Abstract
Obesity represents a major medical and public health problem worldwide. Efforts have been made to develop novel treatments, and among them bariatric surgery is used as an effective treatment to achieve significant, long-term weight loss and alleviate medical problems related to obesity. Alcohol use disorder (AUD) is also a leading cause of morbidity and mortality worldwide. Recent clinical studies have revealed a concern for bariatric surgery patients developing an increased risk for alcohol consumption, raising concerns about development of AUD. A better understanding of the relationship between bariatric surgery and potential later development of AUD is important, given the critical need of identifying patients at high risk for AUD. This paper reviews current clinical and basic science research and discusses potential underlying mechanisms. Special emphasis in this review is given to recent work suggesting that, alterations in alcohol metabolism/pharmacokinetics resulting from bariatric surgery are unlikely to be the primary or at least the only explanation for increased alcohol use and development of AUD, as changes in brain reward processing are also likely to play an important role. Additional studies are needed to clarify the potential role and mechanisms of how bariatric surgery may increase alcohol use and lead to AUD development.
Keywords: alcohol, alcohol use disorder, bariatric surgery, Roux-en-Y gastric bypass, sleeve gastrectomy, gastric banding, gut-liver-brain axis
1. Introduction
1.1. Obesity: An alarming public health problem
Obesity is defined as a body mass index (BMI) ≥30 kg/m2 (Centers for Disease Control and Prevention 2011), where obesity with a BMI ≥40 kg/m2 is the most severe form (Seidell & Flegal 1997). Obesity is also classified as Obesity Class I (BMI: 30 kg/m2 – 34.9 kg/m2), Obesity Class II (BMI: 35 kg/m2 – 39.9 kg/m2) and Obesity Class III or Extreme Obesity (BMI ≥40 kg/m2) (National Heart Lung and Blood Institute 2015; World Health Organization 2015).
According to the World Health Organization (2015), the worldwide rate of obesity has more than doubled in the last 25 years. The majority of people, particularly in developed nations, are above normal weight, with 39% of adults overweight in 2014 and 13% obese. Obesity represents a major medical and public health problem as it may lead to decreased quality of life and increased mortality and morbidity (Flegal et al. 2012; Gilbert & Wolfe 2012; Kuk et al. 2009; Malinoff et al. 2013; Reis et al. 2009). Obesity is associated with increased risk for several medical diseases, including hypertension, dyslipidemia, type 2 diabetes mellitus, cardiovascular diseases and certain cancers (Centers for Disease Control and Prevention 2006).
The development of effective treatments for obesity is of crucial importance. While several treatments are available, they have relatively limited efficacy (Hainer & Aldhoon-Hainerova 2014), which is made worse by the associated reduced patients’ compliance. Therefore, efforts have been made to develop novel, more effective treatments. Among them, bariatric surgery is now used as an effective treatment for obesity.
1.2. Bariatric surgery
In the United States (US), bariatric surgery is indicated in obese patients who have a BMI ≥40 kg/m2 or are more than 100 pounds overweight. Of special importance for this review, the majority of the US population that undergoes this surgical procedure is female, i.e. approximately 80%. Bariatric surgery is also indicated in those obese patients who have a BMI ≥35 kg/m2 associated with at least two obesity-related co-morbidities, e.g. type 2 diabetes mellitus, hypertension, sleep apnea and other respiratory disorders, non-alcoholic fatty liver disease, osteoarthritis, lipid abnormalities, gastrointestinal disorders, or heart disease. While these criteria regulate the US practice, the indications for bariatric surgery may vary globally.
Challenges in achieving weight loss via diet and/or exercise only, combined with increasing evidence of efficacy for bariatric surgery, have made this surgical approach an increasingly used obesity treatment (Fisher & Schauer 2002). Patients receiving bariatric surgery have significant reductions in food intake and weight (Buchwald et al. 2004; Garb et al. 2009; Wittgrove & Clark 2000). Bariatric surgery also results in beneficial effects on weight-associated medical conditions.
Bariatric surgery includes different techniques, some of which can be executed by open and/or laparoscopic abdominal surgery. Common bariatric surgery techniques include Roux-en-Y Gastric Bypass (RYGB), sleeve gastrectomy, vertical banded gastroplasty and gastric banding. The RYGB procedure involves creating a stomach pouch out of a small portion of the stomach and attaching it directly to the small intestine, bypassing a large part of the stomach and the entire duodenum. Not only is the stomach pouch too small (0.5 to 1 ounce) to hold large amounts of food, but by bypassing the duodenum, fat absorption is substantially reduced (Lo Menzo et al. 2015). With the sleeve gastrectomy, more than half of the stomach is removed, leaving a thin vertical sleeve. Vertical banded gastroplasty uses both a band and staples to create a small stomach pouch. In the bottom of the pouch there is a hole, where the pouch contents can move to the stomach and then onto the intestine. Finally, gastric banding involves placing a band around the upper part of the stomach in order to limit the amount of food that can be ingested due to a rapid feeling of fullness after eating small quantities of food.
1.3. Alcohol use disorder
Alcohol dependence and alcohol abuse, now replaced by the common definition of alcohol use disorder (AUD), according to the DSM-5 (American Psychiatric Association 2013), are leading causes of morbidity and mortality worldwide (Grant et al. 2015; Lim et al. 2012). Continued heavy alcohol use may lead to several serious medical consequences (Nutt et al. 2010; Rehm et al. 2013), including cardiovascular disease and liver cirrhosis, and represents a risk factor for several types of cancers (Seitz & Mueller 2015). As such, factors potentially responsible for increasing the risk of AUD warrant serious consideration. Among them, bariatric surgery has recently emerged as a potential risk factor for AUD.
1.4. Bariatric surgery and AUD
As discussed in detail with full citations below, recent studies suggest that bariatric surgery may be associated with significant changes in alcohol drinking habits. Specifically, the majority of the studies suggest that it may lead to an increased alcohol use, and risk for AUD and alcohol problems. Both obesity and AUD represent important clinical and public health problems. Therefore, clarifying the relationship between bariatric surgery and potential later development of AUD is important, given the critical need of identifying risk factors for AUD.
The goal of this paper is to review the literature pertaining to the potential role of bariatric surgery as a risk factor for increased alcohol use and development of AUD, and to offer the authors’ perspective on the work conducted to date and on the potential biological mechanisms that might guide future preclinical and translational research. Special emphasis in this review will be given to recent work suggesting that alterations in alcohol metabolism/pharmacokinetics resulting from bariatric surgery are unlikely to be the primary or at least the only explanation for increased alcohol use and development of AUD, as changes in brain reward processing are also likely to play an important role.
In the present review, sleeve gastrectomy, vertical banded gastroplasty and gastric banding will be discussed under the category of ‘Restrictive Surgeries’ and compared to the RYGB procedure. The rationale for this organization is that several clinical studies have not clearly discriminated among the various procedures of which the exclusive or primary mechanism is the restriction of stomach volume, whereas there is clearer separation in the literature for the RYGB operation. Less commonly used procedures (i.e., biliopancreatic diversion, duodenal switch, mini-gastric bypass, single anastomosis duodeno-ileostomy and other procedures involving ileal transposition) were not included in the subject of the present review.
