Abstract
If a visual object of interest suddenly starts to move, we will try to follow it with a smooth movement of the eyes. This smooth pursuit response aims to reduce image motion on the retina that could blur visual perception. In recent years, our knowledge of the neural control of smooth pursuit initiation has sharply increased. However, stopping smooth pursuit eye movements is less well understood and will be discussed in this paper. The most straightforward way to study smooth pursuit stopping is by interrupting image motion on the retina. This causes eye velocity to decay exponentially towards zero. However, smooth pursuit stopping is not a passive response, as shown by behavioural and electrophysiological evidence. Moreover, smooth pursuit stopping is particularly influenced by active prediction of the upcoming end of the target. Here, we suggest that a particular class of inhibitory neurons of the brainstem, the omnipause neurons, could play a central role in pursuit stopping. Furthermore, the role of supplementary eye fields of the frontal cortex in smooth pursuit stopping is also discussed.
This article is part of the themed issue ‘Movement suppression: brain mechanisms for stopping and stillness’.
Keywords: smooth pursuit, prediction, inhibition, omnipause neurons, supplementary eye fields
1. Smooth pursuit
The repertoire of conjugate eye movements is composed of saccades and smooth pursuit. Saccades allow rapid refixation of the visual axis between different stationary objects. They can be initiated voluntarily and occur at a rate of approximately two to three times per second during spontaneous exploration of the environment. However, if a visual object of interest suddenly starts to move, its image will move on the retina if the eyes remain stationary. In reaction to image motion, a smooth pursuit response is initiated that stabilizes the stimulus on the fovea, where visual acuity is the highest. The smooth pursuit system can be conceptualized as a negative-feedback system: if retinal image motion increases, the eyes accelerate and their velocity increases in order to match target velocity during the initiation period [1]. While saccades can be initiated without a stimulus, it is not possible to initiate smooth pursuit without a stimulus that is visible and moving.
The time course of smooth pursuit can be divided into three periods: initiation, sustained pursuit and pursuit end. During initiation, eye velocity rapidly increases and can briefly overshoot target velocity (figure 1a). The velocity overshoot at the end of this period is part of an oscillatory process that has a frequency of approximately 3–4 Hz. During sustained pursuit, oscillation amplitude progressively decays, and eye velocity settles around target velocity. The ratio of eye velocity to target velocity (or gain) is approximately 0.9–1.0 for target velocities less than 20 deg s−1. As a result, the primary sensory stimulus (retinal slip velocity, the motion of the image relative to the retina) is strongly reduced. The continued maintenance of sustained pursuit therefore suggests a process of integral control, for example using efference copy [1,4]. During smooth pursuit, saccades frequently occur in order to reduce the positional error between the visual axis and the target (figure 1a). Saccades are most often observed at the time of pursuit initiation. However, they also occur during sustained pursuit if pursuit gain is too high (example presented on figure 1a) or too low. Although saccades and smooth pursuit are outcomes of different oculomotor subsystems, they can aim at the same target and a synergy exists between them, allowing optimal orientation [5]. If the pursued target stops, eye velocity rapidly decays following an approximately exponential time course (figure 1b). The aim of this paper is to further discuss smooth pursuit stopping and to suggest a possible neuronal mechanism for its control.
Figure 1.
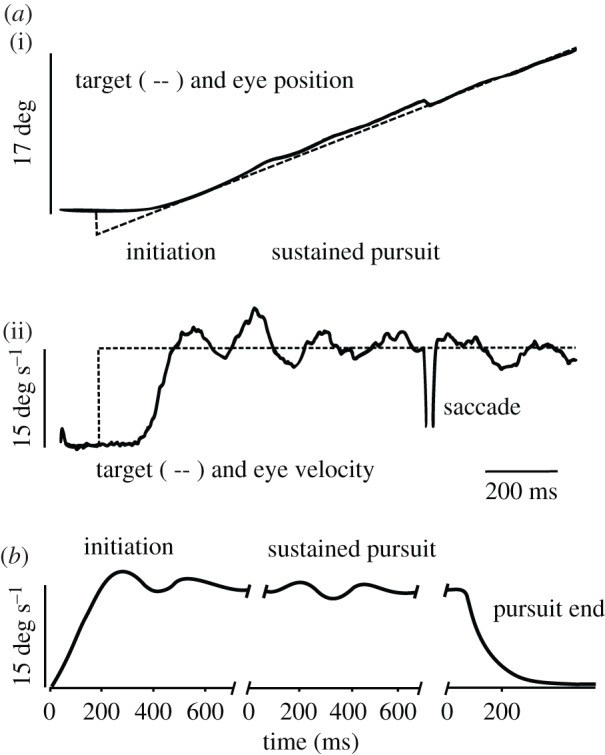
Characteristics of smooth pursuit. (a) (i): example of smooth pursuit in the Rhesus monkey. The target (dashed line) was initially stationary, then stepped to a 3° eccentric position and started to move at a constant velocity (15 deg s−1). This particular stimulus is referred to as a Rashbass step-ramp target motion [2]. Adapted from [3]. (ii): target and eye velocity for the same trajectory. After a delay period of approximately 120 ms, eye velocity rapidly increased and overshot the target velocity (initiation period). Afterwards, during sustained pursuit, oscillations of eye velocity around the target velocity were also observed but with a decaying amplitude. A saccade was triggered to reduce the accumulated error in position. Adapted from [3]. (b) Smooth pursuit in humans shows the same oscillations as observed in monkeys. At the end of the pursuit response, eye velocity rapidly decayed with an approximately exponential time course. Note the absence of overshoot and oscillations at the end of pursuit. Modified from Robinson et al. [1].
2. Neural control of smooth pursuit
In recent years, the knowledge of the neural control of smooth pursuit has rapidly increased. The input to smooth pursuit comes from the motion processing pathway [6,7] consisting of the middle temporal area (MT) [8–10] and medial superior temporal area (MST) [11] that together determine the speed and direction of image motion (see figure 2 for the location of these areas on a schematic lateral view of a Rhesus monkey brain). Both MT and MST project to the frontal eye field (FEF) of the frontal lobe (Brodmann area 8). The FEF contains saccade-related neurons located in the rostral bank of the arcuate sulcus [13], and smooth pursuit-related neurons reside in the floor and posterior bank of that sulcus [14]. Electrical stimulation of the rostral bank of the arcuate sulcus evokes saccades, whereas stimulation of the fundus or posterior bank evokes smooth eye movements. The latter part of the FEF will be referred to as the ‘FEF-SEM’ (FEF for smooth eye movements). It has been shown that activity of FEF-SEM neurons is sufficient to drive a movement [15]. Moreover, if the FEF-SEM is lesioned, smooth pursuit is impaired [16,17]. However, the FEF is not simply a motor relay structure that transforms visual motion signals into an eye movement command. Indeed, lesions of the FEF-SEM impair anticipatory initiation and prediction during pursuit, suggesting that it plays a more cognitive role in pursuit [18]. Accordingly, neurons in the FEF-SEM are active during smooth pursuit based on remembered target information [19]. In addition, the selection process that choses one target to pursue among others is probably achieved by the FEF. If two visual targets are moving concurrently and one must be chosen, electrical stimulation of FEF-SEM biases the choice towards one of the two targets [20].
Figure 2.
