Abstract
A fundamental challenge to the brain is how to prevent intrusive movements when quiet is needed. Unwanted limb movements such as tremor impair fine motor control and unwanted eye drifts such as nystagmus impair vision. A stable platform is also necessary to launch accurate movements. Accordingly, nature has designed control systems with agonist (excitation) and antagonist (inhibition) muscle pairs functioning in push–pull, around a steady level of balanced tonic activity, the set-point. Sensory information can be organized similarly, as in the vestibulo-ocular reflex, which generates eye movements that compensate for head movements. The semicircular canals, working in coplanar pairs, one in each labyrinth, are reciprocally excited and inhibited as they transduce head rotations. The relative change in activity is relayed to the vestibular nuclei, which operate around a set-point of stable balanced activity. When a pathological imbalance occurs, producing unwanted nystagmus without head movement, an adaptive mechanism restores the proper set-point and eliminates the nystagmus. Here we used 90 min of continuous 7 T magnetic field labyrinthine stimulation (MVS) in normal humans to produce sustained nystagmus simulating vestibular imbalance. We identified multiple time-scale processes towards a new zero set-point showing that MVS is an excellent paradigm to investigate the neurobiology of set-point adaptation.
This article is part of the themed issue ‘Movement suppression: brain mechanisms for stopping and stillness’.
Keywords: set-point, adaptation, vestibular, homeostasis, magnetic field
1. Introduction: a rationale for set-point adaptation
(a). Homeostasis and posture
Homeostasis, from the Greek, homeo, meaning unchanging, and stasis, meaning standing, refers to the ability of an organism to regulate its internal conditions so as to maintain a condition (a ‘set-point’) of equilibrium and stability in the face of perturbations, from either the outside world or the body's internal environment. Typical examples are control of the temperature of the body and of appetite. In this paper, we will discuss how homeostasis and its set-points are optimized for movement when it is needed and for stability when it is not. A fundamental challenge to the nervous system is how to prevent intrusive and disruptive movements when quiet is called for. Unwanted movements in the limbs impair tasks requiring fine coordination, as with a tremor of the hand. Unwanted drifts of the eyes during attempted fixation impair vision, as with a pathological spontaneous nystagmus. These abnormalities are evolutionarily disadvantageous so that being able to launch accurate movements from a quiet platform becomes a central goal for motor behaviour.
(b). The design of a postural system
The optimal position of rest is more than just a lack of extraneous activity; it is best developed upon a sustained balanced level of opposing tonic neural activity. In this way, movement is produced by a reciprocal increase and decrease of activity in muscles that act together, one being the agonist and the other the antagonist: a key design recognized by Sherrington [1]. This ‘push–pull’ mechanism serves us well, being optimal for prompt initiation and fast and accurate movements of any size and direction, and providing a substrate for compensation in the face of disease or trauma. For example, when abduction of the eye is impaired because a lateral rectus muscle is paralysed the eye can still move in at least one hemifield of the orbit, by contraction and relaxation of the remaining intact medial rectus muscle. Similarly, consider processing of sensory information from the labyrinth, which is used to drive compensatory eye movements when the head moves. Rotation of the head is sensed by reciprocal changes in activity between pairs of semicircular canals, one in each labyrinth. This difference, relayed centrally to the vestibular nuclei, generates the eye movement command that compensates for head movements when we attempt to stabilize retinal images of objects that are stationary in the environment. This simple reflex, the vestibulo-ocular reflex (VOR), is called the T-VOR when responding to translations, which are sensed by the otolith organs, and the R-VOR when responding to rotations, which are sensed by the semicircular canals. When one labyrinth is destroyed, rotation or translations in all directions can still be detected by an increase or decrease in activity in the remaining labyrinth, but not with the same fidelity as when both labyrinths are working.
(c). Set-point adaptation
To ensure the optimal level of balanced tonic activity the brain has evolved an adaptive capability—‘set-point or bias adaptation’—as a core neurobiological mechanism to maintain states of physical quiet and increase the ability to detect and dynamically respond to small differences in changes in neural activity, i.e. to increase the signal to noise ratio, and also to extend the range of linear behaviour. In this way, one is poised to respond immediately and accurately with the next necessary action or reaction. Change in patterns of sensory inputs may drive this adaptation such as during locomotion, in the transition from crawling to walking, and with exposure to microgravity. Changes in context demand different combinations of optimal set-points for different tasks, such as writing, playing golf or the violin. But there must also be sufficient neural flexibility so the set-points can be immediately changed when faced with a new context.
Comparative analyses in the control of posture by molluscs and lamprey show that set-point adaptation developed early in evolution [2]. In human behaviour, set-points are ubiquitous; from unconscious motor behaviour that compensates for changing mechanical loads at the level of segmental reflex arcs in the spinal cord, to the highest cognitive and emotional levels that maintain equipoise when one is faced with making difficult decisions in stressful and uncertain environments. Here we concentrate on set-point adaptation as it relates to movement, and use specific examples associated with the control of the eyes and head. Compared with the considerable attention paid to adaptation of dynamic motor behaviour to optimize the amplitude, direction and timing of eye and head movements, less is known about the equally important mechanisms underlying ‘set-point’ adaptation, and especially those related to the brain's earliest responses to a challenge to its normal state of quiet.