1.5. Search strategy and selection criteria
References were identified through searches of PubMed, Embase and Web of Science conducted until January 2016, by use of the following search terms:
-
Bariatric surgery or gastric bypass or gastroplasty or jejunoileal bypass or lipectomy or bariatric surgery or gastric bypass or gastroplasty or jejuneileal bypass or lipectomy or Roux-en-Y Gastric bypass or RYGB or vertical banded gastroplasty or jejunoileal bypass or ileojejunal bypass or intestinal bypass or sleeve gastrectomy or gastric banding
AND
Alcoholism or alcoholic or alcoholics or alcohol dependence or alcohol use disorder or alcohol abuse or alcohol drinking or binge drinking or alcohol consumption or ethanol
Peer-reviewed articles resulting from these searches and relevant references cited in those articles were reviewed. There was no restriction on publication date. Only articles published in English were included.
2. Bariatric surgery as a risk for AUD
Both clinical and preclinical studies have been conducted on the potential role of bariatric surgery on alcohol consumption and risk for AUD and alcohol problems.
2.1. Clinical studies
2.1.1. Bariatric surgery (not otherwise specified)
A survey with 318 bariatric surgery patients found that approximately 83% consumed alcohol after surgery. A total of 28.4% of the sample reporting difficulties controlling their alcohol use, compared with 4.5% before surgery. Additionally, 84% of those who consumed alcohol after surgery reported an increase in sensitivity to effects of alcohol (Buffington 2007). Another study based on electronic medical records compared bariatric patients to matched non-bariatric obese patients. Both groups (n = 54 each) were equally likely to have a diagnosis of alcohol dependence, however, bariatric patients were significantly more likely to have a diagnosis of alcohol withdrawal and to report larger amounts of alcohol consumed per drinking day (Saules et al. 2010). Overall, the clinical relevance of these two studies is limited by either methodology (i.e.: survey) or sample limitations.
2.1.2. Roux-en-Y Gastric Bypass
Initial clinical studies examining the relationship between RYGB and AUD were limited by either observational or retrospective designs, or survey-based cross-sectional reports. For example, a study examined the rates of alcohol abuse and dependence in a sample of 70 patients who had received RYGB 6–10 years before (Ertelt et al. 2008). Results indicated that, while only <3% of these RYGB patients developed an AUD, 20% of them reported feelings of alcohol intoxication after consuming lower amounts of alcohol compared to the quantities consumed before the surgery. Additionally, 34.3% of this sample reported feeling intoxicated more rapidly after versus before RYGB. However, this study had important limitations, including the lack of validated measures of alcohol use and intoxication, and the lack of pre-surgery data (Ertelt et al. 2008).
More recently, a retrospective study looking at electronic medical records reported that out of 823 patients seeking treatment for AUD, 4.9% had RYGB (Cuellar-Barboza et al. 2015). In this study, RYGB patients were compared to a smaller sample (n = 122) of non-bariatric obese patients selected among the same population of treatment-seeking AUD population seen during the same timeframe. The main finding was that RYGB patients met AUD criteria at a significantly earlier age compared to controls.
Prospective human laboratory studies have investigated the effects of RYGB on alcohol concentrations. For example, Hagedorn et al. (2007) conducted a pharmacokinetic/pharmacodynamics study with 36 subjects (19 post-gastric bypass and 17 controls) whose breath alcohol concentrations were assessed repeatedly after consuming 5 oz of wine. This study indicated that, while symptoms did not differ between the two groups, the gastric bypass group, compared to controls, had higher peak alcohol levels and longer times to zero out. A more recent small human laboratory study with five female participants who had undergone RYGB 3–4 years before, investigated blood alcohol concentration (BAC) after administration of alcohol (0.3 g/kg). Blood samples were collected every minute for the first five minutes and then at 7.5, 10, 20, and 60 minutes. All participants reported very rapid increases in BACs, with values >0.08% reached within 10 minutes after alcohol administration (Steffen et al. 2013). More recently, another small alcohol pharmacokinetics study in eight female participants found that RYGB increased the rate of delivery of oral alcohol into the systemic circulation (Pepino et al. 2015). This delivery rate resulted in both earlier and higher BAC peaks; participants also reported greater feeling of intoxication (Pepino et al. 2015). Notably, while Hagedorn et al. (2007) enrolled a much larger sample, the other two studies, on the other hand, employed a much more rigorous design, including a more accurate method for alcohol administration and the use of blood alcohol concentrations (Pepino et al. 2015; Steffen et al. 2013).
Some studies have reported reduced alcohol use after RYGB. For example, in a follow-up study with 100 patients assessed 13–15 years after RYGB, there was an increase in alcohol abuse over time (2.6% pre-surgery versus 5.1% post-surgery), while a decline in alcohol dependence (10.3% pre-surgery versus 2.6% post-surgery) (Mitchell et al. 2001). In a more recent study, 155 out of 899 RYGB patients were assessed prospectively for alcohol use before and after surgery. The number of RYGB patients reporting any alcohol use decreased significantly postoperatively (72.3% to 63.2%). However, despite the overall reduction in any alcohol use, 23% of patients who did not use alcohol pre-surgery reported alcohol use post-surgery. In contrast, out of those patients who used alcohol in the year before surgery, 21.4% stopped drinking after surgery (Lent et al. 2013).
In another study with 129 RYGB patients assessed pre- and post-surgery, self-reported alcohol use decreased, however the proportion of participants reporting alcohol disturbance increased after surgery (Alfonsson et al. 2014). In a prospective RYGB study (N = 6165), alcohol consumption significantly declined after surgery in those patients that self-reported occasional to frequent alcohol use before surgery; by contrast, no changes were observed in those individuals that reported no alcohol use before surgery (Davis et al. 2012). Collectively, these results suggest that the potential role of RYGB in affecting alcohol use may depend on the levels of alcohol use before the surgery and that individual differences might explain opposite effects of RYGB on alcohol-related behaviors.
An important issue with alcohol use in RYGB patients is weight regain. In a study with 119 RYGB patients, no significant association was found between alcohol use and weight loss at 1-year follow up (Sears et al. 2008). Other retrospective or follow-up studies suggest that a history of alcohol use may be associated with stronger weight loss following RYGB (Clark et al. 2003; Heinberg & Ashton 2010). However, a survey-based study found that concerns over alcohol use represented an independent predictor of weight regain after RYGB surgery (Odom et al. 2010). In summary, it is still unclear whether alcohol use is a factor that contributes to weight regain.
2.1.3. Restrictive Procedures
Restrictive procedures reviewed here in the context of alcohol use include sleeve gastrectomy, vertical banded gastroplasty and gastric banding.
Literature investigating restrictive procedures solo (i.e. without comparison to RYGB, as detailed in section 2.1.4) is very limited. For example, a sample of 140 severe obese patients (25 with binge eating disorders) was evaluated 18 months after surgery: post-surgery symptoms of alcohol dependence were more prevalent in the binge eaters than in the non-binge eaters (Guisado Macias & Vaz Leal 2003). A few studies have investigated the effects of sleeve gastrectomy on alcohol metabolism and have, however, generated contradictory findings. For example, Gallo et al. (2015) conducted a prospective study with 10 obese patients scheduled for laparoscopic sleeve gastrectomy and all were presented with three alcohol challenges, i.e., preoperatively, and at 3 and 12 months postoperatively. Alcohol intoxication symptoms and BAC levels were assessed over time at each alcohol challenge session. Sleeve gastrectomy did not affect alcohol metabolism at either 3 or 12 months after surgery. Peak BAC and time for BAC to zero out did not significantly change after sleeve gastrectomy, neither did the symptoms of alcohol intoxication (Gallo et al. 2015). Contradictory to Gallo et al. (2015), another alcohol challenge study (Maluenda et al. 2010) was conducted in 12 patients before and 2 months after sleeve gastrectomy. Increased BAC levels and prolonged time for BAC to zero out were found after surgery compared to baseline, while post-surgery changes in alcohol intoxication symptoms were not observed. It is important to note that the patients in this study were different from the typical bariatric population in the United States (e.g.: Gallo et al. (2015)) as 80% were males, while only 20% are typically males in the average bariatric population in the US. This difference may account, at least in part, the conflicting results observed between the two studies, an explanation consistent with the known sex differences in alcohol metabolism (Baraona et al. 2001; Frezza et al. 1990; Maluenda et al. 2010).
