Summary
The metazoan gut harbors complex communities of commensal and symbiotic bacterial microbes. The quantity and quality of these microbes fluctuate dynamically in response to physiological changes. The mechanisms that hosts developed to respond to and manage such dynamic changes and maintain homeostasis remain largely unknown. Here, we identify a dual oxidase (Duox)-regulating pathway that contributes in maintaining homeostasis in the gut of both Aedes aegypti and Drosophila melanogaster. We show that a gut membrane-associated protein, named Mesh, plays an important role in controlling proliferation of gut bacteria by regulating Duox expression through an Arrestin-mediated MAPK JNK/ERK phosphorylation cascade. Expression of both Mesh and Duox is correlated with the gut bacterial microbiome that, in mosquitoes, increases dramatically soon after a blood meal. Ablation of Mesh abolishes Duox induction leading to an increase of the gut microbiome load. Our study reveals that the Mesh-mediated signaling pathway is a central homeostatic mechanism of the insect gut.
Introduction
The intestinal tract of most metazoans harbors complex communities of microbes that contribute to maintaining homeostasis with the gut epithelia. These microbes manipulate a wide range of physiological in the host, including nutrition, development, differentiation and defense1–3. Although these microbial communities maintain homeostasis with the gut epithelia, their quantity and composition dynamically fluctuate in response to the host dietary situations, environmental conditions and physical activities4. The gut epithelia have developed mechanisms to tolerate commensal microorganisms5–10, while the microorganisms have developed mechanisms to evade the host immune response11,12. Nonetheless, the gut epithelia are required to mount a finely tuned immune response of proper strength and duration in response to microbial fluctuation in a timely and appropriate manner. The molecular mechanisms employed by the gut epithelia to manage the dynamic fluctuation of microbes and maintain homeostasis are not well understood.
Similar to the mammalian intestinal tract, the insect gut constantly interacts with its microbial residents. Drosophila and mosquitoes are established models for deciphering the complex interactions between the gut and its microbes13,14. Previous studies revealed that Dual oxidase (Duox)-mediated production of reactive oxygen species (ROS) is a major immune mechanism regulating insect gut-microbe homeostasis6,7,15–17. In Drosophila, Duox-mediated ROS are required for routine control of Saccharomyces cerevisiae, an essential microbial food source6. A reduction in ROS levels in the midgut of the major arboviral vector mosquito Aedes aegypti results in dysbiotic proliferation of the intestinal microbiota15. Duox-mediated ROS also play a pivotal role in regulating homeostasis and the composition of the gut bacterial community in Bactrocera dorsalis16 and Phlebotomine sandflies17. Indeed, under homeostatic conditions, both the expression and activity of Duox are tightly restricted to a level that allows healthy gut-microbe interactions, thereby precluding any pathophysiological effects on the gut epithelia. Induction of Duox gene expression is limited in an off-state status by phospholipase Cβ (PLCβ)-mediated mitogen-activated protein kinase (MAPK) P38 dephosphorylation in the Drosophila gut epithelia7. Modest ROS levels are achieved by activation of basal Duox expression through intracellular Ca2+ mobilization by a G-protein α subunit q protein (Gαq)-PLCβ signal cascade6,18. Thus, the basal expression and activation of Duox are essential to manage symbiotic microbes under healthy conditions in the insect gut.
The complement control protein (CCP) domain is an evolutionarily conserved module essential for complement-mediated immune functions19–22. We have previously demonstrated that the CCP domain plays an important role in insect-microbe interactions23,24. In this study, we show that a CCP-containing protein named Mesh25 regulates commensal bacterial proliferation through regulation of Duox expression in the gut of both Drosophila and A. aegypti, via a signaling cascade involving Arrestin-mediated MAPK JNK/ERK phosphorylation. In both insects, Mesh expression correlates with the gut commensal bacterial load, enabling Duox abundance to be dynamically regulated by microbial fluctuation. Since generation of Duox-mediated ROS is a major gut immune response in maintenance of insect gut homeostasis, our study reveals a fine-tuning mechanism for Duox expression to manage healthy gut-microbe interactions in insects.
Results
Mesh controls Duox expression and gut microbiome load in A. aegypti and Drosophila
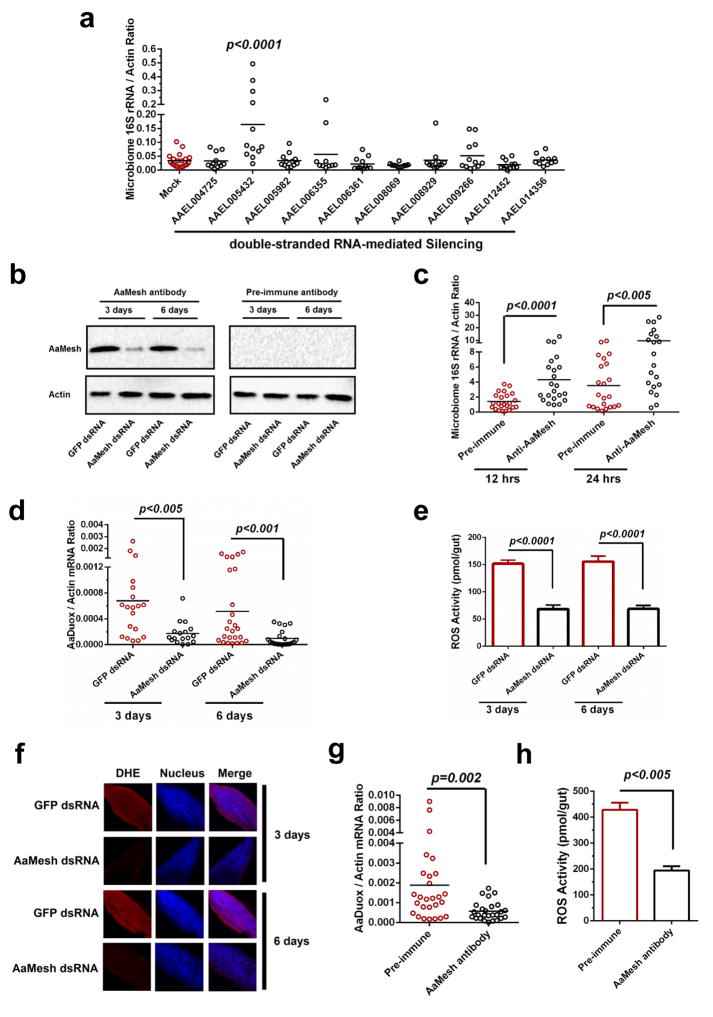
Proteins with CCP domains are key immune factors restricting microbial infection in invertebrates23,24,26. Here, we assessed the roles of CCP-domain proteins23 in maintenance of the gut commensal bacteria in the mosquito A. aegypti. Ten genes encoding CCP domains23 were silenced individually via thoracic microinjection of double-stranded RNA (dsRNA). Six days later, the midguts of dsRNA-treated mosquitoes were dissected for determination of the gut bacterial load by qPCR27. The results showed that knockdown of the AAEL005432 gene, but not other genes, significantly induced 4- to 5-fold proliferation of the gut microbiome in A. aegypti, compared to that found in green fluorescent protein (GFP) dsRNA-treated A. aegypti, suggesting an antibacterial role of this protein in mosquito gut immunity (Fig. 1a). AAEL005432 shares high sequence identity with the Drosophila melanogaster Mesh (CG31004) (DmMesh) that was previously identified as a transmembrane component of the gut septate junctions25 (Supplementary Fig. 1a). Moreover, the AAEL005432-encoded protein is predicted to contain domains that are identical to those of DmMesh (Supplementary Fig. 1b). Therefore, we refer to this gene as A. aegypti Mesh, i.e. AaMesh.
Figure 1. Mesh maintains gut microbiome homeostasis by controlling AaDuox expression in A. aegypti.
(a) The roles of the CCP genes in the maintenance of the gut microbiome in A. aegypti. The genes were individually silenced via thoracic microinjection of dsRNA. The results were combined from 2 independent experiments.
(b) Validation of an AaMesh antibody in the guts of AaMesh-silenced mosquitoes. The amount of AaMesh was assessed via immuno-blotting with a murine AaMesh antibody in the mosquito lysates. Pre-immune serum served as a negative control. A total of 50 μg of protein from mosquito lysates was loaded into each lane.
(c) Immuno-blockade of AaMesh enhanced colonization of gut bacteria in the midgut of A. aegypti. The burden of the gut microbiome was detected by SYBR Green qPCR.
(d–f) Regulation of both Duox expression (d) and ROS activity (e and f) in the guts of AaMesh silencing mosquitoes. The GFP dsRNA-treated mosquitoes served as mock controls. ROS activity was measured by H2O2 assay (e) and Dihydroethidium staining (DHE) (f). (f) Nuclei were stained blue with To-Pro-3 iodide. Images were examined using a 10×objective lens on a Zeiss LSM 780 meta confocal microscope.
