Abstract
The brain networks supporting nausea not yet understood. We previously found that while visual stimulation activated primary (V1) and extrastriate visual cortices (MT+/V5, coding for visual motion), increasing nausea was associated with increasing sustained activation in several brain areas, with significant co-activation for anterior insula (aIns) and mid-cingulate (MCC) cortices. Here, we hypothesized that motion sickness also alters functional connectivity between visual motion and previously identified nausea-processing brain regions. Subjects prone to motion sickness and controls completed a motion sickness provocation task during fMRI/ECG acquisition. We studied changes in connectivity between visual processing areas activated by the stimulus (MT+/V5, V1), right aIns and MCC when comparing rest (BASELINE) to peak nausea state (NAUSEA). Compared to BASELINE, NAUSEA reduced connectivity between right and left V1 and increased connectivity between right MT+/V5 and aIns and between left MT+/V5 and MCC. Additionally, the change in MT+/V5 to insula connectivity was significantly associated with a change in sympathovagal balance, assessed by heart rate variability analysis. No state-related connectivity changes were noted for the control group. Increased connectivity between a visual motion processing region and nausea/salience brain regions may reflect increased transfer of visual/vestibular mismatch information to brain regions supporting nausea perception and autonomic processing. We conclude that vection-induced nausea increases connectivity between nausea-processing regions and those activated by the nauseogenic stimulus. This enhanced low-frequency coupling may support continual, slowly evolving nausea perception and shifts toward sympathetic dominance. Disengaging this coupling may be a target for biobehavioral interventions aimed at reducing motion sickness severity.
Keywords: brain-gut interactions, MT+/V5, brain connectivity, heart rate variability, sympathovagal balance
Introduction
Nausea is a universal human experience. It is a perceptual state that evolves slowly over time and the brain networks supporting this state are not well understood. Non-invasive investigations of human brain connectivity, using functional connectivity MRI (fcMRI), have been applied to evaluate both trait and state spatiotemporal dynamics [1], and are sensitive to networks typically characterized by low frequency signal fluctuations [2]. Hence, this method is well suited to evaluate the neural networks underlying slowly progressing perceptual states such as motion sickness induced nausea.
Recent neuroimaging studies have investigated brain activation in response to nausea. Our previous fMRI study employing a visual stimulus found that while phasic activation in fear conditioning and noradrenergic brainstem regions precipitates transition to strong nausea, sustained activation following this transition occurs in a broader interoceptive, limbic, somatosensory, and cognitive network. This most likely reflects the multiple dimensions of nausea perception [3]. Specifically, while the stimulus activated vision and visual motion processing brain areas (primary visual, V1, and middle temporal area, MT+/V5, respectively), increasing nausea was associated with activation in brain areas such as insula and anterior cingulate cortex. These latter regions are classical targets for interoceptive afference [4-6] as well as autonomic processing [7], which in turn is strongly modulated by motion sickness and nausea [8-10]. Interestingly, a further fMRI study found that these areas differ from those implicated in vection alone [11], suggesting that visually induced illusions of self-motion may be supported by a different circuitry than that supporting a perceptual state in which vection is accompanied by a nausea percept. Functional connectivity response to visual motion stimulation (but not nausea) shows reduced functional connectivity between MT+/V5 and several other striate and extrastriate visual processing areas [12] in comparison to a resting state. Recently, Miyazaki et al. [13] found that a brief (6 minutes) visual stimulation that was accompanied by mild motion sickness resulted in desynchronization between left and right MT+ areas for the high frequency (>0.1 Hz) component of the BOLD fMRI signal. Few other studies exist that have evaluated functional brain connectivity response to motion sickness-induced nausea following sustained stimulation.
In this study, we evaluate brain connectivity response to nausea induced by vection. We focus our analysis on key brain regions robustly activated by our vection-inducing visual stimulation, as well as on brain regions shown to activate with increasing nausea perception [3]. Specifically, we investigate how nausea alters functional connectivity between primary visual (V1), middle temporal (MT+/V5), anterior insula, and anterior cingulate cortices.
