Abstract
Purpose: The objective of study is aiming to investigate the residual tumor rate after Vacuum-assisted Breast Biopsy (VABB) for early breast cancer excision and the efficacy of mammogram and ultrasound in detecting residual tumor.
Methods: Patients who underwent VABB and were confirmed with breast cancer in Sun Yat-sen University Cancer Center from 2010 to 2015 were reviewed retrospectively. The residual tumor rate determined by histological examination was calculated, and then was compared with the results estimated by mammogram and ultrasound which were performed post VABB but before subsequent surgery. Univariate and multivariate analysis (logistic regression) were carried out to identify the independent risk factors associated with residual tumor.
Results: In total, 126 eligible patients with early breast cancer were recruited for this study, of whom 79 (62.7%) had residual tumor and 47 (37.3 %) underwent complete excision. The residual tumor rates for lesions < 10mm, lesions 10 to 20 mm and lesions >20mm in size were 55.0%, 68.9% and 53.1%, respectively. The complete excision rates estimated by mammogram and ultrasound were 76.5% and 73.9%, with a negative predictive value of only 46.2% and 50.6%, respectively. In the multivariate logistic regression analysis, no specific factors were found associated with risk of residual tumor (all P > 0.05).
Conclusions: There was a high residual tumor rate after VABB in early breast cancer. Both mammogram and ultrasound could not effectively detect the residual tumor after VABB.
Keywords: Vacuum-assisted breast biopsy, residual tumor rate, early breast cancer, mammogram and ultrasound.
Introduction
Surgery is one of the main treatments for early breast cancer 1, and modified radical mastectomy and breast conserving surgery (BCS) have remained the standard surgery approaches. However, in recent years, oncoplastic and minimally invasive surgeries for early breast cancer have attracted more attention and played a more and more important role 2-4.
Vacuum-assisted breast biopsy (VABB) device was first developed by Johnson Company of USA in 1994 5, and had a high biopsy accuracy for lesions impalpable, deep-located and less than 1 cm 6-9. It has been reported that 14-gauge and 11-gauge probes using the VABB device could respectively obtain more than two and six times biopsy sample weights per specimen than the traditional automated 14-gauge probe 10, 11. Due to its rich tissue harvesting and less invasive procedure, VABB as a minimally invasive surgery in breast benign tumor excision has been proven to achieve high complete excision rates (90-100%) and few complications by numerous clinical studies 12-15. However, these complete excision rates were only confirmed by the imaging examination findings without further histological results, because there is no need for subsequently extended surgery if benign disease is present. Thus, the efficacy of mammogram and ultrasound in detecting residual tumor after VABB has not been defined under the guidance of histologically golden standard.
Of note, a part of patients receiving VABB were diagnosed with benign disease by ultrasound and mammogram before treatment, but were finally proven to have malignant tumor by histological findings. Therefore, it would be of great importance to determine whether residual tumor existed after VABB for those patients since they might avoid further surgery if no residual tumor was found. However, to the best of our knowledge, no study to date has reported the residual rate of tumor after VABB or investigated the efficacy of ultrasound and mammogram in detecting the residual tumor compared with histological findings in early breast cancer. Based on this premise, we performed this retrospective cohort study to characterize this issue and help guiding clinical treatment.
Materials and methods
Study patient and data collection
We investigated 126 female patients who underwent VABB and were confirmed with breast cancer histologically in Sun Yat-sen University Cancer Center (SYSUCC) from 2010 to 2015. The included patients were diagnosed with breast benign tumor clinically before treatment and received VABB using the EnCor Vacuum Assisted Biopsy System (SenoRx Inc., USA) with a 7-gauge probe. The inclusion criteria were as follows: (I) single suspicious lesion was found by mammogram and ultrasound before VABB; (II) the BI-RADS of mammogram and ultrasound was 3 or 4a, if the BI-RADS between ultrasound and mammogram was inconsistent, then the higher BI-RADS was used; (III) the largest dimension of the lesion was less than 3 cm. Patients with suspicious skin invasion found by mammogram, ultrasound or grossly-viewing and with incomplete information were excluded. The study was approved by the Independent Ethics Committee/Institutional Review Board of SYSUCC and written informed consent about researchable use of the clinical data was obtained from every participant prior to surgery.