(g–h) Immuno-blockade of AaMesh impaired both Duox expression (g) and ROS activity (h) in the mosquito guts. The midguts of were collected at 18 hours after a blood meal. ROS activity was detected by H2O2 assay.
(a, c, d, g) In the mosquito midguts, the gene expression was determined by SYBR Green qPCR and normalized against A. aegypti actin (AAEL011197). The qPCR primers are described in Supplementary Table 6. One dot represents one gut. The horizontal line represents the mean value of the results.
(c, g, h) The murine AaMesh antibody, in a 100-fold serial dilution, was premixed with fresh mouse blood for the mosquito blood meal. Pre-immune serum at the same dilution served as a negative control.
(e, h) The data are presented as the mean ± S.E.M.
(a, c, d, e, g, h) The data were analyzed using the non-parametric Mann-Whitney test.
(b–h) All results were repeated in at least 3 independent experiments.
We assessed AaMesh expression in various tissues of A. aegypti by qPCR. We noted that AaMesh abundance varied among mosquito tissues. The AaMesh transcript in the midgut is 6- to 11-fold higher than that in the Malpighian tubules and salivary glands, and more than 20- fold higher than that in head, hemolymph and ovaries (Supplementary Fig. 2a), which is consistent to the previous reports28,29. Therefore, we speculated that AaMesh expression levels might coincide with the gut bacterial loads. Indeed, oral feeding of mosquitoes with antibiotics repressed AaMesh expression in the midgut (Supplementary Fig. 2b). Conversely, AaMesh expression in the gut was gradually induced after a blood meal over a time course (Supplementary Fig. 2c), most likely as a result of microbial proliferation15,27.
To validate the role of AaMesh in controlling the gut microbiome, we expressed and purified an AaMesh peptide (994 aa-1174 aa) from Escherichia coli cells (Supplementary Fig. 3a) and generated a mouse polyclonal antiserum against this fragment (Supplementary Fig. 3b). The antiserum recognized a protein band corresponding to AaMesh in control mosquito lysates, which was impaired from the AaMesh-silenced mosquitoes demonstrating the specificity of the antiserum (Fig. 1b). We added the antiserum into the A. aegypti blood meal and measured the gut microbiome load 12 and 24 hours later. The result showed that immuno-blockade of AaMesh significantly increased the gut microbiome load at both time points (Fig. 1c).
To investigate the molecular mechanism by which Mesh restricts the gut microbiome proliferation, we collected midguts from AaMesh dsRNA-inoculated (Day 3 and 6) and AaMesh antiserum-fed (18 hours) mosquitoes, respectively. Midguts of GFP dsRNA-treated and pre-immune antiserum-fed mosquitoes served as controls. RNA-Seq and in-depth analysis of immune-related genes identified 48 genes in anti-AaMesh antiserum-treated guts, and 44 genes in 3 day- and 52 genes in the 6 day-AaMesh dsRNA-treated guts as significantly downregulated compared to their respective controls (Supplementary Fig. 4a,b and Supplementary Table 1). Five of these genes were detected in all three experimental groups, including A. aegypti Arrestin a and b (AaArrestin), Duox (AaDuox), haemopexin 7 (AaHPX7) and C-type lysozyme 9 (AaLYSC9) (Supplementary Fig. 4c). Of these, Duox is a member of the ROS-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Generation of Duox-mediated ROS is a major immune mechanism regulating gut-microbe homeostasis in insects6,7.
We validated the regulation of AaDuox (A. aegypti Duox) in the gut of AaMesh-silenced mosquitoes by qPCR. The mRNA abundance of AaDuox was significantly downregulated 4–6 folds at 3 and 6 days post AaMesh silencing, compared to the GFP dsRNA-treated controls (Fig. 1d). In consistence with this finding, ROS activity measured by H2O2 assay (Fig. 1e) and Dihydroethidium (DHE) staining (Fig. 1f) was consistently suppressed in the gut of AaMesh-silenced mosquitoes. We also measured ROS activity after feeding mosquitoes with AaMesh antiserum through the blood meal. The results showed that immuno-blockade of AaMesh dramatically impairs the AaDuox expression (Fig. 1g) and ROS activity (Fig. 1h), respectively.
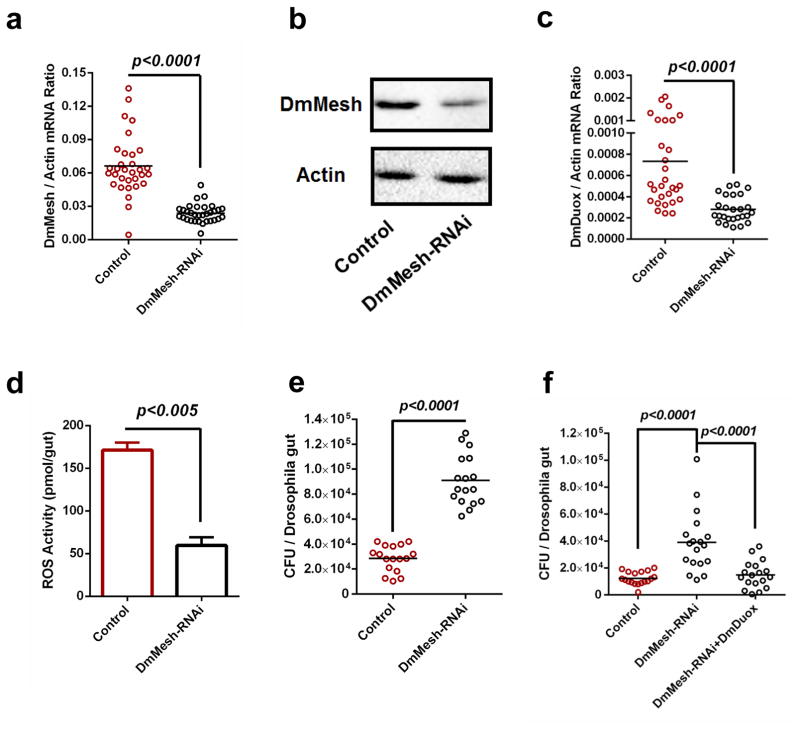
Next, we assessed the conservation of the role of Mesh in controlling the gut microbiome by regulating Duox expression in the model insect D. melanogaster. DmMesh is previously shown to be an essential component of gut development25. We exploited a UAS/GAL4 system to knock down DmMesh expression specifically in the Drosophila midgut, via a specific GAL4 line driven by a midgut-specific NP3084 promoter. Since Mesh was known to involve in the formation of septate junction25, we therefore tested the gut integrity of the DmMesh RNAi flies, via a Smurf assay30. Silencing DmMesh does not impair the gut integrity (Supplementary Fig. 5). Compared to the GFP-RNAi control flies, both DmMesh mRNA and protein levels were consistently impaired in the gut of DmMesh RNAi flies (Fig. 2a,b). We next examined the role of DmMesh in regulating the expression of DmDuox (CG3131). DmDuox expression (Fig. 2c) and ROS activity (Fig. 2d) were much lower in the guts of DmMesh RNAi flies respectively. Consistently, the DmDuox mRNA levels were significantly reduced in early-stage 1st instar DmMesh−/− compared to wild-type larvae (Supplementary Fig. 6a,b). To further assess the regulation of DmDuox by DmMesh, we cloned DmMesh into a pAc5.1/V5-His expression vector (pAc-DmMesh). Ectopic expression of DmMesh in Drosophila S2 cells resulted in more than 3-fold higher expression of the DmDuox (Supplementary Fig. 7). In accordance to the observation in A. aegypti, the gut microbiome load was significantly increased by ~3-fold in DmMesh RNAi Drosophila compared to GFP RNAi controls (Fig. 2e), confirming a key role of Mesh in restricting the proliferation of the gut microbiome in insects. We next rescued the DmDuox into DmMesh-RNAi flies to test whether over-expression of DmDuox can rescue the effects on bacterial burden. The gut bacterial burden was significantly reduced in the DmDuox-rescued DmMesh-RNAi Drosophila, compared to that in the gut of DmMesh RNAi flies (Fig. 2f).
Figure 2. Regulation of commensal microbiome and DmDuox expression in the guts of DmMesh RNAi Drosophila.
(a–b) Knockdown efficiency of DmMesh gene in the guts of DmMesh RNAi Drosophila. The DmMesh RNAi Drosophila strain was generated using a GAL4 line driven by a midgut-specific NP3084 promoter. The NP3084/GFP-RNAi flies served as a negative control. The knockdown efficiency in the guts of DmMesh RNAi flies was assessed via SYBR Green qPCR (a) and immuno-blotting with an AaMesh antibody (b). (b) A total of 50 μg of protein from mosquito lysates was loaded into each lane.
(c–d) Regulation of the DmDuox gene (c) and ROS activity (d) in the guts of DmMesh RNAi Drosophila. (d) ROS activity was measured by H2O2 assay. The data are presented as the mean ± S.E.M.
(e) Regulation of the gut microbiome in the guts of DmMesh RNAi Drosophila. The burden of the gut microbiome was determined by a colony-forming unit (CFU) assay.
