Abstract
Background: The aim of this study is to evaluate the long-term therapeutic gain of induction chemotherapy (IC) in locoregionally advanced nasopharyngeal carcinoma (NPC) in the era of intensity-modulated radiotherapy (IMRT).
Methods: Data on 957 patients with stage T1-2N2-3 or T3-4N1-3 NPC treated with IMRT were retrospectively reviewed. Propensity score matching (PSM) method was adopted to balance influence of various covariates. Patient survival between IC and non-IC groups were compared.
Results: For the 318 pairs selected from the original 957 patients by PSM, the median follow-up duration was 57.13 months (range, 1.27-78.1 months). The 5-year overall survival (OS), distant metastasis-free survival (DMFS), disease-free survival (DFS) and locoregional relapse-free survival (LRRFS) rates for IC group vs. non-IC group were 87.2% vs. 80.8% (P = 0.023), 88.1% vs. 83.2% (P = 0.071), 80.7% vs. 71.4% (P = 0.011) and 92.1% vs. 86.7% (P = 0.081), respectively. Multivariate analysis identify IC as an independent prognostic factor for OS (HR, 0.595; 95% CI, 0.397-0.891; P = 0.012) and DFS (HR, 0.627; 95% CI, 0.451-0.872; P = 0.006). After excluding the patients not receiving concurrent chemotherapy, IC was found to be an independent prognostic factor for OS (HR, 0.566; 95% CI, 0.368-0.872; P = 0.01), DMFS (HR, 0.580; 95% CI, 0.367-0.916; P = 0.02) and DFS (HR, 0.633; 95% CI, 0.444-0.903; P = 0.012).
Conclusions: IC is an effective treatment modality for patients with stage T1-2N2-3 and T3-4N1-3 NPC, and the incorporation of IC with standard CCRT could achieve the best therapeutic gain.
Keywords: Nasopharyngeal carcinoma, induction chemotherapy, locoregionally advanced, intensity-modulated radiotherapy, prognosis.
Introduction
Nasopharyngeal carcinoma (NPC) is a tumor originating from nasopharynx epithelium and has an extremely unbalanced geographic distribution whereby in endemic regions, such as south China, its annual age-standard incidence rate is up to 20-50 per 100,000 males 1. As a result of the anatomic constraints and its high degree of radiosensitivity, radiotherapy (RT) has been the primary and only curative treatment for non-disseminated NPC. NPC also responses well to chemotherapy (CT), and randomized trials have demonstrated that a combination of CT with standard RT could improve the therapeutic outcome of patients with locoregionally advanced NPC 2-5 compared with RT alone. Therefore, concurrent chemoradiotherapy (CCRT) with or without adjuvant chemotherapy (ACT) has been established as the standard treatment for advanced NPC 6-8.
With the advent of intensity-modulated radiotherapy (IMRT), local control of advanced NPC has improved greatly and distant metastasis has emerged as the predominant mode of treatment failure pattern 9, 10. Therefore, there has been a renewed interest in the re-exploration of induction chemotherapy (IC) in advanced NPC 11-16 as it may reduce distant metastasis and improve overall survival. However, results from previous randomized or non-randomized trials 11-18 were controversial with regard to the therapeutic gain of overall survival. Therefore, the prognostic value of IC remains to be addressed. Moreover, due to the insufficient follow-up duration in abovementioned studies, few 5-year survival outcomes were reported in previous studies.
According to previous findings, we conducted this retrospective study to establish the value of IC for patients with locoregionally advanced NPC treated by IMRT based on the 5-year survival outcomes. To balance the influence of covariates, propensity score matching (PSM) method was adopted to compare survival outcomes and decrease potential bias 19.
Materials and Methods
Study Patients
Data on 1811 patients with newly diagnosed stage I-IVB NPC, who were treated between November 2009 and February 2012 at Sun Yat-sen university cancer center, were retrospectively reviewed. The including criteria for this study were as follows: (1) stage T1-2N2-3 or T3-4N1-3 NPC; (2) World Health Organization (WHO) pathology type II/III; (3) with the data of pre-treatment Epstein-Barr virus (EBV) DNA (pre-DNA); (4) age 18 years or older. Finally, 957 (52.8%) patients were recruited for the current study. This study was approved by the Research Ethics Committee of Sun Yat-sen university cancer center. Informed consent was obtained from all the patients.
