Abstract
Tumor necrosis factor-α (TNF-α) inhibitors are increasingly being used as treatment for rheumatoid arthritis (RA). However, the administration of these drugs carries the risk of inducing injection site reaction (ISR). ISR gives rise to patient stress, nervousness, and a decrease in quality of life (QoL). In order to alleviate pain and other symptoms, early countermeasures must be taken against this adverse event. In order to improve understanding of the risk factors contributing to the induction of ISR, we evaluated the association between TNF-α inhibitors and ISR by applying a logistic regression model to age-stratified data obtained from the Food and Drug Administration Adverse Event Reporting System (FAERS) database.
The FAERS database contains 7,561,254 reports from January 2004 to December 2015. Adjusted reporting odds ratios (RORs) (95% Confidence Intervals) were obtained for interaction terms for age-stratified groups treated with etanercept (ETN) and adalimumab (ADA). The adjusted RORs for ETN* ≥ 70 and ADA* ≥ 70 groups were the lowest among the age-stratified groups undergoing the respective monotherapies. Furthermore, we found that crude RORs for ETN + methotrexate (MTX) combination therapy and ADA + MTX combination therapy were lower than those for the respective monotherapies.
This study was the first to evaluate the relationship between aging and ISR using the FAERS database.
Keywords: etanercept, adalimumab, injection site reaction, adverse event reporting system.
Introduction
The treatment of rheumatoid arthritis (RA) and other immune-mediated diseases has benefited from the development of a variety of tumor necrosis factor-α (TNF-α) inhibitors such as etanercept (ETN), adalimumab (ADA), golimumab (GLM), certolizumab (CZM), and infliximab (INF)1-6. These TNF-α inhibitors are effective in reducing the signs and symptoms of RA and in inhibiting structural damage compared to traditional disease-modifying anti-rheumatic drugs7, 8. ETN, ADA, GLM, CZM, and INF are currently available the U.S. Food and Drug Administration (FDA)-approved TNF-α inhibitors1-6. They all appear to possess similar efficacy in clinical practice. ETN, ADA, GLM, and CZM are administered subcutaneously (SC) by the patient. INF, on the other hand, is administered intravenously (IV) by a health care professional.
Patient experience with injectable biologics appears to be an important consideration when selecting a TNF-α inhibitor9. Several studies have found that patients prefer SC injection over IV drug administration and prefer to receive treatment at home10, 11. The adverse events reported in clinical trials of SC TNF-α inhibitors include injection site reactions (ISRs), infections, headaches, etc. ISR, by definition, includes any of the following: erythema, pruritus, pain, inflammation, rash, induration, itching, and edema. The prevalence of these symptoms has been reported as ranging from 12-37% in clinical trials2, 3. Since ISR is often subjective, and may not be a part of routine inquiries by physicians, its prevalence could be underestimated in many rheumatological practices12. Although SC TNF-α inhibitors may be more convenient than IV infusion, they may induce ISR, which may affect patient quality of life (QoL). ISR gives rise to stress, nervousness, and a reduced QoL. In order to alleviate pain and other symptoms, early countermeasures against this adverse event class must be taken. However, at present, even the prevalence and clinical importance of ISR in routine clinical practice is uncertain13.
The FDA Adverse Event Reporting System (FAERS) is a spontaneous reporting system (SRS) and the largest and best-known database in world. Data collected from doctors, nurses, and other concerned clinical practitioners are compiled in this database. FAERS reflects the realities of clinical practice14. SRS can be used to evaluate drug-associated adverse events via disproportionality analysis, which usually involves the crude reporting odds ratio (ROR)15. The crude ROR can be used in a technique that allows for adjustments through logistic regression analyses in order to mitigate the effects of confounding factors16-22.
To the best of our knowledge, the relationship between SC TNF-α inhibitors and ISR has not yet been evaluated with regards to age-stratified patient groups analyzed from SRS. In this study, we evaluated a possible relationship between SC TNF-α inhibitors and ISR from data available in the FAERS database using a logistic regression model and subset analysis. Furthermore, TNF-α inhibitors are often combined with methotrexate (MTX) in RA treatment7, 8. This combination therapy was found in our study to cause fewer ISR cases than monotherapy using a single TNF-α inhibitor.
Methods
Data from January 2004 to December 2015 present in the FAERS database were downloaded from the FDA website (http://www.fda.gov/). The FAERS database structure complies with standards of the International Council on Harmonization (ICH) E2B. DrugBank ver. 3.0 and 4.0 (The Metabolomics Innovation Centre, Canada, http://www.drugbank.ca/) were utilized as dictionaries for batch conversion and compilation of drug names23. We built a database that integrated the FAERS database and DrugBank data using FileMaker Pro 13 software (FileMaker, Inc.). In the FAERS database, adverse events are coded according to the terminology prescribed by the Medical Dictionary for Regulatory Activities (MedDRA) 19.0 (http://www.meddra.org/). The preferred terms (PTs) selected for identification of ISR reporting were 77 terms containing the words “injection site” (Table 1). We followed the FDA's recommendation in adopting the most recent case numbers in order to identify duplicate reports from the same patient and exclude them from analysis24. Additionally, only reports with complete age and sex information were extracted.
