Abstract
Recently, it has been proposed that similar to goal-directed and habitual action mediated by the fronto-striatal circuits, the fronto-striato-subthalamic-pallidal-thalamo-cortical network may also mediate goal-directed and habitual (automatic) inhibition in both the motor and non-motor domains. Within this framework, some of the clinical manifestations of Parkinson's disease, dystonia, Tourette syndrome and obsessive–compulsive disorder can be considered to represent an imbalance between goal-directed and habitual action and inhibition. It is possible that surgical interventions targeting the basal ganglia nuclei, such as deep brain stimulation of the subthalamic nucleus or the internal segment of the globus pallidus, improve these disorders by restoring a functional balance between facilitation and inhibition in the fronto-striatal networks. These proposals require investigation in future studies.
This article is part of the themed issue ‘Movement suppression: brain mechanisms for stopping and stillness’.
Keywords: inhibition, basal ganglia, Parkinson's disease, dystonia, Tourette syndrome, obsessive–compulsive disorder
1. Introduction
Inhibiting behaviour that is inappropriate in a particular context or socially unacceptable is a necessary feature of adaptive behaviour. Our daily lives are dotted with such inhibitory control: when dieting exerting, self-control and not eating donuts made available at tea breaks; censoring and not expressing one's true opinion to a senior colleague; not jumping the red light on the drive home. While the contribution of such inhibitory control to adaptive behaviour is perhaps underestimated because non-occurrence of behaviour is difficult to observe and quantify, nevertheless, it is likely that inhibitory processes operate across all domains: motor, cognitive and emotional. Inhibition of motor responses that is suppression of a movement or motor response that has been prepared or close to initiation and execution is perhaps the easiest to measure and for this reason the most widely investigated to date with the go no-go and the stop-signal reaction time tasks. Behavioural inhibition of inappropriate impulses or urges, cognitive inhibition (e.g. mental suppression of irrelevant information from memory) and emotional inhibition (e.g. inhibition of anxiety-provoking mental images) are more difficult to measure and study, but are also relevant to understanding inhibitory control for adaptive behaviour.
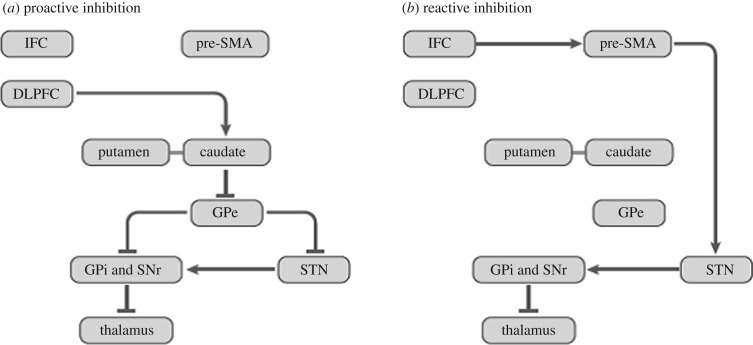
Several types of behavioural inhibition have been distinguished [1–4]. In contrast with automatic inhibition, volitional inhibition is intentional, controlled and effortful [3,4]. However, the automatic–volitional inhibition distinction is not fixed, as volitional inhibition has been shown to be triggered by masked ‘no-go’ stimuli of which the person is not overtly aware [5] and through learning, volitional inhibition can become automatic [6]. Another common distinction is between reactive inhibition triggered by external stimuli (e.g. stopping car at red traffic light) and proactive inhibition, which is prospective and necessary for achieving goals and self-control over drinking, eating, smoking, etc. [1–3]. Somewhat overlapping with and encompassing the above two sets of distinctions are the concepts of goal-directed versus habitual/automatic inhibition [4]. Goal-directed inhibition (e.g. not smoking when trying to quit) is mainly volitional, intentional and proactive in the service of a specific goal achievement, whereas habitual inhibition (for instance, not swearing in public) is developed through practice and education, largely stimulus-driven and automatic [4]. Global (e.g. freezing when faced with a grizzly bear in a wood) versus selective (e.g. stopping singing while continuing to play the piano) inhibition has been a further distinction [1]. The indirect and hyperdirect cortico-basal ganglia pathways considered to, respectively, mediate proactive and reactive inhibition are shown in figure 1.
Figure 1.
(a,b) The cortico-basal ganglia pathways involved in proactive and reactive inhibition, with proactive and reactive inhibition mediated, respectively, via the indirect fronto-striato-pallido-thalamo-cortical pathway and the hyperdirect cortico-subthalamic-pallidal-thalamocortical pathways. IFC, inferior frontal cortex; pre-SMA, pre-supplementary motor area; DLPFC, dorsolateral prefrontal cortex; GPe, external segment of globus pallidus; GPi, internal segment of globus pallidus; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus. Some connections are not shown. Connections ending in arrows: excitatory; connections ending in line/dash: inhibitory. (With permission from [4]).
One of the executive functions of the frontal cortex is inhibitory control, with the inferior frontal cortex [7], the dorsolateral prefrontal cortex [8], the orbitofrontal cortex [9,10] and the supplementary motor area (SMA) [11,12] playing a role in inhibition. A wealth of information is now available supporting the role of the basal ganglia—particularly the subthalamic nucleus (STN) and the striatum—in inhibition, including evidence from lesion [13,14] and electrophysiological [15–17] studies in animals, functional imaging studies in humans [11,12,18–21], behavioural and imaging investigations in Parkinson's disease (PD) [22–25], assessment of the effects of surgical treatment of PD with subthalamotomy [26,27] or deep brain stimulation (DBS) of the STN [28–34] and recordings of local field potentials from the implanted electrodes after surgery [35–37]. From a review of this evidence, Jahanshahi et al. [4] proposed that the fronto-striato-subthalamic-pallidal network is involved in goal-directed and habitual inhibition. This parallels the role of these circuits in goal-directed and habitual action, respectively, mediated by the associative and motor fronto-striatal circuits [38,39]. With repetition and practice, both initiation and inhibition of action can shift from goal-directed to habitual/automatic, which frees up attentional resources and allows for concurrent engagement in other tasks. Goal-directed but not habitual actions are sensitive to outcome devaluation and it needs to be established if outcome sensitivity is also a criterion that distinguishes goal-directed inhibition from habitual inhibition and to identify other relevant criteria.