Mammogram and ultrasound examinations were ordered for the included patients post VABB but before subsequent surgery to estimate residual tumor status. The images of mammogram and ultrasound were reviewed by a radiologist and an ultrasound doctor respectively. A standard hook wire was placed to localize the residual cavity under guidance of ultrasound. Then patients underwent BCS or mastectomy according to the surgeon's clinical judgment and patients' preference. Any suspicious lesion detected by mammogram, ultrasound or grossly-viewing and the entire surrounding rim of the residual cavity about 1 cm in thickness were resected to block for paraffin sections. Hematoxylin-eosin staining and immunochemical staining were performed for specimen to examine for residual tumor, and the final histological findings would be adopted as the golden standard to calculate residual tumor rate. All the slides from VABB and resection specimens were reviewed by two independent pathologists in SYSUCC without knowledge of patients' mammogram or ultrasound examination findings.
Two investigators independently collected the clinic-pathological data of the study population, including age, menstrual status, family history, dominant feature (mass only or mass with calcification), BI-RADS of ultrasound and mammogram, the largest tumor dimension, mammogram and ultrasound examination findings post VABB but before subsequent surgery, histological type, surgery methods, residual tumor after VABB (yes or no), axillary lymph node status, TNM stage, hormonal receptor, human epidermal growth factor receptor 2 (HER-2) and Ki67 status. Any divergence of data collection was solved through consensus by the two investigators.
Statistical analyses
Continuous data, such as age, were described by median and range. Categorical data, such as family history and dominant feature, were described by numbers and percentages. The calculation of sensitivity, specificity, negative predictive value and positive predictive value of mammogram and ultrasound in detecting residual tumor was shown in Table 4. Univariate and multivariate analysis (logistic regression) were carried out to identify the independent risk factors associated with residual tumor. Multivariate analysis was adjusted for covariates of age, dominant feature (mass only or mass with calcification), the largest tumor dimension (≤10mm, 10-20mm or >20mm in size), histological type and lymph node status (negative or positive). A two-tailed P value of <0.05 in logistic regression analysis was considered statistically significant. All the statistical analysis was performed by the SPSS, version 19.0 (SPSS Inc., Chicago, IL, USA).
Table 4.
Efficacy of mammogram and ultrasound in detecting residual tumor.
| Histological findings | Sensitivity e (%) | Specificity f (%) | Negative predictive value g (%) | Positive predictive value h (%) | ||
|---|---|---|---|---|---|---|
| Residual tumor No. (%) | No residual tumor No. (%) | |||||
| Mammogram findings (n=53) | 36.3 | 90.0 | 46.2 | 85.7 | ||
| Residual tumor | 12 (22.6) (a) | 2 (3.8) (b) | ||||
| No residual tumor | 21(39.6) (c) | 18 (34.0) (d) | ||||
| Ultrasound findings (n=115) | 38.2 | 91.5 | 50.6 | 86.7 | ||
| Residual tumor | 26 (22.6) (a') | 4 (3.5) (b') | ||||
| No residual tumor | 42 (36.5) (c') | 43 (37.4) (d') | ||||
e Sensitivity = a/ (a + c) for mammogram or a'/ (a' + c') for ultrasound.
f Specificity = d/ (b + d) for mammogram or d'/ (b' + d') for ultrasound.
g Negative predictive value = d/ (c + d) for mammogram or d'/ (c' + d') for ultrasound.
h Positive predictive value = a/ (a + b) for mammogram or a'/ (a' + b') for ultrasound.
Results
Baseline characteristics
The baseline clinic-pathological characteristics of the included 126 consecutive early breast cancer patients were summarized in Table 1. The TNM stages of the study population were between stage 0 and stage ⅡB. The median age was 42 years (range, 23-77 years), and 88 (69.8%) patients were younger than 45 years old. 97 (77.0%) patients chose mastectomy, and 83 (65.9%) patients had histologic type of invasive ductal carcinoma (IDC). 101 (80.2%) patients were BI-RADS 3 and 25 (19.8%) were BI-RADS 4a. 22 (17.5%) patients were confirmed to have metastatic axillary lymph nodes by the final histological findings. Of note, 11 (8.7%), 15 (11.9%) and 21 (16.7%) patients had unknown estrogen receptor (ER), progesterone receptor (PR) and her-2 status respectively because these patients refused the immunochemical staining.