Finally, alcohol use in the context of gastric banding has also been investigated and compared to RYGB, as detailed next.
2.1.4. Potential differences in alcohol use due to differences between surgery techniques
Different surgical techniques result in different changes in the anatomy of the gastrointestinal tract, and this difference in procedure has posed the question if these anatomical changes can result in technique-specific changes in alcohol use. As such, some studies have compared potential differences in alcohol-related behaviors between bariatric surgery techniques.
As for the Restrictive Procedures, there has been only one study comparing sleeve gastrectomy to gastric banding. In this study, the effects of gastric banding (n = 9) vs. sleeve gastrectomy (n = 7) on alcohol metabolism were assessed longitudinally (Changchien et al. 2012). This study reported that either technique was not associated with significant changes in peak BAC or time to sober at the 3-month and 6-month post-surgery follow-ups. Furthermore, there were no significant changes in drinking amounts or symptoms of alcohol intoxication post-surgery. While gastric banding does not result in changes in the stomach surface area, the authors hypothesized that after the sleeve gastrectomy there is still enough stomach surface area to remain unchanged by alcohol metabolism. The lack of changes in alcohol metabolism after sleeve gastrectomy is consistent with Gallo et al. (2015), but is in conflict with Maluenda et al. (2010), as discussed above (section 2.1.3). However, it is important to note that the majority of this sample (15/16) were social drinkers or non-drinkers, and no patients in this study had a history of alcohol abuse or dependence before or after surgery (Changchien et al. 2012).
The most relevant clinical literature is represented by several studies that have compared RYGB and gastric banding. For example, a retrospective study used electronic charts of bariatric surgery patients, among which 562 had gastric banding and 97 had RYGB. There was a significant reduction in alcohol use, independent from the type of surgery (de Araujo Burgos et al. 2015), i.e., alcohol use decreased from 24.2% to 9.4% in the 2-year post-surgery follow-up. An interview-based study with 541 bariatric surgery patients where alcohol use was assessed before and after surgery, found a small minority of patients reporting high-risk drinking at both 1 and 2 years post-surgery (either gastric bypass or gastric banding). On the other hand, more than half of the patients who reported high-risk alcohol consumption before surgery reduced drinking. However, none of these changes were statistically significant, nor did the two surgical techniques differ each other, although there was a trend toward a higher proportion of RYGB patients, compared to gastric banding, that reduced alcohol use at the year 1 follow-up (Wee et al. 2014).
In conflict with the studies mentioned above (de Araujo Burgos et al. 2015; Wee et al. 2014), other studies comparing different bariatric techniques found increased drinking, particularly after RYGB surgery. For example, three prospective studies (Conason et al. 2013; King et al. 2012; Suzuki et al. 2012) have shown an effect of RYGB, but not gastric banding in increasing alcohol use after 2+ years follow-up. These studies represent, indeed, the most robust work conducted, until now, on this topic. Suzuki et al. (2012) found that ~10% of 51 bariatric surgery patients undergoing either RYGB or gastric banding met criteria for alcohol abuse or dependence 2–5 years post-surgery; none of these individuals had met criteria at the pre-surgery evaluation. Notably, all individuals who met AUD criteria after surgery had undergone RYGB, while none of them had undergone gastric banding (Suzuki et al. 2012). Additionally, this study indicated that the lifetime prevalence of AUD in this group was comparable to the general population, therefore suggesting that RYGB patients should be evaluated not only for current, but also for lifetime AUD history (Suzuki et al. 2012). One additional fact that can be gleaned from the literature is that the age of onset of AUD after bariatric surgery is significantly younger than in the general population, which points strongly to surgery having had some kind of contribution to the onset of AUD in these patients. A prospective longitudinal study with 155 bariatric surgery patients (Conason et al. 2013) indicated that the frequency of alcohol use increased in those patients that underwent RYGB. Specifically, while alcohol use decreased immediately after RYGB surgery (probably due to the recovery from the surgery and medical advice received), the RYGB patients reported a significant increase in alcohol consumption at the 2-year post-surgery follow-up. Again, this increase in alcohol use was specific for RYGB, as similar findings were not observed in those patients who underwent gastric banding (Conason et al. 2013).
A prospective large cohort study with 2,458 bariatric surgery patients found that AUD symptoms, as measured by an Alcohol Use Disorders Identification Test (AUDIT) score ≥8, were significantly higher two years after surgery (King et al. 2012). This was a large multi-site study conducted in the U.S. and represents one of the most important clinical evidence on the potential role of RYGB on subsequent at-risk use of alcohol. Notably, in this study, RYGB doubled the likelihood of post-surgery AUD compared with gastric banding. Several baseline factors were also associated with post-surgery alcohol use, with pre-surgery alcohol use being the strongest one (King et al. 2012).
Finally, two studies (Ostlund et al. 2013; Svensson et al. 2013), both conducted in Sweden, compared RYGB, gastric banding and vertical banded gastroplasty. In a population-based retrospective study of 11,115 obese patients undergoing bariatric surgery, the post-surgery incidence of inpatient treatment for AUD was assessed (Ostlund et al. 2013). There was no difference in inpatient treatment of alcohol abuse among the three groups before the bariatric surgery. After surgery, RYGB patients had more than double the risk of inpatient treatment for AUD, compared to the patients who underwent either gastric banding or vertical banded gastroplasty. In a prospective controlled study, data on alcohol use were collected in 2,010 obese patients undergoing vertical banded gastroplasty (68%), gastric banding (19%) or RYGB (13%) and compared to 2,037 matched obese controls. It was observed that, while alcohol use was not different in obese patients who underwent gastric banding, patients receiving either RYGB or vertical banded gastroplasty, compared to controls, were more likely to report at least medium risk alcohol consumption (defined as ≥ 40g of pure alcohol per day in men and ≥ 20g in women), as well as increased risk of diagnoses of alcohol abuse and alcohol problems. Finally, RYGB patients had a significantly increased risk for alcohol abuse diagnosis, at least medium risk alcohol consumption, and self-reported alcohol problems compared to both vertical banded gastroplasty and gastric banding (Svensson et al. 2013). One additional noteworthy finding from the latter study was that the risk of onset of AUD continued to climb in a linear fashion for the whole 10-year post-op follow up period, supporting the notion of an increased risk for addiction in RYGB patients. This large study conducted in Sweden represents very important clinical evidence on the potential role of RYGB on subsequent at-risk use of alcohol.