(d). Challenges and responses to quiet and stable platforms: velocity and position biases and neural integrators
Challenges to stable postures include velocity biases, which cause unwanted motion or drift when there should be none. A typical example is a spontaneous eye nystagmus from a vestibular imbalance due to a sudden loss of labyrinthine function in one ear. Eliminating this visually disabling bias quickly is a major priority for the nervous system and the brain ‘pulls out all the stops’ in its immediate attempts to eliminate this threat to survival. Adaptation can counteract the velocity bias by suppressing activity in central vestibular nuclei neurons that receive afferent information from the intact labyrinth. Oppositely directed biases or drifts can also be introduced to counteract the initial pathological one. As an example, neural networks that normally hold eccentric positions of gaze steady can be become ‘leaky’ with diminished time constants of integration so as to introduce centripetal drift that in one direction will be a counteracting bias that helps to create one position of the eyes in the orbit where the effect of the pathological bias is diminished [3].
Position biases also occur after a unilateral loss of labyrinthine function and include vertical ocular misalignment (skew deviation) causing diplopia, and ocular torsion (counterroll) causing the visual world to appear tilted. These abnormalities reflect imbalance in otolith inputs just as nystagmus reflects imbalance in semicircular canal inputs. Position biases are also used to shift the neutral or null position when there are new requirements imposed by a sustained change in the habitual working range in which the movements are generated. An example is given by the rebound phenomenon (or rebound nystagmus) that occurs when the eyes are returned to a straight-ahead position after being held in an eccentric position for a period of time (figure 1) [4]. Normally, a sustained tonic eye position signal must be generated to oppose the elastic restoring forces of orbital tissues that would passively pull the eye back to its initial position. During sustained eccentric gaze the brain attempts to shift its null position towards the new eccentric position in the orbit in which the eyes are now spending most of their time. This would presumably provide some relief to the central circuits that must provide the commands to maintain the high level of sustained activity required to keep the eye steady in a far eccentric position, and also restore a range for push–pull linear behaviour. On return to the original, straight-ahead position, a rebound nystagmus occurs, with the eyes drifting toward the eccentric position from which they had just returned. The rebound nystagmus slowly dissipates as the adaptive response decays and brings the neutral or ‘null position’ back to the original straight-ahead. A similar rebound phenomenon occurs after the head is held in a sustained eccentric position on the torso and then returned to a centre position on the torso [5,6]. In both these examples of a rebound phenomenon, the shift of the null position is adaptive, but with neurological disease a constant shift or continuous fluctuation in the null position is counterproductive and leads to unwanted postures (e.g. wry neck), adventitious movements (e.g. head tremor) and impaired dynamic motor performance (dysmetria). Binocular visual functions, too, rely on set-point adaptation to establish the basic deviation between the two lines of sight (called the phoria) from which convergence or divergence can be most effectively driven [7]. In strabismus, abnormal set-points can lead to sustained deviation of the visual axes producing both confusing diplopia with loss of depth perception as well as mislocalization of objects as each fovea receives a different image.
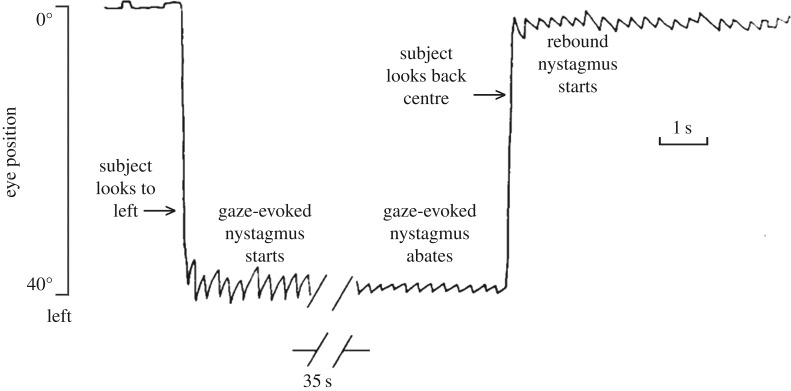
Figure 1.
An example of the rebound phenomenon shown by an individual with spinocerebellar ataxia type 6. On looking towards a visual target located on the far left, gaze-evoked nystagmus begins, with inwardly directed, centripetal drifts of the eyes towards the central position and outwardly directed, centrifugal corrective quick phases. After 35 s of this sustained effort at maintaining eccentric gaze, the slow-phase drift velocity is reduced. When the eyes are returned to the central position, the nystagmus reverses direction (rebound nystagmus) and slowly dissipates reflecting the adaptation after-effect. Modified from [4].