Lack of behavioural inhibition can manifest itself as disinhibition, impulsivity and perseveration, all of which are characteristic of patients with frontal lesions. Impulsivity can manifest itself as delay aversion or an inability to take time to reflect or defer decisions, actions or gratification [40]. Impulsivity is among the diagnostic criteria for attention-deficit hyperactivity disorder, substance abuse and trichotillomania and can also feature in mania. In this paper, we consider the relevance of inhibition to some of the clinical manifestations of a number of primarily basal ganglia disorders, namely, PD, dystonia, Tourette syndrome (TS) and obsessive–compulsive disorder (OCD). For each disorder, we will consider the specific clinical manifestations that reflect failure of inhibitory processes and then the empirical evidence for deficits in inhibition on experimental tasks or from imaging studies.
2. Parkinson's disease
PD is a movement disorder, characterized by the primary motor symptoms of akinesia, bradykinesia, tremor, rigidity, postural instability and gait problems, and a host of non-motor symptoms including cognitive deficits, psychiatric problems such as depression, apathy, anxiety, hallucinations and delusions. According to the classical models, dopamine depletion in PD is associated with underactivity of the direct and overactivity of the indirect pathway, which results in excessive inhibitory output from the basal ganglia to motor, premotor and prefrontal cortical areas and to the brainstem [41,42]. This excessive inhibitory output from the basal ganglia is considered responsible for akinesia, characterized by the reduction or absence of a range of normally automatic movements such as blinking, facial expression, gesturing, turning in bed, arm swinging and handwriting in PD, which represents over-inhibition of habitual movements. Lesioning or DBS of the STN or the internal segment of the globus pallidus (GPi) reduces this excessive inhibitory output and improves akinesia [43,44]. There is some evidence in support of this. In an fMRI study [45], the delay in simple reaction times of PD patients relative to healthy controls was associated with the pre-go signal activation increases in the caudate, precuneus and thalamus: nodes of the proactive inhibitory network. This was interpreted as reflecting that PD patients were locked in a proactive inhibitory mode even in situations that did not call for such action restraint. A subsequent study by the same group showed that STN-DBS re-established the voluntary release of proactive inhibitory control in a cued reaction time task in PD patients [46].
Patients with PD have deficits in inhibitory control on a host of cognitive tasks requiring inhibition of prepotent or habitual responses such as the Stroop, random number generation and the Hayling sentence completion test [23]. Deficits in inhibitory control are also present in PD relative to age-matched controls on go no-go, stop-signal reaction time tasks, anti-saccade and Eriksen flanker tasks [22,23,47–49]. On the stop-signal task requiring motor inhibition, medicated or de novo never-medicated patients with PD have prolonged stop-signal reaction times indicative of delayed inhibition and this is associated with reduced task-related activation of the inhibition network, including the inferior frontal gyrus [24,25].
With progression of the illness, freezing of gait, episodes marked by a temporary motor block during walking, can be experienced by people with PD, triggered by turning, fatigue, confined spaces and stressful situations [50]. During freezing episodes patients are unable to initiate a step despite the intention to do so, and they report that their feet feel as if they are glued to the floor. Such freezing represents excessive motor inhibition [4,51]. It is possible that inhibitory output from the substantia nigra pars reticulata (SNr) and SMA to the mesencephalic locomotor region could be involved, as these areas can suppress locomotion in animal models [52]. From a review of the imaging studies of freezing of gait in PD [53], disruptions in the ‘executive attention’ network and tissue loss in the premotor cortex, inferior frontal gyrus, precentral gyrus, parietal and occipital areas of the right hemisphere were noted, areas that are involved not only in visuospatial function but also in inhibitory control. In addition, involvement of the caudate nucleus and the locomotor centres in the brainstem in freezing of gait was also identified. These findings, particularly grey matter atrophy in the inferior frontal gyrus [54], hypoactivation of the SMA and posterior parietal regions and overactivation of the mesencaphalic locomotor regions during motor imagery of walking [55] in patients with freezing of gait, are consistent with excessive inhibition of locomotion triggered by conflictual visual information. The hypothesis that the episodic motor blocks during walking characteristic of freezing of gait reflect excessive inhibition due to dysfunction of the cortical (SMA, inferior prefrontal cortex) and subcortical (caudate, SNr) areas engaged in inhibitory control, particularly triggered in situations when concurrent executive control of attention and gait are necessary, requires direct investigation in future studies. There is some preliminary support for this proposal from studies showing deficits in inhibitory control in patients with PD and freezing of gait on the stop-signal task [56,57], which becomes more pronounced with the addition of a cognitive load [57].
Apathy is a common psychiatric problem in PD [58,59]. It is a multidimensional syndrome with cognitive, emotional and behavioural components, whereby patients lack motivation, interest, concern, emotional reactivity and show no initiative or spontaneous activity and often report being devoid of thoughts and emotions. The biological basis of apathy remains unclear, with both dopaminergic and serotonergic systems implicated [60,61]. It is possible that excessive inhibition in the motor and non-motor domains mediated through the fronto-basal ganglia circuits contributes to the development of apathy in PD [4,62]. In support of this proposal, there is imaging evidence of decreased grey matter volume in the right SMA, the right inferior frontal gyrus, the left orbitofrontal cortex, left inferior and superior parietal areas and the nucleus accumbens bilaterally in non-depressed PD patients with apathy relative to those without apathy [63]. Other imaging evidence also implicates the right caudate or ventral striatum and the inferior frontal gyrus in apathy in PD. Compared with PD patients without apathy, de novo untreated non-depressed PD patients with apathy had lower dopamine transporter levels in the striatum, particularly the right caudate [64], and reduced metabolism in the right ventral striatum prior to surgery predicted development of apathy following STN-DBS surgery [65]. Furthermore, imaging has identified an association between apathy in PD and right hemispheric metabolism in the inferior frontal gyrus, middle frontal gyrus and the anterior insula [66], and in another study reduced grey matter volume in the inferior frontal gyrus bilaterally was among the areas that correlated with high apathy scores in PD [67]. Some of these areas overlap with the brain inhibitory network, and their volume reduction may contribute to the reduction of spontaneous and self-generated activity and motivational deficits in PD patients with apathy. Also of interest is the finding from a longitudinal study that in de novo PD patients, reduced performance on the Stroop interference task, which requires inhibitory control and engages medial prefrontal areas, predicted subsequent development of apathy [61].
