Abstract
Buttercup (Ranunculus spp.) flowers are exceptional because they feature a distinct gloss (mirror-like reflection) in addition to their matte-yellow coloration. We investigated the optical properties of yellow petals of several Ranunculus and related species using (micro)spectrophotometry and anatomical methods. The contribution of different petal structures to the overall visual signal was quantified using a recently developed optical model. We show that the coloration of glossy buttercup flowers is due to a rare combination of structural and pigmentary coloration. A very flat, pigment-filled upper epidermis acts as a thin-film reflector yielding the gloss, and additionally serves as a filter for light backscattered by the strongly scattering starch and mesophyll layers, which yields the matte-yellow colour. We discuss the evolution of the gloss and its two likely functions: it provides a strong visual signal to insect pollinators and increases the reflection of sunlight to the centre of the flower in order to heat the reproductive organs.
Keywords: flower colour, epidermis, structural coloration, thin-film interference, pigment, starch
1. Introduction
Many flowering plants display brightly coloured flowers to distinguish themselves from their environment and to attract pollinators [1–4]. In virtually all plant species floral coloration is pigmentary, i.e. the visual signal is the result of light scattering by cells and organelles containing pigments that absorb light in a wavelength-specific way [5–7]. Diffusely reflected light with wavelengths outside the absorption range of the pigment thus determines the colour of the petals. The absorption range depends on the nature of the pigment, e.g. blue-absorbing carotenoids yield yellow colours (reviewed by [6]).
Structural colours arise in regularly ordered, nano-sized structures composed of materials with different refractive indices [8], and are common among animals, such as birds, butterflies and beetles [9–11]. Structural colours require (quasi-)regularly patterned structures with typical distances of the order of the wavelength of light (several hundred nanometres) [8,12]. The reflecting structure can be a single layer acting as a thin film, such as in bird feathers and butterfly wing scales [13–16], a multilayer, as in beetle elytra and fish scales [10,17], or a complex photonic crystal, as in peacock feathers [18] and some butterfly species [9]. Typical for structural coloration with long-range spatial periodicity is that it is highly directional and angle dependent (iridescent).
Structural coloration is exceedingly rare among flowers, because the interiors and surfaces of flowers are very irregularly shaped and ordered [19,20]. In those plant species with flowers that do have structures with periodicity of the order of the wavelength of light, structural coloration and iridescence can only be observed by applying very local and directional illumination [19]. Consequently, under natural viewing and illumination conditions the coloration of flowers is generally pigmentary and not structural [19,21].
The flowers of many buttercups (Ranunculus spp.) are a clear exception to this rule, as they feature a distinct gloss (i.e. a mirror-like reflection) in addition to their overall yellow coloration [19,22,23]. Previous studies suggested that the gloss is due to the very flat and smooth epidermal layer that acts as a mirror [19,22], possibly enhanced by an air layer immediately below the epidermis [24]. Vignolini et al. [24] applied optical multilayer theory to model the reflectance spectra, but stated that an optical model of the whole complex petal structure was missing. A comprehensive overview that quantifies the contribution of different petal components to the visual signal of Ranunculus flowers has not yet been developed, leaving the complex nature of the flowers' coloration unknown.
Inspired by the likely structural origin of the buttercup's glossiness [19,23,24], we present here an in-depth study of the reflection characteristics of Ranunculus flowers. The genus Ranunculus comprises more than 500 species with a virtually cosmopolitan distribution [25]. We investigated the detailed spectral characteristics of three annual, herbaceous meadow buttercups, i.e. Ranunculus repens L, R. acris L. and R. lingua L, as well as the glossy flowers of the related lesser celandine Ficaria verna Huds. (formerly known as R. ficaria) and the non-glossy flowers of the kingcup (also called marsh marigold) Caltha palustris L. (all Ranunculaceae). We applied various spectrophotometric methods, anatomical studies and a recently developed optical model [26] to assess the contributions of different petal components to the buttercup flowers' bright-yellow coloration. We conclude that the coloration of buttercup flowers is due to a very rare combination of structural and pigmentary coloration, and we discuss the functional significance of the gloss.
2. Material and methods
2.1. Plant material, photography and spectrophotometry
Flower samples were obtained from meadows around Groningen, The Netherlands. Flowers were photographed with a Nikon D70 digital camera having an F Micro-Nikkor (60 mm, f2.8) macro-objective (Nikon, Tokyo, Japan). Petal details were photographed with an Olympus SZX16 stereomicroscope equipped with an Olympus DP70 digital camera (Olympus, Tokyo, Japan) and a Zeiss Universal Microscope (Zeiss, Oberkochen, Germany) with a Mueller DCM510 camera (Mueller Optronic, Erfurt, Germany). To visualize the contribution of the gloss, photographs of pieces of petal were made by using white light with both a parallel and a crossed analyser on the microscope.
The overall reflectance and transmittance spectra of the dominant-coloured (central) area of the petals of at least five different plants were measured with an integrating sphere. For reflectance measurements, the flower was directionally illuminated from within the integrated sphere; the illuminated area had a diameter of about 5 mm. For transmittance measurements, the petal was illuminated from outside the sphere with an optical fibre at an area with diameter approximately 1 mm (for equipment details see [26]). The spectral characteristics of very small petal areas (5–10 µm diameter) were measured with a microspectrophotometer (MSP), following [26]. For measurements of the absorbance spectrum of the buttercup petal's pigment, small pieces of petal were immersed in water. Transmittance spectra were measured on the isolated tissues with the MSP, from which absorbance spectra were calculated. For details about imaging scatterometry, see the electronic supplementary material (see also [19]).
2.2. Cryo-electron microscopy
Cryo-electron microscopy (cryoSEM) was applied to study the anatomy of R. acris petals. Petals were glued in a special copper holder and quickly frozen in nitrogen slush. The samples were examined in a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan) equipped with an Oxford CT1500 Cryostation for cryoSEM. Electron micrographs were acquired from uncoated frozen samples.
