Abstract
Background: As the most predominant tumor-infiltrating immune cells, tumor-associated macrophages (TAMs) are associated with poor outcome in multiple solid cancers and play important roles in cancer progression. Cancer stem cells (CSCs) may account for metastasis and recurrence after cancer therapy. However, the association between TAMs and CSCs is not clarified in gastric cancer (GC). The aim of the present study was to evaluate the effects of TAMs on CSCs in GC and find out the risk factors to predict recurrence and prognosis.
Material and methods: This study included consecutive 236 patients with histologically confirmed primary GC. TAMs marker CD163 and CSCs-related proteins were detected by immunohistochemistry (IHC) in GC tissues and their prognostic values were all investigated.
Results: High expression of CD163+ TAMs was found in patents with aggressive characteristics, especially for patents with recurrence. There existed a significant correlation between high expression of CD163 and CSCs-related markers in GC tissues. In patients with recurrence, high-expression of CD163 TAMs was an independent worse prognostic factor.
Conclusion: High infiltration of TAMs was related to aggressive behavior, associated with aberrant expression of CSC markers, and an independent worse prognostic factor in GC. Targeting TAMs may be a potential treatment strategy for GC, including patients with recurrence.
Keywords: Gastric cancer, Tumor-associated macrophages (TAMs), Cancer stem cells (CSCs), Recurrent, Prognosis.
Background
Gastric cancer (GC) is one of the most aggressive malignancies with poor prognosis 1. The prevalence of GC in China is amongst the highest in the world, along with Japan and Korea 1,2. Despite recent advances in diagnostic and therapeutic strategies, the prognosis of GC patients remains poor due to tumor recurrence and metastasis.
Cancer stem cells (CSCs), defined as a small subpopulation of cancer cells with the ability of self-renewal and pluripotency, may account for tumor progression, metastasis and recurrence after therapy 3-5. Recent data suggest that CSCs rely on a specialized tumor microenvironment (TME) or niche 6. However, the effect of TME on cancer cell stemness remains unclear on GC. Tumor-associated macrophages (TAMs), the most abundant immune-related stromal cells in TME 7, are pivotal orchestrators of TME and directly affect cancer cell growth, neoangiogenesis, and extracellular matrix remodeling 8. TAMs of GC often display an alternatively activated phenotype, promoting tumor invasion and metastasis, and are associated with poor prognosis in cancer patients 9-11.
There have been few studies on the correlation between infiltration of TAMs and expression of CSCs markers in GC, and little is known on how TAMs affect CSCs properties in GC. We speculated that TAMs might induce cancer cell stemness and consequently promote GC recurrence and metastasis. The purpose of the present study was to examine the correlation of infiltrated polarized CD163+ TAMs with expression of CSCs markers in GC tumors, using immunohistochemistry (IHC). The clinicopathological characteristics of GC, recurrence patterns, risk factors to predict recurrence and prognosis were all analyzed.
Materials and Methods
Patients and Specimens
This study included consecutive 236 patients with histologically confirmed primary GC, all of whom underwent radical gastrectomy with curative intend between 2007 and 2009 at the Nanjing Drum Tower Hospital and the 101th Hospital of People Liberation Army. They included 150 men and 86 women, ranging from 20 to 84 years of age (mean, 57.8 years). Patients lost during follow up were excluded. All specimens were pathologically reassessed independently by two gastrointestinal pathologists, according to the WHO criteria 12. None of the patients received neoadjuvant or adjuvant chemotherapy before the operation.
This retrospective study protocol was approved by the clinical research ethics committee of the Nanjing Drum Tower Hospital and the 101th Hospital which complies with the Declaration of Helsinki.