Methods
Subjects
We recruited right-handed subjects [Edinburgh Inventory [14]] through public advertisement. All subjects underwent prescreening to determine propensity to motion sickness as evaluated through the Motion Sickness Susceptibility Questionnaire (MSSQ) [15]. The MSSQ consists of two separate sections related to childhood experiences (below 12 years of age) of travel and motion sickness and to experiences of travel and motion sickness over the last 10 years. The MSSQ score has been shown to be a significant predictor of severity and tolerance of nausea and vomiting in response to controlled nauseogenic motion stimuli exposure, with an average MSSQ score of 24 ± 13.3 (mean ± s.d.) in a population of 106 healthy subjects not suffering from migraine [15]. A complete medical history and physical examination was performed by a gastroenterologist. Two (2) subjects reported a history of migraines in the nausea group, while 1 subject reported a history of migraines in the control group (Fisher's Exact test, two-tailed: p=1). Subjects with irritable bowel syndrome or upper gastrointestinal disorders were excluded from both groups. Upon clinical examination, subjects had no history of balance or vestibular disorders. Additional confirmation of susceptibility to motion sickness was obtained through subjective nausea intensity ratings from a mock MRI session which included a visual nauseogenic stimulus (see below). In the nausea group, we excluded subjects with an MSSQ score >60 who reported low (<2 on an integer scale from 0 to 4) maximum nausea rating in response to the stimulus. All subjects with an MSSQ score >60 and maximum nausea rating >1 were allocated to the motion sickness (nausea) group, and all subjects with an MSSQ score <60 and maximum nausea rating <2 were allocated to a control group. A cutoff of 60 in MSSQ score was chosen because it has been shown that subjects with MSSQ scores >60 experienced moderate nausea to visual nauseogenic stimulation more reliably and more quickly than those with scores below this inflection point [15]. This resulted in a nausea group of sixteen subjects (female, age: 27.5 ± 8.6 years, mean ± SD) and a control group of eight subjects (female, age: 25 ± 1.1 years years, mean ± SD). All twenty-four subjects agreed to continue to the experimental MRI session. Subject who needed corrective vision were allowed to wear contact lenses. Given the increased risk of vomiting during the experimental session, for safety reasons all subjects were asked to refrain from food and water intake for 12h and from cigarettes and alcohol for 24 h prior to fMRI. While alcohol and nicotine withdrawal can produce nausea in addicted individuals, the baseline nausea rating for all subjects was 0 (no nausea - see below), suggesting that subjects in our study did not experience withdrawal associated nausea. All experiments took place between 7 AM and 12 PM at the A.A. Martinos Center for Biomedical Imaging in Charlestown, MA. Written informed consent was obtained from all participants, and the protocol was approved by the Human Research Committee of Massachusetts General Hospital.
Experimental Protocol
Scanner and screen configuration
Subjects were placed, supine, in a 1.5 T Siemens Avanto MRI scanner (Siemens Medical Systems) equipped with a specialized 23-channel head coil constructed at the Martinos Center for Biomedical Imaging [16] which provided an extra-wide field of view. This was necessary for unobstructed administration of visual stimuli during fMRI. We used video projection onto a screen which comprised a central section (30.48 cm wide, 40.64 cm high), and 2 side wings (10.16 cm wide, semicircular) inclined at 45° with respect to the central section. This screen was positioned approximately 10 cm in front of their eyes. Thus, assuming a single central visual focus, the field of view was 165.7°, covering both central and peripheral visual fields.
Stimulus design and nausea rating
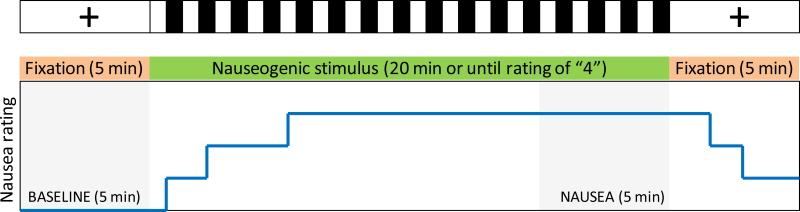
Subjects were instructed to lie still and look directly at a crosshair which was projected onto the center of the screen for an initial baseline period of 5 min (Figure 1). After baseline, the nauseogenic stimulus was projected as follows: alternating black and white stripes (black stripes 1.2 cm, 6.9° viewing angle; white stripes 1.85 cm, 10.6° viewing angle) with left-to-right circular motion at 62.5°/s. The horizontal translation induces vection, i.e. a false sensation of translation of the subject itself directed to their left. During and after the stimulus, all subjects rated the intensity of the nausea they were experiencing on a scale ranging from “0” to “4” (0 = no nausea, followed by 1=mild/2=moderate/3=strong/4=severe nausea) using a button box. Ratings were verbally instructed and practiced during the mock scanner session and performed without predetermined timings/cues. The stimulus was immediately terminated when a rating of 4 was reached (corresponding to the urge to vomit according to past experience) - see Figure 1. The experimental procedure ended with a second 5-min period of crosshair fixation.