2.3. Thin-film optics
We calculated the reflectance of a thin film with thickness d and refractive index n in air using the classical Airy formulae. With normal illumination the reflectance spectrum has extrema at wavelengths λe = 4nd/u, with u an integer; the reflectance is maximal when u is odd and minimal when u is even [14,27]. The wavenumbers of the extrema (ke = 2π/λe) hence are a linear function of u: ke = su, with slope s = π/(2nd). We assumed an upper epidermis with a refractive index of n = 1.4 [24], which faced air on both sides and contained β-carotene with peak absorbance A = 1.4 (measured at 448 nm; see below). In our modelling, we considered a number of cases where the thin film had a Gaussian-distributed varying thickness with mean 2.7 µm and standard deviation σ = 0, 25, 50, 75, 100 and 125 nm.
2.4. Modelling petal reflectance and transmittance
The diffuse coloration of flowers is due to the random pathways of light propagating in the petal interior [26,28]. We modelled petal reflectance and transmittance by applying Kubelka & Munk [29] theory—which is often used to model the optical properties of plant leaves [30–35]—combined with a calculation procedure for a stack of absorbing and scattering layers (electronic supplementary material). By implementing model parameters found in real flowers, the relative contribution of different floral elements to the overall visual signal can be quantified [7,26,28].
The parameters of the model were based on our anatomical findings. We assumed for the upper epidermis a Gaussian-distributed varying thickness with mean d = 2.7 µm and standard deviation 125 nm, for the starch layer a thickness of 38 µm [36], and for the mesophyll and lower epidermis thicknesses of 100 and 40 µm, respectively. For the pigment of the upper epidermis, we estimated a peak absorption coefficient κ = A/(log10e d) = 1.4/(0.4343 × 2.7) = 1.2 µm−1; for the mesophyll and lower epidermal layers we assumed peak absorption coefficients of 0.02 and 0.04 µm−1, respectively. By comparing the experimental and simulated data, the scattering coefficients of the starch layer, mesophyll and lower epidermis were estimated to be 10, 5 and 2 mm−1, respectively. Finally, the surface reflectance of the lower epidermis (which is solely due to differences in refractive indices between the very last layer and air) was assumed to be 0.1 (and hence the transmittance was 0.9).
3. Results
3.1. Spectral measurements and anatomy
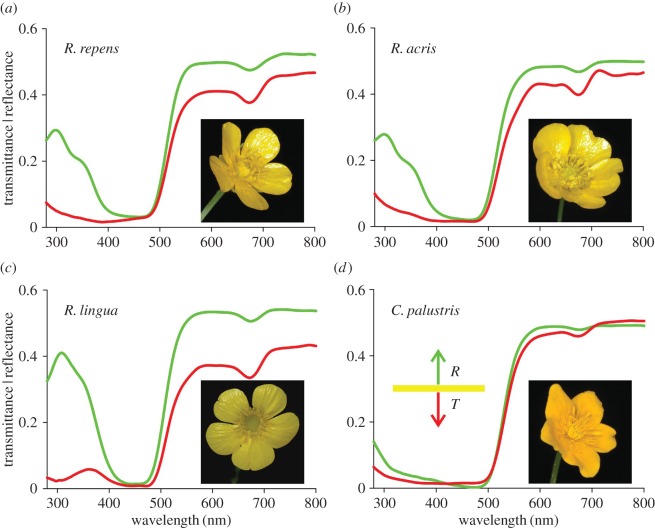
For all investigated buttercup flowers, the reflectance and transmittance was low in the blue wavelength range and high above 500 nm (figure 1). The long-wavelength reflectance was markedly higher than the transmittance. This is distinctly different from the majority of flowers, where the (long-wavelength) reflectance is lower than the transmittance [7]. The transmittance spectra showed a slight depression at approximately 680 nm, which suggests the presence of α-chlorophyll. The flowers of C. palustris exhibited a very low reflectance in the ultraviolet (UV) compared with the Ranunculus species. Whereas for the kingcup the reflectance and transmittance spectra were rather similar, for the buttercups these spectra differed from each other in the UV, suggesting that their petals are asymmetric in pigmentation and/or anatomy.
Figure 1.
Reflectance and transmittance spectra of petals of four Ranunculaceae species. (a) Ranunculus repens, (b) R. acris, (c) R. lingua and (d) Caltha palustris. Green curves: reflectance, R; red curves: transmittance, T.
