Abstract
Rapid movements to a target are ballistic; they usually do not last long enough for visual feedback about errors to influence them. Yet, the brain is not simply precomputing movement trajectory. Classical models of movement control involve a feedback loop that subtracts ‘where we are now’ from ‘where we want to be’. That difference is an internal motor error. The feedback loop reduces this error until it reaches zero, stopping the movement. However, neurophysiological studies have shown that movements controlled by the cerebrum (e.g. arm and head movements) and those controlled by the brain stem (e.g. tongue and eye movements) are also controlled, in parallel, by the cerebellum. Thus, there may not be a single error control loop. We propose an alternative to feedback error control, wherein the cerebellum uses adaptive, velocity feedback, integral control to stop the movement on target.
This article is part of the themed issue ‘Movement suppression: brain mechanisms for stopping and stillness’.
Keywords: eye movements, cerebellum, brainstem, omnipause neuron
1. Introduction
How a movement's target is selected, how a movement is initiated and how it is driven are subjects of other papers in this special volume. Here, we deal with the critical question of what stops a rapid movement. The question is not trivial when considering ballistic movements performed with maximal velocity and acceleration, and hence brief durations. Ballistic movements are common whenever an ultrafast and powerful action is required. For example, the tongue projection of a salamander to capture her prey [1], and human movements such as striking, kicking or throwing. These rapid movements are too brief to be corrected by exteroceptive feedback.
The archetypal ballistic movement is the saccade, a rapid movement that turns the eye so that the image of a peripheral target lands on the fovea. The fovea, the central area of the retina that enables the best visual acuity, is very small (2° in diameter), so saccades must be very accurate. To minimize interference with vision, saccades must be of very short duration. A saccade has an amplitude-dependent duration of 30–120 ms, with most saccades being smaller than 15° and shorter than 60 ms [2,3]. However, in non-human primates visual information takes about 40–50 ms to reach the superior colliculus [4] and about 50–90 ms to reach the first cortical visual area (V1) [5]. Times should be even longer in human subjects. Thus, movements less than about 40° must be ballistic, because they do not last long enough for visual feedback to influence them. Yet, if saccades are stopped mid-movement by stimulating the pause cell region in the brain stem, saccades resume and land on target [6]. This suggests that the brain is not simply precomputing movement trajectory, but rather is using feedback control [7]. How, then, does the brain stop a saccade so that the eye lands on the target?
2. Classical model of local feedback control of motor error
In his seminal work, Robinson [2] showed that the dynamics of the globe, orbital tissues and muscles (the so-called plant) were dominated by muscle viscosity. Thus, making a saccade requires a pulse–slide–step change in innervation to the extraocular muscles, which has indeed been recorded from abducens neurons [8]. The brief, high frequency pulse generates a large force to overcome the plant's short-time constant viscosities. The slide slowly changes from the pulse to the step level of activity, overcoming the plant's long-time constant viscosities. The step is a constant level of activity, which holds the final eye orientation against orbital elastic restoring forces. The slide and the step can be obtained from the pulse by low-pass filtering and integration. Thus, Robinson [9] argued that all one needed to make a saccade was a properly formed, pre-programmed pulse (i.e. with the correct intensity and duration for the desired change in eye orientation). Later, he developed an internal feedback loop model that constructed this pulse automatically from the current and desired eye orientations [10,11]. This was the seminal model for all subsequent models of saccades, because its feedback loop automatically got the eye on target without pre-programming the movement.
In the Robinson model, the internal signals encoded the eye's orientation: where the eye was in the head. Subsequently, however, neurophysiological recordings showed that all internal signals in the saccadic system encoded changes in eye orientation, not absolute eye orientation. Therefore, Jürgens et al. [12] restructured the internal feedback loop to use a desired eye displacement, or change in eye orientation. In doing so, they added a second integrator to track the current displacement (DI). This model is the core of all classical saccadic models (figure 1).
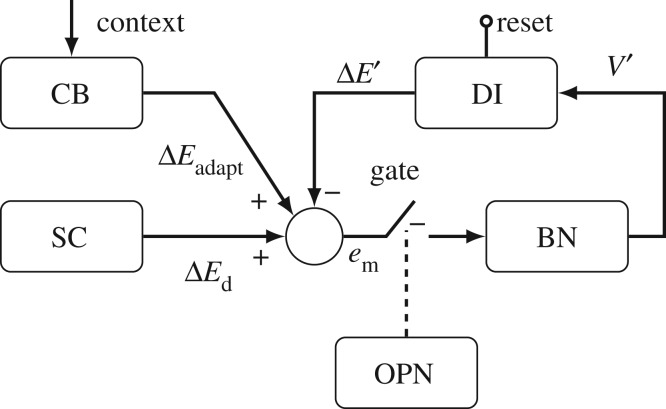
Figure 1.