The central neural networks that elaborate the commands that normally hold positions steady are commonly conceptualized as mathematical integrators or holding networks [8–10]. Because integrating circuits rely on various types of feedback of neural activity, be it derived from afferent signals reflecting changing external circumstances or from internal copies of efferent neural commands, they can become unstable when feedback is excessive or even of the wrong sign, producing unwelcome oscillatory movements. Examples include both congenital and acquired forms of pendular eye nystagmus, in which unwanted drifts alternate in opposite directions, often with sinusoidal wave forms, and ‘jerk’ eye nystagmus, with ‘runaway’ slow phases that have an increasing velocity [4,11–14]. Similar patterns of instability can be seen in the integrators that hold the position of the head on the torso, which may cause different forms of oscillations, such as head tremor in patients with cervical dystonia [5,6]. Other examples of oscillations produced by unstable integrators are periodic alternating nystagmus (from a vestibular velocity bias) [15] and periodic alternating skew deviation (from a vestibular position bias) [16].
2. Set-point adaptation and the vestibulo-ocular reflex
(a). Translational vestibulo-ocular reflex
Set-point adaptation for movements may also respond to specific perceptual needs. Consider the T-VOR, which helps to stabilize gaze when the head is translating—such as for up-and-down or side-to-side motion during locomotion. The geometry of head translations (figure 2) indicates that eye rotations that hold the image of a near target steady on the retina are much larger than the eye rotations required to hold images of the distant background steady. It follows that the T-VOR cannot simultaneously hold images of far and near targets still on the retina. One solution would be for the brain to generate eye movements during head translations that minimize relative motion of the near object of regard with respect to the background [17]. This conceptualization accounts for the widely reported finding that the T-VOR does not generate eye rotations that perfectly stabilize the image of a near object on the fovea [18,19]. Evidence to support this type of adaptive, ‘set-point’ property of T-VOR comes from experiments in which subjects look through optical devices that require larger eye movements to hold images steady compared with normal viewing [20]. Under such circumstances, the T-VOR does generate larger eye movements compared with normal viewing but the magnitude of these movements is still less than that required to hold images of the fixation target steady on the retina [21]. In other words, although the T-VOR is capable of generating larger and faster eye movements than are observed during natural viewing, the brain deliberately chooses a set-point that minimizes relative image motion of the object of regard with respect to its background. Thus, the brain must decide whether to stabilize a particular image of a near object on the fovea or to minimize image motion relative to the background; both are not possible at once. We suggest the brain chooses a compromise set-point for the gain of the T-VOR (the amplitude of compensatory movement for a head translation at a given viewing distance) that is optimal for overall visual performance during locomotion, taking into account retinal image slip of the target with respect to the background.
Figure 2.
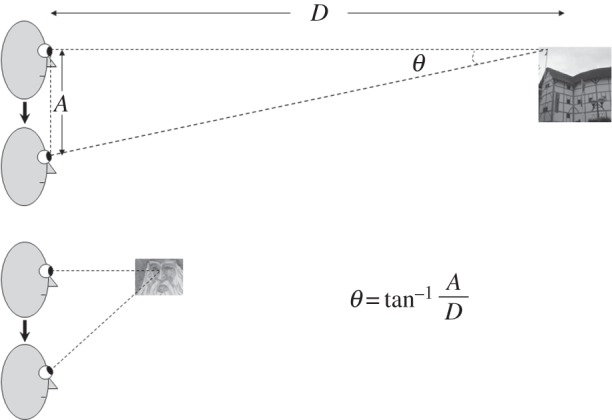
Schematic of geometry of the linear or translational vestibulo-ocular reflex (T-VOR). During vertical head translations (in the Z-axis, bob or heave), vertical eye rotations are required to hold the eyes on target. The magnitude (θ) of eye movements required to hold the foveal line of sight on the target is given by the equation at bottom, where D is the target distance and A is the amplitude of the head translation. Note that during fixation on a near target (lower panel), larger eye rotations are required than while viewing far objects (upper panel). This geometry indicates that the T-VOR cannot generate eye rotations that simultaneously hold images of far and near targets still on the retina.
(b). Rotational vestibulo-ocular reflex
As noted above, the VOR works in a push–pull, excitation–inhibition fashion around tonic levels of activity. How do adaptive mechanisms create a new set-point to keep the eyes still when the head is still when there is a sustained imbalance in tonic activity (and a consequent unwanted drift of the eyes)? Such an adaptation process is clearly evident in the archetypical clinical problem of a human suddenly losing function of one labyrinth. Devoid of activity from the affected labyrinth, the subject initially has a strong spontaneous nystagmus due to the central imbalance in tone that is created by the asymmetry in the levels of afferent activity from the semicircular canals transmitted to the vestibular nuclei from the two labyrinths. The nystagmus subsides, but usually taking days to weeks to completely resolve, as central balance is restored and a new stable set-point is achieved [22]. A striking example of this remarkable adaptive capability is Bechterew's phenomenon. Here a patient who has previously compensated for a unilateral loss of function suddenly loses function in the remaining labyrinth and develops again the clinical syndrome of a loss of labyrinthine function [23]. Despite being bereft of activity from both labyrinths, the patient shows a strong spontaneous nystagmus similar to but oppositely directed from that brought on by the original loss of function on one side.