(f) Reduction of burden of gut microbiome by rescuing DmDuox into the DmMesh RNAi flies. The burden of the gut microbiome was determined by a CFU assay.
(a, c) The gene expression was determined by SYBR Green qPCR and normalized against D. melanogaster actin (CG12051). The qPCR primers are described in Supplementary Table 6. One dot represents one fly gut. The horizontal line represents the mean value of the results.
(a–f) All results were repeated in at least 3 independent experiments.
We next determined whether the Mesh-Duox signaling axis changes the gut microbial composition in Drosophila and A. aegypti. The midguts were isolated from the mosquitoes (AaMesh dsRNA vs GFP dsRNA) and Drosophila (DmMesh RNAi vs GFP RNAi) for bacterial 16S rDNA pyrosequencing. Intriguingly, dsRNA-mediated AaMesh knockdown enhanced the composition of Firmicutes and Proteobacteria, but decreased that of Actinobacteria and Bacteroidetes in the midgut of A. aegypti (Supplementary Fig. 8a,b and Supplementary Table 2,3). In Drosophila, enhancement of Proteobacteria and impairment of Bacteroidetes and Firmicutes in bacterial composition were determined in the gut of DmMesh RNAi flies (Supplementary Fig. 8c,d and Supplementary Table 4,5), suggesting that the Mesh-Duox axis-based regulation pathway is specific for particular bacterium.
Mesh regulates Duox, but not Nox expression, for ROS production
In insect intestine, ROS is produced via the catalytic action of NADPH oxidases, including Nox31 and Duox7,32. A particular commensal bacteria genus in the Drosophila guts, Lactobacillus, can stimulate Nox-dependent ROS production to facilitate cellular proliferation in intestinal stem cells. A specific Nox-dependent ROS generation in the gut epithelial cells may act as an important role in ROS-mediated intestinal homeostasis33. Nevertheless, many previous studies indicated the pivotal roles of Duox in regulating homeostasis and the composition of the gut commensal bacterial community in Drosophila6, Aedes aegypti15 and Bactrocera dorsalis16. To address this, we therefore generated both DmNox (CG34399) RNAi and DmDuox RNAi GAL4 Drosophila lines driven by a midgut-specific NP3084 promoter. Compared to that in the GFP RNAi control flies, the burden of gut commensal bacteria was enhanced in both the DmNox RNAi and the DmDuox RNAi Drosophila gut (Supplementary Fig. 9a,b). We further silenced either A. aegypti Nox (AaNox) or AaDuox by dsRNA inoculation in A. aegypti, respectively. In consistent with the studies in flies, knockdown of both NADPH oxidase genes also enhanced the burden of gut microbiota in the mosquito midgut (Supplementary Fig. 9c,d), suggesting both NADPH oxidases are important for the ROS-mediated intestinal homeostasis.
We next explored Nox regulation in the Mesh-mediated signaling pathway. In contrast to the significant DmDuox reduction in the DmMesh RNAi fly guts, expression of the DmNox gene was not regulated by silencing DmMesh (Supplementary Fig. 9e). Furthermore, there was no influence of AaNox transcript in the gut of dsRNA-mediated AaMesh-silenced mosquitoes (Supplementary Fig. 9f), suggesting that Mesh controls insect gut-microbe homeostasis by regulating the expression of Duox, but not Nox gene.
Mesh regulates Duox expression via Arrestin-mediated MAPK phosphorylation
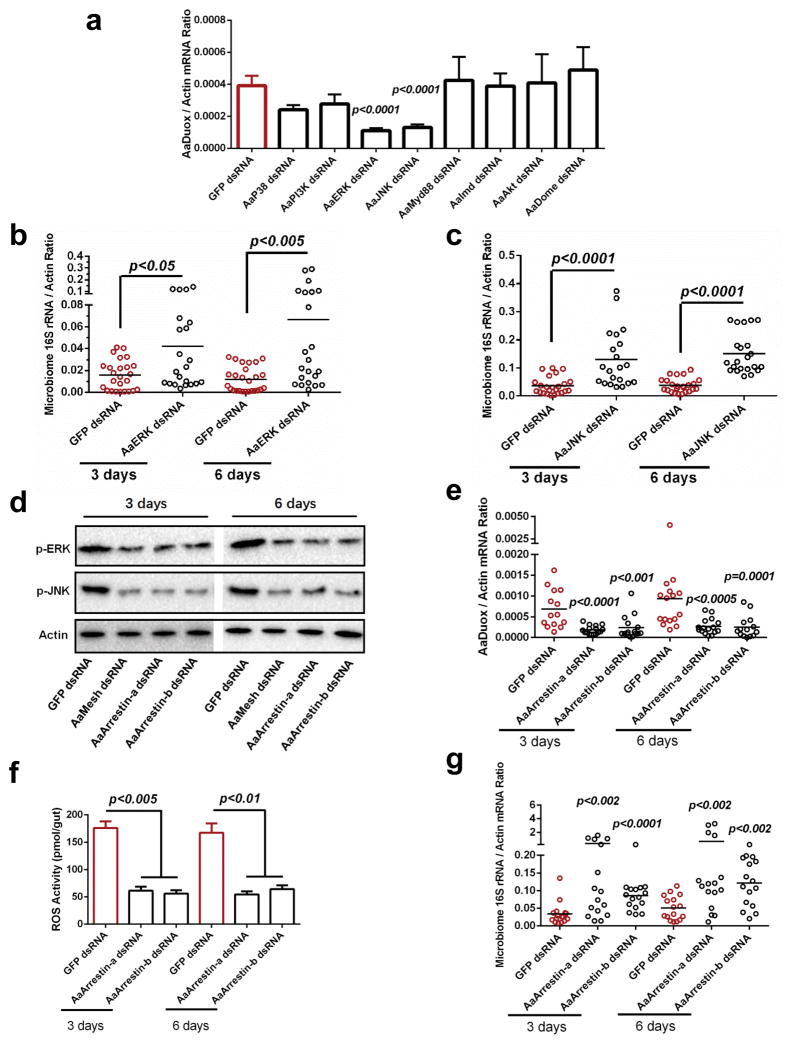
We examined the roles of major immune signaling pathways in AaDuox and AaMesh expression in the mosquito gut through dsRNA-mediated knockdown of key pathway modulators, including components of the MAPK pathways (AaP38, AaERK and AaJNK), AaMyd88 of the Toll pathway, AaImd of the Immune deficiency (Imd) pathway, AaDome of (Janus kinase (JAK)-signal transduction and activators of transcription (STAT) pathway), phosphatidylinositol 3-kinase (AaPI3K) and AaAkt of the PI3K-AKT-mTOR pathway (Supplementary Fig. 10a–h). Silencing either AaERK or AaJNK, but not any of the other immune signaling modulators, significantly reduced AaDuox expression in the mosquito midgut (Fig. 3a). However, none of the above-mentioned gene knockdowns affected AaMesh mRNA levels (Supplementary Fig. 10i), suggesting that AaERK and AaJNK may act downstream of AaMesh, but upstream of AaDuox. Indeed, knockdown of either AaERK or AaJNK reduced ROS production in the A. aegypti gut (Supplementary Fig. 11a,b), and increased the gut microbiome load (Fig. 3b,c).
Figure 3. Mesh regulates AaDuox expression via Arrestin-mediated MAPK phosphorylation in A. aegypti.
(a) The role of immune signaling pathways in regulating Duox expression in the midgut of A. aegypti. The genes of immune signaling components were knocked down by dsRNA thoracic inoculation. Six days later, the abundance of the Duox gene was determined in the midgut of A. aegypti by SYBR Green qPCR.
(b–c) Knockdown of AaERK and AaJNK enhanced the burden of the gut microbiome in A. aegypti. Both AaERK and AaJNK were silenced by dsRNA thoracic inoculation in A. aegypti. The midguts were isolated 3 and 6 days after gene silencing, and the burden of the gut microbiome was subsequently determined by SYBR Green qPCR.
(d) Genetic suppression of AaMesh and AaArrestins impaired the phosphorylation of AaERK and AaJNK in the mosquito guts. AaMesh and AaArrestins were silenced by dsRNA thoracic inoculation in A. aegypti, respectively. The midguts were isolated 3 and 6 days after gene silencing, and the phosphorylation of AaERK (p-ERK) and AaJNK (p-JNK) were subsequently determined by western blotting. A total of 50 μg of protein from mosquito gut lysates was loaded into each lane.
(e–f) Reduction of Duox expression (e) and ROS activity (f) in the guts of AaArrestins-silenced mosquitoes. The midguts from the dsRNA-treated mosquitoes were isolated for the detection of Duox expression (e) by SYBR Green qPCR and for measuring ROS activity using a H2O2 assay (f). (f) The data are presented as the mean ± S.E.M.
(g) Silencing AaArrestins increased the load of the gut microbiome in A. aegypti.
