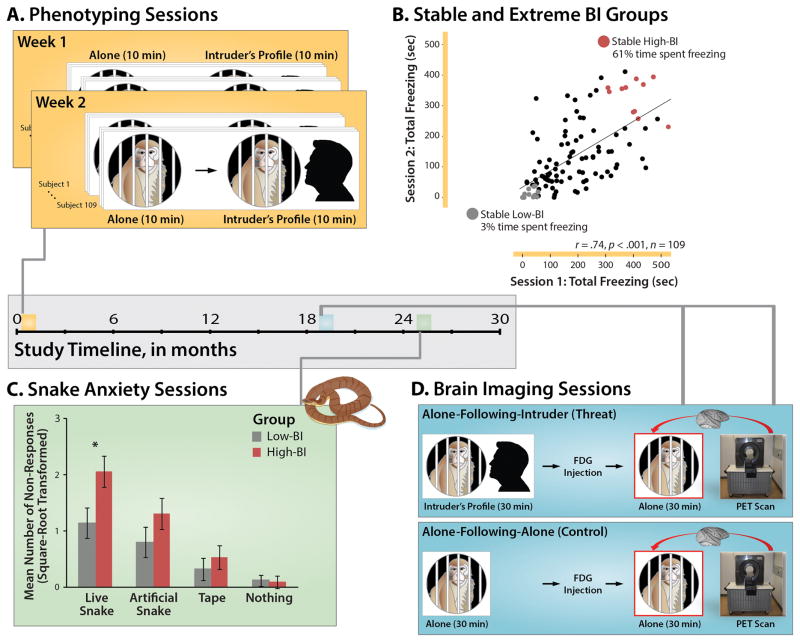
Figure 1. Overview of longitudinal phenotyping and brain imaging procedures.
The horizontal axis at the center of the figure depicts the relative timing of the longitudinal assessments. A. Phenotyping sessions. Individual differences in freezing elicited by diffuse threat (i.e. 10 min exposure to the testing cage) and potential threat (i.e. 10 min exposure to the human intruder’s profile) were assessed in 109 monkeys twice, one week apart using the ‘Alone’ and ‘No Eye Contact’ conditions of the Human Intruder Paradigm7, 35. The Alone condition was always administered first to circumvent carry-over from the higher-intensity intruder challenge. B. Formation of stable and extreme BI groups. Groups were formed from individuals who consistently showed extreme freezing in response to the intruder’s profile across the two phenotyping sessions. Age- and sex-matched High-BI and Low-BI groups are depicted in red and gray, respectively. For illustrative purposes, the x- and y-axes indicate the total number of seconds spent freezing during the 10-min intruder challenges. Inferential statistics employed log10-transformed freezing. C. Snake anxiety sessions. More than 2 years after initial assessment, subjects were trained to retrieve highly-preferred foods in the Wisconsin General Testing Apparatus. During the snake anxiety assessment, foods were placed on top of a clear enclosure containing a live snake, an artificial snake, a roll of tape, or nothing. As shown in the bar plot, the stable High-BI group shows significantly greater passive avoidance in the presence of the live snake (Group × Stimulus, p<.05). Asterisk indicates significant pairwise group difference. Error bars depict SE. D. Brain imaging sessions. Approximately 1.5 years after the initial assessment, subjects were scanned. FDG-PET scanning was conducted in two sessions (order pseudo-randomized). Subjects were acclimated to behavioral testing procedures for 5 days prior to the first scanning session. In the threat condition (upper blue panel), subjects were placed in a testing cage and exposed to the human intruder’s profile for 30-min. To minimize habituation, the intruder presented his profile for 10 min, exited the testing environment, returned after 5 min, presented his profile for 5 min, exited for 5 min, and then presented for a final 5 min. At the end of the 30-min challenge, subjects received an injection of the radiotracer 18FDG and were returned to the testing cage. At the end of this 30-min ‘recovery’ period, subjects were anesthetized and positioned in the high-resolution, small-bore PET scanner. The control condition (lower blue panel) differed only in the absence of threat exposure during the initial 30-min. Comparison of the physically-identical ‘recovery’ periods of the threat and control conditions (red boxes) enabled us to assess group differences in regional brain activity following the intruder encounter. Portions of this figure were adapted with permission from Refs.83, 84.
A key advantage of the nonhuman primate model is that it permits concurrent measures of regional brain metabolism and naturalistic defensive responses. Here, the extreme BI groups were imaged on two occasions, more than 1.5 years after the initial phenotyping (Figure 1d). In the threat condition, subjects were placed in a testing cage and exposed to the human intruder’s profile for 30 min. Next, they received an injection of the radiotracer 18FDG and were returned to the testing cage, where they were allowed to recover from the threat encounter for 30 min. At the end of this ‘recovery’ period, subjects were anesthetized and placed in the PET scanner. The control condition differed only in the absence of threat exposure during the initial 30-min. Comparison of the physically-identical ‘recovery’ phases of the threat and control conditions enabled us to assess persistent group differences in freezing and accompanying brain activity. In contrast to conventional functional MRI techniques, FDG-PET, which provides a measure of regional brain metabolism integrated over the 30-minute ‘recovery’ period, is uniquely well suited for assessing sustained neural responses36. Establishing the neural systems that support persistent, contextually inappropriate anxiety is important for understanding the mechanisms that contribute to the development and maintenance of psychopathology, for guiding the development of improved prevention and treatment strategies, and for understanding the neural bases of childhood temperament.
