Abstract
The anti-saccade task has emerged as an important tool for investigating the complex nature of voluntary behaviour. In this task, participants are instructed to suppress the natural response to look at a peripheral visual stimulus and look in the opposite direction instead. Analysis of saccadic reaction times (SRT: the time from stimulus appearance to the first saccade) and the frequency of direction errors (i.e. looking toward the stimulus) provide insight into saccade suppression mechanisms in the brain. Some direction errors are reflexive responses with very short SRTs (express latency saccades), while other direction errors are driven by automated responses and have longer SRTs. These different types of errors reveal that the anti-saccade task requires different forms of suppression, and neurophysiological experiments in macaques have revealed several potential mechanisms. At the start of an anti-saccade trial, pre-emptive top-down inhibition of saccade generating neurons in the frontal eye fields and superior colliculus must be present before the stimulus appears to prevent express latency direction errors. After the stimulus appears, voluntary anti-saccade commands must compete with, and override, automated visually initiated saccade commands to prevent longer latency direction errors. The frequencies of these types of direction errors, as well as SRTs, change throughout the lifespan and reveal time courses for development, maturation, and ageing. Additionally, patients diagnosed with a variety of neurological and/or psychiatric disorders affecting the frontal lobes and/or basal ganglia produce markedly different SRT distributions and types of direction errors, which highlight specific deficits in saccade suppression and inhibitory control. The anti-saccade task therefore provides valuable insight into the neural mechanisms of saccade suppression and is a valuable tool in a clinical setting.
This article is part of the themed issue ‘Movement suppression: brain mechanisms for stopping and stillness’.
Keywords: superior colliculus, frontal cortex, parietal cortex, basal ganglia, development, ageing
1. Introduction
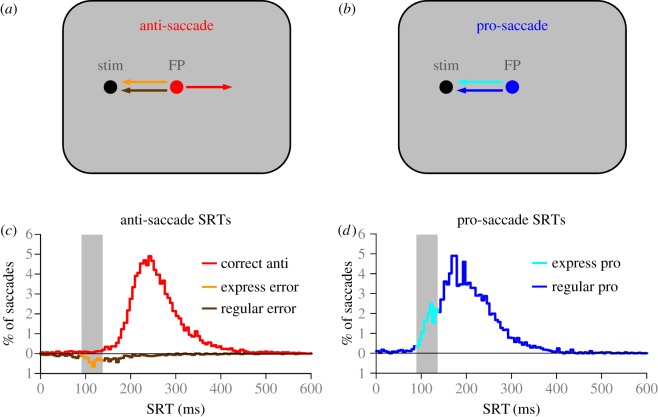
The anti-saccade task (figure 1a), first developed by Peter Hallett [1], has been used extensively to investigate mechanisms of voluntary saccade control. Typically, participants start each trial by fixating a central visual stimulus (often referred to as a fixation point) on a computer screen. A peripheral visual stimulus then appears and participants must first suppress the impulse to look toward the visual stimulus and then make a saccade to the diametrically opposite position. The task was initially developed as a way to dissociate between the stimulus location and the goal of the saccade. This creates competition between: (i) stimulus induced signals that can be predominantly automated saccadic commands (pro-saccade), and (ii) voluntary driven signals that are presumed to be goal-directed saccadic commands (anti-saccade). If the voluntary signals override the automated signals, correct anti-saccades are performed. If, however, the automated signals override the voluntary signals then saccades are made toward the stimulus, which are referred to as direction errors. The frequency and timing of direction errors provide important insight for understanding the processes of saccade inhibition required for the suppression of automated signals.
Figure 1.
Basic design for the (a) anti- and (b) pro-saccade tasks. Typically, participants start each trial fixating a central fixation point (FP) whose colour provides the instruction for (a) an anti-saccade or (b) a pro-saccade. (c,d) Behaviour of healthy adult subjects, 19–39 years old, performing the anti- and pro-saccade tasks. Values above zero on ordinate indicate correct anti- (c) and pro- (d) saccade reaction times (SRT) and values below zero represent the SRT for direction errors.
In this article, we review key aspects of human behaviour in the anti-saccade task. We focus on two kinds of direction errors, delineated by SRT (figure 1c), that indicate two distinct kinds of failures to stop saccadic eye movements. We will review the evidence for these different forms of saccade suppression from different experimental settings. First, we review findings from macaque neurophysiological experiments that have illustrated how different saccade suppression signals interact with different saccade generation signals. We then describe how these abilities evolve over the lifespan in healthy humans. Finally, we review clinical literature that has used the anti-saccade task to identify biomarkers of disease.
2. Basic behaviour in the anti-saccade task
Typically, anti-saccade performance is compared with pro-saccade task performance (figure 1b), where stimulus conditions are nearly identical, but the instruction is to simply look at the peripheral stimulus when it appears. We first describe the pro- (figure 1d) and the anti- (figure 1c) saccade behaviour of healthy young adult participants (ages: 19–39 years, n = 71), which was published previously [2,3]. For these data, the anti-saccade task and the pro-saccade task were performed in separate blocks of trials. The distribution of pro-SRTs (figure 1d) is typically not normal but skewed to the right and is often multimodal. The fastest visually triggered pro-saccades create the earliest mode in the distribution (figure 1d, light blue trace) and are termed express latency saccades [4–6]. In humans, the range of SRTs that defines the express saccade epoch (vertical grey bars in figure 1c,d) typically spans 90–140 ms and in macaques it is earlier (70–110 ms). The precise time of the express-latency epoch is dependent upon the timing of the initial visual response to the stimulus that propagates through the oculomotor system (see §3). This depends greatly upon laboratory conditions, especially the contrast of the target relative to background [7–9]. Other experimental conditions can also be manipulated to change the frequency of express latency saccades [6,10]; for example, removing the fixation point 200 ms before stimulus appearance (i.e. gap condition) or increasing the probability of the stimulus appearing at a specific location [11,12] increases express saccade frequency. During the pro-saccade task, the automated drive to saccade towards a visual stimulus and the voluntary desire to make a goal-directed eye movement work together to facilitate pro-saccades. Here, we describe automated saccadic signals as being triggered by the appearance of the stimulus, and propagating a well-learned motor plan to make a saccade toward the visual stimuli. The overlapping reaction time histograms of express, automated, and voluntary saccades can make these saccades difficult to tease apart, but these three types have been defined behaviourally [13], have been manipulated experimentally using target size [14] and have been modelled statistically to show the effect of training, specifically on the automated (or ‘fast-regular’) saccades [15].