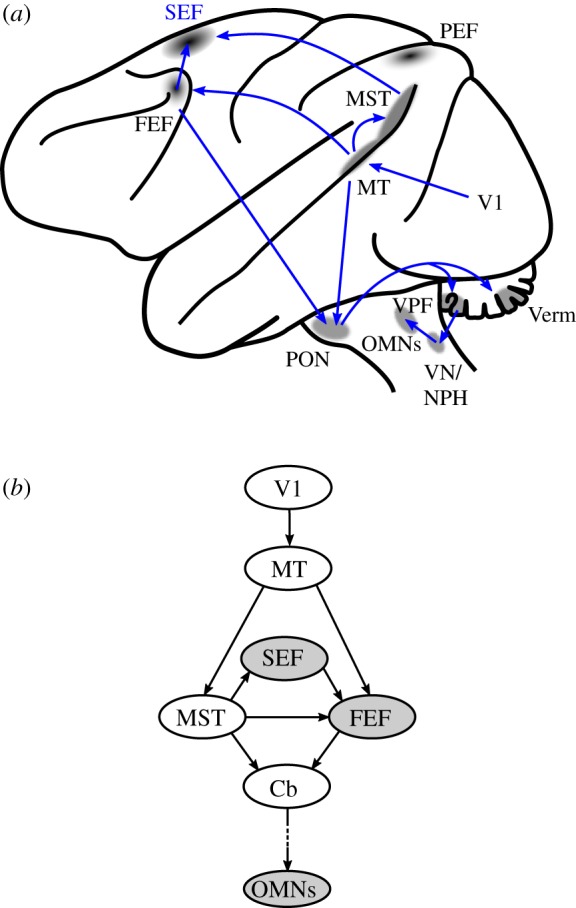
The smooth pursuit pathways in the Rhesus monkey. (a) Lateral view of the left hemisphere with areas involved in motion processing and smooth pursuit preparation. FEF, frontal eye field; MT, middle temporal area; MST, medial superior temporal area; PEF, parietal eye fields; OMNs: oculomotor motoneurons; SEF, dorsomedial frontal cortex; V1: primary visual area; PON, pontine nuclei; VPF, flocculus and ventral paraflocculus; Verm, oculomotor vermis; VN, vestibular nuclei; NPH, nucleus prepositus hypoglossi. Adapted from [12]. For clarity, connections of the PEF with FEF and SEF are not shown and are not essential for the argument presented in this paper. Neurons in the vermis could project to OMNs through the caudal fastigial nucleus. These projections are not shown for the sake of clarity. (b) Simplified schematic diagram of the flow of neural signals in pursuit areas from the visual cortex to premotor neurons. Cb, cerebellum. The role of the shaded areas will be further discussed in this paper.
The FEF is not the only frontal area devoted to smooth pursuit control. The supplementary eye field of the dorsomedial frontal cortex (SEF), located in area 6aβ at the rostral pole of the supplementary motor area, is another cortical region involved in saccade control [21,22] and smooth pursuit [23–25]. Smooth pursuit eye movements can be electrically evoked from the SEF in anaesthetized monkeys [26]. In awake animals smooth eye movements can also be evoked electrically but only if there is a strong expectation of upcoming target motion [27]. The SEF shares reciprocal and bilateral connections with the FEF [28], but the relative role of the SEF and FEF in smooth pursuit control is still a matter of speculation. However, the FEF seems to be more directly connected with visual structures of the brain than the SEF. This has led to the hypothesis that the FEF sets the gain of the visuomotor transformation between visual motion signals and eye movement commands [29]. But the connectivity of the SEF with the rest of the pursuit system is different. The dorsomedial region of MST (MSTd) [11] is part of the motion processing system [30], and sends direct projections to the SEF but not to the FEF [31]. Area MT projects to the FEF directly, but not to the SEF [28,31]. Moreover, it has been shown in a memory-based smooth pursuit task that more SEF neurons are likely to encode visual memory of target motion or memory of an instructional cue than FEF neurons, where movement preparation neurons are more frequent [32,33]. Anatomically, the SEF is better connected to prefrontal structures that are probably involved in working memory [31]. In summary, the SEF appears more involved in the planning of smooth pursuit than in generating oculomotor control signals that drive eye velocity and acceleration. Consistent with a role in planning, the SEF is involved in prediction and anticipation of future target motion [24,34] (see below further discussion of this topic). Finally, smooth pursuit-related neuronal activity has been found in the parietal cortex (labelled ‘PEF’ in figure 2). Electrical stimulation of the parietal cortex can also evoke smooth eye movements [35]. It is usually accepted that the parietal cortex could play a role in differentiating retinal motion caused by an external stimulus from retinal motion caused by a movement of the eye itself. A neural representation of the position of the eye in the orbit has also been found in that region (see [36]).
From the cortical afferents described above, the cerebellum and brainstem pathways progressively elaborate a motor command that drives the eyes at the appropriate velocity (see reviews in [12,36,37]; figure 2). Cortical areas related to smooth pursuit project to the pontine nuclei (labelled PON in figure 2), which then project to the flocullus/paraflocullus complex (VPF) and the posterior vermis (Verm) of the cerebellum. Pursuit areas of the cerebellum project to ocular motoneurons (OMNs) through the medial vestibular nuclei (VN) and nucleus prepositus hypoglossi (NPH) [38,39].
These cortico-subcortical connections have been well established on the basis of anatomical and physiological studies. However, recent research has put the emphasis on the synergy between saccades and smooth pursuit in gaze orientation [12,40], hypothesizing that certain groups of neurons participate in the control of both. During a saccade, OMNs show a high-frequency transient discharge that accelerates the eyes. Afterwards, a sustained activity at a lower frequency is observed that allows the eyes to fixate at a new location. The high-frequency activity of motoneurons originates in premotor excitatory burst neurons (EBNs) that project monosynaptically to them [41,42]. EBNs encode saccade dynamics [43], and are kept under inhibitory control by omnipause neurons (OPNs) of the nucleus raphe interpositus (RIP) [41,44]. The inhibition of the OPNs on EBNs ceases about 10–20 ms before saccade onset, after which burst neurons are released and the saccade is executed. Figure 3a shows the activity of a typical OPN recorded in the Rhesus monkey during small horizontal saccades. During the initial fixation period, neuronal activity was high and constant but dropped to zero (‘paused’) during saccades. During the next fixation period, the activity of OPNs returns to a steady level.
Figure 3.