Classical local feedback loop model of the saccadic burst generator. The burst neuron (BN) is driven by the internal motor error (em), the difference between the output of the superior colliculus (SC), desired change in eye orientation (ΔEd), and the movement's progress so far (ΔE′). Progress is tracked by the displacement integrator (DI), which integrates the velocity drive signal (V′) coming from the BN. The DI must be reset after each saccade. The inhibitory omnipause neurons (OPN) act as a gate, preventing the system from oscillating between saccades. The cerebellum (CB) adapts saccade amplitude by adding another drive signal (ΔEadapt) based on the movement's context.
The key element in this circuit compares how much the eye's orientation needs to change (ΔEd) with how much it has already changed (ΔE′), thus computing an internal motor error (em). The DI computes ΔE′ during the movement from an efference copy of the velocity command, and must be reset to zero before every movement. The drive signal decays as the eye approaches the target, i.e. when the DI's output approaches the desired change of orientation, the motor error approaches zero. When the saccadic drive becomes zero the saccade stops. In some circumstances, however, the saccade does not land on target, e.g. because of muscle weakness. In these cases, the cerebellum is assumed to adapt another input to the feedback loop to compensate for the expected error [13,14]. This additional component (ΔEadapt) is a feed-forward correction, and must be learned from errors over many saccades made in the same context. Here, context means any factor that can influence the desired saccade, such as the same eye position, the same vergence state, etc. It also refers to information about the target. For example, if the target is moving, the target has both a retinal eccentricity and a retinal slip. If the target moves toward (or away from) the fovea, then the subsequent saccade will be smaller (larger) than a saccade to a stationary target [15].
Robinson [11] recognized that if there were any delays or noise in the neuronal system, the very high gain of the bursting element would cause the system to oscillate around the target with of a series of overshooting saccades. Therefore, he added a gate that would prevent oscillations by removing the input to the burst neurons generating the saccade. To make a saccade, the gate would have to close. The omnipause neurons (OPN) discovered by Luschei & Fuchs [16] act like this gate; they fire steadily, inhibiting the burst neurons, except during a saccade. Additionally, when OPN are stimulated during a saccade, the saccade stops abruptly [6].
It should be noted that this type of model describes functions that the saccadic system might perform, but it does not shed much light on its anatomical basis, i.e. on what parts of the brain contribute to those functions. These limitations can be overcome by building a model based on the anatomy and physiology of the brain, i.e. a neuromimetic model. The neuromimetic model can answer the four critical questions about movement control raised by the model in figure 1: (i) where is the desired change in orientation encoded; (ii) where is the motor error signal computed; (iii) where is the displacement integrator, and (iv) what stops the saccade?
3. Saccadic system anatomy and physiology
The major neurons and areas we consider as critical for generating visually guided saccades are shown in figure 2. (Cortical areas and the basal ganglia are also important for making saccades, but they are not included here because they are more involved in target selection than saccade generation). The motor neurons for horizontal eye movements consist of the abducens nuclei motor neurons, which innervate the lateral rectus muscles, and some of the oculomotor nuclei motor neurons, those that innervate the medial rectus muscles. These motor neurons are driven by premotor burst neurons, which are silent except during a saccade, when they burst vigorously. The premotor burst neurons are driven by upstream areas, which determine the frequency and duration of the burst, and thus the amplitude, velocity and duration of the saccade. The saccadic circuitry has been extensively reviewed [13,17–19], so here we give a minimal description.
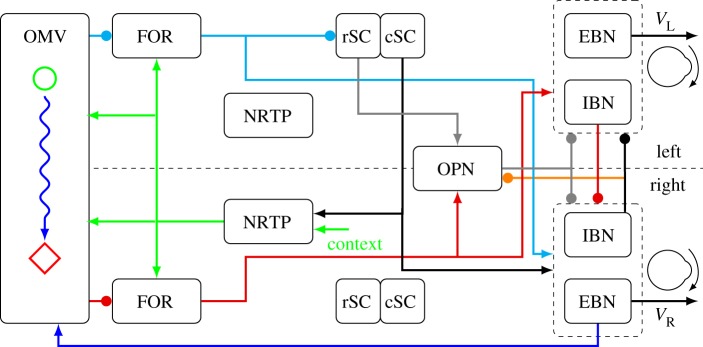
Figure 2.
Schematic diagram of the dual-pathway model of saccadic control. The model is symmetric, but only connections for making a rightward saccade are shown. EBN, excitatory burst neurons; IBN, inhibitory burst neurons; (r,c)SC, rostral, caudal superior colliculus; NRTP, nucleus reticularis tegmenti pontis; FOR, fastigial oculomotor region; OMV, oculomotor vermis; OPN, omnipause neuron; VR, VL are the velocity drive commands from the EBN to the ocular motor nuclei. Arrowheads are excitatory, disks are inhibitory. See §§3 and 4 for explanation of function and colour code.