Correct SRTs in the anti-saccade task (figure 1c, red trace) are typically later than pro-saccades (figure 1d, blue trace) [16,17]. Participants also occasionally generate direction errors (figure 1c; traces below zero on ordinate), where the first saccade after stimulus appearance is directed toward the peripheral stimulus. Similar to the distribution of pro-SRTs (figure 1d), direction errors can be triggered at express (90–140 ms) or regular (more than 140 ms) latencies, and their precise timing reveals that there are different saccade suppression mechanisms at play. The first form of suppression is pre-emptive in nature, and must take place before the peripheral visual stimulus can propagate a saccadic command. If this suppression fails, then express latency direction errors are triggered. The second form of suppression occurs later when the voluntary saccadic signals must override automated saccadic signals. If this form of suppression fails, then regular latency direction errors are initiated. Thus, both the initial visual-motor transformation and the subsequent automated saccadic signals are involuntary signals, as they are both initiated by the appearance of the peripheral stimulus, but occur at different times and indicate the need for different suppression mechanisms, or pathways, through oculomotor system (figure 2). These concepts are explained in more detail using the neurophysiology of the oculomotor circuit.
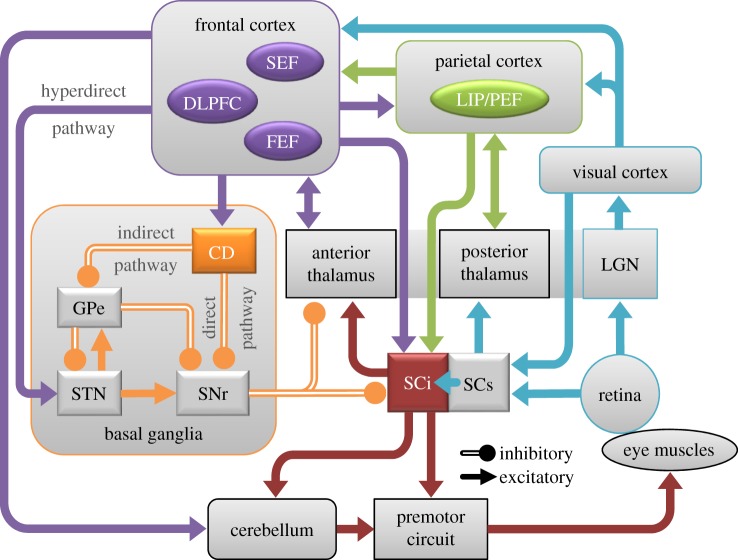
Figure 2.
Oculomotor circuit through the brain highlighting areas involved in anti- and pro-saccade control. CD, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; FEF, frontal eye fields; GPe, external segment of globus pallidus; LGN, lateral geniculate nucleus; LIP, lateral intraparietal area; PEF, parietal eye fields; SCi, intermediate layers of superior colliculus; SCs, superficial layers of superior colliculus; SEF, supplementary eye fields; SNr, substantia nigra pars reticulate; STN, subthalamic nucleus.
3. The oculomotor circuit
An extensive body of literature describing lesion studies, human behavioural testing, functional neuroimaging, animal neurophysiology, and detailed anatomy has identified several brain areas that are involved in controlling visual fixation and saccadic eye movements, including regions within the cerebral cortex, basal ganglia, thalamus, superior colliculus (SC), brainstem reticular formation, and cerebellum [18–22]. Figure 2 shows the circuit of brain areas important for performance on the anti-saccade task. Visual inputs to the system arise from the retino-geniculo-cortical pathway to primary visual cortex and from the retinotectal pathway to the superficial layers of the SC (SCs). Visual information is processed through several extrastriate visual areas before it impinges on motor structures to effect action. The lateral intraparietal area (LIP in monkey; PEF, parietal eye field in human) in the parietal cortex is one area at the interface between sensory and motor processing. LIP/PEF projects to both the intermediate layers of the SC (SCi) and frontal cortical oculomotor areas including the frontal eye fields (FEF), the supplementary eye fields (SEF), and the dorsolateral prefrontal cortex (DLPFC). The FEF play a critical role in executing voluntary saccades. The SEF play an important role in internally guided decision-making and sequencing of saccades. The DLPFC is involved in ‘domain-general’ functions (i.e. improvements in individual functions also improve other related functions) such as executive function, spatial working memory, and suppressing automated or reflexive responses. These frontal oculomotor regions project to the SCi, which is a critical node in the premotor circuit where cortical and subcortical signals converge and are integrated. The SCi projects directly to the premotor circuit in the brainstem reticular formation to provide the necessary input to guide saccades.
There are also important pathways through the basal ganglia [19,21,23]. Frontal cortical oculomotor areas project to the caudate nucleus (CN). Via the direct pathway, GABAergic neurons in the CN project directly to the substantia nigra pars reticulata (SNr). Neurons in the SNr form the major output of the basal ganglia circuit: they are GABAergic and they project to the SCi and nuclei in the thalamus that project to frontal cortex. Cortical inputs to the direct pathway lead to disinhibition of the SC and thalamus because these signals pass through two inhibitory synapses. There is also an indirect pathway through the basal ganglia, in which a separate set of GABAergic neurons in the CN project to the external segment of the globus pallidus (GPe). GABAergic neurons in GPe then project to the subthalamic nucleus (STN). Neurons in the STN send excitatory projections to the GPe, which then projects via GABAergic projections to the SNr. There is also a hyperdirect pathway in which regions of cerebral cortex project to the STN, which then projects directly to SNr. These complex sets of excitatory and inhibitory projections within the basal ganglia provide a rich set of control mechanisms to help guide voluntary behaviour [19].
