Abstract
Introduction
A clearer understanding of the factors affecting the cure rate of Helicobacter pylori infection might lead to the development of novel prevention strategies and therapeutic targets.
Areas covered
This review covers two important issues that affect the eradication of H. pylori: bacterial and host factors. Several virulence factors have been shown to be predictors for gastroduodenal diseases. Successful treatment of H. pylori infection also depends on host genetic factors such as cytochrome P450 2C19 (CYP2C19) and interleukin (IL)-1B. The latest evidence on host genetic factors is discussed.
Expert opinion
The authors identify three main targets for achieving effective eradication therapy. The first therapeutic target is to identify counter measures for antibiotic-resistant H. pylori strains. Thus, antibiotic susceptibility should be checked in all patients, ideally, before the start of eradication treatment. The second therapeutic target is the inhibition of acid suppression. Maintaining a high intragastric pH for 24 hours increases the effectiveness of some antibiotics and the eradication effects for H. pylori. The third therapeutic target is to identify high-risk groups; the CYP2C19 and IL-1B polymorphisms are candidates for significant risk factors. A personalized medical approach will likely increase the cure rate of H. pylori infection.
Keywords: antibiotic resistance, cytochrome P450 2C19, Helicobacter pylori, interleukin 1B, virulence factors
1. Introduction
Helicobacter pylori is a gram-negative bacterium that colonizes the gastric epithelium of humans. It is one of the most important human pathogens that are involved in the pathogenesis of atrophic gastritis, gastroduodenal ulcers, gastric cancer, MALT lymphoma, idiopathic thrombocytopenic purpura, iron deficiency anemia, and vitamin B12 deficiency. The prevalence of H. pylori infection is >50% worldwide and 63–94% in developing countries [1]. Although it has decreased in developed countries through advanced diagnosis and eradication therapy, the rate of infected patients remains at 27.5–32.5% [2,3]. More progress towards worldwide H. pylori elimination needs to be made. In February 2013, the Japanese government approved diagnostic testing and eradication therapy for all H. pylori-positive patients confirmed by endoscopy [4].
Although triple therapy with a proton pump inhibitor (PPI), clarithromycin, and amoxicillin or metronidazole have been used as the first line treatment for H. pylori infections, the American College of Gastroenterology suggested that the cure rates were 70–85% in 2007 [5]. Additionally, recent systematic review showed that the cure rates of sequential and standard triple therapy were 84.1% and 75.1%, respectively [6]. The most important factor affecting H. pylori cure rates is the antibiotic resistance of H. pylori strains. The number of H. pylori strains that are resistant to antibiotics is increasing. The cure rate of patients were co-infected with clarithromycin-and metronidazole-resistant strains has been reported to be around 37% (16.2–60.7%) [7].
H. pylori contains several virulence factors, including cytotoxin-associated gene A product (CagA), vacuolating cytotoxin A (VacA), duodenal ulcer promoting gene A product (DupA), outer inflammatory protein A (OipA), and blood group antigen binding adhesin (BabA). These factors affect gastric mucosal inflammation and injury by activating inflammatory cell infiltration. They are predictors of gastric atrophy, intestinal metaplasia, and severe clinical outcomes [8]. Virulence factors also play important roles in gastric mucosal injury and are thus thought to affect the cure rates of H. pylori infection [9].
In addition, successful treatment of H. pylori infection depends on host genetic factors such as cytochrome P450 2C19 (CYP2C19), interleukin 1B (IL-1B), and multidrug-resistant transporter-1 (MDR1). Although PPIs are indispensable for eradication, the effect of PPIs is related to CYP2C19 genetic polymorphisms [10]. In this review, we summarize eradication therapy strategies for H. pylori infection from the viewpoint of bacterial and host factors.
2. Bacterial factors
2.1 Antibiotic resistance
Clarithromycin-containing triple therapy (PPI twice daily in combination with 2 antibiotics: 200–500 mg clarithromycin and 750–1000 mg amoxicillin or 400 mg metronidazole) for 7–14 days is recommended by several guidelines [5,11,12]. However the cure rates of H. pylori infection have declined to 75% in the United States and Europe and 70–75% in China and Korea [13]. Moreover, although prolonged duration of the therapy became 14-days, the cure rate was still poor (70%) [14].
Increasing antibiotic resistance rates of H. pylori strains due to the improper usage of antibiotics are thought to be one of the main reasons for the decrease in cure rates. The frequent use of clarithromycin results in resistant bacteria. In Europe, the highest clarithromycin resistance rates; more than 30%, have been reported in Austria, Hungary and Portugal. In contrast, low resistance rate of < 10% have been observed in Northern Europe [15].
This might be due to differences in prescriptions for infectious diseases in these countries. High resistance rates to clarithromycin have been reported in Japan and China (22.7% and 32%, respectively). The resistance rates in both countries increased to >10% in the last decade [16,17]. To address the increased prevalence of clarithromycin resistance, new guidelines have been published in Europe. These recommend choosing eradication therapies based on resistance rates [18]. In regions with low clarithromycin resistance rates (≤20%), standard therapy containing clarithromycin is still allowed as first line therapy; however, it should be avoided in regions with high clarithromycin resistance rates (>20%) [18].
The antimicrobial effects of clarithromycin are mediated through binding of the compound to the 50S ribosomal subunit, preventing the bacterial ribosome from translating its messenger RNAs to synthesize new proteins. Three point mutations in the peptidyltransferase region of domain V of the 23S ribosomal RNA (rRNA) are responsible for more than 90% of clarithromycin–resistant strains. They include substitutions of adenine to guanine at position 2143 (A2143G) and those from adenine to guanine or cytosine at position 2142 (A2142G or A2142C) [19]. Novel mutations related to single mutations in infB or ribosomal protein L 22 (rpl22) (either a 9 base pairs [bp] insertion or a 3 bp deletion) have been shown to have synergic effects with mutations in 23S rRNA [20]. In Japan, tailored eradication treatment has been attempted. Clarithromycin susceptibility was analyzed by PCR before the start of eradication therapy; based on these results, the patients were treated by a suitable regimen containing either clarithromycin or metronidazole [21]. The overall cure rate in this study was 96.7% (95% CI 92.5–98.9), and the one of patients infected with clarithromycin-resistant H. pylori strains was over 95% after checking the susceptibility since metronidazole was used in their treatment regimen.
The number of metronidazole-resistant H. pylori strains has also increased. For the past decades, the prevalence of metronidazole-resistant strains has been around 50% in Latin America [22]. The highest resistance rate (83%) was observed in Colombia. In the US and all Europe countries, resistance rates of 20–30% and 28.6–3.8% were reported, respectively [15,23,24]. High resistance rates have also been reported in Asia, in particular in China and Korea (63.9% and 49.6%, respectively) [17,25].
Fluoroquinolones have been the leading choice to solve H. pylori antibiotic resistance [18,26,27]. The resistance rate of H. pylori strains to this antibiotic is low compared to the two above-mentioned drugs. However, fluoroquinolone-containing regimens cannot replace all eradication therapies or first line therapies, because resistance rates differ by region [28].
The key factor to achieve successful eradication is assessing antibiotic susceptibility. Regional differences in antibiotic susceptibility have been reported. As the prescriptions of antibiotics differ by area, age, country, and background, resistant bacteria strains also differ. The use of inappropriate antibiotics contributes to the creation of novel resistant pathogens. Thus, the best way to achieve effective eradication is to select an appropriate drug by checking the presence or absence of resistant bacteria in individual patients. If this is not possible, the antibiotic should be selected in consideration of the resistance characteristics in the patient’s region of residence.
2.2 Virulence factors
Characterizing the pathogenesis of H. pylori infections, in particular, the interaction between virulence factors in the organism and host genetics, is crucial. Although gastric cancer and duodenal ulcer are at the opposite ends of the disease spectrum, H. pylori infection promotes the development of both diseases. A clearer understanding of how H. pylori infection remains latent in the majority of infected patients and why only the minority ever develops a severe disease might lead to the development of new preventive approaches and novel therapeutic targets.