(a). Premotor burst neurons
The premotor burst neurons consist of two types: excitatory and inhibitory burst neurons (EBN and IBN). EBN and IBN have very high gains and are assumed to have post-inhibitory rebound (PIR) [20,21]. Thus, they can fire at very high frequency to generate brief bursts; also, they can fire just if their inhibition is removed. The EBN provide the drive for agonist eye muscles, whereas the IBN project to the contralateral EBN and inhibit the drive to the antagonist muscles. The IBN show a few spikes or a brief burst of activity at the end of off-direction saccades [20,22,23]. Importantly, the IBN project to the contralateral IBN [24], which means that the left and right IBN mutually inhibit each other. This reciprocal inhibition can lead to instability and saccadic oscillations [21].
(b). Omnipause neurons
The EBN and IBN are tonically inhibited by omnipause neurons (OPN) [25], which are active except during saccades, but pause during saccades in any direction [16,26]. The OPN play a key role in Robinson's original feedback model, as explained in §2, because they gate the input to the premotor burst neurons, preventing them from oscillating when the motor error reaches zero at the end of a saccade. Indeed, if the OPN are held off (e.g. during large, off vertical saccades or blinks), the eyes do oscillate [27,28].
Is the resumption of OPN activity what normally stops the saccade? In normal saccades, the pause begins approximately 20 ms before saccade start, and ends essentially synchronously with saccade end [29]. As there is about a 10 ms delay between EBN activity and eye movement [26], the OPN reactivate too late to stop the saccade. Moreover, Soetedjo et al. [30] found that lesions of the OPN slowed saccades, but did not change their accuracy, as would be expected if OPN stopped saccades on target. Finally, Rucker et al. [31] showed that when vergence movements hold off OPN, saccades still stop on target. Thus, OPN prevent the onset of saccades, but they do not normally play a role in stopping saccades on target.
(c). Superior colliculus
The superior colliculus (SC) is important for saccades. It contains a retinotopic map of the visual scene and a motorotopic map of saccades [4,32]. The SC is divided into two mutually inhibitory zones: the rostral SC (rSC) with activity related to fixation and small saccades, and the caudal SC (cSC) with activity related to large saccades [33–37]. The rSC drives the OPN [38], and the cSC drives the contralateral EBN and IBN [39]. During fixation, the rSC is active, and the cSC is silent. Just before the saccade, the cSC becomes active, and the rSC shuts off. Appearance of a target will activate cells in the cSC at a site corresponding to the target's retinal location, and electrical stimulation of the cSC at that same locus will evoke a saccade to that target location.
Might the rostral pole of the SC be what stops a saccade? The rSC projects to the OPN region with decreasing strength out to about 10° [40]. Stimulation of the rSC during fixation can delay or prevent saccades [34]. Rostral cells pause about 36 ms before saccade onset, but they restart tightly time-locked to the end of the saccade, with a latency of about −2 ms for contraversive and +10 ms for ipsiversive saccades [35]. Stimulation of the rostral pole during a saccade causes a transient slowing, with a latency of just 12 ms [34]. This short delay means that rostral cells pause about 24 ms before the saccadic drive signal starts, and resume firing almost 10 ms after the saccadic drive signal has ended. This is consistent with the finding that electrical stimulation of the rSC more than 40 ms before saccade start, or within 10 ms of saccade end, has no effect on the saccade [34]. From these studies, we conclude that activity in the rSC does not normally play a role in stopping a saccade on target.
(d). Cerebellum
The midline cerebellum plays a pivotal role in saccades. In the cerebellar cortex, the posterior parts of the vermis, lobules VI and VII, also called the oculomotor vermis (OMV), are related to saccades [41–43]. The OMV projects to the ipsilateral caudal fastigial nucleus, called the fastigial oculomotor region (FOR). The vermis and fastigial nuclei receive mossy fibre inputs from the SC (via the nuclei reticularis tegmenti pontis, NRTP) and the EBN [44]. The OMV Purkinje cells tonically inhibit the FOR. The FOR excite the contralateral rSC [45–48]. They also excite the contralateral OPN, EBN and IBN [45,47–50]. The fastigial nuclei must also disfacilitate the ipsilateral cSC [19,51–53].
It has long been known that the activity in the fastigial nuclei occurs early on the contraversive side, accelerating saccades, and late on the ipsiversive side, decelerating saccades [54]. Therefore, the cerebellum seems to be a good candidate for stopping a saccade. For a rightward saccade, saccades start when the left vermis pauses, allowing the left fastigial nucleus to fire [55]. This drives the right EBN and IBN. Later, the right vermis pauses, allowing the right fastigial nucleus to fire.
We previously hypothesized that the late drive from the ipsiversive fastigial nucleus drives the contraversive EBN and IBN on, which chokes off the drive of the ipsiversive EBN, thus stopping the saccade [52,53]. What causes the pause in the ipsiversive vermis remains unknown. Mossy fibres carry the input signals to the vermis, but their latencies align with saccade onset, not saccade end [56]. We have hypothesized that the initial pause in activity in the contraversive vermis spreads (in proportion to an efference copy of the velocity signal carried on EBN) from the contraversive to the ipsiversive vermis, where it causes the activation of the ipsiversive FOR [52,53]. This is possible because the vermis is continuous across the midline. Thus, the vermis acts as a spatial integrator, and would thus be the only part of the brain that knows when to stop the saccade to get on target.