METHODS AND MATERIALS
Subjects
Peri-adolescent rhesus monkeys (Macaca mulatta; n=109; 63.3% female; M (SD)=2.19 years (.50); range=1.45–3.42 years) were tested as part of a larger program of research to understand the mechanisms underlying early-life anxiety6, 37–39. Data were collected between September 2004 and November 2006. Hypothesis testing focused on data obtained from 23 animals selected on the basis of stable and extreme levels of freezing (High-BI: n=11; Low-BI: n=12; Table 1). We focused on freezing because, in contrast to other components of anxious temperament phenotype (e.g. cortisol), it affords the temporal resolution needed to dissociate recovery from the acute impact of exposure to the intruder’s profile (cf. Figure 1d). The procedures used for forming extreme groups are detailed below. In contrast to post-hoc dichotomization, the use of extreme groups is scientifically and statistically appropriate, given our focus on stable and extreme levels of BI40. Prior work by our group using similar extreme-groups designs indicates adequate power to detect mean differences with 11–12 animals per group (e.g. 92.9% power to detect d=1.5 using two-tailed α=.05)41, 42. All procedures were in accord with guidelines established by the local Institutional Animal Care and Use Committee.
Table 1.
Descriptive statistics for the extreme behavioral inhibition (BI) groups.
| High-BIa | Low-BIb | |||
|---|---|---|---|---|
| Age (Years)c | M | SD | M | SD |
| First screening session | 2.31 | .57 | 2.14 | .53 |
| First FDG-PET session | 3.94 | .60 | 3.76 | .56 |
| Second FDG-PET session | 3.99 | .60 | 3.80 | .57 |
| Snake anxiety assessment | 4.45 | .62 | 4.26 | .56 |
Consisted of 8 females and 3 males.
Consisted of 9 females and 3 males.
The groups did not differ in sex or age, ps>.44.
General Procedures
All techniques have been described in detail in prior publications by our group6, 37–39. Experimental personnel were blind to group status at the time of data collection. For additional details, see the Supplement.
Formation of Groups with Stable and Extreme BI
Research in children indicates that those who consistently express high levels of BI (e.g. more persistent freezing, fewer vocalizations) across repeated assessments are at the greatest risk for the development of psychopathology1, 43. Accordingly, groups with stable, extreme levels of the BI phenotype were formed by organizing animals into quartiles based on log10-transformed freezing during exposure to the human intruder’s profile for the first, second, and mean of the two phenotyping sessions (Figure 1a). Individual differences in intruder-elicited freezing were continuously distributed without obvious gaps or discontinuities (Supplementary Figure S1), consistent with prior work by our group28. Subjects who remained in the same quartile across the three metrics were deemed stable. Age- and sex-matched groups were formed using the most extreme individuals with stable phenotypes. During the initial phenotyping sessions, freezing was also assessed in the absence of potential threat (i.e. the ‘Alone’ condition of the Human Intruder Paradigm7, 35; Figure 1a). The Alone condition was always administered first to circumvent carry-over from the higher-intensity intruder challenge
Snake Anxiety
To clarify the stability and generality of the BI phenotype, snake anxiety was assessed using standard techniques42 (see the Supplement) ~2 years after initial phenotyping (Figure 1c). Subjects were pre-trained to retrieve preferred foods. During the assessment, foods were placed on top of a transparent enclosure containing a live snake, comparison stimuli (i.e. artificial snake, roll of tape), or nothing (6 trials/condition; order counterbalanced). Because many subjects refused to respond in the presence of the live snake (55% and 17% of the High- and Low-BI groups, respectively), anxiety was assessed using the total number of non-responses (square-root transformed), circumventing the need to omit subjects with incomplete response time (RT) data and maximizing statistical power.
FDG-PET and MRI Data Acquisition
Brain FDG metabolism was measured on two occasions (i.e. the threat and control conditions depicted in Figure 1d) ~1.5 years after the phenotyping sessions (M (SD) interval=1.62 (.06) years; Table 1). The order of the two FDG-PET sessions was pseudo-randomized across subjects and balanced across groups. Imaging sessions occurred within 28 days of one another (M=2.20 weeks, SD=1.10). Subjects were acclimated to the imaging procedures prior to the first FDG-PET session (see the Supplement). FDG-PET reflects the amount of regional FDG uptake and metabolism between the injection and PET scan, with majority of uptake occurring in the first 30 min. Anatomical scans were collected using a GE Signa 3T MRI scanner, standard quadrature coil, and a 3D T1-weighted, inversion-recovery, gradient-echo prescription (TI/TR/TE/Flip/NEX/FOV/Matrix/Bandwidth/Slices/Gap:600ms/8.648ms/1.888ms/10°/2/140mm/256×224/61.0547kHz/128/-0.5mm; reconstructed to 0.2734×0.2734×0.5 mm). Brain activity during the first half of the session (Figure 1d), prior to FDG administration, was not measured.
Brain Imaging Data Processing Pipeline
As detailed in the Supplement, T1-weighted anatomical images and FDG-PET data were normalized to a study-specific rhesus template (0.625-mm3) using standard techniques. Anatomical images were segmented using FAST (http://www.fmrib.ox.ac.uk/fsl/fast4). PET and gray matter probability maps were smoothed 4mm.