(a, b, c, e, g) The GFP dsRNA-treated mosquitoes served as mock controls. The gene expression was determined by SYBR Green qPCR and normalized against A. aegypti actin (AAEL011197). The qPCR primers are described in Supplementary Table 6. One dot represents one gut. The horizontal line represents the mean value of the results.
(a, f) The data are presented as the mean ± S.E.M.
(a–c, e–g) The data were analyzed using the non-parametric Mann-Whitney test.
(a–g) All results were repeated in at least 3 independent experiments.
Besides AaDuox, the expression levels of two A. aegypti Arrestin genes (AaArrestin-a and AaArrestin-b) were also downregulated in both anti-AaMesh antiserum-treated and AaMesh dsRNA-treated guts (Supplementary Fig. 4c and Supplementary Fig. 12a,b). In mammals, β-arrestin, the orthologue of insect Arrestins, is essential for the MAPK signaling cascade by forming a transduction scaffold for phosphorylation of MAP kinases, especially ERK and JNK33. Therefore, we assessed the role of AaArrestins in the “MAPK-Duox” cascades. Genetic suppression of either AaMesh or AaArrestins expression (Supplementary Fig. 12c,d) impaired the phosphorylation of both AaERK and AaJNK MAPK kinases in the mosquito gut (Fig. 3d). Knockdown of AaMesh did not significantly affect the expression of AaERK and AaJNK (Supplementary Fig. 13a,b). In addition, both the AaDuox expression (Fig. 3e) and ROS activity (Fig. 3f) were consistently reduced in the AaArrestins-silenced guts, resulting in expansion of the gut microbiome 3 and 6 days after AaArrestins knockdown (Fig. 3g). These data suggested that a Mesh-Arrestin-ERK/JNK signaling cascade might control Mesh-mediated Duox expression.
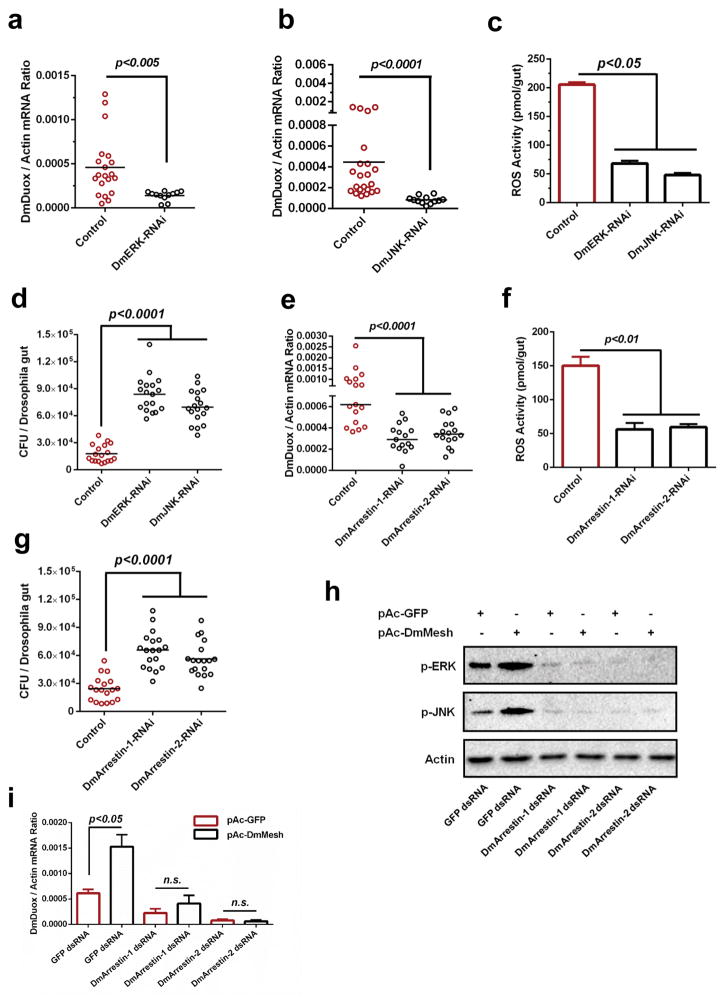
We next tested our findings in the Drosophila gut by exploiting the UAS/GAL4 system to knockdown DmERK (CG12559) and DmJNK (CG5680) via GAL4 lines driven by the NP3084 promoter. Both MAPK genes were stably knocked down in the Drosophila gut (Supplementary Fig. 14a,b), resulting in a significant downregulation of DmDuox expression (Fig. 4a,b), a reduction of ROS activity (Fig. 4c) and an increase of the gut bacterial load (Fig. 4d). We next exploited DmArrestin-1 (CG5711) and DmArrestin-2 (CG5962) RNAi flies for investigation. Silencing DmArrestin-1 and DmArrestin-2 showed the consistent phenotypes in regulation of DmDuox expression (Fig. 4e), ROS activity (Fig. 4f) and gut bacterial burden (Fig. 4g). To further examine the role of the Mesh-Arrestin-ERK/JNK signaling cascade in Duox expression, we silenced both of the DmArrestin genes by dsRNA transfection in Drosophila S2 cells (Supplementary Fig. 15a,b) that ectopically express DmMesh, and then examined the DmDuox mRNA abundance. Indeed, ectopic expression of DmMesh induced ERK/JNK phosphorylation (Fig. 4h) and Duox expression (Fig. 4i), which were abolished following knockdown of either of the DmArrestins, further validating that Mesh regulates Duox expression via Arrestin-mediated MAPK phosphorylation. We next introduced the DmDuox gene into DmArrestins RNAi flies respectively to test whether DmDuox over-expression can rescue the effects on gut bacterial burden. The gut bacterial burden was significantly decreased in the DmDuox-rescued DmArrestin-RNAi Drosophila, compared to that in the gut of DmArrestin RNAi flies (Supplementary Fig. 16a,b).
Figure 4. The role of Mesh-Arrestin-ERK/JNK-MAPK signaling cascade in DmDuox regulation in Drosophila.
(a–b) Regulation of the DmDuox gene in the guts of DmERK (a) and DmJNK (b) RNAi flies.
(c) Regulation of ROS activity in the guts of DmERK and DmJNK RNAi flies.
(d) Enhancement of the gut microbiome in the guts of DmERK and DmJNK RNAi flies.
(e–f) Silencing DmArrestins impaired expression of the DmDuox gene (e) and ROS activity (f) in the Drosophila guts.
(g) Increasing the burden of gut microbiome in the guts of DmArrestins RNAi flies.
(h–i) Assessing the role of the “Arrestin-ERK/JNK” cascade in AaMesh-mediated Duox expression in Drosophila. Both DmArrestin-1 and DmArrestin-2 were silenced by dsRNA transfection in the pAc-DmMesh-trnasfected Drosophila S2 cells. (h) The phosphorylation of DmERK (p-ERK) and DmJNK (p-JNK) was detected by western blotting. (i) The abundance of the DmDuox gene was determined by SYBR Green qPCR and normalized by Drosophila actin (CG12051).
(a–g) GFP RNAi flies served as a negative control.
(a–b, e) The gene expression was determined by SYBR Green qPCR and normalized against D. melanogaster actin (CG12051). The qPCR primers are described in Supplementary Table 6. One dot represents one fly gut. The horizontal line represents the mean value of the results.
(c, f) The ROS activity was detected by a H2O2 assay. The data are presented as the mean ± S.E.M.
(d, g) The burden of gut microbes was determined by a CFU assay.
(a–g, i) The data were analyzed using the non-parametric Mann-Whitney test.
(a–i) The results were reproduced by at least 3 independent experiments.
Mesh-mediated Duox expression correlates with the gut commensal bacterial load
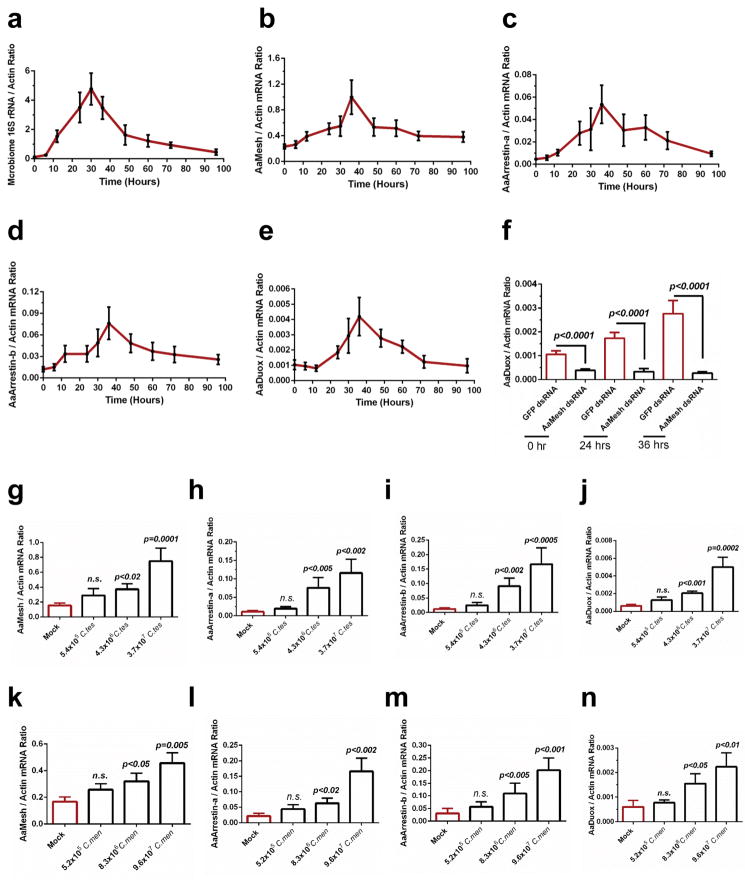
In hematophagous insects such as mosquitoes, gut commensal bacteria proliferate rapidly during blood meal digestion. We investigated the correlation between the Mesh-mediated Duox expression and the microbiome load in the Aedes gut following a blood meal. The gut microbiome load increased drastically reaching a peak at about 30 hours post blood feeding (Fig. 5a). The expression levels of AaMesh, AaArrestins and AaDuox were also significantly and progressively upregulated after blood feeding, coinciding with the microbiome expansion (Fig. 5b–e). Silencing AaMesh completely abolished the induction of AaDuox expression after a blood meal (Fig. 5f).