Table 1.
Preferred terms associated with injection site reaction in MedDRA.
| PTa) CODE | PT NAME | PTa) CODE | PT NAME |
|---|---|---|---|
| 10022044 | injection site abscess | 10064111 | injection site joint inflammation |
| 10022045 | injection site abscess sterile | 10053979 | injection site joint movement impairment |
| 10022046 | injection site anaesthesia | 10049261 | injection site joint pain |
| 10022048 | injection site atrophy | 10049260 | injection site joint swelling |
| 10022052 | injection site bruising | 10049262 | injection site joint warmth |
| 10054812 | injection site calcification | 10067253 | injection site laceration |
| 10050057 | injection site cellulitis | 10057665 | injection site lymphadenopathy |
| 10050082 | injection site coldness | 10067255 | injection site macule |
| 10022055 | injection site cyst | 10022081 | injection site mass |
| 10022056 | injection site dermatitis | 10056250 | injection site movement impairment |
| 10065600 | injection site discharge | 10022082 | injection site necrosis |
| 10051572 | injection site discolouration | 10022083 | injection site nerve damage |
| 10054266 | injection site discomfort | 10057880 | injection site nodule |
| 10067252 | injection site dryness | 10022085 | injection site oedema |
| 10069124 | injection site dysaesthesia | 10022086 | injection site pain |
| 10066221 | injection site eczema | 10066041 | injection site pallor |
| 10022059 | injection site erosion | 10066044 | injection site papule |
| 10022061 | injection site erythema | 10022088 | injection site paraesthesia |
| 10068689 | injection site exfoliation | 10022090 | injection site phlebitis |
| 10022062 | injection site extravasation | 10053396 | injection site photosensitivity reaction |
| 10022064 | injection site fibrosis | 10073174 | injection site plaque |
| 10022065 | injection site granuloma | 10022093 | injection site pruritus |
| 10022066 | injection site haematoma | 10054994 | injection site pustule |
| 10022067 | injection site haemorrhage | 10022094 | injection site rash |
| 10073418 | injection site hyperaesthesia | 10022095 | injection site reaction |
| 10022071 | injection site hypersensitivity | 10066797 | injection site recall reaction |
| 10075313 | injection site hypertrichosis | 10066210 | injection site scab |
| 10022072 | injection site hypertrophy | 10059009 | injection site scar |
| 10074586 | injection site hypoaesthesia | 10066778 | injection site streaking |
| 10022075 | injection site induration | 10053425 | injection site swelling |
| 10022076 | injection site infection | 10022104 | injection site thrombosis |
| 10022078 | injection site inflammation | 10022105 | injection site ulcer |
| 10066083 | injection site injury | 10022107 | injection site urticaria |
| 10022079 | injection site irritation | 10067995 | injection site vasculitis |
| 10048648 | injection site ischaemia | 10022111 | injection site vesicles |
| 10073459 | injection site joint discomfort | 10022112 | injection site warmth |
| 10064494 | injection site joint effusion | 10073752 | lack of injection site rotation |
| 10076327 | injection site joint erythema | 10025478 | malabsorption from injection site |
| 10064111 | injection site joint inflammation |
a) Preferred Term
From the selected reports, we calculated both the crude ROR and the adjusted ROR. Patients using TNF-α inhibitors were categorized by age (< 20, 20-29, 30-39, 40-49, 50-59, 60-69, and ≥ 70-year-old groups). Crude ROR was calculated using a two-by-two contingency table in the form of (a*d)/(b*c) (Table 2). A “case” was defined as patients reporting adverse events relating to “ISR”, while “non-cases” were defined as patients without adverse events relating to “ISR”24. RORs were each expressed as a point estimate with 95% confidence intervals (CIs). The safety signal was defined as the lower limit of the 95% CI for each ROR exceeding 115.
Table 2.
2×2 contingency table.
| Adverse event of interest | All other adverse events of interest | Total | ||||
|---|---|---|---|---|---|---|
| Drug of interest | a | b | a + b | |||
| All other drugs of interest | c | d | c + d | |||
| Total | a + c | b + d | a + b + c + d |
RORa) = a*d/b*c.
95% CIb) = eln(ROR)±1.96√(1/a+1/b+1/c+1/d).
a) Reporting Odds Ratio, b) Confidence Interval.