Table 1.
Baseline characteristics of 126 patients with early breast cancer.
| Variable | No. (%) |
|---|---|
| Age (years, median) | 42 |
| ≤45 | 88(69.8) |
| >45 | 38(30.2) |
| Menopause | |
| No | 108(85.7) |
| Yes | 18(14.3) |
| Family history | |
| No | 97(77.0) |
| BC or OC | 12(9.5) |
| others | 17(13.5) |
| Dominant feature | |
| Mass only | 103(81.7) |
| Mass with calcification | 23(18.3) |
| BI-RADS classification | |
| 3 | 101(80.2) |
| 4a | 25(19.8) |
| Largest tumor dimension/mm | |
| ≤10 | 20(15.9) |
| 10-20 | 74(58.7) |
| >20 | 32(25.4) |
| Surgical method | |
| Breast conserving surgery | 29(23.0) |
| Mastectomy | 97(77.0) |
| Histological type | |
| IDC | 83(65.9) |
| DCIS | 26(20.6) |
| Others a | 17(13.5) |
| Lymph node status | |
| LN- b | 104(82.5) |
| LN+ c | 22(17.5) |
| TNM stage | |
| 0 | 26(20.6) |
| Ⅰ | 61(48.4) |
| ⅡA | 29(23.0) |
| ⅡB | 10(7.9) |
| ER (missing 11) | |
| Positive | 92(80.0) |
| Negative | 23(20.0) |
| PR (missing 15) | |
| Positive | 83(74.8) |
| Negative | 28(25.2) |
| Her-2 (missing 21) | |
| Positive | 30(28.6) |
| Negative | 75(71.4) |
Abbreviations: BC: breast cancer; OC: ovarian cancer; IDC: invasive ductal carcinoma; DCIS: ductal carcinoma in situ; ER: estrogen receptor; PR: progesterone receptor; HER-2: human epidermal growth factor receptor 2.
a Others included invasive lobular carcinoma (2 cases), mucinous carcinoma (9 cases), phylloides tumor (2 cases), signet-ring cell carcinoma (1 case), medullary carcinoma (1 case), metaplastic carcinoma (1 case), intraductal papillary carcinoma (1 case).
b No metastatic axillary lymph node.
c One or more metastatic axillary lymph node.
Residual tumor rate after VABB
Altogether, 79 (62.7%) patients were confirmed by post-surgery histological examination with residual tumor after undergoing VABB (Table 2). Therefore, only 47 (37.3%) patients experienced histological complete excision. After Stratified by BI-RADS (3 or 4a), the largest tumor dimension, histological type and axillary lymph node status (negative or positive), the residual tumor rates for BI-RADS 3 and 4a were 62.4% and 64.0%, respectively. As for lesions <10mm, lesions 10 to 20 mm and lesions > 20 mm in size, the residual tumor rates were 55.0%, 68.9% and 53.1%, respectively. Histological type of IDC, ducal carcinoma in situ (DCIS) and other types have residual tumor rates of 69.9%, 42.3% and 58.8%. With regard to patients with positive axillary lymph nodes, the residual tumor rate was as high as 81.8%, which was almost 20% higher than that of those with negative lymph nodes (58.7%). Table 3 displays the logistic regression analysis of factors associated with residual tumor. In the univariate analysis, histological type of DCIS was associated with significantly lower risk of residual tumor compared with IDC (OR, 0.32; 95% CI, 0.13-0.78; P = 0.013), and positive axillary lymph node status was associated with higher risk of residual tumor (OR, 3.17; 95% CI, 1.00-10.03; P = 0.049). However, no specific factors were identified to be associated with the risk of residual tumor by multivariate analysis (all P > 0.05).
Table 2.