Figure 5. The AaMesh-mediated AaDuox regulatory pathway in response to commensal bacteria in the guts of A. aegypti.
Responses of the AaMesh-mediated AaDuox regulatory pathway to the blood meal-mediated augmentation of gut commensal bacteria (a–f), and oral introduction of the commensal microbiome C. testosteroni (C. tes, g–j) and C. meningosepticum (C. men, k–n), in the gut of A. aegypti.
(a) Regulation of the gut microbiome after a blood meal in the mosquito guts.
(b–e, g–n) Regulation of the AaMesh (b, g, k), AaArrestin-a (c, h, l), AaArrestin-b (d, i, m) and AaDuox (e, j, n) genes in the mosquito midguts.
(f) Silencing AaMesh abolished the induction of the AaDuox gene after a blood meal.
(g–n) These bacteria were introduced into the guts of antibiotic-treated mosquitoes. Measurement of the average bacterial number introduced into each mosquito gut was presented by Supplementary Fig. 18. The genes were determined at 12 hours post blood-mediated bacterial introduction.
(a–n) Both the burden of the gut microbes and the gene abundance were determined by SYBR Green qPCR and normalized against A. aegypti actin (AAEL011197). The qPCR primers are described in Supplementary Table 6. The data are presented as the mean ± S.E.M. All results were repeated by 3 independent experiments. The data were analyzed using the non-parametric Mann-Whitney test.
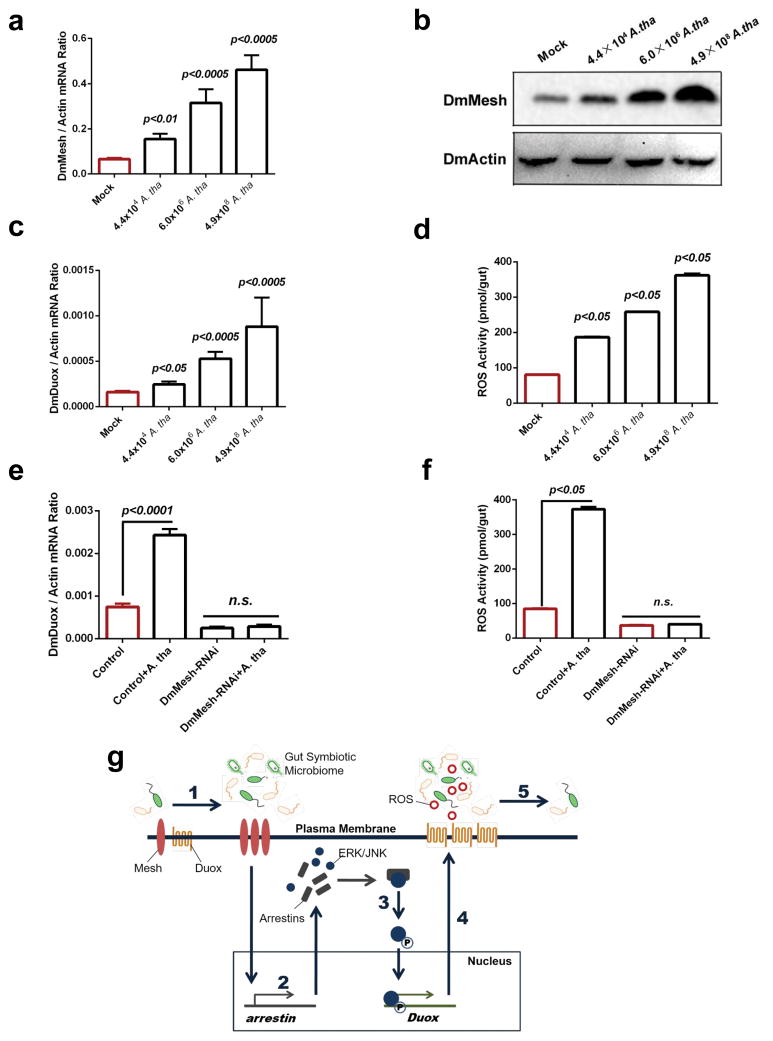
We examined whether the upregulation of AaMesh, AaArrestins and AaDuox observed after blood feeding can be correlated with specific members of the mosquito gut microbiome. Both Comamonas testosteroni and Chryseobacterium meningosepticum have been routinely identified as cultivable gut commensals in Aedes mosquitoes12,34. These 2 bacteria robustly proliferated in response to a blood meal and by suppressing the Mesh-mediated signaling (Supplementary Fig. 17). We next cultured these bacteria12 and orally fed these bacteria to antibiotic-treated mosquitoes through the blood meal (Supplementary Fig. 18a,b). The AaMesh, AaArrestins and AaDuox genes were upregulated by oral introduction of C. testosteroni (Fig. 5g–j) and C. meningosepticum (Fig. 5k–n) in a dose-dependent manner. These findings were also tested in Drosophila. We identified Acetobacter thailandicus as a cultivable bacterium from the Drosophila gut35. Moreover, composition of A. thailandicus was dramatically regulated in the gut of DmMesh RNAi flies (Supplementary Fig. 8d). Oral introduction of serial quantities of A. thailandicus cells (Supplementary Fig. 18c) in the germ-free fly guts (Supplementary Fig. 19) induced the expression of DmMesh in both mRNA transcript (Fig. 6a) and protein (Fig. 6b) levels. In consistent, the DmDuox expression (Fig. 6c) and ROS activity (Fig. 6d) were dramatically induced by the bacterial oral introduction in a dose-dependent manner. Both DmDuox induction (Fig. 6e) and the ROS enhancement (Fig. 6f) were abolished in DmMesh RNAi flies.
Figure 6. The DmMesh-mediated DmDuox regulation in response to commensal bacteria in Drosophila.
(a–d) Oral introduction of A. thailandicus (A. tha) induced the DmMesh expression and DmDuox-mediated ROS in the gut of aseptic Drosophila. A. thailandicus was orally introduced into the germ-free DmMesh RNAi flies by food ingestion. Measurement of the average bacterial number introduced into each fly gut was presented by Supplementary Figure 18. The mRNA expression levels of DmMesh (a) and DmDuox (c) were determined by SYBR Green qPCR. (b) The protein level of DmMesh was determined by Western-blot with an anti-AaMesh murine antibody. (d) Detection of the ROS activity in the Drosophila midguts.
(e–f) The DmMesh-mediated DmDuox regulatory pathway in response to A. thailandicus. The DmMesh RNAi Drosophila strain was generated using a GAL4 line driven by a NP3084 promoter. The germ-free NP3084/GFP-RNAi flies that fed on the same bacteria served as negative controls. 6×106 A. thailandicus was orally introduced into the germ-free DmMesh RNAi flies by food ingestion. (e) Measurement of DmDuox expression and (f) the ROS activity in the Drosophila midguts.
(g) Schematic representation of the AaMesh-mediated Duox regulatory pathway in response to symbiotic gut microbes. The expression of Mesh can be upregulated by the proliferation of the symbiotic microbiome in insect guts (1). Because Mesh is a positive regulator of Arrestin expression, induction of Mesh results in a higher abundance of Arrestins (2), and subsequently accelerates the phosphorylation of the ERK and JNK MAPK proteins (3). The activation of ERK and JNK leads to the downstream Duox transcription (4). Thus, higher DUOX-mediated ROS are generated in response to the gut microbial proliferation, thereby moderating the proliferation of symbiotic microbes in gut-microbe homeostasis (5).
(a, c, e) The Duox expression was assessed by SYBR Green qPCR and normalized against Drosophila actin. The qPCR primers are described in Supplementary Table 6.
(d, f) The ROS activity was detected by a H2O2 assay.