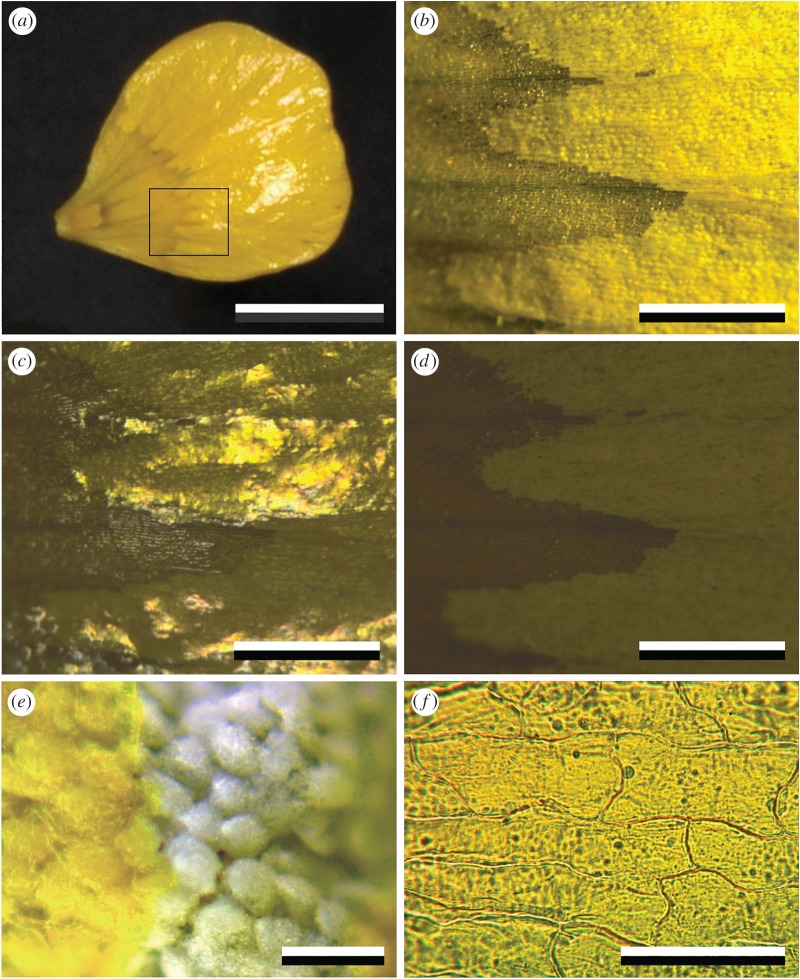
The buttercups are glossy, as is clearly visible in the main petal area (figures 1a–c and 2a,c). When observed with a low-power microscope, applying oblique illumination, the gloss was avoided and neat rows of effectively scattering granules were then discernible in the main petal area (figure 2b). While applying epi-illumination with polarized light, the gloss was prominently visible with a parallel polarizer and analyser (figure 2c), but it was fully extinguished by a crossed analyser; then only the depolarized backscattering from the inner petal structures remained (figure 2d). The starch granules below the epidermis are white (figure 2e), indicating that the yellow colour of the petals is due to a short-wavelength absorbing pigment concentrated in the upper epidermis. Indeed, the isolated upper epidermis, obtained by gently peeling, shows a bright-yellow colour, clearly due to a homogeneously distributed blue-absorbing pigment (figure 2f).
Figure 2.
Coloration of a glossy R. acris petal. (a) The glossiness is restricted to the main distal area of the upper (adaxial) side. (b) Close-up of the area indicated by the rectangle in (a), showing granular rows in the main petal area with oblique illumination. (c) The same area with normal epi-illumination. (d) The same area and illumination, but with crossed polarizer and analyser. (e) Close-up of a petal with disrupted upper epidermis, showing white starch cells. (f) Isolated upper epidermis observed in transmitted light. Scale bars: (a) 1 cm, (b–d) 1 mm, (e) 100 µm, (f) 50 µm.
The mesophyll and lower epidermal layers markedly contribute to the light scattering. Focusing at the lower epidermal layer reveals yellow reflections with bright contours shining through. The cell contours became distinctly visible when focusing at the mesophyll layer (figure 3a,b), showing that the boundaries of the mesophyll cells are strongly scattering. When the air gaps are filled with water, the reflectance is about twofold smaller (electronic supplementary material, figure S2), because the interior of the flower is then more homogeneous and the refractive index contrast between the normally air-filled gaps and the surrounding cells is diminished.
Figure 3.
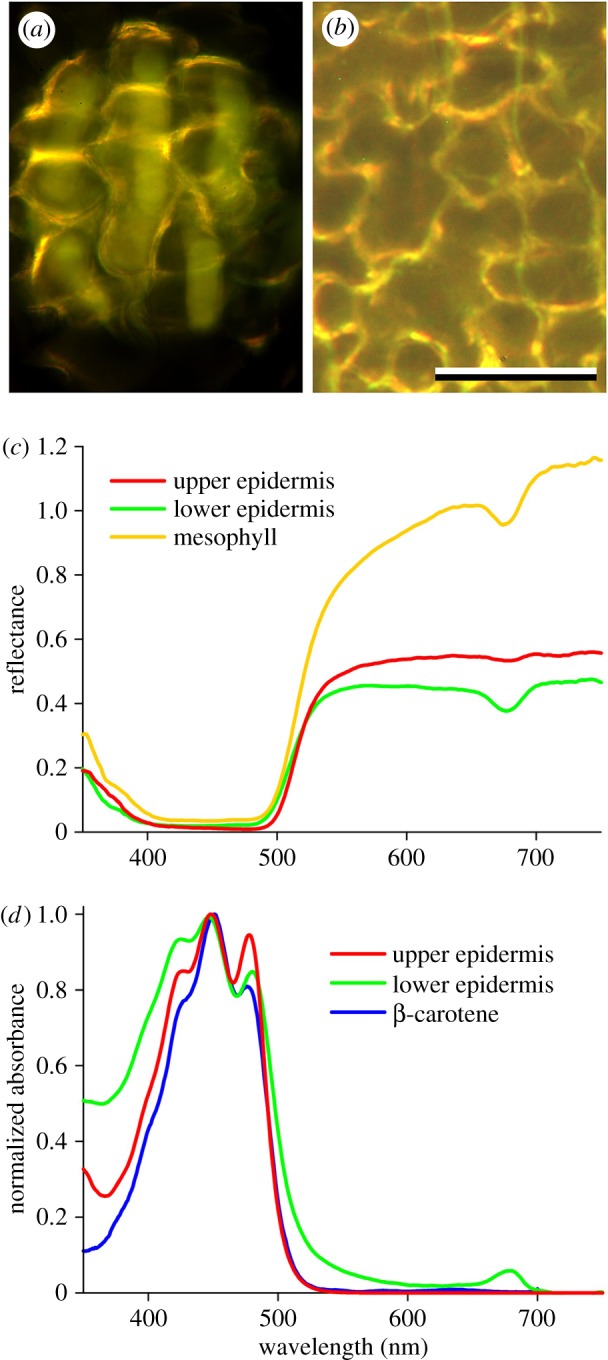
Coloration of the abaxial side of R. acris petals and pigmentation. (a) A small area observed with bright-field illumination and focus at the lower epidermis. (b) Dark-field illumination with focus at the mesophyll layer; scale bar: (a,b) 50 µm. (c) Reflectance spectra measured from 5–10 µm sized areas with the MSP of the lower and isolated upper epidermis and a boundary area of mesophyll cells. (d) Normalized absorbance spectra measured with the MSP from an isolated upper and lower epidermis, together with the spectrum of β-carotene in hexane. (Adapted from http://omlc.org/spectra/PhotochemCAD/data/041-abs.txt.)