Hypothesis Testing Strategy
Brain and behavioral data were carefully inspected to ensure that inferential test assumptions were adequately satisfied. As detailed in the Supplement, we used a series of voxelwise general linear models (GLM) to identify regions (i) where the Group (High-BI, Low-BI) × Condition (Alone-Following-Intruder, Alone-Following-Alone) interaction was significant and (ii) where the High-BI group showed significantly more metabolism than the Low-BI group during the critical 30-min ‘recovery’ period following the threat encounter (Alone-Following-Intruder). Given our a priori focus on the contributions of the extended amygdala, each test was thresholded at p<.05, corrected for a region-of-interest (ROI) encompassing the amygdala, BNST, and substantia innominata. To identify regions satisfying both of these key criteria, thresholded maps were combined using a minimum conjunction (logical AND)44, as in prior work by our group28. To provide additional information about specificity, clusters lying outside of the ROI that survived the small-volume threshold are depicted visually and detailed in the supplementary tables (i.e. were not masked). On an exploratory basis, we also assessed whether they survived a whole-brain, cluster-extent threshold. The code and scripts used to conduct these analyses is available upon request.
RESULTS
Identifying Stable and Extreme BI Groups
Across the two phenotyping sessions (Figure 1a), the screening sample of 109 individuals spent more than an order of magnitude more time freezing in response to the human intruder’s profile compared to when they were alone in the testing cage (Intruder: M (SD)=161.6 seconds (112.6); Alone: M (SD)=14.1 seconds (24.5)), F(1,108)=484.09, p<.001). For detailed results, see the Supplement. Individual differences in intruder-elicited freezing were stable over the one week interval, r=.74, p<.001 (Figure 1b). Using these data, we formed sex- and age-matched groups with stable and extreme levels of the BI phenotype (High-BI, Low-BI; see Tables 1 and S1).
When compared to the Low-BI group, the High-BI group showed an 18-fold increase in freezing when the human intruder was present. Additional analyses revealed a 13-fold increase when the High-BI individuals were alone in the testing cage at the beginning of the session ((F(1,21)=8.56, p=.008; see Figure 1a and Table S1). In other words, although BI is more strongly expressed in response to the intruder’s profile (Group × Context interaction: F(1,21)=13.73, p=.001; note: this p-value should be interpreted with caution, as this test is not independent of the group-selection procedures), it also manifests in response to the diffusely threatening testing cage.
The BI Phenotype Shows Continuity Across Time and Contexts
To gauge the long-term stability and generality of the BI phenotype, we performed two additional analyses. First, we tested whether the High-BI group continued to show elevated freezing during the first 30 minutes of the imaging sessions, prior to FDG administration (see Figure 1d). Analyses revealed that subjects froze more in response to the intruder’s profile (F(1,21)=9.41, p=.006) and that the High-BI group froze substantially more than the Low-BI group, (F(1,21)=10.12, p=.004; Table S2). In fact, the High-BI group froze more than 8 times longer than the Low-BI group when the intruder was present (F(1,21)=10.61, p=.004) and nearly 5 times longer when they were alone in the testing cage (F(1,21)=4.99, p=.04), despite extensive acclimation to both the cage and testing procedures (see the Supplement). Across groups, individual differences in the BI phenotype (i.e. intruder-elicited freezing) exhibited substantial test-retest reliability (ICC=.85) over the ~1.5 years separating the phenotyping from the brain imaging sessions (Figure 1).
As a second test of phenotypic continuity, we assessed snake anxiety ~2 years after the initial phenotyping sessions (Figure 1c). Analyses revealed a significant effect of Stimulus on anxiety (F(3,63)=30.11, p<.001). In particular, the frequency of non-responses followed the expected linear pattern: Live Snake > Artificial Snake > Tape > Nothing (F(1,21)=62.56, p<.001) and all of the pairwise differences between stimuli were in the expected direction and significant (ps<.03). Importantly, the Group × Stimulus interaction was also significant (F(1,21)=2.83, p<.05). The High-BI group was twice as likely to refrain from reaching for a highly preferred food reward in the presence of the live snake compared to the Low-BI group (F(1,21)=5.76, p=.03). Other differences were not significant (ps>.20). Analyses of observer ratings of freezing behavior during the snake anxiety assessment revealed a similar pattern (Supplement and Table S2). Across groups, reticence in the presence of the live snake was prospectively predicted by individual differences in freezing to the intruder’s profile during the phenotyping sessions more than 2 years earlier (ρSpearman=.44, p=.04, n=23). Collectively, these results indicate that the monkey BI phenotype represents an enduring predisposition to respond to a range of potentially threatening cues and contexts with heightened anxiety.
The High-BI Group Shows Persistently Elevated Freezing Following the Intruder Encounter
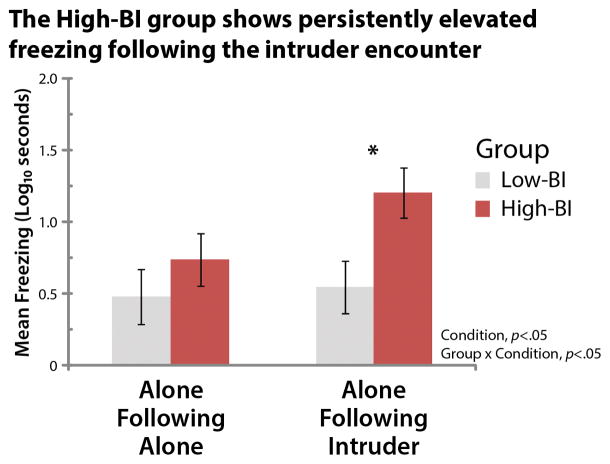
Next, we tested whether the High-BI group exhibits persistently elevated freezing following the encounter with the human intruder. Analyses revealed that, on average, subjects froze longer following the intruder encounter (F(1,21)=8.20, p=.009; Fig. 2b and Table S2). Consistent with expectation, the Group × Condition interaction was significant (F(1,21)=4.62, p=.04). Pairwise contrasts revealed that the High-BI group froze significantly longer during the Alone-Following-Intruder condition compared to the Alone-Following-Alone condition (F(1,21)=12.04, p=.002), whereas the Low-BI group did not show significant differences in freezing (p=.61). This effect was specific to the ‘recovery’ period following the termination of threat: the High-BI group froze an order of magnitude longer than the Low-BI group during the Alone-Following-Intruder condition (F(1,21)=6.61, p=.02), whereas the groups did not significantly differ during the Alone-Following-Alone condition (p=.35). The main effect of Group was not significant (p=.08). These results demonstrate that individuals with stable and extreme BI show sustained levels of heightened anxiety during the half-hour following the intruder encounter.