At the other extreme, some medication-related complications of PD can be considered as reflecting disinhibition of the networks involved in motor and non-motor inhibition. Dopaminergic medication used in the treatment of PD can give rise to levodopa-induced dyskinesias (LIDs) and impulse control disorders (ICDs). Both LIDs and ICDs may represent failures of habitual or automatic inhibition [4,62]. With LIDs, failure of automatic inhibition may result in fragments of movement and stereotyped behaviours being released. Recent imaging evidence has implicated the inferior frontal gyrus and the SMA, two key nodes in the motor inhibitory network, in dyskinesias [68–70], leading to the proposal that dysfunction of the neural network mediating motor inhibition may also induce LIDs. For example, in the on-medication state, for patients with LIDs the right inferior frontal cortex showed decreased functional connectivity with the motor cortex and increased connectivity with the putamen compared with patients with no LIDs [70]. This was interpreted as reflecting reduced inhibitory control from the right inferior frontal gyrus over the motor cortex in the on state, giving rise to LIDs. Furthermore, it has been shown that in PD patients with LIDs, intake of levodopa medication tended to make inhibitory control and monitoring of failures of inhibition during a stop-signal motor inhibition task worse, which fMRI showed to be associated with decreased activity of the right inferior frontal cortex during motor inhibition, whereas patients without LIDs showed the reverse [71].
A range of ICDs, including hypersexuality, pathological gambling and shopping, overeating and punding, develop in approximately 25% of people with PD treated with dopamine agonists or levodopa [72]. As a result of deficient habitual/automatic inhibition, patients with ICDs respond excessively to rewarding stimuli associated with sex, food, money or shopping [4]. PD patients with ICDs show impulsive choice on delay discounting tasks, preferring small immediate rewards to larger delayed rewards [73]. Imaging has revealed that dopaminergic medication results in differential patterns of activation in brain areas implicated in response inhibition and impulse control, particularly the lateral orbitofrontal cortex, rostral cingulate, amygdala and external segment of the globus pallidus (GPe) in PD patients with (decreased dopamine-induced activation) or without (increased dopamine-induced activation) ICDs such as pathological gambling [74]. Enhanced sexual desire and increased activation in the ventral striatum, anterior cingulate cortex and orbitofrontal cortex were reported in response to sexual cues for PD patients with hypersexuality relative to patients without such ICDs [75]. Thus it is possible that ICDs in PD reflect impulsive choice and behaviour due to failure of habitual inhibition [4]. Cognitive behaviour therapy has some success in helping PD patients with ICDs to proactively impose goal-directed inhibition over their impulsive behaviour, but response to this can be variable [76].
3. Dystonia
Dystonia is a hyperkinetic movement disorder. The latest expert classification has defined it as ‘a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation.’ [77, p. 863] The abnormal contractions can affect muscles of the eyes (blepharospasm), face and jaw (oromandibular dystonia), larynx (spasmodic dysphonia), neck (cervical dystonia) and the hand during writing (writer's cramp). Dystonia can be focal, affecting only one part of the body, segmental (two adjacent body parts affected), multifocal, hemi-dystonia (one side of body) or generalized (involving the trunk and two other body parts).
The exact cause of dystonia is not known. Dystonia is considered a movement disorder associated with dysfunction of the basal ganglia [78,79], and more recently, cerebellar involvement in dystonia has also been documented [80–82]. Many genes causing various forms of dystonia in association with environmental triggers have been identified [83].
According to the classical DeLong [42] and Albin et al. [41] models, hyperkinetic movement disorders such as dystonia are associated with reduced inhibitory output from the GPi, which gives rise to increased thalamic and cortical activation. There is some support for this model from hyperkinetic transgenic mice model of DYT1 dystonia [84] and neuronal recordings from the GPi of patients with dystonia undergoing DBS surgery [85], but the improvement of dystonia with GPi DBS [86], which reduces the activity of the GPi, is not consistent with the classical ‘rate’ models.
Imaging studies have shown movement-related overactivity of the dorsolateral prefrontal cortex, the anterior cingulate cortex, the SMA and the lenticular nuclei, and underactivation of the primary motor cortex in dystonia [87,88]. Using different transcranial magnetic stimulation (TMS) protocols, reduced cortico-cortical inhibition [89,90] and increased plasticity [91] have been demonstrated in dystonia. This loss of inhibition, particularly loss of ‘surround inhibition’, is considered responsible for loss of selectivity and overflow of activation to other muscles. The essence of ‘surround inhibition’ is that muscles that are not actively involved in producing a movement are inhibited during the movement. Surround inhibition can be demonstrated with TMS and in focal hand dystonia, it has been shown that surround inhibition is deficient [92–94]. This loss of selectivity in muscle activation has been demonstrated even when patients imagined making specific movements [95]. The loss of inhibition in dystonia is also associated with a delay in the ability to inhibit preplanned responses and patients required longer warning times than healthy controls to be able to do so [96]. It has been suggested that some of the sensory deficits such as increased spatial [97] and temporal [98] discrimination thresholds may reflect deficient inhibition. In fact, recent neurophysiological evidence has shown that increased somatosensory temporal discrimination thresholds in dystonia are related to reduced activity of inhibitory circuits within the primary somatosensory cortex [99,100]. These deficits and impairment of sensorimotor integration [101,102] in dystonia, as well as the increased plasticity, may all result from the loss of inhibition that could itself be due to a reduced number of inhibitory interneurons [103]. The latter hypotheses require verification in future investigations.
4. Tourette syndrome
TS is characterized by motor and/or vocal tics, the latter often consisting of obscene words. TS is frequently accompanied by comorbidities such as attention-deficit hyperactivity disorder, depression, obsessive compulsive behaviours and self-injurious behaviours. Tics are involuntary movements or vocalizations, but are often preceded by a premonitory sensation and urge, and patients describe a sense of relief after performing the tic. Patients with TS can suppress the tics temporarily and thus can impose goal-directed intentional inhibition to suppress their involuntary tics for brief periods.
TS has been considered a basal ganglia disorder of inhibition [104]. Mink [104] proposed that repeated inappropriate activation of striatal neurons leads to inhibition of the GPi and the SNr, the output pathways of the basal ganglia, which would normally be tonically active to prevent unwanted movements, and that this GPi/SNr inhibition leads to a disinhibition of thalamo-cortical targets and generates tics. Evidence from imaging and neuropathological studies implicates the cortico-basal ganglia circuits and abnormal distribution of inhibitory interneurons in the basal ganglia in TS [105–107]. On behavioural tasks that assess inhibitory control, the evidence in TS is inconsistent, with some suggesting failure of inhibition [108,109] but others not finding such deficits [110] and even reporting enhanced cognitive and inhibitory control in TS [111] due to development of compensatory mechanisms to counteract the lack of inhibition. The neural correlates of intentional suppression of tics in TS have been investigated in several imaging studies. Success in intentional inhibition of tics by TS patients was found to be associated with a significant increase in a measure of local connectivity in the inferior frontal gyrus [112]. In another study, comparison of tic suppression with a ‘free-ticcing’ state was associated with significant activation of the prefrontal cortex, anterior cingulate cortex, caudate and putamen [113]. Compared with age-matched healthy controls, people with TS showed greater activation of the frontal cortex and caudate during suppression of the urge to blink [114]. These results indicate the central role of the basal ganglia and the frontal cortex in volitional and intentional tic suppression in TS. By exerting voluntary effort, goal-directed inhibition mediated by the frontal cortex, anterior cingulate cortex and the caudate can be mobilized by patients with TS to suppress tics. DBS of the GPi or the nucleus accumbens [115–117] may control tics by restoring the balance between facilitation and inhibition in the cortico-basal ganglia circuits. It was proposed that spontaneous tics emerge either by overactivity in the generation of habitual actions or reduced activation of the mechanisms of habitual/automatic inhibition [4].