Residual tumor rate after VABB as a minimally invasive surgery for breast cancer.
| Residual tumor No. (%) | No residual tumor No. (%) | Residual tumor rate (%) | |
|---|---|---|---|
| Total | 79 | 47 | 62.7 |
| BI-RADS classification | |||
| 3 | 63(79.7) | 38(80.9) | 62.4 |
| 4a | 16(20.3) | 9(19.1) | 64.0 |
| Largest tumor dimension/mm | |||
| ≤10 | 11(13.9) | 9(19.2) | 55.0 |
| 10-20 | 51(64.6) | 23(48.9) | 68.9 |
| >20 | 17(21.5) | 15(31.9) | 53.1 |
| Histological type | |||
| IDC | 58(73.4) | 25(53.2) | 69.9 |
| DCIS | 11(13.9) | 15(31.9) | 42.3 |
| Others b | 10(12.7) | 7(14.9) | 58.8 |
| Lymph node status | |||
| LN- a | 61(77.2) | 43(91.5) | 58.7 |
| LN+ b | 18(22.8) | 4(8.5) | 81.8 |
a No metastatic axillary lymph node.b One or more metastatic axillary lymph node.
Table 3.
Logistic regression analysis of factors associated with residual tumor.
| Variable | Unadjusted OR (95% CI) | P | Adjusted OR* (95% CI) | P |
|---|---|---|---|---|
| Age a | 1.02(0.98-1.06) | 0.299 | 1.03(0.99-1.07) | 0.206 |
| Dominant feature | ||||
| Mass only | 1.00 | 1.00 | ||
| Mass with calcification | 1.45(0.55-3.84) | 0.453 | 1.84(0.62-5.45) | 0.270 |
| Largest tumor dimension/mm | ||||
| ≤10 | 1.00 | 1.00 | ||
| 10-20 | 1.81(0.66-4.98) | 0.247 | 1.62(0.54-4.89) | 0.391 |
| >20 | 0.93(0.30-2.85) | 0.895 | 0.59(0.17-2.08) | 0.413 |
| Histological type | ||||
| IDC | 1.00 | 1.00 | ||
| DCIS | 0.32(0.13-0.78) | 0.013 | 0.44(0.16-1.17) | 0.098 |
| Others b | 0.62(0.21-1.80) | 0.376 | 0.83(0.26-2.65) | 0.83 |
| Lymph node status | ||||
| LN- c | 1.00 | 1.00 | ||
| LN+ d | 3.17(1.00-10.03) | 0.049 | 3.51(0.98-12.54) | 0.053 |
Abbreviations: IDC: invasive ductal carcinoma; DCIS: ductal carcinoma in situ; OR: odds ratio; CI: confidence interval.
a Continuous variable.
b Others included invasive lobular carcinoma (2 cases), mucinous carcinoma (9 cases), phylloides tumor (2 cases), signet-ring cell carcinoma (1 case), medullary carcinoma (1 case), metaplastic carcinoma (1 case), intraductal papillary carcinoma (1 case).