2.2.1 Cytotoxin-associated gene A product (CagA)
CagA is located at one end of the cag pathogenicity island (PAI) and encodes CagA, a 120–145-kDa immunodominant protein [29]. CagA is injected into gastric epithelial cells through the type IV secretion system (T4SS), which is an extracellular structure for transferring nucleic acids and proteins that is encoded by the cag PAI. CagA mimics a host cell protein and binds and activates multiple signaling factors [30]. Tyrosine phosphorylation by Src family kinases, which undergoes translocated CagA, is characterized by the presence of a Glu-Pro-Ile-Tyr-Ala (EPIYA) motif. Different tyrosine phosphorylation motifs are associated with different binding abilities of the Src homology 2 domain of the Src homology 2-containing protein-tyrosine phosphatase (SHP-2) and induced morphological changes in epithelial cells [31].
Based on proprietary cagA gene, H. pylori can be categorized as cagA-positive or -negative. In transgenic mice, wild-type CagA induced gastric epithelial hyperplasia, gastric polyps, and adenocarcinoma [32]. Similarly, Mongolian gerbils infected with wild-type CagA developed inflammation, gastric dysplasia, and gastric cancer [33]. CagA expression in cagA-positive H. pylori strains has shown to be associated with the host inflammatory response and an increased risk for clinical outcomes [34]. Recently, a meta-analysis of 16 studies showed that patients infected with cagA-positive strains were at increased risk for non-cardiac gastric cancer (OR 2.01, 95%CI 1.21–3.32) [35].
The key between cagA and eradication is gastric mucosal inflammation. Increased blood flow caused by severe mucosal inflammation is thought to improve the flow of antibiotics [36]. In 1997, it was first reported that the presence of cagA gene caused a difference in cure rates (Table 1). The study indicated that the cure rate of patients infected with cagA-positive strains was significantly higher than that of patients infected with cagA-negative strains (cagA-positive, 73%; cagA-negative, 52%; p = 0.017). However, several important issues are pointed out in Table 1: 1) Sample sizes were small in several studies; 2) Different regions were studied; 3) Different treatment regimens were used or the duration of treatment was different; and 4) The background was different. However, even when these issues are taken into account and based on the data presented in the Table, the cure rates in patients infected with cagA-negative H. pylori strains were still lower than that of patients infected with cagA-positive strains (cagA-positive, 83%; cagA-negative, 69%; p < 0.01) (Table 1).
Table 1.
Cure rates for H. pylori infections by cagA status
| Authors | Year | Country |
cagA diagnosis |
Regimen, duration (d) |
Cure ratecagA+ (n) |
Cure ratecagA− (n) |
P value |
|---|---|---|---|---|---|---|---|
| van der Hulst et al. | 1997 | Netherlands | PCR | OA, 14 | 73% (89/122) | 52% (17/33) | 0.02* |
| Greenberg et al. | 1999 | USA | WB | OC, 14 | 65% (22/34) | 100% (10/10) | 0.08 |
| Lopez-Brea et al. | 1999 | Spain | PCR | BAM | 75% (6/8) | 75% (18/24) | >0.05 |
| van Doorn et al. | 2000 | Netherlands | PCR | LBTM, 4 or 5 | 81% (48/59) | 50% (19/38) | <0.01* |
| Broutet et al. | 2001 | France | PCR | PAC | 76% (64/84) | 63% (45/72) | 0.07 |
| Sarc et al. | 2001 | Turkey | IgG | LAC, 7 | 87% (111/127) | 72% (41/57) | 0.02* |
| Rudi et al. | 2002 | Germany | PCR | PPI+AC or CM, 7 | 89% (73/82) | 79% (26/33) | 0.15 |
| Queiroz et al. | 2002 | Brazil | PCR | PCF, 7 | One arm | 75% (17/20) | One arm |
| Scholte et al. | 2002 | Netherland | PCR | OAC, 7 | 100% (10/10) | 81% (13/16) | 0.15 |
| Treiber et al. | 2002 | Germany | PCR | PPI+ACM, 3 or 5 | 91% (147/161) | 87% (61/70) | 0.33 |
| De Francesco et al. | 2002 | Italy | PCR | RA+RCT, 10 | 87% (27/31) | 86% (24/28) | >0.05 |
| Chaudhuri et al. | 2003 | India | PCR | OAC, 10 | 60% (25/42) | 60% (3/5) | >0.05 |
| Russo et al. | 2003 | Italy | PCR | LAC, 7 | 76% (69/91) | 42% (8/19) | <0.01* |
| Xia et al. | 2003 | Australia | IgG | OAC, 7 | 88% (63/72) | One arm | One arm |
| De Francesco et al. | 2004 | Italy | PCR | RA+RCT or RAC, 10 | 93% (68/73) | 77% (12/22) | <0.05* |
| Zhao et al. | 2007 | China | PCR | EAC | 93% (54/58) | 38% (3/8) | <0.01* |
| Total | 83% (813/982) | 69% (300/435) | <0.01* | ||||
Data are based on per-protocol (PP) analyses.
Significantly different
Total: Excluding single-arm study
Eradication duration were not shown
P >0.05: No detailed data were available; the author described the results as not significant.
A, amxicillin; R, rabeprazole; AC, amoxicillin and clarithromycin; ACM, bismuth, clarithromycin, and metronidazole; BAM, bismuth, amoxicillin, and metronidazole; CM, clarithromycin and metronidazole; CT, clarithromycin and tinidazole; EAC, esomeprazole, amoxicillin, and clarithromycin; LAC, lansoprazole, amoxicillin, and clarithromycin; LBTM, lansoprazole, bismuth, tetracycline, and metronidazole; OA, omeprazole and amoxicillin; OAC, omeprazole, amoxicillin, and clarithromycin; OC, omeprazole and clarithromycin; PAC, pantoprazole, amoxicillin, and clarithromycin; PAM, pantoprazole, amoxicillin, and metronidazole; PFC, pantoprazole, furazolidone, and clarithromycin; PPI, proton pump inhibitor; RA, rabeprazole and amoxicillin; RAC, rabeprazole, amoxicillin, and clarithromycin; RCT, rabeprazole, clarithromycin, and tinidazole
2.2.2 Vacuolating cytotoxin A (VacA)
VacA is a cytotoxin secreted by bacteria. It enters host cells through endocytosis, thereby inducing multiple cellular activities such as the alteration of membrane permeability, ultimately resulting in apoptosis with vacuolation via autophagy [37]. All H. pylori strains possess the vacA gene, but vacuolating activity differs among strains. The gene encoding vacA displays allelic diversity, including signal (s) regions s1 and s2 and middle (m) regions m1 and m2. Based on in vitro experiments, s1m1 strains are the most cytotoxic strains as they consistently induce cell vacuolation. They are followed by s1m2 strains (cell vacuolation not consistently induced) and s2m2 strains that have no cytotoxic activity because they fail to induce cell vacuolation [38]. In agreement with in vitro data, significant associations between vacA s1 and vacA m1 genotypes and peptic ulcer or gastric carcinoma prevalence was shown in several countries [39].
VacA s1 strains have a significant correlation with the presence of cagA (r = 0.87) [40]. In spite of the concordance a theory that a high cure rate is related with highly virulence factors, several studies indicated that H. pylori vacA s2 strains were difficult to cure with significant differences (Table 2). Based on all the data reported in Table 2, the overall cure rate of patients infected with s2 strains was significantly lower than that of those infected with s1 strains (s2, 81%; s1; 72%; P = 0.02). On the other hand, there was no significant difference in the cure rate of patients infected with m1 and m2 strains. Table 2 includes a list of previous studies based on the vacA status and H. pylori eradication success.
Table 2.