Figure 1.
Experimental protocol design and analysis window definition. Connectivity analysis employed data from a 5-min BASELINE window and data from a 5-min NAUSEA window (last 5 minutes of the nauseogenic stimulus).
MRI and ECG data collection
Whole-brain blood oxygen level-dependent (BOLD) fMRI data were collected in a 1.5T scanner using using a gradient echo T2*-weighted pulse sequence (repetition time [TR]/echo time [TE] = 3 s/30 ms, slice thickness = 3.0 mm, interslice gap = 0.6 mm, matrix = 64 × 64, FOV = 200 mm, flip angle [FA] = 90°). The fMRI acquisition ran continuously throughout the experimental protocol, resulting in a maximum of 600 volumes, unless stimulation was interrupted as a consequence of severe nausea rating (see above). Prior to fMRI, we also acquired a high-resolution T1-weighted structural image, using a standard MPRAGE sequence (TR/TE/TI = 2730/3.39/1000 ms, slice thickness = 1.33 mm, FOV = 156 mm, FA = 7°). Concurrently with MRI data acquisition, ECG signals were collected at a sampling frequency of 400 Hz using Chart Data Acquisition Software (ADInstruments) on a laptop equipped with a 16-channel Powerlab DAQ System (ADInstruments) using an MRI-compatible Patient Monitoring system (Model 3150, Invivo Research, Inc.) through MRI-compatible electrodes (VerMed, Bellows Falls) placed on the chest.
Data Preprocessing
Heart Rate Variability (HRV) analysis
R–R intervals were obtained by feeding ECG data into automated processing (WaveForm DataBase Software Package, PhysioNet, MIT) followed by manual adjustments by an expert observer (RB) in order ensure correct peak detection. We then applied a point process method used to develop local likelihood heart rate (HR) estimation and compute instantaneous HRV estimates [17]. The model assumes a history-dependent inverse Gaussian process to generate the next R peak, and derives an explicit probability density directly from a physiologically based integrate-and-fire model. Additionally, contrary to classical time-frequency decomposition approaches used in HRV analysis (which may entail short term Fourier transforms, wavelets [18], splines, or quadratic distributions [19]), this framework does not require any type of interpolation of RR series. Modeling the mean of the R–R interval lengths as a linear function of the last k R–R intervals allows us to subsequently estimate the dependence of such intervals on the recent history of parasympathetic and sympathetic inputs to the sinoatrial (SA) node of the heart. The estimation of the total spectral power and further extraction of the high frequency (HF, 0.15–0.5 Hz, representative of parasympathetic activity) and low frequency (LF, 0.04–0.15 Hz, a mixture of sympathetic and parasympathetic activity) spectral components is then performed based on the estimated set of coefficients (see [17] for details). Sympathovagal balance (LF/HF) was also computed. The model allows estimation at arbitrary time-resolution, hence enabling precise alignment with fMRI volumes. In this paper, all series were estimated at a time resolution of 2 ms using a fixed model order k = 8, low-pass filtered at 0.33 Hz, and resampled at the time points coinciding with fMRI volumes as derived from timestamps in DICOM headers.
fMRI preprocessing
BOLD images were preprocessed using the FMRIB Software Library (FSL v5.0) [20] to correct for magnetic field inhomogeneities, skull stripping, motion correction, spatial smoothing (full-width at half-maximum = 5 mm) and spatial normalization to Montreal Neurological Institute (MNI) space. Any residual head motion after the steps above was corrected by performing probabilistic independent component analysis and removing components related to motion artifacts (e.g., positive/negative fMRI response on opposing edges of the brain, independent component time series spikes consistent with prior motion correction time series spikes) as well as physiological noise components (e.g. cardiac and respiratory CSF pulsations and signal from vessels outside the brain), as in our previous analyses [3, 10].