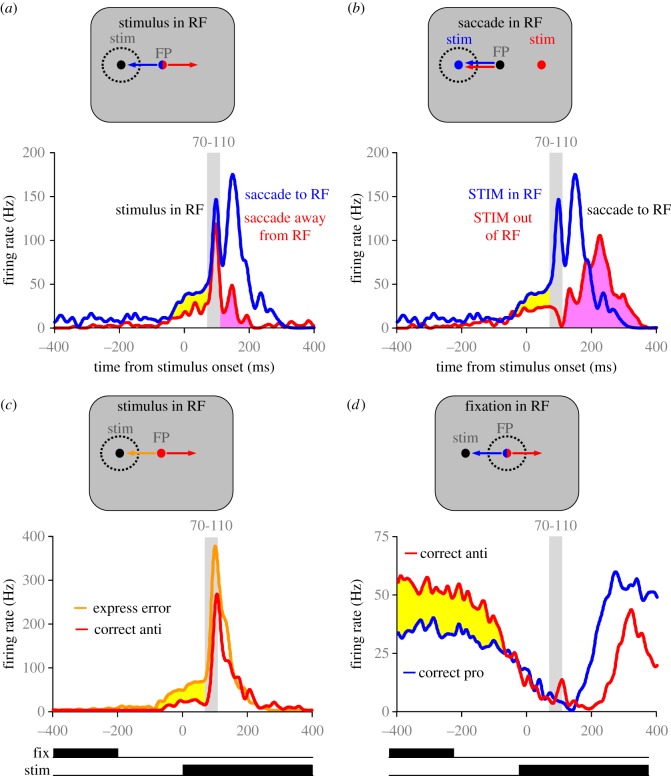
The behaviour from macaques that have been trained to perform anti-saccades is similar to that of humans [24,25]. Neurophysiological experiments in macaques have provided valuable information regarding the role of saccade suppression signals in successful completion of the anti-saccade task. The appearance of the visual stimulus in the periphery triggers a transient visual response that enters via the retino-geniculo-striate pathway to V1 and the retinotectal pathway to the SCs. This visual response propagates through the oculomotor regions of the circuit including LIP, FEF and the SCi. Indeed, many neurons with saccade responses in the SCi [26] have robust visual responses in addition to saccade responses. Figure 3a,b highlights the responses of a typical visuomotor neuron in the SCi when the monkey performs correct pro-saccades (blue traces) and anti-saccades (red traces). The monkey started each trial fixating a central FP. The colour of the FP conveyed whether a pro-saccade or an anti-saccade should be made following the appearance of the peripheral stimulus. Figure 3a illustrates the neural responses when the stimulus was located in the neuron's response field and figure 3b illustrates the responses when the saccade was made toward the neuron's response field. The response field is defined as the region of visual space to which this neuron responds. Please note that the same pro-saccade curves are presented in figure 3a,b, but the anti-saccade curves represent anti-saccades in different directions. In the pro-saccade task, the neuron discharged two bursts: the first burst was aligned with the appearance of the visual stimulus, while the second burst was aligned with the onset of the saccade (blue traces in figure 3a,b). In the anti-saccade task, the neuron still discharged a visually aligned burst, because the stimulus appeared in the neuron's response field (red traces in figure 3a). However, this neuron must be inhibited, so that the saccade neurons on the opposite side of the brain can then discharge the motor burst to drive the correct anti-saccade (red traces in figure 3b). Note that even after extensive training in the anti-saccade task, the initial visual burst is not eliminated. Instead, the visuomotor neurons in the SCi have a reduced discharge rate prior to stimulus appearance on anti-saccade trials compared with pro-saccade trials (yellow shading in figure 3a,b). When this pre-stimulus inhibition is insufficient, the visually aligned response is strong enough to propagate a saccadic command and trigger a direction error at express latency (orange trace in figure 3c) [27]. Thus, the goal of the enhanced pre-stimulus inhibition on anti-saccade trials serves to prevent the initial visually aligned response from triggering express latency direction errors.
Figure 3.
Representative neurons recorded from the SCi that highlight specific elements of neural responses for pro- (blue traces) and anti- (red traces) saccade trials. (a,b) Discharges of a single visuomotor neuron in the SCi for correct pro- and anti-saccade trials when either the stimulus (a) or the saccade (b) was directed into the response field (RF). (c) Discharge of a visuomotor neuron for correct anti-saccades (red trace) and direction errors triggered at express latency (orange trace). (d) Discharge of a fixation neuron recorded from the SCi when the monkey made correct pro- and anti-saccades. Yellow shading highlights evidence of top-down global suppression required to block express errors. Pink shading in (a) shows the brief activity that could reflect the automated pro-saccade signals that were quenched whereas the pink shading in (b) shows the much larger voluntary anti-saccade command that produced longer-latency anti-saccades. Figure panels adapted from [27,28].
What are the potential sources of pre-stimulus inhibition during anti-saccade trials? First, the rostrolateral pole of the SCi contains neurons that are active during fixation. These neurons, referred to initially as fixation neurons, are tonically active during visual fixation and they cease firing during the execution of saccades greater than 2° of visual angle [29–31]. Many of these neurons also participate in the generation of microsaccades (e.g. saccades less than 2°) [32]. There is some debate about whether these are true fixation neurons, or saccade neurons for small vectors (i.e. microsaccades), or in fact whether these neurons could subserve both functions. The complex nature of what fixation entails is reviewed elsewhere in this issue [33,34]. These fixation neurons, have discharge patterns that are reciprocal to saccade neurons, and it has been hypothesized that a network of inhibitory connections participates in shaping the reciprocal discharges of fixation and saccade neurons [35–37]. In support of this hypothesis, fixation neurons in the SCi have higher discharges during anti-saccade trials than during pro-saccade trials (red trace above blue trace in figure 3d). This type of pre-stimulus activity during anti-saccade trials that is displayed by SCi fixation neurons (figure 3d) has also been observed in oculomotor regions of the frontal cortex and basal ganglia. Specifically, neurons with enhanced pre-stimulus activity during anti-saccade trials compared with pro-saccade trials have also been observed in DLPFC [38], the FEF [39], the CN [40], and the external segment of the globus pallidus [41]. Thus, it appears that this form of top-down inhibition that is required for the anti-saccade task is widely distributed through the brain and is the signal required to inhibit saccade neurons in the SCi prior to stimulus appearance and prevent the initial visual transient response from triggering a direction error (figure 3c).
The successful completion of the anti-saccade task also requires that the participant generate the voluntary anti-saccade in the direction opposite of the stimulus. This requires activation of saccade neurons in oculomotor regions ipsilateral to the stimulus and cessation of the automated motor command in oculomotor regions contralateral to the stimulus (pink shading in figure 3a,b). This form of inhibition cannot be global because a spatially specific saccade command must eventually emerge from the SCi. Therefore, the growing anti-saccade command on the ipsilateral side (red trace in figure 3b) must outcompete the automated motor command on the contralateral side (red trace in figure 3a) to prevent longer latency direction errors.
What are the potential sources of an enhanced voluntary motor command to help overcome the automated motor command? Again, there are several possible sources for such a signal. The SEF contain neurons with enhanced activity on anti-saccade trials [42], which could serve to provide this voluntary command to the FEF and SCi. In addition, there is growing evidence of the competition of automated and voluntary commands within the basal ganglia (figure 2) that could provide an important source of the signal to SCi. Specifically, there are some saccade neurons in the CN that discharge preferentially for the automated saccade and there are others that discharge selectively for the voluntary anti-saccade [43]. There are also neurons within GPe that are selectively activated for anti-saccades [41]. Therefore, there are ways for automated and voluntary saccade commands to also interact within the competing pathways through the basal ganglia. For example, there can be interactions between the traditional direct (CD to SNr) versus indirect (CD to GPe to STN to SNr) pathways and between the hyperdirect (cortex to STN to SNr) versus the STN to GPe to SNr route [19,41]. Then, from the SNr there are projections to both the ipsilateral and contralateral SCi [44] that ultimately influence SCi activity [19,23].