(a, c–f) The data are presented as the mean ± S.E.M. All results were repeated by 3 independent experiments. The data were analyzed using the non-parametric Mann-Whitney test.
In addition to commensal gut bacteria, the gut epithelia may be also face infected by pathogenic bacteria. We next determined the role of Mesh-Duox axis in response to pathogen infection. A Drosophila pathogen, Erwinia carotovora carotovora 15 (Ecc15), was fed to both DmMesh RNAi and control flies. Since Ecc15 can trigger a strong systemic immune response following oral infection32, the DmDuox gene was dramatically up-regulated in the midgut of both DmMesh RNAi and control flies (Supplementary Fig. 20). These data suggest that the Mesh-Duox axis may only play a fine-tuning role in conventional condition (Fig. 6g).
Discussion
The quantity and quality of the gut symbiotic bacteria fluctuate dynamically with gut activities such as alimentary flow, food ingestion and other physiological changes36. The host senses these changes and fine-tunes its gut immune system to respond with proper strength and duration to the dynamic changes in commensal microbes. In mammals, ROS plays an important role in controlling the normal gut microbiota. The previous studies indicated that Duox2, an important source of hydrogen peroxide, can be induced by normal gut microbiota in mice37. In addition to Duox2-induced ROS generation, NADPH oxidase 1 (Nox1) also acts as a key player for ROS responses in the murine intestine, particularly induced by members of the genus Lactobacillus31, revealing an important role of commensal bacteria-mediated ROS generation in maintenance of homeostasis in the mammalian intestine.
It is largely unknown how the insect gut epithelium might sense the dynamic changes of the microbiome and adjust its response. Several studies in Drosophila and other insects have revealed that the gut epithelia rely on basal production of Duox-dependent ROS for maintaining gut-microbe homeostasis16,17,38. Here, we identified Mesh as an indispensable factor of the A. aegypti and D. melanogaster gut, which fine-tunes the expression of Duox and production of ROS, thereby regulating the gut bacterial load. The expression level of Mesh correlates with the load of gut bacteria, which in mosquitoes are induced dramatically after a blood meal. Mechanistic studies have demonstrated that Mesh mediates constitutive Duox expression in the gut via an Arrestin-MAP kinase signaling cascade. First, silencing Mesh abolishes bacterially induced Duox expression, while its ectopic overexpression increases Duox levels. Second, depletion of the gut microbiome reduces Mesh expression, while reconstitution of the microbiome recovers Mesh and Duox expression level in a microbial burden-dependent manner. Third, silencing either of the Arrestins, JNK or ERK abolishes Mesh-mediated Duox expression. Taken together, these results suggest that Mesh, directly or indirectly, senses the dynamic fluctuation of the gut microbiome thereby transmitting a signal to an intracellular signaling cascade to fine-tune the antibacterial immune responses and restore homeostasis.
Mesh has a complement control protein (CCP) domain, which is an evolutionarily conserved immune module that recognizes microbial ligands. Scavenger Receptor-C (SR-C), a Drosophila membrane receptor that also contains 2 CCP domains, is capable of recognizing both Gram-positive and Gram-negative bacteria acting as a pattern recognition receptor for phagocytosis in hemocytes26. Moreover, the CCP module of various A. aegypti immune factors mediates direct recognition of Dengue viral particles restricting viral infection23,24. Our preliminary data suggest that neither of Aedes or Drosophila Mesh (expressed as full-length proteins in Drosophila S2 cells) can directly interact with C. testosteroni, C. meningosepticum or A. thailandicus, which are common members of the insect microbiome. In addition to surface bacterial components, metabolites generated by gut microbes can also contribute to the regulation of gut immunity. It is known that bacterial-derived uracil acts as a modulator to boost the ROS activity in Drosophila38. Future work should aim to investigate bacterial ligands for the Mesh-mediated Duox expression.
RNA-Seq and in-depth analysis of immune-related genes identified that 5 genes were consistently down-regulated in both AaMesh-silenced and AaMesh-immuno-blockaded mosquito guts. In these 5 genes, LYSC9 is an enzyme with bactericidal activity39,40. Duox is a member of the ROS-generating NADPH oxidases. Besides the Duox gene that we focused on this study, expression of a C-type lysozyme 9 (LYSC9) was also significantly impaired by immuno-blocking/silencing AaMesh, suggesting expression of LYSC9 might be controlled by Mesh-mediated signaling. Recent studies showed that a C. elegans lysozyme, known as invertebrate-type lysozyme-3 (ilys-3), can be up-regulated by ERK-MAPK-dependent signaling in the worm intestine, during challenge with Gram-positive pathogens41. Indeed, our studies have demonstrated that Mesh mediates down-stream gene (Duox) expression via the ERK/JNK MAPK signaling cascade. We therefore speculate that LYSC9 might also be counted as one of the Mesh-mediated MAPK signaling-regulated genes, and contribute to fine-tuning the gut-microbe homeostasis in insects. The role of LYSC9 in the regulation of microbiota will be investigated in our further study.
In addition to commensal gut bacteria in Drosophila and mosquitoes, the gut epithelia also face invasion from allochthonous microorganisms with pathogenic properties. The proliferation of these pathogens and their metabolites stimulates the epithelia to generate ROS at a much higher level than under conventional conditions7,38. Induction of the Duox in the Drosophila gut is mediated by the MAP kinase kinase kinase (MEKK1)-MAP kinase kinase 3 (MKK3)-P38-ATF2 pathway. Gαq-mediated PLC-β directly activates MEKK1, thereby leading to the MAPK P38-dependent Duox induction7. However, under healthy conditions, the expression of Duox is off-set by MAPK P38 dephosphorylation via PLC-β-MAP kinase phosphatase-3 (MKP3) signaling7, indicating that the MAPK P38 signaling cascade plays a central role in regulating Duox expression during pathogenic microbial infections. Moreover, MAPK P38 pathway deficient flies can survive with conventional rearing, suggesting that the MAPK P38-mediated cascade is dispensable for the maintenance of basal Duox expression in healthy gut-microbe interactions7,18. Here, we show that indeed Mesh constitutively regulates the basal Duox expression via phosphorylation of the MAP kinases JNK and ERK but not P38. We also show that these phosphorylation events are mediated by Arrestins, the expression of which is also controlled by the pathway. Indeed, previous studies have shown that the mammalian β-arrestin acts as a signal transduction scaffold for MAP kinases, and is thereby essential for the MAPK signaling cascade33.
The current findings suggest that Mesh-mediated Duox induction is a bacterium-specific response. However, three important questions remain unanswered: 1) is this pathway responsive to all the commensal bacteria or specific classes? 2) how does Mesh sense bacteria, the bacterial surface PAMPs or secreted metabolites? and 3) is this pathway applicable to the mammalian system? Addressing these questions in our future endeavors may not only provide a complete picture of delicate interactions between microbiota and host, but also a conceptual advancement in general.
Methods
Mosquitoes, Drosophila and bacteria
Aedes aegypti (the Rockefeller strain) was maintained in the laboratory in a low-temperature illuminated incubator (model 818, Thermo Electron Corporation, Waltham, MA, USA) at 26°C and 80% humidity according to standard rearing procedures23,42. The 5-day old female mosquitoes were used for the experiment. D. melanogaster were maintained on standard cornmeal-agar medium at 25°C in 60% relative humidity. Drosophila S2 cells were cultured at 28°C in Schneider’s Drosophila medium, which was supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, and 100 U/ml each of penicillin and streptomycin. Both C. testosteroni and C. meningosepticum from the midguts of A. aegypti, and A. thailandicus from the midguts of D. melanogaster were isolated, and subsequently cultured on LB plates without any antibiotics at 37°C.
Antibody
A fragment of the AaMesh gene (2980 bp-3522 bp) was amplified from A. aegypti cDNA and cloned into a pET-28a (+) expression vector. The cloning primers are presented in Supplementary Table 6. The recombinant peptide was expressed in the E. coli BL21 DE3 strain, with the insoluble form in inclusion bodies. The proteins were resolved with 8 M urea and purified using a purification kit (Clontech, Cat. No# 635515). Polyclonal antibodies were produced by 3 boosting immunizations in C57BL/6 mice. The antibodies for the phosphorylated DmERK and DmJNK were purchased from Sigma Aldrich (Cat. No# M9692) and Thermo Fisher (Cat. No# MA5-14943), respectively. These antibodies for DmERK and DmJNK can efficiently detect the phosphorylated AaERK and AaJNK in A. aegypti. The antibodies for the tags were purchased from the Medical & Biological Lab (MBL, Japan).
Genetic manipulation in the Drosophila S2 cells
DmMesh was amplified from the Drosophila cDNA library and then cloned into the pAc5.1-V5/His A vector (Invitrogen, Cat. No# V4110-20). The GFP gene was cloned into the same plasmid as a mock control. The recombinant plasmids were designated pAc-DmMesh and pAc-GFP, respectively. For gene silencing study, the dsRNA was synthesized as previously described43. The S2 cells were seeded at 2×106 cells/mL per well in a 6-well plate. Then, dsRNA or recombinant plasmids were premixed with Effectene® (Qiagen, Cat. No# 301425), according to the manufacturer’s instructions, and consequently used for transfection.