We visualized the spatial reflection properties of the gloss and the backscattering from the petal interior with an imaging scatterometer (electronic supplementary material, figure S1a). The scatterogram of the adaxial side of the Ranunculus flowers yielded a bright spot created by the specular upper epidermis together with a wide-field yellow light distribution due to the backscattering petal interior (electronic supplementary material, figure S1b). The abaxial side of the petals did not show the gloss, which is due to the convex surface of the epidermal cells (electronic supplementary material, figure S1c,d). Similarly, the flowers of C. palustris, which feature conically shaped epidermal cells, featured a matte-yellow coloration. Owing to their irregular shape, the epidermal cones effectively scatter light into a wide angular space (electronic supplementary material, figure S1e,f; see also [19]).
We further investigated the pigmentation of the epidermal layers by measuring the reflectance and transmittance spectra of very small petal areas with an MSP. The shapes of the reflectance spectra measured abaxially from the bright contours and in between the contours were different (compare figure 3c, mesophyll and lower epidermis), which is likely to be due to wavelength-dependent scattering of the mesophyll cells. Both spectra showed a distinctly depressed reflectance at approximately 680 nm, characteristic for α-chlorophyll, which is absent in the spectra measured from non-glossy, diffusely reflecting areas at the adaxial side (figure 3c, upper epidermis), indicating an asymmetrical pigment deposition in the petal. Transmittance measurements on the isolated upper and lower epidermal layers of R. acris yielded the absorbance spectrum of a blue-absorbing pigment (figure 3d) very similar to the spectrum reported for β-carotene dissolved in hexane; yet, the peaks in the measured buttercup spectra (at 425, 448 and 478 nm) were more pronounced (figure 3d). For the upper epidermis, the estimated absorbance value at the peak wavelength 448 nm was 1.4 ± 0.3, whereas for the lower epidermis the absorbance was more variable: 0.8 ± 0.5. The distinct peak at approximately 680 nm in the absorbance spectrum of the lower epidermis corroborated our previous finding of the presence of a minor amount of α-chlorophyll (figure 3d; see also the electronic supplementary material, figure S2a, dry).
Cryo-electron microscopy (CryoSEM) on R. acris petals showed that petals consist of differently structured layers. The upper epidermis has a very constant thickness of approximately 2.7 µm and a very smooth surface (see also [19]). Air spaces separated the plate-like upper epidermis and the underlying starch granule layer; locally the upper epidermis touched the starch granules, providing mechanical support. The size of the air spaces is variable, which is mostly due to the irregularly shaped starch cells (figure 4a). We combined these new anatomical data with previous findings [36–38] to conceive a diagram of the inner structure of the Ranunculus petals (figure 4b). The very thin upper epidermis (which contains highly concentrated carotenoid pigment) faces slanted palisade parenchymal cells [36,37] that are filled with grainy starch. The mesophyll and lower epidermis form additional layers; both contain carotenoid pigment and α-chlorophyll, mostly in the boundary areas. In between the cells variably sized air spaces exist that contribute to the overall scattering.
Figure 4.
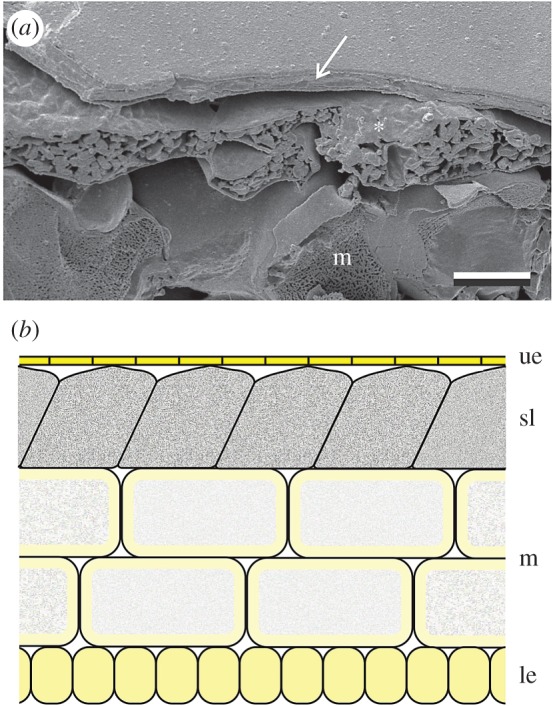
Anatomy of an R. acris petal. (a) Low-temperature scanning electron micrograph of a cut petal. The upper epidermis (arrow) is a flat layer with thickness of a few micrometres. Below the epidermis are the randomly distributed starch cells (asterisk). The size of the air spaces beneath the epidermis is variable and large air spaces exist between the starch cells and cells of the mesophyll layer. Scale bar: 10 µm. (b) Diagram of a Ranunculus petal; ue: upper epidermis; sl: starch layer; m: mesophyll; le: lower epidermis.