Connectivity estimation
Functional connectivity analyses were carried out using the CONN-fMRI functional connectivity toolbox v14 [21] (http://www.nitrc.org/projects/conn). This entailed comparing connectivity between the initial resting 5-minute fixation period (“BASELINE” state, see Figure 1) and the 5-minute period when subjects reported peak nausea levels (NAUSEA state). Nausea perception during this period ranged from 2 to 3 (on a scale of 0 to 4, where 4 is severe nausea on the verge of vomiting), and was previously reported in detail as part of a separate analysis [8, 10]. Briefly, the average rating (median/interquartile range) increased from 0/0-0 (BASELINE) to 2.56/2.08-3 (NAUSEA) in nausea-prone subjects, and from 0/0-0 (BASELINE) to 0.65/0.48-1 (NAUSEA) in non-nausea prone controls. Prior to further analysis, the fMRI signal was high pass filtered (fhigh > 0.007Hz) and the block design (fixation-stimulus-fixation, see Figure 1) was regressed out within each subject in order to minimize the influence of stimulus-related, unspecific co-activation on connectivity estimation. Spherical regions of interest (ROIs, diameter: 8mm) were centered on right (MNI X,Y,Z=36,−72,18 mm) and left (X,Y,Z=−42,−70,10 mm) MT+/V5, right (X,Y,Z=18,−64,6 mm) and left (X,Y,Z=−16,−68,8 mm) V1, and previously identified regions most closely linked with nausea-processing (right anterior insula (X,Y,Z=40,32,6 mm) and MCC (X,Y,Z=4,8,30 mm)) in our previous fMRI analysis [3]. Successively, condition-specific, between-ROI connectivity estimates were obtained by computing pairwise correlations (seed-to-seed) between all six of these ROIs. State-related connectivity differences (BASELINE vs. NAUSEA) were assessed through a general linear model (GLM) which modeled mean group connectivity (i.e. Fisher r-to-Z transformed Pearson correlation coefficients) as a between-subject factor and state-related connectivity as a within subject factor. Type I error was controlled through the use of cluster-level false discovery rate (FDR) correction across all pairs of ROIs (p<0.05). Changes in connectivity were considered statistically significant when associated with a p-value < 0.05.
Correlation analysis
In order to explore the autonomic and behavioral correlates of nausea-related changes in connectivity, we computed the following metrics within the BASELINE and NAUSEA analysis window for each subject: mean nausea rating (NAUSEA window only), mean HF, mean LF, mean LF/HF, as well as fractional changes defined as (NAUSEA-BASELINE)/BASELINE, for HF, LF and LF/HF. Detailed methods for this procedure were reported in our previous publications [8, 10]. Outcome metrics were used in the current study for correlational analyses with brain connectivity outcomes. These metrics were entered along with effect sizes (i.e. statistically significant changes in connectivity in NAUSEA with respect to BASELINE) and MSSQ score into bivariate non-parametric (Spearman-Rank) correlation analyses.
Results
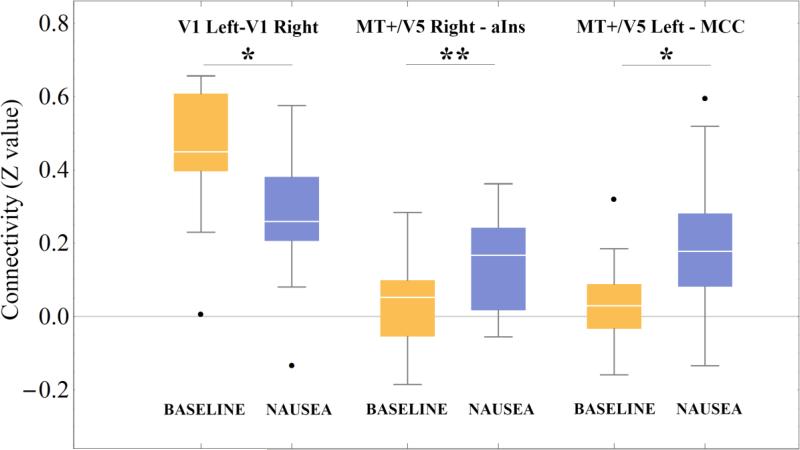
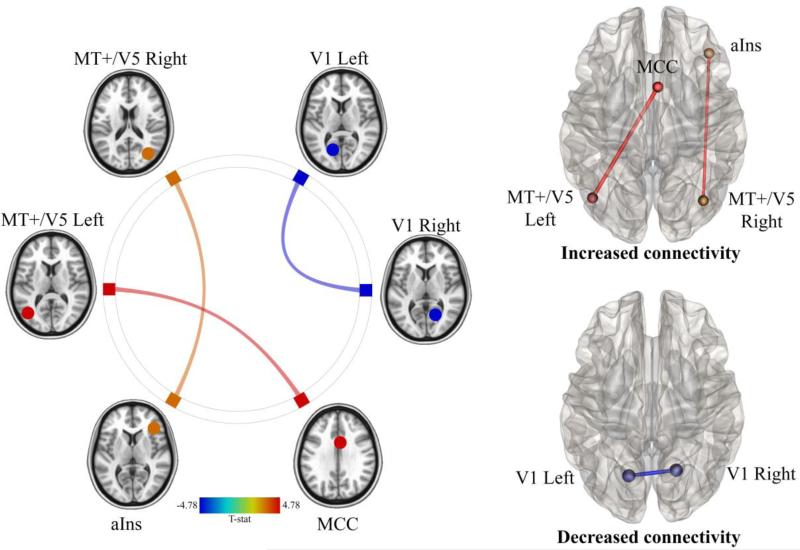
Compared to BASELINE, the NAUSEA state reduced connectivity between right and left V1 (p=0.045) and increased connectivity between right MT+/V5 and anterior insula (p=0.009) and between left MT+/V5 and MCC (p=0.03) (Figure 2). Figure 3 shown the anatomical localization of connections showing statistically significant stimulus-related changes in connectivity.
