Abstract
Human enteroviruses (HEV), especially coxsackievirus serotype B (CVB) and echovirus (E), have been associated with diseases of both the exocrine and endocrine pancreas, but so far evidence on HEV infection in human pancreas has been reported only in islets and ductal cells. This study aimed to investigate the capability of echovirus strains to infect human exocrine and endocrine pancreatic cells. Infection of explanted human islets and exocrine cells with seven field strains of E6 caused cytopathic effect, virus titer increase and production of HEV protein VP1 in both cell types. Virus particles were found in islets and acinar cells infected with E6. No cytopathic effect or infectious progeny production was observed in exocrine cells exposed to the beta cell-tropic strains of E16 and E30. Endocrine cells responded to E6, E16 and E30 by upregulating the transcription of interferon-induced with helicase C domain 1 (IF1H1), 2′-5′-oligoadenylate synthetase 1 (OAS1), interferon-β (IFN-β), chemokine (C–X–C motif) ligand 10 (CXCL10) and chemokine (C–C motif) ligand 5 (CCL5). Echovirus 6, but not E16 or E30, led to increased transcription of these genes in exocrine cells. These data demonstrate for the first time that human exocrine cells represent a target for E6 infection and suggest that certain HEV serotypes can replicate in human pancreatic exocrine cells, while the pancreatic endocrine cells are permissive to a wider range of HEV.
Keywords: acinar cells, echovirus, enterovirus, inflammation, islet of Langerhans, pancreas, tropism
1. Introduction
Human enteroviruses (HEV), in particular the coxsackievirus serotypes B (CVB) and echoviruses (E), have long been linked to diseases of both the exocrine and endocrine pancreas such as pancreatitis and type 1 diabetes, respectively [1,2]. Several independent studies have shown that members of both CVB and E replicate in explanted human pancreatic islets, causing functional beta cells impairment and local virus-induced production of pro-inflammatory cytokines and chemokines [3,4,5,6]. Thus, the exposure to virus strains targeting beta cells and inducing innate and adaptive immune responses may represent the mechanism through which HEV infection is involved in the pathogenesis of type 1 diabetes.
Despite the well-documented tropism of HEV toward human pancreatic islets, little is known about the human exocrine pancreas as a HEV target. To date, there is no direct evidence of HEV infection in the exocrine component of the pancreas. Evidence supporting the concept of a HEV etiology in the development of pancreatitis derives from serological studies [7,8] and case reports [9,10,11]. However, the assumption of exocrine infection by HEV in humans has not been confirmed by ex vivo studies.
Animal studies have shown that CVB replicates to high titers in mouse pancreas with subsequent acinar cell destruction, immune infiltration [12], and severe acinar pancreatitis [13]. Furthermore, virus particles, HEV capsid protein VP1 and RNA of CVB have been identified in acinar cells by electron microscopy [14], immunohistochemistry [15] and in situ hybridization [16]. In contrast with data in mice, studies on pancreases from patients with type 1 diabetes demonstrated the presence of HEV-positive cells in pancreatic islets whereas neither CVB-specific hybridization signal nor HEV genomes and HEV capsid protein VP1 were detectable in exocrine cells [3,17,18]. Likewise, tropism analysis of two CVB5 strains revealed virus growth in human explanted pancreatic islets but not in exocrine cell clusters, supporting the overall view that, in humans, CVB tropism in vitro is restricted to pancreatic endocrine cells [19].
However, most of the aforementioned studies have focused mainly on CVB, and there has been no attempt to assess the possible tropism of echovirus towards exocrine cells. Although we previously demonstrated that strains of E16 and E30 with a proven association with islet autoimmunity were able to replicate in explanted human islets and β cell-derived lines [20,21,22], their effect on human exocrine cells has never been tested. Interestingly, maternal infection with E6, a closely related echovirus strain, was associated with the induction of coexisting neonatal type 1 diabetes and exocrine pancreatic insufficiency in a Finnish report [23]. Further evidence supporting the idea that E6 may induce exocrine pancreatic damage in humans is the finding of significantly risen antibodies titers against E6 in sera from patients with acute pancreatitis [8]. Thus, the existence of specific HEV with a tropism for both human exocrine and endocrine pancreas cannot be completely ruled out.
Therefore, the aims of this study were to investigate the capability of E6 to infect explanted human exocrine and endocrine pancreatic cells and to assess the tropism of E16 and E30 toward human exocrine cells. This study also evaluated the virus-induced expression of innate immunity genes in both exocrine and endocrine pancreatic cells and examined to what extent the virus infection affects the expression of the genes encoding amylase and insulin.