5. Obsessive compulsive disorder
People with OCD experience intrusive unwanted thoughts and images (obsessions) and repetitive behaviours (compulsions), which are driven by doubt, exaggerated perceptions of danger and anxiety. Different types of repetitive behaviour may be engaged in, including washing, checking or hoarding. Imaging studies have established hyperactivity of the orbitofrontal cortex–caudate circuit in people with OCD, which is increased by symptom provocation and conversely reduced by successful treatment [118–120]. The orbitofrontal cortex not only plays a role in detecting motivational value and salience of stimuli [121], but also mediates inhibition as its lesioning in animals results in perseveration [9,10].
Other imaging evidence also supports inhibitory failure in OCD. During performance of a ‘go no-go’ task that requires action restraint/inhibition on no-go trials, a negative association between severity of OCD symptoms and inhibition-related activation of the orbitofrontal cortex was found [122]. A meta-analysis of imaging studies in OCD revealed that these patients show reduced activation in the caudate and putamen during performance of inhibition and interference tasks and reduced activation of the inferior frontal gyrus, anterior cingulate cortex, medial frontal cortex, dorsolateral prefrontal cortex and caudate during switching tasks [120]. A recent imaging study [123] demonstrated that during exposure to symptom-provoking stimuli, OCD patients had deactivation of the caudate–prefrontal circuits together with hyperactivation of the STN and putamen, which were interpreted as reflecting a dissociation between areas involved in goal-directed and habitual behaviours, respectively. This study also showed hyperactivity of the putamen during symptom provocation and subsequent putaminal deactivation during avoidance of the provoking stimuli and relief, indicating that the putamen may play a significant role in habit formation in OCD [123]. OCD patients were more prone to ‘slips of action’ indicative of over-reliance on habits and were deficient in goal-directed control on an instrumental learning task [124]. OCD patients also develop excessive avoidance habits on a shock avoidance task [125]. On the basis of these results, OCD has been formulated as a shift from goal-directed to habitual responding [123–125]. However, in the light of the fact that patients with OCD can temporarily exert goal-directed inhibition to control their compulsive behaviours, it is likely that failure of habitual/automatic inhibition of unwanted and intrusive thoughts and images and emotions may play a role in the genesis of obsessions and compulsions [4]. OCD is characterized by an imbalance between goal-directed and habitual action and inhibition [4]. Successful treatment of OCD with DBS of several targets including the ventral internal capsule and ventral striatum [126], the nucleus accumbens [127] or the STN [128] has been documented. In OCD patients with bilateral STN-DBS, stimulation prolonged stop-signal reaction times resulting in delayed inhibition, and dynamic causal modelling of EEG suggested that STN stimulation significantly decreased the connection from the basal ganglia to the right inferior frontal gyrus [129]. In an imaging study of operated OCD patients, acute DBS in the ventral caudate and ventral striatum was associated with activation of the prefrontal cortex, anterior cingulate cortex, putamen and globus pallidus [130]. Thus, DBS may improve OCD symptoms by modulating the dysfunctional network in the disorder that overlaps with the inhibitory network, which may in turn restore the balance between goal-directed and habitual action and inhibition [4].
6. Conclusion
The basal ganglia are phylogenetically ancient structures whose basic organizational principle has remained unchanged for many millions of years [131]. In the lamprey, the basal ganglia have inhibitory projections to brainstem nuclei that appear to form the basis for action selection through release of inhibition, and it has been suggested that such an organizational arrangement has been co-opted in higher primates to control multiple cognitive, emotional and motor functions in a broad range of behaviours [131]. Although the work above suggests that this basic tenet is correct, the range of control has expanded considerably, to distinguish, e.g. proactive and reactive control or habitual and goal-directed inhibitory control of behaviour. The complexities that these additional functions require may explain the wide variety of pathologies of control that can be observed. It remains to be clarified how the recently elucidated ‘pause then cancel’ model of stopping, with subthalamic-SNr ‘pause’ and arkypallidal-striatal ‘cancel’ components [132] contribute to some of the clinical manifestations of basal ganglia disorders outlined above.
Authors' contributions
M.J. wrote the first draft and J.C.R. reviewed and contributed to the final draft.
Competing interests
We declare we have no competing interests.
Funding
Both authors are funded by HEFCE.