RORs can be adjusted using logistic regression analysis and then used to analyze the effects of interaction terms in detail16-18, 21, 22. The FAERS database included information relating to confounding factors that affect the crude ROR. The logistic model used to calculate the adjusted ROR was as follows:
| Log (odds) = intercept + β1Y + β2S + β3A + β4T + β5M + β6 T*M + β7 T*A |
(Y = reporting year, S = sex, A = age-stratified group, T = TNF-α inhibitor, M = MTX)
The 20-29-year-old group was used as a reference group to calculate RORs adjusted for age variations. A likelihood ratio test can be used to evaluate the effect of adding a particular term. Because the difference in -2 log-likelihood follows a chi-square distribution with one degree of freedom, adding an interaction term, in this case, was statistically significant (p < 0.05). These analyses were performed using JMP 11 (SAS Institute Inc., Cary, NC, U.S.A.).
The data subsets strategy may help to mitigate the effect of confounding factors on signal detection by limiting the analysis to a population of patients that are thought to share common risk factors and diseases20, 25-27. We evaluated the intra-class RORs of the ETN-treatment subset as well as the ADA-treatment subset.
Because the FAERS database does not contain information on the severity of ISR, this information was not taken into account in our analysis.
Results
The FAERS database contains 7,561,254 reports from January 2004 to December 2015. After excluding duplicate reports, 6,157,897 reports remained. Following selection for reports containing sex and age information, a final total of 3,839,264 reports were analyzed in this study. In total, 137,535 reports corresponded to case PTs (Tables 3, 4). The number of reports of ISRs for ETN, ADA, GLM, CZM, and INF were 57,428, 32,223, 235, 565, and 271, respectively. Since the number of extracted reports of ISRs for GLM and CZM were low, and since INF is an IV-administered TNF-α inhibitor, we did not further investigate reports relating to these drugs. Other biological disease-modifying antirheumatic drugs (DMARDs), abatacept (T-cell action blocker) and tocilizumab (IL-6 inhibitor) were not included in our study.
Table 3.
Crude and Adjusted ROR of etanercept for injection site reaction.
| Total | Casea) | Crude RORb)(95% CIc)) | Adjusted RORb) (95% CIc)) | Likelihood ratio test | |
|---|---|---|---|---|---|
| Total | 3,839,264 | 137,535 | |||
| Female | 2,344,951 | 108,144 | 2.41 (2.38-2.44) | 1.99 (1.96-2.02) | <0.0001 |
| Reporting year | 1.02 (1.02-1.02) | <0.0001 | |||
| Age (y.o.) | |||||
| <20 | 234,685 | 5,433 | 0.62 (0.61-0.64) | 0.58 (0.55-0.60) | <0.0001 |
| 20-29 | 265,823 | 9,716 | 1.02 (1.00-1.04) | 1 | |
| 30-39 | 367,427 | 16,538 | 1.31 (1.28-1.33) | 1.11 (1.07-1.14) | <0.0001 |
| 40-49 | 534,967 | 25,776 | 1.45 (1.43-1.47) | 1.18 (1.15-1.22) | <0.0001 |
| 50-59 | 773,826 | 37,337 | 1.50 (1.48-1.52) | 1.13 (1.10-1.16) | <0.0001 |
| 60-69 | 775,032 | 27,157 | 0.97 (0.96-0.98) | 0.89 (0.86-0.91) | <0.0001 |
| ≥70 | 887,504 | 15,578 | 0.41 (0.41-0.42) | 0.54 (0.52-0.55) | <0.0001 |
| ETNd) | 216,147 | 57,428 | 16.00 (15.81-16.19) | 20.48 (19.48-21.52) | <0.0001 |
| MTXe) | 102,712 | 12,569 | 4.03 (3.95-4.11) | 3.70 (3.60-3.80) | <0.0001 |
| ETN*MTX | 27,451 | 6,759 | 9.19 (8.94-9.46) | 18.30 (17.29-19.36) | <0.0001 |
| ETN*Age | |||||
| ETN*<20 | 6,960 | 2,300 | 13.49 (12.83-14.19) | 19.95 (18.86-21.11) | <0.0001 |
| ETN*20-29 | 9,384 | 3,219 | 14.37 (13.76-15.00) | 20.48 (19.48-21.52) | <0.0001 |
| ETN*30-39 | 20,665 | 6,897 | 14.14 (13.73-14.56) | 19.85 (19.10-20.63) | <0.0001 |
| ETN*40-49 | 37,823 | 11,314 | 12.43 (12.15-12.71) | 16.88 (16.32-17.45) | <0.0001 |
| ETN*50-59 | 64,097 | 17,610 | 11.55 (11.34-11.76) | 14.78 (14.34-15.24) | <0.0001 |
| ETN*60-69 | 51,481 | 11,587 | 8.44 (8.27-8.63) | 11.28 (10.92-11.66) | <0.0001 |
| ETN*≥70 | 25,737 | 4,501 | 5.86 (5.68-6.06) | 8.09 (7.77-8.43) | <0.0001 |
a) Number of patients with injection site reaction, b) Reporting Odds Ratio, c) Confidence Interval, d) etanercept, e) methotrexate.