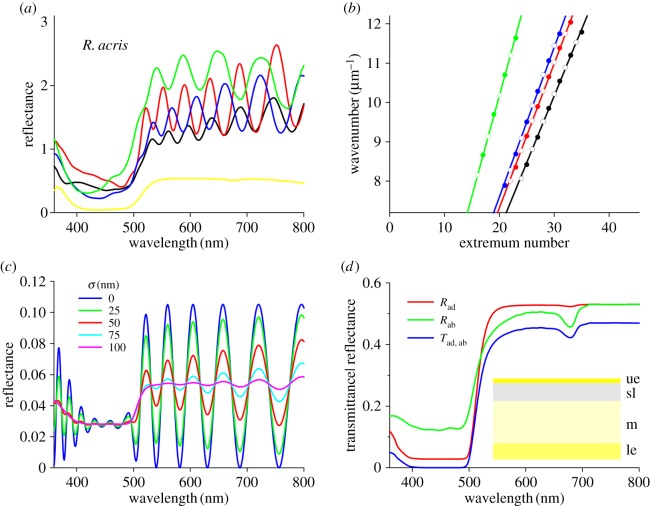
The electron micrographs demonstrated that the upper epidermis is essentially a thin plate separated from the starch layer by (mostly) air space, and it may thus act as an optical thin film in air. Indeed, the adaxial reflectance spectra of very small petal areas feature characteristic thin-film oscillations (figure 5a and electronic supplementary material, figure S3a,c). The oscillations were absent in the blue wavelength range, where the carotenoid pigment strongly suppressed the reflectance (figure 5a and electronic supplementary material, figure S3a,c). However, in the partially unpigmented glossy petals of Ficaria verna oscillations are also visible in the blue wavelength range (electronic supplementary material, figure S4; white glossy Ficaria and Ranunculus morphs are found occasionally [22]), corroborating our findings of an optical thin film. By fitting a linear function to the wavelengths of the reflectance extrema converted into wavenumbers, we derived from R. repens, R. acris and R. lingua average thickness values of 2.3 ± 0.3 µm, 2.7 ± 0.4 µm and 3.1 ± 0.5 µm, respectively, which is in good agreement with the anatomy (figure 4a).
Figure 5.
Measurements and modelling of an R. acris petal. (a) Reflectance spectra of small areas measured in the main distal area of the petal. The oscillating spectra are from areas with gloss; the yellow curve was obtained from a matte area, that is, where the surface reflections were outside the aperture of the MSP's objective. (b) Wavenumber values of the oscillation extrema. The linear fits to the extrema wavenumbers (closed symbols: maxima; open symbols: minima) yielded thickness values of the upper epidermis. (c) Modelling the reflectance of the upper epidermis with varying thickness. (d) Modelling reflectance (R) and transmittance (T) spectra of a buttercup petal considered as a stack of layers with different absorption and scattering coefficients (ad: adaxial; ab: abaxial; ue: upper epidermis; sl: starch layer; m: mesophyll; le: lower epidermis).
3.2. Optical modelling
We performed optical modelling to gain further understanding of the upper epidermis acting as a thin film. In the UV and long-wavelength range the reflectance featured strong oscillations, depending on the variation in the thickness, but in the blue wavelength range all calculated reflectance spectra exhibited a distinct trough, due to the absorbing β-carotene (figure 5c). The oscillations vanished when the standard deviation of the thickness exceeded approximately 5% (figure 6c, σ ≥ 100 nm; see also electronic supplementary material, figure S5), explaining why clear oscillations are only measurable from very small areas. To investigate the possible contributions of additional petal layers, we also considered an optical model incorporating a narrow air layer in between the epidermis and the starch layer, thus causing a multilayer (sensu [24]). This yielded oscillating spectra with a similar periodicity to the single thin film but with slightly higher peak values (electronic supplementary material, figure S5). However, because the air space size is highly variable (figure 4a) and the starch layer will act as a wide-field scatterer rather than as a plane reflector, we conclude that multilayer interference is unrealistic.
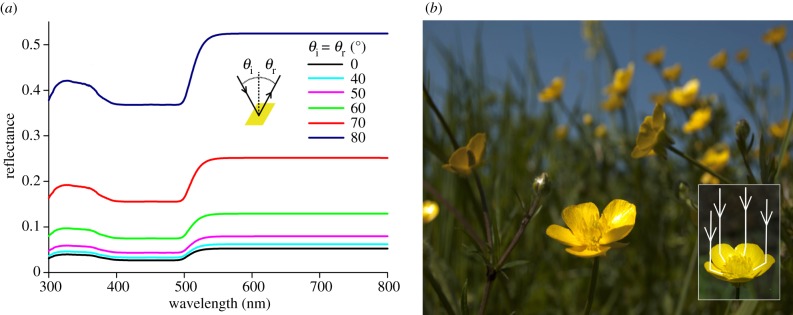
Figure 6.
Increased light reflection by buttercup petals under large angles. (a) The thin-film reflectance of the petal's epidermis for unpolarized light calculated for a few angles of incidence. The angles of illumination and reflection (θi and θr) were identical. (b) A meadow with many flowering R. acris plants. The flowers have an approximately paraboloid shape and are collectively facing the sun. Inset: simplified visualization of light reflection by the petals of an R. acris flower to the reproductive organs. Light reflected from the distal and central parts of the petal is reflected towards the reproductive organs.
Quantitative insight into flower coloration can be gained by treating the petals as a stack of layers, where each layer is characterized by its specific reflectance and transmittance spectra [26]. Our anatomical studies showed that the buttercup's upper epidermis can be considered as a homogeneous medium with negligible scattering, and that the starch, mesophyll and lower epidermal layers strongly scatter. We applied a Kubelka–Munk-layer-stack model (Material and methods, electronic supplementary material, [26]) to an R. acris petal treated as a four-layer stack. This yielded distinctly different adaxial and abaxial reflectance spectra (figure 5d). The adaxial reflectance (Rad) had a deeper trough in the blue wavelength range than the abaxial reflectance (Rab), due to the higher concentration of carotenoid pigment in the upper epidermis compared with the lower cell layers, whereas the abaxial reflectance spectrum had a pronounced dip at approximately 680 nm, due to the exclusive abaxial presence of chlorophyll (figure 5d).
4. Discussion
4.1. The coloration toolkit: a pigmented thin film and underlying backscattering layers
This study describes the special combination of buttercup flowers' pigmented thin-film reflector and strongly scattering underlying starch and mesophyll layers. Pigmentary filtering of structural coloration is common in various animal taxa, such as butterflies (e.g. [14,39]) and birds (e.g. [40]), but is very rare among flowers. In flowers of the vast majority of plant species, specular reflections are absent, because of the presence of conical epidermal cells and/or epidermal microstructures [5,20] that scatter light into a wide angular space [19]. When directionally illuminated, buttercup flowers display a strong gloss in addition to an overall matte-yellow colour (figures 1 and 2).