After the eyes stop, the drive from the now disinhibited contraversive EBN (because of the ipsiversive FOR activation) must also be stopped, otherwise the eyes would move backward, or start oscillating because of post-inhibitory rebound of EBN and IBN. Thus, at the end of the saccade the OPN must be reactivated to shut down the EBN and IBN and prevent further movements. OPN might be reactivated by the ipsiversive fastigial nucleus at the end of the saccade by a collateral projection of the efference sent to the brainstem burst neurons. The reactivated OPN would then prevent saccadic oscillations.
(e). Cerebellar deficits
In patients with oculomotor cerebellar deficits, saccades often overshoot the target. That error will generate a backward corrective saccade, leading to a sequence of macrosaccadic oscillations (with each saccade followed by an approximately normal inter-saccadic interval) [57]. If the overshoot is less than 100%, the sequence of oscillations decreases in amplitude and stops. If the overshoot is greater than 100%, the sequence increases in amplitude for several saccades. After a few of these growing saccades, the amplitude begins to decrease (for reasons unknown, but possibly central fatigue), creating a spindle-shaped set of oscillations.
If both fastigial nuclei are lesioned in a monkey, saccades become hypermetric in both directions [14,22], like in many human cerebellar patients. The fact that saccades in these subjects are hypermetric is consistent with our hypothesis that it is the ipsiversive fastigial nucleus that stops the saccade on target. However, although saccades overshoot the target, they do eventually stop.
What mechanism might then stop a hypermetric saccade? One possibility is that the cSC activity runs down, stopping the saccade because there is no longer a drive signal to the premotor burst neurons. Also, when the cSC runs down, the rSC would be disinhibited, and it could reactivate, exciting the OPN. The OPN would then inhibit the EBN and IBN, ending the saccade and holding the eyes still. However, if both the fastigial nuclei and the OPN were held off, the eyes would not stay still at the end of the saccade. As said above, the intrinsic instability of the reciprocal inhibition between the left and right IBN and their PIR would cause ocular oscillations, without an interval between the saccades, until the drive signal ran down from fatigue. Such a mechanism also may explain other oscillatory disorders, e.g. opsoclonus and flutter.
4. How a neuromimetic model makes a rightward saccade
The neuromimetic model (figure 2) [52,53] takes into account the neuronal populations in the brainstem and cerebellum for which a saccade-related activity has been clearly demonstrated.
Let us consider the model's actions during a rightward saccade. Before the movement, the rSC and the OPN are active (grey lines), holding the premotor burst neurons (dashed boxes) off. The eye movement cortical areas (e.g. frontal eye fields and lateral intraparietal sulcus, not shown) activate the left cSC at a locus corresponding to the target's retinotopic location. The left cSC excites the right premotor burst neurons (EBN and IBN, black line). The left cSC also inhibits the rSC and the OPN (via right long-lead IBN, orange line). The left cSC projects to the right NRTP, which sends the retinotopic locus of the target to the vermis and the fastigial nuclei (green lines). The cSC signal is combined with contextual information (e.g. target velocity) to determine where in the vermis a pause of activity should occur (green circle). Accuracy is assured by adaptation of the starting place in the vermis, learned from prior errors in that specific movement context.
The pause of the left vermis releases the left fastigial nucleus from inhibition, which then drives the right EBN and IBN (cyan lines). The left fastigial nucleus also disfacilitates the SC, causing its activity to decrease throughout the movement (this is essential to allow the cerebellum to steer the movement). While the eye is moving, an efference copy of EBN activity (i.e. the eye velocity command) is fed back to the vermis (blue line). The feedback causes a wave of inhibition to spread across the vermis (blue wavy line). The speed of the spread is proportional to the feedback velocity, making the vermis a spatial integrator representing the progress of the movement.
The inhibitory wave spreads until it reaches the right side of the vermis (red diamond). The pause in the vermis on the right side then releases the right fastigial nucleus from inhibition, which then sends a drive signal to the left EBN and IBN (red lines) and the OPN. We assume that although the right IBN holds off the left EBN and IBN, the right fastigial nucleus is strong enough to turn on the left IBN. The left IBN then inhibits the right EBN and IBN (red line), which chokes off the drive to the motor nuclei, stopping the saccade. The OPN can be turned back on by the rSC and the fastigial nucleus.
5. Distributed motor error
The evidence that movements require the coordination of parallel pathways challenges the model of a feedback loop with a single motor error controller (figure 1). We have shown above (§3) that the saccadic system involves at least two parallel pathways [23], with the saccadic drive to the premotor burst neurons coming from both the SC and the fastigial nuclei [52]. Thus, the drive comes from two sources and computing the motor error is problematic [58]. To explicate this problem we must consider a target that dissociates its retinotopic location from the desired saccade. This is easily accomplished by using moving targets.