Silencing genes in the A. aegypti mosquitoes
The synthesis of dsRNA was performed as previously described43. The primers are shown in Supplementary Table 6. We described the detailed procedures used for gene silencing in mosquitoes elsewhere23,42,43. Briefly, the mosquitoes were cold-anesthetized on a cold tray (BioQuip, USA), and 1 μg/300 nl of dsRNA was subsequently microinjected into the mosquito thoraxes. The injected mosquitoes were allowed to recover under standard rearing conditions and were used for the subsequent investigations.
Generation of the homozygous DmMesh−/− flies
Using a two-component CRISPR/Cas9-mediated gene targeting system44, a Mesh mutation line with mutations and deletions was generated via the non-homologous end joining (NHEJ) pathway. Briefly, we co-injected Cas9-mRNA and Mesh-specific gRNAs into w1118 early embryos. The recovered male F0 flies were then crossed to w; atm/TM6B.Act5c-GFP flies to obtain the F1 progeny with germline-transmitted mutations. It was balanced with a GFP-expressing Tm6B balancer to distinguish homozygous DmMesh−/− flies from heterozygous flies. The mutation in the Mesh locus was consequentially verified by PCR and DNA sequencing45. Primers used for gRNA, PCR and sequencing are listed in Supplementary Table 6.
RNAi Drosophila strains
Drosophila DmMesh RNAi (VDRC, Cat. No# 108297), DmArrestin-1 RNAi (VDRC, Cat. No# 109860), and DmArrestin-2 RNAi (VDRC, Cat. No# 20989) lines were provided by the Vienna Drosophila RNAi Center. DmERK RNAi (THFC, Cat. No# THU5780), DmJNK RNAi (THFC, Cat. No# THU1781), DmDuox RNAi (THFC, Cat. No# THU1093), DmNox RNAi (THFC, Cat. No# THU0880) and Ubi-GAL4 (THFC, Cat. No# TB00152) were obtained from the Tsinghua Fly Center (THFC, Beijing, China). UAS-GFP RNAi (VDRC, Cat. No# 41557) was purchased from the Bloomington Drosophila Stock Center (Bloomington, IN, USA) and NP3084 (DGRC, Cat. No# 113094) was purchased from the KYOTO Stock Center (DGRC) (KYOTO, Japan).
Rescuing DmDuox into DmMesh RNAi and DmArrestins RNAi Drosophila strain
The entire open reading frame of the DmDuox-cDNA were subcloned into the pWALIUM20 vector46 to obtain the UAS-DmDuox-cDNA construct, respectively. These constructs were then used to generate transgenic rescue flies through the φC31-mediated recombination between attB and the corresponding pseudo attP sites. The UAS-DmDuox-cDNA was built on the second chromosome. The transgenic flies (UAS-DmDuox-cDNA) were used to rescue the phenotypic defects of DmMesh RNAi, DmArrestin-1 RNAi and DmArrestin-2 RNAi flies, respectively. In briefly, the transgenic flies with DmDuox over-expression (UAS-DmDuox-cDNA strain) were used to crossed with UAS-DmMesh-RNAi flies to obtain the final strains carrying one copy of the UAS-DmMesh-RNAi construct with one copy of the UAS-DmDuox-cDNA. As well, we obtained the final strains carrying one copy of either DmArrestin-1 RNAi or DmArrestin-2 RNAi construct with one copy of the UAS-DmDuox-cDNA flies.
Generation of germ-free Drosophila and bacterial oral feeding
To generate germ-free flies, eggs were washed in sterile deionized water, immersed in 2.7% sodium hypochlorite solution for 2 minutes. Embryos were subsequently washed twice in 70% ethanol, followed by three washes with sterile water. Embryos were transferred to axenic standard fly food vials for rearing. The bacteria cells, which were collected in exponential growth phase, were mixed with the standard Drosophila food to feed the germ-free flies.
Antibiotic treatment mosquito and bacterial oral feeding
Mosquitoes were treated using cotton balls moistened with a 10% sucrose solution including 20 units of penicillin and 20 μg of streptomycin per milliliter for 3 days47,48. Then, the mosquitoes were starved for 24 hours to allow the antibiotics metabolized prior to bacterial challenge. The mosquitoes were decontaminated in 70% ethanol and rinsed in sterile PBS, and the midguts were dissected under aseptic conditions. The removal of the microbes was confirmed by qPCR using a universal bacteria primer27 and a colony forming units (CFU) assay12. We furthermore used the specific primers12 for C. testosteroni, C. meningosepticum and S. marcescens, which are found to be ones of most abundant cultivable bacteria species in the A. aegypti midgut12, to ensure the removal by antibiotic treatment. After the antibiotic treatment, the mosquitoes were fed a mixture containing either C. testosteroni or C. meningosepticum with fresh mouse blood (1:1 v/v) via the Hemotek® feeding system (6W1, Hemotek Limited, England). All supplies used in the mosquito feeding experiment (feeders, membrane, tips, etc.) were aseptic. The bacteria-fed mosquitoes were sacrificed for the gut isolation at several hours after blood feeding. Because of the short time interval, the mosquitoes will not receive a new portion of food that may introduce bacterial contamination.
Counting the oral-introduced bacterial number per insect gut
To determine the number of acquired bacteria in the gut of individual insect, a serial numbers of bacteria was exploited to feed the antibiotic-treated mosquitoes or germ-free flies. Twelve fed insects in each group were randomly selected for the gut isolation under aseptic conditions, and were ground in sterile PBS. The lysates of different dilutions were applied on LB plates without antibiotics. After overnight culture at 37°C, the number of bacteria on the plate was counted to calculate the number of acquired bacteria by per insect. For acquired bacteria counting in the mosquito midguts, a serial number of either C. testosteroni or C. meningosepticum (0.5 OD, 5 OD and 50 OD) were exploited to feed the antibiotic-treated mosquitoes. Twelve fed mosquitoes in each group were randomly selected for the gut isolation, and subsequently we did a bacterial count using a CFU assay. The number of bacteria ingested by a mosquito was approximately 5.4×105 cells in 0.5 OD, 4.3×106 cells in 5 OD, and 3.7×107 cells in 50 OD C. testosteroni-fed mosquitoes, and 5.2×105 cells in 0.5 OD, 8.3×106 cells in 5 OD, and 9.6×107 cells in 50 OD C. meningosepticum-fed mosquitoes (Supplementary Fig. 18a,b). For bacteria counting in the fly guts, a serial amounts of A. thailandicus (1 OD, 50 OD and 250 OD), gradually mixed with the standard Drosophila food respectively, was exploited to feed the germ-free flies. Twelve fed flies in each group were randomly selected to isolate the guts for bacterial counting. The quantity of bacteria ingested by a fly was approximately 4.4×104 cells in 1 OD, 6.0×106 cells in 50 OD, and 4.9×108 cells in 250 OD bacteria-fed flies (Supplementary Fig. 18c).
Bacterial isolation from the insect guts
The insect surface was sterilized with 70% ethanol and washed twice with sterile PBS. The guts of mosquitoes or flies were carefully removed from the abdomen into PBS buffer, and ground adequately under aseptic conditions to reduce contamination from surrounding bacteria. The gut lysates were plated on LB plate without antibiotics, and cultured at 37°C. We identified C. testosteroni, C. meningosepticum from Aedes aegypti, and A. thailandicus from Drosophila melanogaster.
Measurement of gut microbiome by 16S rRNA qPCR
The total RNA of the guts was extracted from the sample with the RNeasy Mini Kit (Qiagen, Cat. No# 74106). cDNA was randomly reverse-transcribed by an iScript cDNA Synthesis Kit (Bio-Rad, Cat. No# 1708891). A commercial matrix was used for qPCR assay (Bio-Rad Cat. No#172-5121). The 16S rRNA was amplified using universal primers27 and bacterial specific primers12. The burden of the gut microbiome was normalized to A. aegypti actin (AAEL011197) or D. melanogaster actin (CG12051).
H2O2 assay for determination of ROS activity
The insect guts were dissected in PBS with 2 mg/ml of the catalase inhibitor 3-amino-1,2,4-triazole (A8056-10G, Sigma) at different time points. After homogenization, the samples were filtered through a spin filter with a 10K molecular weight cutoff (Corning, Corning Spin-XUF, Cat. No# 431486). The eluate from each experimental group was then collected and tested using a hydrogen peroxide assay kit (BioVision, Cat. No# K265-200). The fluorescence intensity was measured at an excitation wavelength of 550 nm and an emission wavelength of 590 nm using a fluorescence microplate reader according to the manufacturer’s instructions.