H. pylori cure rates by vacA
| Authors | Year | Country |
vacA Diagnosis |
Regimen, duration (d) |
vacAtype | Cure rate (n) | P value |
|---|---|---|---|---|---|---|---|
| Lopez-Brea et al. | 1999 | Spain | PCR | BAM** | s1 vs. s2 | 50% (3/6) vs. 80% (21/26) | >0.05 |
| van Doorn et al. | 2000 | Netherlands | PCR | LBTM, 4 or 5 | s1 vs. s2 | 75% (56/75) vs. 50% (11/22) | 0.03* |
| Rudi et al. | 2002 | Germany | PCR | PPI+AC or CM, 7 | s1 vs. s2 | 87% (80/92) vs. 83% (19/23) | 0.59 |
| Scholte et al. | 2002 | Netherland | PCR | OAC, 7 | s1 vs. s2 | 100% (11/11) vs. 85% (11/13) | 0.17 |
| Chaudhuri et al. | 2003 | India | PCR | OAC, 10 | s1 vs. s2 | 62% (26/42) vs. 40% (2/5) | >0.05 |
| Russo et al. | 2003 | Italy | PCR | LAC, 7 | s1 vs. s2 | 77% (67/87) vs. 43% (9/21) | <0.01* |
| De Francesco et al. | 2004 | Italy | PCR | RA+RCT or RAC, 10 |
s1 vs. s2 | 91% (40/44) vs. 90% (46/51) | >0.05 |
| Zhao et al. | 2007 | China | PCR | EAC** | s1 vs. s2 | 93% (53/57) vs. 44% (4/9) | <0.05* |
| Rudi et al. | 2002 | Germany | PCR | PPI+AC or CM,7 | m1 vs. m2 | 90% (44/49) vs. 83% (55/66) | 0.32 |
| Scholte et al. | 2002 | Netherland | PCR | OAC,7 | m1 vs. m2 | 100% (5/5) vs. 83% (16/19) | 0.34 |
| Chaudhuri et al. | 2003 | India | PCR | OAC, 10 | m1 vs. m2 | 46% (11/24) vs. 74% (17/23) | <0.05* |
| De Francesco et al. | 2004 | Italy | PCR | RA+RCT or RAC, 10 |
m1 vs. m2 | 89% (33/37) vs. 90% (52/58) | >0.05 |
| Zhao et al. | 2007 | China | PCR | EAC** | m1 vs. m2 | 94% (17/18) vs. 83% (40/48) | <0.05* |
| Total | s1 vs. s2 | 81% (336/414) vs. 72% (123/170) |
0.02* | ||||
| m1 vs. m2 | 83% (110/133) vs. 84% (180/214) |
0.73 | |||||
Data are based on per-protocol (PP) analyses.
Significantly different
Eradication duration were not shown
P >0.05: No detailed data were available; the author described the results as not significant.
A, amoxicillin; R, rabeprazole; AC, amoxicillin and clarithromycin; ACM, bismuth, clarithromycin, and metronidazole; BAM, bismuth, amoxicillin, and metronidazole; CM, clarithromycin and metronidazole; CT, clarithromycin and tinidazole; EAC, esomeprazole, amoxicillin, and clarithromycin; LAC, lansoprazole, amoxicillin, and clarithromycin; LBTM, lansoprazole, bismuth, tetracycline, and metronidazole; OA, omeprazole and amoxicillin; OAC, omeprazole, amoxicillin, and clarithromycin; OC, omeprazole and clarithromycin; PAC, pantoprazole, amoxicillin, and clarithromycin; PAM, pantoprazole, amoxicillin, and metronidazole; PFC, pantoprazole, furazolidone, and clarithromycin; PPI, proton pump inhibitor; RA, rabeprazole and amoxicillin; RAC, rabeprazole, amoxicillin, and clarithromycin; RCT, rabeprazole, clarithromycin, and tinidazole
It was recently shown that multi component vaccines consisting CagA, VacA and neutrophil-activating protein (NAP) with aluminum hydroxide, administered intramuscularly or parenterally induced antibody responses and interferon-γ (IFN-γ) production in almost all individuals, and still detectable several months after the last immunization. Preliminary data show that this vaccine is equally safe and highly immunogenic in H. pylori-infected individuals [41].
2.2.3 Outer inflammatory protein A (OipA), duodenal ulcer promoting gene A product (DupA), and blood group antigen-binding adhesion (BabA)
OipA, an outer membrane protein that is involved in IL-8 production in the gastric mucosa, is independent of the cag PAI, and leads actin dynamics via the phosphorylation from multiple pathways [42]. Variations in the number of CT repeats lead to an altered functional status of OipA (switch “on” and “off” states). Strains with an oipA “on” status have been more frequently found in patients with gastric cancer than in those with gastritis [33]. The oipA “on” status is strongly correlated with the presence of cagA (r = 0.82) and is an independent predictor of duodenal ulcer (OR 5.0, 95% CI 2.1–11.9) [40]. Although we expected oipA “off” be less exposed with antibiotic due to the less ability to attach, a study reported that the oipA “off” status showed a higher cure rate in patients treated with short-term quadruple therapy than the oipA “on” status (94.6% vs. 86.8%. respectively; P = 0.02) [43]. Further studies between oipA and eradication are expected in the near future.
DupA is an H. pylori virulence factor that is located in the plasticity region of the H. pylori genome. It has been shown to be associated with an increased risk for duodenal ulcer but was protective against gastric atrophy, intestinal metaplasia, and gastric cancer [44]. The dupA gene is classified as either dupA1 (long-type, 1884 bp) or dupA2 (the truncated version by the mutations) [45]. An in vitro study of dupA mutants showed that dupA substantially increased H. pylori-induced IL-12p40 and IL-12p70 production by CD14+ mononuclear cells [46]. DupA1 (without frameshift mutations) was significantly correlated with gastric ulcer and gastric cancer than gastritis (OR 3.35, 95% CI1.55–7.24 and OR 4.14, 95% CI 1.23–13.94, respectively), even after adjusting for age, sex, and cagA [47]. It was reported that the secretion of gastric acid in dupA-positive patients was significantly higher than that in dupA-negative patients [48]. The authors concluded that dupA might affect intragastric pH. The presence of dupA might render H. pylori eradication difficult. The cure rate in patients infected with dupA-positive H. pylori strains was significantly lower than that infected with dupA-negative strains with clarithromycin resistance (28.6% vs. 69%, respectively; P = 0.04) [49]. In the study’s multivariate analysis, dupA presence was independent risk factor for eradication failure (OR 3.71, 95% CI 1.07–12.83). High gastric secretion might be become the reason the difficulty of eradication in the patients with dupA-positive strain.
BabA was detected on the bacterial cell outer membrane of the CCUG17875 strain, which contains one silent babA1 and one expressed babA2 gene [50]. The sequences of these two genes differ only by the presence of a 10 bp deletion in the signal peptide sequence of babA1 which eliminates its translational initiation codon [51]. A recent study reported that the babA mutant was less capable of inducing DNA double-strand breaks (DSBs) in primary and transformed murine and human epithelial and mesenchymal cells, suggesting that bacterial adhesion via babA is required to induce DSBs. The induction of DSBs contributes to the genetic instability and frequent chromosomal aberrations that are the hallmarks of gastric cancer [52]. Moreover, BabA expression is a major determinant of the density of H. pylori colonization. The absence of Lewis b expression was shown to be inversely proportional with the density of colonization and Lewis x or Lewis a expression [53]. Colonization density might adversely affect the efficacy of eradication and ulcer healing [54]. Eradication and ulcer cure rates decrease from 88% for low-density colonization to 69% and 63%, respectively, for high-density colonization. Thus, although the role of BabA in eradication has only been indirectly shown right now, this bacterial adherence factor could provide the basis for vaccine therapy and might be become a future target for therapeutic interventions.
3. Host factors
3.1 Impact of acid inhibition on H. pylori eradication
It is well known that acid inhibition affects the cure rate of H. pylori and that inadequate usage of acid inhibitors leads to eradication failure. There are several reasons why acid inhibitors required for eradication therapy are. 1) Acid inhibitors create an optimal environment for antibiotics such as macrolides or quinolones as they maintain a pH ≥7.4 in the gastric submucusal layer [55]; 2) A higher pH increases the benefits of amoxicillin by more than 10 times [56]; 3) H. pylori changes from a non-dividing to a growth state, the latter of which is effective for antibiotics such as clarithromycin or amoxicillin by elevating the pH of the gastric surface [57]. Thus, PPIs, which rapidly and potently neutralize intragastric pH, are needed for successful eradication. Several studies showed the importance of a high intragastric pH for eradication success. The median 24-hour intragastric pH was significantly higher in the group with successful eradication than in the group with failure eradication (pH 6.4, range 5.0–7.6 vs. pH 5.2, range 2.2–6.2; p = 0.013) [58]. In a meta-analysis comparing high-dose with standard-dose PPI, cure rates of 82% and 74% were achieved, respectively (RR 1.09; 95% CI 1.01–1.17) [59]. Maintaining an intragastric pH > 6 is important for eradication therapy [60]. The one of the successful key to eradicate the patients infected with clarithromycin-resistant H. pylori strains may maintain high pH levels [58].