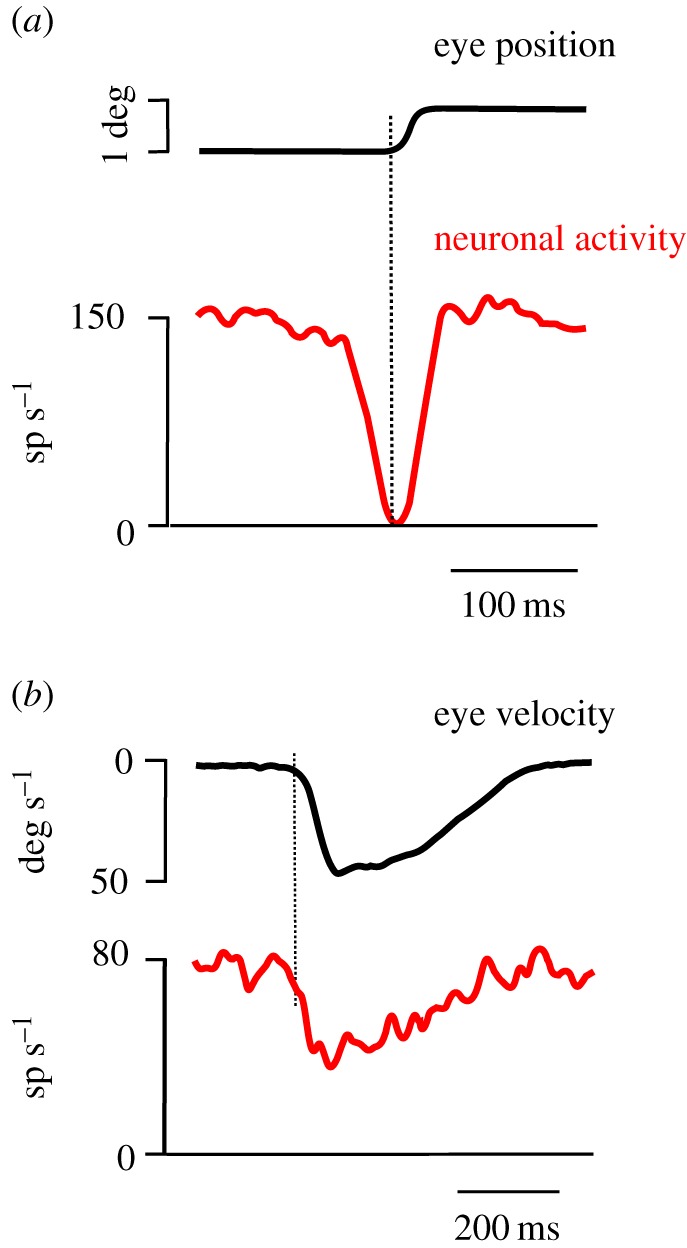
Activity of omnipause neurons (OPNs) during saccades and smooth pursuit. (a) Average eye position and spike density (neuronal activity) of a single OPN during saccades (n = 10 trials). Note that the activity abruptly started to decrease approximately 15 ms before saccade onset and recovered 5 ms after saccade end. During saccades, no activity was observed. (b) Average eye velocity and spike density during pursuit trials without saccades in a single OPN (n = 14 trials). The spike density decreased around the time of pursuit onset. However, the activity did not drop to zero (‘pause’) as observed during saccades. Reproduced with permission from Missal & Keller [45].
Recently, ‘saccadic’ premotor burst neurons have been shown to be active during smooth pursuit [40,46]. Moreover, some OPNs reduce their activity during pursuit to a level between the high frequency observed during fixation and the absence of activity during saccades (figure 3b). Therefore, some OPNs could also play an inhibitory role in smooth pursuit [45]. These neurons could be involved in terminating both saccades and smooth pursuit to allow a swift transition from an ongoing movement to stable fixation.
3. Stopping smooth pursuit
Stopping target motion during smooth pursuit causes eye velocity to exponentially decay after a latency of ≈120 ms [47–52] (figure 1b). If the smooth pursuit system were linear, then pursuit offset should exhibit the same dynamics as pursuit onset. However, the velocity overshoot and oscillations observed when pursuit begins are absent when pursuit stops, suggesting that fixation is not pursuit at zero velocity [1]. Stopping pursuit could be interpreted as an exponential transition from sustained pursuit to fixation of a new stationary target.
It appears that the time constant of the velocity decay during pursuit offset depends of the experimental conditions used. There are different ways to stop smooth pursuit: the target might either come to rest and remain immobile for a while (as depicted in figure 1b) or vanish or be stabilized on the retina. If a moving target vanishes after a short period of pursuit, eye velocity decays towards zero with a time constant of 0.10–0.15 s in 400 to 500 ms. But if the target is stabilized on the fovea after a short period of pursuit, eye velocity decays towards zero with a longer time constant of 400–800 ms. The mere presence of a visual target changes the dynamics of pursuit offset probably because an internal positive feedback loop remains active in this condition, whereas it is disconnected if the target vanishes [49].
Several alternative categories of smooth pursuit termination models have been proposed to explain these observations. The first category of models postulates that a different system, the fixation system, is used to stop smooth pursuit [1,48,50]. In these models, pursuit termination is characterized as a transition between actively pursuing a moving object and holding gaze steady. The second category of models posits that a single system is responsible for oscillations during pursuit initiation, and exponential decay during pursuit offset [52,53]. These models are based on the observation that the visuomotor processing driving smooth pursuit is subject to online gain control. In support of this, it has been shown that a brief sinusoidal perturbation of target velocity caused a larger deviation of eye velocity during pursuit than during fixation [54]. Furthermore, oscillations during pursuit offset can be suppressed theoretically by setting the visuomotor gain element in the smooth pursuit models to zero. This suggests that pursuit onset and offset rely on the same gain adjustment in the visuomotor pathway that translates target motion into an eye movement. There is evidence that such online gain control is exerted by the frontal eye fields [20,29].
Although the primary stimulus for smooth pursuit is retinal motion, the position of the target relative to the fovea during pursuit offset could also play also a significant role [49,55,56]. This suggests that the smooth pursuit system is not only a servomechanism that reduces target motion on the retina but in particular circumstances it can also use target position. Although the details of these modelling approaches are beyond the scope of this paper, they all converge on one point: offset dynamics do not reflect properties of the eye plant, but change with stimulus condition and context.
4. Role of prediction
Smooth pursuit is not only a reflexive motion-driven system. Prediction plays a major role before pursuit initiation, during sustained pursuit and in pursuit termination. Before the expected motion of a stationary target, smooth eye velocity of a few degrees per second that anticipates target motion is often observed, despite the target being stationary [57–59]. This anticipatory pursuit is an important aspect of motor control, because it compensates for delays present in the sensorimotor pursuit system of approximately 100 ms. For a target moving at 20 deg s−1, this corresponds to a 2° displacement. This much retinal motion could compromise visual acuity, since it could easily displace the target from the fovea, given that the fovea subtends less than 1° of visual angle. Anticipatory pursuit minimizes this error at pursuit initiation. This movement relies upon both a perception of elapsed time [60,61] and spatial information that can be provided in advance by visual cues [34,62].
During sustained pursuit, prediction also occurs if the target transiently disappears. This experimental condition mimics what is often experienced in nature: a pursued target (an animal, a soccer ball) is transiently occluded by another object. During transient target disappearance, eye velocity decays to a level approximately two-thirds that of the previous pursuit response [63]. This high residual velocity is due to expectation of target reappearance at the end of the delay period. Without the expectation that the target will reappear, eye velocity rapidly returns to zero.