Early attempts to study the initial phases of these types of adaptive phenomena for the R-VOR in normal human subjects used constant acceleration stimuli in rotatory chairs to simulate an acute lesion by virtue of the sustained nystagmus that is produced with this type of stimulus [24–26]. The idea was that the brain would infer that the sustained nystagmus was the result of a lesion (and a tone imbalance) that would challenge adaptive mechanisms to remove the nystagmus and restore balance. But the time for which the sustained nystagmus could be maintained in these experiments was limited by how long a human could be safely subjected to a constantly increasing rotational velocity. Hence these early investigations could only study adaptation mechanisms that were incomplete and had decay time constants in the order of a few minutes.
3. Magneto-hydrodynamic vestibular stimulation: an experimental paradigm for set-point adaptation
Recent studies have shown that the strong magnetic fields of MRI machines stimulate the labyrinth and induce a nystagmus [27–29]. The is due to static magneto-hydrodynamic (Lorentz) forces in which ion-containing fluids (endolymph in this case) interact with a strong magnetic field to produce a force that acts on the endolymph within the semicircular canals and pushes the cupula to a maintained new position. This experimental stimulus mimics a constant head acceleration (which also pushes the cupula to a maintained new position) inducing a sustained nystagmus similar to an acute natural vestibular lesion in one labyrinth, with the caveat, of course, that with MVS in normal subjects both labyrinths are still intact. MVS is especially suited to study vestibular set-point adaptation because it produces a sustained nystagmus, comfortably and effortlessly, in animals or humans, lasting hours—much longer than can be accomplished with rotational stimulation or other artificial ways of stimulating the labyrinth such as with galvanic or caloric stimulation [30,31].
(a). Magneto-hydrodynamic vestibular stimulation-induced nystagmus: basic characteristics and simulations of the adaptive response
We placed subjects in a 7 T MRI for up to 90 min, during which they showed a sustained nystagmus [31]. Because of properties of the labyrinth, and a central velocity-storage mechanism that normally perseverates peripheral vestibular activity, the slow-phase velocity induced by a constant acceleration should rise to a constant value with a time constant of 10–15 s [32]. During this period of sustained MVS, however, slow-phase velocity, after rising relatively quickly to its maximum value, slowly decays back towards a new, but non-zero, baseline though if the MVS could have been sustained for longer periods the nystagmus would presumably have eventually faded away completely. This gradual fading of the nystagmus induced by MVS represents the action of adaptive processes that monitor and adjust set-points. In this case, the brain infers that a sustained unchanging nystagmus is pathological (as it always is in natural circumstances) and tries to eliminate the bias and unwanted eye drift. When the adaptive stimulus is abruptly removed, as when the subject exits the MRI bore, an after-effect appears, revealing the prior adaptation, with oppositely directed slow phases that slowly fade away.
A typical example of MVS-induced nystagmus and the attempt of an adaptive mechanism to rid itself of it is shown in figure 3 (blue data points). The subject was placed in the MRI magnetic field for 90 min. We identified a process that occurred over multiple time courses and simulations suggested that three integrators of varying dynamic properties could account for it. Our ideas were based on the hypothesis that set-point adaptation was implemented by adaptation operators, representing mathematical integrators of varying fidelity that feed back their output to be subtracted from the input signal (figure 4). The greater the integrator time constant, the slower the pace of learning toward the new set-point but the reversal nystagmus (the after-effect) lasts longer. As noted above, both Young & Oman [24] and Malcomb & Jones [25] studied short-term adaptation using constant acceleration chair rotations to induce a sustained nystagmus. They simulated their results with a single adaptation operator but did not have results from long-enough accelerations to look for slower time courses of adaptation. In our paradigm, we were able to examine this issue and developed a new model with multiple adaptation operators (figure 4) that nicely simulated our data (figure 3, purple solid line). In our scheme, the leak variable of an adaptation operator, reflecting how faithfully it acts as a mathematical integrator, represents the degree to which the adapted state approximates the environment; the smaller the leak, the closer the adaptation operator reaches the new set-point (figure 5). Slower dynamic properties shift the system in stages towards a new zero set-point by gradually eliminating the intrusive bias. Furthermore, more slowly changing (lower-frequency) stimuli beget slower but more enduring adaptation as reflected in a longer-lasting after-effect as the period of exposure to the unwanted bias is increased. This is represented as adaptation operators with slower dynamic properties that are less leaky and eliminate bias over longer periods of time. In figure 5, the shaded areas reflect the relative effects of the different adaptation operators. The purple dots are the slow-phase velocity.
Figure 3.
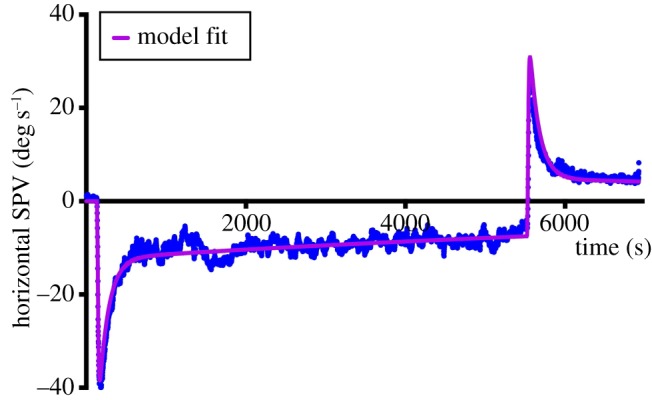
Response of a single subject to 90 min of MVS. Blue points reflect individual slow-phase velocities. Purple line is the simulation result for an optimized three-time constant adaptation model (see also figure 4). Modified from [31]. SPV, slow-phase velocity.