Clinical Staging Work
The conventional staging workups included a complete history and clinical examinations of the head and neck region, direct fibre-optic nasopharyngoscopy, magnetic resonance imaging (MRI), chest radiography, whole-body bone scan and abdominal sonography, as well as positron emission tomography (PET)-CT if necessary. Tumour-related markers like pre-DNA were quantified. All patients received a dental evaluation before radiotherapy.
All patients were restaged according to the 7th edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) system 20. All MRI materials and clinical records were reviewed to minimize heterogeneity in restaging. Two radiologists (L.Z.L. and L.T.) employed at our hospital separately evaluated all of the scans and disagreements were resolved by consensus.
Real-time quantitative EBV DNA PCR
Measurement of the plasma EBV DNA load was performed before treatment, and plasma DNA was extracted and assayed using real-time quantitative PCR which was described previously 21. The real-time quantitative PCR system was developed for plasma EBV DNA detection, and targeted the BamHI-W region of the EBV genome using primers 5'-GCCAGAGGTAAGTGGACTTT-3' and 5'-TACCACCTCCTCTTCTTGCT-3'. The dual fluorescence-labelled oligomer 5'-(FAM) CACACCCAGGCACACACTACACAT (TAMRA)-3' served as a probe. Sequence data for the EBV genome were obtained from the GeneBank sequence database.
Clinical Treatment
All patients received IMRT at Sun Yat-sen university cancer center. The prescribed doses were 66-72Gy at 2.12-2.43Gy/fraction to the planning target volume (PTV) of the primary gross tumour volume (GTVnx), 64-70Gy to the PTV of the GTV of the metastatic lymph nodes (GTVnd), 60-63Gy to the PTV of the high-risk clinical target volume (CTV1), and 54-56Gy to the PTV of the low-risk clinical target volume (CTV2). IC consisted of cisplatin (80 mg/m2) with 5-fluorouracil (1000 mg/m2) (PF), docetaxel (75 mg/m2) with cisplatin (75 mg/m2) (TP) every three weeks for two or more cycles. Concurrent chemotherapy was cisplatin weekly (30-40 mg/m2) or on weeks 1, 4 and 7 (80-100 mg/m2) of radiotherapy.
Follow-Up and Statistical Analysis
Follow-up was measured from first day of therapy to last examination or death, and patients were followed by MRI and plasma EBV DNA at least every 3 months during first 2 years, then every 6 months thereafter (or until death).
Propensity scores were computed by logistic regression for each patient using the following covariates: age, gender, concurrent chemotherapy (CRT), smoking, drink, T category, N category, overall stage and pre-DNA. The cut-off value of pre-DNA was evaluated by receiver operating characteristic (ROC) analysis. Chi-square test or Fisher's exact test and non-parametric test were adopted to compare categorical and continuous variables. Overall survival (OS), distant metastasis-free survival (DMFS), disease-free survival (DFS) and locoregional relapse-free survival (LRRFS) curves were estimated using Kaplan-Meier analysis and compared using the log-rank test. The multivariate Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs); age, gender, CRT, smoking, drinking, T category, N category, overall stage, pre-DNA and IC were included as variables. All tests were two-sided; P < 0.05 was considered significant. Stata Statistical Package 12 (StataCorp LP, College Station, TX, USA) was used for all analyses.
Results
Cut-off Value of Pre-treatment EBV DNA
Among the whole cohort, 158/957 (16.5%) patients had undetectable pre-DNA and the median pre-DNA load was 5200 copies/ml (interquartile range, 517-30950). According to the ROC curve analysis, the cut-off value of pre-DNA was 1595 copies/ml for OS (area under curve [AUC], 0.611; sensitivity, 0.791; specificity, 0.383).