Figure 2.
Connectivity (Fisher r-to-Z transformed Pearson correlation coefficients) associated with baseline (BASELINE) as well as stimulus (NAUSEA) conditions for connections between right and left V1, between right MT+/V5 and anterior insula (aIns), and between left MT+/V5 and MCC. *=p<0.05, **=p<0.01, FDR corrected across all pairs of ROIs. Black dots: outliers.
Figure 3.
Changes in connectivity in the NAUSEA state with respect to the BASELINE state for motion sickness prone subjects. Red indicates increased connectivity. Blue indicates decreased connectivity.
No state-related changes in connectivity were noted for the control group, who experienced the same stimulation but did not report significant nausea.
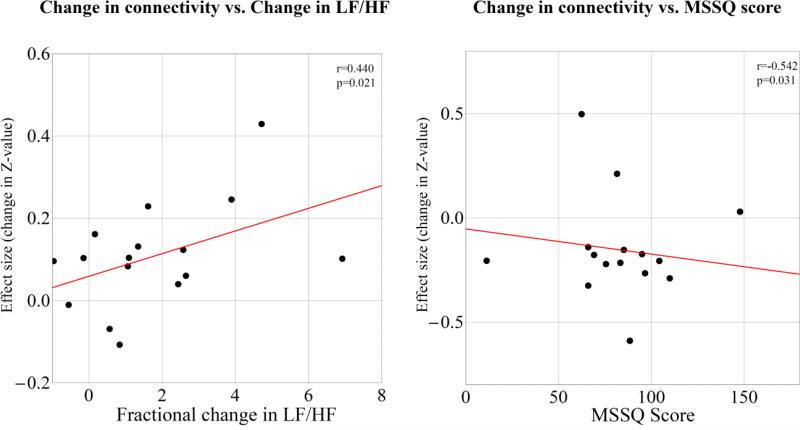
Table 1 shows a summary of correlation analysis results. We found that effect size (i.e. increase in connectivity) between right MT+/V5 and anterior insula significantly correlated with NAUSEA vs. BASELINE fractional change in LF/HF ratio (Spearman Rank Correlation coefficient: 0.44, p=0.021). Additionally, MSSQ score was negatively correlated (Spearman Rank Correlation coefficient: −0.542, p=0.031) with the NAUSEA-induced change in connectivity between left and right V1 (Figure 4).
Table 1.
Spearman Rank Correlations between subject specific metrics related to stimulus response as well as autonomic activity and effect sized in pairs of regions where a statistically significant, stimulus-related variation in connectivity was detected.
| V1/Left - V1-Right | MT+/V5 Right-aIns | MT+/V5 Left-MCC | |
|---|---|---|---|
| MSSQ score | −0.54* | 0.13 | 0.39 |
| Time to peak nausea | 0.35 | 0.10 | 0.21 |
| Median Nausea Rating | 0.03 | −0.27 | −0.01 |
| Mean HF (Fractional change) | 0.23 | −0.10 | −0.22 |
| Mean LF (Fractional change) | 0.24 | 0.20 | −0.08 |
| Mean LF/HF (Fractional change) | −0.04 | 0.44* | 0.17 |
Statistically significant correlations (p<0.05) are marked with an asterisk (*). “Fractional Change” in a variable X refers to [X(Stimulus) - X(Baseline)]/X(Baseline).