2.1.5. Summary of clinical studies
Human studies investigating the potential effects of bariatric surgery on alcohol consumption and risk for AUD and alcohol problems have generated conflicting results. RYGB has been the most studied technique, and perhaps for this reason has also been the one generating the most conflicting results. Nonetheless, considering the overall literature published to date (Table 1), the majority of the clinical studies suggest that bariatric surgery represents a potential risk for increased alcohol use. Further supporting our overall conclusion is the fact that, while the clinical studies conducted to date have been very heterogeneous in terms of study designs and sample sizes, the two most rigorous published studies conducted to date (King et al. 2012; Svensson et al. 2013) provide consistent evidence of a role of RYGB in increasing alcohol use and risk of alcohol-related problems. Both also use a prospective longitudinal design and enroll large samples. Notably, these two studies were conducted by two independent teams and in two different geocultural areas, United States (King et al. 2012) and Sweden (Svensson et al. 2013). Nonetheless, the overall conflicting data suggest not only that additional clinical research is needed, but also that it is important to shed light on the possible mechanisms of how bariatric surgery may affect alcohol use. Initial preclinical work on the latter direction has been conducted, as is described next.
Table 1.
Summary of preclinical [P] and clinical [C] studies that have investigated the potential effects of bariatric surgery on alcohol consumption, risk for alcohol use disorder (AUD) and alcohol problems
| Studies that indicate increased alcohol consumption and/or risk for AUD and/or alcohol problems | Studies that indicate decreased alcohol consumption and/or risk for AUD and/or alcohol problems | Studies that indicate no change in alcohol consumption and/or risk for AUD and/or alcohol problems | |
|---|---|---|---|
|
Bariatric surgery (not otherwise specified) |
Buffington 2007 (C) Saules et al. 2010 (C) |
None | None |
| Roux-en-Y gastric bypass (RYGB) |
Mitchell et al. 2001 (C)* Ertelt et al. 2008 (C) Hajnal et al. 2012 (P) Ivezaj et al. 2012 (C) King et al. 2012 (C) Suzuki et al. 2012 (C) Thanos et al. 2012 (P) Conason et al. 2013 (C) Culler-Barboza et al. 2014 (C) Davis et al. 2013 (P) Östlund et al. 2013 (C) Polston et al. 2013 (P) Svensson et al. 2013 (C) Wee et al. 2013 (C) Pepino et al. 2015 (C) |
Mitchell et al. 2001 (C)* Davis et al. 2012 (P,C) Lent et al. 2013 (C) Wee et al. 2013 (C) Alfonsson et al. 2014 (C) de Araujo Burgos et al. 2015 (C) |
None |
| Restrictive Procedures |
Svensson et al. 2013 (C) Guisado Macias et al. 2003 (C) Östlund et al. 2013 (C) Wee et al. 2013 (C) |
Wee et al. 2013 (C) de Araujo Burgos et al. 2015 (C) |
Changchien et al. 2012 (C) King et al. 2012 (C) Conason et al. 2013 (C) Svensson et al. 2013 (C) |
Michell et al. (2001) reported increase in alcohol abuse but decrease in alcohol dependence with RYGB.
2.2. Preclinical studies
There have been a few preclinical studies examining the effects of RYGB on alcohol consumption in rats. A study assessed the effects of RYGB on ethanol consumption and reward in EtOH preferring (P) rats (Davis et al. 2012). The findings showed that RYGB resulted in reduced alcohol use in the EtOH preferring rats following RYGB surgery (Davis et al. 2012). In this study, the authors also investigated the potential role of feeding peptides in mediating these effects, i.e., glucagon-like peptide-1 (GLP-1) and ghrelin. The authors concluded that EtOH-induced increase in plasma GLP-1 levels may have a role in reduced EtOH consumption in P rats following RYGB. Nevertheless, a role for ghrelin could not be excluded as pharmacologic replacement of ghrelin restored EtOH drinking behavior in P rats that received RYGB (Davis et al. 2012).
In another set of studies from an independent laboratory, non-alcohol preferring outbred obese rats underwent RYGB and were habituated along with their sham-operated obese controls, and with lean rats, to increasing concentrations of EtOH in a two-bottle free-choice paradigm (Thanos et al. 2012). In contrast with Davis et al. (2012), this study found that RYGB rats’ daily consumption of EtOH was twice as high as sham-operated obese controls and 50% higher than normal-diet lean controls. Obese controls drank significantly less (approximately 50%) grams of ethanol per body weight, as compared to lean controls. Furthermore, the RYGB rats displayed a higher EtOH preference and intake during reinstatement of the higher concentration (8%) alcohol following periods of abstinence. An additional finding was that food restriction was not sufficient to increase EtOH consumption in the control (obese) rats. Furthermore, the reduced EtOH intake in obese versus lean rats suggests that adaptations during obesity may interfere with EtOH’s reinforcing effects and that their recovery after RYGB may underlie the increased risk for alcohol abuse after surgery. The fact that the highest EtOH intake in the RYGB animals was seen for the low concentrations (2%, 4%) may reflect enhanced sensitivity to the rewarding effects of low EtOH concentrations. This effect could also be due to faster EtOH absorption and a reduced metabolism of EtOH after the RYGB surgery.
In a follow up study directly investigating changes in alcohol reward (Hajnal et al., 2012), high fat diet-induced obese rats underwent RYGB or sham operation and were tested 4 months after surgery on a progressive ratio schedule of reinforcement operant task for 2, 4, and 8% EtOH. Compared to sham-operated controls, RYGB rats made significantly more efforts to earn access to alcohol reward, and also consumed more when it was available. This study also found that pretreatment with a single peripheral injection of a ghrelin receptor antagonist preferentially reduced seeking and consumption of EtOH in the RYGB rats, with the same doses of the drug being ineffective in the sham-operated obese group. This observation strongly suggests that increased sensitivity of the reward system to ghrelin following surgery may contribute to the effects of RYGB on alcohol drinking (Hajnal et al. 2012). Consistent with this study, an additional study that measured brain-glucose metabolism indicated activation of brain areas involved in reward expectation and sensory processing during anticipation of a palatable fatty food (Thanos et al. 2015). Thus, it appears that while the surgery ultimately reduces intake of rich (very sweet and fatty) foods, an effect reliably demonstrated both in humans and animals (Behary & Miras 2015; Brown et al. 1982; Halmi et al. 1981; Le Roux et al. 2011; Olbers et al. 2006), the brain areas activating anticipatory responses to rewarding cues remain on ‘high alert’. In fact, recent studies investigating taste functions and taste reward supports this notion, and suggests that reduced sweet and fat preferences following RYGB surgery are rather due to learned aversions to certain foods, than a primarily reduced reward function (Mathes et al. 2015a; Mathes et al. 2015b; Mathes et al. 2012). A similar mechanism may underlie improved food preferences (moving away from highly stimulating foods) and remission of food addiction (Pepino et al. 2014) following RYGB, and at the same time explain why an individual or an animal with persisting ‘hunger’ chose alternative (non-food) rewards such as alcohol to maintain stimulation of the reward system at a pre-operative level.
As changes in the gastrointestinal anatomy may affect EtOH’s absorption and metabolism (Hagedorn et al. 2007; Holt 2011; Klockhoff et al. 2002; Woodard et al. 2011), a question posed in the preclinical literature was whether this could be a factor playing a role on the changes in EtOH consumption seen in these animal models of RYGB. As such, a study was designed to investigate the effects of RYGB on EtOH administration given intravenously (IV), therefore bypassing the stomach (Polston et al. 2013). In this study, diet-induced obese male Sprague Dawley rats could administer IV EtOH approximately 2 months after RYGB or sham surgery, using both fixed and progressive ratio schedules of reinforcement. RYGB rats made significantly more licks on an empty active spout to earn EtOH from a second spout during the fixed ratio schedule, and also reached significantly higher breakpoints (i.e., completed more cycles) during the progressive ratio schedule sessions. These results suggest that RYGB increases the rewarding effects of EtOH independently from its effects on EtOH absorption (Polston et al. 2013).