c No metastatic axillary lymph node.
d One or more metastatic axillary lymph node.
* Each covariate adjusted for all others.
Efficacy of mammogram and ultrasound in detecting residual tumor
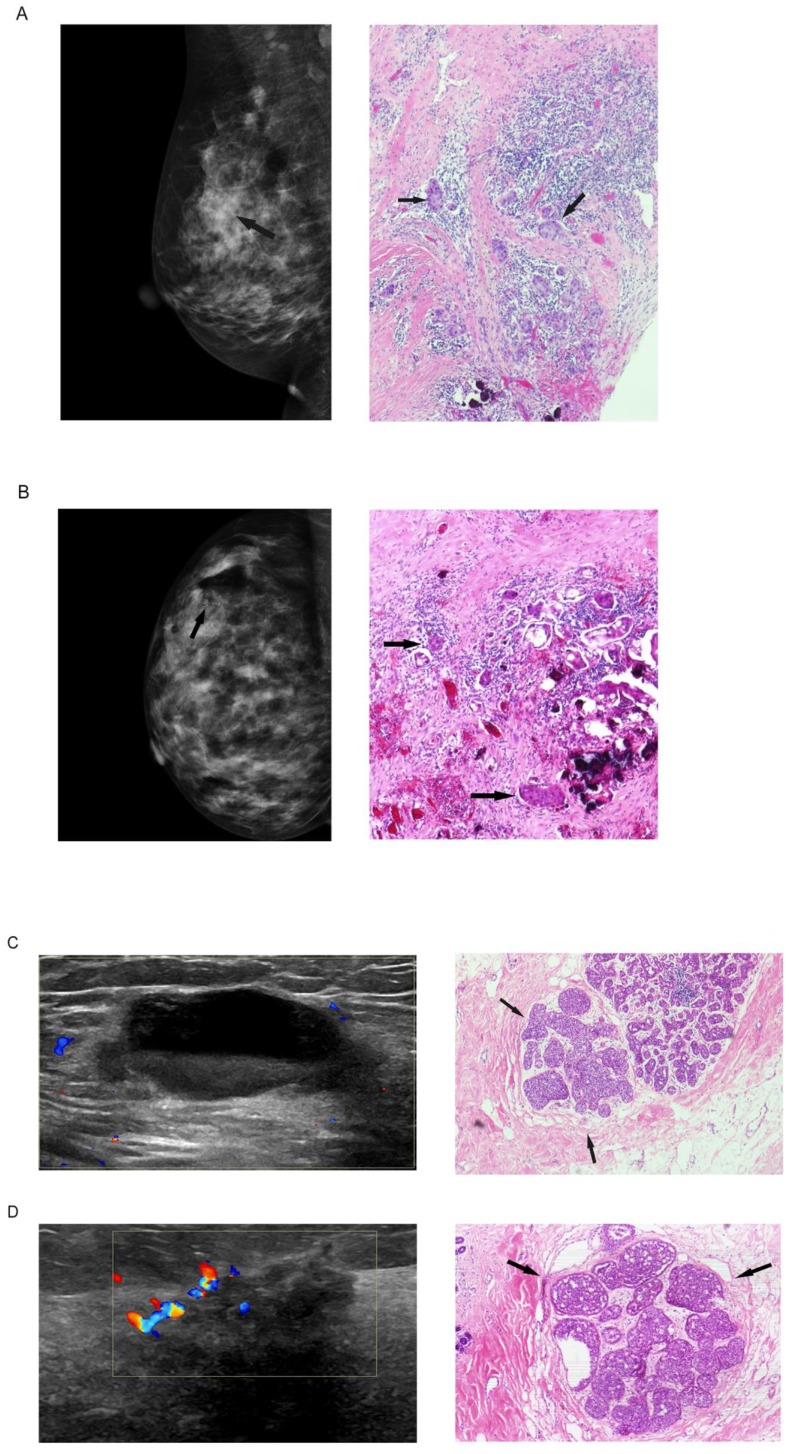
In total, 53 (42.1%) patients received mammogram and 115(91.3%) patients received ultrasound examination post VABB but before subsequent surgery (Table 4, Figure 1). Among these patients, 39 (76.5%) and 85 (73.9%) were estimated with no residual tumor by mammogram and ultrasound examinations, respectively. However, when compared with the histological findings, mammogram and ultrasound only had a sensitivity of 36.3% and 38.2%, which resulted in a negative predictive value of 46.2% and 50.6%. Namely, the probability for a patient free of histologically residual tumor was only 50% or even less when mammogram or ultrasound suggested no residual tumor after VABB. In addition, 12 (22.6%) patients were estimated with no residual tumor by mammogram and 26 (22.6%) by ultrasound, both with a high specificity of 90% or more, which resulted in a positive predictive value of 85.7% and 86.7%.
Figure 1.
Mammogram and ultrasound findings post biopsy but before subsequent surgery and the corresponding histological findings: (A) mammogram finding suggested no residual tumor at the biopsy site of a 45-year-old woman, inconsistent with the final histological finding with residual tumor; (B) mammogram finding suggested residual tumor (residual microcalcification shown by arrow) of a 30-year-old woman, consistent with the final histological finding; (C) ultrasound finding suggested no residual tumor of a 35-year-old woman, inconsistent with the final histological finding; (D) ultrasound finding suggested residual tumor of a 35-year-old woman, consistent with the final histological finding.