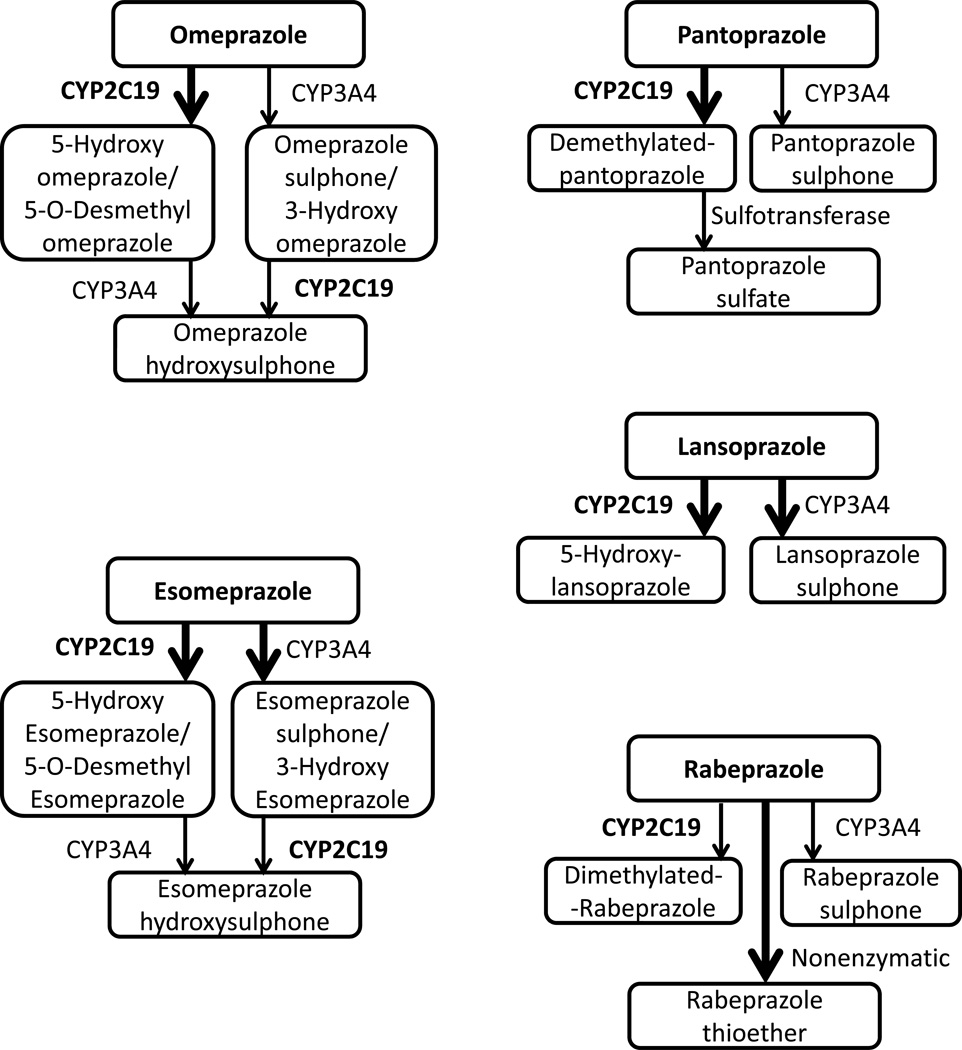
Drug metabolizing enzymes (for example, CYP), drug resistance genes (for example, MDR1), and inflammation-related cytokine genes (for example, IL-1 and TNF-α) contribute to gastric acid secretion. Eradication could target these genes since their activities depend on genetic polymorphisms. PPIs are the most popular acid inhibitors. They are mainly metabolized by CYP2C19 (Figure 1). Inflammatory cells infiltrating the gastric mucosa in patients with H. pylori infection induce several proinflammatory cytokines. IL-1 and TNF-α are well known as inhibitors of gastric acid secretion, on another hand, MDR1 is one of the most important transporters for drug disposition in humans.
Figure 1. All proton pump inhibitors are metabolized by cytochrome P 2C19.
Esomeprazole, omeprazole, pantoprazole, lansoprazole, and rabepazole depend on CYP2C19 polymorphisms.
3.2 Cytochrome P 2C19 (CYP2C19)
PPI has prominent role to inhibit gastric acid suppression against upper gastrointestinal diseases, such as gastroesophageal reflux disease (GERD), gastroduodenal ulcers, and Zollinger-Ellison syndrome. The prophylactic use of PPIs is recommended in patients taking drugs that potentially induce peptic ulcers (e.g., non-steroidal anti-inflammatory drugs and anti-platelet drugs such as low-dose aspirin) [61,62]. In addition, PPIs are indispensable drugs for H. pylori eradication therapy, as the rapid and potent neutralization of gastric acid improves the cure rate of H. pylori [63]. Moreover, PPIs increase the intragastric concentration of amoxicillin by strongly inhibiting acid secretion [56]. Since omeprazole (OPZ), a substituted benzimidazoles, was found to inhibit gastric acid secretion by blocking H+/K+-ATPase (a proton pump), a number of PPIs have been developed later. PPIs are mainly metabolized by CYP in the liver, and CYP2C19 is the most essential metabolize enzyme [10] (Figure 1). CYP2C19 polymorphisms involved on the largest part of interindividual variability in pharmacokinetics and pharmacodynamics. The principal CYP2C19 genotypes are classified as extensive metabolizers (EM), intermediate metabolizers (IM), and poor metabolizers (PM) based on the combination of two SNPs in CYP2C19*2 in exon 5 and CYP2C19*3 in exon 4 [64]. Racial differences in the proportion of genotypes have been identified. The incidences of EMs are 98% in black American [65], 68.8% in Caucasian [66], and 34.9% in Asian [67]. It was also reported that acid inhibition by OPZ is associated with the CYP2C19 genotype [68]. EMs eliminate PPIs from systemic circulation faster than IMs or PMs. Thus, PPI plasma levels of EMs are the lowest in CYP2C19 genotypes. The 24-hour intragastric pH is also lower in EMs than IMs or PMs. Different host CYP2C19 genotypes are associated with different cure rates of H. pylori infection. In a study using standard therapy, the cure rate of EMs was significantly lower than that of IMs and PMs (72.2%, 92.1%, and 97.8%, respectively). Moreover, 70% of patients who failed eradication were EMs [69]. To overcome the problem for EM, a high intragastric pH should be maintained for 24 hours. Since the key factor is that PPI inhibits gastric acid secretion in a dose-dependent manner, high-dose multiple-dividing PPI therapy was developed. When PPIs were administered 4 times rather than once daily, maintaining a 24-hour high intragastric pH could achieve sufficient acid inhibition through maintaining appropriate PPI plasma levels, even in patients with EMs [70]. Changing the dosing schedules in consideration of CYP2C19 polymorphism was also found to be important. Using specific dosing schedules (for example, once daily for PMs, twice daily for IMs, and 4 times daily for Ems) was successful at maintaining a pH of at least 4 [71]. High-dose multiple-dividing PPI therapy could achieve eradication rates over 90%, even in EM patients [72].
3.3 Interleukin 1 (IL-1)
IL-1β is a member of the inflammatory cytokines that were identified as chemoattractants for neutrophils, lymphocytes, and monocytes. Mucosal IL-1β expression and production is upregulated during H. pylori infection and plays an important role in initiating and amplifying the inflammatory response to the infection [73,74]. In an animal model, increasing IL-1β levels in the gastric mucosa suppressed gastric acid secretion. For example, IL-1β reduced gastric acid secretion by 80% in a rat model that was intravenously infused with pentagastrin. IL-1β was 100 times more potent than PPIs and 6,000 time more potent than H2 receptor antagonist (H2RA) [75]. It is thought that IL-1β in the gastric mucosa of H. pylori-infected individuals is one of the mediators for the inhibition of gastric acid secretion and induces inflammation [76]. In a Mongolian gerbil model of H. pylori infection, gastric inflammation and IL-1β levels were significantly increased in the gastric mucosa [77].