4. Anti-saccade behaviour across the lifespan
Several aspects of oculomotor control vary systematically across the lifespan; these include reaction time, accuracy and ability to exert cognitive control [45–50]. Over several years our laboratory has conducted pro- and anti-saccade studies with healthy human participants who varied in age from 5 to 90 years [2]. In these studies, the pro- and anti-saccade tasks were run in separate blocks of trials. Figure 4 contrasts the cumulative distributions of SRTs for correct anti-saccades (values above zero on ordinate) and direction errors (values below zero on ordinate). The colour curves contrast different age cohorts performing the task across development (figure 4a) and normal healthy ageing (figure 4b).
Figure 4.
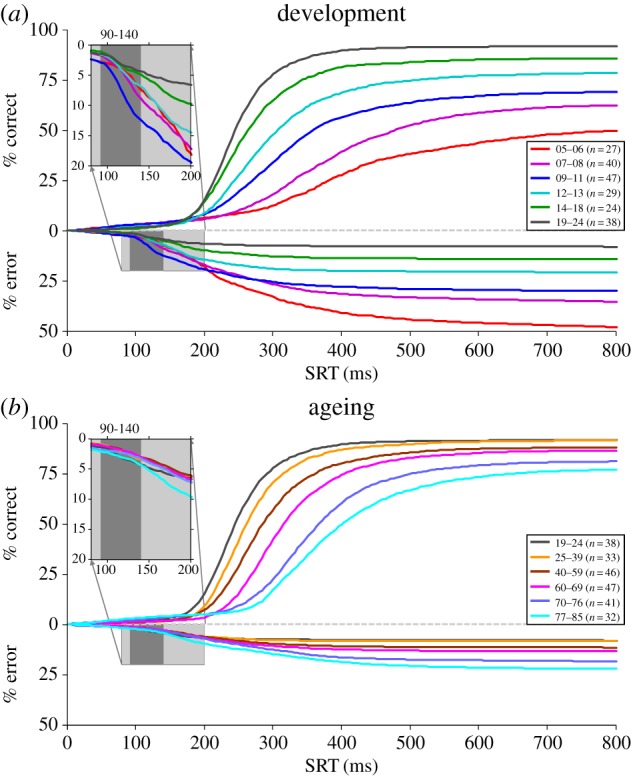
Cumulative distributions of reaction times for correct anti-saccades (values above zero on ordinate) and direction errors (values below zero on ordinate). Different colour curves represent different cohorts of healthy human subjects across different ages to highlight dramatic changes across development (a) and ageing (b). The light grey inserts highlight the triggering of express direction errors in the different groups. The express epoch is shaded dark grey.
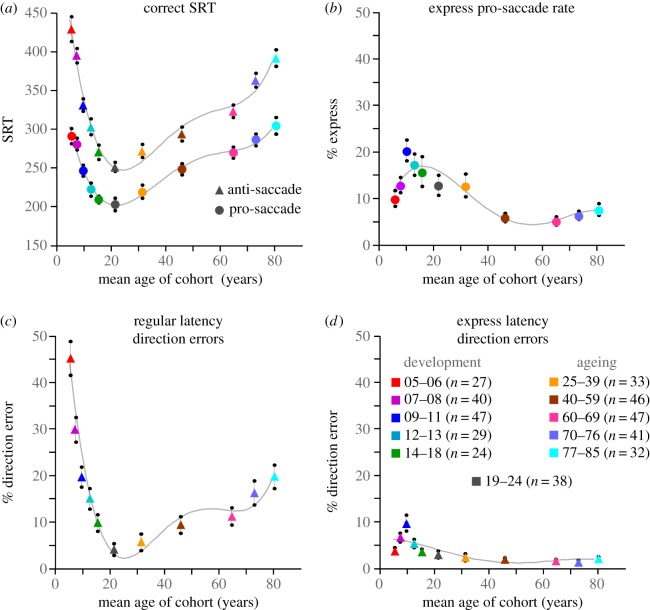
Performance on the anti-saccade task changes dramatically through child and adolescent development (figures 4a and 5). The youngest children (5–6 years old (yo)) tended to perform very poorly on the anti-saccade task, generating almost 50% errors. As participant age increases to 20 yo, correct anti-SRTs (figure 5a) and the percentage of regular latency direction errors (figure 5c) drop considerably. Express latency direction errors, however, actually increase to peak at 9–11 yo, then drop as participants approach 20 yo (figures 5d and 4a inset). The correct express pro-saccade data follow a very similar pattern (figure 5b). This change in the pattern of the timing of direction errors reveals the maturation of the automated pathways by 9–11 yo, but the delayed maturation of top-down suppression signals. Beyond 11 yo, regular latency error rates continue to diminish, and express latency error rates then start to improve. This reveals the delayed maturation of top-down suppression required to block the visual transient response from triggering express direction errors.
Figure 5.
Summary of pro- and anti-saccade behaviour across the lifespan for the same cohorts of healthy subjects illustrated in figure 4. (a) Mean SRT for correct pro (circles) and anti (triangles) saccade trials. (b) Percentage of trials with express latency saccades in the pro-saccade task. (c,d) Percentage of anti-saccade trials with direction errors in the express (d) or regular (c) latency epoch.
The important developmental milestones identified in the anti-saccade task have direct correlates with development of frontal lobe function, as revealed by combining eye tracking and functional brain imaging as participants perform the anti-saccade task. Several imaging studies have revealed how blood oxygen level dependent (BOLD) signal increases through development during anti-saccade task preparation in key frontal lobe areas (e.g. DLPFC, FEF, SEF) [45,51–54]. There are also changes in effective connectivity that support the improved task performance [55].
During adulthood and the ageing process, however, the anti-saccade task reveals a separate pattern of behavioural changes (figures 4b and 5). Young adults (19–24 yo) had the fastest correct anti-SRTs (figure 5a) and they generated the fewest regular latency direction errors (figure 5c). Correct anti-SRTs increased progressively throughout adulthood (figure 5a). The regular latency direction error rate rose very slowly throughout adulthood before increasing beyond 60 years of age. Most of the direction errors made by elderly were initiated at longer latency, well beyond the express epoch for all but the 77–85 yo cohort (see insert in figures 4b and 5d), and could be more indicative of working memory performance than of inhibitory control. The changes in task performance that occur in the elderly have also been related to changes in functional connectivity in frontal cortex [56,57]. In summary, the different trajectories for the frequencies of the two types of direction errors in the anti-saccade task (figure 5c,d) provide a fascinating view of brain development and ageing across the lifespan.