Another study tested EtOH consumption using the two-bottle free-choice paradigm in Long-Evans rats that were maintained on a high-fat diet for an 8-week period to induce obesity (Davis et al. 2013). In conflict with Davis et al. (2012), but consistent with Hajnal et al. (2012), this study reinforced the notion that RYGB increases new onset EtOH intake, and that this effect is not merely a consequence of the significant weight loss (Davis et al. 2013). In fact, both rats that were obese prior to surgery and weight loss controls lost significant amounts of body weight; however, only rats undergoing RYGB surgery consumed larger amounts of EtOH compared to rats that received sham surgery (Davis et al. 2013). Additionally, no differences in BAC levels, measured 30 minutes following oral gavage of ethanol, were observed between RYGB and sham groups. This study also showed that the RYGB procedure was associated with changes in brain regions that regulate alcohol intake via orexin signaling; orexin, also called hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. Specifically, in the lateral hypothalamus, RYGB surgery significantly increased prepro-orexin expression while the expression of orexin 2 receptors was increased in the weight loss control condition, relative to both RYGB and sham surgery conditions. No significant effect of surgery or weight loss was observed in the expression of orexin 1 receptor, dopamine D1 receptor, or dopamine D2 receptor in the lateral hypothalamus. As recent evidence suggests that orexin peptides increase EtOH consumption in rodents (Lawrence et al. 2006), the authors hypothesized that the increased intake observed following RYGB may be regulated, at least in part, by an increased central orexin signaling. The authors also reported significant reductions in D1 receptor expression in the nucleus accumbens of animals subjected to RYGB, relative to sham surgery or weight-matched controls (Davis et al. 2013). Alcohol stimulates dopamine release in the nucleus accumbens (Di Chiara & Imperato 1986) and dopamine signaling in this region has been associated with the regulation of EtOH reward and dependence (Boileau et al. 2003; Koob et al. 1998). Together, these data suggest that RYGB induces transcriptional alterations within brain circuits known to regulate dopamine function, alcohol intake, and reward processes. Notably, decreases in the dopamine signaling in the mesoaccumbens dopamine pathway have been linked to increases in appetitive behaviors, as well as increases in subjective pleasure from the use of dopamine-enhancing substances (Bello et al. 2011; Bulwa et al. 2011; Volkow et al. 1999).
The preclinical studies investigating the potential effects of bariatric surgery on alcohol consumption have generated consistent results from independent laboratories demonstrating that RYGB surgery increases alcohol consumption in dietary obese rats with no prior history of alcohol exposure. In contrast, rats bred for preferring alcohol showed an opposite, reduced alcohol preference following the surgery. Although these differences are likely due to the different genetic background of the animal models used, it warrants evidence for expanding clinical research to include screening for genetic susceptibility factors (e.g. the presence of Taq1 A allele, which is associated with reduced dopamine signaling in reward processing, e.g.: (Blum et al. 2011)) in bariatric patients. In summary, the preclinical data is consistent with the majority of the clinical literature (section 2.1), and support the notion that bariatric surgery may represent a potential risk for increased alcohol use.
3. Possible mechanisms of action
As suggested by some clinical work (e.g.: (Hagedorn et al. 2007); Ostlund et al. (2013); (Woodard et al. 2011)), the increased alcohol use after bariatric surgery may be due to altered pharmacokinetics and metabolism after RYGB (Hagedorn et al. 2007; Holt 2011; Klockhoff et al. 2002; Woodard et al. 2011), which results in patients becoming intoxicated more quickly with less alcohol following surgery and taking longer to return to sobriety than before surgery (Woodard et al. 2011). It is also possible that the lower alcohol tolerance following surgery leads individuals to experience the rewarding properties of alcohol sooner and more frequently, which may then contribute to increased risk of AUD post-RYGB surgery. A number of mechanisms for changes in alcohol pharmacokinetics have been hypothesized. The rapid emptying of the gastric pouch may facilitate faster absorption of alcohol. Additionally, the reduced volume of the stomach results in less alcohol dehydrogenase, which partially metabolizes alcohol (Hagedorn et al. 2007).
Notably, RYGB-treated obese rats increase IV alcohol administration, thus using a procedure where the stomach is bypassed, suggesting that RYGB increases the rewarding effects of alcohol independent of its direct gastric effects or change in alcohol absorption after the surgery (Polston et al. 2013). In this regard, studies have shown that alcohol, drugs, and food trigger similar responses in the brain (for review, see: Volkow et al. (2011)). This seems consistent with the ‘symptom substitution theory’, according to which the elimination of one behavior, without treating the underlying cause, may lead to a replacement behavior (Kazdin 1982). The switch from excessive food intake to the escalation of drinking behavior could be related to an isocaloric set point in all patients. RYGB patients may be compensating for their inability to consume as many calories from food by accelerated drinking. Additionally, bariatric surgery may result in changes in reward processing and dopamine signaling that could play a role in the mechanisms involved in how the surgery alters alcohol-seeking behaviors. Foods that are high in sugar or fat cause an increase in dopamine, although not as dramatically as alcohol and drugs (Volkow & Wise 2005). Imaging studies with RYGB patients have demonstrated a decrease in dopamine D2 receptor availability in the ventral striatum and caudate nucleus after surgery (Dunn et al. 2010). Notably, these are brain areas involved with alcohol’s rewarding effects (Thanos et al. 2005) and associated with risk for alcohol abuse (Thanos et al. 2004; Thanos et al. 2001; Volkow et al. 2006).
The possibility that altered reward processing may play a key role, regardless of changes in alcohol absorption and pharmacokinetics/pharmacodynamics, becomes even more compelling taking into account recent literature suggesting that feeding-related peptides, mainly produced in the stomach and/or intestine, play a role in alcohol reward and dopamine signaling. For example, ghrelin is mainly produced by the stomach. Consistent with its main site of production, a significant reduction in peripheral (blood) ghrelin levels right after RYGB has been reported both in obese patients and in a rat model of RYGB (Korner et al. 2009; Shin et al. 2010). Ghrelin has also been implicated in reward-related behaviors and, more specifically, in the modulation of food intake and reward (Dickson et al. 2011). Additionally, preclinical work has shown that ghrelin may play a role in alcohol reward and consumption, and that ghrelin receptor antagonism blocks these behaviors (Gomez et al. 2015; Gomez & Ryabinin 2014; Jerlhag et al. 2009; Kaur & Ryabinin 2010; Landgren et al. 2012; Stevenson et al. 2015; Suchankova et al. 2013). Consistent with this preclinical work, clinical studies have reported changes in blood ghrelin levels in alcoholic patients versus controls and a positive correlation between blood levels of ghrelin and alcohol craving (Addolorato et al. 2006; Badaoui et al. 2008; Koopmann et al. 2012; Leggio et al. 2012). Notably, a recent human laboratory study with heavy drinking alcohol-dependent subjects demonstrated a causality link by showing that IV ghrelin infusion, compared to placebo, resulted in an acute increase in cue-induced craving for alcohol (Leggio et al. 2014). Taking into account this body of literature, it is possible to hypothesize that ghrelin signaling plays a role on the mechanisms how bariatric surgery and RYGB, in particular, may result in increased alcohol use. Consistent with this hypothesis, subthreshold doses of ghrelin receptor antagonism reduced alcohol consumption only in RYGB animals, while no similar effect was found in the sham-operated control group (Hajnal et al. 2012). Another appetitive peptide whose pathway has been recently implicated in alcohol-seeking behaviors is GLP-1 (Egecioglu et al. 2013; Engel & Jerlhag 2014a, b; Shirazi et al. 2013; Suchankova et al. 2015). In that regard, the study by Davis and colleagues suggests that GLP-1 signaling may also be involved in the mechanisms of how RYGB affects alcohol drinking (Davis et al. 2012). Recent studies in humans (Allen et al. 2013; Madsbad et al. 2014; Nguyen & Korner 2014) and rodents, however, suggest that altered GLP-1 signaling is not required either for diabetes resolution or weight loss (Mokadem et al. 2014; Ye et al. 2014), or altered food reward (Mathes et al. 2012). Figure 1 outlines a potential schematic of how ghrelin and GLP-1 may play a role in a plausible increase of reward-sensitivity following RYGB surgery in the context of increased alcohol use. However, future studies are needed on the effect of bariatric surgery on alcohol consumption and risk of AUD in order to address numerous unanswered questions, including (but not limited to): a) better understanding of the potential role and interplay of gut-liver-brain axis pathways like ghrelin and GLP-1; b) comparison of the effects of sleeve gastrectomy versus RYGB on hormones like ghrelin and GLP-1 and investigations on how different effects on these hormones may result in different effects on excessive alcohol use; c) research on the potential role of other gut-brain peptides (e.g. leptin, cholecystokinin, and others; for an extensive review: see: (Kenna et al. 2012)); and d) better understanding of the potential role of sex differences.