In humans, the IL-1B gene contains three polymorphisms (located −511, −31, and +3954 bp from the transcriptional start site) [78]. The IL-1 receptor antagonist gene (IL-RN) has a variable number of identical 86 bp tandem repeats in intron 2 [79]. The ratio of gastric atrophy, gastritis, peptic ulcer, and gastric cancer is associated with these polymorphisms [76,80]. Mucosal IL-1β levels in the IL-1B-511 T/T g or IL-1RN*2 genotypes were significantly higher than in carriers of the IL-1B-511C or IL-1RN*1 alleles (IL-1β 511: antrum p <0.05, corpus p <0.01; IL-1RN: antrum p = 0.01, corpus p = 0.056) [81]. Furthermore, the IL-1B-511 T/T genotype exhibited an increased histological score, such as inflammatory cell infiltration atrophy, more frequency than C allele carriers, but not the difference in IL-1RN polymorphism [80,81]. IL-1β polymorphisms have also shown to be associated with the risk of gastric cancer (OR for gastric cancer 2.6, 95% CI 1.7–3.9 for the IL-1β −511 T/T genotype when compared with the C/C genotype; OR 2.5, 95% CI 1.6–3.8 for the IL-1β −31 T/T genotype when compared with the C/C genotype, and OR 3.7, 95 % CI 2.4–5.7 for the IL-1RN*2 genotype when compared with the *1 genotype) [76]. This, the polymorphism in the IL-B-511T/T and IL-RN*2 genotypes increase IL-1β levels, suppress gastric acid secretion, induce histological inflammatory cell infiltration, and cause the development of gastroduodenal diseases such as gastric atrophy and gastric cancer.
The cure rate in individuals harboring the IL-1B-511 T/T genotype was significantly higher than that of individuals harboring the C/C genotype (T/T genotype: 94.7%, C/C genotype: 77.3%; P = 0.002) (Table 3). In particular, the cure rate of individuals with the T/T genotype was higher than that of carriers of the C genotype infected with clarithromycin-resistant H. pylori strains (T/T genotype: 78%, C/T genotype: 50%, C/C genotype: 35%). Another study reported that the IL-1β −511 T/T and C/T genotypes showed very similar eradication rates (88.2% and 87.2%, respectively), whereas the C/C genotype had a much lower eradication rate (72.2%) (Table 3) [82]. In contrast, no difference in cure rates has been observed for the IL-1B-31, +3954, and IL-1RN polymorphisms (Table 3).
Table 3.
H. pylori cure rates and IL-1
| Gene | Authors | Year | Country | Patients (n) |
Regimen | Genotype | Cure rates (n) | P value |
|---|---|---|---|---|---|---|---|---|
| IL-1B −511 | Take et al. | 2003 | Japan | 231 | OAC, LAC, RAC |
C/T + T/T vs. C/C |
80% (138/172) vs. 80% (47/59) |
0.25 |
| Furuta et al | 2004 | Japan | 336 | OAC, LAC | C/C vs. C/T vs. T/T |
77% (75/97) vs. 90% (147/164) vs. 95% (71/75) |
0.05* (C/C vs. C/T) <0.01* (C/C vs. T/T) |
|
| Sugimoto et al. | 2006 | Japan | 360 | OAC, LAC, RAC |
C/C vs. C/T vs. TT |
72% (70/97) vs. 88% (164/187) vs. 88% (67/76) |
<0.01* (C/C vs. C/T) <0.01* (C/C vs. T/T) |
|
| Zhang et al. | 2010 | China | 224 | OAC, RAC | C/C vs. C/T vs. TT |
87% (39/45) vs. 88% (68/77) vs. 89% (91/102) |
0.91 | |
|
IL-1B +3954 |
Zhang et al. | 2010 | China | 224 | OAC, RAC | C/C vs. C/T vs. TT |
89% (183/206) vs. 83% (15/18) vs. none |
0.45 |
| Gawronska-Szklarz et al. | 2010 | Poland | 139 | PAM | C/C vs. C/T vs. TT |
75% (9/12) vs. 81% (38/47) vs. 70% (56/80) |
0.40 | |
| IL-1B −31 | Ishida et al. | 2006 | Japan | 67 | LAC | C/C vs. C/T vs. TT |
100% (14/14) vs. 83% (30/36) vs. 77% (13/17) |
>0.05 |
| Zhang et al. | 2010 | China | 224 | OAC, RAC | C/C vs. C/T vs. TT |
92% (22/24) vs. 92% (57/62) vs. 86% (119/138) |
0.44 | |
| IL-1B–RN | Sugimoto et al. | 2006 | Japan | 360 | OAC, LAC, RAC |
*2 carrier vs. non-carrier |
76% vs. 85%** | >0.05 |
| Zhang et al. | 2010 | China | 224 | OAC, RAC | 1/1 vs. 1/2 vs. 1/4 vs. 2/2 |
87% (143/164) vs. 92% (45/49) vs. 100% (1/1) vs. 90% (9/10) |
0.67 |
Treatment duration was 7 days in all studies.
Data are based on per-protocol (PP) analyses.
Significantly different
Raw data were not shown.
P >0.05: No detailed data were available; the author described the results as not significant.
Zambon et al showed that there was no difference between eradication rate and IL-1β −31, IL-1RN; however, no detailed data were provided.
IL, interleukin; LAC, lansoprazole, amoxicillin, and clarithromycin; OAC, omeprazole, amoxicillin, and clarithromycin; PAM, pantoprazole, amoxicillin, and clarithromycin; RAC, rabeprazole, amoxicillin, and clarithromycin
3.4 Multi-drug resistance gene 1 (MDR1)
MDR1 is one of the most important transporters for drug disposition in humans. MDR1 , more commonly referred to as P-glycoprotein (P-gp), which is an integral membrane protein weighing 170 kDa [83]. The drug is usually influenced by ATP-dependent efflux transporter; P-gp [84]. PPIs are substrates for and inhibitors of P-gp [85]. Although the majority of single nucleotide polymorphisms (SNPs) have no effect on humans, it was reported that the MDR1 3435 T/T genotype in exon 26 was associated with significantly lower intestinal P-gp expression and increased bioavailability of some drugs after oral administration [86].
The involvement of the MDR1 3435 T/T exon 26 SNP for eradication is controversial (Table 4). In Japan, it was reported that 82% and 81% of patients with the MDR1 3435 C/C and C/T genotypes, respectively, showed successful H. pylori eradication. In contrast, only 67% of patients with the T/T genotype showed successful eradication (P = 0.001 for all) (Table 4). Two reports from Korea and Poland reported that the cure rate was independent of the MDR1 genotype (Table 4).
Table 4.
H. pylori cure rates MDR1 and TNF-α
| Gene | Authors | Year | Country | Patients (n) |
Regimen | Genotype | Cure rates (n) | P value |
|---|---|---|---|---|---|---|---|---|
| MDR1 3435 | Gawronska-Szklarz et al. |
2005 | Poland | 70 | OAC, PAM | C allele carrier vs T/T |
40% (21/52) vs 72.2% (13/18) |
<0.05* |
| Gawronska-Szklarz et al. |
2005 | Poland | 70 | OAC, PAM | C/C vs. C/T vs. T/T |
41% (11/27) vs 40% (10/25) vs 72% (13/18) |
<0.05* |
|
| Furuta et al. | 2007 | Japan | 314 | LAC | C/C vs. C/T vs. T/T |
82% (83/101)vs 81% (112/139) vs 67% (44/73) |
<0.01* (T/T vs. C/T) <0.01* (T/T vs. C/C) |
|
| Oh et al. | 2009 | Korea | 210 | PAC | C/C vs. C/T vs. T/T |
83% (62/75)vs 84% (92/102) vs.77% (20/26) |
0.66 | |
| Gawronska-Szklarz, et al. |
2010 | Poland | 139 | PAM | C/C vs. C/T vs. T/T |
75% (33/44) vs 74% (45/61) vs 74% (25/34) |
0.99 | |
| TNF-α 1031 | Sugimoto et al. | 2006 | Japan | 360 | OAC, LAC, RAC |
C allele carrier vs T/T |
84% vs. 83%** | >0.05 |
| Ishida et al. | 2006 | Japan | 67 | LAC | C/C vs. C/T vs. T/T |
100% (2/2)*** vs. 71% (10/14) vs. 85% (45/51) |
>0.05 | |
| TNF-α 857 | Sugimoto et al. | 2006 | Japan | 360 | OAC, LAC, RAC |
T allele carrier vs. C/C |
82% vs. 84%** | >0.05 |
| TNF-α 863 | Sugimoto et al. | 2006 | Japan | 360 | OAC, LAC, RAC |
A allele carrier vs C/C |
85% vs. 83%** | >0.05 |
Treatment duration was 7 days in all studies.