The glossy petals of Ranunculus have fascinated scientists for more than a century [5,22,23,38]. Pioneering work by Parkin [36,37] showed that the starch cells are arranged in a slanted manner and occur in many Ranunculus species. More recently, Vignolini et al. [24] discovered that the epidermal and starch layers are separated by an air layer, a feature that had not been described in any plant system before. By combining previous and new anatomical findings, spectroscopy and optical models, we show that buttercup flowers feature a special coloration system.
The clearly oscillating reflectance spectra measured from very small petal areas of Ranunculus and Ficaria species demonstrated that the epidermal layer acts as a thin film. The upper epidermis locally has a constant thickness, and optical modelling showed that this thickness—independent of the air space and starch layer—determines the periodicity of the oscillations. The thickness of the upper epidermis can thus be directly derived from measurements of the petal reflectance (figure 5 and electronic supplementary material, figures S3 and S4). Reflectance measurements from various areas of the same petal yielded thicknesses varying by more than 10%, resulting in smooth reflectance spectra when measured from larger areas (cf. figures 1 and 5c). The upper epidermis is only locally flat; over larger areas the surface is wrinkled, so that the gloss is restricted to those areas that have the right mirror position with respect to the light source and observer (figure 2a,c).
The upper epidermis contains a high concentration of carotenoid pigment and therefore it serves as an effective spectral filter for the light backscattered by the underlying starch and mesophyll layers. Mesophyll cells commonly occur in flowers [7], but starch cells are almost exclusively found in buttercups [5,22,25], where they act as strong, diffuse reflectors, enhancing the buttercup's brilliance (figures 2b,e, 5d and electronic supplementary material, figure S2).
An intriguing question concerns the radiation of petal glossiness. Phylogenetic reconstruction using recently published phylogenies [25] shows that glossiness is predominant among Ranunculus species and also occurs in related genera (electronic supplementary material, figure S6). The most conservative interpretation would be that glossiness—due to a thin-film epidermis—is the ancestral state for Ranunculus, and also for Ficaria, Oxygraphis and Halerpestes (electronic supplementary material, figure S6), which are more distantly related genera [25,41]. In several species glossiness has been lost over the course of evolution (electronic supplementary material, figure S6). The starch layer is also found in most Ranunculus species [25]. Large-scale sampling of Ranunculaceae species will elucidate the ancestral origin and the anatomical variation between species.
4.2. Functionally glossy?
The glossiness presumably has two non-mutually exclusive functions: enhancing the visual signal to pollinators and increasing the light reflection to the reproductive organs. For nearly normally incident light, the reflectance amplitude of the gloss is no more than approximately 5% (figure 6a), but the reflectance amplitude considerably increases with an increasing angle of incidence, i.e. away from normally incident light (figure 6a). Therefore, pollinators that approach the flower under a large mirror angle will perceive the gloss as a flash. The spectral modulation of the gloss signal is nonetheless rather low (i.e. a glare without a distinct hue), especially under a large incident angle (figures 5a and 6a); hence the colour contrast, which is considered important for pollinators to detect flowers [1,42–44], is limited. We conclude that the bright flash may provide a long-distance signal to pollinators, whereas at short distances the diffuse UV-yellow coloration will constitute the flower's visual signal.
Buttercup flowers are heliotropic [45–49], and when ambient temperatures are low they have approximately the shape of a paraboloid (figure 6b). Under these circumstances, incident sunlight that reaches the petal surface under a large angle will not be reflected to the outside but towards the central flower area where the reproductive structures are located (see also [50]). This will cause increased floral temperatures, which enhances seed and pollen maturation and is preferred by pollinators [45–53]. Thus, due to the combined effect of near-closure and heliotropism of the flowers, the petals' thin film will enhance light reflection to the reproductive organs. Indeed, under natural conditions, the centre of the paraboloid-shaped glossy flowers of the arctic buttercup, R. adoneus, were found to be several degrees warmer than the ambient air [54].
Abiotic factors, including sunlight, have been previously documented to impose selective pressures on flower anatomy and coloration [53,55,56]. For example, Koski & Ashman [56] recently reported that exhaustive amounts of internal UV reflections can be deleterious for plant reproduction. Based on a large geographical sample, they showed that the size of the UV-absorbing area of a (quasi)paraboloid-shaped flower depends on the amount of sunlight. Experiments confirmed that high levels of solar irradiance (as in regions close to the Equator) favour phenotypes with larger UV-absorbing petal areas, so as to reduce (harmful) UV reflectance to the pollen and prevent DNA damage [56]. Future experiments, e.g. manipulation of the internal light reflections, will illuminate the functional significance of the buttercup's remarkable anatomy.
Supplementary Material
Acknowledgements
The authors are grateful to Hein Leertouwer, Marten Staal and Wanda Reen for support and three referees for suggestions for improvements. Drs Beverley Glover, Silvia Vignolini and Bodo Wilts kindly commented on an early version of this manuscript.
Authors' contributions
All authors performed measurements; C.J.v.d.K. and D.G.S. analysed results; C.J.v.d.K. and D.G.S. drafted the manuscript, which was approved by all authors.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the AFOSR/EOARD (award no. FA9550-15-1-0068).