Figure 1. Scheme illustrating plausible mechanisms of increased alcohol reward following Roux-en-Y Gastric Bypass (RYGB) surgery.
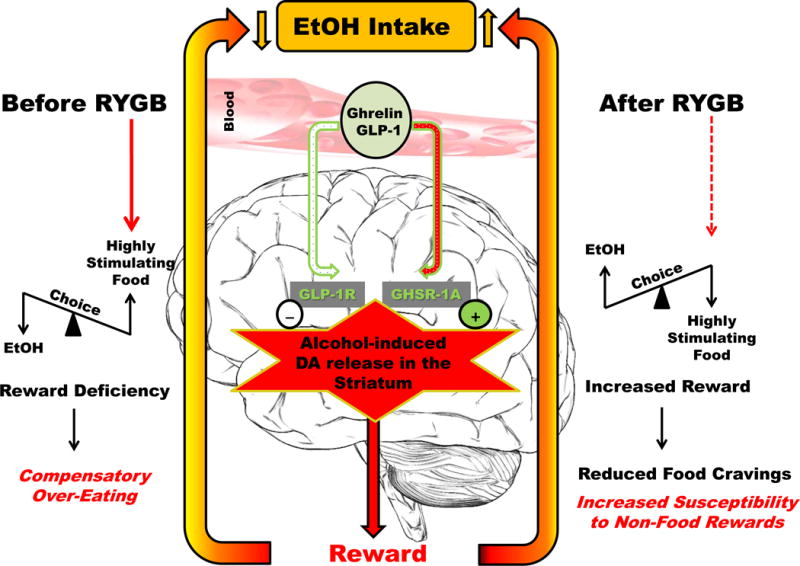
Consistent with preclinical evidence (see: Section 2.2 and Section 3), we propose that RYGB may reverse obesity-induced dysregulation of the dopamine (DA) reward processing, and in turn, may improve reward sensitivity. According to the widely accepted current theories on the cause of reward dysregulation in obesity (the ‘Reward Deficiency Syndrome’ and the ‘Hypodopaminergia Theory’), chronic stimulation of the dopamine reward signaling (e.g., by eating highly palatable foods and larger meals) may result in down-regulated signaling causing reduced reward sensitivity, driving the animals towards richer foods as compensation to maintain DA stimulation. Prior to RYGB (left), obese subjects prefer richer (i.e., more stimulating) foods, and consume larger quantities due to the reduced postprandial release of anorexigenic gastrointestinal hormones, e.g.: glucagon-like peptide-1 (GLP-1). Chronically reduced ghrelin levels (or a plausible inefficient signaling due to down-regulated ghrelin receptors) may also contribute to obesity-related DA deficits. Studies in dietary obese rats have found increased seeking of higher concentrations of sucrose solutions, but also shown reduced preference for, and intake of, low concentrations of sucrose and alcohol. Dietary obese animals also have reduced ghrelin levels, a finding that is consistent with reduced alcohol preference. Therefore, such conditions result in a finely-balanced relationship favoring highly stimulating food over alcohol in high-fat diet-induced obese rats. This relationship is depicted as a see-saw on the left in Fig 1. This balance is reversed following RYGB as shown in the figure (right). Thus, increased reward sensitivity, in part due to increased ghrelin signaling, together with increased anorexigenic hormone feed-back (e.g., increased postprandial GLP-1), and learned food aversion (e.g., due to malabsorption and gastrointestinal discomfort ‘dumping’) may help improving food choices and reducing cravings for rich foods after RYGB (right). In contrast, RYGB may create an increased sensitivity to alternative (non-food) rewards, such as alcohol, that are not controlled by the same distributed anorexigenic homeostatic systems. Consistent with this hypothesis, human studies also show a specific effect of ghrelin depending on the population being studied. In fact, while studies with obese patients show effects on ghrelin in increasing appetite and food intake, a recent human study in non-obese (BMI < 30 kg/m2) heavy drinking alcohol-dependent subjects shows that intravenous administration of ghrelin increases the urge to drink alcohol (assessed as cue-induced alcohol craving), with no effect on the urge to drink a non-alcoholic sweet beverage or on food craving. Please note, this figure only illustrates possible mechanisms of action inferred based on peripheral changes in gut-liver-brain hormones. Although it is reasonable to assume an effect of GLP-1 regulation of DA reward processing opposite to ghrelin, the effect of GLP-1 on the ventral tegmental area (VTA) is likely to be indirect via the nucleus of the tract solitary, and is unlikely to be related to well-characterized changes of GLP-1 levels in the periphery.
4. Conclusions
Most of the published literature on the role of bariatric surgery in alcohol use and AUD suggest that bariatric surgery patients, especially after RYGB, may be at risk of alcohol use problems. The American Society for Metabolic and Bariatric Surgery guidelines recommend that high risk patients (i.e., those with a history of substance abuse, regular alcohol use pre-surgery, or RYGB patients) abstain from alcohol use after gastric bypass given the altered metabolism of alcohol, as well as the potential for alcohol use disorders after surgery.
However, many questions remain unanswered. Among them, a critical question is whether the increase in alcohol consumption is a consequence of an adjustment for caloric intake, and/or whether this increase may be due to higher alcohol reward substituting for reduced reward from previously highly-preferred, rich food items. From a clinical standpoint, even before the underlying mechanism is fully understood, the priority of future research is to identify the risk factors for the development of AUD among post-bariatric surgery patients. Adequate screening, assessment, and pre-operative preparation could help mitigate this risk. Unfortunately to date, the most significant clinical limitation is the lack of a good understanding of who is at risk for developing alcohol-related problems after RYGB, and what care is needed for this unique population. In humans, it is also important to consider social factors (e.g., smoking and other substance use disorders, depression, anxiety, and quality of life) that may play a role in the increased ethanol consumption following RYGB. In addition to the crucial need for additional translational research on this topic, it is important to carefully screen candidates for bariatric surgery in order to identify patients that may be potentially at risk of increased risk of AUD after surgery. Additionally, investigating the potential mechanisms of how bariatric surgery may result in increased alcohol use will be important, not only to better understand the relationship between bariatric surgery and alcohol use, but also to identify new possible pathways that may represent new pharmacological targets for the treatment of AUD.