Immunohistochemistry
All specimens were fixed in 10% buffered neutral formalin, embedded in paraffin wax, and cut into 4-μm-thick sections. After deparaffinization in xylene and rehydration in a graded series of ethanol, the sections were washed twice (5 min per wash) in phosphate buffered saline (PBS). Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 15 min. After washing in PBS, the slides were heated at 120°C in an autoclave for 10 min for antigen retrieval. A nonspecific staining blocking agent (Dako, Kyoto, Japan) was used to prevent nonspecific binding. Slides were incubated with the mouse monoclonal anti-CD163 antibody and monoclonal antibodies against CSCs markers, CD133 and CD44, overnight at 4°C, and washed in PBS for 10 min. The information on antibody manufacturers, clones and concentrations was listed in Table 1. Subsequently, the slides were treated with a biotinylated secondary antibody for 10 min at room temperature. After washing in PBS, the slides were reacted with 3-3'-diamino-benzidine (DAB) solution for 5 min and counterstained with hematoxylin. Positive controls consisted of each staining run and consisted of GCs known to express each of the antigens. Negative controls were normal mouse serum instead of the primary antibody.
Table 1.
The list of manufacturers, origin, clones and concentrations of the antibodies.
| Parameters | Manufacturer | Origin | Clone | Concentration |
|---|---|---|---|---|
| Anti-CD163 antibody | Abcam, Cambridge, MA, USA | Mouse | mono-clone | 1: 100 |
| Anti-CD44 antibody | Abcam, Cambridge, MA, USA | Mouse | mono-clone | 1: 100 |
| Anti-CD133 antibody | Abcam, Cambridge, MA, USA | Mouse | mono-clone | 1: 100 |
Evaluation of Immunohistochemical Staining
All slices were independently evaluated by two pathologists who were blinded to the clinical data. The percentage of immunoreactive cells was scored on a scale of 0 to 4 from no staining as 0, 1 to 10% of cells stained as score 1, 11 to 50% as 2, 51 to 80% as 3, and 81 to 100% as 4. The staining intensities were graded from 0 as negative, to 1 as weak, 2 as moderate, and 3 as strong, respectively. The final immunohistochemical score (IHS) was obtained by calculating the product of the staining intensity and the positive cell percentage. Cases with a score of less than 5 were considered as low expression, and those with ≥6 were regarded as high expression.
Stained sections were microscopically scrutinized at ×200 magnification. TAMs density identified by CD163 expression were estimated (per mm2) at ×400 magnification in 5 fields per case. The average number of positive cells in each case was determined. Low density was defined as the number of positive cells smaller than the average; otherwise, high density was considered.
Follow-up
All GC patients underwent radical gastrectomy with nodal dissection. No major perioperative complications occurred in GC patients who were all discharged home after a short hospital stain period. The closing date for follow-up was January 31, 2016. All patients were followed up every 3 months during the first 2 years after discharge and every 6 months thereafter. GC recurrence was confirmed by detection of significantly increased levels of tumor seral biomarkers, including Carcinoembryonic antigen (CEA), Alpha Fetal Protein (AFP), CA19-9 and CA125, and imaging detection of tumor in distant organs by chest X-Ray, barium meal, abdominal ultrasonography (US), computed tomography (CT), and upper endoscopy, during the follow-up period. The locations and times of tumor recurrence were recorded. The follow-up time was calculated from the day of surgery to the day of detection of tumor recurrence or death. Overall survival (OS) was defined as the interval between surgery and last visit or death of any causes. Disease-free survival was measured in patients in whom a tumor was present and calculated as the time from end of treatment to date at which recurrence was detected or most recent follow-up in which recurrence was not detected (censored).
Statistical Analysis
All statistical analyses were performed with SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). The correction between expression of CD163, CD44 and CD133 and clinicopathological features was analyzed by the χ2 and Fisher's exact tests. The Spear-man's rank correlation coefficient was used for analyzing the association of CD133 and CD44 expression levels with CD163 expression levels. Post-operative survival curves were estimated using the Kaplan-Meier method with the log-rank test. For multivariate analysis, the prognostic factors were analyzed using the Cox's proportional hazard model. Overall and disease-specific survivals were the designated endpoints. Differences with a p value of 0.05 or less were considered statistically significant.
Results
Patient Characteristics
The detailed clinicopathological characteristics of GC patients after resection were shown in Table 2. The median follow-up period after surgery was 33.14 months. When the follow-up was over, 86 patients were alive without recurrence and additional 12 with GC recurrence, while 138 were dead (63 died of other diseases without recurrence and 75 deceased due to tumor recurrence). The mean OS of 236 patients was 39.42±19.68 months, and the 5-year survival rate of all enrolled patients was 22.4%.