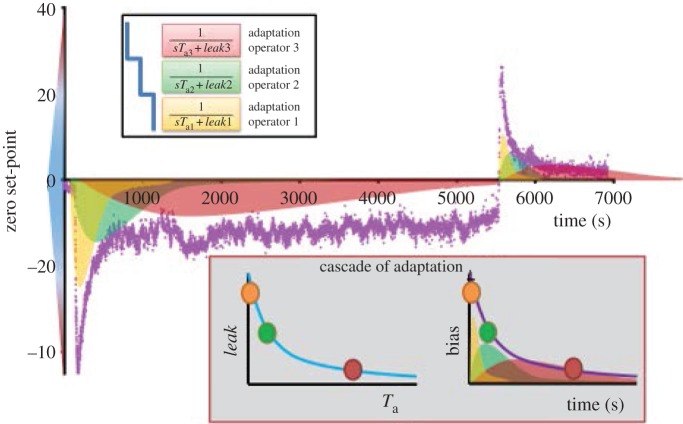
Figure 4.
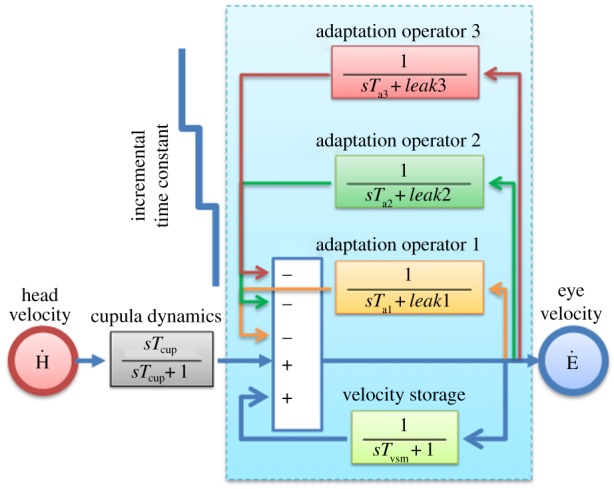
Vestibular set-point adaptation model using three adaptation operators (integrators of varying dynamic properties acting as low-pass filters and feeding back their output to be subtracted from the input signal). Adaptation operators are variably leaky integrators (modifiable leak and time constant parameters) with progressively different dynamics (Ta3 > Ta2 > Ta1). Cupula dynamics are represented by a high-pass filter. The Laplace transform operator is denoted by ‘s’. Modified from [31] in which the quantified parameters for time constant and leak are presented. s, LaPlace transform operator; Tcup, cupula time constant; Tvsm, velocity storage mechanism time constant; Ḣ, head velocity, Ė, eye velocity; Ta1, adaptation time constant (1); Ta2, adaptation time constant (2); Ta3, adaptation time constant (3).
Figure 5.
A cascade of adaptation operators (integrators) with progressively slower dynamic properties shifts the system towards a new zero set-point by eliminating bias. Slowly changing, lower-frequency stimuli beget slower but more enduring adaptation as reflected in the persistence of the after-effect. This progression is represented as adaptation operators with slower dynamic properties that are less leaky and eliminate bias over a longer period of time. Shaded areas reflect the relative effects of the different adaptation operators. Purple dots are the slow-phase velocity nystagmus response. Modified from [31].
4. The underlying neurophysiology of vestibulo-ocular reflex set-point adaptation: error signals and the implementation of the adaptive response
What are the error signals that drive VOR set-point adaptation, and how does the brain act upon them? Vision including the slip of images on the retina, which is the consequence of a spontaneous nystagmus when the head is still, is not necessary. Set-point adaptation proceeds, and the spontaneous nystagmus measured in the dark recedes, regardless of whether the subject remains in the dark or if the nystagmus is suppressed by fixation in the light [33]. This type of static, set-point VOR adaptation contrasts with adaptation for the dynamic properties of the VOR response to head movement, which needs visual error signals, or internally generated surrogates, to apprise the brain of a need for and the type of adaptive change [34,35]. The cerebellum is commonly thought to be central to this process, receiving retinal-slip error signals about the performance of the VOR during head movements and readjusting the response accordingly.
By contrast, for VOR set-point adaptation the brain must have a mechanism to monitor the relative and absolute levels of spontaneous neural activity in the vestibular nuclei to know if and what type of readjustment is needed to restore the optimal level of balanced activity. The vestibular commissural system could be a conduit for rebalancing activity between vestibular neurons on the two sides of the brainstem [36,37], though this mechanism is probably only effective for small degrees of imbalance such as might occur with a mild lesion in the labyrinth or with the challenges posed by natural development and ageing [38].