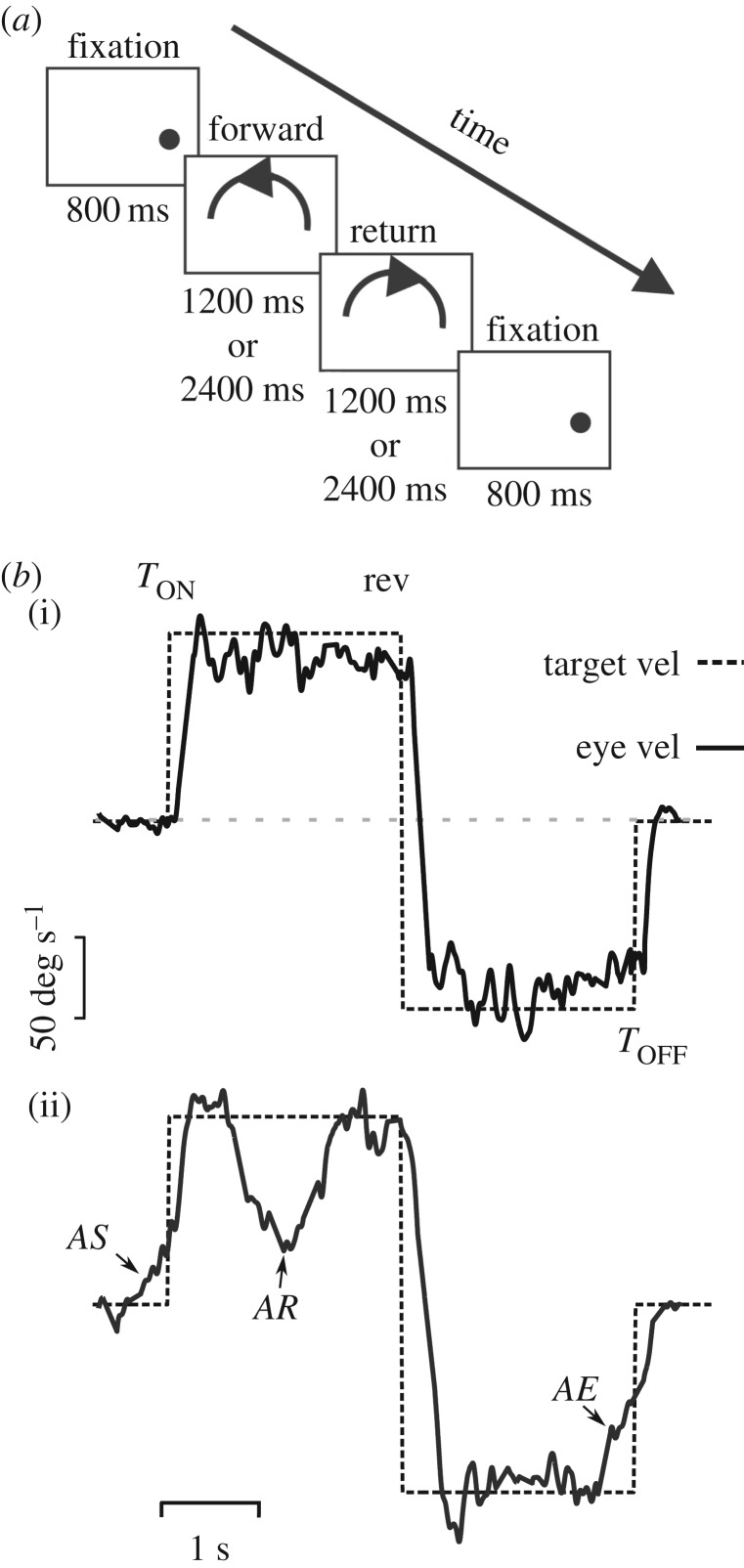
Another type of prediction during sustained pursuit is observed when a target moves with a periodic trajectory (see review in [64]). In this condition, there is initially a phase lag between the eye and the target because of sensorimotor processing delays, but this phase lag is reduced sometimes in less than a cycle. Often the eye changes direction before target reversal. In pursuit of a periodic waveform, it is particularly efficient to use previous information gathered during a preceding cycle of the signal. However, in a complex environment, moving objects rarely move periodically: instead, they constantly change their speed and direction, either predictably (a car announcing a lane change on the highway) or unpredictably (an erratically flying Drosophila). Predictable changes of trajectory are frequent given that some cues can be used for prediction. In a laboratory environment, this situation can be tested by having a pursuit target change trajectory. Figure 4a shows a simple pursuit paradigm where a target follows a curved trajectory and then suddenly reverses direction after a period of either 1200 or 2400 ms randomly [65]. Figure 4b(i) shows eye velocity of a human subject pursuing this stimulus for the first time. The trajectory of the target lasted for 2400 ms during both ways. The second example (figure 4b(ii)) shows that after just a few repetitions, the subject anticipated target motion onset (AS), reversal (AR) and end (AE). During AS, eye velocity increased in order to reduce retinal slip at target motion onset. During AR, given that trajectory reversal could occur after either 1200 or 2400 ms, eye velocity started to decay around the time of the shortest duration (1200 ms) and dropped from 150 to 20 deg s−1 before the direction change. Eye velocity decayed at a time appropriate for the short 1200 ms reversal, because the current long trial (2400 ms) had been preceded by the short one, which was stored in short-term memory. This example clearly shows that when needed, prediction can override a strong opposing motion signal [65]. Note that non-motion cues like a sound can also be used to predict a reversal of trajectory [66]. It is thought that smooth pursuit direction change is a combination of terminating pursuit in one direction and initiating pursuit in another, implemented using a short-term velocity memory that, when released, overrides the opposing retinal motion signal [64]. During AE, prediction also operates to decrease eye velocity in expectation of a target stopping [1,59] (figure 4b). The time course of eye deceleration depends on the previously experienced target trajectory, and is not under voluntary control [67]. As do other types of prediction, anticipatory termination of smooth pursuit relies on both spatial (e.g. approaching limit of the test screen) and temporal information. It is a preprogrammed response that is quickly recalibrated if a target following a repeated trajectory suddenly changes where or when it will stop [68,69].
Figure 4.
Prediction during smooth pursuit. (a) Schematic of the experimental paradigm. After an initial fixation period of 800 ms, the target moved along the forward path for either 1200 or 2400 ms unpredictably (labelled forward). Afterwards, the target retraced its movement back to the starting position (labelled return). The trial ended with a final fixation period. This paradigm allows us to examine anticipation of target motion onset, reversal and end within the same trial. The influence of spatial cues was reduced by randomizing the initial target position. Reproduced with permission from de Hemptinne et al. [65]. (b) Target velocity (dashed curve) and eye velocity (continuous curve) during pursuit of the stimulus depicted in (a). (i): single pursuit trial without significant anticipation. (ii): single pursuit trial with three different anticipatory responses: AS, anticipatory pursuit start before target motion onset (TON); AR, anticipatory deceleration in expectation of a target reversal 1200 ms after motion onset; AE, anticipatory deceleration before the target ends (TOFF). Rev, reversal of the trajectory. Reproduced with permission from de Hemptinne et al. [65].
In general, motor response timing can be either explicit or implicit. Explicit timing is defined as voluntarily keeping track of elapsed time [70]. For instance, without watching a clock, you can decide to stop reading the present article in approximately 1 min (however unlikely it is that this will happen!). Explicit temporal estimation depends on the integrity of nigrostriatal projections in the basal ganglia [71]. It is affected by experimental dopaminergic manipulations and is impaired in Parkinson's disease. Implicit timing is also important for motor control. For instance, catching a flying ball is done without an explicit estimate of the time that the ball will take to hit your hand. Predictive smooth pursuit termination also uses implicit timing. Indeed, we cannot control the onset of the predictive component of smooth pursuit deceleration, even if told to continue to pursue [68]. Implicit timing in predictive pursuit termination was tested with the same paradigm of trajectory reversal as described in figure 3 but in untreated early Parkinson's disease patients [65]. Interestingly, although they anticipated less often (probably an oculomotor form of hypokinesia), the timing of their anticipatory pursuit was not significantly different from that in age-matched controls. This negative result supports the hypothesis that implicit timing does not depend on the integrity of nigrostriatal projections.