Table 2.
The relationship between expression of CD163 expression and clinicopathological features of gastric cancer.
| Parameters | N | High CD163 positive (%) | χ2-value | P value |
|---|---|---|---|---|
| Age(yr) | 2.097 | 0.148 | ||
| <60 | 129 | 65 (50.4) | ||
| ≥60 | 107 | 64 (59.8) | ||
| Gender | 0.929 | 0.335 | ||
| Male | 151 | 79 (52.3) | ||
| Female | 85 | 50 (58.8) | ||
| Tumor location | 0.752 | 0.861 | ||
| Upper third | 43 | 23 (53.5) | ||
| Middle third | 54 | 29 (53.7) | ||
| Lower third | 122 | 66 (54.1) | ||
| Diffused | 17 | 11 (64.7) | ||
| Differentiation | 4.734 | 0.030 | ||
| Well/Moderate | 86 | 39 (45.3) | ||
| Poor | 150 | 90 (60.0) | ||
| Lauren type | 0.311 | 0.577 | ||
| Intestinal | 110 | 58 (52.7) | ||
| Diffuse | 126 | 71 (56.3) | ||
| Tumor size(cm) | 4.623 | 0.032 | ||
| <5 | 99 | 46 (46.5) | ||
| ≥5 | 137 | 83 (60.6) | ||
| Borrmann type | 3.755 | 0.289 | ||
| Ⅰ | 8 | 3 (37.5) | ||
| Ⅱ | 49 | 22 (44.9) | ||
| Ⅲ | 103 | 59(57.3) | ||
| Ⅳ | 76 | 45 (59.2) | ||
| Depth of invasion | 14.577 | 0.002 | ||
| pT1 | 15 | 3 (20.0) | ||
| pT2 | 35 | 13 (37.1) | ||
| pT3 | 88 | 52 (59.1) | ||
| pT4 | 98 | 61 (62.2) | ||
| Lymph node status | 5.089 | 0.024 | ||
| N0/N1 | 77 | 34 (44.2) | ||
| N2/N3 | 159 | 95 (59.7) | ||
| Vascular invasion | 4.408 | 0.036 | ||
| Absent | 74 | 33 (44.6) | ||
| Present | 162 | 96 (59.3) | ||
| Recurrence | 11.377 | 0.001 | ||
| Absent | 149 | 69 (46.3) | ||
| Present | 87 | 60 (69.0) | ||
| TNM stage | 5.814 | 0.016 | ||
| Ⅰ-Ⅱ | 80 | 35 (43.75) | ||
| Ⅲ-Ⅳ | 156 | 94 (60.3) | ||
| Serum CEA level (μg /L) | 1.956 | 0.162 | ||
| <5 | 90 | 44 (48.9) | ||
| ≥5 | 146 | 85 (58.2) |
Infiltration of TAMs and Association with Clinicopathologic Features of Tumors
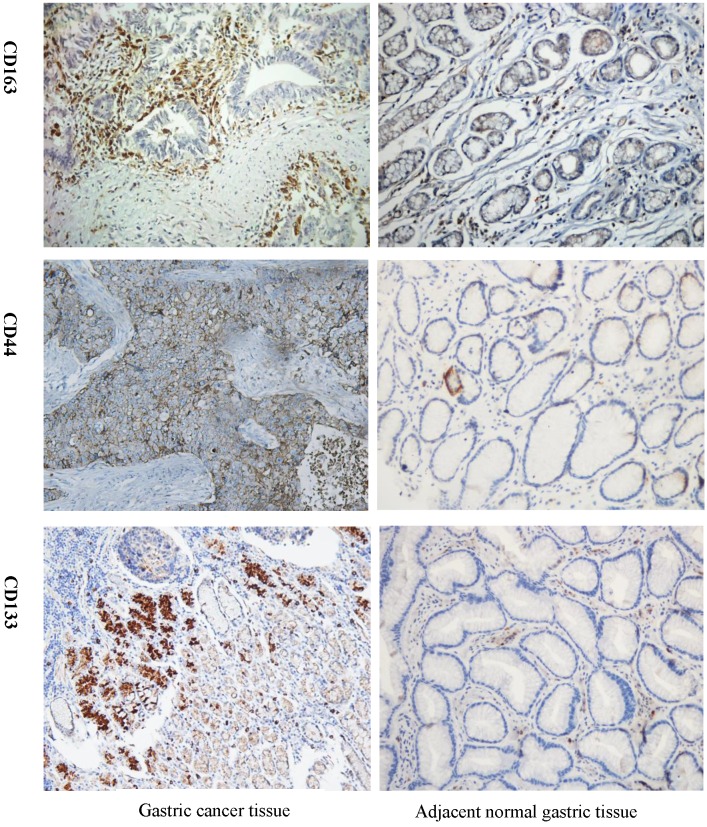
As shown in Figure 1, expression of CD163 in GC was significantly higher than that in the adjacent benign gastric mucosa. Immunoreactivity of CD44 was observed primarily in the cytoplasm and membrane of tumor cells, but weak or negative in the majority of gastric mucosa (Fig. 1). CD133 stained the membrane of tumor cells, but was negative for most gastric mucosa (Fig. 1). High expression of CD163 was found in 67.8% (59/87) of GC cases with recurrence, significantly higher than that without recurrence (47.0%, 70/149). CD163 expression was significantly related to the TNM stage, nodal metastasis, the depth of invasion, tumor differentiation, size, lymphovascular invasion, but not to the patient age, gender, tumor Lauren type, Borrmann type, location, and serum levels of CEA (Table 2).