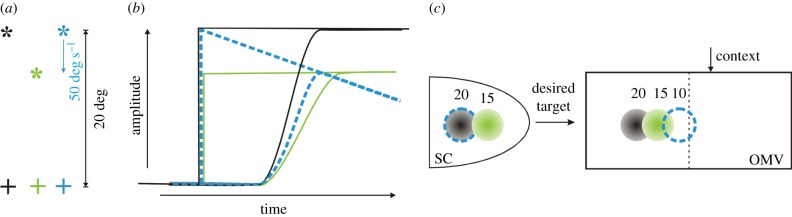
For example, figure 3a shows three saccadic stimuli: a target appearing at 20° (black asterisk), a target appearing at 15° (green), and a target appearing at 20° but moving toward the fixation point (plus) at 50° s−1 (blue). Figure 3b shows the time course of simulated movements. Figure 3c shows the initial loci of activity in the SC and OMV.
Figure 3.
There is no single motor error signal if two pathways contribute to making a saccade. (a) Three saccadic stimuli. The plus symbols are the fixation points, and the asterisks are the targets. (b) Simulated target and eye orientation traces. (c) Schematic diagram of contributions of SC and OMV to the drive signal. See explanation in §5.
When a saccade is made to a stationary target, the loci in SC and vermis are at corresponding points (black or green discs for 20° or 15°, respectively). However, for a moving target, the locus of activity in the SC always corresponds to the retinotopic location where the target first appeared [59] (blue dashed circle), but the vermis receives contextual information and specifies a different starting place in the vermis corresponding to the required contribution to the drive based on context (blue dashed circle). If the SC is contributing enough drive for a 20° saccade in this last case, the vermis must initiate activity closer to the midline, say at a site corresponding to a 10° saccade, so that deceleration occurs sooner. This will make the resultant 15° saccade slightly faster than a 15° saccade made to a stationary target, because the SC's drive for a 20° saccade is larger than for a 15° saccade. This prediction has been confirmed experimentally [60].
Thus, target information is distributed across both the SC and the OMV. The goal is reached when the spatial integration in the vermis has reached the ipsiversive side. If the movement does not end on target, a learning mechanism in the cerebellum updates the starting locus in the vermis (e.g. the blue dashed circle) for the next time that context occurs. Thus, rather than compute a motor error to drive the saccade velocity command, the saccadic system could reach its goal by integrating velocity feedback to spread activity from its context-dependent starting point to the opposite side. Note that the output of the vermal integration is not an efference copy of the movement, nor can it be subtracted from the desired change in orientation to obtain an efference copy of the motor error.
6. Assumptions and predictions
Despite the complexity of this hypothetical mechanism for stopping saccades, it is well supported by anatomical and physiological data. Nonetheless, it also includes many assumptions. A key assumption is that the activity in the contraversive IBN at the end of the saccade is sufficient to choke off the activity in the ipsiversive premotor burst neurons. This might be tested with new optogenetic techniques that could turn off contraversive IBN just before the end of the saccade.
Another major assumption is that premotor burst neurons exhibit post-inhibitory rebound. PIR has been found in cerebellar nuclei [61,62], but it has not been looked for in EBN, IBN or OPN.
Another important assumption of this model is that the OMV has a wave of spreading depression that crosses from the contralateral to the ipsilateral side (or at least a contraversive site close to the midline that projects to the ipsiversive FOR). This assumption could be tested by recording from multiple electrodes across the OMV, or by imaging the activity of the OMV with voltage sensitive dyes.
Another assumption is that for hypermetric, abnormal saccades the onset of the OPN do stop the saccade. Alternatively, the very high firing rates in the cSC or EBN may cause them to fatigue, stopping the saccade because they run down. Experimental tests are needed to resolve this. For example, it should be possible to record from SC, OPN, EBN and IBN during hypermetric movements caused by reversible inactivation of the cFN.
A prediction of the model is that changing the context of the movement (e.g. saccades to moving targets) changes the initially active site in the OMV. This also could be tested by imaging the vermal activity. Some of this contextual information also may be processed in cortex or basal ganglia, which are not included in this model.
7. Conclusion
Our four questions can now be answered. (i) Where is the desired change in orientation encoded? The desired change in orientation is distributed, with the SC encoding the retinotopic location of the target and the locus of the initial pause in the vermis encoding any compensation required by the movement's context. (ii) Where is the motor error signal computed? No motor error signal is computed. (iii) Where is the displacement integrator? The vermis spatially integrates the velocity command to track the progress of the movement, but it is not an efference copy of the displacement. (iv) What stops the saccade? The saccade stops because the spreading inhibition in the vermis crosses the midline and activates the ipsiversive fastigial nucleus, which activates the contraversive IBN, which choke off the drive from the ipsiversive EBN. The FOR also activates the OPN, which hold the eyes on target.