Figure 2. The stable High-BI group shows persistently elevated freezing during the ‘recovery’ period following the intruder encounter (1.5 years later).
See Figure 1d for an overview of the paradigm. The groups did not significantly differ in the Alone-Following-Alone control condition (p=.35). The High-BI group froze longer following the encounter (Alone-Following-Intruder) compared to the control condition (Alone-Following-Alone) (F(1,21)=12.04, p=.002). The y-axis indicates the log10-transformed freezing duration, averaged across six consecutive 5-min bins. Asterisk indicates significant pairwise group differences. Error bars depict SE.
The High-BI Group Shows Elevated Activity in the BST Region Following the Intruder Encounter
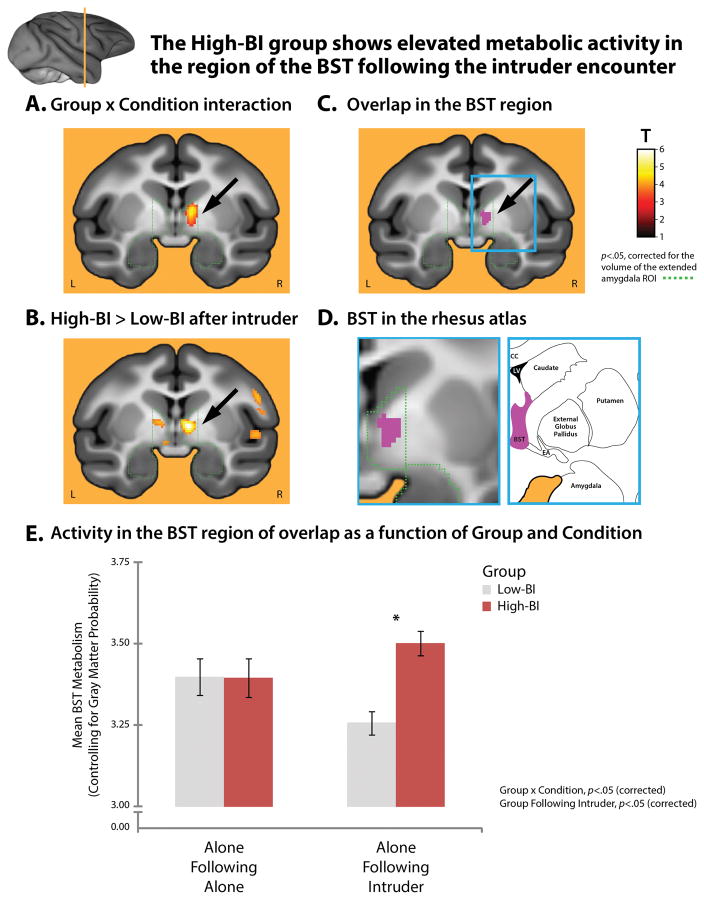
To identify the neural systems underlying group differences in freezing following the intruder encounter, we used a whole-brain voxelwise GLM to identify regions where the critical Group × Condition interaction was significant in the period following FDG administration (Figure 1d). Given our a priori focus on the extended amygdala, the interaction was thresholded at p<.05 (corrected for a 4,188-voxel region-of-interest [ROI] encompassing the amygdala and BST; depicted in green in Figure 3). This analysis revealed a cluster in the region of the BST (Figure 3a; Table S3; Supplementary Figure S2). We used a second whole-brain analysis to identify regions where the High-BI group showed significantly more activity than the Low-BI group during the Alone-Following-Intruder condition (p<.05, corrected). This revealed an overlapping cluster within the a priori ROI (Figure 3b; Table S4). Although several clusters were significant in one or the other of these two statistical maps, the region of overlap in the region of the BST was the only cluster in the entire brain to satisfy both of these critical tests (Figures 3c–e and Supplementary Figure S3). This effect was specific to the period following threat; like freezing behavior, the High- and Low-BI groups did not show significant differences in activity in the BST or other parts of the extended amygdala during the control condition (Alone-Following-Alone; cf. Figure 3e).
Figure 3. The High-BI group shows elevated metabolic activity in the region of the BST following the intruder encounter.
The a priori extended amygdala ROI is indicated by the dashed green line. The imaging paradigm is depicted in Figure 1d. A. Regions showing a significant Group × Condition interaction. The cluster in the region of the BST is indicated by the black arrow (p<.05, small-volume corrected). Data extracted from this cluster is depicted in Supplementary Figure S1. B. Regions where the stable High-BI group showed significantly more activity than the Low-BI group during the 30-min ‘recovery’ period following the intruder encounter. Clusters visually depicted outside the ROI (green) were not significant using a whole-brain threshold. C. Region of overlap in the BST region. A minimum conjunction44 (logical AND) of the whole-brain contrasts shown in panels A and B revealed a cluster in the vicinity of the BST (purple). No other clusters were identified. D. BST in the corresponding region of the rhesus brain atlas. The left image shows a magnified view of the region marked by the cyan rectangle in panel C. The arrow is omitted for clarity. The right image depicts corresponding region of the rhesus atlas. Additional views of the BST clusters can be found in Supplementary Figure S2. E. Activity in the BST region of overlap as a function of Group and Condition. Figure shows mean activity from the purple cluster depicted in panel C controlling for nuisance variance in mean-centered gray matter probability. Asterisk indicates the significant pairwise group differences. Error bars depict SE. Portions of this figure were adapted with permission from plate 54 in the atlas of Paxinos and colleagues73. Abbreviations—CC: corpus callosum, EA: extended amygdala, LV: lateral ventricle.