References
- 1.Chittka L, Menzel R. 1992. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J. Comp. Physiol. A 171, 171–181. ( 10.1007/BF00188925) [DOI] [Google Scholar]
- 2.Dyer AG, Boyd-Gerny S, McLoughlin S, Rosa MG, Simonov V, Wong BB. 2012. Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proc. R. Soc. B 279, 3606–3615. ( 10.1098/rspb.2012.0827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papiorek S, et al. 2016. Bees, birds and yellow flowers: pollinator-dependent convergent evolution of UV patterns. Plant Biol. 18, 46–55. ( 10.1111/plb.12322) [DOI] [PubMed] [Google Scholar]
- 4.van der Kooi CJ, Pen I, Staal M, Stavenga DG, Elzenga JTM. 2016. Competition for pollinators and intra-communal spectral dissimilarity of flowers. Plant Biol. 18, 56–62. ( 10.1111/plb.12328) [DOI] [PubMed] [Google Scholar]
- 5.Kay QON, Daoud HS, Stirton CH. 1981. Pigment distribution, light reflection and cell structure in petals. Bot. J. Linn. Soc. 83, 57–83. ( 10.1111/j.1095-8339.1981.tb00129.x) [DOI] [Google Scholar]
- 6.Grotewold E. 2006. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 57, 761–780. ( 10.1146/annurev.plant.57.032905.105248) [DOI] [PubMed] [Google Scholar]
- 7.van der Kooi CJ, Elzenga JTM, Staal M, Stavenga DG. 2016. How to colour a flower: on the optical principles of flower coloration. Proc. R. Soc. B 283, 20160429 ( 10.1098/rspb.2016.0429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasarao M. 1999. Nano-optics in the biological world: beetles, butterflies, birds, and moths. Chem. Rev. 99, 1935–1962. ( 10.1021/cr970080y) [DOI] [PubMed] [Google Scholar]
- 9.Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424, 852–855. ( 10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 10.Seago AE, Brady P, Vigneron JP, Schultz TD. 2009. Gold bugs and beyond: a review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Soc. Interface 6, S165–S184. ( 10.1098/rsif.2008.0354.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavenga DG, Leertouwer HL, Marshall NJ, Osorio D. 2010. Dramatic colour changes in a bird of paradise caused by uniquely structured breast feather barbules. Proc. R. Soc. B 278, 2098–2104. ( 10.1098/rspb.2010.2293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita S. 2008. Structural colors in the realm of nature. Singapore: World Scientific. [Google Scholar]
- 13.Nakamura E, Yoshioka S, Kinoshita S. 2008. Structural color of rock dove's neck feather. J. Phys. Soc. Jpn 77, 124801 ( 10.1143/JPSJ.77.124801) [DOI] [Google Scholar]
- 14.Stavenga DG. 2014. Thin film and multilayer optics cause structural colors of many insects and birds. Mater. Today 1, 109–121. ( 10.1016/j.matpr.2014.09.007) [DOI] [Google Scholar]
- 15.Doucet SM, Shawkey MD, Hill GE, Montgomerie R. 2006. Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 209, 380–390. ( 10.1242/jeb.01988) [DOI] [PubMed] [Google Scholar]
- 16.Maia R, Caetano JVO, Báo SN, Macedo RH. 2009. Iridescent structural colour production in male blue-black grassquit feather barbules: the role of keratin and melanin. J. R. Soc. Interface 6, S203–S211. ( 10.1098/rsif.2008.0460.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka S, Matsuhana B, Tanaka S, Inouye Y, Oshima N, Kinoshita S. 2011. Mechanism of variable structural colour in the neon tetra: quantitative evaluation of the Venetian blind model. J. R. Soc. Interface 8, 56–66. ( 10.1098/rsif.2010.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zi J, et al. 2003. Coloration strategies in peacock feathers. Proc. Natl Acad. Sci. USA 100, 12 576–12 578. ( 10.1073/pnas.2133313100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Kooi CJ, Wilts BD, Leertouwer HL, Staal M, Elzenga JTM, Stavenga DG. 2014. Iridescent flowers? Contribution of surface structures to optical signaling. New Phytol. 203, 667–673. ( 10.1111/nph.12808) [DOI] [PubMed] [Google Scholar]
- 20.Papiorek S, Junker RR, Lunau K. 2014. Gloss, colour and grip: multifunctional epidermal cell shapes in bee-and bird-pollinated flowers. PLoS ONE 9, e112013 ( 10.1371/journal.pone.0112013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Kooi CJ, Dyer AG, Stavenga DG. 2015. Is floral iridescence a biologically relevant cue in plant-pollinator signaling? New Phytol. 205, 18–20. ( 10.1111/nph.13066) [DOI] [PubMed] [Google Scholar]
- 22.Parkin J. 1928. The glossy petals of Ranunculus. Ann. Bot. 42, 739–755. [Google Scholar]
- 23.Galsterer S, Musso M, Asenbaum A, Fürnkranz D. 1999. Reflectance measurements of glossy petals of Ranunculus lingua (Ranunculaceae) and of non-glossy petals of Heliopsis helianthoides (Asteraceae). Plant Biol. 1, 670–678. ( 10.1111/j.1438-8677.1999.tb00279.x) [DOI] [Google Scholar]
- 24.Vignolini S, et al. 2012. Directional scattering from the glossy flower of Ranunculus: how the buttercup lights up your chin. J. R. Soc. Interface 9, 1295–1301. ( 10.1098/rsif.2011.0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hörandl E, Emadzade K. 2012. Evolutionary classification: a case study on the diverse plant genus Ranunculus L. (Ranunculaceae). Perspect. Plant Ecol. Evol. Syst. 14, 310–324. ( 10.1016/j.ppees.2012.04.001) [DOI] [Google Scholar]
- 26.Stavenga DG, van der Kooi CJ. 2016. Coloration of the Chilean Bellflower, Nolana paradoxa, interpreted with a scattering and absorbing layer stack model. Planta 243, 171–181. ( 10.1007/s00425-015-2395-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh P. 2005. Optical waves in layered media. Hoboken, NJ: Wiley-Interscience. [Google Scholar]
- 28.van der Kooi CJ. 2015. The coloration toolkit of flowers. Filtering pigments, scattering structures and biological significance. PhD thesis, University of Groningen, Groningen, The Netherlands.