Figure 4.
Changes in connectivity in the NAUSEA state with respect to the BASELINE state as a function of fractional change in LF/HF ratio (left) and MSSQ score (right). “Effect size” is the difference in Z-values (Fisher r-to-Z transformed Pearson correlation coefficients) between the NAUSEA and the BASELINE state. Linear regression line is shown in red for visualization purposes.
Discussion
We demonstrated that visual stimulus vection-induced nausea resulted in alterations in functional connectivity between previously identified nausea processing and visual motion processing brain regions. Specifically, we found that a nausea state increased connectivity between right MT+/V5 and anterior insula, and between left MT+/V5 and middle cingulate cortex. Additionally, nausea reduced connectivity between right and left primary visual cortices. Interestingly, the change in MT+/V5 to insula connectivity was associated with a nausea-induced fractional change in LF/HF ratio, which was estimated using heart rate variability analysis in the same subjects. Thus, subjects who experienced a greater shift toward sympathetic outflow in sympathovagal balance also demonstrated increased connectivity between MT+/V5 and anterior insula. In addition, MSSQ score was negatively correlated with nausea-induced change in connectivity between left and right V1 – i.e. subjects who are more prone to motion sickness (greater MSSQ score), responded to visual stimulus vection-induced nausea with greater reduction in inter-hemispheric connectivity between primary visual areas that are highly connected at rest.
Our previous study found that a visual stimulus that produces motion sickness results in activation in bilateral MT+/V5 and V1, while increasing nausea during sustained stimulation by this stimulus was reflected in increasing activation of anterior insula [3]. A follow-up diffusion tensor imaging study noted altered microstructure along the inferior fronto-occipital fasciculus, the white matter pathway connecting MT+/V5 with anterior insula, in subjects reporting high susceptibility to motion sickness [22]. In addition, a recent combined autonomic/fMRI analysis noted that greater sympathetic modulation (indexed by skin conductance response) during nausea perception was associated with fMRI signal in anterior insula [10]. This confluence of evidence supporting insular and extrastriate cortical involvement in motion sickness perception and concomitant autonomic response is now extended in our current analysis, which demonstrated that not only does nausea increase functional connectivity between anterior insula and MT+/V5, but that greater increase in connectivity is specifically associated with nausea-induced increase in LF/HF ratio, a heart rate variability metric estimating sympathovagal balance. The anterior insula has been linked with sympathetic modulation by prior neuroimaging meta-analyses [7], and this region is known to play an important role in interoception [4-6], defined as conscious awareness of internal body states [4]. Our current study suggests that visually-induced motion sickness may produce nausea perception and autonomic modulation through greater information exchange between brain regions activated by such visual motion stimuli (i.e. MT+/V5) and interoceptive processing areas such as the insula. Also, we found significant correlations between effect size (i.e. stimulus-induced change in connectivity) and nausea-induced fractional change in LF/HF ratio, but not HF or LF individually. Our previous study also demonstrated that LF/HF ratio was most sensitive to the nauseogenic stimulus and the only parameter (amongst HF, LF, and LF/HF) to show statistically significant alterations in response to our nauseogenic stimulus [8]. However, future studies should confirm this result, as HF, in particular, is well suited as a metric for combined HRV-fMRI analyses.