Basic characteristics
In total, 542 (56.7%) patients received IC. From the original 957 NPC patients, 318 pairs were selected by PSM (Table 1). The median pre-DNA was 4260 copies/ml (interquartile range, 398-21900) and 5500 copies/ml (interquartile range, 544-26825) for the non-IC and IC groups (P = 0.551), respectively. No significant difference was found with regard to the host, tumor and treatment factors between the IC and non-IC groups (P > 0.05 for all rates). For the selected cohort, the male (n=488)-to-female (n=148) ratio was 3.0:1, and the median age was 45 (range, 18-78) years-old.
Table 1.
Baseline characteristics of all 318 pairs of stage III-IVB NPC patients (except T3-4N0) with or without IC.
| IC | Non-IC | ||
|---|---|---|---|
| Characteristics | No. (%) | No. (%) | P |
| Age (median, y) | 46 | 44 | 0.293a |
| Gender | 0.851b | ||
| Male | 245 (77.0) | 243 (76.4) | |
| Female | 73 (23.0) | 75 (23.6) | |
| Concurrent chemotherapy | 0.232b | ||
| Yes | 283 (89.0) | 273 (85.8) | |
| No | 35 (11.0) | 45 (14.2) | |
| Smoking | 0.807b | ||
| Yes | 123 (38.7) | 126 (39.6) | |
| No | 195 (61.3) | 192 (60.4) | |
| Drinking | 0.802b | ||
| Yes | 37 (11.6) | 35 (11.0) | |
| No | 281 (88.4) | 283 (89.0) | |
| T category c | 0.267b | ||
| T1 | 13 (4.1) | 14 (4.4) | |
| T2 | 23 (7.2) | 12 (3.8) | |
| T3 | 214 (67.3) | 227 (71.4) | |
| T4 | 68 (21.4) | 65 (20.4) | |
| N category c | 0.984b | ||
| N1 | 209 (65.7) | 211 (66.4) | |
| N2 | 76 (23.9) | 75 (23.6) | |
| N3 | 33 (10.4) | 32 (10.0) | |
| Overall stage c | 0.931b | ||
| III | 224 (70.4) | 225 (70.8) | |
| IVA-IVB | 94 (29.6) | 93 (29.2) | |
| Pre-DNA (median, copies/ml) | 5500 | 4260 | 0.551a |
Abbreviations: NPC = nasopharyngeal carcinoma; IC = induction chemotherapy; Pre-DNA = pre-treatment Epstein-Barr virus DNA.
a P-values were calculated by Non-parametric test.
b P-values were calculated by Chi-square test or Fisher exact test if indicated.
c According to the 7th AJCC/UICC staging system.
Failure Patterns
The median follow-up duration for the selected 318 pairs was 57.13 months (range, 1.27-78.1 months). Up to the last follow-up, 14/318 (4.4%) patients in IC group and 23/318 (7.2%) patients in non-IC group developed local recurrence; 15/318 (4.7%) patients in IC group and 17/318 (5.3%) patients in non-IC group experienced regional recurrence; and 36/318 (11.3%) patients in IC group and 51/318 (16.0%) patients in non-IC group experienced distant metastasis. Moreover, 39/318 (12.6%) patients and 60/318 (15.8%) patients died in IC and non-IC group, respectively.