For ETN, the crude RORs for ISRs stratified by age are summarized in Table 3. The < 20, 20-29, 30-39, 40-49, 50-59, 60-69, and ≥ 70-year-old groups contained 6,960, 9,384, 20,665, 37,823, 64,097, 51,481, and 25,737 reports relating to all adverse events, respectively. The < 20, 20-29, 30-39, 40-49, 50-59, 60-69, and ≥ 70-year-old groups contained 2,300, 3,219, 6,897, 11,314, 17,610, 11,587, and 4,501 reports relating to ISRs, respectively. The crude RORs (95% CIs) were 13.49 (12.83-14.19), 14.37 (13.76-15.00), 14.14 (13.73-14.56), 12.43 (12.15-12.71), 11.55 (11.34-11.76), 8.44 (8.27-8.63), and 5.86 (5.68-6.06), respectively. The adjusted RORs (95% CIs) for interaction terms for ETN* < 20, ETN* 20-29, ETN* 30-39, ETN* 40-49, ETN* 50-59, ETN* 60-69, and ETN* ≥ 70-year-old groups were 19.95 (18.86-21.11), 20.48 (19.48-21.52), 19.85 (19.10-20.63), 16.88 (16.32-17.45), 14.78 (14.34-15.24), 11.28 (10.92-11.66), and 8.09 (7.77-8.43), respectively (Table 3). All interaction terms were statistically significant (p < 0.0001) (Table 3). The crude RORs for ISRs in groups stratified based on therapy type are summarized in Table 3. The adjusted RORs (95% CIs) for ETN monotherapy, MTX monotherapy, and ETN + MTX combination therapy were 20.48 (19.48-21.52), 3.70 (3.60-3.80), and 18.30 (17.29-19.36), respectively.
For ADA, the crude RORs for ISRs stratified by age are summarized in Table 4. For age-stratified groups, the < 20, 20-29, 30-39, 40-49, 50-59, 60-69, and ≥ 70-year-old groups contained 6,204, 15,007, 20,422, 28,644, 38,915, 29,453, and 16,059 reports relating to all adverse events, respectively. The < 20, 20-29, 30-39, 40-49, 50-59, 60-69, and ≥ 70-year-old groups were 1,582, 4,032, 4,904, 6,405, 7,962, 5,178, and 2,160 reports relating to ISRs, respectively. Crude RORs (95% CIs) for each of the same groups were 9.31 (8.79-9.86), 10.16 (9.79-10.53), 8.78 (8.50-9.07), 8.08 (7.86-8.31), 7.29 (7.11-7.47), 5.93 (5.75-6.11), and 4.23 (4.04-4.43), respectively. The adjusted RORs (95% CIs) for interaction terms for ADA* < 20, ADA* 20-29, ADA* 30-39, ADA* 40-49, ADA* 50-59, ADA* 60-69, and ADA* ≥ 70-year-old groups were 16.07 (15.08-17.13), 16.18 (15.46-16.92), 13.91 (13.34-14.51), 12.77 (12.29-13.28), 11.30 (10.89-11.73), 9.39 (9.02-9.79), and 6.90 (6.54-7.27), respectively (Table 4). All interaction terms were statistically significant (p < 0.0001) (Table 4). The crude RORs for ISRs in groups stratified based on therapy type are summarized in Table 4. The adjusted RORs (95% CIs) for ADA monotherapy, MTX monotherapy, and ADA + MTX combination therapy were 16.18 (15.46-16.92), 3.59 (3.50-3.68), and 15.05 (14.24-15.91), respectively.
Table 4.
Crude and Adjusted ROR of adalimumab for injection site reaction.