References
- 1.Aron AR. 2011. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry 69, e55–e68. ( 10.1016/j.biopsych.2010.07.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffard M, Longcamp M, Velay J-L, Anton J-L, Roth M, Nazarian B, Boulinguez P. 2008. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage 42, 1196–1206. ( 10.1016/j.neuroimage.2008.05.041) [DOI] [PubMed] [Google Scholar]
- 3.Verbruggen F, Logan GD. 2008. Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 137, 649–672. ( 10.1037/a0013170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. 2015. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 16, 719–732. ( 10.1038/nrn4038) [DOI] [PubMed] [Google Scholar]
- 5.van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VA. 2010. Unconscious activation of the prefrontal no-go network. J. Neurosci. 30, 4143–4150. ( 10.1523/JNEUROSCI.2992-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbruggen F, Best M, Bowditch WA, Stevens T, McLaren IP. 2014. The inhibitory control reflex. Neuropsychologia 65, 263–278. ( 10.1016/j.neuropsychologia.2014.08.014) [DOI] [PubMed] [Google Scholar]
- 7.Aron AR, Robbins TW, Poldrack RA. 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends Cognit. Sci. 18, 177–185. ( 10.1016/j.tics.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 8.Diamond A. 1996. Evidence for the importance of dopamine for prefrontal cortex functions early in life. Phil. Trans. R. Soc. Lond. B 29, 1483–1493. ( 10.1098/rstb.1996.0134) [DOI] [PubMed] [Google Scholar]
- 9.Iversen SD, Mishkin M. 1970. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp. Brain Res. 11, 376–386. ( 10.1007/BF00237911) [DOI] [PubMed] [Google Scholar]
- 10.Dias R, Robbins T, Roberts A. 1996. Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380, 69–72. ( 10.1038/380069a0) [DOI] [PubMed] [Google Scholar]
- 11.Aron AR, Poldrack RA. 2006. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433. ( 10.1523/JNEUROSCI.4682-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. 2007. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 27, 3743–3752. ( 10.1523/JNEUROSCI.0519-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. 2008. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex 18, 178–188. ( 10.1093/cercor/bhm044) [DOI] [PubMed] [Google Scholar]
- 14.Baunez C, Nieoullon A, Amalric M. 1995. In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. J. Neurosci. 15, 6531–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida A, Tanaka M. 2009. Enhanced modulation of neuronal activity during antisaccades in the primate globus pallidus. Cereb. Cortex 19, 206–217. ( 10.1093/cercor/bhn069) [DOI] [PubMed] [Google Scholar]
- 16.Isoda M, Hikosaka O. 2008. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J. Neurosci. 28, 7209–7218. ( 10.1523/JNEUROSCI.0487-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. 2013. Canceling actions involves a race between basal ganglia pathways. Nat. Neurosci. 16, 1118–1124. ( 10.1038/nn.3456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rae CL, Hughes LE, Anderson MC, Rowe JB. 2015. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J. Neurosci. 35, 786–794. ( 10.1523/JNEUROSCI.3093-13.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zandbelt BB, Bloemendaal M, Hoogendam JM, Kahn RS, Vink M. 2013. Transcranial magnetic stimulation and functional MRI reveal cortical and subcortical interactions during stop-signal response inhibition. J. Cognit. Neurosci. 25, 157–174. ( 10.1162/jocn_a_00309) [DOI] [PubMed] [Google Scholar]
- 20.Chikazoe J, Jimura K, Hirose S, Yamashita K-I, Miyashita Y, Konishi S. 2009. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 29, 15 870–15 877. ( 10.1523/JNEUROSCI.3645-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schel MA, Kühn S, Brass M, Haggard P, Ridderinkhof KR, Crone EA. 2014. Neural correlates of intentional and stimulus-driven inhibition: a comparison. Front. Hum. Neurosci. 8, 27–30. ( 10.3389/fnhum.2014.00027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauggel S, Rieger M, Feghoff TA. 2004. Inhibition of ongoing responses in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 75, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obeso I, et al. 2011. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp. Brain Res. 212, 371–384. ( 10.1007/s00221-011-2736-6) [DOI] [PubMed] [Google Scholar]
- 24.Ye Z, et al. 2014. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease. Brain 137, 1145–1155. ( 10.1093/brain/awu032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vriend C, Gerrits NJHM, Berendse HW, Veltman DJ, van den Heuvel OA, van der Werf YD. 2015. Failure of stop and go in de novo Parkinson's disease—a functional magnetic resonance imaging study. Neurobiol. Aging 36, 470–475. ( 10.1016/j.neurobiolaging.2014.07.031) [DOI] [PubMed] [Google Scholar]
- 26.Obeso I, et al. 2014. The subthalamic nucleus and inhibitory control: impact of subthalamotomy in Parkinson's disease. Brain 137, 1470–1480. ( 10.1093/brain/awu058) [DOI] [PubMed] [Google Scholar]
- 27.Bickel S, et al. 2010. Cognitive and neuropsychiatric effects of subthalamotomy for Parkinson's disease. Parkinsonism Relat. Disord. 16, 535–539. ( 10.1016/j.parkreldis.2010.06.008) [DOI] [PubMed] [Google Scholar]
- 28.Jahanshahi M, et al. 2000. The impact of deep brain stimulation on executive function in Parkinson's disease. Brain 123, 1142–1154. ( 10.1093/brain/123.6.1142) [DOI] [PubMed] [Google Scholar]
- 29.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. 2007. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318, 1309–1312. ( 10.1126/science.1146157) [DOI] [PubMed] [Google Scholar]
- 30.Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, Wouwe NC, van der Wildenberg WPM. 2010. Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson's disease. Brain 133, 3611–3624. ( 10.1093/brain/awq239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. 2004. Stimulation of STN impairs aspects of cognitive control in PD. Neurology 62, 1110–1114. ( 10.1212/01.WNL.0000118202.19098.10) [DOI] [PubMed] [Google Scholar]
- 32.Ballanger B, et al. 2009. Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol. 66, 817–824. ( 10.1002/ana.21795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obeso I, Wilkinson L, Rodríguez-Oroz MC, Obeso JA, Jahanshahi M. 2013. Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict-induced slowing in Parkinson's disease. Exp. Brain Res. 226, 451–462. ( 10.1007/s00221-013-3457-9) [DOI] [PubMed] [Google Scholar]