5. Clinical studies
The anti-saccade task was first used in a clinical study by Guitton et al. [58] who demonstrated that patients with large lesions of DLPFC had profound difficulties performing the anti-saccade task. Specifically, they triggered more direction errors than normal healthy adults, and this was especially true when they were under pressure to respond quickly. Many of these direction errors were triggered at express latencies. This seminal study demonstrated the clinical usefulness of the anti-saccade task to reveal deficits in inhibitory control and executive function. Since this initial study demonstrating the clinical usefulness of the anti-saccade task, the application of the anti-saccade task as a research tool in clinical studies has proliferated [18,59].
Our laboratory has investigated anti-saccade behaviour in many disorders of the frontal cortex and/or basal ganglia including attention deficit hyperactivity disorder (ADHD) [60,61], fetal alcohol spectrum disorders (FASD) [62,63], Huntington's disease [64], Parkinson's disease (PD) [65–67], Alzheimer's disease [68], mild cognitive impairment [68], amyotrophic lateral sclerosis (ALS) [69], and bipolar disease [70]. Other studies have demonstrated anti-saccade deficits in schizophrenia [71–73], obsessive compulsive disorder [74,75], Tourette syndrome [75], multiple sclerosis [76], depression [77,78], frontotemporal dementia [79,80], PD [81,82], mild cognitive impairment [83], and Alzheimer's disease [84].
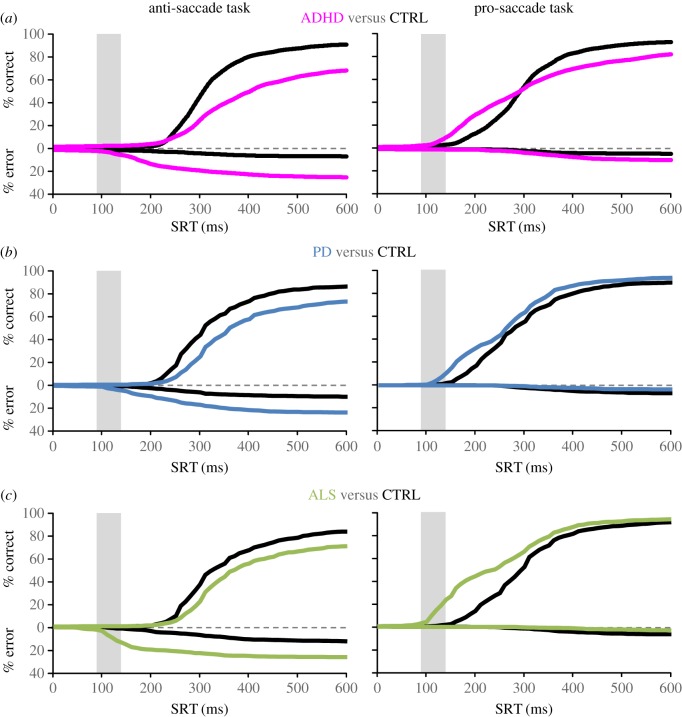
A review of the clinical literature has revealed there are different types of direction errors than can be initiated [85]. Here, we highlight the results of three specific patient groups which our laboratory studied (ADHD, ALS and PD), that performed the same interleaved pro- and anti-saccade task with simultaneous functional magnetic resonance imaging and eye tracking. Despite the identical experimental conditions, each patient group produced a unique pattern of abnormal behaviour that reveals different mechanisms of saccade suppression. Figure 6 contrasts the cumulative distribution of SRTs of correct and error responses in ADHD, ALS and PD. Relative to age-matched controls, all three patient groups generate more direction errors in the anti-saccade task. However, ADHD participants generated more express and regular latency errors (figure 6a, left) [61]. ALS participants made more express-latency errors (figure 6c, left) [69]. PD patients made more longer latency errors (figure 6b, left) [66]. These different patterns of abnormal behaviour correlated with different patterns of abnormal BOLD signal from frontal oculomotor regions and the CN measured with functional magnetic resonance imaging. This dramatic difference in the timing of direction errors between these patient groups reveals different mechanisms of saccade suppression that are present in the anti-saccade task and that these different mechanisms are impacted differentially in disease.
Figure 6.
Cumulative distributions of correct and error trials from three specific different disease groups performing an interleaved pro-/anti-saccade task highlighting different patterns of abnormal behaviour. (a) The attention deficit hyperactivity disorder (ADHD) group makes both short and long latency direction errors. Adapted from [61]. (b) Patients with Parkinson's disease (PD) make more long latency direction errors. Adapted from [66]. (c) Patients with amyotrophic lateral sclerosis (ALS) make more express latency errors. Adapted from [69].
6. Conclusion
In summary, analysis of behaviour in the anti-saccade task has revealed different forms of saccade suppression required for successful task completion. The first competition is pre-emptive in nature, requiring a preparatory suppression signal that must be present prior to stimulus appearance so that when the visual transient response travels through the oculomotor areas of the brain, it does not trigger a reflexive orienting response. The second competition is reactionary in nature and is based upon internal goals. It requires the active suppression of an automated saccade plan, and the generation of a voluntary saccade to an abstract location. The saccadic suppression described here may be different than stopping ongoing movements as discussed elsewhere in the issue [86–88], but involve very similar neural circuitry. Additionally, we posit that the two types of saccade suppression we discussed are also different from the need to cancel a voluntarily planned saccade, which is better studied using the countermanding and stop-signal tasks that are also reviewed elsewhere in this issue [85,89–91]. Both the preparatory and the reactionary mechanisms of saccade suppression are independent, as demonstrated by anti-saccade behaviour across the lifespan, but both require an intact frontal cortex and basal ganglia.
Data accessibility
No new data were collected for this manuscript.
Authors' contributions
Both authors contributed equally to this manuscript.
Competing interests
We have no competing interests.
Funding
This research was funded by Canadian Institutes of Health Research (grant no. MOP-FDN-148418).