| Total | Casea) | Crude RORb)(95% CIc)) | Adjusted RORb)(95% CIc)) | Likelihood ratio test | |
|---|---|---|---|---|---|
| Total | 3,839,264 | 137,535 | |||
| Female | 2,344,951 | 108,144 | 2.41 (2.38-2.44) | 2.23 (2.20-2.26) | <0.0001 |
| Reporting year | 1.06 (1.05-1.06) | <0.0001 | |||
| Age (y.o.) | |||||
| <20 | 234,685 | 5,433 | 0.62 (0.61-0.64) | 0.81 (0.78-0.85) | <0.0001 |
| 20-29 | 265,823 | 9,716 | 1.02 (1.00-1.04) | 1 | |
| 30-39 | 367,427 | 16,538 | 1.31 (1.28-1.33) | 1.51 (1.46-1.56) | <0.0001 |
| 40-49 | 534,967 | 25,776 | 1.45 (1.43-1.47) | 1.77 (1.71-1.82) | <0.0001 |
| 50-59 | 773,826 | 37,337 | 1.50 (1.48-1.52) | 1.85 (1.79-1.90) | <0.0001 |
| 60-69 | 775,032 | 27,157 | 0.97 (0.96-0.98) | 1.37 (1.33-1.41) | <0.0001 |
| ≥70 | 887,504 | 15,578 | 0.41 (0.41-0.42) | 0.72 (0.70-0.75) | <0.0001 |
| ADAd) | 154,704 | 32,223 | 8.94 (8.82-9.07) | 16.18 (15.46-16.92) | <0.0001 |
| MTXe) | 102,712 | 12,569 | 4.03 (3.95-4.11) | 3.59 (3.50-3.68) | <0.0001 |
| ADA*MTX | 26,441 | 5,048 | 6.55 (6.35-6.76) | 15.05 (14.24-15.91) | <0.0001 |
| ADA*Age | |||||
| ADA*<20 | 6,204 | 1,582 | 9.31 (8.79-9.86) | 16.07 (15.08-17.13) | <0.0001 |
| ADA*20-29 | 15,007 | 4,032 | 10.16 (9.79-10.53) | 16.18 (15.46-16.92) | <0.0001 |
| ADA*30-39 | 20,422 | 4,904 | 8.78 (8.50-9.07) | 13.91 (13.34-14.51) | <0.0001 |
| ADA*40-49 | 28,644 | 6,405 | 8.08 (7.86-8.31) | 12.77 (12.29-13.28) | <0.0001 |
| ADA*50-59 | 38,915 | 7,962 | 7.29 (7.11-7.47) | 11.30 (10.89-11.73) | <0.0001 |
| ADA*60-69 | 29,453 | 5,178 | 5.93 (5.75-6.11) | 9.39 (9.02-9.79) | <0.0001 |
| ADA*≥70 | 16,059 | 2,160 | 4.23 (4.04-4.43) | 6.90 (6.54-7.27) | <0.0001 |
a) Number of patients with injection site reaction, b) Reporting Odds Ratio, c) Confidence Interval, d) adalimumab, e) methotrexate.
Discussion
RA is an autoimmune disease and a chronic inflammatory disorder. Despite novel therapies such as TNF-α inhibitors entering the market, RA treatment strategy has generally comprised of case control, pain mitigation, and increasing QoL for patients7, 8. This is largely because ISR has been found to be a relatively common side effect of injectable TNF-α inhibitors28-31. ISR usually occurs within the first month of treatment, lasting for 3 to 5 days32. Although most cases of ISR resolve themselves, symptomatic eruptions are treated with corticosteroids, antihistamines, acetaminophen, or cold compresses32. The effect of ISR on clinical outcomes for RA is unknown, but research involving multiple sclerosis patients that shows pain during and after injections has been found to affect adherence to drug regimen33. Side effects such as ISR may similarly be an important factor involved in the patient preference for SC TNF-α inhibitors and treatment adherence.
Our results suggest that ETN and ADA monotherapy does induce ISRs, while MTX combination therapy with ETN or ADA decreases the incidence of this adverse event. Our findings also suggest that aging may influence the adjusted RORs for ETN or ADA therapy based on the logistic regression analysis performed using the FAERS database.
ISR commonly occurs in biologic DMARDs such as TNF-α inhibitors administered via SC injections, and is becoming more clinically important as the number of biologic therapies available for RA therapy increases. ETN and ADA were both found to be associated with ISR in the FAERS database. The adjusted RORs for ADA treatment were lower than those for ETN treatment. ETN was administered to RA patients once or twice per week1, 2. On the other hand, ADA was administered once every two weeks3. The difference in administration frequency between ETN and ADA may be the cause for the high crude and adjusted RORs for ETN therapy when compared to RORs for ADA therapy (Tables 3, 4). Perhaps since GLM is a biologic agent which is administered once a month4, the number of reports regarding adverse events induced by GLM was found to be low. Multiple studies of drug adherence across a variety of medications have reported an inverse relationship between dosing frequency and drug adherence34-36, and patients have been found to prefer longer dosing intervals when given the option37-39. Another plausible reason for the differences in RORs is the varying composition of different TNF-α inhibitors, which can range from a complex composition with strong buffering capacity3 to a more simple composition with weak buffering capacity5. Further work to better understand potential mechanisms of ISR and the consequent clinical implications is needed.