- 34.Pote I, Torkamani M, Kefalopoulou Z-M, Zrinzo L, Limousin-Dowsey P, Foltynie T, Speekenbrink M, Jahanshahi M. 2016. Subthalamic nucleus deep brain stimulation induces impulsive action when patients with Parkinson's disease act under speed pressure. Exp. Brain Res. 234, 1837–1848. ( 10.1007/s00221-016-4577-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray NJ, et al. 2012. The role of the subthalamic nucleus in response inhibition: evidence from local field potential recordings in the human subthalamic nucleus. Neuroimage 60, 271–278. ( 10.1016/j.neuroimage.2011.12.035) [DOI] [PubMed] [Google Scholar]
- 36.Alegre M, et al. 2013. The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson's disease. Exp. Neurol. 239, 1–12. ( 10.1016/j.expneurol.2012.08.027) [DOI] [PubMed] [Google Scholar]
- 37.Benis D, et al. 2014. Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson's disease. Neuroimage 91, 273–281. ( 10.1016/j.neuroimage.2013.10.070) [DOI] [PubMed] [Google Scholar]
- 38.Balleine BW, O'Doherty JP. 2010. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69. ( 10.1038/npp.2009.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graybiel AM. 2008. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359–387. ( 10.1146/annurev.neuro.29.051605.112851) [DOI] [PubMed] [Google Scholar]
- 40.Evenden J. 1999. Impulsivity: a discussion of clinical and experimental findings. J. Psychopharamcol. 13, 180–192. ( 10.1177/026988119901300211) [DOI] [PubMed] [Google Scholar]
- 41.Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. ( 10.1016/0166-2236(89)90074-X) [DOI] [PubMed] [Google Scholar]
- 42.DeLong MR. 1990. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. ( 10.1016/0166-2236(90)90110-V) [DOI] [PubMed] [Google Scholar]
- 43.Wichmann T, DeLong MR, Guridi J, Obeso JA. 2011. Milestones in research on the pathophysiology of Parkinson's disease. Mov. Disord. 26, 1032–1041. ( 10.1002/mds.23695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obeso JA, Jahanshahi M, Alvarez L, Macias R, Pedroso I, Wilkinson L, Pavon N. 2009. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson's disease. Exp. Neurol. 220, 283–292. ( 10.1016/j.expneurol.2009.08.030) [DOI] [PubMed] [Google Scholar]
- 45.Criaud M, et al. 2016. Slowness in movement initiation is associated with proactive inhibitory network dysfunction in Parkinson's disease. J. Parkinsons Dis. 6, 433–440. ( 10.3233/JPD-150750) [DOI] [PubMed] [Google Scholar]
- 46.Favre E, Ballanger B, Thobois S, Broussolle E, Boulinguez P. 2013. Deep brain stimulation of the subthalamic nucleus, but not dopaminergic medication, improves proactive inhibitory control of movement initiation in Parkinson's disease. Neurotherapeutics 10, 154–167. ( 10.1007/s13311-012-0166-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beste C, Willemssen R, Saft C, Falkenstein M. 2009. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia 48, 366–373. ( 10.1016/j.neuropsychologia.2009.09.023) [DOI] [PubMed] [Google Scholar]
- 48.Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. 2005. Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia 43, 784–796. ( 10.1016/j.neuropsychologia.2004.06.026) [DOI] [PubMed] [Google Scholar]
- 49.Praamstra P, Plat FM. 2001. Failed suppression of direct visuomotor activation in Parkinson's disease. J. Cognit. Neurosci. 13, 31–43. ( 10.1162/089892901564153) [DOI] [PubMed] [Google Scholar]
- 50.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. 2008. The factors that induce or overcome freezing of gait in Parkinson's disease. Behav. Neurol. 19, 127–136. ( 10.1155/2008/456298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis SJG, Shine JM. 2016. The next step: a common neural mechanism for freezing of gait. Neuroscientist 22, 72–82. ( 10.1177/1073858414559101) [DOI] [PubMed] [Google Scholar]
- 52.Snijders AH, et al. 2016. Physiology of freezing of gait. Ann. Neurol. 80, 644–659. ( 10.1002/ana.24778) [DOI] [PubMed] [Google Scholar]
- 53.Fasano A, Herman T, Tessitore A, Strafella AP, Bohnen NI. 2015. Neuroimaging of freezing of gait. J. Parkinson Dis. 5, 241–254. ( 10.3233/JPD-150536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kostic VS, et al. 2012. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology 78, 409–416. ( 10.1212/WNL.0b013e318245d23c) [DOI] [PubMed] [Google Scholar]
- 55.Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. 2011. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain 134, 59–72. ( 10.1093/brain/awq324) [DOI] [PubMed] [Google Scholar]
- 56.Bissett PG, Logan GD, van Wouwe NC, Tolleson CM, Phibbs FT, Claassen DO, Wylie SA. 2015. Generalized motor inhibitory deficit in Parkinson's disease patients who freeze. J. Neural Transm. 122, 1693–1701. ( 10.1007/s00702-015-1454-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgiades MJ, Gilat M, Ehgoetz Martens KA, Walton CC, Bissett PG, Shine JM, Lewis SJG. 2016. Investigating motor initiation and inhibition deficits in patients with Parkinson's disease and freezing of gait using a virtual reality paradigm. Neuroscience 337, 153–162. ( 10.1016/j.neuroscience.2016.09.019) [DOI] [PubMed] [Google Scholar]
- 58.Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, Cummings JL. 1999. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 67, 492–496. ( 10.1136/jnnp.67.4.492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. 2015. Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 14, 518–531. ( 10.1016/S1474-4422(15)00019-8) [DOI] [PubMed] [Google Scholar]
- 60.Santangelo G, et al. 2015. Relationship between apathy and cognitive dysfunctions in de novo, untreated Parkinson's disease patients: a prospective longitudinal study. Eur. J. Neurol. 22, 253–260. ( 10.1111/ene.12467) [DOI] [PubMed] [Google Scholar]
- 61.Maillet A, et al. 2016. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain 139, 2486–2502. ( 10.1093/brain/aww162) [DOI] [PubMed] [Google Scholar]
- 62.Jahanshahi M, Obeso I, Baunez C, Alegre M, Krack P. 2015. Parkinson's disease, the subthalamic nucleus, inhibition, and impulsivity. Mov. Disord. 30, 128–140. ( 10.1002/mds.26049) [DOI] [PubMed] [Google Scholar]
- 63.Marinez-Horta S, Sampedro F, Pagonabarraga J, Fernandez-Bobadilla R, Marin J, Riba J, Kulisevsky J. 2016. Non-demented Parkinson's disease patients with apathy show decreased grey matter volume in key executive and reward-related nodes. Brain Imaging Behav. 233, 823–829. ( 10.1007/s11682-016-9607-5) [DOI] [PubMed] [Google Scholar]
- 64.Santangelo G, et al. 2015. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson's disease patients. Parkinsonism Relat. Disord. 21, 489–493. ( 10.1016/j.parkreldis.2015.02.015) [DOI] [PubMed] [Google Scholar]
- 65.Robert GH, et al. 2014. Preoperative factors of apathy in subthalamic stimulated Parkinson disease: a PET study. Neurology 83, 1620–1626. ( 10.1212/WNL.0000000000000941) [DOI] [PubMed] [Google Scholar]
- 66.Robert GH, et al. 2012. Apathy in patients with Parkinson disease without dementia or depression: a PET study. Neurology 79, 1155–1160. ( 10.1212/WNL.0b013e3182698c75) [DOI] [PubMed] [Google Scholar]