References
- 1.Hallett PE. 1978. Primary and secondary saccades to goals defined by instructions. Vision Res. 18, 1279–1296. ( 10.1016/0042-6989(78)90218-3) [DOI] [PubMed] [Google Scholar]
- 2.Munoz DP, Broughton JR, Goldring JE, Armstrong IT. 1998. Age-related performance of human subjects on saccadic eye movement tasks. Exp. Brain Res. 121, 391–400. ( 10.1007/s002210050473) [DOI] [PubMed] [Google Scholar]
- 3.Peltsch A, Hemraj A, Garcia A, Munoz DP. 2011. Age-related trends in saccade characteristics among the elderly. Neurobiol. Aging 32, 669–679. ( 10.1016/j.neurobiolaging.2009.04.001) [DOI] [PubMed] [Google Scholar]
- 4.Fischer B, Boch R. 1983. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 260, 21–26. ( 10.1016/0006-8993(83)90760-6) [DOI] [PubMed] [Google Scholar]
- 5.Fischer B, Ramsperger E. 1984. Human express saccades: extremely short reaction times of goal directed eye movements. Exp. Brain Res. 57, 191–195. ( 10.1007/BF00231145) [DOI] [PubMed] [Google Scholar]
- 6.Fischer B, Weber H. 1993. Express saccades and visual attention. Behav. Brain Sci. 16, 553 ( 10.1017/S0140525X00031575) [DOI] [Google Scholar]
- 7.Bell AH, Meredith MA, Van Opstal AJ, Munoz DP. 2006. Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Exp. Brain Res. 174, 53–59. ( 10.1007/s00221-006-0420-z) [DOI] [PubMed] [Google Scholar]
- 8.Marino RA, Munoz DP. 2009. The effects of bottom-up target luminance and top-down spatial target predictability on saccadic reaction times. Exp. Brain Res. 197, 321–335. ( 10.1007/s00221-009-1919-x) [DOI] [PubMed] [Google Scholar]
- 9.Marino RA, Trappenberg TP, Dorris M, Munoz DP. 2012. Spatial interactions in the superior colliculus predict saccade behavior in a neural field model. J. Cogn. Neurosci. 24, 315–336. ( 10.1162/jocn_a_00139) [DOI] [PubMed] [Google Scholar]
- 10.Munoz DP, Dorris MC, Paré M, Everling S. 2000. On your mark, get set: brainstem circuitry underlying saccadic initiation. Can. J. Physiol. Pharmacol. 78, 934–944. ( 10.1139/y00-062) [DOI] [PubMed] [Google Scholar]
- 11.Sparks D, Rohrer WH, Zhang Y. 2000. The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res. 40, 2763–2777. ( 10.1016/S0042-6989(00)00133-4) [DOI] [PubMed] [Google Scholar]
- 12.Dorris MC, Munoz DP. 1998. Saccadic probability influences motor preparation signals and time to saccadic initiation. J. Neurosci. 18, 7015–7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer B, Weber H, Biscaldi M, Aiple F, Otto P, Stuhr V. 1993. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Exp. Brain Res. 92, 528–541. ( 10.1007/BF00229043) [DOI] [PubMed] [Google Scholar]
- 14.Ploner CJ, Ostendorf F, Dick S. 2004. Target size modulates saccadic eye movements in humans. Behav. Neurosci. 118, 237–242. ( 10.1037/0735-7044.118.1.237) [DOI] [PubMed] [Google Scholar]
- 15.Gezeck S, Fischer B, Timmer J. 1997. Saccadic reaction times: a statistical analysis of multimodal distributions. Vision Res. 37, 2119–2131. ( 10.1016/S0042-6989(97)00022-9) [DOI] [PubMed] [Google Scholar]
- 16.Fischer B, Weber H. 1992. Characteristics of ‘anti’ saccades in man. Exp. Brain Res. 89, 415–424. ( 10.1007/BF00228257) [DOI] [PubMed] [Google Scholar]
- 17.Dafoe JM, Armstrong IT, Munoz DP. 2007. The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Exp. Brain Res. 179, 563–570. ( 10.1007/s00221-006-0817-8) [DOI] [PubMed] [Google Scholar]
- 18.Munoz DP, Everling S. 2004. Look away: the anti-saccade task and the voluntary control of eye movement. Nat. Rev. Neurosci. 5, 218–228. ( 10.1038/nrn1345) [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Munoz DP. 2011. Probing basal ganglia functions by saccade eye movements. Eur. J. Neurosci. 33, 2070–2090. ( 10.1111/j.1460-9568.2011.07691.x) [DOI] [PubMed] [Google Scholar]
- 20.Schall JD. 2004. On the role of frontal eye field in guiding attention and saccades. Vision Res. 44, 1453–1467. ( 10.1016/j.visres.2003.10.025) [DOI] [PubMed] [Google Scholar]
- 21.Hikosaka O, Nakamura K, Nakahara H. 2006. Basal ganglia orient eyes to reward. J. Neurophysiol. 95, 567–584. ( 10.1152/jn.00458.2005) [DOI] [PubMed] [Google Scholar]
- 22.Scudder CA, Kaneko CR, Fuchs AF. 2002. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp. Brain Res. 142, 439–462. ( 10.1007/s00221-001-0912-9) [DOI] [PubMed] [Google Scholar]
- 23.Hikosaka O, Takikawa Y, Kawagoe R. 2000. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 80, 953–978. [DOI] [PubMed] [Google Scholar]
- 24.Amador N, Schlag-Rey M, Schlag J. 1998. Primate antisaccades. I. Behavioral characteristics. J Neurophysiol. 80, 1775–1786. [DOI] [PubMed] [Google Scholar]
- 25.Bell AH, Everling S, Munoz DP. 2000. Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J. Neurophysiol. 84, 2595–2604. [DOI] [PubMed] [Google Scholar]
- 26.Mohler CW, Wurtz RH. 1976. Organization of monkey superior colliculus: intermediate layer cells discharging before eye movements. J. Neurophysiol. 39, 722–744. [DOI] [PubMed] [Google Scholar]
- 27.Everling S, Dorris MC, Munoz DP. 1998. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J. Neurophysiol. 80, 1584–1589. [DOI] [PubMed] [Google Scholar]
- 28.Everling S, Dorris MC, Klein RM, Munoz DP. 1999. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J. Neurosci. 19, 2740–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz DP, Wurtz RH. 1993. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J. Neurophysiol. 70, 576–589. [DOI] [PubMed] [Google Scholar]
- 30.Munoz DP, Wurtz RH. 1993. Superior colliculus and visual fixation. Biomed. Res. 14, 75–79. [Google Scholar]
- 31.Munoz DP, Wurtz RH. 1992. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J. Neurophysiol. 67, 1000–1002. [DOI] [PubMed] [Google Scholar]
- 32.Hafed ZM, Krauzlis RJ. 2012. Similarity of superior colliculus involvement in microsaccade and saccade generation. J. Neurophysiol. 107, 1904–1916. ( 10.1152/jn.01125.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Conde S, Macknik SL. 2017. Unchanging visions: the effects and limitations of ocular stillness. Phil. Trans. R. Soc. B 372, 20160204 ( 10.1098/rstb.2016.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krauzlis RJ, Goffart L, Hafed ZM. 2017. Neuronal control of fixation and fixational eye movements. Phil. Trans. R. Soc. B 372, 20160205 ( 10.1098/rstb.2016.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munoz DP, Istvan PJ. 1998. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J. Neurophysiol. 79, 1193–1209. [DOI] [PubMed] [Google Scholar]