Dihydroethidium staining
The mosquito midguts were dissected in PBS containing the catalase inhibitor 3-amino-1,2,4-triazole (A8056, Sigma). Immediately after dissection, the midguts were incubated with 2 μM dihydroethidium (DHE) (D7008, Sigma) in PBS at RT for 30 min in the dark. Then, the midguts were fixed with 4% PFA for 30 min and incubated for an additional 30 min with Triton X-100. Nuclei were stained blue with To-Pro-3 iodide (Thermo-Fisher Scientific, Cat. No# T3605). Slides were imaged using a 10×objective lens on a Zeiss LSM 780 meta confocal microscope (Carl Zeiss, Germany) in a multi-track mode.
Smurf assay
The dyes Blue dye no. 1 or Red dye no. 40 purchased from Sigma-Aldrich (2.5% wt/vol) were premixed with Drosophila standard food respectively. Flies were reared on the medium with dyes for 12 hours. The dissemination of dyes were subsequently recorded in the fed Drosophila bodies.
RNA-Seq analysis of mosquito midguts
Total RNA was extracted with TRIzol (Ambion Cat. No# 15596018) from pools of 60 midguts of the AaMesh dsRNA-inoculated (Day 3 and Day 6) and AaMesh antiserum-fed (18 hours) female A. aegypti mosquitoes. The samples were delivered to the Beijing Genomics Institute (Shenzhen, China) for commercial RNA-Seq services and data analysis. Clean reads were mapped to the A. aegypti transcript database using SOAPaligner/SOAP2 mismatches. The number of clean reads for each gene was calculated and then normalized to Reads per Kb per Million reads (RPKM), which associates read numbers with gene expression levels. The log2 ratio (read number in AaMesh-suppressed midgut/read number in control midgut) was exploited to determine gene regulation. Immune genes with log2 ratio ≤ −0.4 were selected for further analysis38,48. A rigorous algorithm was used to screen for differentially expressed genes in each group. The sequencing data were deposited in the Short Read Archive (NCBI) with accession number GSE79070.
Statistics
Animals were randomly allocated into different groups. Mosquitoes and flies that died before measurement were excluded from analysis. The investigators were not blinded to the allocation during the experiments or to the outcome assessment. Descriptive statistics have been provided in the figure legends. Given the nature of the experiments and the type of samples, differences in continuous variables were assessed with the non-parametric Mann-Whitney test. All analyses were performed using GraphPad Prism statistical software.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please see methods for specific data sets.
Supplementary Material
Acknowledgments
This work was funded by the grants from the National Key Research and Development Plan of China (2016YFD0500400, 2016YFC1201000, 2016ZX10004001-008), the grants from the National Natural Science Foundation of China (81422028, 81571975, 61472205, 31171278, and 31271542), and the grant from National Institute of Health of the United States (AI103807). We thank Professor Won-Jae Lee from Seoul National University to provide Erwinia carotovora carotovora 15 for the study. We thank that Professor George K. Christophides from Imperial College London provided critical suggestions for this manuscript. G.C. is a Newton Advanced Fellow awarded by the Academy of Medical Sciences and the Newton Fund, and a Janssen Investigator of Tsinghua University. We thank the technical supports from the Core Facility of Center for Life Sciences and Center of Biomedical Analysis (Tsinghua University).
Footnotes
The authors declare that they have no competing financial interests.
Author Contributions
G.C. designed the experiments and wrote the manuscript; X.X. performed the majority of the experiments and analyzed data; X.P., R.Z. and Y.Z. helped with the RNA isolation and qPCR detection; L.Y. and G.G. provided the Drosophila systems and contributed in the investigations in Drosophila. P.W. contributed experimental suggestions and strengthened the writing of manuscript. All authors reviewed, critiqued and provided comments to the text.
References
- 1.Koropatnick TA, et al. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 3.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 4.Clark RI, et al. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12:1656–1667. doi: 10.1016/j.celrep.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 6.Ha EM, et al. Regulation of DUOX by the Galphaq-phospholipase C beta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009a;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Ha EM, et al. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol. 2009b;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- 8.Lhocine N, et al. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe. 2008;4:147–158. doi: 10.1016/j.chom.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity. 2011;35:770–779. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Ryu JH, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 11.Cullen TW, et al. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 2015;347:170–175. doi: 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang XJ, et al. Mosquito C-type lectins maintain gut microbiome homeostasis. Nature microbiology. 2016 doi: 10.1038/nmicrobiol.2016.23. Article number: 16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde S, Rasgon JL, Hughes GL. The microbiome modulates arbovirus transmission in mosquitoes. Curr Opin Virol. 2015;15:97–102. doi: 10.1016/j.coviro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanellopoulos J. Studying host-microbiota interactions in Drosophila melanogaster. Biomed J. 2015;38:275. doi: 10.4103/2319-4170.162482. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira JH, et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Z, et al. The dual oxidase gene BdDuox regulates the intestinal bacterial community homeostasis of Bactrocera dorsalis. ISME J. 2015;10:1037–1050. doi: 10.1038/ismej.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz-Albiter H, Sant’Anna MR, Genta FA, Dillon RJ. Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the sand phlebotomine fly Lutzomyia longipalpis. J Biol Chem. 2012;287:23995–24003. doi: 10.1074/jbc.M112.376095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Waterhouse RM, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 21.Casasnovas JM, Larvie M, Stehle T. Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J. 1999;18:2911–2922. doi: 10.1093/emboj/18.11.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dörig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 23.Xiao X, et al. Complement-related proteins control the flavivirus infection of Aedes aegypti by inducing antimicrobial peptides. PLoS Pathog. 2014;10:e1004027. doi: 10.1371/journal.ppat.1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, et al. A neuron-specific antiviral mechanism prevents lethal flaviviral infection of mosquitoes. PLoS Pathog. 2015;11:e1004848. doi: 10.1371/journal.ppat.1004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi Y, Yanagihashi Y, Furuse M. A novel protein complex, Mesh-Ssk, is required for septate junction formation in the Drosophila midgut. J Cell Sci. 2012;125:4923–4933. doi: 10.1242/jcs.112243. [DOI] [PubMed] [Google Scholar]
- 26.Rämet M, et al. Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity. 2001;15:1027–1038. doi: 10.1016/s1074-7613(01)00249-7. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 29.Robinson SW, Herzyk P, Dow JA, Leader DP. FlyAtlas: database of gene expression in the tissues of Drosophila melanogaster. Nucleic Acids Res. 2013;41:D744–750. doi: 10.1093/nar/gks1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RM, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. Embo J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakrabarti S, Poidevin M, Lemaitre B. The Drosophila MAPK p38c regulates oxidative stress and lipid homeostasis in the intestine. PLoS Genet. 2014;10:e1004659. doi: 10.1371/journal.pgen.1004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez JL, et al. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl Trop Dis. 2012;6:e1561. doi: 10.1371/journal.pntd.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nittaya Pitiwittayakul PY, Winai Chaipitakchonlatarn YY, Theeragool G. Acetobacter thailandicus sp. nov., for a strain isolated in Thailand. Ann Microbiol. 2015;65:1855–1863. [Google Scholar]
- 36.Chaston JM, Newell PD, Douglas AE. Metagenome-wide association of microbial determinants of host phenotype in Drosophila melanogaster. MBio. 2014;5:e01631. doi: 10.1128/mBio.01631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer F, Backhed F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015;8:372–379. doi: 10.1038/mi.2014.74. [DOI] [PubMed] [Google Scholar]
- 38.Lee KA, et al. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Wang WX, et al. Molecular and functional characterization of a c-type lysozyme from the Asian corn borer, Ostrinia furnacalis. J Insect Sci. 2009;9:17–29. doi: 10.1673/031.009.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu LP, Sun BG, Li J, Sun L. Characterization of a c-type lysozyme of Scophthalmus maximus: expression, activity, and antibacterial effect. Fish Shellfish Immunol. 2013;34:46–54. doi: 10.1016/j.fsi.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Gravato-Nobre MJ, Vaz F, Filipe S, Chalmers R, Hodgkin J. The invertebrate lysozyme effector ILYS-3 is systemically activated in response to danger signals and confers antimicrobial protection in C. elegans. PLoS Pathog. 2016;12:e1005826. doi: 10.1371/journal.ppat.1005826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, et al. Transmission-blocking antibodies against mosquito C-type lectins for dengue prevention. PLoS Pathog. 2014;10:e1003931. doi: 10.1371/journal.ppat.1003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng G, et al. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell. 2010;142:714–725. doi: 10.1016/j.cell.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Z, et al. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics. 2013;195:289–291. doi: 10.1534/genetics.113.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HJ, Lee HJ, Kim H, Cho SW, Kim JS. Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome Res. 2009;19:1279–1288. doi: 10.1101/gr.089417.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni JQ, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods. 2011;8:405–407. doi: 10.1038/nmeth.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y, Ng FS, Jackson FR. Comparison of larval and adult Drosophila astrocytes reveals stage-specific gene expression profiles. G3 (Bethesda) 2015;5:551–558. doi: 10.1534/g3.114.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please see methods for specific data sets.