- 67.Reijnders JS. 2010. Neuroanatomical correlates of apathy in Parkinson's disease: a magnetic resonance imaging study using voxel-based morphometry. Mov. Disord. 25, 2318–2325. ( 10.1002/mds.23268) [DOI] [PubMed] [Google Scholar]
- 68.Herz DM, Haagensen BN, Christensen MS, Madsen KH, Rowe JB, Løkkegaard A, Siebner HR. 2014. The acute brain response to levodopa heralds dyskinesias in Parkinson disease. Ann. Neurol. 75, 829–836. ( 10.1002/ana.24138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cerasa A, et al. 2012. Prefrontal alterations in Parkinson's disease with levodopa-induced dyskinesia during fMRI motor task. Mov. Disord. 27, 364–371. ( 10.1002/mds.24017) [DOI] [PubMed] [Google Scholar]
- 70.Cerasa A, et al. 2015. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain 138, 414–427. ( 10.1093/brain/awu329) [DOI] [PubMed] [Google Scholar]
- 71.Cerasa A. 2015. The motor inhibition network in Parkinson's disease with levodopa-induced dyskinesias. Mov. Disord. 30, 1912–1920. ( 10.1002/mds.26378) [DOI] [PubMed] [Google Scholar]
- 72.Weintraub D, David AS, Evans AH, Grant JE, Stacy M. 2015. Clinical spectrum of impulse control disorders in Parkinson's disease. Mov. Disord. 30, 121–127. ( 10.1002/mds.26016) [DOI] [PubMed] [Google Scholar]
- 73.Voon V, et al. 2010. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl.) 207, 645–659. ( 10.1007/s00213-009-1697-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Eimeren T, et al. 2010. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology 75, 1711–1716. ( 10.1212/WNL.0b013e3181fc27fa) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Politis M, Loane C, Wu K, O'Sullivan SS, Woodhead Z, Kiferle L, Lawrence AD, Lees AJ, Piccini P. 2013. Neural response to visual sexual cues in dopamine treatment-linked hypersexuality in Parkinson's disease. Brain 136, 400–411. ( 10.1093/brain/aws326) [DOI] [PubMed] [Google Scholar]
- 76.Okai D, Askey-Jones S, Samuel M, David AS, Brown RG. 2015. Predictors of response to a cognitive behavioral intervention for impulse control behaviors in Parkinson's disease. Mov. Disord. 30, 736–739. ( 10.1002/mds.26108) [DOI] [PubMed] [Google Scholar]
- 77.Albanese A. 2013. Phenomenology and classification of dystonia: a consensus update. Mov. Disord. 28, 863–873. ( 10.1002/mds.25475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. 1998. The pathophysiology of primary dysonia. Brain 121, 1195–1212. ( 10.1093/brain/121.7.1195) [DOI] [PubMed] [Google Scholar]
- 79.Marsden CD. 1976. Blepharospasm–oromandibular dystonia syndrome (Brueghel's syndrome). A variant of adult-onset torsion dystonia? J. Neurol. Neurosurg. Psychiatry 39, 1204 ( 10.1136/jnnp.39.12.1204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delmaire C. 2007. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology 69, 376–380. ( 10.1212/01.wnl.0000266591.49624.1a) [DOI] [PubMed] [Google Scholar]
- 81.Jinnah HA, Hess EJ. 2006. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology 67, 1740–1741. ( 10.1212/01.wnl.0000246112.19504.61) [DOI] [PubMed] [Google Scholar]
- 82.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. 2008. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain 131, 2499–2509. ( 10.1093/brain/awn168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fuchs T, Ozelius LJ. 2011. Genetics of dystonia. Semin. Neurol. 31, 441–448. () [DOI] [PubMed] [Google Scholar]
- 84.Chiken S, Shashidharan P, Nambu A. 2008. Cortically evoked long-lasting inhibition of pallidal neurons in a transgenic mouse model of dystonia. J. Neurosci. 28, 13 967–13 977. ( 10.1523/jneurosci.3834-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vitek JI. 1999. Pallidotomy and deep brain stimulation as a treatment for dystonia. Neurology 52, 294. [Google Scholar]
- 86.Vidailhet M, et al. 2007. Bilateral, pallidal, deepbrain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 6, 223–229. ( 10.1016/S1474-4422(07)70035-2) [DOI] [PubMed] [Google Scholar]
- 87.Ceballos-Baumann AO. 1995. Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann. Neurol. 37, 363–372. ( 10.1002/ana.410370313) [DOI] [PubMed] [Google Scholar]
- 88.Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos-Baumann AO. 2006. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain 129, 36–46. ( 10.1093/brain/awh665) [DOI] [PubMed] [Google Scholar]
- 89.Edwards MJ, Huang Y, Wood NW, Rothwell JC, Bhatia KP. 2003. Different patterns of electrophysiological deficits in manifesting and nonmanifesting carriers of the DYT1 gene mutation. Brain 126, 2074–2080. ( 10.1093/brain/awg209) [DOI] [PubMed] [Google Scholar]
- 90.Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. 1995. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J. Neurol. Neurosurg. Neuropsychiatry 59, 493 ( 10.1136/jnnp.59.5.493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quartarone A, et al. 2003. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain 126, 2586–2596. ( 10.1093/brain/awg273) [DOI] [PubMed] [Google Scholar]
- 92.Sohn YH, Hallett M. 2004. Distirbed surround inhibition in focal hand dystonia. Ann. Neurol. 56, 595–599. ( 10.1002/ana.20270) [DOI] [PubMed] [Google Scholar]
- 93.Beck S. 2008. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J. Neurosci. 28, 10 363–10 369. ( 10.1523/JNEUROSCI.3564-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stinear CM, Byblow WD. 2004. Impaired modulation of corticospinal excitability following subthreshold rTMS in focal hand dystonia. Hum. Mov. Sci. 23, 527–538. ( 10.1016/j.humov.2004.08.022) [DOI] [PubMed] [Google Scholar]
- 95.Quartarone A, et al. 2005. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain 128, 1943–1950. ( 10.1093/brain/awh527) [DOI] [PubMed] [Google Scholar]
- 96.Stinear CM, Byblow WD. 2004. Impaired inhibition of a pre-planned response in focal hand dystonia. Exp. Brain Res. 158, 207–212. ( 10.1007/s00221-004-1891-4) [DOI] [PubMed] [Google Scholar]
- 97.Tinazzi M. 2002. Deficits of temporal discrimination in dystonia are independent from the spatial distance between the loci of tactile stimulation. Mov. Disord. 17, 333–338. ( 10.1002/mds.10019) [DOI] [PubMed] [Google Scholar]
- 98.Fiorio M, et al. 2007. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: a new endophenotype of dystonia? Brain 130, 134–142. ( 10.1093/brain/awl283) [DOI] [PubMed] [Google Scholar]
- 99.Rocchi L, Casula E, Tocco P, Berardelli A, Rothwell J. 2016. Somatosensory temporal discrimination threshold involves inhibitory mechanisms in the primary somatosensory area. J. Neurosci. 36, 325–335. ( 10.1523/JNEUROSCI.2008-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antelmi E, Erro R, Rocchi L, Liguori R, Tinazzi M, Stasio FD, Berardelli A, Rothwell JC, Bhatia KP. 2016. Neurophysiological correlates of abnormal somatosensory temporal discrimination in dystonia. Mov. Disord. ( 10.1002/mds.26804) [DOI] [PubMed] [Google Scholar]