- 36.Trappenberg TP, Dorris MC, Munoz DP, Klein RM. 2001. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J. Cogn. Neurosci. 13, 256–271. ( 10.1162/089892901564306) [DOI] [PubMed] [Google Scholar]
- 37.Munoz DP, Fecteau JH. 2002. Vying for dominance: dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Prog. Brain Res. 140, 3–19. ( 10.1016/S0079-6123(02)40039-8) [DOI] [PubMed] [Google Scholar]
- 38.Johnston K, Everling S. 2006. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J. Neurosci. 26, 12 471–12 478. ( 10.1523/JNEUROSCI.4101-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Everling S, Munoz DP. 2000. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci. 20, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe M, Munoz DP. 2010. Presetting basal ganglia for volitional actions. J. Neurosci. 30, 10 144–10 157. ( 10.1523/JNEUROSCI.1738-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida A, Tanaka M. 2016. Two types of neurons in the primate globus pallidus external segment play distinct roles in antisaccade generation. Cereb. Cortex 26, 1187–1199. ( 10.1093/cercor/bhu308) [DOI] [PubMed] [Google Scholar]
- 42.Schlag-Rey M, Amador N, Sanchez H, Schlag J. 1997. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390, 398–401. ( 10.1038/37114) [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Munoz DP. 2009. Neural correlates of conflict resolution between automatic and volitional actions by basal ganglia. Eur. J. Neurosci. 30, 2165–2176. ( 10.1111/j.1460-9568.2009.06998.x) [DOI] [PubMed] [Google Scholar]
- 44.Jiang H, Stein BE, McHaffie JG. 2003. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature 423, 982–986. ( 10.1038/nature01698) [DOI] [PubMed] [Google Scholar]
- 45.Luna B, et al. 2001. Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13, 786–793. ( 10.1006/nimg.2000.0743) [DOI] [PubMed] [Google Scholar]
- 46.Fischer B, Gezeck S, Hartnegg K. 1997. The analysis of saccadic eye movements from gap and overlap paradigms. Brain Res. Protoc. 2, 47–52. ( 10.1016/S1385-299X(97)00027-5) [DOI] [PubMed] [Google Scholar]
- 47.Fukushima J, Hatta T, Fukushima K. 2000. Development of voluntary control of saccadic eye movements: I. Age-related changes in normal children. Brain Dev. 22, 173–180. ( 10.1016/S0387-7604(00)00101-7) [DOI] [PubMed] [Google Scholar]
- 48.Klein C, Foerster F. 2001. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology 38, 179–189. ( 10.1111/1469-8986.3820179) [DOI] [PubMed] [Google Scholar]
- 49.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. 2004. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 75, 1357–1372. ( 10.1111/j.1467-8624.2004.00745.x) [DOI] [PubMed] [Google Scholar]
- 50.Kramer AF, de Sather JC, Cassavaugh ND. 2005. Development of attentional and oculomotor control. Dev. Psychol. 41, 760–772. ( 10.1037/0012-1649.41.5.760) [DOI] [PubMed] [Google Scholar]
- 51.Luna B, Velanova K, Geier CF. 2008. Development of eye-movement control. Brain Cogn. 68, 293–308. ( 10.1016/j.bandc.2008.08.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alahyane N, Brien DC, Coe BC, Stroman PW, Munoz DP. 2014. Developmental improvements in voluntary control of behavior: effect of preparation in the fronto-parietal network? Neuroimage 98, 103–117. ( 10.1016/j.neuroimage.2014.03.008) [DOI] [PubMed] [Google Scholar]
- 53.Marek S, Hwang K, Foran W, Hallquist MN, Luna B. 2015. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 13, e1002328 ( 10.1371/journal.pbio.1002328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. 2015. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 38, 151–170. ( 10.1146/annurev-neuro-071714-034054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang K, Velanova K, Luna B. 2010. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J. Neurosci. 30, 15 535–15 545. ( 10.1523/JNEUROSCI.2825-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pa J, et al. 2014. The functional oculomotor network and saccadic cognitive control in healthy elders. Neuroimage 95, 61–68. ( 10.1016/j.neuroimage.2014.03.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirsky JB, Heuer HW, Jafari A, Kramer JH, Schenk AK, Viskontas IV, Miller BL, Boxer AL. 2011. Anti-saccade performance predicts executive function and brain structure in normal elders. Cogn. Behav. Neurol. 24, 50–58. ( 10.1097/WNN.0b013e318223f6c6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guitton D, Buchtel HA, Douglas RM. 1985. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp. Brain Res. 58, 455–472. ( 10.1007/BF00235863) [DOI] [PubMed] [Google Scholar]
- 59.Everling S, Fischer B. 1998. The antisaccade: a review of basic research and clinical studies. Neuropsychologia 36, 885–899. ( 10.1016/S0028-3932(98)00020-7) [DOI] [PubMed] [Google Scholar]
- 60.Munoz DP, Armstrong IT, Hampton KA, Moore KD. 2003. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J. Neurophysiol. 90, 503–514. ( 10.1152/jn.00192.2003) [DOI] [PubMed] [Google Scholar]
- 61.Hakvoort Schwerdtfeger RM, Alahyane N, Brien DC, Coe BC, Stroman PW, Munoz DP. 2013. Preparatory neural networks are impaired in adults with attention-deficit/hyperactivity disorder during the antisaccade task. NeuroImage Clin. 2, 63–78. ( 10.1016/j.nicl.2012.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green CR, Munoz DP, Nikkel SM, Reynolds JN. 2007. Deficits in eye movement control in children with fetal alcohol spectrum disorders. Alcohol Clin. Exp. Res. 31, 500–511. ( 10.1111/j.1530-0277.2006.00335.x) [DOI] [PubMed] [Google Scholar]
- 63.Paolozza A, Titman R, Brien D, Munoz DP, Reynolds JN. 2013. Altered accuracy of saccadic eye movements in children with fetal alcohol spectrum disorder. Alcohol Clin. Exp. Res. 37, 1491–1498. ( 10.1111/acer.12119) [DOI] [PubMed] [Google Scholar]
- 64.Peltsch A, Hoffman A, Armstrong I, Pari G, Munoz DP. 2008. Saccadic impairments in Huntington's disease. Exp. Brain Res. 186, 457–469. ( 10.1007/s00221-007-1248-x) [DOI] [PubMed] [Google Scholar]
- 65.Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. 2005. Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia 43, 784–796. ( 10.1016/j.neuropsychologia.2004.06.026) [DOI] [PubMed] [Google Scholar]