Data are based on per-protocol (PP) analyses.
Significantly different
Raw data were not shown.
n = 2
IL, interleukin; LAC, lansoprazole, amoxicillin, and clarithromycin; MDR1, multidrug-resistant transporter-1; OAC, omeprazole, amoxicillin, and clarithromycin; PAC, pantoprazole, amoxicillin, and clarithromycin; PAM, pantoprazole, amoxicillin, and clarithromycin; RAC, rabeprazole, amoxicillin, and clarithromycin; TNF, tumor necrosis factor
3.5 Tumor necrosis factor-α (TNF-α) and other cytokines
TNF-α encoded by TNF-A on chromosome 6 is a proinflammatory cytokine that is involved in several pathways of gastric acid inhibition. For example, TNF-α inhibits basal acid and stimulates acid through histamine and gastrin [87]. It is produced by macrophages, monocytes, neutrophils, T cells, and NK cell after stimulation. H. pylori infection elevates TNF-α in tissue, inducing cytotoxicity and apoptosis of gastric epithelial cells [88]. TNF-α has five biallelic SNPs in its promotor region; -G238A, -G308A, -C857T, -C863A, and T1031C. Some polymorphism in TNF-Aare thought to be related to its expression. For example, TNF-A-308A has been shown to increase TNF-α production [89]. The TNF-A-G238A and -G308A polymorphisms are relevant to different transcriptional intra-individual activities and are associated with an increased risk for gastric cancer and gastric and duodenal ulcer [90,91]. Furthermore, the TNF-A-308A polymorphism was more strongly associated with H. pylori cagA-positive strains when compared with cagA-negative strains or controls (p = 0.019 and p = 0.011, respectively) [92]. However, no significant associations were shown between the TNF-A (−1031, −857, and −863 polymorphisms and and eradication (Table 4).
IL-8 and IL-10 are important mediators in gastric physiology and are thought to have important roles in the etiology of gastric cancer. For example, IL-8 stimulates the proliferation of endothelial cells, and IL-10 downregulates cytotoxic responses. In spite of some studies, only one report showed a significant correlation between the H. pylori eradication rate and these factors [93].
4. Other factors implicated in H. pylori eradication
Several factors such as smoking, food, economic status, treatment compliance, treatment duration, dosages, and methods of treatment (e.g., concomitant, hybrid, or sequential therapy) have been shown to be associated with the cure rate of H. pylori infection. However, a discussion of these factors is not within the main scope of this review.
5. Future aspects of H. pylori eradication therapy
Multi-dividing therapy for 7 days after assessing clarithromycin resistance resulted in an overall cure rate of 97.4% (148/152 study participants; 95% CI 93.4–99.3%) in the PP analysis and 96.7% (148/153 study participants; 95% CI 92.5–98.9%) in the ITT analysis [21]. The cure rates for patients infected with clarithromycin-resistant strains were 98.4% and 98.4% in the PP and ITT analysis, respectively.
Recently, new system that can simultaneously analyze CYP2C19 and clarithromycin susceptibility within 30 min was developed [94]. The susceptibility targets the A2142G and A2143G point mutations in the H. pylori 23S rRNA and the fully automated DNA analyzer measures the polymorphisms in gastric juice sample collected by aspiration during gastroduodenoscopy. Although this automated machine is rapid and effective for increase eradication rate, the procedure is costly (around 150 US dollars), need a special automated machine and requires patients to undergo an upper endoscopy [94].
6. Conclusion
In this review, we discuss the treatment of H. pylori infection in relation to therapeutic targets. We mention the increase in antibiotic-resistant strains and provide a list of virulence factors. Antibiotic-resistant H. pylori strains are difficult to cure, and developing a treatment strategy targeting these strains is crucial. Virulence factors not only involved in the progression of gastroduodenal disease but might be also related to cure therapy. In the section on host factors, we describe the importance of acid inhibition, the appropriate usage of PPIs, and list several polymorphisms. CYP2C19 must be useful in eradication therapy. We expect that the most suitable eradication therapy will use these targets in near future.
7. Expert opinion
In summary, there are three main targets to achieve effective eradication therapy for H. pylori infection.
The first therapeutic target is to identify counter measures for antibiotic-resistant H. pylori strains. Susceptibility to antibiotics should be assessed in all patients, ideally, before the start of eradication treatment so that physicians can prescribe the appropriate antibiotics. Physicians should also know the resistance rates of H. pylori in their country of residence as the ratio of resistant strains differ by country (e.g., Southern vs. Northern European countries). The number of clarithromycin- and metronidazole-resistant H. pylori strains have increased recently. Thus far, the resistance rate to fluoroquinolones has been relatively low, but it has seen a rapid increase in some countries. Although fluoroquinolone-containing regimens or novel antibiotics might become important options of treatment, these do not adapt in all countries. Susceptibility to antibiotics should be checked to enable the administration of effective antibiotics and to avoid the development of novel resistant pathogens. Moreover, effective eradication can be achieved if each patient is checked for the absence or presence of resistant bacteria.
The second therapeutic target is inhibiting acid suppression since insufficient acid inhibition leads to eradication failure. Maintaining a high intragastric pH for 24 hours increases the effectiveness of some antibiotics and makes H. pylori easier to cure. Thus, physicians should maintain a high intragastric pH (pH ≥ 6) to achieve successful eradication. Intragastric pH levels are associated with the PPI dose and regimen. Hence, a high-dose and multiple-dividing PPI therapy should be selected for effective eradication. This will not change until the development of more powerful and long-acting acid inhibitors.
Although until now we could not modify the patient’s genotypes, identifying high-risk groups is very important to become the third therapeutic target. Candidates for one of the most significant risk factors are EMs on CYP2C19 polymorphisms. As EMs metabolize PPIs quickly, it is difficult to elevate intragastric pH in patients with this compared to other genotypes. So, EMs may need to increase dosage and frequency of PPI. Fortunately, as some studies showed the frequency of EMs, it may not need to measure CYP2C19 genotypes if the countries, area or race have the high frequency of EMs. In these cases, special PPI therapy (e.g. high-dose multiple therapy) should be applied without measuring CYP2C19. On the other hand, it may be needed to know the genotypes for saving cost and solution of troublesome for patients in low frequency of EMs. Some studies showed that the presence of cagA and vacA s2 could be risk factors for eradication. Moreover, the cure rate in individuals with the IL-1B-511 C/C genotype was significantly lower than in those having other genotypes. In summary, the CYP2C19, IL-1β, and cagA polymorphisms are relevant to clinical practice. By analyzing these polymorphisms before eradication, high-risk groups can be identified, and effective eradication can be achieved in these patients. Novel markers that are more sensitive and effective for eradication will likely be identified in future studies.
We herein described H. pylori eradication therapy utilizing the three targets antibiotic resistance, adequate acid inhibition, and the identification of high-risk groups. Our ultimate goal is to achieve a cure rate of nearly 100%. To achieve this aim, it is necessary to administer precise care for each patient, including assessments of antibiotic resistance and/or genetic polymorphisms. However, the usefulness of utilizing several targets (e.g., virulence factors and genetic polymorphisms except CYP2C19) for increasing the eradication rate still needs to be confirmed in clinical practice. Furthermore, as examining both antibiotic resistance and gene polymorphisms requires specialized laboratories and equipment (e.g., PCR machines), it might be difficult to apply this approaches in clinical practice worldwide. To get a gastric mucosal sample, an upper endoscopy is also sometimes required. Therefore, these evidence-based medicines are necessary for some condition including their cost. However, we believe that personalized medicine will increase the H. pylori cure rate, and that the costs can be offset by achieving a higher cure rate. At least, physicians should know the local peculiarity in susceptibility to antibiotics or the frequency of some genotypes. Further studies are required to establish a complete eradication therapy utilizing these or new targets.
Highlights Box.