The role of the cerebellum in VOR set-point adaptation is not established, though it has been suggested that in the early attempt to decrease the spontaneous nystagmus the cerebellum inhibits neurons in the vestibular nuclei that receive afferent activity from the intact labyrinth (the cerebellar clamp) [39,40]. Whether or not the cerebellum participates in the short-term adaptive mechanisms we have identified with the MVS paradigm is not known. There are hints, however, from other types of movements, such as those that stabilize posture and prevent abnormal turning, that the cerebellum is important for adjusting biases [41]. Likewise, the cerebellum has been implicated in adjusting biases in the control systems for steady fixation. If the posterior fastigial nucleus of a monkey is experimentally inactivated with chemical toxins, the animal develops an inappropriate static offset in its straight-ahead position [42]. A similar phenomenon occurs in human patients with Wallenberg's syndrome, which is due to a brainstem infarction that interrupts climbing fibre inputs to the cerebellum leading to excessive inhibition upon the fastigial nucleus. When such patients close their eye lids, the eyes deviate away from straight-ahead and towards the side of the lesion (ipsipulsion) [43]. If the dorsal vermis of monkeys is lesioned bilaterally the animals develop abnormal alignment of the eyes; the eyes turn in (esotropia) when looking at a distant target [44]. This is a pattern of misalignment similar to that shown by some human patients with cerebellar disease [4]. Fixation itself is unsteady with cerebellar lesions; excessive drift and a ‘wandering’ null point appear [40,45].
Finally, we must ask how the brain implements VOR set-point adaptation with multiple time courses. Using the engineering, circuit approach one can envision a neural network with multiple clusters of differentially leaky integrators similar to that suggested for the velocity-to-position integrator that holds gaze steady during attempted fixation [46–48]. For more profound disturbances to the VOR set-point, such as from an acute complete loss of the function of a labyrinth, the brain enlists a cascade of responses over time courses extending to days, weeks and months. Changes in synaptic activity, neurotransmitter levels, gene expression, inflammatory responses, neurogenesis and many other cellular and cognitive mechanisms are important [22,49]. The challenge represented by multiple time courses of adaptation, even in its early phases as we have shown with MVS, is to identify their underlying biological mechanisms, anatomical substrates and especially potential sites of intervention to promote recovery from disease and trauma. MVS set-point adaptation has become a biological model with many potential applications to basic neurophysiological research, in both humans and experimental animals, into how the brain prevents movement when there should be none and provides a stable platform from which accurate movements can be launched [29,31].
Acknowledgement
The authors acknowledge the assistance from Dale Roberts, MS, Bryan Ward, MD, Jorge Otero-Millan, PhD and Michael Schubert, PhD.
Ethics
All experiments were performed in accordance with protocols approved by the Johns Hopkins Human Subject experimentation committee.
Authors' contributions
All authors contributed to the conceptualization of this report, collection and interpretation of the experimental data and writing and approval of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Brain-Science Institute of The Johns Hopkins University and The Johns Hopkins Medicine Discovery Fund.
References
- 1.Sherrington CS. 1915. Postural activity of muscle and nerve. Brain 39, 191–234. ( 10.1093/brain/38.3.191) [DOI] [Google Scholar]
- 2.Deliagina TG, Beloozerova IN, Orlovsky GN, Zelenin PV. 2014. Contribution of supraspinal systems to generation of automatic postural responses. Front. Integr. Neurosci. 8, 76 ( 10.3389/fnint.2014.00076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson DA, Zee DS, Hain TC, Holmes A, Rosenberg LF. 1984. Alexander's law: its behavior and origin in the human vestibulo-ocular reflex. Ann. Neurol. 16, 714–722. ( 10.1002/ana.410160614) [DOI] [PubMed] [Google Scholar]
- 4.Leigh RJ, Zee DS. 2015. The neurology of eye movements, 5th edn New York, NY: Oxford University Press. [Google Scholar]