Survival Analysis
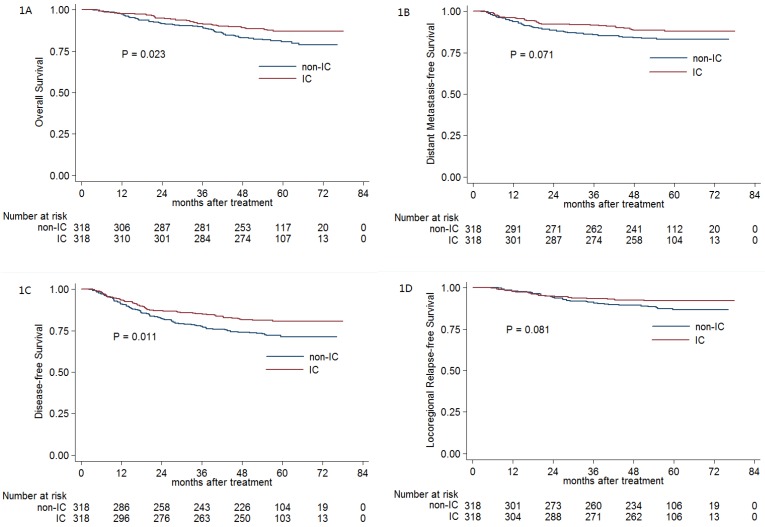
For the selected 318 pairs, the 5-year OS, DMFS, DFS and LRRFS rates were 83.9%, 85.7%, 76.1% and 89.5%, respectively. The 5-year OS (87.2% vs. 80.8%; P = 0.023; Figure 1A) and DFS (80.7% vs. 71.4%; P = 0.011; Figure 1C) rates for patients receiving IC were significantly higher than the corresponding rates for patients not receiving IC. Moreover, the difference in the DMFS rate (88.1% vs. 83.2%; P = 0.071; Figure 1B) and LRRFS rate (92.1% vs. 86.7%; P = 0.081; Figure 1D) between the IC and non-IC groups nearly reached statistical significance.
Figure 1.
Kaplan-Meier OS (A), DMFS (B), DFS (C) and LRRFS (D) curves for the 318 pairs of stage III-IVB NPC with or without IC. Abbreviations: OS = overall survival; DMFS = distant metastasis-free survival; DFS = disease-free survival; LRRFS = locoregional relapse-free survival; IC = induction chemotherapy.
Multivariate analysis was performed to adjust for various prognostic factors, and consistent with the results of univariate analysis, it revealed that IC could improve the therapeutic outcomes of OS (HR, 0.595; 95% CI, 0.397-0.891; P = 0.012) and DFS (HR, 0.627; 95% CI, 0.451-0.872; P = 0.006) (Table 2). Patients receiving IC also had a lower risk of distant metastasis (HR, 0.684; 95% CI, 0.446-1.048; P = 0.081) and locoregional relapse (HR, 0.634; 95% CI, 0.378-1.063; P = 0.084), and this difference was marginally statistically significant.
Table 2.
Multivariate analysis of prognostic factors for all 318 pairs of stage III-IVB NPC patients (except T3-4N0) with or without IC.
| Endpoints | Variable | HR (95% CI) | Pa |
|---|---|---|---|
| OS | Age | 1.849 (1.206-2.834) | 0.005 |
| N category, N2 vs. N1 | 2.007 (1.274-3.161) | 0.003 | |
| Overall stage | 2.299 (1.448-3.652) | < 0.001 | |
| Pre-DNA | 1.848 (1.155-2.957) | 0.01 | |
| IC | 0.595(0.397-0.891) | 0.012 | |
| DMFS | N category, N2 vs. N1 | 1.809 (1.113-2.941) | 0.017 |
| Overall stage | 1.814 (1.084-3.037) | 0.023 | |
| Pre-DNA | 2.850 (1.630-4.985) | < 0.001 | |
| IC | 0.684 (0.446-1.048) | 0.081 | |
| DFS | Age | 1.408 (1.006-1.971) | 0.046 |
| N category, N2 vs. N1 | 1.623 (1.116-2.360) | 0.011 | |
| Overall stage | 1.743 (1.181-2.573) | 0.005 | |
| Pre-DNA | 1.591 (1.103-2.293) | 0.013 | |
| IC | 0.627(0.451-0.872) | 0.006 | |
| LRRFS | IC | 0.634(0.378-1.063) | 0.084 |
Abbreviations: NPC = nasopharyngeal carcinoma; IC = neoadjuvant chemotherapy; HR = hazard ratio; CI = confidence interval; OS = overall survival; DMFS = distant metastasis-free survival; DFS = disease-free survival; LRRFS = locoregional relapse-free survival; Pre-DNA = pre-treatment Epstein-Barr virus DNA.
a: Multivariate P-values were calculated using an adjusted Cox proportional-hazards model with backward elimination and the following parameters: age (> 45y vs. ≤ 45y), gender (male vs. female), concurrent chemotherapy (yes vs. no), smoking (yes vs. no), drinking (yes vs. no), T category (T1-2 vs. T3-4), N category (N2 vs. N1, N3 vs. N1), overall stage (III vs. IVA-B), pre-DNA (≥ 1595 copies/ml vs. < 1595 copies/ml) and IC (yes vs. no).