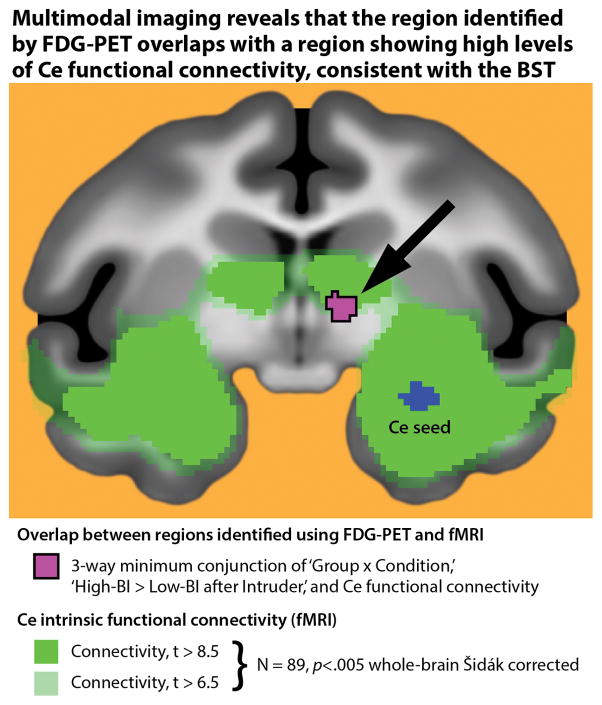
Compared to other brain regions implicated in BI and anxiety, the BST is small and lacks distinct macroscopic boundaries33, making it challenging to definitively localize using conventional brain imaging techniques. To better understand the region showing elevated activity in the High-BI group (purple cluster in Figure 3c), we capitalized on work demonstrating that the BST and Ce show robust anatomical33 and functional connectivity45 . Here we used previously published45 fMRI data obtained from an independent sample of 89 young monkeys to demonstrate that the hyper-metabolic cluster identified using FDG-PET overlaps with a region expressing high levels of ‘resting-state’ functional connectivity with the Ce (Figure 4; significant connectivity depicted in green; overlap depicted in purple), enhancing our confidence that it includes the BST.
Figure 4. Multimodal imaging reveals that the hypermetabolic region identified by FDG-PET overlaps a region expressing high levels of Ce functional connectivity, consistent with the BST.
Shown in purple is the overlap (i.e. minimum conjunction44) between voxels identified using FDG-PET (purple cluster in Figure 3c) and those showing significant functional connectivity with the Ce (green) in an independent sample of 89 monkeys (detailed in Ref. 45). The substantial overlap enhances our confidence that the hyper-metabolic region identified using PET encompasses portions of the BST. The Ce seed region used in the functional connectivity analyses is depicted in blue.
To clarify the consequences of elevated BST metabolism, we extracted activity from the region of overlap identified using FDG-PET (Figure 3c) for each subject and assessed brain-behavior relations after controlling for nuisance variance in mean-centered gray matter probability. Consistent with the group mean differences described above, individual differences in BST activity following the intruder encounter (a) were prospectively predicted by intruder-elicited freezing during the initial phenotyping sessions (partial ρ=.58, p=.005), (b) predicted concurrent freezing (partial ρ=.46, p=.03), and (c) discriminated High-BI individuals with good sensitivity (90.9%) and specificity (83.3%), χ2(1)=15.51, p<.001. These relations were specific to the period following the encounter with potential threat; significant associations were not obtained using BST activity associated with the Alone-Following-Alone control condition (ps>.20). Collectively, these findings suggest that elevated activity in the BST plays a key role in promoting extreme early-life anxiety.
DISCUSSION
The present study, which combines longitudinal phenotyping with high-resolution FDG-PET imaging in young nonhuman primates, provides unique insights into the neural bases of extreme early-life anxiety. Consistent with research in children and adolescents, this work demonstrates that BI represents a trait-like tendency to respond to a range of potentially threatening cues and contexts with increased signs of anxiety. Drawing from a pool of 109 young monkeys, we formed age- and sex-matched groups characterized by stable and extreme freezing in response to an unfamiliar human intruder (Figures 1a, b). Compared to the Low-BI group, the High-BI group showed nearly twenty times more freezing to the intruder during the two screening sessions and an order of magnitude more freezing when they re-encountered the intruder ~1.5 years later. The High-BI group also showed increased reticence when exposed to a snake ~2 years after the initial phenotyping sessions (Figure 1c and Supplement). In short, the BI phenotype shows continuity across time and across different types of ethologically relevant threats in young monkeys. Importantly, the High-BI group also showed increased freezing when alone in the testing cage during the initial phenotyping sessions and continued to show exaggerated wariness and inhibition in the testing cage during the first 30 min of the brain imaging sessions (i.e. prior to FDG administration), despite repeated exposure to this context during the intervening year-and-a-half. These observations suggest that extreme BI manifests in amplified caution and anxiety in situations where threat is diffuse, weak, or uncertain. Consistent with this perspective, the High-BI group showed persistently elevated freezing during the 30-min recovery period following the intruder encounter (Figure 2b)–more than an order of magnitude greater than the Low-BI group–and this was associated with increased metabolic activity in the BST region (Figures 3, 4). Hyper-metabolism was associated with exaggerated freezing and discriminated High- and Low-BI individuals with good sensitivity and specificity. Collectively, these observations provide compelling evidence that sustained levels of heightened anxiety in the absence of immediate threat reflect elevated activity in the BST region.