Interestingly, we found that motion sickness reduces connectivity between left and right V1 seeds. A study of functional connectivity in response to visual motion (but not nausea) demonstrated that compared to a resting state, visual motion stimulation reduced functional connectivity between MT+/V5, which was activated by this task, and several visual processing areas, including cuneus and lingual gyrus [12]. More recently, Miyazaki et al. reported inter-hemispheric desynchronization between left and right MT+ during a brief (6-minute) visual stimulation that induced mild motion sickness [13]. This study compared connectivity during a global motion stimulus with that during a control, local motion stimulus, and also noted a trend towards decreased inter-hemispheric correlation in the primary visual cortices (V1) (though not statistically significant). While our study found a significant reduction in left to right V1 connectivity, it is worth noting that Miyazaki et al. investigated temporal correlation in the high frequency domain of the BOLD fMRI signal (f > 0.1 Hz). It is therefore possible that reduced correlation between left and right MT+ is mainly related to faster dynamics, while the desynchronization between left and right V1 is reflected in both low and high BOLD signal frequencies. Additionally, our analysis compared a nausea state during a translating stripes stimulation with a resting baseline state during crosshair fixation, raising the possibility that reduced interhemispheric V1 connectivity was due to differences luminance, image contrast, etc. Ultimately, this result is supported by numerous studies with other stimulus modalities showing reduced intrinsic functional connectivity for task-activated brain areas – i.e. when an area is activated by a stimulus, during sustained stimulation that region reduces its connectivity to areas it is typically connected to at rest. This phenomenon has been found for sustained auditory/cognitive [23], working memory [24], and pain [25, 26] stimuli. Additionally, connectivity between activated regions and brain areas outside that region's intrinsic network, may be upregulated during sustained stimulation. For instance, sustained pain was found to increase cortical representation specific S1 connectivity to salience network regions (i.e. insula) [26]. Similarly, in the current study we found that a visual motion stimulus that activates MT+/V5 increases connectivity between this regions and both insula and anterior cingulate cortex (i.e. salience network areas) during sustained stimulation state that induced nausea perception.
Several limitations should be noted. Firstly, while we applied a hypothesis-driven ROI approach to evaluate brain connectivity response to motion sickness induced nausea, other brain areas may also demonstrate altered connectivity in response to the stimulus. For instance, vection and motion sickness may also modulate connectivity for other activated areas such as prefrontal cortex, intraparietal sulcus, posterior insula, and caudate, as well as target brainstem nuclei, demonstrated to be activated by our own study [3] and others [27]. Secondly, due to limitations of the customized head coil we used for vection induction our data was acquired at 1.5T. Future studies will extend our approach to higher field, where the improved sensitivity should allow for more precise localization of both brain activation and connectivity response to vection-induced nausea.
In conclusion, vection-induced nausea increases connectivity between nausea processing brain regions and those that activate in response to the visual stimulus itself. This enhanced low-frequency coupling may support continual, and slowly evolving, nausea perception, and autonomic shifts toward sympathetic dominance. Disengaging this coupling may be a target for biobehavioral interventions aimed at reducing motion sickness severity.
Highlights.
We use fMRI to study brain connectivity changes under a nauseogenic stimulus
The stimulus reduced V1 (L) to V1 (R) connectivity
The stimulus increased MT+/V5 (R) to aIns and MT+/V5 (L) to MCC connectivity
Change in MT+/V5 to aIns connectivity was associated with change in sympathovagal balance
Nausea increases connectivity between stimulus response regions and nausea-processing regions
Acknowledgements
This work was supported by the National Institutes of Health (grant numbers P01-AT006663, R01-AT007550, and R01-AR064367 to VN, K23-DK069614 to BK, and R21-AR057920 to VN, BK and RB); the National Center for Research Resources (P41RR14075; CRC 1 UL1 RR025758, Harvard Clinical and Translational Science Center); the MGH Department of Anesthesia, Critical Care and Pain Medicine and the International Foundation of Functional Gastrointestinal Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geerligs L, Rubinov M, Cam C, Henson RN. State and Trait Components of Functional Connectivity: Individual Differences Vary with Mental State. J Neurosci. 2015;35(41):13949–61. doi: 10.1523/JNEUROSCI.1324-15.2015. doi: 10.1523/JNEUROSCI.1324-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31(21):7910–9. doi: 10.1523/JNEUROSCI.1296-11.2011. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napadow V, Sheehan JD, Kim J, Lacount LT, Park K, Kaptchuk TJ, Rosen BR, Kuo B. The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex. 2013;23(4):806–13. doi: 10.1093/cercor/bhs073. doi: 10.1093/cercor/bhs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–66. doi: 10.1038/nrn894. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 5.Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18(4):442–7. doi: 10.1097/01.wco.0000168079.92106.99. doi: 00019052-200508000-00015 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95. doi: 10.1038/nn1176. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 7.Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaCount LT, Barbieri R, Park K, Kim J, Brown EN, Kuo B, Napadow V. Static and dynamic autonomic response with increasing nausea perception. Aviat Space Environ Med. 2011;82(4):424–33. doi: 10.3357/asem.2932.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muth ER. Motion and space sickness: intestinal and autonomic correlates. Auton Neurosci. 2006;129(1-2):58–66. doi: 10.1016/j.autneu.2006.07.020. doi: 10.1016/j.autneu.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Sclocco R, Kim J, Garcia RG, Sheehan JD, Beissner F, Bianchi AM, Cerutti S, Kuo B, Barbieri R, Napadow V. Brain Circuitry Supporting Multi-Organ Autonomic Outflow in Response to Nausea. Cereb Cortex. 2016;26(2):485–97. doi: 10.1093/cercor/bhu172. doi: 10.1093/cercor/bhu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs G, Raabe M, Greenlee MW. Neural correlates of visually induced self-motion illusion in depth. Cereb Cortex. 2008;18(8):1779–87. doi: 10.1093/cercor/bhm203. doi: 10.1093/cercor/bhm203. [DOI] [PubMed] [Google Scholar]
- 12.Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15(8):1315–9. doi: 10.1097/01.wnr.0000129997.95055.15. doi: 00001756-200406070-00020 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki J, Yamamoto H, Ichimura Y, Yamashiro H, Murase T, Yamamoto T, Umeda M, Higuchi T. Inter-hemispheric desynchronization of the human MT+ during visually induced motion sickness. Exp Brain Res. 2015;233(8):2421–31. doi: 10.1007/s00221-015-4312-y. doi: 10.1007/s00221-015-4312-y. [DOI] [PubMed] [Google Scholar]
- 14.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. doi. [DOI] [PubMed] [Google Scholar]
- 15.Golding JF. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bull. 1998;47(5):507–16. doi: 10.1016/s0361-9230(98)00091-4. doi. [DOI] [PubMed] [Google Scholar]
- 16.Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56(1):216–23. doi: 10.1002/mrm.20925. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- 17.Barbieri R, Matten EC, Alabi AA, Brown EN. A point-process model of human heartbeat intervals: new definitions of heart rate and heart rate variability. Am J Physiol Heart Circ Physiol. 2005;288(1):H424–35. doi: 10.1152/ajpheart.00482.2003. doi: 10.1152/ajpheart.00482.2003. [DOI] [PubMed] [Google Scholar]
- 18.Calandra-Buonaura G, Toschi N, Provini F, Corazza I, Bisulli F, Barletta G, Vandi S, Montagna P, Guerrisi M, Tinuper P, Cortelli P. Physiologic autonomic arousal heralds motor manifestations of seizures in nocturnal frontal lobe epilepsy: implications for pathophysiology. Sleep Med. 2012;13(3):252–62. doi: 10.1016/j.sleep.2011.11.007. doi: 10.1016/j.sleep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Romigi A, Albanese M, Placidi F, Izzi F, Mercuri NB, Marchi A, Liguori C, Campagna N, Duggento A, Canichella A, Ricciardo Rizzo G, Guerrisi M, Marciani MG, Toschi N. Heart rate variability in untreated newly diagnosed temporal lobe epilepsy: Evidence for ictal sympathetic dysregulation. Epilepsia. 2016;57(3):418–26. doi: 10.1111/epi.13309. doi: 10.1111/epi.13309. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 22.Napadow V, Sheehan J, Kim J, Dassatti A, Thurler AH, Surjanhata B, Vangel M, Makris N, Schaechter JD, Kuo B. Brain white matter microstructure is associated with susceptibility to motion-induced nausea. Neurogastroenterol Motil. 2013;25(5):448–50. e303. doi: 10.1111/nmo.12084. doi: 10.1111/nmo.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbabshirani MR, Havlicek M, Kiehl KA, Pearlson GD, Calhoun VD. Functional network connectivity during rest and task conditions: a comparative study. Hum Brain Mapp. 2013;34(11):2959–71. doi: 10.1002/hbm.22118. doi: 10.1002/hbm.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–45. doi: 10.1016/j.neuropsychologia.2006.06.017. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner F, Garcia RG, Kim H, Barbieri R, Wasan AD, Edwards RR, Napadow V. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015;67(5):1395–405. doi: 10.1002/art.39043. doi: 10.1002/art.39043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Loggia ML, Edwards RR, Wasan AD, Gollub RL, Napadow V. Sustained deep-tissue pain alters functional brain connectivity. Pain. 2013;154(8):1343–51. doi: 10.1016/j.pain.2013.04.016. doi: 10.1016/j.pain.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keshavarz B, Riecke BE, Hettinger LJ, Campos JL. Vection and visually induced motion sickness: how are they related? Front Psychol. 2015;6:472. doi: 10.3389/fpsyg.2015.00472. doi: 10.3389/fpsyg.2015.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]