5. Countermanding smooth pursuit
In a changing environment, it is often necessary to cancel a planned movement. Schematically, imagine yourself waiting in your car at a traffic light. The traffic light goes from red to green (go signal) and you prepare to go ahead. However, sometimes and unexpectedly the red light reappears (stop signal). In this case, you have to cancel your previously prepared motor command to accelerate the car. The behaviour and physiology of how we respond to events such as the faulty traffic light are researched using the countermanding paradigm, a laboratory test of the ability to suppress a preplanned motor response. Several variants of the countermanding paradigm have been developed [72]. In the oculomotor domain, cancelling a saccade is a particularly demanding task that requires integrity of frontal pathways [73]. Beyond a fixed delay between when the go signal appears, and when the stop signal is issued, a saccade can no longer be cancelled (e.g. you accelerate the car although the traffic light is red). Kornylo et al. [74] compared the ability to stop saccades and smooth pursuit with a modified countermanding paradigm. They suggested that the same inhibitory neurons could be used for stopping saccades and smooth pursuit although some details of the neural processes involved are different. Also inspired by the saccadic countermanding paradigm, Jarrett & Barnes [75] showed that anticipatory pursuit could be stopped at will with an auditory cue. Presenting a stop signal during anticipatory pursuit at various times during 20% of the trials, they found that the internally generated anticipatory pursuit response can be halted voluntarily, even though anticipatory smooth pursuit cannot be initiated at will.
6. Neural control of smooth pursuit stopping
Historically, most of the research on smooth pursuit has been devoted to understanding neural processes that convert visual motion signals arising at the retina to a command initiating pursuit [7,76–78]. Little work has been devoted to how smooth pursuit is terminated and, as already mentioned, the mechanism guiding this process does more than cause the eyes to simply drift to a stop when a target stops moving. Indeed, in the Rhesus monkey, premotor signals suggesting an active control of smooth pursuit offset have been recorded: the firing rate of motoneurons decreases with a time course similar to eye velocity decay during pursuit offset [79,80] and gaze-velocity Purkinje cells in the flocculus of the cerebellum have a temporal discharge profile that correlates with eye velocity during the same period [53]. In sum, neural activity is generated throughout the period of stopping in the final pursuit pathway. These neurophysiological observations confirm that the offset of pursuit is not a passive delay, because it results from a decrease in the neural drive to eye muscles. Pursuit offset seems to be actively controlled.
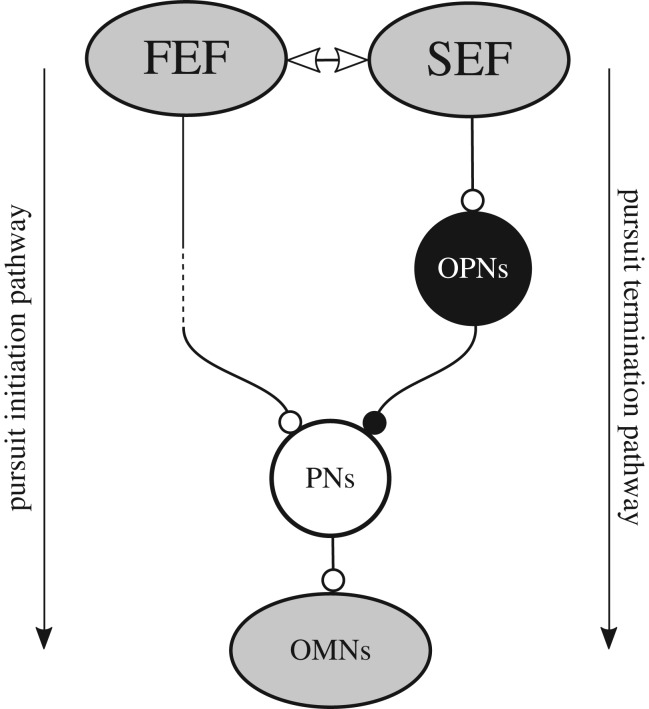
We suggest that the active control of pursuit offset could be exerted by OPNs. As shown in figure 5a, stimulation of OPNs during sustained pursuit (red curves) evoked a pronounced deceleration of the eyes that lasted for the duration of the stimulation train (represented by the shaded area; see [45] for details). How could OPNs' activation evoke a reduction of eye velocity during pursuit? As shown above (figure 2), the final premotor pathway for smooth pursuit eye movements involves the medial VN and the NPH. Premotor neurons in these structures encode eye and head velocity during pursuit [38]. Some additional neurons located in the paramedian pontine reticular formation and receiving input from the cerebellar vermis could also be involved (not shown; see [45] for details). Altogether, premotor pursuit neurons projecting to OMNs will be referred to as ‘PNs’. We suggested that OPNs could inhibit PNs. Indeed, OPNs' activity decreases during pursuit, and stimulation of these neurons evokes a reduction of pursuit eye velocity in all directions [46]. However, to the best of our knowledge, there is no demonstrated anatomical or physiological evidence for a direct connection between OPNs and PNs.
Figure 5.
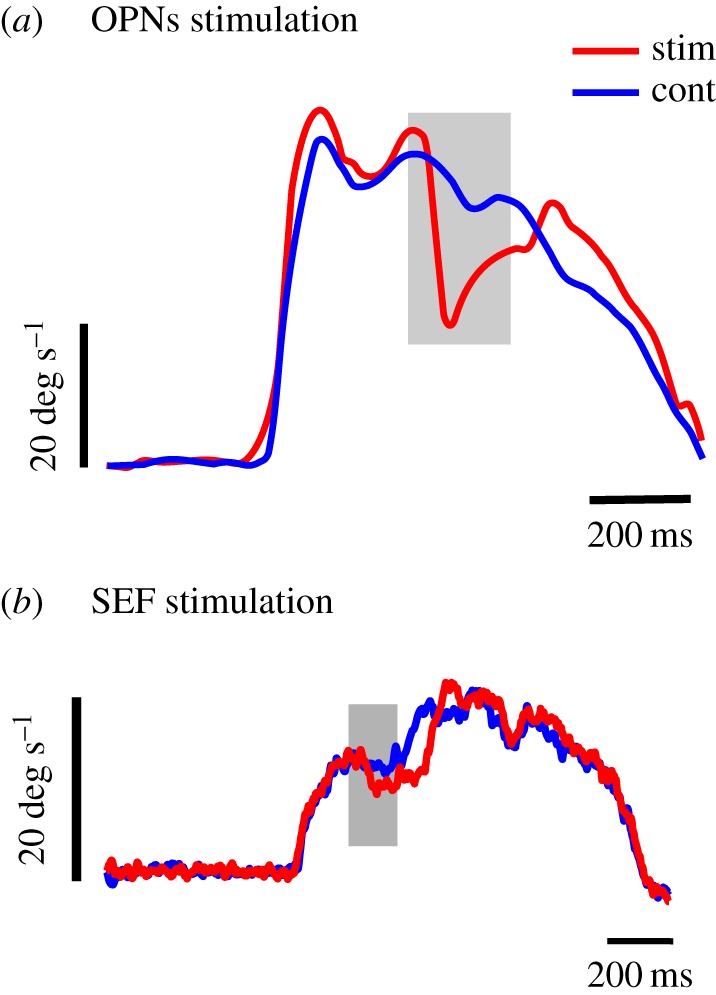
Smooth pursuit inhibition. (a) Smooth pursuit deceleration induced by electrical stimulation in the OPNs’ region (15 µA, 400 Hz) during the period indicated with a grey rectangle. Average eye velocity during trials without stimulation (blue trace; n = 8) and during electrical stimulation (n = 9; red trace). Redrawn from Missal & Keller [45]. (b) Smooth pursuit deceleration induced by electrical stimulation in the SEF region (n = 10 trials, 50 µA, 200 Hz) during the period indicated with a grey rectangle (S. J. Heinen 2017, personal communication). Same conventions as in (a).