Acknowledgments
The authors would like to thank Ms. Karen Smith, National Institutes of Health (NIH) Library for bibliographic assistance.
ANB and LL were supported by NIH intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Leggio) jointly supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program of the National Institute on Drug Abuse (NIDA). AH was supported by NIH grant AA024490, and by a grant from the Pennsylvania Department of Health using Tobacco CURE Funds (SAP # 4100068724).
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Authors Contribution
ANB was responsible for drafting the manuscript. AH and LL provided critical revision of the manuscript, contributing important intellectual content. All authors critically reviewed content and approved final version for publication.
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest related to this research.
References
- Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol Clin Exp Res. 2006;30:1933–1937. doi: 10.1111/j.1530-0277.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Alfonsson S, Sundbom M, Ghaderi A. Is age a better predictor of weight loss one year after gastric bypass than symptoms of disordered eating, depression, adult ADHD and alcohol consumption? Eat Behav. 2014;15:644–647. doi: 10.1016/j.eatbeh.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Allen RE, Hughes TD, Ng JL, Ortiz RD, Ghantous MA, Bouhali O, et al. Mechanisms behind the immediate effects of Roux-en-Y gastric bypass surgery on type 2 diabetes. Theor Biol Med Model. 2013;10:45. doi: 10.1186/1742-4682-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Press; Washington, D.C.: 2013. [Google Scholar]
- Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Starkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. Eur J Clin Invest. 2008;38:397–403. doi: 10.1111/j.1365-2362.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–507. [PubMed] [Google Scholar]
- Behary P, Miras AD. Food preferences and underlying mechanisms after bariatric surgery. Proc Nutr Soc. 2015;74:419–425. doi: 10.1017/S0029665115002074. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen AL, Oscar-Berman M, Chen TJ, Lubar J, White N, et al. Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: selecting appropriate phenotypes for reward dependence behaviors. International Journal of Environmental Research and Public Health. 2011;8:4425–4459. doi: 10.3390/ijerph8124425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Brown EK, Settle EA, Van Rij AM. Food intake patterns of gastric bypass patients. J Am Diet Assoc. 1982;80:437–443. [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- Buffington CK. Alcohol Use and Health Risks: Survey Results. Bariatric Times. 2007;4:21–23. [Google Scholar]
- Bulwa ZB, Sharlin JA, Clark PJ, Bhattacharya TK, Kilby CN, Wang Y, et al. Increased consumption of ethanol and sugar water in mice lacking the dopamine D2 long receptor. Alcohol. 2011;45:631–639. doi: 10.1016/j.alcohol.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. State-specific prevalence of obesity among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:985–988. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Defining Adult Overweight and Obesity. 2011. [Google Scholar]
- Changchien EM, Woodard GA, Hernandez-Boussard T, Morton JM. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J Am Coll Surg. 2012;215:475–479. doi: 10.1016/j.jamcollsurg.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Clark MM, Balsiger BM, Sletten CD, Dahlman KL, Ames G, Williams DE, et al. Psychosocial factors and 2-year outcome following bariatric surgery for weight loss. Obes Surg. 2003;13:739–745. doi: 10.1381/096089203322509318. [DOI] [PubMed] [Google Scholar]
- Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148:145–150. doi: 10.1001/2013.jamasurg.265. [DOI] [PubMed] [Google Scholar]
- Cuellar-Barboza AB, Frye MA, Grothe K, Prieto ML, Schneekloth TD, Loukianova LL, et al. Change in consumption patterns for treatment-seeking patients with alcohol use disorder post-bariatric surgery. J Psychosom Res. 2015;78:199–204. doi: 10.1016/j.jpsychores.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol Psychiatry. 2012;72:354–360. doi: 10.1016/j.biopsych.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Magrisso IJ, Grayson BE, Seeley RJ, et al. Roux en Y gastric bypass increases ethanol intake in the rat. Obes Surg. 2013;23:920–930. doi: 10.1007/s11695-013-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo Burgos MG, Cabral PC, Maio R, Oliveira BM, Dias MS, de Figueiredo Melim DB, et al. Prevalence of Alcohol Abuse Before and After Bariatric Surgery Associated With Nutritional and Lifestyle Factors: A Study Involving a Portuguese Population. Obes Surg. 2015;25:1716–1722. doi: 10.1007/s11695-015-1609-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann N Y Acad Sci. 1986;473:367–381. doi: 10.1111/j.1749-6632.1986.tb23629.x. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Research. 2010;1350:123–130. doi: 10.1016/j.brainres.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013;38:1259–1270. doi: 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E. Alcohol: mechanisms along the mesolimbic dopamine system. Prog Brain Res. 2014a;211:201–233. doi: 10.1016/B978-0-444-63425-2.00009-X. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E. Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014b;28:875–886. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surgery for Obesity and Related Diseases. 2008;4:647–650. doi: 10.1016/j.soard.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184:9S–16S. doi: 10.1016/s0002-9610(02)01173-x. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Gallo AS, Berducci MA, Nijhawan S, Nino DF, Broderick RC, Harnsberger CR, et al. Alcohol metabolism is not affected by sleeve gastrectomy. Surg Endosc. 2015;29:1088–1093. doi: 10.1007/s00464-014-3790-5. [DOI] [PubMed] [Google Scholar]
- Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009;19:1447–1455. doi: 10.1007/s11695-009-9927-2. [DOI] [PubMed] [Google Scholar]
- Gilbert EW, Wolfe BM. Bariatric surgery for the management of obesity: state of the field. Plast Reconstr Surg. 2012;130:948–954. doi: 10.1097/PRS.0b013e318262f566. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Cunningham CL, Finn DA, Young EA, Helpenstell LK, Schuette LM, et al. Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence. Neuropharmacology. 2015;97:182–193. doi: 10.1016/j.neuropharm.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Ryabinin AE. The effects of ghrelin antagonists [D-Lys(3) ]-GHRP-6 or JMV2959 on ethanol, water, and food intake in C57BL/6J mice. Alcohol Clin Exp Res. 2014;38:2436–2444. doi: 10.1111/acer.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado Macias JA, Vaz Leal FJ. Psychopathological differences between morbidly obese binge eaters and non-binge eaters after bariatric surgery. Eat Weight Disord. 2003;8:315–318. doi: 10.1007/BF03325032. [DOI] [PubMed] [Google Scholar]
- Hagedorn JC, Encarnacion B, Brat GA, Morton JM. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis. 2007;3:543–548. doi: 10.1016/j.soard.2007.07.003. discussion 548. [DOI] [PubMed] [Google Scholar]
- Hainer V, Aldhoon-Hainerova I. Tolerability and safety of the new anti-obesity medications. Drug Saf. 2014;37:693–702. doi: 10.1007/s40264-014-0206-3. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, et al. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One. 2012;7:e49121. doi: 10.1371/journal.pone.0049121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5:457–464. [PubMed] [Google Scholar]
- Heinberg LJ, Ashton K. History of substance abuse relates to improved postbariatric body mass index outcomes. Surg Obes Relat Dis. 2010;6:417–421. doi: 10.1016/j.soard.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Holt PR. Changes in alcohol metabolism after gastric bypass surgery. Lancet. 2011;378:767–768. doi: 10.1016/S0140-6736(11)61372-X. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Symptom substitution, generalization, and response covariation: implications for psychotherapy outcome. Psychol Bull. 1982;91:349–365. [PubMed] [Google Scholar]