Figure 1.
Detection of CD163, CSCs markers CD44 and CD133 expression in GC tissue and adjacent normal tissue by IHC. Strong CD163 immunoreactivity was identified in poorly differentiated cancer. CD44 and CD133 expression was barely seen in normal tissue but was observed in GC tissue. GC=gastric cancer, IHC=immunohistochemistry.
As shown in Table 3, high expressions of CD163 was significantly associated with poor differentiation, large size, deep invasion depth, advanced TNM stage, node metastasis, lymphovascular invasion; expression of CSCs markers was significantly more frequently associated with overall recurrence, whereas patient age, gender, tumor histological type, location, Borrmann type, and serum levels of CEA level were.
Table 3.
Characteristics of gastric cancer with recurrence compared with patients without recurrence.
| Parameters | Recurrence (n=87) |
No recurrence (n=149) |
χ2-value | P value |
|---|---|---|---|---|
| Age(yr) | 3.157 | 0.076 | ||
| <60 | 41 | 88 | ||
| ≥60 | 46 | 61 | ||
| Gender | 0.561 | 0.454 | ||
| Male | 53 | 98 | ||
| Female | 34 | 51 | ||
| Tumor location | 2.732 | 0.435 | ||
| Upper third | 19 | 24 | ||
| Middle third | 22 | 32 | ||
| Lower third | 39 | 83 | ||
| Diffused | 7 | 10 | ||
| Differentiation | 4.664 | 0.031 | ||
| Well/Moderate | 24 | 62 | ||
| Poor | 63 | 87 | ||
| Lauren type | 0.015 | 0.903 | ||
| Intestinal | 41 | 69 | ||
| Diffuse | 46 | 80 | ||
| Tumor size(cm) | 4.201 | 0.040 | ||
| <5 | 29 | 70 | ||
| ≥5 | 58 | 79 | ||
| Borrmann type | 2.664 | 0.508 | ||
| Ⅰ | 2 | 6 | ||
| Ⅱ | 14 | 35 | ||
| Ⅲ | 42 | 61 | ||
| Ⅳ | 29 | 47 | ||
| Depth of invasion | 6.030 | 0.110 | ||
| pT1 | 2 | 13 | ||
| pT2 | 12 | 23 | ||
| pT3 | 30 | 58 | ||
| pT4 | 43 | 55 | ||
| Lymph node status | 8.933 | 0.003 | ||
| N0/N1 | 18 | 59 | ||
| N2/N3 | 69 | 90 | ||
| Vascular invasion | 4.482 | 0.034 | ||
| Absent | 20 | 54 | ||
| Present | 67 | 95 | ||
| TNM stage | 10.729 | 0.001 | ||
| Ⅰ-Ⅱ | 18 | 62 | ||
| Ⅲ-Ⅳ | 69 | 87 | ||
| CD163 | 9.622 | 0.002 | ||
| Low | 28 | 79 | ||