Prognostic Value of IC in Patients Receiving CRT
Given the truth that CCRT has been the basic treatment for patients with advanced NPC, we therefore exclude the patients not receiving CCRT and re-evaluate the prognostic value of IC. Overall, 163 (17.0%) patients not receiving CRT from the originally entire cohort were excluded, and 276 pairs were selected by PSM (Table 3).
Table 3.
Baseline characteristics of all 276 pairs of stage III-IVB NPC patients (except T3-4N0) receiving CCRT with or without IC.
| IC | Non-IC | P | |
|---|---|---|---|
| Characteristics | No. (%) | No. (%) | |
| Age (median, y) | 47 | 44 | 0.301a |
| Gender | 1.000b | ||
| Male | 216 (78.3) | 216 (78.3) | |
| Female | 60 (21.7) | 60 (21.7) | |
| Smoking | 0.794b | ||
| Yes | 109 (39.5) | 112 (40.6) | |
| No | 167 (60.5) | 164 (59.4) | |
| Drinking | 0.900b | ||
| Yes | 36 (13.0) | 37 (13.4) | |
| No | 240 (87.0) | 239 (86.6) | |
| T category c | 0.305b | ||
| T1 | 10 (3.6) | 14 (5.1) | |
| T2 | 19 (6.9) | 10 (3.6) | |
| T3 | 185 (67.0) | 192 (69.6) | |
| T4 | 62 (22.5) | 60 (21.7) | |
| N category c | 1.000b | ||
| N1 | 180 (65.2) | 180 (65.2) | |
| N2 | 66 (23.9) | 66 (23.9) | |
| N3 | 30 (10.9) | 30 (10.9) | |
| Overall stage c | 1.000b | ||
| III | 190 (68.8) | 190 (68.8) | |
| IVA-IVB | 86 (31.2) | 86 (31.2) | |
| Pre-DNA (median, copies/ml) | 6760 | 4590 | 0.430a |
Abbreviations: NPC = nasopharyngeal carcinoma; IC = induction chemotherapy; CCRT = concurrent chemoradiotherapy; Pre-DNA = pre-treatment Epstein-Barr virus DNA.
a P-values were calculated by Non-parametric test.
b P-values were calculated by Chi-square test or Fisher exact test if indicated.
c According to the 7th AJCC/UICC staging system.
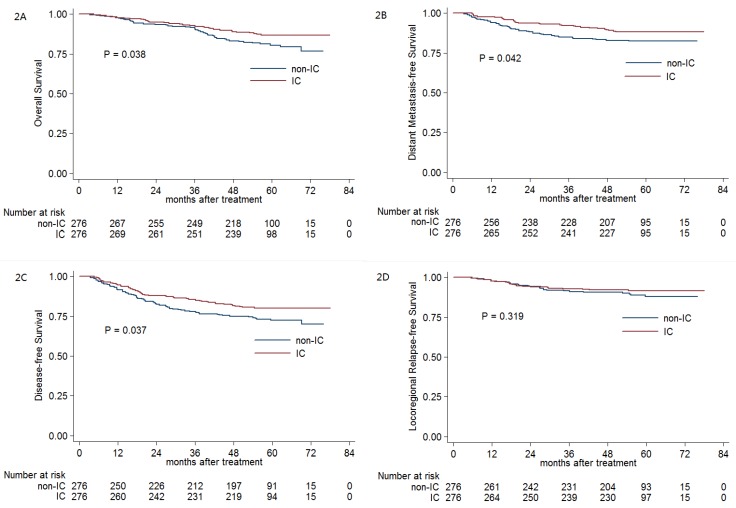
The 5-year OS, DMFS, DFS and LRRFS rates for IC group vs. non-IC group were 86.7% vs. 80.5% (P = 0.038, Figure 2A), 88.3% vs. 82.4% (P = 0.042, Figure 2B), 80.1% vs. 72.4% (P = 0.037, Figure 2C) and 91.6% vs. 88.1% (P = 0.319, Figure 2D), respectively. When entered into the multivariate analysis, IC was still found to be an independent prognostic factor for OS (HR, 0.566; 95% CI, 0.368-0.872; P = 0.01), DMFS (HR, 0.580; 95% CI, 0.367-0.916; P = 0.02) and DFS (HR, 0.633; 95% CI, 0.444-0.903; P = 0.012), respectively.