In children, anxious temperament and the narrower BI phenotype are heritable early-life risk factors for the development of anxiety, depression, and co-morbid substance abuse1, 2, 5 and our results provide a neurobiologically-grounded framework for understanding the mechanisms underlying this liability. In particular, our results provide evidence that individuals with consistently high levels of the BI phenotype early in development show persistent wariness in the period following exposure to potential threat and that this is associated with increased engagement of the BST. These observations complement evidence that threat-elicited activity in the BST is heritable and genetically correlated with individual differences in anxious temperament in young monkeys29. The relevance of the BST to sustained anxiety is consistent with anatomical tracing studies in rodents and monkeys showing that this region sends dense projections to brainstem and subcortical effector sites33, 46. Our imaging results are also consistent with mechanistic studies in rodents showing that the BST exhibits persistent excitability following direct stimulation, supports passive avoidance during sustained exposure (5–20 min.) to diffusely threatening contexts (e.g. elevated-plus maze), and contributes to the over-generalization of anxiety to safety cues (CS-)11, 25, 26, 47, 48.
Our observations provide an important extension of recent imaging work documenting that: (a) individuals with extreme anxiety show elevated activation in the vicinity of the BST during exposure to uncertain threat and punishment49–52 and (b) individual differences in BST function predict anxious mood, freezing, skin conductance, and cortisol elicited by uncertain or diffuse threat6, 28, 29, 38, 53–56. Extending earlier work in rodents, monkeys, and humans, our results reflect the comparison of two physically-identical conditions (i.e. Alone-Following-Intruder and Alone-Following-Alone; Figure 1d), neural activity integrated over an extended 30 min ‘recovery’ period following the offset of threat, concurrent measures of naturalistic defensive responses in individuals selected on the basis of stable and extreme phenotypic risk, and a multi-modal imaging approach to identifying the BST region. These features enhance our confidence in the translational significance of these results.
While the BST has not been previously linked to childhood BI, our findings dovetail with emerging evidence that, like anxious adults9, 11–13, children and adolescents with a history of extreme BI and elevated anxiety are prone to contextually inappropriate defensive behaviors14–18, 57. Moreover, contextually inappropriate responses in the laboratory prospectively predict heightened real-world anxiety in children and avoidance of threat-related contexts in adults19, 20. Among adults, heightened apprehension and defensive responses in safe contexts are generally more discriminative of pathological anxiety than that elicited by overt threat11, 12. This maladaptive phenotype appears to be amplified by early adversity58 and prospectively predicts the onset of anxiety disorders in adolescence59 as well as the intensification of anxious symptoms in young adults60. Collectively, these findings underscore the potential etiological significance of heightened BST activity.
The BST is increasingly conceptualized as a key promoter of ‘sustained’ defensive responses to uncertain, psychologically diffuse, or temporally remote threat and our results are broadly consistent with this perspective. Nonetheless, it is likely that the BST makes a broader contribution to phenotypic risk and anxiety, one that encompasses exaggerated defensive responses during both sustained and transient exposure to uncertain threat33, 61. Mechanistic work in rodents suggests that BST engagement can begin quite early, between 4 and 60 s following the onset of cues associated with uncertain danger11, and contributes to the overgeneralization of conditioned fear to 30 s auditory cues25. In human fMRI studies, BST activation has also been observed in response to acute aversive challenges (e.g. 4-s tarantula video clip; see Refs. 62–65). In fact, a recent meta-analysis demonstrated that imaging studies of fear and anxiety consistently reveal activation in the region of the central extended amygdala, including the BST as well as the Ce, across a broad spectrum of populations, paradigms, and time-scales33. An important avenue for future research will be to clarify the boundary conditions and mechanistic importance of BST engagement. Likewise, while our results highlight the importance of the BST, it will be important to understand how functional interactions between the BST and other regions sensitive to potential threat (e.g. Ce, periaqueductal gray, orbitofrontal cortex, insula, and anterior cingulate) control the expression of persistent anxiety, support variation in early phenotypic risk, and ultimately contribute to the development and maintenance of psychopathology in humans24, 29, 54, 66–68. Given the higher prevalence of anxiety disorders and depression among women69, 70, understanding potential sex differences in the function of these circuits and their relevance to risk represents another key challenge.
As we and other commentators have recently noted, understanding the BST is challenging33, 71. From a technical standpoint, the BST is small; it is functionally and anatomically heterogeneous; and it lacks clear boundaries in vivo, even when using ultra-high field-strength MRI techniques33, 72, 73.74, 75. Furthermore, most imaging software for automated cluster labeling does not include the BST, although standardized segmentation protocols75 and masks have recently become available (https://afni.nimh.nih.gov/afni/community/board/read.php?1,149436,149436). The upshot is that researchers may not recognize that a cluster encompasses the BST or may be hesitant to label it as such. Regardless of the label, there is value to discussing the potential role of the BST and other neighboring regions of the basal forebrain (e.g. accumbens), even in cases where a cluster cannot be unambiguously ascribed to the BST. Using multi-band imaging sequences and high-precision spatial normalization techniques would provide enhanced spatial resolution29, 76. Multi-modal ‘double-labeling’ strategies, as in the present study (i.e. FDG-PET paired with resting-state fMRI) and other recent work by our group29 (i.e. FDG-PET paired with in vivo dopamine receptor imaging) provide other tools for ascertaining whether a cluster is likely to include the BST.