What cortical structure could exert control over the timing of OPNs' activity during pursuit? Our work provides evidence that the timing of smooth pursuit initiation and termination is under the control of the SEF in the Rhesus monkey [24,34]. The activity of pursuit initiation neurons in the SEF builds up and reaches a peak that occurs within ±500 ms of target motion initiation. SEF pursuit termination neurons exhibit a similar profile of activity, but peak at around ±500 ms of target motion offset. This pattern of activity suggests that these SEF cells are involved in starting and stopping pursuit at a time appropriate for predictable target motion onset and offset. SEF pursuit initiation neurons could participate in movement initiation through the FEF and PNs, whereas termination neurons could activate OPNs and slow down pursuit. Indeed, Shook et al. [81] have shown anatomically that the SEF projects to the nucleus RIP of the brainstem that contains OPNs. According to Büttner-Ennever & Büttner. [41], the FEF also projects to the RIP, though this has not been confirmed by other investigators [28,31]. Therefore, it seems that the SEF has a preferential direct access to OPNs. Consistent with SEF projections to the OPNs, electrical microstimulation in that structure during sustained pursuit could also cause the eyes to decelerate (figure 5b; from S. J. Heinen 2016, unpublished data). Another important result comes from the work of Fukushima et al. [33], who found that a large proportion (24%) of cells in the SEF are active when trained monkeys must refrain from performing smooth pursuit in a go/no-go task (see also [82] for a similar observation using a different paradigm). Although these ‘no-go’ neurons appear to be complex instruction-related neurons, this finding supports the hypothesis of a top–down inhibitory role of the SEF. Moreover, comparison between inhibition during OPNs’ (figure 5a) and SEF stimulation (figure 5b) suggests that these neuronal structures belong to the same inhibitory pathway.
7. Conclusion
Both smooth pursuit initiation and termination depend on image motion on the retina. However, image motion on the retina is not enough to explain all the characteristics of smooth pursuit, particularly during movement termination where prediction can override or ‘neglect’ retinal motion information. Stopping smooth pursuit is a finely tuned behaviour, and not simply stopping a premotor drive. Figure 6 proposes a schematic representation of our current hypothesis about smooth pursuit termination. Towards the end of the movement, SEF ‘termination’ neurons become active [24]; our suggestion is that these neurons, providing a hypothetical inhibitory pathway from SEF via OPNs to the premotor neurons, are probably responsible for smooth pursuit termination by inhibition of PNs. This suggests that the control of ‘stopping movement’ is as important as the control of ‘initiating movement’. Actually, this right of ‘veto’ is particularly well illustrated in smooth pursuit. Indeed, we cannot initiate smooth pursuit at will…but we can stop it at will.
Figure 6.
Hypothetical neuronal pathway for smooth pursuit stopping. The FEF projects indirectly (dashed line) to premotor neurons driving pursuit (PNs) that activates OMNs. The SEF interacts with the FEF during anticipation and pursuit initiation (double-headed arrow). Pursuit stopping could occur when termination neurons of the SEF increase their activity. These neurons are connected with OPNs through a direct excitatory synapse (open circle). OPNs inhibit premotor pursuit neurons (PNs; filled circle). In this schematic diagram, the inhibitory connection between OPNs and PNs is hypothetical but is supported by results presented in figure 5. Shaded areas correspond to the shaded areas in figure 2b.
Acknowledgement
The authors thank Jeremy Badler for additional data analysis needed for the current paper.
Authors' contributions
M.M. drafted the manuscript. S.J.H. helped draft the manuscript. Both authors gave their final approval for publication.
Competing interests
The authors have declared that no competing interests exist.
Funding
This work was supported by a grant of the Fonds de la Recherche Scientifique (FRS-FNRS) ‘Crédit de recherche’ number 26043414 (www.fnrs.be/) attributed to M.M. M.M. was also supported by the Université catholique de Louvain. S.J.H. was supported by NIH grant no. 1R01 EY021286 and the Smith-Kettlewell Eye Research Institute. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Robinson DA, Gordon JL, Gordon SE. 1986. A model of the smooth pursuit eye movements system. Biol. Cybern. 55, 43–57. ( 10.1007/BF00363977) [DOI] [PubMed] [Google Scholar]
- 2.Rashbass C. 1961. The relationship between saccadic and smooth tracking eye movements. J. Physiol. 159, 326–338. ( 10.1113/jphysiol.1961.sp00681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchland MM, Lisberger SG. 2001. Experimental and computational analysis of monkey smooth pursuit eye movements. J. Neurophysiol. 86, 741–759. [DOI] [PubMed] [Google Scholar]
- 4.Young LR, Forster JD, van Houtte N. 1968. A revised stochastic sampled data model for eye tracking movements. In Fourth Ann NASA-University Conference on Manual Control, (NASA SP-192). National Aeronautics and Space Administration.
- 5.Orban de Xivry JJ, Lefèvre P. 2007. Saccades and pursuit: two outcomes of a single sensorimotor process. J. Physiol. 584, 11–23. ( 10.1113/jphysiol.2007.139881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisberger SG, Movshon JA. 1999. Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. J. Neurosci. 19, 2224–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lisberger SG. 2010. Visual guidance of smooth pursuit eye movements: sensation, action, and what happens in between. Neuron 66, 477–491. ( 10.1016/j.neuron.2010.03.027). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maunsell JH, Van Essen DC. 1983. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J. Neurophysiol. 49, 1127–1147. [DOI] [PubMed] [Google Scholar]
- 9.Albright TD. 1984. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 52, 1106–1130. [DOI] [PubMed] [Google Scholar]
- 10.Zeki S. 2015. Area V5—a microcosm of the visual brain. Front. Integr. Neurosci. 9, 21 ( 10.3389/fnint.2015.00021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desimone R, Ungerleider LG. 1986. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J. Comp. Neurol. 248, 164–189. ( 10.1002/cne.902480203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krauzlis RJ. 2004. Recasting the smooth pursuit eye movement system. J. Neurophysiol. 91, 591–603. ( 10.1152/jn.00801.2003) [DOI] [PubMed] [Google Scholar]
- 13.Bruce CJ, Goldberg ME. 1985. Primate frontal eye fields. I. Single neurons discharging before saccades. J. Neurophysiol. 53, 603–635. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb JP, MacAvoy MG, Bruce CJ. 1994. Neural responses related to smooth-pursuit eye movements and their correspondence with elicited smooth eye movements in the primate frontal eye field. J. Neurophysiol. 72, 1634–1653. [DOI] [PubMed] [Google Scholar]