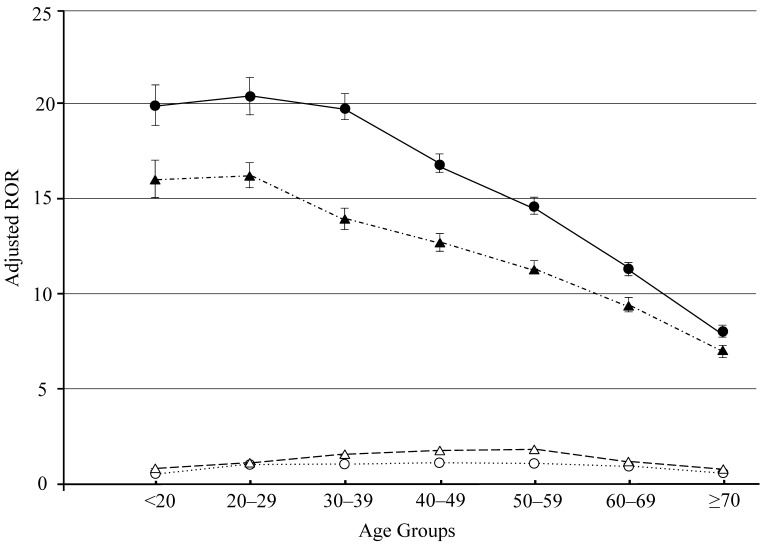
Analysis using a logistic model allows for adjustment of confounding factors included in crude ROR calculation. The 95% CIs lower limits for crude and adjusted RORs for both ETN and ADA therapy were above 1 (Tables 3, 4, Fig 1). Adjusted RORs for ETN* ≥ 70 and ADA* ≥ 70 were the lowest in each age-stratified group (Tables 3, 4, Fig 1). Therefore, in our study, we have demonstrated that adjusted RORs decreased with an increase in age.
Figure 1.
Adjusted reporting odds ratios and 95% confidence intervals. Open circles and triangles: adjusted reporting odds ratios and 95% confidence intervals for age analyzed by TNF-α inhibiter drug, etanercept and adalimumab, respectively. Filled circles and triangles: adjusted reporting odds ratios and 95% confidence intervals for etanercept- and adalimumab-associated injection site reaction, respectively.
We do not have a conclusive explanation for these data. To the best of our knowledge, systematic studies of ISR that are stratified by age group are rare. Additionally, the biological mechanisms that mediate ISR by SC TNF-α inhibitors are not well understood. Therefore, ISR may be related to inflammatory mediators that are released during non-immune-stimulated mast cell degranulation40. Immune function decreases with age owing to the reduced function of T lymphocytes41. A plausible reason for our result may therefore be related to the differences in sensitivity to medication between younger and older patients.
Regarding effectiveness, several clinical studies have suggested that treatment of RA with TNF-α inhibitor drugs should be in combination with MTX42-46. The guidelines also suggest that ETN + MTX combination therapy is more effective than ETN monotherapy7, 8. From the perspective of safety, the reporting ratio of other severe adverse events, such as infection, for ETN + MTX combination therapy is similar to that for ETN monotherapy42-46. The adjusted RORs of ISRs for ETN + MTX combination therapy and ADA + MTX combination therapy were lower than those for their respective monotherapies (Tables 3, 4). Therefore, our result strengthens the credibility of ETN + MTX combination therapy in the clinical setting.
There are several limitations to this study, and the results obtained from SRSs, such as the FAERS database, as well as specifically from our own study, should be interpreted with caution. SRS is a passive reporting system and is therefore subject to biases such as under-reporting, over-reporting, and confounding by comorbidities24. Most notably, there is a lack of comparison groups as well as missing data relating to patient characteristics15, 24. Cases reported in the FAERS database do not always contain sufficient information regarding patient background, dose response and mode of administration to allow for proper evaluation. For example, it is worthwhile to note that a possible association has been found between a patient's mental health and his/her pain sensitivity in context of adverse events. It has been reported that ISR reporting was higher among patients with more severe RA, and also among those with fibromyalgia and depression12, 47. At least 7% of patients develop “recall ISR” or reaction at a prior treatment site following subsequent injections32. Moreover, patients may not complain about ISR if they perceive it to be a trade-off required to achieve the benefit of these medications. Our analysis also did not evaluate the association between ISR and the duration of therapy with ETN or ADA. We could not incorporate patient prior experiences and information regarding other concurrently administered medications into our analysis.
Because of these limitations, crude RORs without logistic regression analysis do not indicate the risk of adverse event occurrence in absolute terms, and can only offer a rough indication of signal strength15. For this reason, we partially refined the results with a dedicated correction in order to detect possible confounders present in the database using logistic regression and subset analysis. Consequently, we believe that despite the limitations, it may be acceptable to compare the adjusted RORs of a particular adverse event derived from stratified analysis within a particular context. However, further research is needed in order to more accurately determine the specific associations between TNF-α inhibitors and ISR.