Saccades may be a prototype for other rapid movements (e.g. tongue, head, trunk and limb) [63]. These movements become dysarthric or dysmetric during cerebellar disease [64]. It is reasonable to assume that they all share a similar dual-pathway structure with saccades; one part of the brain encodes the movement goal and initiates the movement (a feed-forward pathway), and a parallel cerebellar pathway keeps track of the movement's displacement and stops it on target (a feedback pathway) [63,65].
Furthermore, stopping a movement is not the same as holding still. Stopping a saccade by choking off its drive signal does not ensure that the eyes remain still on the target, because the saccadic system is unstable. What keeps the eyes still is not simply a passive lack of drive, but an active inhibition from the reactivation of the OPN, which stabilizes the brain stem circuits. Somatomotor systems are also prone to tremor, with oscillatory circuits in the thalamus, spinal cord and cortex. Thus, the saccadic model could represent a general model for stopping rapid movements in all parts of the body and also preventing tremor and oscillation.
Competing interests
We have no competing interests.
Funding
This work was funded by the Intramural Research Program of the National Eye Institute, NIH, DHHS.
References
- 1.Deban SM, Wake DB, Roth G. 1997. Salamander with a ballistic tongue. Nature 389, 27–28. ( 10.1038/37898).9288962 [DOI] [Google Scholar]
- 2.Robinson DA. 1964. The mechanics of human saccadic eye movement. J. Physiol. 174, 245–264. ( 10.1113/jphysiol.1964.sp007485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahill AT, Adler D, Stark L. 1975. Most naturally occurring human saccades have magnitudes of 15 degrees or less. Invest. Ophthalmol. 14, 468–469. [PubMed] [Google Scholar]
- 4.Goldberg ME, Wurtz RH. 1972. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J. Neurophysiol. 35, 542–559. [DOI] [PubMed] [Google Scholar]
- 5.Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. 1998. Signal timing across the macaque visual system. J. Neurophysiol. 79, 3272–3278. [DOI] [PubMed] [Google Scholar]
- 6.Keller EL. 1977. Control of saccadic eye movements by midline brain stem neurons. In Control of gaze by brain stem neurons (eds Baker R, Berthoz A), pp. 327–336. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 7.Fuchs AF, Kaneko CR, Scudder CA. 1985. Brainstem control of saccadic eye movements. Annu. Rev. Neurosci. 8, 307–337. ( 10.1146/annurev.ne.08.030185.001515). [DOI] [PubMed] [Google Scholar]
- 8.Sylvestre PA, Cullen KE. 1999. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J. Neurophysiol. 82, 2612–2632. [DOI] [PubMed] [Google Scholar]
- 9.Robinson DA. 1973. Models of the saccadic eye movement control system. Kybernetik 14, 71–83. ( 10.1007/BF00288906) [DOI] [PubMed] [Google Scholar]
- 10.Zee DS, Optican LM, Cook JD, Robinson DA, Engel WK. 1976. Slow saccades in spinocerebellar degeneration. Arch. Neurol. 33, 243–251. ( 10.1001/archneur.1976.00500040027004) [DOI] [PubMed] [Google Scholar]
- 11.Robinson D. 1975. Oculomotor control signals. In Basic mechanisms of ocular motility and their clinical implications (eds Lennerstrand G, Bach-y-Rita P), pp. 337–374. Oxford, UK: Pergamon. [Google Scholar]
- 12.Jurgens R, Becker W, Kornhuber HH. 1981. Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol. Cybern. 39, 87–96. ( 10.1007/BF00336734) [DOI] [PubMed] [Google Scholar]
- 13.Scudder CA, Kaneko CS, Fuchs AF. 2002. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp. Brain Res. 142, 439–462. ( 10.1007/s00221-001-0912-9) [DOI] [PubMed] [Google Scholar]
- 14.Optican LM, Robinson DA. 1980. Cerebellar-dependent adaptive control of primate saccadic system. J. Neurophysiol. 44, 1058–1076. [DOI] [PubMed] [Google Scholar]
- 15.Gellman RS, Carl JR. 1991. Motion processing for saccadic eye movements in humans. Exp. Brain Res. 84, 660–667. ( 10.1007/BF00230979) [DOI] [PubMed] [Google Scholar]
- 16.Luschei ES, Fuchs AF. 1972. Activity of brain stem neurons during eye movements of alert monkeys. J. Neurophysiol. 35, 445–461. [DOI] [PubMed] [Google Scholar]