| High | 59 | 70 | ||
| CD44 | 6.246 | 0.012 | ||
| Low | 27 | 71 | ||
| High | 60 | 78 | ||
| CD133 | 9.028 | 0.003 | ||
| Low | 28 | 78 | ||
| High | 59 | 71 | ||
| Serum CEA level (μg /L) | 1.127 | 0.288 | ||
| <5 | 37 | 53 | ||
| ≥5 | 50 | 96 |
Time to Recurrence and Recurrence Patterns
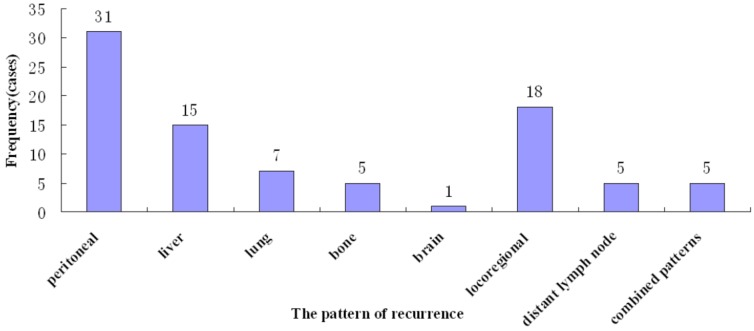
Tumor recurrence occurred in 87 (36.8%) patients, most (53, 60.9%) within 2 years. The median time to recurrence was 18.0 months (range, 6 - 78). Various recurrence patterns were noted, including peritoneal relapse (31, 35.6%), hematogeneous spread (28, 31.5%) to the liver (n=15), lung (n=7), bone (n=5), and brain (n=1). Locoregional recurrence was found in 18 (20.7%), distant lymph node metastasis in 5 (5.7%), and combined patterns in 5 (5.7%) (Figure 2).
Figure 2.
Pattern of recurrence of after curative gastrectomy.
Association of TAMs with Expression of Cancer Stem Cell Markers
As shown in Table 4, high expression of neoplastic cells in GC were found in over half cases for CD44 (58.5%, 138/236) and CD133 (55.1%, 130/236). There existed a significant correlation between high expression of CD163 with high expression of CD44 (r=0.303, P<0.001), and CD133 (r=273, P<0.001).
Table 4.
The association between CD163 with CSCs markers: CD44 and CD133.
| CD163 | r | P | ||
|---|---|---|---|---|
| Low | High | |||
| CD44 | 0.303 | <0.001 | ||
| Low | 62 | 36 | ||
| High | 45 | 93 | ||
| CD133 | 0.273 | <0.001 | ||
| Low | 64 | 42 | ||
| High | 43 | 87 | ||
Prognostic value of TAM Infiltration in Patients with Recurrent Gastric Carcinoma
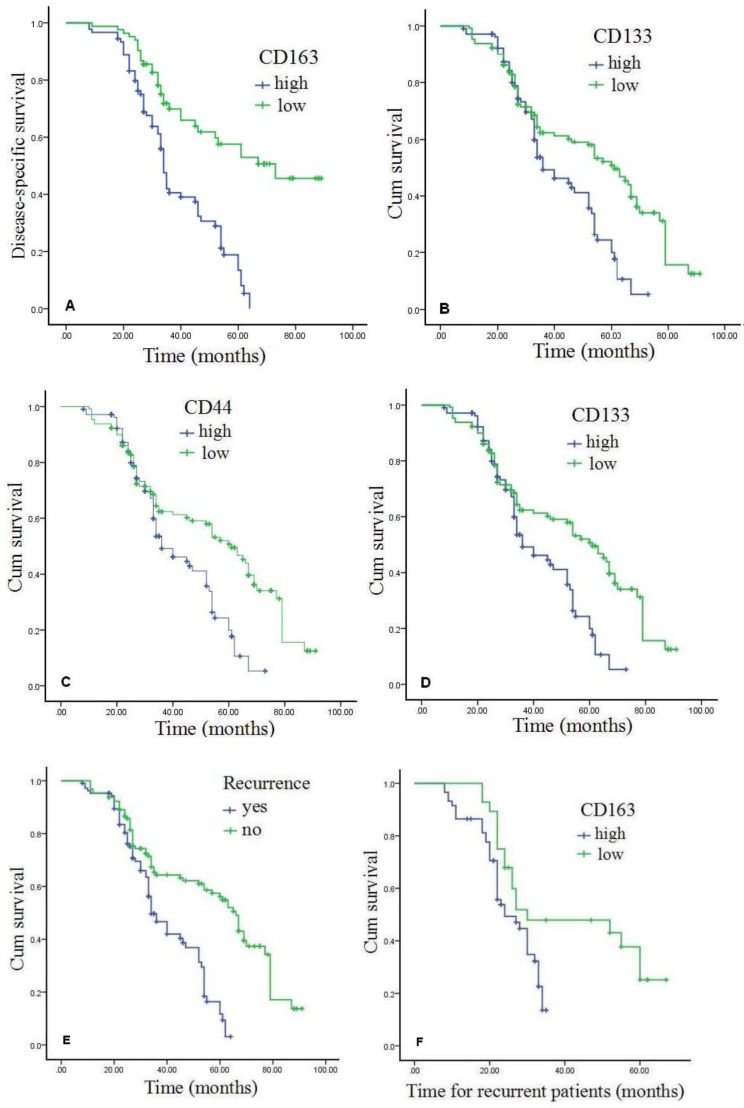
As shown in the Kaplan-Meier survival curves (Figure 3A-B, Table 5), overall DSS and OS rates for the cohort were 56.6% and 41.5%, respectively, which were significantly shorter for patients with high CD163 expression than those with low CD163 expression (Figures 3A and 3B). High expression of cancer stem cell markers, CD44 and CD133, was also statistically correlated with poor OS (P<0.05) (Figure 3C-D). Moreover, as exhibited in Table 4, multivariate Cox analysis showed that high expression of CD163 and over-expression of CD44 were independent worse prognostic factors for DSS and OS rates in GC patients with recurrence (P<0.05). Compared with GC patients without recurrence, DSS and OS rates for patients with recurrence were significantly lower (P<0.001) (Figures 3E). In patients with recurrence, high-expression of CD163 was significantly correlated with poor survival (Figures 3F) (P<0.001), and TAM infiltration was a worse prognostic factor.