When discussing SC TNF-α inhibitor treatment for RA, ISR may be an important factor for patient comfort and safety as well as therapeutic efficacy. Our results show that younger patients should be closely monitored for ISR when they are administered a SC TNF-α inhibitor. It is important to provide patients with information regarding the tolerability of SC TNF-α inhibitors. We hope that our research may make a valuable contribution to the information available to clinicians in order to improve the management of RA and to allow patients to make a more informed treatment choice.
Conclusions
This study was the first to evaluate the correlation between aging and induction of ISRs by TNF-α inhibitors using SRS analysis strategy. We demonstrated that overall, the adjusted RORs in younger patients were comparatively high. Therefore, we can infer that younger RA patients receiving ETN or ADA treatment should be closely monitored for ISR symptoms. We also provided evidence of higher efficacy of combined therapy comprising ETN and MTX administration. Our results may potentially be used in clinical practice to improve the management of RA and ISRs adverse events.
Acknowledgments
This research was partially supported by JSPS KAKENHI Grant Number, 24390126.
References
- 1.Enbrel: PRODUCT MONOGRAPH. Mississauga, Canada: AMGEN CANADA INC; [2015]. https://www.amgen.ca/Enbrel_PM.pdf. [Google Scholar]
- 2.Enbrel: HIGHLIGHTS OF PRESCRIBING INFORMATION. Silver spring, Maryland, USA: US Food and Drug administration (FDA); [2015]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103795s5548lbl.pdf. [Google Scholar]
- 3.Humila: HIGHLIGHTS OF PRESCRIBING INFORMATION. Silver spring, Maryland, USA: US Food and Drug administration (FDA); [2015]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125057s394lbl.pdf. [Google Scholar]
- 4.Simponi: HIGHLIGHTS OF PRESCRIBING INFORMATION. Silver spring, Maryland, USA: US Food and Drug administration (FDA); [2016]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125289s127lbl.pdf. [Google Scholar]
- 5.Cimzia: HIGHLIGHTS OF PRESCRIBING INFORMATION. Silver spring, Maryland, USA: US Food and Drug administration (FDA); [2016]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125160s241lbl.pdf. [Google Scholar]
- 6.Remicade: HIGHLIGHTS OF PRESCRIBING INFORMATION. Silver spring, Maryland, USA: US Food and Drug administration (FDA); [2015]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103772s5373lbl.pdf. [Google Scholar]
- 7.Jasvinder AS, Saag KG, Bridges SL. et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 8.Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennifer LB. Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence. 2009;3:335–44. doi: 10.2147/ppa.s5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams EL, Edwards CJ. Patient preferences in choosing anti-TNF therapies-R1. Rheumatology (Oxford) 2006;45:1575–6. doi: 10.1093/rheumatology/kel369. [DOI] [PubMed] [Google Scholar]
- 11.Chilton F, Collett RA. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care. 2008;6:1–14. doi: 10.1002/msc.110. [DOI] [PubMed] [Google Scholar]
- 12.Curtis JR, Hobar C, Hansbrough K. Injection-site burning and stinging in patients with rheumatoid arthritis using injectable biologics. Curr Med Res Opin. 2011;27:71–8. doi: 10.1185/03007995.2010.534959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salt E, Frazier SK. Adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a narrative review of the literature. Orthop Nurs. 2010;29:260–75. doi: 10.1097/NOR.0b013e3181e5c2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahabreh IJ, Kent DM. Can the learning health care system be educated with observational data? JAMA. 2014;312:129–30. doi: 10.1001/jama.2014.4364. [DOI] [PubMed] [Google Scholar]
- 15.Van Puijenbroek EP, Bate A, Leufkens HG. et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002;11:3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 16.Van Puijenbroek EP, Egberts AC, Meyboom RH. et al. Signalling possible drug-drug interactions in a spontaneous reporting system: delay of withdrawal bleeding during concomitant use of oral contraceptives and itraconazole. Br J Clin Pharmacol. 1999;47:689–93. doi: 10.1046/j.1365-2125.1999.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Puijenbroek EP, Egberts AC, Heerdink ER. et al. Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2000;56:733–8. doi: 10.1007/s002280000215. [DOI] [PubMed] [Google Scholar]