Ideally susceptibility to antibiotics resistance should be assessed in all patients before the start of eradication treatment to avoid the development of novel resistant pathogens and to obtain more effective H. pylori eradication. At least, physicians should know the resistance rates of H. pylori in their region.
Physicians should maintain a high intragastric pH to achieve successful H. pylori eradication with a high-dose and multiple-dividing PPI therapies.
The extensive metabolizers on CYP2C19 polymorphisms may need increasing dosage and frequency of PPI. Particular methods of PPI therapy should be apply without measuring CYP2C19.
The presence of cagA and vacA s2 genotype could be risk factors for eradication. Moreover, the cure rate in individuals with the IL-1B-511 C/C genotype was significantly lower than in those having other genotypes.
By identifying high-risk groups before eradication, an effective eradication could be achieved.
Acknowledgments
This report was based on work supported in part by grants from the National Institutes of Health (DK62813) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (24406015, 24659200, 25293104 and 26640114). This work was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology from Japan Science and Technology Agency (JST).
Abbreviations
- BabA
blood group antigen binding adhesion
- bp
base pair
- CagA
cytotoxin-associated gene A product
- CYP2C19
cytochrome P450 2C19
- DSB
double strand break
- DupA
duodenal ulcer promoting gene A product
- EM
extensive metabolizer
- GERD
gastroesophageal reflux disease
- H. pylori
Helicobacter pylori
- H2RA
H2 receptor antagonist
- IFN
interferon
- IL
interleukin
- IL-RN
IL-1 receptor antagonist gene
- IM
intermediate metabolizer
- ITT
intention-to-treat
- MDR1
multidrug-resistant transporter 1
- NSAID
non-steroidal anti-inflammatory drug
- OipA
outer inflammatory protein
- OPZ
omeprazole
- Rpl22
ribosomal protein L 22
- rRNA
ribosomal RNA
- PAI
pathogenicity island
- PM
poor metabolizer
- PP
per-protocol
- PPI
protein pump inhibitor
- SHP2
Src homology 2-containing protein-tyrosine phosphatase
- SNP
single nucleotide polymorphism
- T4SS
type IV secretion system
- VacA
vaculating cytotoxin A
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(Suppl 1):1–5. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 2.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. American journal of epidemiology. 2012;175(1):54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirayama Y, Kawai T, Otaki J, Kawakami K, Harada Y. Prevalence of Helicobacter pylori infection with healthy subjects in Japan. Journal of gastroenterology and hepatology. 2014;29(Suppl 4):16–19. doi: 10.1111/jgh.12795. [DOI] [PubMed] [Google Scholar]
- 4.Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. Journal of gastroenterology. 2014;49(1):1–8. doi: 10.1007/s00535-013-0897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chey WD, Wong BC. Practice Parameters Committee of the American College of G. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. The American journal of gastroenterology. 2007;102(8):1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 6.Feng L, Wen MY, Zhu YJ, Men RT, Yang L. Sequential Therapy or Standard Triple Therapy for Helicobacter pylori Infection: An Updated Systematic Review. American journal of therapeutics. 2015 doi: 10.1097/MJT.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 7.Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. Bmj. 2013;347:f4587. doi: 10.1136/bmj.f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors Nature reviews. Gastroenterology & hepatology. 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy--results of an individual data analysis of 2751 patients. Alimentary pharmacology & therapeutics. 2003;17(1):99–109. doi: 10.1046/j.1365-2036.2003.01396.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Alimentary pharmacology & therapeutics. 1999;13(Suppl 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim SG, Jung HK, Lee HL, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Journal of gastroenterology and hepatology. 2014;29(7):1371–1386. doi: 10.1111/jgh.12607. [DOI] [PubMed] [Google Scholar]
- 12.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of gastroenterology and hepatology. 2009;24(10):1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12(4):275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 14.Zagari RM, Bianchi-Porro G, Fiocca R, Gasbarrini G, Roda E, Bazzoli F. Comparison of 1 and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for Helicobacter pylori eradication: the HYPER Study. Gut. 2007;56(4):475–479. doi: 10.1136/gut.2006.102269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-Year First-Line Eradication Rate of Helicobacter pylori Based on Triple Therapy with Proton Pump Inhibitor, Amoxicillin and Clarithromycin. Journal of clinical biochemistry and nutrition. 2010;47(1):53–58. doi: 10.3164/jcbn.10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W, Cheng H, Hu F, et al. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15(5):460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 18.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 19.Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J Gastroenterol. 2013;19(45):8168–8180. doi: 10.3748/wjg.v19.i45.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binh TT, Shiota S, Suzuki R, et al. Discovery of novel mutations for clarithromycin resistance in Helicobacter pylori by using next-generation sequencing. The Journal of antimicrobial chemotherapy. 2014;69(7):1796–1803. doi: 10.1093/jac/dku050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto M, Uotani T, Sahara S, et al. Efficacy of tailored Helicobacter pylori eradication treatment based on clarithromycin susceptibility and maintenance of acid secretion. Helicobacter. 2014;19(4):312–318. doi: 10.1111/hel.12128. [DOI] [PubMed] [Google Scholar]
- 22.Camargo MC, Garcia A, Riquelme A, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. The American journal of gastroenterology. 2014;109(4):485–495. doi: 10.1038/ajg.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146(8):556–563. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 24.Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med. 2001;161(9):1217–1220. doi: 10.1001/archinte.161.9.1217. [DOI] [PubMed] [Google Scholar]
- 25.Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Journal of clinical gastroenterology. 2006;40(8):683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Alimentary pharmacology & therapeutics. 2006;23(1):35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Huang X, Yao L, Shi R, Zhang G. Advantages of Moxifloxacin and Levofloxacin-based triple therapy for second-line treatments of persistent Helicobacter pylori infection: a meta analysis. Wiener klinische Wochenschrift. 2010;122(13–14):413–422. doi: 10.1007/s00508-010-1404-3. [DOI] [PubMed] [Google Scholar]
- 28.Molina-Infante J, Gisbert JP. Levofloxacin in first-line eradication regimens for Helicobacter pylori: better test antibiotic susceptibility before treating. Gut. 2011;60(11):1605. doi: 10.1136/gut.2010.233015. author reply 1605–1606. [DOI] [PubMed] [Google Scholar]
- 29.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer science. 2011;102(1):36–43. doi: 10.1111/j.1349-7006.2010.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein Nature reviews. Cancer. 2004;4(9):688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(3):1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer research. 2008;68(2):379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crabtree JE, Taylor JD, Wyatt JI, et al. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 35.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125(6):1636–1644. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 36.Maeda S, Yoshida H, Ikenoue T, et al. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut. 1999;44(3):336–341. doi: 10.1136/gut.44.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3(4):320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 38.Rhead JL, Letley DP, Mohammadi M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133(3):926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(9):835–842. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123(2):414–424. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 41.Ruggiero P, Peppoloni S, Rappuoli R, Del Giudice G. The quest for a vaccine against Helicobacter pylori: how to move from mouse to man? Microbes and infection / Institut Pasteur. 2003;5(8):749–756. doi: 10.1016/s1286-4579(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 42.Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126(4):1030–1043. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 43.Treiber G, Wittig J, Ammon S, Walker S, van Doorn LJ, Klotz U. Clinical outcome and influencing factors of a new short-term quadruple therapy for Helicobacter pylori eradication: a randomized controlled trial (MACLOR study) Archives of internal medicine. 2002;162(2):153–160. doi: 10.1001/archinte.162.2.153. [DOI] [PubMed] [Google Scholar]
- 44.Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128(4):833–848. doi: 10.1053/j.gastro.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussein NR, Argent RH, Marx CK, Patel SR, Robinson K, Atherton JC. Helicobacter pylori dupA is polymorphic, and its active form induces proinflammatory cytokine secretion by mononuclear cells. The Journal of infectious diseases. 2010;202(2):261–269. doi: 10.1086/653587. [DOI] [PubMed] [Google Scholar]
- 46.Hussein NR. The association of dupA and Helicobacter pylori-related gastroduodenal diseases. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2010;29(7):817–821. doi: 10.1007/s10096-010-0933-z. [DOI] [PubMed] [Google Scholar]