- 5.Shaikh AG, Wong AL, Zee DS, Jinnah HA. 2013. Keeping your head on target. J. Neurosci. 33, 11 281–11 295. ( 10.1523/JNEUROSCI.3415-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh AG, Zee DS, Crawford JD, Jinnah HA. 2016. Cervical dystonia: a neural integrator disorder. Brain 39, 2590–2599. ( 10.1093/brain/aww141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi B. 1986. Heterophoria: a vergence adaptive position. Ophthalmic Physiol. Opt. 6, 151–156. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter RH. 2011. What Sherrington missed: the ubiquity of the neural integrator. Ann. NY Acad. Sci. 1233, 208–213. ( 10.1111/j.1749-6632.2011.06110.x) [DOI] [PubMed] [Google Scholar]
- 9.Robinson DA. 1989. Integrating with neurons. Annu. Rev. Neurosci. 12, 33–45. ( 10.1146/annurev.ne.12.030189.000341) [DOI] [PubMed] [Google Scholar]
- 10.Barreiro AK, Bronski JC, Anastasio TJ. 2009. Bifurcation theory explains waveform variability in a congenital eye movement disorder. J. Comput. Neurosci. 26, 321–329. ( 10.1007/s10827-008-0113-7) [DOI] [PubMed] [Google Scholar]
- 11.Zee DS, Yamazaki A, Butler PH, Gucer G. 1981. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J. Neurophysiol. 46, 878–899. [DOI] [PubMed] [Google Scholar]
- 12.Optican LM, Zee DS. 1984. A hypothetical explanation of congenital nystagmus. Biol. Cybern. 50, 119–134. ( 10.1007/BF00337159) [DOI] [PubMed] [Google Scholar]
- 13.Arnold DB, Robinson DA, Leigh RJ. 1999. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vision Res. 39, 4286–4295. ( 10.1016/S0042-6989(99)00142-X) [DOI] [PubMed] [Google Scholar]
- 14.Das VE, Oruganti P, Kramer PD, Leigh RJ. 2000. Experimental tests of a neural-network model for ocular oscillations caused by disease of central myelin. Exp. Brain Res. 133, 189–197. ( 10.1007/s002210000367) [DOI] [PubMed] [Google Scholar]
- 15.Leigh RJ, Robinson DA, Zee DS. 1981. A hypothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann. NY Acad. Sci. 374, 619–635. ( 10.1111/j.1749-6632.1981.tb30906.x) [DOI] [PubMed] [Google Scholar]
- 16.Colen CB, Ketko A, George E, Van Stavern GP. 2008. Periodic alternating nystagmus and periodic alternating skew deviation in spinocerebellar ataxia type 6. J. Neuroophthalmol. 28, 287–288. ( 10.1097/WNO.0b013e318183bf5a) [DOI] [PubMed] [Google Scholar]
- 17.Liao K, Walker MF, Joshi A, Reschke M, Leigh RJ. 2008. Vestibulo-ocular responses to vertical translation in normal human subjects. Exp. Brain Res. 185, 553–562. ( 10.1007/s00221-007-1181-z) [DOI] [PubMed] [Google Scholar]
- 18.Ramat S, Straumann D, Zee DS. 2005. Interaural translational VOR: suppression, enhancement, and cognitive control. J. Neurophysiol. 94, 2391–2402. ( 10.1152/jn.01328.2004) [DOI] [PubMed] [Google Scholar]
- 19.Liao K, Walker MF, Joshi A, Reschke M, Wang Z, Leigh RJ. 2008. A reinterpretation of the purpose of the translational vestibulo-ocular reflex in human subjects. Prog. Brain Res. 171, 295–302. ( 10.1016/S0079-6123(08)00643-2) [DOI] [PubMed] [Google Scholar]
- 20.Rushton DN. 1989. Geometrical optics of the retinal image stabilisation device. J. Neurol. Neurosurg. Psychiatry 52, 137–138. ( 10.1136/jnnp.52.1.137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao K, Schneider RM, Yaniglos SS, Bertolini G, Glendinning P, Sawyer RN Jr, Reschke M, Leigh RJ. 2011. Visual and vestibular determinants of the translational vestibulo-ocular reflex. Ann. NY Acad. Sci. 1233, 263–270. ( 10.1111/j.1749-6632.2011.06148.x) [DOI] [PubMed] [Google Scholar]
- 22.Lacour M, Helmchen C, Vidal PP. 2016. Vestibular compensation: the neuro-otologist's best friend. J. Neurol. 263, 54–64. ( 10.1007/s00415-015-7903-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zee DS, Preziosi TJ, Proctor LR. 1982. Bechterew's phenomenon in a human patient. Ann. Neurol. 12, 495–496. ( 10.1002/ana.410120519) [DOI] [PubMed] [Google Scholar]
- 24.Young LR, Oman CM. 1969. Model for vestibular adaptation to horizontal rotation. Aerosp. Med. 40, 1076–1080. [PubMed] [Google Scholar]
- 25.Malcomb R, Jones GM. 1970. A quantitative study of vestibular adaptation in humans. Acta Otolaryngol. 70, 126–135. ( 10.3109/00016487009181867) [DOI] [PubMed] [Google Scholar]
- 26.Furman JM, Hain TC, Paige GD. 1989. Central adaptation models of the vestibulo-ocular and optokinetic systems. Biol. Cybern. 61, 255–264. ( 10.1007/BF00203172) [DOI] [PubMed] [Google Scholar]
- 27.Roberts DC, Marcelli V, Gillen JS, Carey JP, la Santina CC, Zee DS. 2011. MRI magnetic field stimulates rotational sensors of the brain. Curr. Biol. 21, 1635–1640. ( 10.1016/j.cub.2011.08.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antunes A, Glover PM, Li Y, Mian OS, Day BL. 2012. Magnetic field effects on the vestibular system: calculation of the pressure on the cupula due to ionic current-induced Lorentz force. Phys. Med. Biol. 57, 4477–4487. ( 10.1088/0031-9155/57/14/4477) [DOI] [PubMed] [Google Scholar]