Figure 3.
Expression of CD163 and associated CSCs proteins predict poor prognosis of GC. Patients that high expression of CD163 demonstrated shorter DSS and OS than those with low expressed (P<0.001). Patients with high expression levels of CSCs markers CD44 and CD133 had a worse OS than those with low CSCs markers (P<0.005). As for cases with recurrent, patients with high positive CD163 exhibited poor survival rates compared with those who were negative or low for CD163 (P<0.005). GC=gastric cancer, DSS=disease-specific survival, OS=overall survival.
Table 5.
Multivariate analysis of significant prognostic factors for disease-specific and overall survivals in patients with gastric cancer.
| Parameters | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Disease-specific survival | |||
| TNM stage | 5.359 | 2.700-10.636 | <0.001 |
| CD163 | 3.318 | 1.304-8.442 | 0.007 |
| CD44 | 1.778 | 1.048-3.017 | 0.026 |
| Lymph node status | 1.708 | 1.066-2.738 | 0.033 |
| Overall survival | |||
| TNM stage | 3.087 | 1.264-7.538 | 0.001 |
| CD163 | 2.643 | 1.637-4.269 | 0.003 |
| CD44 | 1.806 | 1.277-2.555 | 0.014 |
Discussion
The CSC hypothesis contributes to tumorigenesis, metastasis and recurrence following therapy 13. A number of studies have been focused on CSCs and their niche because signaling from the niche regulates CSC function 14. Recent reports show that TAMs play a key role in maintaining the undifferentiated state of CSCs and promote cancer progression 15. Targeting or reprogramming TAMs can inhibit cancer progression and metastasis, and may represent a promising target of cancer treatment 16, 17. TAMs may migrate along lymphatic channels to pre-metastatic lymph nodes 10. High levels of infiltrating TAMs were associated with poor prognosis and may be involved in the epithelial-mesenchymal transition phenomenon in human GC 9. Infiltration of polarized TAMs has been identified as an independent prognostic factor, which, along with the TNM stage, may refine a more precise prognosis stratification system 11.