Figure 2.
Kaplan-Meier OS (A), DMFS (B), DFS (C) and LRRFS (D) curves for the 276 pairs of stage III-IVB NPC patients receiving CCRT with or without IC. Abbreviations: OS = overall survival; DMFS = distant metastasis-free survival; DFS = disease-free survival; LRRFS = locoregional relapse-free survival; IC = induction chemotherapy; CCRT = concurrent chemoradiotherapy.
Discussion
To the best of our knowledge, this is the first study with the largest sample and longest follow-up duration to established the prognostic value of IC in the era of IMRT, and the findings of this current study revealed IC was associated with significantly improved 5-year OS and DFS for patients with locoregionally advanced NPC. Moreover, after excluding the patients not receiving CRT, we further proved that IC could also improve therapeutic outcomes of 5-year DMFS. However, IC was not associated with better 5-year LRRFS no matter CRT was delivered or not.
Unlike previous studies which include all locoregionally advanced NPC (stage III-IVB) 13, 15, 17, 18, 22, we excluded patients with stage T3-4N0 disease because patients with negative lymph node metastasis stage have lower distant tumor burden and could not really benefit from IC. This recruiting criteria was also adopted in our previous work which appraised the contribution of adjuvant chemotherapy additional to CCRT in locoregionally advanced NPC 8. For the selected 318 pairs, 80 (12.6%) patients did not accepted CRT. Refusal by patients after IC was the primary and main reason for uncompleted CRT. In addition, a part of patients (26/80 patients with age more than 60 years) avoided CRT as well because they could not tolerate CRT after IC according to clinicians' decisions.
With the adoption of CRT and IMRT being the standard RT technique, the control of locoregional is satisfactory and distant metastasis has been the predominant mode of treatment failure 9, 10. Therefore, much attention had been paid to IC, and many combined regimens had been applied such as BEC (bleomycin, epirubicin and cisplatin) 14, BFC (bleomycin, fluorouracil and cisplatin) 15, TP (docetaxel and cisplatin) 13 and GCP (gemcitabine, carboplatin and paclitaxel) 16. However, only one phase II trial by Hui et al. 13 showed an OS benefit. Of note, the sample in this study was too small (n = 65) and the results may not be solid conclusive. Moreover, other two phase III trials showed a benefit of DFS 12, 14 but not OS. However, the treatment modality was not the currently standard pattern. Furthermore, the follow-up duration in these three studies was insufficient. These shortages made the prognostic value of IC inconclusive. After making up for these deficiencies, our current study substantially established the efficacy of IC and the outcomes were similar to the meta-analysis by Ouyang et al. 23.
Since CCRT has been the mainly standard treatment for advanced NPC, we therefore excluded patients not receiving CRT and re-evaluated the prognostic value of IC. Consistent with the results of primary analysis, it revealed IC was still associated with significantly improved OS and DFS. Intriguingly, a significantly better 5-year DMFS was observed in patients receiving IC plus CCRT compared with patients receiving CCRT alone. As expected, IC did not influence the 5-year LRRFS, and this should be attributed to use of IMRT. These results indicate that IC prior to RT is an effective treatment strategy for the eradication of micro-metastasis and improving therapeutic outcomes in locoregionally advanced NPC. Therefore, IC combined with CCRT should be a promising treatment modality in advanced NPC with high risk of distant metastasis.
The major strength of our study is the use of PSM and multivariate analysis to establish the prognostic value of IC in advanced NPC; this addressed the potential limitations of divergent confounders, treatment heterogeneity, and selection bias associated with the direct retrospective analysis of observational data 19. As the most important prognostic biomarker, plasma EBV DNA 24-27 was also well balanced between IC and non-IC groups. Therefore, the results of our study should be reliable. With regard to the limitations, first, the data was retrospectively collected from one single center. Second, the IC regimens were not uniform. However, this would not affect the outcomes of this study because no evidence has proven the efficacy difference of these two regimens so far. Future prospective trials are warrant to address this issue.