- Kenna GA, Swift RM, Hillemacher T, Leggio L. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev. 2012;22:211–228. doi: 10.1007/s11065-012-9209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–2525. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockhoff H, Naslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54:587–591. doi: 10.1046/j.1365-2125.2002.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K, et al. The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology. 2012;37:980–986. doi: 10.1016/j.psyneuen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk JL, Ardern CI, Church TS, Hebert JR, Sui X, Blair SN. Ideal weight and weight satisfaction: association with health practices. Am J Epidemiol. 2009;170:456–463. doi: 10.1093/aje/kwp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats. Addict Biol. 2012;17:86–94. doi: 10.1111/j.1369-1600.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, et al. Gastric bypass reduces fat intake and preference. American Journal of Physiology РRegulatory, Integrative and Comparative Physiology. 2011 doi: 10.1152/ajpregu.00139.2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17:452–464. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, et al. Intravenous Ghrelin Administration Increases Alcohol Craving in Alcohol-Dependent Heavy Drinkers: A Preliminary Investigation. Biol Psychiatry. 2014;76:734–741. doi: 10.1016/j.biopsych.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lent MR, Hayes SM, Wood GC, Napolitano MA, Argyropoulos G, Gerhard GS, et al. Smoking and alcohol use in gastric bypass patients. Eat Behav. 2013;14:460–463. doi: 10.1016/j.eatbeh.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Menzo E, Szomstein S, Rosenthal RJ. Changing Trends in Bariatric Surgery. Scand J Surg. 2015;104:18–23. doi: 10.1177/1457496914552344. [DOI] [PubMed] [Google Scholar]
- Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152–164. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- Malinoff RL, Elliott MN, Giordano LA, Grace SC, Burroughs JN. Obesity utilization and health-related quality of life in Medicare enrollees. J Ambul Care Manage. 2013;36:61–71. doi: 10.1097/JAC.0b013e31826746bf. [DOI] [PubMed] [Google Scholar]
- Maluenda F, Csendes A, De Aretxabala X, Poniachik J, Salvo K, Delgado I, et al. Alcohol absorption modification after a laparoscopic sleeve gastrectomy due to obesity. Obes Surg. 2010;20:744–748. doi: 10.1007/s11695-010-0136-9. [DOI] [PubMed] [Google Scholar]
- Mathes CM, Bohnenkamp RA, Blonde GD, Letourneau C, Corteville C, Bueter M, et al. Gastric bypass in rats does not decrease appetitive behavior towards sweet or fatty fluids despite blunting preferential intake of sugar and fat. Physiol Behav. 2015a;142:179–188. doi: 10.1016/j.physbeh.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes CM, Bohnenkamp RA, le Roux CW, Spector AC. Reduced sweet and fatty fluid intake after Roux-en-Y gastric bypass in rats is dependent on experience without change in stimulus motivational potency. Am J Physiol Regul Integr Comp Physiol. 2015b;309:R864–874. doi: 10.1152/ajpregu.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol. 2012;302:R751–767. doi: 10.1152/ajpregu.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Lancaster KL, Burgard MA, Howell LM, Krahn DD, Crosby RD, et al. Long-term follow-up of patients’ status after gastric bypass. Obes Surg. 2001;11:464–468. doi: 10.1381/096089201321209341. [DOI] [PubMed] [Google Scholar]
- Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2014;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute. Classification of overweight and obesity by BMI, waist circumference, and associated disease risks 2015 [Google Scholar]
- Nguyen KT, Korner J. The sum of many parts: potential mechanisms for improvement in glucose homeostasis after bariatric surgery. Curr Diab Rep. 2014;14:481. doi: 10.1007/s11892-014-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, Independent Scientific Committee on Drugs Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349–356. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148:374–377. doi: 10.1001/jamasurg.2013.700. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K, Klein S. Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4. JAMA Surg. 2015;150:1096–1098. doi: 10.1001/jamasurg.2015.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Stein RI, Eagon JC, Klein S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston JE, Pritchett CE, Tomasko JM, Rogers AM, Leggio L, Thanos PK, et al. Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats. PLoS One. 2013;8:e83741. doi: 10.1371/journal.pone.0083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. Journal of Hepatology. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Reis JP, Araneta MR, Wingard DL, Macera CA, Lindsay SP, Marshall SJ. Overall obesity and abdominal adiposity as predictors of mortality in u.s. White and black adults. Ann Epidemiol. 2009;19:134–142. doi: 10.1016/j.annepidem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Saules KK, Wiedemann A, Ivezaj V, Hopper JA, Foster-Hartsfield J, Schwarz D. Bariatric surgery history among substance abuse treatment patients: prevalence and associated features. Surg Obes Relat Dis. 2010;6:615–621. doi: 10.1016/j.soard.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Sears D, Fillmore G, Bui M, Rodriguez J. Evaluation of gastric bypass patients 1 year after surgery: changes in quality of life and obesity-related conditions. Obes Surg. 2008;18:1522–1525. doi: 10.1007/s11695-008-9604-x. [DOI] [PubMed] [Google Scholar]
- Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53:238–252. doi: 10.1093/oxfordjournals.bmb.a011611. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Mueller S. Alcohol and cancer: an overview with special emphasis on the role of acetaldehyde and cytochrome P450 2E1. Adv Exp Med Biol. 2015;815:59–70. doi: 10.1007/978-3-319-09614-8_4. [DOI] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One. 2013;8:e61965. doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen KJ, Engel SG, Pollert GA, Li C, Mitchell JE. Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:470–473. doi: 10.1016/j.soard.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Buirkle JM, Buckley LE, Young KA, Albertini KM, Bohidar AE. GHS-R1A antagonism reduces alcohol but not sucrose preference in prairie voles. Physiol Behav. 2015;147:23–29. doi: 10.1016/j.physbeh.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PLoS One. 2013;8:e71284. doi: 10.1371/journal.pone.0071284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry. 2015;5:e583. doi: 10.1038/tp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes Surg. 2012;22:201–207. doi: 10.1007/s11695-010-0346-1. [DOI] [PubMed] [Google Scholar]
- Svensson PA, Anveden A, Romeo S, Peltonen M, Ahlin S, Burza MA, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity (Silver Spring) 2013;21:2444–2451. doi: 10.1002/oby.20397. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Subrize M, Miller ML, Bellezza R, Cooney RN, et al. Roux-en-Y Gastric Bypass Alters Brain Activity in Regions that Underlie Reward and Taste Perception. PLoS One. 2015;10:e0125570. doi: 10.1371/journal.pone.0125570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H, et al. Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. 2005;77:130–139. doi: 10.1016/j.lfs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Subrize M, Delis F, Cooney RN, Culnan D, Sun M, et al. Gastric bypass increases ethanol and water consumption in diet-induced obese rats. Obes Surg. 2012;22:1884–1892. doi: 10.1007/s11695-012-0749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, et al. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wee CC, Mukamal KJ, Huskey KW, Davis RB, Colten ME, Bolcic-Jankovic D, et al. High-risk alcohol use after weight loss surgery. Surg Obes Relat Dis. 2014;10:508–513. doi: 10.1016/j.soard.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–239. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- Woodard GA, Downey J, Hernandez-Boussard T, Morton JM. Impaired alcohol metabolism after gastric bypass surgery: a case-crossover trial. J Am Coll Surg. 2011;212:209–214. doi: 10.1016/j.jamcollsurg.2010.09.020. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO | 10 facts on obesity 2015 [Google Scholar]
- Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306:R352–362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