- 18.Qian Y, Ye X, Du W. et al. A computerized system for detecting signals due to drug-drug interactions in spontaneous reporting systems. Br J Clin Pharmacol. 2010;69:67–73. doi: 10.1111/j.1365-2125.2009.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe J, Umetsu R, Mataki K. et al. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database. J Pharm Heal Care Sci. 2016;2:14. doi: 10.1186/s40780-016-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umetsu R, Abe J, Ueda N. et al. Association between selective serotonin reuptake inhibitor therapy and suicidality: Analysis of U.S. food and drug administration adverse event reporting system data. Biol Pharm Bull. 2015;38:1689–99. doi: 10.1248/bpb.b15-00243. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Suzuki H, Umetsu R. et al. Analysis of the Interaction between Clopidogrel, Aspirin, and Proton Pump Inhibitors Using the FDA Adverse Event Reporting System Database. Biol Pharm Bull. 2015;38:680–6. doi: 10.1248/bpb.b14-00191. [DOI] [PubMed] [Google Scholar]
- 22.Abe J, Umetsu R, Kato Y. et al. Evaluation of Dabigatran- and Warfarin-Associated Hemorrhagic Events Using the FDA-Adverse Event Reporting System Database Stratified by Age. Int J Med Sci. 2015;12:312–21. doi: 10.7150/ijms.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wishart DS, Knox C, Guo AC. et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–72. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poluzzi E, Raschi E, Piccinni C, Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS) INTECH; 2012. pp. 265–302. [Google Scholar]
- 25.Grégoire F, Pariente A, Fourrier-Reglat A. et al. A signal of increased risk of hypoglycaemia with angiotensin receptor blockers caused by confounding. Br. J Clin Pharmacol. 2008;66:142–5. doi: 10.1111/j.1365-2125.2008.03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almenoff JS, Pattishall EN, Gibbs TG. et al. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82:157–66. doi: 10.1038/sj.clpt.6100258. [DOI] [PubMed] [Google Scholar]
- 27.Raschi E, Piccinni C, Poluzzi E. et al. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 2013;50:569–77. doi: 10.1007/s00592-011-0340-7. [DOI] [PubMed] [Google Scholar]
- 28.Weinblatt ME, Keystone EC, Furst DE. et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 29.Weinblatt ME, Kremer JM, Bankhurst AD. et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–9. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 30.Koike T, Harigai M, Inokuma S. et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36:898–906. doi: 10.3899/jrheum.080791. [DOI] [PubMed] [Google Scholar]
- 31.Moreland LW, Schiff MH, Baumgartner SW. et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–86. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 32.Clelland S, Hunek JR. Etanercept injection site reaction. Dermatol Nurs. 2005;17:375. [PubMed] [Google Scholar]
- 33.Cohen BA, Rieckmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract. 2007;61:1922–30. doi: 10.1111/j.1742-1241.2007.01561..x. [DOI] [PubMed] [Google Scholar]
- 34.Coleman CI, Limone B, Sobieraj DM. et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18:527–39. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iskedjian M, Einarson TR, MacKeigan LD. et al. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther. 2002;24:302–16. doi: 10.1016/s0149-2918(02)85026-3. [DOI] [PubMed] [Google Scholar]
- 36.Saini SD, Schoenfeld P, Kaulback K. et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22–33. [PubMed] [Google Scholar]
- 37.Granger AL, Fehnel SE, Hogue SL. et al. An assessment of patient preference and adherence to treatment with Wellbutrin SR: A web-based survey. J Affect Disord. 2006;90:217–21. doi: 10.1016/j.jad.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Kendler D, Kung AW, Fuleihan Gel-H. et al. Patients with osteoporosis prefer once weekly to once daily dosing with alendronate. Maturitas. 2004;48:243–51. doi: 10.1016/j.maturitas.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: Understanding the relationship. Bone. 2006;38:S2–6. doi: 10.1016/j.bone.2006.01.150. [DOI] [PubMed] [Google Scholar]
- 40.Lamour S, Bracher M, Nesbitt A. The PEG Component of Certolizumab Pegol Inhibits Degranulation by Stimulated Mast Cells. Arthritis Rheum. 2009;15:16–21. [Google Scholar]
- 41.Vasto S, Malavolta M, Pawelec G. Age and immunity. Immun ageing. 2006;3:2. doi: 10.1186/1742-4933-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kameda H, Kanbe K, Sato E. et al. Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol. 2011;38:1585–92. doi: 10.3899/jrheum.110014. [DOI] [PubMed] [Google Scholar]
- 43.Van der Heijde D, Klareskog L, Landewé R. et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:3928–39. doi: 10.1002/art.23141. [DOI] [PubMed] [Google Scholar]
- 44.Klareskog L, Van der Heijde D, De Jager JP. et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 45.Emery P, Breedveld F, Van der Heijde D. et al. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum. 2010;62:674–82. doi: 10.1002/art.27268. [DOI] [PubMed] [Google Scholar]
- 46.Emery P, Breedveld F, Hall S. et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 47.Katona C, Peveler R, Dowrick C. et al. Pain symptoms in depression: definition and clinical significance. Clin Med (Lond) 2005;5:390–5. doi: 10.7861/clinmedicine.5-4-390. [DOI] [PMC free article] [PubMed] [Google Scholar]