Discussion
Different with the previous studies concerning about VABB in the use of breast benign tumors, our study focused on breast cancer and estimated the efficacy of mammogram and ultrasound in detecting residual tumor compared with the golden standard of histological findings. To the best of our knowledge, this is the first study to characterize this issue. Of note, all the patients recruited for this study were diagnosed with benign disease before treatment and they were therefore permitted to receive VABB. In the present study, we retrospectively reviewed the histological slides of tumor specimens obtained from VABB and subsequent surgery of 126 early breast cancer patients to determine the residual tumor rate after VABB. The efficacy of mammogram and ultrasound in detecting residual tumor, and the correlation between clinic-pathological factors and the risk of residual tumor were analyzed. We mainly found that: (1) the residual tumor rate after VABB in early breast cancer patients was as high as 62.7%; (2) no specific factors were associated with the risk of residual tumor; (3) both mammogram and ultrasound were ineffective to detect the residual tumor.
For the last decade, numerous studies about VABB in breast benign tumor excision have been conducted, and the reported complete excision rates of VABB were about 50% to 90% 15-17. Fine et al 12 observed that the complete excision rates were reversely related to the lesion size, with 94% for lesions < 5mm, 91% for lesions 5 to10mm and 83% for lesions >10mm in size, but we did not observe such a reversely relationship in our study patients. However, as for malignant tumors, the complete excision rates were around 33% to 54.5% 14, 18, similar to the value of 37.3% in our study. The big difference in the complete excision rates between malignant tumors and benign tumors might be caused by the different essential biological characteristic. Malignant tumors have the capability of invasion and metastasis, so they are more likely to spread along the lymphatic vessels, blood vessels and mesenchyme, resulting in a larger invisible range which could not be detected through ultrasound or mammogram. Thus, they could not be completely excised as benign tumors did, because mammogram and ultrasound were less likely to have a truly presentation of tumors with irregular shapes, angular margins and ductal extension 19.
Previous studies suggested that preoperative diagnosis, age, extensive intraductal component, lymphovascular invasion, lobular histology, multifocality and tumor size were related to the risk of positive lumpectomy surgical margins 20-23. In the current study, we observed a higher residual tumor rate of 81.8% in patients with positive axillary lymph nodes and a lower rate of 42.3% in DCIS, but no significant relationship between age, dominant feature, tumor size, IDC histology, positive axillary lymph node status and risk of residual tumor were observed after the multivariate analysis. Since high residual tumor rates (42.3% - 81.8%) were observed in different subgroups, it is reasonable to speculate that presence of residual tumor might be related to the operative procedure of VABB, but not the clinic-pathological features. Because the diagnosis of breast cancer could not be obtained preoperatively, the excision range would be inadequate if malignant tumors underwent the same operative procedure as benign ones. In addition, VABB was seldom applied in breast cancer excision; this technic still needs exploration and improvement. The residual tumor rate of breast cancer is expected to decrease if larger extent resection is performed with VABB and the margins can be obtained to evaluate the residual tumor status, which requires proof from further studies.
As from mammogram and ultrasound evidence, the complete excision rates after VABB in breast cancer were 76.5% and 73.9%, much higher than the histologically determined complete excision rate of 37.3%. Though their specificities in detecting residual tumor were as high as 90%, the sensitivities were no more than 40%, a bit lower than the reported values from 42.7% to 62% 24-26. There is no doubt that a positive result of histological examination would be achieved when residual tumor was detected morphologically by mammogram and ultrasound. On the contrary, when residual tumor was present histologically, it could not always be detected morphologically because both mammogram and ultrasound imaging had difficult in accurately identifying lesions less than 5mm 27. Therefore, it would be inappropriate to estimate the complete excision rates of VABB in breast cancer through morphological findings without further histological examination.
The limitations of this study should be characterized: the data was retrospectively collected from a single center, and the sample may be insufficient since only a small part of patients with breast cancer would choose to receive the VABB. Moreover, VABB was performed by different operators which might result in deviation of surgical outcomes since this operative procedure is highly correlated with the skill of operators. Of note, patients recruited for this study were diagnosed with benign disease based on the findings of ultrasound and mammogram before histological outcomes were available, and all the operators have followed the strategy of benign tumor treatment that resecting all the visible lesions under guidance of ultrasound. Therefore, although different operators may have different skills, this uniform standard could help minimizing the deviation.