In conclusion, our findings suggest that IC prior to RT is an advantage for patients with stage III-IVB NPC (except T3-4N0) and receiving CRT. Based on the present evidence, it is recommended that IC combined with CCRT should be delivered to patients with advanced NPC, although future randomized trials are warrant to define the optimal IC regimen.
Conclusion
IC before RT is an effective treatment pattern for patients with stage III-IVB NPC (except T3-4N0), and the incorporation of IC with standard CCRT could achieve the best therapeutic gain. Further randomized trials are warrant to define the optimal IC regimen.
Table 4.
Multivariate analysis of prognostic factors for all 276 pairs of stage III-IVB NPC patients (except T3-4N0) receiving CCRT with or without IC.
| Endpoints | Variable | HR (95% CI) | Pa |
|---|---|---|---|
| OS | Age | 2.110 (1.334-3.336) | 0.001 |
| Gender | 0.460(0.244-0.870) | 0.017 | |
| Overall stage | 1.890 (1.136-3.145) | 0.014 | |
| IC | 0.566(0.368-0.872) | 0.010 | |
| DMFS | Age | 1.643(1.029-2.622) | 0.037 |
| N category, N3 vs. N1 | 2.217 (1.223-4.018) | 0.009 | |
| Pre-DNA | 1.996 (1.133-3.518) | 0.017 | |
| IC | 0.580 (0.367-0.916) | 0.02 | |
| DFS | Age | 1.584 (1.096-2.288) | 0.014 |
| Overall stage | 1.729(1.215-2.461) | 0.002 | |
| Pre-DNA | 1.493 (1.003-2.223) | 0.048 | |
| IC | 0.633(0.444-0.903) | 0.012 | |
| LRRFS | Overall stage | 2.033(1.166-3.546) | 0.012 |
| IC | 0.751(0.429-1.132) | 0.314 |
Abbreviations: NPC = nasopharyngeal carcinoma; IC = neoadjuvant chemotherapy; CCRT = concurrent chemoradiotherapy; HR = hazard ratio; CI = confidence interval; OS = overall survival; DMFS = distant metastasis-free survival; DFS = disease-free survival; LRRFS = locoregional relapse-free survival; Pre-DNA = pre-treatment Epstein-Barr virus DNA.
a: Multivariate P-values were calculated using an adjusted Cox proportional-hazards model with backward elimination and the following parameters: age (> 45y vs. ≤ 45y), gender (male vs. female), smoking (yes vs. no), drinking (yes vs. no), T category (T1-2 vs. T3-4), N category (N2 vs. N1, N3 vs. N1), overall stage (III vs. IVA-B), pre-DNA (≥ 1595 copies/ml vs. < 1595 copies/ml) and IC (yes vs. no).
Acknowledgments
This work was supported by grants from the Health & Medical Collaborative Innovation Project of Guangzhou City, China (No. 201400000001), the Sun Yat-sen University Clinical Research 5010 Program (No. 2012011), the Science and Technology Project of Guangzhou City, China (No. 14570006).
Abbreviations
- NPC
nasopharyngeal carcinoma
- RT
radiotherapy
- CT
chemotherapy
- CCRT
concurrent chemoradiotherapy
- ACT
adjuvant chemotherapy
- IMRT
intensity-modulated radiotherapy
- IC
induction chemotherapy
- PSM
propensity score matching
- WHO
World Health Organization
- EBV
Epstein-Barr virus
- Pre-DNA
pre-treatment Epstein-Barr virus DNA
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- UICC/AJCC
International Union against Cancer/American Joint Committee on Cancer
- PTV
planning target volume
- GTV
gross tumour volume
- GTVnx
primary gross tumour volume
- GTVnd
gross tumour volume of the metastatic lymph nodes
- CTV1
high-risk clinical target volume
- CTV2
low-risk clinical target volume
- PF
cisplatin with 5-fluorouracil
- TP
docetaxel with cisplatin
- OS
overall survival
- DMFS
distant metastasis-free survival
- DFS
disease-free survival
- LRRFS
locoregional relapse-free survival
- CRT
concurrent chemotherapy
- HR
hazard ratio
- CI
confidence interval
- AUC
area under curve
- BEC
bleomycin with epirubicin and cisplatin
- BFC
bleomycin with fluorouracil and cisplatin
- GCP
gemcitabine with carboplatin and paclitaxel.