The results from the present study supported the hypothesis that infiltration of CD163+TAMs correlates with aberrant expressions of CSCs markers, predicts worse OS of patients with recurrent GC. Multivariate analysis confirmed that the polarized TAMs emerged as an independent risk factor for GC recurrence, and also an independent worse prognostic factor for GC patients, especially for those with recurrence. High expression of CSC markers, CD44 and CD133, is also significantly associated with GC recurrence and may be used as a useful risk prediction marker for GC recurrence, if confirmed by futures studies with larger samples 18, 19.
In the current study, high levels of TAM infiltration was found in over half GC patients and also as an independent poor prognostic factor, significantly associated with aggressive characteristics of cancer. Overexpression of CD163 in TAMs was positively correlated with high expressions of CSC markers, CD44 and CD133 and predicted GC recurrence and poor prognosis when combined with with CD44 expression, suggesting that CSCs in GC may function as a promoter for tumor progression and aggressiveness, and polarized CD163+TAMs may help maintain CSCs in GC tumor. If confirmed by future studies in larger samples, the results may be useful in GC patient management.
In a recent meta-analysis, Liu et al concluded that TAMs was not an independent predictor for worse survival of GC patients on the basis of analysis of 11 published papers 20. In that paper, the sample size remains small and importantly, the most important recent paper by Zhang J, et al. was not included 9. In that study by Zhang et al, the findings suggested that the high level of infiltration TAMs was associated with aggressive features of GC and was an independent poor prognostic factor in GC patients. In addition, publication bias may impact the reliability of the analysis results. Regardless, further prospective, clinical and basic researches are needed to elucidate the prognostic significance and molecular mechanism of TAMs in GC patients.
In our series, there are large variations in tumor size, differentiation, lymph node metastasis, lymphovascular invasion, and TNM stage between the GC group with recurrence and that without. By multivariant analysis, patients presenting at more advanced TNM stages, coupled with high expression of CD163 along with CD44 and CD133 most frequently had GC recurrence, which showed in three main patterns: locoregional, peritoneal, and hematogeneous metastases. Peritoneal relapse and hematogeneous metastases appear to be the predominant patterns and account for most recurrences, which is similar to those reported previously 21, 22.
GC patient survivals after recurrence have rarely been documented in previous studies 23, 24. In our previously study, the majority of GC patients with early recurrence (within two years) died within two years, and CD44 was an independent predictor of early recurrence 25. The results of this study, consistent with the data of our previously study, demonstrated that GC patients with recurrence, especially for those with high-expression of CD163, had a significant correlation with poor survival (P<0.005).
Our data have several limitations. First, our definition of polarized TAMs by using CD163 was limited because macrophages display high plasticity in response to different stimuli, such as cytokines and easily change their phenotype, which should be assessed using reliable surface markers 26. Second, this was a retrospective study with a limited sample size. Finally, we did not study the detailed molecular mechanisms on how TAMs affect GC progression and recurrence.
In conclusion, high infiltration of TAMs was associated with high expression of CSC markers in GC, and related to aggressive behavior, including recurrence. Polarized CD163+TAMs emerged as an independent risk factor for recurrence and worse prognosis for GC patients, especially for those with recurrence.
Table 6.
Multivariate analysis of significant prognostic factors for overall survival in patients with recurrent gastric cancer.
| Parameters | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| TNM stage | 1.549 | 1.049-2.288 | 0.043 |
| CD163 | 2.609 | 1.282-5.310 | 0.024 |
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81201909, 81572338 and 81672380), the Fundamental Research Funds for the Central Universities (No. 021414380031), Nanjing Medical Science and Technology Development program (Nos. YKK12072 and YKK15062). This work was also a C class sponsored project of Jiangsu provincial Six Talent Peaks (WSN-078).