- 15.Schoppik D, Nagel KI, Lisberger SG. 2008. Cortical mechanisms of smooth eye movements revealed by dynamic covariations of neural and behavioral responses. Neuron 58, 248–260. ( 10.1016/j.neuron.2008.02.015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating EG. 1991. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp. Brain Res. 86, 311–323. ( 10.1007/BF00228954) [DOI] [PubMed] [Google Scholar]
- 17.Keating EG, Pierre A, Chopra S. 1996. Ablation of the pursuit area in the frontal cortex of the primate degrades foveal but not optokinetic smooth eye movements. J. Neurophysiol. 76, 637–641. [DOI] [PubMed] [Google Scholar]
- 18.MacAvoy MG, Gottlieb JP, Bruce CJ. 1991. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb. Cortex 1, 95–102. ( 10.1093/cercor/1.1.95) [DOI] [PubMed] [Google Scholar]
- 19.Fukushima K, Yamanobe T, Shinmei Y, Fukushima J. 2002. Predictive responses of periarcuate pursuit neurons to visual target motion. Exp. Brain Res. 145, 104–120. ( 10.1007/s00221-002-1088-7) [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Lisberger SG. 2002. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. II. Relation to vector averaging pursuit. J. Neurophysiol. 87, 2700–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlag J, Schlag-Rey M. 1987. Evidence for a supplementary eye field. J. Neurophysiol. 57, 179–200. [DOI] [PubMed] [Google Scholar]
- 22.Schall JD. 1991. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J. Neurophysiol. 66, 530–558. [DOI] [PubMed] [Google Scholar]
- 23.Heinen SJ. 1995. Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp. Brain Res. 104, 357–361. ( 10.1007/BF00242022) [DOI] [PubMed] [Google Scholar]
- 24.Heinen SJ, Liu M. 1997. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis. Neurosci. 14, 853–865. ( 10.1017/S0952523800011597) [DOI] [PubMed] [Google Scholar]
- 25.Missal M, Heinen SJ. 2001. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J. Neurophysiol. 86, 2413–2425. [DOI] [PubMed] [Google Scholar]
- 26.Tian JR, Lynch JC. 1995. Slow and saccadic eye movements evoked by microstimulation in the supplementary eye field of the cebus monkey. J. Neurophysiol. 74, 2204–2210. [DOI] [PubMed] [Google Scholar]
- 27.Missal M, Heinen SJ. 2004. Supplementary eye fields stimulation facilitates anticipatory pursuit. J. Neurophysiol. 92, 1257–1262. ( 10.1152/jn.01255.2003). [DOI] [PubMed] [Google Scholar]
- 28.Huerta MF, Krubitzer LA, Kaas JH. 1987. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J. Comp. Neurol. 265, 332–361. ( 10.1002/cne.902650304) [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Lisberger SG. 2002. Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J. Neurophysiol. 87, 802–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celebrini S, Newsome WT. 1994. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J. Neurosci. 14, 4109–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huerta MF, Kaas JH. 1990. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J. Comp. Neurol. 293, 299–330. ( 10.1002/cne.902930211) [DOI] [PubMed] [Google Scholar]
- 32.Shichinohe N, Akao T, Kurkin S, Fukushima J, Kaneko CRS, Fukushima K. 2009. Memory and decision-making in the frontal cortex during visual motion-processing for smooth pursuit eye movements. Neuron 62, 717–732. (doi:0.1016/j.neuron.2009.05.010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukushima J, Akao T, Shichinohe N, Kurkin S, Kaneko CRS, Fukushima K. 2011. Neuronal activity in the caudal frontal eye fields of monkeys during memory-based smooth-pursuit eye movements: comparison with the supplementary eye fields. Cereb. Cortex 21, 1910–1924. ( 10.1093/cercor/bhq261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Hemptinne C, Lefèvre P, Missal M. 2008. Neuronal bases of directional expectation and anticipatory pursuit. J. Neurosci. 28, 4298–4310. ( 10.1523/jneurosci.5678-07.2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurylo DD, Skavenski AA. 1991. Eye movements elicited by electrical stimulation of area PG in the monkey. J. Neurophysiol. 65, 1243–1253. [DOI] [PubMed] [Google Scholar]
- 36.Ilg UJ, Thier P. 2008. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain Cogn. 68, 229–240. ( 10.1016/j.bandc.2008.08.014) [DOI] [PubMed] [Google Scholar]
- 37.Keller EL, Heinen SJ. 1991. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci. Res. 11, 79–107. ( 10.1016/0168-0102(91)90048-4) [DOI] [PubMed] [Google Scholar]
- 38.McFarland JL, Fuchs AF. 1992. Discharge pattern in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J. Neurophysiol. 68, 319–336. [DOI] [PubMed] [Google Scholar]
- 39.Evinger C. 1988. Extraocular motor nuclei: location, morphology, and afferents. In Neuroanatomy of the oculomotor system (ed. Büttner-Ennever JA.), pp. 81–118. Amsterdam, The Netherlands: Elsevier. [PubMed] [Google Scholar]
- 40.Missal M, de Brouwer S, Lefèvre P, Olivier E. 2000. Activity of mesencephalic vertical burst neurons during saccades and smooth pursuit. J. Neurophysiol. 83, 2080–2092. [DOI] [PubMed] [Google Scholar]
- 41.Büttner-Ennever JA, Büttner U. 1988. The reticular formation. In Neuroanatomy of the oculomotor system (ed. Büttner-Ennever JA.), pp. 119–176. Amsterdam, The Netherlands: Elsevier. [PubMed] [Google Scholar]
- 42.Strassman A, Highstein SM, McCrea RA. 1986. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J. Comp. Neurol. 249, 337–357. ( 10.1002/cne.902490303) [DOI] [PubMed] [Google Scholar]
- 43.Van Gisbergen JAM, Robinson DA, Gielen S. 1981. A quantitative analysis of generation of saccadic eye movements by burst neurons. J. Neurophysiol. 45, 417–442. [DOI] [PubMed] [Google Scholar]
- 44.Keller EL. 1974. Participation of medial pontine reticular formation in eye movement generation in monkey. J. Neurophysiol. 37, 316–332. [DOI] [PubMed] [Google Scholar]
- 45.Missal M, Keller EL. 2002. Common inhibitory mechanism for saccades and smooth-pursuit eye movements. J. Neurophysiol. 88, 1880–1892. [DOI] [PubMed] [Google Scholar]
- 46.Keller EL, Missal M. 2003. Shared brainstem pathways for saccades and smooth-pursuit eye movements. Ann. N.Y. Acad. Sci. 1004, 29–39. ( 10.1196/annals.1303.004) [DOI] [PubMed] [Google Scholar]
- 47.Mitrani MJ, Dimitrov G. 1978. Pursuit movements of a disappearing moving target. Vision Res. 18, 537–539. ( 10.1016/0042-6989(78)90199-2) [DOI] [PubMed] [Google Scholar]
- 48.Luebke AE, Robinson DA. 1988. Transition dynamics between pursuit and fixation suggest different systems. Vision Res. 28, 941–946. ( 10.1016/0042-6989(88)90103-4) [DOI] [PubMed] [Google Scholar]
- 49.Pola J, Wyatt HJ. 1997. Offset dynamics of human smooth pursuit eye movements: effects of target presence and subject attention. Vis Res 37, 2579–2595. ( 10.1016/S0042-6989(97)00058-8) [DOI] [PubMed] [Google Scholar]