- 29.Kubelka P, Munk F. 1931. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. 12, 593–601. [Google Scholar]
- 30.Allen WA, Richardson AJ. 1968. Interaction of light with a plant canopy. J. Opt. Soc. Am. 58, 1023–1028. ( 10.1364/JOSA.58.001023) [DOI] [Google Scholar]
- 31.Gausman H, Allen W. 1973. Optical parameters of leaves of 30 plant species. Plant Physiol. 52, 57–62. ( 10.1104/pp.52.1.57) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyfried M, Fukshansky L. 1983. Light gradients in plant tissue. Appl. Opt. 22, 1402–1408. ( 10.1364/AO.22.001402) [DOI] [PubMed] [Google Scholar]
- 33.Yamada N, Fujimura S. 1991. Nondestructive measurement of chlorophyll pigment content in plant leaves from three-color reflectance and transmittance. Appl. Opt. 30, 3964–3973. ( 10.1364/AO.30.003964) [DOI] [PubMed] [Google Scholar]
- 34.Vogelmann TC. 1993. Plant tissue optics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 231–251. ( 10.1146/annurev.pp.44.060193.001311) [DOI] [Google Scholar]
- 35.Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R. 2009. Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 50, 684–697. ( 10.1093/pcp/pcp034) [DOI] [PubMed] [Google Scholar]
- 36.Parkin J. 1935. The structure of the starch layer in the glossy petal of Ranunculus II. The British species examined. Ann. Bot. 49, 283–289. [Google Scholar]
- 37.Parkin J. 1931. The structure of the starch layer in the glossy petal of Ranunculus. Ann. Bot. 45, 201–205. [Google Scholar]
- 38.Brett D, Sommerard A. 1986. Ultrastructural development of plastids in the epidermis and starch layer of glossy Ranunculus petals. Ann. Bot. 58, 903–910. [Google Scholar]
- 39.Wilts BD, Matsushita A, Arikawa K, Stavenga DG. 2015. Spectrally tuned structural and pigmentary coloration of birdwing butterfly wing scales. J. R. Soc. Interface 12, 20150717 ( 10.1098/rsif.2015.0717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Alba L, Kieffer L, Shawkey MD. 2012. Relative contributions of pigments and biophotonic nanostructures to natural color production: a case study in budgerigar (Melopsittacus undulatus) feathers. J. Exp. Biol. 215, 1272–1277. ( 10.1242/jeb.064907) [DOI] [PubMed] [Google Scholar]
- 41.Cossard G, et al. 2016. Subfamilial and tribal relationships of Ranunculaceae: evidence from eight molecular markers. Plant Syst. Evol. 302, 419–431. ( 10.1007/s00606-015-1270-6) [DOI] [Google Scholar]
- 42.Giurfa M, Vorobyev M, Brandt R, Posner B, Menzel R. 1997. Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Phys. A 180, 235–243. ( 10.1007/s003590050044) [DOI] [Google Scholar]
- 43.Lunau K, Papiorek S, Eltz T, Sazima M. 2011. Avoidance of achromatic colours by bees provides a private niche for hummingbirds. J. Exp. Biol. 214, 1607–1612. ( 10.1242/jeb.052688) [DOI] [PubMed] [Google Scholar]
- 44.Hempel de Ibarra N, Vorobyev M, Menzel R. 2014. Mechanisms, functions and ecology of colour vision in the honeybee. J. Comp. Phys. A 200, 411–433. ( 10.1007/s00359-014-0915-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanton ML, Galen C. 1989. Consequences of flower heliotropism for reproduction in an alpine buttercup (Ranunculus adoneus). Oecologia 78, 477–485. ( 10.1007/BF00378737) [DOI] [PubMed] [Google Scholar]
- 46.Totland Ø. 1996. Flower heliotropism in an alpine population of Ranunculus acris (Ranunculaceae): effects on flower temperature, insect visitation, and seed production. Am. J. Bot. 83, 452–458. ( 10.2307/2446214) [DOI] [Google Scholar]
- 47.Luzar N, Gottsberger G. 2001. Flower heliotropism and floral heating of five alpine plant species and the effect on flower visiting in Ranunculus montanus in the Austrian Alps. Arct. Antarct. Alp. Res. 33, 93–99. ( 10.2307/1552282) [DOI] [Google Scholar]
- 48.Galen C, Stanton ML. 2003. Sunny-side up: flower heliotropism as a source of parental environmental effects on pollen quality and performance in the snow buttercup, Ranunculus adoneus (Ranunculaceae). Am. J. Bot. 90, 724–729. ( 10.3732/ajb.90.5.724) [DOI] [PubMed] [Google Scholar]
- 49.Ida TY, Totland Ø. 2014. Heating effect by perianth retention on developing achenes and implications for seed production in the alpine herb Ranunculus glacialis. Alp. Bot. 124, 37–47. ( 10.1007/s00035-014-0129-8) [DOI] [Google Scholar]
- 50.Kevan PG. 1975. Sun-tracking solar furnaces in high arctic flowers: significance for pollination and insects. Science 189, 723–726. ( 10.1126/science.189.4204.723) [DOI] [PubMed] [Google Scholar]
- 51.Dyer AG, Whitney HM, Arnold SE, Glover BJ, Chittka L. 2006. Behavioural ecology: bees associate warmth with floral colour. Nature 442, 525 ( 10.1038/442525a) [DOI] [PubMed] [Google Scholar]
- 52.Norgate M, Boyd-Gerny S, Simonov V, Rosa MG, Heard TA, Dyer AG. 2010. Ambient temperature influences Australian native stingless bee (Trigona carbonaria) preference for warm nectar. PLoS ONE 5, e12000 ( 10.1371/journal.pone.0012000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Kooi CJ. 2016. Plant biology: flower orientation, temperature regulation and pollinator attraction. Curr. Biol. 26, R1143–R1145. ( 10.1016/j.cub.2016.08.071) [DOI] [PubMed] [Google Scholar]
- 54.Cooley JR. 1995. Floral heat rewards and direct benefits to insect pollinators. Ann. Entomol. Soc. Am. 88, 576–579. ( 10.1093/aesa/88.4.576) [DOI] [Google Scholar]
- 55.Arista M, Talavera M, Berjano R, Ortiz PL. 2013. Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. J. Ecol. 101, 1613–1622. ( 10.1111/1365-2745.12151) [DOI] [Google Scholar]
- 56.Koski MH, Ashman T-L. 2015. Floral pigmentation patterns provide an example of Gloger's rule in plants. Nat. Plants 1, 14007 ( 10.1038/nplants.2014.7) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.