- 66.Cameron IGM, Pari G, Alahyane N, Brien DC, Coe BC, Stroman PW, Munoz DP. 2012. Impaired executive function signals in motor brain regions in Parkinson's disease. Neuroimage 60, 1156–1170. ( 10.1016/j.neuroimage.2012.01.057) [DOI] [PubMed] [Google Scholar]
- 67.Cameron IGM, Watanabe M, Pari G, Munoz DP. 2010. Executive impairment in Parkinson's disease: response automaticity and task switching. Neuropsychologia 48, 1948–1957. ( 10.1016/j.neuropsychologia.2010.03.015) [DOI] [PubMed] [Google Scholar]
- 68.Peltsch A, Hemraj A, Garcia A, Munoz DP. 2014. Saccade deficits in amnestic mild cognitive impairment resemble mild Alzheimer's disease. Eur. J. Neurosci. 39, 2000–2013. ( 10.1111/ejn.12617) [DOI] [PubMed] [Google Scholar]
- 69.Witiuk K, Fernandez-Ruiz J, McKee R, Alahyane N, Coe BC, Melanson M, Munoz DP. 2014. Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. J. Neurosci. 34, 14 260–14 271. ( 10.1523/JNEUROSCI.1111-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soncin S, Brien DC, Coe BC, Marin A, Munoz DP. 2016. Contrasting emotion processing and executive functioning in attention-deficit/hyperactivity disorder and bipolar disorder. Behav. Neurosci. 130, 531–543. ( 10.1037/bne0000158) [DOI] [PubMed] [Google Scholar]
- 71.Levy DL, Mendell NR, Holzman PS. 2004. The antisaccade task and neuropsychological tests of prefrontal cortical integrity in schizophrenia: empirical findings and interpretative considerations. World Psychiatry 3, 32–40. [PMC free article] [PubMed] [Google Scholar]
- 72.Zanelli J, Simon H, Rabe-Hesketh S, Walshe M, McDonald C, Murray RM, MacCabe JH. 2005. Eye tracking in schizophrenia: does the antisaccade task measure anything that the smooth pursuit task does not? Psychiatry Res. 136, 181–188. ( 10.1016/j.psychres.2004.12.008) [DOI] [PubMed] [Google Scholar]
- 73.Cutsuridis V, Smyrnis N, Evdokimidis I, Perantonis S. 2007. A neural model of decision-making by the superior colicullus in an antisaccade task. Neural Netw. 20, 690–704. ( 10.1016/j.neunet.2007.01.004) [DOI] [PubMed] [Google Scholar]
- 74.Lennertz L, et al. 2012. Antisaccade performance in patients with obsessive-compulsive disorder and unaffected relatives: further evidence for impaired response inhibition as a candidate endophenotype. Eur. Arch. Psychiatry Clin. Neurosci. 262, 625–634. ( 10.1007/s00406-012-0311-1) [DOI] [PubMed] [Google Scholar]
- 75.Jahanshahi M, Rothwell JC. 2017. Inhibitory dysfunction contributes to some of the motor and non-motor symptoms of movement disorders and psychiatric disorders. Phil. Trans. R. Soc. B 372, 20160198 ( 10.1098/rstb.2016.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clough M, Millist L, Lizak N, Beh S, Frohman TC, Frohman EM, White OB, Fielding J. 2015. Ocular motor measures of cognitive dysfunction in multiple sclerosis I: inhibitory control. J. Neurol. 262, 1130–1137. ( 10.1007/s00415-015-7645-3) [DOI] [PubMed] [Google Scholar]
- 77.Carvalho N, Noiret N, Vandel P, Monnin J, Chopard G, Laurent E. 2014. Saccadic eye movements in depressed elderly patients. PLoS ONE 9, e105355 ( 10.1371/journal.pone.0105355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malsert J, Guyader N, Chauvin A, Polosan M, Poulet E, Szekely D, Bougerol T, Marendaz C. 2012. Antisaccades as a follow-up tool in major depressive disorder therapies: a pilot study. Psychiatry Res. 200, 1051–1053. ( 10.1016/j.psychres.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 79.Burrell JR, Hornberger M, Carpenter RHS, Kiernan MC, Hodges JR. 2012. Saccadic abnormalities in frontotemporal dementia. Neurology 78, 1816–1823. ( 10.1212/WNL.0b013e318258f75c) [DOI] [PubMed] [Google Scholar]
- 80.Boxer AL, et al. 2012. Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer disease. Arch. Neurol. 69, 509–517. ( 10.1001/archneurol.2011.1021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amador SC, Hood AJ, Schiess MC, Izor R, Sereno AB. 2006. Dissociating cognitive deficits involved in voluntary eye movement dysfunctions in Parkinson's disease patients. Neuropsychologia 44, 1475–1482. ( 10.1016/j.neuropsychologia.2005.11.015) [DOI] [PubMed] [Google Scholar]
- 82.Antoniades CA, Demeyere N, Kennard C, Humphreys GW, Hu MT. 2015. Antisaccades and executive dysfunction in early drug-naive Parkinson's disease: the discovery study. Mov. Disord. 30, 843–847. ( 10.1002/mds.26134) [DOI] [PubMed] [Google Scholar]
- 83.Heuer HW, Mirsky JB, Kong EL, Dickerson BC, Miller BL, Kramer JH, Boxer AL. 2013. Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology 81, 1235–1243. ( 10.1212/WNL.0b013e3182a6cbfe) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaufman LD, Pratt J, Levine B, Black SE. 2012. Executive deficits detected in mild Alzheimer's disease using the antisaccade task. Brain Behav. 2, 15–21. ( 10.1002/brb3.28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cutsuridis V. 2017. Behavioural and computational varieties of response inhibition in eye movements. Phil. Trans. R. Soc. B 372, 20160196 ( 10.1098/rstb.2016.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roseberry T, Kreitzer A. 2017. Neural circuitry for behavioural arrest. Phil. Trans. R. Soc. B 372, 20160197 ( 10.1098/rstb.2016.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Optican LM, Pretegiani E. 2017. What stops a saccade? Phil. Trans. R. Soc. B 372, 20160194 ( 10.1098/rstb.2016.0194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pouget P, Murthy A, Stuphorn V. 2017. Cortical control and performance monitoring of interrupting and redirecting movements. Phil. Trans. R. Soc. B 372, 20160201 ( 10.1098/rstb.2016.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schall JD, Palmeri TJ, Logan GD. 2017. Models of inhibitory control. Phil. Trans. R. Soc. B 372, 20160193 ( 10.1098/rstb.2016.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noorani I. 2017. Towards a unifying mechanism for cancelling movements. Phil. Trans. R. Soc. B 372, 20160191 ( 10.1098/rstb.2016.0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt R, Berke JD. 2017. A Pause-then-Cancel model of stopping: evidence from basal ganglia neurophysiology. Phil. Trans. R. Soc. B 372, 20160202 ( 10.1098/rstb.2016.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were collected for this manuscript.