- 50.Huebner WP, Leigh RJ, Seidman SH, Thomas CW, Billian CD, Disceenna AO, Dell'Osso L. 1992. Experimental tests of a superposition hypothesis to explain the relationship between the vestibuloocular reflex and smooth pursuit during horizontal combined eye–head tracking in humans. J. Neurophysiol. 68, 1775–1792. [DOI] [PubMed] [Google Scholar]
- 51.Krauzlis RJ, Lisberger SG. 1994. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J. Comput. Neurosci. 1, 265–283. ( 10.1007/BF00961876) [DOI] [PubMed] [Google Scholar]
- 52.Krauzlis RJ, Miles FA. 1996. Transitions between pursuit eye movements and fixation in the monkey: dependence on context. J. Neurophysiol. 76, 1622–1638. [DOI] [PubMed] [Google Scholar]
- 53.Krauzlis RJ, Lisberger SG. 1994. Simple spike responses of gaze velocity Purkinje cells in the floccular lobe of the monkey during the onset and offset of pursuit eye movements. J. Neurophysiol. 72, 2045–2050. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz JD, Lisberger SG. 1994. Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Vis. Neurosci. 11, 411–424. ( 10.1017/S0952523800002352) [DOI] [PubMed] [Google Scholar]
- 55.Robinson DA. 1965. The mechanics of human smooth pursuit eye movements. J. Physiol. 180, 569–591. ( 10.1113/jphysiol.1965.sp007718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pola J, Wyatt HJ. 2001. The role of target position in smooth pursuit deceleration and termination. Vision Res. 41, 655–669. ( 10.1016/S0042-6989(00)00280-7) [DOI] [PubMed] [Google Scholar]
- 57.Kowler E, Martins AJ, Pavel M. 1984. The effect of expectations on slow oculomotor control–IV. Anticipatory smooth eye movements depend on prior target motions. Vision Res. 24, 197–210. ( 10.1016/0042-6989(84)90122-6) [DOI] [PubMed] [Google Scholar]
- 58.Kowler E. 1989. Cognitive expectations, not habits, control anticipatory smooth oculomotor pursuit. Vision Res. 29, 1049–1057. ( 10.1016/0042-6989(89)90052-7) [DOI] [PubMed] [Google Scholar]
- 59.Boman DK, Hotson JR. 1988. Stimulus conditions that enhance anticipatory slow eye movements. Vision Res. 28, 1157–1165. ( 10.1016/0042-6989(88)90142-3) [DOI] [PubMed] [Google Scholar]
- 60.Heinen SJ, Badler JB, Ting W. 2005. Timing and velocity randomization similarly affect anticipatory pursuit. J. Vis. 5, 493–503. ( 10.1167/5.6.1) [DOI] [PubMed] [Google Scholar]
- 61.de Hemptinne C, Nozaradan S, Duvivier Q, Lefèvre P, Missal M. 2007. How do primates anticipate uncertain future events? J. Neurosci. 27, 4334–4341. ( 10.1523/jneurosci.0388-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Hemptinne C, Lefevre P, Missal M. 2006. Influence of cognitive expectation on the initiation of anticipatory and visual pursuit eye movements in the rhesus monkey. J. Neurophysiol. 95, 3770–3782. ( 10.1152/jn.00007.2006) [DOI] [PubMed] [Google Scholar]
- 63.Becker W, Fuchs AF. 1985. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp. Brain Res. 57, 562–575. ( 10.1007/BF00237843) [DOI] [PubMed] [Google Scholar]
- 64.Barnes GR. 2008. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 68, 309–326. ( 10.1016/j.bandc.2008.08.020) [DOI] [PubMed] [Google Scholar]
- 65.de Hemptinne C, Ivanoiu A, Lefèvre P, Missal M. 2013. How does Parkinson's disease and aging affect temporal expectation and the implicit timing of eye movements? Neuropsychologia 51, 340–348. ( 10.1016/j.neuropsychologia.2012.10.001) [DOI] [PubMed] [Google Scholar]
- 66.Jarrett C, Barnes G. 2005. The use of non-motion-based cues to pre-programme the timing of predictive velocity reversal in human smooth pursuit. Exp. Brain Res. 164, 423–430. ( 10.1007/s00221-005-2260-7) [DOI] [PubMed] [Google Scholar]
- 67.Collins CJ, Barnes GR. 2009. Predicting the unpredictable: weighted averaging of past stimulus timing facilitates ocular pursuit of randomly timed stimuli. J. Neurosci. 29, 13 302–13 314. ( 10.1523/jneurosci.1636-09.2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnes GR, Collins CJ, Arnold LR. 2005. Predicting the duration of ocular pursuit in humans. Exp. Brain Res. 160, 10–21. ( 10.1007/s00221-004-1981-3) [DOI] [PubMed] [Google Scholar]
- 69.de Hemptinne C, Barnes GR, Missal M. 2010. Influence of previous target motion on anticipatory pursuit deceleration. Exp. Brain Res 207, 173–184. ( 10.1007/s00221-010-2437-6) [DOI] [PubMed] [Google Scholar]
- 70.Ameqrane I, Pouget P, Wattiez N, Carpenter R, Missal M. 2014. Implicit and explicit timing in oculomotor control. PLoS ONE 9, e93958 ( 10.1371/journal.pone.0093958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buhusi CV, Meck WH. 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. ( 10.1038/nrn1764) [DOI] [PubMed] [Google Scholar]
- 72.Logan GD, Cowan WB. 1984. On the ability to control thoughts and action: a theory of an act of control. Psychol. Rev. 91, 295–327. ( 10.1037/0033-295X.91.3.295) [DOI] [Google Scholar]
- 73.Curtis CE, Cole MW, Rao VY, D'Esposito M. 2005. Canceling planned action: an FMRI study of countermanding saccades. Cereb. Cortex 15, 1281–1289. ( 10.1093/cercor/bhi011) [DOI] [PubMed] [Google Scholar]
- 74.Kornylo K, Dill N, Saenz M, Krauzlis RJ. 2003. Cancelling of pursuit and saccadic eye movements in humans and monkeys. J. Neurophysiol. 89, 2984–2999. ( 10.1152/jn.00859.2002) [DOI] [PubMed] [Google Scholar]
- 75.Jarrett CB, Barnes GR. 2003. The volitional inhibition of anticipatory ocular pursuit using a stop signal. Brain Res. Cogn. Brain Res. 17, 759–769. ( 10.1016/S0926-6410(03)00200-3) [DOI] [PubMed] [Google Scholar]
- 76.Lisberger SG, Westbrook LE. 1985. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J. Neurosci. 5, 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lisberger SG, Morris EJ, Tychsen L. 1987. Visual motion processing and sensory–motor integration for smooth pursuit eye movements. Annu. Rev. Neurosci. 10, 97–129. ( 10.1146/annurev.ne.10.030187.000525) [DOI] [PubMed] [Google Scholar]
- 78.Tychsen L, Lisberger SG. 1986. Visual motion processing for the initiation of smooth-pursuit eye movements in humans. J. Neurophysiol. 56, 953–968. [DOI] [PubMed] [Google Scholar]
- 79.Eckmiller R, Mackeben M. 1980. Pre-motor single unit activity in the monkey brain stem correlated with eye velocity during pursuit. Brain Res. 17, 210–214. ( 10.1016/0006-8993(80)90600-9) [DOI] [PubMed] [Google Scholar]
- 80.Eckmiller R, Mackeben M. 1978. Pursuit eye movements and their neural control in the monkey. Pflugers Arch. 377, 15–23. ( 10.1007/BF00584369) [DOI] [PubMed] [Google Scholar]
- 81.Shook BL, Schlag-Rey M, Schlag J. 1988. Direct projection from the supplementary eye field to the nucleus raphe interpositus. Exp. Brain Res. 73, 215–218. ( 10.1007/BF00279675) [DOI] [PubMed] [Google Scholar]
- 82.Heinen SJ, Hwang H, Yang SN. 2011. Flexible interpretation of a decision rule by supplementary eye field neurons. J. Neurophysiol. 106, 2992–3000. ( 10.1152/jn.01134.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]