In conclusion, our study suggested that VABB as a minimally invasive surgery in breast cancer excision was invalid due to a high residual tumor rate, especially for those with positive axillary lymph nodes. Furthermore, the residual tumor could not be effectively detected by mammogram or ultrasound. Therefore, there is a need for those breast cancer patients who only underwent VABB to receive further extended surgery, even though mammogram or ultrasound indicated no residual tumor. Additional studies of large cohorts are warranted to further confirm our findings.
Acknowledgments
We would like to thank Shu-Jing Wang and Xi Lin, for their reviewing the images of mammogram and ultrasound, respectively; and Yi-Jun Zhang and Peng Sun, for their reviewing of the histological slides.
Funding
This study was supported by funds from the National Natural Science Foundation of China (81472575, 8127251, 81372133), the Key Program of the National Natural Science Foundation of China (31030061), the Science and Technology Planning Project of Guangdong and Guangzhou (2013B060300009, 2014J4100169).
Abbreviations
- BCS
breast conserving surgery
- VABB
vacuum-assisted breast biopsy
- SYSUCC
Sun Yat-sen University Cancer Center
- BI-RADS
Breast Imaging - Reporting and Data System
- HER-2
human epidermal growth factor receptor 2
- IDC
invasive ductal carcinoma
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- PR
progesterone receptor.
References
- 1.Coates AS, Winer EP, Goldhirsch A. et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–46. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clough KB, Nos C, Salmon RJ. et al. Conservative treatment of breast cancers by mammaplasty and irradiation: a new approach to lower quadrant tumors. Plast Reconstr Surg. 1995;96:363–70. doi: 10.1097/00006534-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Clough KB, Lewis JS, Couturaud B. et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003;237:26–34. doi: 10.1097/00000658-200301000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kepple J, Van Zee KJ, Dowlatshahi K. et al. Minimally invasive breast surgery. J Am Coll Surg. 2004;199:961–75. doi: 10.1016/j.jamcollsurg.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Parker SH, Burbank F. A practical approach to minimally invasive breast biopsy. Radiology. 1996;200:11–20. doi: 10.1148/radiology.200.1.8657896. [DOI] [PubMed] [Google Scholar]
- 6.Meyer JE, Smith DN, Lester SC. et al. Large-core needle biopsy of nonpalpable breast lesions. JAMA. 1999;281:1638–41. doi: 10.1001/jama.281.17.1638. [DOI] [PubMed] [Google Scholar]
- 7.Grady I, Gorsuch H, Wilburn-Bailey S. Ultrasound-guided, vacuum-assisted, percutaneous excision of breast lesions: an accurate technique in the diagnosis of atypical ductal hyperplasia. J Am Coll Surg. 2005;201:14–7. doi: 10.1016/j.jamcollsurg.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Velanovich V, Lewis FR, Nathanson SD. et al. Comparison of mammographically guided breast biopsy techniques. Ann Surg. 1999;229:625–30. doi: 10.1097/00000658-199905000-00004. discussion 630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon JR, Kalbhen CL, Cooper RA. et al. Accuracy and complication rates of US-guided vacuum-assisted core breast biopsy: initial results. Radiology. 2000;215:694–7. doi: 10.1148/radiology.215.3.r00jn37694. [DOI] [PubMed] [Google Scholar]
- 10.Burbank F, Parker SH, Fogarty TJ. Stereotactic breast biopsy: improved tissue harvesting with the Mammotome. Am Surg. 1996;62:738–44. [PubMed] [Google Scholar]
- 11.Burbank F, Forcier N. Tissue marking clip for stereotactic breast biopsy: initial placement accuracy, long-term stability, and usefulness as a guide for wire localization. Radiology. 1997;205:407–15. doi: 10.1148/radiology.205.2.9356621. [DOI] [PubMed] [Google Scholar]