Our results also have implications for theories of temperament and personality9, 77. There is ample evidence that individuals who are most at risk for developing a mood or anxiety disorder are prone to persistently elevated distress in contexts where threat is distant or absent78, 79. In fact, longitudinal experience-sampling studies suggest that the vast majority of negative affect experienced by adults with an anxious disposition cannot be attributed to clear-cut stressors in the immediate environment80. Although this has been described as a tonic or endogenous effect of temperament78, our results suggest that it may reflect increased reactivity to stressors that are weak, diffuse (cf. the test cage) or temporally remote (cf. the Alone-Following-Intruder condition). Our results and others33 motivate the hypothesis that this pervasive anxiety partially reflects alterations in BST function.
CONCLUSIONS
The present study demonstrates that individuals with stable and extreme BI respond to a range of potentially threatening cues and contexts with exaggerated defensive responses. Young monkeys with elevated levels of BI consistently over-reacted to both overt and more diffuse kinds of threat during more than two years of longitudinal study. Concurrent measures of FDG metabolism provide unique evidence that heightened defensive responses following an encounter with potential threat reflects increased engagement of the BST, a key component of the central extended amygdala. The present study has several features that enhance our confidence in the translational significance of these results, including the use of a well-validated primate model of early-life anxiety, physically identical ‘recovery’ conditions81, 82, concurrent measures of evolutionarily-conserved emotional behaviors, and a multi-modal approach to identifying the BST. Translational brain imaging strategies, like that featured in the present study, provide a powerful tool for bridging the gap separating the mechanistic insights afforded by nonhuman animal models from the complexity of human emotions, temperament, and psychopathology and accelerating therapeutic development. Developing more effective interventions is particularly important for minimizing the cumulative damage associated with extreme BI and anxiety early in development.
Supplementary Material
Acknowledgments
Authors acknowledge assistance and critical feedback from A. Alexander, A. Converse, L. Friedman, D. Grupe, R. Hoks, T. Johnson, S. Mansavage, K. Meyer, L. Pessoa, D. Pine, P. Rudebeck, W. Shelledy, M. Stockbridge, T. Johnstone, E. Zao, and the staffs of the Harlow Center for Biological Psychology, HealthEmotions Research Institute (HERI), and Wisconsin National Primate Center. We are particularly grateful for the contributions of Helen Van Valkenberg to this work. This work was supported by the National Institutes of Health (HD003352, HD008352, MH018931, MH046729, MH069315, MH081884, MH084051, MH091550, MH107444, OD011106, and RR000167), HERI, Meriter Hospital, and University of Maryland.
Footnotes
CONTRIBUTIONS
N.H.K. and S.E.S. designed the study. R.J.D. provided theoretical guidance. S.E.S. collected data. A.S.F. processed data. A.J.S., A.S.F., T.R.O., and N.H.K. analyzed data. A.S.F. and T.R.O. developed analytical tools. A.J.S., A.S.F., N.H.K., J.A.O., and R.J.D. contributed to data interpretation. A.J.S., A.S.F., and N.H.K. wrote the paper. A.J.S. and A.S.F. created figures and tables. N.H.K. supervised the study. All authors reviewed and revised the paper
CONFLICTS OF INTEREST
Authors declare no conflicts of interest.
References
- 1.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pine DS, Fox NA. Childhood antecedents and risk for adult mental disorders. Annu Rev Psychol. 2015;66:459–485. doi: 10.1146/annurev-psych-010814-015038. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR. Next-generation treatments for mental disorders. Science translational medicine. 2012;4:155ps119. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- 4.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 5.Fox AS, Kalin NH. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oler JA, Fox AS, Shackman AJ, Kalin NH. The central nucleus of the amygdala is a critical substrate for individual differences in anxiety. In: Amaral DG, Adolphs R, editors. Living without an amygdala. Guilford; NY: 2016. [Google Scholar]
- 8.Buss KA, Kiel EJ. Temperamental risk factors for pediatric anxiety disorders. In: Vasa RA, Roy AK, editors. Pediatric anxiety disorders: A clinical guide. Springer; NY: 2013. pp. 47–68. [Google Scholar]
- 9.Shackman AJ, Stockbridge MD, LeMay EP, Fox AS. The psychological and neurobiological bases of dispositional negativity. In: Fox AS, Lapate RC, Shackman AJ, Davidson RJ, editors. The nature of emotion. Fundamental questions. 2. Oxford University Press; NY: in press. [Google Scholar]
- 10.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 11.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015:239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- 13.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker TV, Reeb-Sutherland BC, Fox NA. Individual differences in fear potentiated startle in behaviorally inhibited children. Dev Psychobiol. 2014;56(1):133–141. doi: 10.1002/dev.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Perez-Edgar K, Henderson HA, Lissek S, et al. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters AM, Nazarian M, Mineka S, Zinbarg RE, Griffith JW, Naliboff B, et al. Context and explicit threat cue modulation of the startle reflex: preliminary evidence of distinctions between adolescents with principal fear disorders versus distress disorders. Psychiatry Res. 2014;217:93–99. doi: 10.1016/j.psychres.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, et al. Development of fear acquisition and extinction in children: effects of age and anxiety. Neurobiol Learn Mem. 2014;113:135–142. doi: 10.1016/j.nlm.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reznick JS, Kagan J, Snidman N, Gersten M, Baak K, Rosenberg A. Inhibited and uninhibited children: A follow-up study. Child Dev. 1986;57:660–680. [Google Scholar]