- 29.Ward BK, Roberts DC, la Santina CC, Carey JP, Zee DS. 2015. Vestibular stimulation by magnetic fields. Ann. NY Acad. Sci. 1343, 69–79. ( 10.1111/nyas.12702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glover PM, Li Y, Antunes A, Mian OS, Day BL. 2014. A dynamic model of the eye nystagmus response to high magnetic fields. Phys. Med. Biol. 59, 631–645. ( 10.1088/0031-9155/59/3/631) [DOI] [PubMed] [Google Scholar]
- 31.Jareonsettasin P, Otero-Millan J, Ward BK, Roberts DC, Schubert MC, Zee DS. 2016. Multiple time courses of vestibular set-point adaptation revealed by sustained magnetic field stimulation of the labyrinth. Curr. Biol. 26, 1359–1366. ( 10.1016/j.cub.2016.03.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raphan T, Matsuo V, Cohen B. 1979. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp. Brain Res. 35, 229–248. ( 10.1007/BF00236613) [DOI] [PubMed] [Google Scholar]
- 33.Fetter M, Zee DS, Proctor LR. 1988. Effect of lack of vision and of occipital lobectomy upon recovery from unilateral labyrinthectomy in rhesus monkey. J. Neurophysiol. 59, 394–407. [DOI] [PubMed] [Google Scholar]
- 34.Jones GM, Berthoz A, Segal B. 1984. Adaptive modification of the vestibulo-ocular reflex by mental effort in darkness. Exp. Brain Res. 56, 149–153. [DOI] [PubMed] [Google Scholar]
- 35.Eggers SD, De PN, Walker MF, Shelhamer M, Zee DS. 2003. Short-term adaptation of the VOR: non-retinal-slip error signals and saccade substitution. Ann. NY Acad. Sci. 1004, 94–110. ( 10.1111/j.1749-6632.2003.tb00245.x) [DOI] [PubMed] [Google Scholar]
- 36.Galiana HL, Flohr H, Jones GM. 1984. A reevaluation of intervestibular nuclear coupling: its role in vestibular compensation. J. Neurophysiol. 51, 242–259. [DOI] [PubMed] [Google Scholar]
- 37.Olabi B, Bergquist F, Dutia MB. 2009. Rebalancing the commissural system: mechanisms of vestibular compensation. J. Vestib. Res. 19, 201–207. [DOI] [PubMed] [Google Scholar]
- 38.Fetter M, Zee DS. 1988. Recovery from unilateral labyrinthectomy in rhesus monkey. J. Neurophysiol. 59, 370–393. [DOI] [PubMed] [Google Scholar]
- 39.McCabe BF, Ryu JH, Sekitani T. 1972. Further experiments on vestibular compensation. Laryngoscope 82, 381–396. ( 10.1288/00005537-197203000-00005) [DOI] [PubMed] [Google Scholar]
- 40.Haddad GM, Friendlich AR, Robinson DA. 1977. Compensation of nystagmus after VIIIth nerve lesions in vestibulo-cerebellectomized cats. Brain Res. 135, 192–196. ( 10.1016/0006-8993(77)91066-6) [DOI] [PubMed] [Google Scholar]
- 41.Earhart GM, Fletcher WA, Horak FB, Block EW, Weber KD, Suchowersky O, Jones GM. 2002. Does the cerebellum play a role in podokinetic adaptation? Exp. Brain Res. 146, 538–542. ( 10.1007/s00221-002-1238-y) [DOI] [PubMed] [Google Scholar]
- 42.Robinson FR, Straube A, Fuchs AF. 1993. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J. Neurophysiol. 70, 1741–1758. [DOI] [PubMed] [Google Scholar]
- 43.Hagstrom L, Hornsten G, Silfverskiold BP. 1969. Oculostatic and visual phenomena occurring in association with Wallenberg's syndrome. Acta Neurol. Scand. 45, 568–582. ( 10.1111/j.1600-0404.1969.tb01267.x) [DOI] [PubMed] [Google Scholar]
- 44.Takagi M, Tamargo R, Zee DS. 2003. Effects of lesions of the cerebellar oculomotor vermis on eye movements in primate: binocular control. Prog. Brain Res. 142, 19–33. ( 10.1016/S0079-6123(03)42004-9) [DOI] [PubMed] [Google Scholar]
- 45.Leech J, Gresty M, Hess K, Rudge P. 1977. Gaze failure, drifting eye movements, and centripetal nystagmus in cerebellar disease. Br. J. Ophthalmol. 61, 774–781. ( 10.1136/bjo.61.12.774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon SC, Robinson DA. 1985. An improved neural-network model for the neural integrator of the oculomotor system: more realistic neuron behavior. Biol. Cybern. 53, 93–108. ( 10.1007/BF00337026) [DOI] [PubMed] [Google Scholar]
- 47.Arnold DB, Robinson DA. 1997. The oculomotor integrator: testing of a neural network model. Exp. Brain Res. 113, 57–74. ( 10.1007/BF02454142) [DOI] [PubMed] [Google Scholar]
- 48.Miri A, Daie K, Arrenberg AB, Baier H, Aksay E, Tank DW. 2011. Spatial gradients and multidimensional dynamics in a neural integrator circuit. Nat. Neurosci. 14, 1150–1159. ( 10.1038/nn.2888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cullen KE, Minor LB, Beraneck M, Sadeghi SG. 2009. Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. J. Vestib. Res. 19, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]