- 17.Leigh RJ, Zee DS. 2015. The neurology of eye movements. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Büttner-Ennever JA. 2006. Neuroanatomy of the oculomotor system. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 19.Daye PM, Optican LM, Roze E, Gaymard B, Pouget P. 2013. Neuromimetic model of saccades for localizing deficits in an atypical eye-movement pathology. J. Transl. Med. 11, 125 ( 10.1186/1479-5876-11-125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K, Optican LM. 2006. Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. J. Comput. Neurosci. 20, 25–41. ( 10.1007/s10827-006-4258-y) [DOI] [PubMed] [Google Scholar]
- 21.Ramat S, Leigh RJ, Zee DS, Optican LM. 2005. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp. Brain Res. 160, 89–106. ( 10.1007/s00221-004-1989-8) [DOI] [PubMed] [Google Scholar]
- 22.Robinson FR, Straube A, Fuchs AF. 1993. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J. Neurophysiol. 70, 1741–1758. [DOI] [PubMed] [Google Scholar]
- 23.Schiller PH, True SD, Conway JL. 1979. Effects of frontal eye field and superior colliculus ablations on eye movements. Science 206, 590–592. ( 10.1126/science.115091) [DOI] [PubMed] [Google Scholar]
- 24.Strassman A, Highstein SM, McCrea RA. 1986. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J. Comp. Neurol. 249, 358–380. ( 10.1002/cne.902490304) [DOI] [PubMed] [Google Scholar]
- 25.Strassman A, Evinger C, McCrea RA, Baker RG, Highstein SM. 1987. Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp. Brain Res. 67, 436–440. ( 10.1007/BF00248565) [DOI] [PubMed] [Google Scholar]
- 26.Keller EL. 1974. Participation of medial pontine reticular formation in eye movement generation in monkey. J. Neurophysiol. 37, 316–332. [DOI] [PubMed] [Google Scholar]
- 27.Zee DS, Robinson DA. 1979. A hypothetical explanation of saccadic oscillations. Ann. Neurol. 5, 405–414. ( 10.1002/ana.410050502) [DOI] [PubMed] [Google Scholar]
- 28.Hain TC, Zee DS, Mordes M. 1986. Blink-induced saccadic oscillations. Ann. Neurol. 19, 299–301. ( 10.1002/ana.410190315) [DOI] [PubMed] [Google Scholar]
- 29.Pare M, Guitton D. 1998. Brain stem omnipause neurons and the control of combined eye-head gaze saccades in the alert cat. J. Neurophysiol. 79, 3060–3076. [DOI] [PubMed] [Google Scholar]
- 30.Soetedjo R, Kaneko CR, Fuchs AF. 2002. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J. Neurophysiol. 87, 679–695. [DOI] [PubMed] [Google Scholar]
- 31.Rucker JC, Ying SH, Moore W, Optican LM, Buttner-Ennever J, Keller EL, Shapiro BE, Leigh RJ. 2011. Do brainstem omnipause neurons terminate saccades? Annu. N Y Acad. Sci. 1233, 48–57. ( 10.1111/j.1749-6632.2011.06170.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DA. 1972. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 12, 1795–1808. ( 10.1016/0042-6989(72)90070-3) [DOI] [PubMed] [Google Scholar]
- 33.Munoz DP, Wurtz RH. 1995. Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J. Neurophysiol. 73, 2334–2348. [DOI] [PubMed] [Google Scholar]
- 34.Munoz DP, Wurtz RH. 1993. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J. Neurophysiol. 70, 576–589. [DOI] [PubMed] [Google Scholar]
- 35.Munoz DP, Wurtz RH. 1993. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J. Neurophysiol. 70, 559–575. [DOI] [PubMed] [Google Scholar]
- 36.Munoz DP, Wurtz RH. 1995. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J. Neurophysiol. 73, 2313–2333. [DOI] [PubMed] [Google Scholar]
- 37.Munoz DP, Istvan PJ. 1998. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J. Neurophysiol. 79, 1193–1209. [DOI] [PubMed] [Google Scholar]
- 38.Buttner-Ennever JA, Horn AK, Henn V, Cohen B. 1999. Projections from the superior colliculus motor map to omnipause neurons in monkey. J. Comp. Neurol. 413, 55–67. ( 10.1002/(SICI)1096-9861(19991011)413:1%3C55::AID-CNE3%3E3.0.CO;2-K) [DOI] [PubMed] [Google Scholar]
- 39.May PJ. 2006. The mammalian superior colliculus: laminar structure and connections. Prog. Brain Res. 151, 321–378. ( 10.1016/S0079-6123(05)51011-2) [DOI] [PubMed] [Google Scholar]
- 40.Gandhi NJ, Keller EL. 1997. Spatial distribution and discharge characteristics of superior colliculus neurons antidromically activated from the omnipause region in monkey. J. Neurophysiol. 78, 2221–2225. [DOI] [PubMed] [Google Scholar]
- 41.Ron S, Robinson DA. 1973. Eye movements evoked by cerebellar stimulation in the alert monkey. J. Neurophysiol. 36, 1004–1022. [DOI] [PubMed] [Google Scholar]
- 42.Yamada J, Noda H. 1987. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J. Comp. Neurol. 265, 224–241. ( 10.1002/cne.902650207) [DOI] [PubMed] [Google Scholar]
- 43.Takagi M, Zee DS, Tamargo RJ. 1998. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J. Neurophysiol. 80, 1911–1931. [DOI] [PubMed] [Google Scholar]