- 12.Fine RE, Israel PZ, Walker LC. et al. A prospective study of the removal rate of imaged breast lesions by an 11-gauge vacuum-assisted biopsy probe system. Am J Surg. 2001;182:335–40. doi: 10.1016/s0002-9610(01)00723-1. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AT, Henry-Tillman RS, Smith LF. et al. Percutaneous excisional breast biopsy. Am J Surg. 2002;184:550–4. doi: 10.1016/s0002-9610(02)01099-1. discussion 554. [DOI] [PubMed] [Google Scholar]
- 14.Chen SC, Yang HR, Hwang TL. et al. Intraoperative ultrasonographically guided excisional biopsy or vacuum-assisted core needle biopsy for nonpalpable breast lesions. Ann Surg. 2003;238:738–42. doi: 10.1097/01.sla.0000094439.93918.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZL, Liu G, Huang Y. et al. Percutaneous excisional biopsy of clinically benign breast lesions with vacuum-assisted system: comparison of three devices. Eur J Radiol. 2012;81:725–30. doi: 10.1016/j.ejrad.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 16.Jackman RJ, Marzoni FA, Nowels KW. Percutaneous removal of benign mammographic lesions: comparison of automated large-core and directional vacuum-assisted stereotactic biopsy techniques. AJR Am J Roentgenol. 1998;171:1325–30. doi: 10.2214/ajr.171.5.9798873. [DOI] [PubMed] [Google Scholar]
- 17.Liberman L, Smolkin JH, Dershaw DD. et al. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251–60. doi: 10.1148/radiology.208.1.9646821. [DOI] [PubMed] [Google Scholar]
- 18.Cangiarella J, Gross J, Symmans WF. et al. The incidence of positive margins with breast conserving therapy following mammotome biopsy for microcalcification. J Surg Oncol. 2000;74:263–6. doi: 10.1002/1096-9098(200008)74:4<263::aid-jso4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Stavros AT, Thickman D, Rapp CL. et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–34. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 20.Beron PJ, Horwitz EM, Martinez AA. et al. Pathologic and mammographic findings predicting the adequacy of tumor excision before breast-conserving therapy. AJR Am J Roentgenol. 1996;167:1409–14. doi: 10.2214/ajr.167.6.8956568. [DOI] [PubMed] [Google Scholar]
- 21.Dillon MF, Hill AD, Quinn CM. et al. A pathologic assessment of adequate margin status in breast-conserving therapy. Ann Surg Oncol. 2006;13:333–9. doi: 10.1245/ASO.2006.03.098. [DOI] [PubMed] [Google Scholar]
- 22.Smitt MC, Horst K. Association of clinical and pathologic variables with lumpectomy surgical margin status after preoperative diagnosis or excisional biopsy of invasive breast cancer. Ann Surg Oncol. 2007;14:1040–4. doi: 10.1245/s10434-006-9308-1. [DOI] [PubMed] [Google Scholar]
- 23.Caughran JL, Vicini FA, Kestin LL. et al. Optimal use of re-excision in patients diagnosed with early-stage breast cancer by excisional biopsy treated with breast-conserving therapy. Ann Surg Oncol. 2009;16:3020–7. doi: 10.1245/s10434-009-0628-9. [DOI] [PubMed] [Google Scholar]
- 24.Homer MJ, Schmidt-Ullrich R, Safaii H. et al. Residual breast carcinoma after biopsy: role of mammography in evaluation. Radiology. 1989;170:75–7. doi: 10.1148/radiology.170.1.2909123. [DOI] [PubMed] [Google Scholar]
- 25.Graham RA, Homer MJ, Sigler CJ. et al. The efficacy of specimen radiography in evaluating the surgical margins of impalpable breast carcinoma. AJR Am J Roentgenol. 1994;162:33–6. doi: 10.2214/ajr.162.1.8273685. [DOI] [PubMed] [Google Scholar]
- 26.Lee CH, Carter D. Detecting residual tumor after excisional biopsy of impalpable breast carcinoma: efficacy of comparing preoperative mammograms with radiographs of the biopsy specimen. AJR Am J Roentgenol. 1995;164:81–6. doi: 10.2214/ajr.164.1.7998574. [DOI] [PubMed] [Google Scholar]
- 27.Berg WA, Blume JD, Cormack JB. et al. Lesion detection and characterization in a breast US phantom: results of the ACRIN 6666 Investigators. Radiology. 2006;239:693–702. doi: 10.1148/radiol.2393051069. [DOI] [PubMed] [Google Scholar]