- 19.Buss KA, Davis EL, Kiel EJ, Brooker RJ, Beekman C, Early MC. Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. J Clin Child Adolesc Psychol. 2013;42:603–616. doi: 10.1080/15374416.2013.769170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- 21.Houben M, Van Den Noortgate W, Kuppens P. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychol Bull. 2015 doi: 10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- 22.Newman MG, Fisher AJ. Mediated moderation in combined cognitive behavioral therapy versus component treatments for generalized anxiety disorder. J Consult Clin Psychol. 2013;81:405–414. doi: 10.1037/a0031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Leemput IA, Wichers M, Cramer AO, Borsboom D, Tuerlinckx F, Kuppens P, et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci U S A. 2014;111(1):87–92. doi: 10.1073/pnas.1312114110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 25.Duvarci S, Bauer EP, Pare D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–1500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc Natl Acad Sci U S A. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, et al. Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Acadademy of Sciences USA. 2015;112:9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 32.Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Sytems. Vol. 4. Elsevier; NY: 2007. pp. 3–34. [Google Scholar]
- 33.Fox AS, Oler JA, Tromp DP, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.deCampo DM, Fudge JL. Amygdala projections to the lateral bed nucleus of the stria terminalis in the macaque: comparison with ventral striatal afferents. J Comp Neurol. 2013;521:3191–3216. doi: 10.1002/cne.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 36.Rilling JK, Winslow JT, O’Brien D, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in rhesus monkeys. Biol Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- 37.Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahn AL, Fox AS, Abercrombie HC, Shelton SE, Oakes TR, Davidson RJ, et al. Subgenual prefrontal cortex activity predicts individual differences in hypothalamic-pituitary-adrenal activity across different contexts. Biol Psychiatry. 2010;67:175–181. doi: 10.1016/j.biopsych.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, et al. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29:9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preacher KJ, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: A critical reexamination and new recommendations. Psychol Methods. 2005;10:178–192. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- 41.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, et al. Evolutionarily-conserved dysfunction of prefrontal-amygdalar connectivity in early-life anxiety. Mol Psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 47.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 48.Nagy FZ, Pare D. Timing of impulses from the central amygdala and bed nucleus of the stria terminalis to the brain stem. J Neurophysiol. 2008;100:3429–3436. doi: 10.1152/jn.90936.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munsterkotter AL, Notzon S, Redlich R, Grotegerd D, Dohm K, Arolt V, et al. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depress Anxiety. doi: 10.1002/da.22382. in press. [DOI] [PubMed] [Google Scholar]
- 53.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, et al. Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Translational psychiatry. 2015;5:e591. doi: 10.1038/tp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Dev Psychol. 2011;47:804–819. doi: 10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolitzky-Taylor K, Vrshek-Schallhorn S, Waters AM, Mineka S, Zinbarg R, Ornitz E, et al. Adversity in early and mid-adolescence is associated with elevated startle responses to safety cues in late adolescence. Clin Psychol Sci. 2014;2:202–213. doi: 10.1177/2167702613495840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Craske MG, Wolitzky-Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek-Schallhorn S, et al. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: evidence from a longitudinal investigation. J Abnorm Psychol. 2012;121(2):315–324. doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenaert B, Boddez Y, Griffith JW, Vervliet B, Schruers K, Hermans D. Aversive learning and generalization predict subclinical levels of anxiety: a six-month longitudinal study. J Anxiety Disord. 2014;28(8):747–753. doi: 10.1016/j.janxdis.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 61.LeDoux JE. Anxious. Using the brain to understand and treat fear and anxiety. Viking; NY: 2015. [Google Scholar]
- 62.Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Acadademy of Sciences USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage. 2012;59:1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SE, Oosting RS, et al. Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol Psychiatry. 2015;78:582–589. doi: 10.1016/j.biopsych.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 66.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mobbs D, Hagan CC, Dalgleish T, Silston B, Prevost C. The ecology of human fear: survival optimization and the nervous system. Front Neurosci. 2015;9:55. doi: 10.3389/fnins.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 71.Avery SN, Clauss JA, Blackford JU. The human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mai JK, Paxinos G, Voss T. Atlas of the human brain. 3. Academic Press; San Diego, CA: 2007. [Google Scholar]
- 73.Paxinos G, Huang X, Petrides M, Toga A. The rhesus monkey brain in stereotaxic coordinates. 2. Academic Press; San Diego: 2009. [Google Scholar]
- 74.Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. Neuroimage. 2014;91:311–323. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torrisi S, O’Connell K, Davis A, Reynolds R, Balderston N, Fudge JL, et al. Resting state connectivity of the bed nucleus of the stria terminalis at ultra-high field. Hum Brain Mapp. 2015;36:4076–4088. doi: 10.1002/hbm.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caspi A, Roberts BW, Shiner RL. Personality development: stability and change. Annu Rev Psychol. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- 78.Gross JJ, Sutton SK, Ketelaar T. Relations between affect and personality: Support for the affect-level and affective reactivity views. Personality and Social Psychology Bulletin. 1998;24:279–288. [Google Scholar]
- 79.Suls J, Martin R. The daily life of the garden-variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. J Pers. 2005;73:1485–1509. doi: 10.1111/j.1467-6494.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 80.Bolger N, Schilling EA. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. J Pers. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 81.Luck SJ. Ten simple rules for designing ERP experiments. In: Handy TC, editor. Event-related potentials: A methods handbook. MIT Press; Cambridge, MA: 2005. pp. 17–32. [Google Scholar]
- 82.Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- 83.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.