- 44.Gerrits NM, Voogd J. 1986. The nucleus reticularis tegmenti pontis and the adjacent rostral paramedian reticular formation: differential projections to the cerebellum and the caudal brain stem. Exp. Brain Res. 62, 29–45. ( 10.1007/BF00237401) [DOI] [PubMed] [Google Scholar]
- 45.Batton RR, Jayaraman A, Ruggiero D, Carpenter MB. 1977. Fastigial efferent projections in monkey—autoradiographic study. J. Comp. Neurol. 174, 281–305. ( 10.1002/cne.901740206) [DOI] [PubMed] [Google Scholar]
- 46.May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. 1990. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience 36, 305–324. ( 10.1016/0306-4522(90)90428-7) [DOI] [PubMed] [Google Scholar]
- 47.Noda H, Sugita S, Ikeda Y. 1990. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J. Comp. Neurol. 302, 330–348. ( 10.1002/cne.903020211) [DOI] [PubMed] [Google Scholar]
- 48.Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. 2012. Visuomotor cerebellum in human and nonhuman primates. Cerebellum 11, 392–410. ( 10.1007/s12311-010-0204-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanton GB. 2001. Organization of cerebellar and area ‘y’ projections to the nucleus reticularis tegmenti pontis in macaque monkeys. J. Comp. Neurol. 432, 169–183. ( 10.1002/cne.1095) [DOI] [PubMed] [Google Scholar]
- 50.Voogd J, Barmack NH. 2006. Oculomotor cerebellum. Prog. Brain Res. 151, 231–268. ( 10.1016/S0079-6123(05)51008-2) [DOI] [PubMed] [Google Scholar]
- 51.Niemi-Junkola UJ, Westby GW. 2000. Cerebellar output exerts spatially organized influence on neural responses in the rat superior colliculus. Neuroscience 97, 565–573. ( 10.1016/S0306-4522(00)00044-0) [DOI] [PubMed] [Google Scholar]
- 52.Lefevre P, Quaia C, Optican LM. 1998. Distributed model of control of saccades by superior colliculus and cerebellum. Neural Netw. 11, 1175–1190. ( 10.1016/S0893-6080(98)00071-9) [DOI] [PubMed] [Google Scholar]
- 53.Quaia C, Lefevre P, Optican LM. 1999. Model of the control of saccades by superior colliculus and cerebellum. J. Neurophysiol. 82, 999–1018. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs AF, Robinson FR, Straube A. 1993. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J. Neurophysiol. 70, 1723–1740. [DOI] [PubMed] [Google Scholar]
- 55.Ohtsuka K, Noda H. 1995. Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J. Neurophysiol. 74, 1828–1840. [DOI] [PubMed] [Google Scholar]
- 56.Kase M, Miller DC, Noda H. 1980. Discharges of Purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J. Physiol. 300, 539–555. ( 10.1113/jphysiol.1980.sp013178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selhorst JB, Stark L, Ochs AL, Hoyt WF. 1976. Disorders in cerebellar ocular motor control. II. Macrosaccadic oscillation. An oculographic, control system and clinico-anatomical analysis. Brain 99, 509–522. ( 10.1093/brain/99.3.509) [DOI] [PubMed] [Google Scholar]
- 58.Optican LM, Quaia C. 2002. Distributed model of collicular and cerebellar function during saccades. Annu. N Y Acad. Sci. 956, 164–177. ( 10.1111/j.1749-6632.2002.tb02817.x) [DOI] [PubMed] [Google Scholar]
- 59.Keller EL, Gandhi NJ, Weir PT. 1996. Discharge of superior collicular neurons during saccades made to moving targets. J. Neurophysiol. 76, 3573–3577. [DOI] [PubMed] [Google Scholar]
- 60.Guan Y, Eggert T, Bayer O, Buttner U. 2005. Saccades to stationary and moving targets differ in the monkey. Exp. Brain Res. 161, 220–232. ( 10.1007/s00221-004-2070-3) [DOI] [PubMed] [Google Scholar]
- 61.Bengtsson F, Ekerot CF, Jorntell H. 2011. In vivo analysis of inhibitory synaptic inputs and rebounds in deep cerebellar nuclear neurons. PLoS ONE 6, e18822 ( 10.1371/journal.pone.0018822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aizenman CD, Linden DJ. 1999. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J. Neurophysiol. 82, 1697–1709. [DOI] [PubMed] [Google Scholar]
- 63.Daye PM, Optican LM, Blohm G, Lefevre P. 2014. Hierarchical control of two-dimensional gaze saccades. J. Comput. Neurosci. 36, 355–382. ( 10.1007/s10827-013-0477-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diener HC, Dichgans J. 1992. Pathophysiology of cerebellar ataxia. Mov. Disord. 7, 95–109. ( 10.1002/mds.870070202) [DOI] [PubMed] [Google Scholar]
- 65.Kiehn O, Dougherty K. 2013. Locomotion: circuits and physiology. In Neuroscience in the 21st Century (ed. Pfaff DW.), pp. 1209–1236. New York, NY: Springer. [Google Scholar]