References
- 1.Jemal A, Bray F, Center MM. et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sarraf M, LeBlanc M, Giri PG. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Lau WH, Tung SY. et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–75. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 4.Lin JC, Jan JS, Hsu CY. et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–7. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 5.Wee J, Tan EH, Tai BC. et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–8. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 6.Baujat B, Audry H, Bourhis J, et al Chemotherapy in locally advanced nasopharyngeal carcinoma. an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Chen YP, Wang ZX, Chen L. et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015;26:205–11. doi: 10.1093/annonc/mdu507. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Hu CS, Chen XZ. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–71. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Su S, Chen C. et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Kam MK, Teo PM, Chau RM. et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440–50. doi: 10.1016/j.ijrobp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Chua DT, Sham JS, Choy D. et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer. 1998;83:2270–83. [PubMed] [Google Scholar]
- 12.Hong RL, Ting LL, Ko JY. et al. Induction chemotherapy with mitomycin, epirubicin, cisplatin, fluorouracil, and leucovorin followed by radiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:4305–13. doi: 10.1200/JCO.2001.19.23.4305. [DOI] [PubMed] [Google Scholar]
- 13.Hui EP, Ma BB, Leung SF. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242–9. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 14.International Nasopharynx Cancer Study Group. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV (> or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. Int J Radiat Oncol Biol Phys. 1996;35:463–9. doi: 10.1016/s0360-3016(96)80007-1. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Mai HQ, Hong MH. et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:1350–7. doi: 10.1200/JCO.2001.19.5.1350. [DOI] [PubMed] [Google Scholar]
- 16.Tan T, Lim WT, Fong KW. et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;91:952–60. doi: 10.1016/j.ijrobp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Lim AM, Corry J, Collins M. et al. A phase II study of induction carboplatin and gemcitabine followed by chemoradiotherapy for the treatment of locally advanced nasopharyngeal carcinoma. Oral Oncol. 2013;49:468–74. doi: 10.1016/j.oraloncology.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Xu T, Zhu G, He X. et al. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral Oncol. 2014;50:71–6. doi: 10.1016/j.oraloncology.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661–77. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Shao JY, Zhang Y, Li YH. et al. Comparison of Epstein-Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res. 2004;24:4059–66. [PubMed] [Google Scholar]
- 22.Kong L, Hu C, Niu X. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer. 2013;119:4111–8. doi: 10.1002/cncr.28324. [DOI] [PubMed] [Google Scholar]
- 23.OuYang PY, Xie C, Mao YP. et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol. 2013;24:2136–46. doi: 10.1093/annonc/mdt146. [DOI] [PubMed] [Google Scholar]
- 24.An X, Wang FH, Ding PR. et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117:3750–7. doi: 10.1002/cncr.25932. [DOI] [PubMed] [Google Scholar]
- 25.Lin JC, Wang WY, Chen KY. et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461–70. doi: 10.1056/NEJMoa032260. [DOI] [PubMed] [Google Scholar]
- 26.Lin JC, Wang WY, Liang WM. et al. Long-term prognostic effects of plasma epstein-barr virus DNA by minor groove binder-probe real-time quantitative PCR on nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1342–8. doi: 10.1016/j.ijrobp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Peng H, Guo R, Chen L. et al. Prognostic Impact of Plasma Epstein-Barr Virus DNA in Patients with Nasopharyngeal Carcinoma Treated using Intensity-Modulated Radiation Therapy. Sci Rep. 2016;6:22000. doi: 10.1038/srep22000. [DOI] [PMC free article] [PubMed] [Google Scholar]