References
- 1.Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Sun YF. et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57(4):1458–1468. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 4.Finkel KA, Warner KA, Kerk S. et al. IL-6 Inhibition with MEDI5117 Decreases the Fraction of Head and Neck Cancer Stem Cells and Prevents Tumor Recurrence. Neoplasia. 2016;18(5):273–81. doi: 10.1016/j.neo.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adorno-Cruz V, Kibria G, Liu X. et al. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75(6):924–9. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borovski T. et al. Cancer stem cell niche: the place to be. Cancer Res. 2011;71(3):634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 7.Allavena P, Sica A, Solinas G. et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Solinas G, Marchesi F, Garlanda C. et al. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29(2):243–8. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Yan Y, Yang Y. et al. High Infiltration of Tumor-Associated Macrophages Influences Poor Prognosis in Human Gastric Cancer Patients, Associates with the Phenomenon of EMT. Medicine (Baltimore) 2016;95(6):e2636. doi: 10.1097/MD.0000000000002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go Y, Tanaka H, Tokumoto M. et al. Tumor-Associated Macrophages Extend Along Lymphatic Flow in the Pre-metastatic Lymph Nodes of Human Gastric Cancer. Ann Surg Oncol. 2016;23(Suppl 2):230–5. doi: 10.1245/s10434-015-4458-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Wang X, Shen Z. et al. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18(4):740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 12.Bosman FT, Carneiro F, Hruban RH, WHO Classification of Tumours of the Digestive System 4th ed. IARC Press, Lyon; 2010. [Google Scholar]
- 13.Valent P, Bonnet D, De Maria R. et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 14.Takakura N. Formation and regulation of the cancer stem cell niche. Cancer Sci. 2012;103(7):1177–1181. doi: 10.1111/j.1349-7006.2012.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ries CH, Cannarile MA, Hoves S. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Georgoudaki AM, Prokopec KE, Boura VF. et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Rep. 2016;15(9):2000–11. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Hua R, Wang X. et al. Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget. 2016;7(9):9815–31. doi: 10.18632/oncotarget.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K, Dan Z, Nie YQ. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J Gastroenterol. 2014;20(18):5420–6. doi: 10.3748/wjg.v20.i18.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JY, Yang XJ, Geng XF. et al. Prognostic significance of tumor-associated macrophages density in gastric cancer: a systemic review and meta-analysis. Minerva Med. 2016;107(5):314–21. [PubMed] [Google Scholar]
- 21.Wu J, Liu X, Cai H. et al. Prediction of tumor recurrence after curative resection in gastric carcinoma based on bcl-2 expression. World J Surg Oncol. 2014;12:40. doi: 10.1186/1477-7819-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Zhang R, Liang H. et al. The pattern and risk factors of recurrence of proximal gastric cancer after curative resection. J Surg Oncol. 2013;107(2):130–5. doi: 10.1002/jso.23252. [DOI] [PubMed] [Google Scholar]
- 23.Otsuji E, Kuriu Y, Ichikawa D. et al. Time to death and pattern of death in recurrence following curative resection of gastric carcinoma: analysis based on depth of invasion. World J Surg. 2004;28:866–869. doi: 10.1007/s00268-004-7359-7. [DOI] [PubMed] [Google Scholar]
- 24.Sakar B, Karagol H, Gumus M. et al. Timing of death from tumor recurrence after curative gastrectomy for gastric cancer. Am J Clin Oncol. 2004;27(2):205–9. doi: 10.1097/01.coc.0000092703.12189.a2. [DOI] [PubMed] [Google Scholar]
- 25.Xu GF, Zhang WJ, Sun Q. et al. Combined epithelial-mesenchymal transition with cancer stem cell-like marker as predictors of recurrence after radical resection for gastric cancer. World J Surg Oncol. 2014;12:368. doi: 10.1186/1477-7819-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantano F, Berti P, Guida FM. et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17(11):1415–21. doi: 10.1111/jcmm.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]