- 101.Kaji R, et al. 1995. Physiological study of cervical dystonia. Task-specific abnormality in contingent negative variation. Brain 118, 511–522. ( 10.1093/brain/118.2.511) [DOI] [PubMed] [Google Scholar]
- 102.Murase N, Kaji R, Shimazu H, Katayama-Hirota M. 2000. Abnormal premovement gating of somatosensory input in writer's cramp. Brain 123, 1813–1829. ( 10.1093/brain/123.9.1813) [DOI] [PubMed] [Google Scholar]
- 103.Hallett M. 2011. TMS3.4 Cortical inhibitory circuits, surround inhibition, and focal hand dystonia. Clin. Neurophys. 122, 56–57. ( 10.1016/S1388-2457(11)60190-9) [DOI] [Google Scholar]
- 104.Mink JW. 2001. Neurobiology of basal ganglia circuits in Tourette syndrome: faulty inhibition of unwanted motor patterns? Adv. Neurol. 85, 113–122. [PubMed] [Google Scholar]
- 105.McNaught KS, Mink JW. 2011. Advances in understanding and treatment of Tourette syndrome. Nat. Rev. Neurol. 7, 667–676. ( 10.1038/nrneurol.2011.167) [DOI] [PubMed] [Google Scholar]
- 106.Kalanithi PS, et al. 2005. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl Acad. Sci. USA 102, 13 307–13 312. ( 10.1073/pnas.0502624102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Worbe Y, et al. 2015. Altered structural connectivity of cortico–striato–pallido–thalamic networks in Gilles de la Tourette syndrome. Brain 138, 472–482. ( 10.1093/brain/awu311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Channon S, Sinclair E, Waller D, Healey L, Robertson MM. 2004. Social cognition in Tourette's syndrome: intact theory of mind and impaired inhibitory functioning. J. Autism Dev. Disord. 34, 669–677. ( 10.1007/s10803-004-5287-x) [DOI] [PubMed] [Google Scholar]
- 109.Wylie SA, Claassen DO, Kanoff KE, Ridderinkhof KR, van den Wildenberg WP. 2013. Impaired inhibition of prepotent motor actions in patients with Tourette syndrome. J. Psychiatry Neurosci. 38, 349–356. ( 10.1503/jpn.120138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roessner V, Albrecht B, Dechent P, Baudewig J, Rothenberger A. 2008. Normal response inhibition in boys with Tourette syndrome. Behav. Brain Funct. 4, 29 ( 10.1186/1744-9081-4-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. 2006. Enhanced cognitive control in young people with Tourette's syndrome. Curr. Biol. 16, 570–573. ( 10.1016/j.cub.2006.01.064) [DOI] [PubMed] [Google Scholar]
- 112.Ganos C, et al. 2014. The neural correlates of tic inhibition in Gilles de la Tourette syndrome. Neuropsychologia 65, 297–301. ( 10.1016/j.neuropsychologia.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 113.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. 1998. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch. Gen. Psychiatry 55, 326–333. ( 10.1001/archpsyc.55.4.326) [DOI] [PubMed] [Google Scholar]
- 114.Mazzone L, Yu S, Blair C, Gunter BC, Wang Z, Marsh R, Peterson BS. 2010. An fMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. Am. J. Psychiatry 167, 341–349. ( 10.1176/appi.ajp.2009.08121831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Welter ML, et al. 2008. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch. Neurol. 65, 952–957. ( 10.1001/archneur.65.7.952) [DOI] [PubMed] [Google Scholar]
- 116.Neuner I, Podoll K, Lenartz D, Sturm V, Schneider F. 2009. Deep brain stimulation in the nucleus accumbens for intractable Tourette's syndrome: follow-up report of 36 months. Biol. Psychiatry 65, e5–e6. ( 10.1016/j.biopsych.2008.09.030) [DOI] [PubMed] [Google Scholar]
- 117.Kefalopoulou Z, et al. 2015. Bilateral globus pallidus stimulation for severe Tourette syndrome: a double- blind, randomized crossover trial. Lancet Neurol. 14, 595–605. ( 10.1016/S1474-4422(15)00008-3) [DOI] [PubMed] [Google Scholar]
- 118.Milad MR, Rauch SL. 2012. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cognit Sci. 16, 43–51. ( 10.1016/j.tics.2011.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. 2005. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 29, 399–419. ( 10.1016/j.neubiorev.2004.11.006) [DOI] [PubMed] [Google Scholar]
- 120.Eng GK, Sim K, Chen SH. 2015. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: an integrative review. Neurosci. Biobehav. Rev. 52, 233–257. ( 10.1016/j.neubiorev.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 121.Kringelbach ML. 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691 ( 10.1038/nrn1747) [DOI] [PubMed] [Google Scholar]
- 122.Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. 2007. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol. Psychiatry 62, 901–909. ( 10.1016/j.biopsych.2006.12.007) [DOI] [PubMed] [Google Scholar]
- 123.Banca P, Voon V, Vestergaard MD, Philipiak G, Almeida I, Pocinho F, Relvas J, Castelo-Branco M. 2015. Imbalance in habitual versus goal directed neural systems during symptom provocation in obsessive-compulsive disorder. Brain 138, 798–811. ( 10.1093/brain/awu379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gillan CM, et al. 2011. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am. J. Psychiatry 168, 718–726. ( 10.1176/appi.ajp.2011.10071062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gillan CM, et al. 2014. Enhanced avoidance habits in obsessive-compulsive disorder. Biol. Psychiatry 75, 631–638. ( 10.1016/j.biopsych.2013.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Greenberg BD, et al. 2010. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol. Psychiatry 15, 64–79. ( 10.1038/mp.2008.55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huff W, et al. 2010. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: outcomes after one year. Clin. Neurol. Neurosurg. 112, 137–143. ( 10.1016/j.clineuro.2009.11.006) [DOI] [PubMed] [Google Scholar]
- 128.Mallet L, et al. 2008. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 359, 2121–2134. ( 10.1056/NEJMoa0708514) [DOI] [PubMed] [Google Scholar]
- 129.Kibleur A, et al. 2016. Modulation of motor inhibition by subthalamic stimulation in obsessive-compulsive disorder. Transl. Psychiatry 6, e922 ( 10.1038/tp.2016.192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rauch SL, et al. 2006. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J. Neurosurg. 104, 558–565. ( 10.3171/jns.2006.104.4.558) [DOI] [PubMed] [Google Scholar]
- 131.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. 2011. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol. 21, 1081–1091. ( 10.1016/j.cub.2011.05.001) [DOI] [PubMed] [Google Scholar]
- 132.Schmidt R, Berke JD. 2017. A Pause-then-Cancel model of stopping: evidence from basal ganglia neurophysiology. Phil. Trans. R. Soc. B 372, 20160202 ( 10.1098/rstb.2016.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]