- 47.Shiota S, Matsunari O, Watada M, Hanada K, Yamaoka Y. Systematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomes. Gut pathogens. 2010;2(1):13. doi: 10.1186/1757-4749-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imagawa S, Ito M, Yoshihara M, Eguchi H, Tanaka S, Chayama K. Helicobacter pylori dupA and gastric acid secretion are negatively associated with gastric cancer development. Journal of medical microbiology. 2010;59(Pt 12):1484–1489. doi: 10.1099/jmm.0.021816-0. [DOI] [PubMed] [Google Scholar]
- 49.Shiota S, Nguyen LT, Murakami K, et al. Association of helicobacter pylori dupA with the failure of primary eradication. Journal of clinical gastroenterology. 2012;46(4):297–301. doi: 10.1097/MCG.0b013e318243201c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilver D, Arnqvist A, Ogren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 51.Yamaoka Y. Roles of Helicobacter pylori BabA in gastroduodenal pathogenesis. World journal of gastroenterology : WJG. 2008;14(27):4265–4272. doi: 10.3748/wjg.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toller IM, Neelsen KJ, Steger M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14944–14949. doi: 10.1073/pnas.1100959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheu BS, Sheu SM, Yang HB, Huang AH, Wu JJ. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut. 2003;52(7):927–932. doi: 10.1136/gut.52.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai YC, Wang TH, Huang SH, et al. Density of Helicobacter pylori may affect the efficacy of eradication therapy and ulcer healing in patients with active duodenal ulcers. World journal of gastroenterology : WJG. 2003;9(7):1537–1540. doi: 10.3748/wjg.v9.i7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grayson ML, Eliopoulos GM, Ferraro MJ, Moellering RC., Jr Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 1989;8(10):888–889. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 56.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111(2):358–367. doi: 10.1053/gast.1996.v111.pm8690200. [DOI] [PubMed] [Google Scholar]
- 57.Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43(Suppl 1):S56–S60. doi: 10.1136/gut.43.2008.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugimoto M, Furuta T, Shirai N, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12(4):317–323. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 59.Villoria A, Garcia P, Calvet X, Gisbert JP, Vergara M. Meta-analysis: high-dose proton pump inhibitors. vs. standard dose in triple therapy for Helicobacter pylori eradication. Alimentary pharmacology & therapeutics. 2008;28(7):868–877. doi: 10.1111/j.1365-2036.2008.03807.x. [DOI] [PubMed] [Google Scholar]
- 60.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 61.Nishino M, Sugimoto M, Kodaira C, et al. Preventive effects of lansoprazole and famotidine on gastric mucosal injury induced by low-dose aspirin in Helicobacter pylori-negative healthy volunteers. Journal of clinical pharmacology. 2011;51(7):1079–1086. doi: 10.1177/0091270010376194. [DOI] [PubMed] [Google Scholar]
- 62.Uotani T, Sugimoto M, Nishino M, et al. Ability of rabeprazole to prevent gastric mucosal damage from clopidogrel and low doses of aspirin depends on CYP2C19 genotype. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10(8):879–885. e872. doi: 10.1016/j.cgh.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 63.de Boer W, Driessen W, Jansz A, Tytgat G. Effect of acid suppression on efficacy of treatment for Helicobacter pylori infection. Lancet. 1995;345(8953):817–820. doi: 10.1016/s0140-6736(95)92962-2. [DOI] [PubMed] [Google Scholar]
- 64.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269(22):15419–15422. [PubMed] [Google Scholar]
- 65.Marinac JS, Balian JD, Foxworth JW, et al. Determination of CYP2C19 phenotype in black Americans with omeprazole: correlation with genotype. Clin Pharmacol Ther. 1996;60(2):138–144. doi: 10.1016/S0009-9236(96)90129-0. [DOI] [PubMed] [Google Scholar]
- 66.Ishizaki T, Sohn DR, Kobayashi K, et al. Interethnic differences in omeprazole metabolism in the two S-mephenytoin hydroxylation phenotypes studied in Caucasians and Orientals. Therapeutic drug monitoring. 1994;16(2):214–215. doi: 10.1097/00007691-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 67.Inoue K, Yamazaki H, Shimada T. Linkage between the distribution of mutations in the CYP2C18 and CYP2C19 genes in the Japanese and Caucasian. Xenobiotica. 1998;28(4):403–411. doi: 10.1080/004982598239506. [DOI] [PubMed] [Google Scholar]
- 68.Furuta T, Ohashi K, Kosuge K, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clinical pharmacology and therapeutics. 1999;65(5):552–561. doi: 10.1016/S0009-9236(99)70075-5. [DOI] [PubMed] [Google Scholar]
- 69.Furuta T, Shirai N, Takashima M, et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clinical pharmacology and therapeutics. 2001;69(3):158–168. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 70.Furuta T, Shirai N, Xiao F, Ohashi K, Ishizaki T. Effect of high-dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clinical pharmacology and therapeutics. 2001;70(5):484–492. doi: 10.1067/mcp.2001.119721. [DOI] [PubMed] [Google Scholar]
- 71.Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clinical pharmacology and therapeutics. 2004;76(4):290–301. doi: 10.1016/j.clpt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Sugimoto M, Furuta T, Shirai N, et al. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert opinion on pharmacotherapy. 2007;8(16):2701–2717. doi: 10.1517/14656566.8.16.2701. [DOI] [PubMed] [Google Scholar]
- 73.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scandinavian journal of gastroenterology. 1994;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 74.Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, −1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. Journal of gastroenterology and hepatology. 1997;12(7):473–480. doi: 10.1111/j.1440-1746.1997.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 75.Wallace JL, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. The American journal of physiology. 1991;261(4 Pt 1):G559–G564. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- 76.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 77.Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48(6):765–773. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stokkers PC, van Aken BE, Basoski N, Reitsma PH, Tytgat GN, van Deventer SJ. Five genetic markers in the interleukin 1 family in relation to inflammatory bowel disease. Gut. 1998;43(1):33–39. doi: 10.1136/gut.43.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tarlow JK, Blakemore AI, Lennard A, et al. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Human genetics. 1993;91(4):403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- 80.Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123(1):92–105. doi: 10.1053/gast.2002.34156. [DOI] [PubMed] [Google Scholar]
- 81.Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123(6):1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 82.Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, Ishizaki T. Influences of proinflammatory and anti-inflammatory cytokine polymorphisms on eradication rates of clarithromycin-sensitive strains of Helicobacter pylori by triple therapy. Clinical pharmacology and therapeutics. 2006;80(1):41–50. doi: 10.1016/j.clpt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Higgins CF. ABC transporters: from microorganisms to man. Annual review of cell biology. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 84.Higgins CF, Gottesman MM. Is the multidrug transporter a flippase? Trends in biochemical sciences. 1992;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 85.Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn-Schmiedeberg’s archives of pharmacology. 2001;364(6):551–557. doi: 10.1007/s00210-001-0489-7. [DOI] [PubMed] [Google Scholar]
- 86.Cascorbi I, Gerloff T, Johne A, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clinical pharmacology and therapeutics. 2001;69(3):169–174. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 87.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42(2):227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shibata J, Goto H, Arisawa T, et al. Regulation of tumour necrosis factor (TNF) induced apoptosis by soluble TNF receptors in Helicobacter pylori infection. Gut. 1999;45(1):24–31. doi: 10.1136/gut.45.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(7):3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125(2):364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 91.Lanas A, Garcia-Gonzalez MA, Santolaria S, et al. TNF and LTA gene polymorphisms reveal different risk in gastric and duodenal ulcer patients. Genes and immunity. 2001;2(8):415–421. doi: 10.1038/sj.gene.6363798. [DOI] [PubMed] [Google Scholar]
- 92.Yea SS, Yang YI, Jang WH, Lee YJ, Bae HS, Paik KH. Association between TNF-alpha promoter polymorphism and Helicobacter pylori cagA subtype infection. Journal of clinical pathology. 2001;54(9):703–706. doi: 10.1136/jcp.54.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zambon CF, Fasolo M, Basso D, et al. Clarithromycin resistance, tumor necrosis factor alpha gene polymorphism and mucosal inflammation affect H. pylori eradication success. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 11(11):1506–1514. doi: 10.1007/s11605-007-0246-4. discussion 1514 (2007) [DOI] [PubMed] [Google Scholar]
- 94.Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81(4):521–528. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]