2. Material and Methods
2.1. Viruses
For these purposes, we selected seven field strains of E6 isolated from the stools of sporadic cases of viral meningitis in Cuba during the years 1991 (E6/91), 1992 (E6/92), 1993 (E6/93), 1994 (E6/94), 1996 (E6/96), 2011(E6/11) and 2012 (E6/12) [24]. Clinical strains of E16 and E30 isolated during the Cuban meningitis epidemic in the years 2000 and 2001, respectively, from stool of subjects that developed islet cells antibodies during the convalescence stage of the infection were also included in the study [25,26]. Stool samples from the subjects were collected and processed according to standard procedures recommended by the World Health Organization (WHO) [27].
All viruses were propagated on 90% confluent green monkey kidney (GMK) cells in Eagle’s minimum essential medium (EMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 u/mL penicillin and 0.1 mg/mL streptomycin. Crude virus preparations were clarified from cell debris by centrifugation at 400 g for 10 min. The viral titer was measured by the cytopathic effect in microtitration assays and expressed as a 50% cell culture infectious dose (CCID50) per mL according to the Kärber formula [28]. The identity of all the isolates was confirmed by neutralization tests with type-specific antisera and partial VP1 sequences by primer pairs 187 (VP1; 5′-ACIGCIGYIGARACIGGNCA-3′) and 011 (2A; 5′-GCICCIGAYTGITGICCRAA-3′) (Thermo Fisher Scientific Waltham, MA, USA) and comparison with published sequences [29].
2.2. Tissue Source and Purity
Human pancreases from 14 organ donors free of any pancreatic disease were obtained from the Nordic Network for Islets Transplantation, Uppsala University, Uppsala, Sweden and human tissue laboratory of Lund University Diabetes Center, Malmö, Sweden [30], using a protocol described elsewhere [31]. For our purposes, we were able to obtain pancreatic islets from four donors, exocrine cell clusters from seven donors and both islets and exocrine cell clusters from the remaining three donors. Islets and exocrine cell clusters were kept in culture bags (Baxter Medical AB, Kista, Sweden) with 200 mL CMRL-1066 (ICN Biomedicals, Costa Mesa, CA, USA) supplemented with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM l-glutamine, 50 mg/mL gentamycin, 0.25 mg/mL fungizone (Gibco BRL; Invitrogen Ltd., Paisley, UK), 20 mg/mL ciprofloxacin (Bayer Healthcare AG, Leverkusen, Germany), and 10% heat-inactivated human serum at 37 °C in 5% CO2 and humidified air for 1–7 days.
In order to obtain a highly purified population for each cell type, islets and exocrine cell clusters were further handpicked with a micropipette under an inverted light microscope. The isolated and handpicked cell preparations used were highly pure (>95%) as measured by dithizone and insulin staining. All islets were intensely stained, whereas the exocrine cells displayed just focal and weak staining in few sections. All experiments were performed on 70 islets and 70 acinar cluster per well, and cultured in non-attach six-well plates (Sarstedt, Numbrecht, Germany) in 2 mL Roswell Park Memorial Institute (RPMI) medium containing 5.5 mM glucose (SVA, Uppsala, Sweden), supplemented with 10% FBS and 2 mM l-glutamine.
2.3. Virus Replication and Cytopathic Effect
Free-floating exocrine cell clusters and islets from each donor were divided into parallel aliquots and either infected with a 1000 CCID50/0.2 mL of each viral strain or mock-infected as a control. All the cells were incubated at 37 °C and examined under a light microscope for a period of 3–5 days, depending of the appearance of cytopathic effect/islet dissociation or not. Virus replication was determined by CCID50 titration method. Briefly, 0.2 mL of 10-fold serial dilutions (1:10 to 1:108) of samples of the culture medium collected on day 0, and day 3 post infection, were added in triplicate to GMK cells cultured in 96-well plates. Cytopathic effect was read on day 5 and CCID50 titer was calculated using the Kärber formula [28]. The virus replication extent was expressed as the difference between the CCID50 titer at day 3 and at 0 day post-infection (samples of culture medium collected directly after infection).
2.4. Gene Expression
Isolated human pancreatic islets and exocrine cells from three donors were washed in phosphate-buffered saline (PBS) and lysed by using RLT buffer (Qiagen, Sollentuna, Sweden) on QIAshredder spin columns (Qiagen). Total RNA was extracted with RNAeasy Mini kit. RNA quantity and quality were determined by Nanodrop (Thermo Scientific, Braunschwig, Germany). Fifty nanograms of total RNA per sample were primed with random hexamer and reverse transcribed to cDNA with SuperScript IITM RT (Invitrogen) according to the manufacturer’s instructions. The reaction was carried out at 25 °C for 10 min, 42 °C for 55 min and for 15 min at 75 °C. Real-time PCRs were run with SYBER Green master mix (Applied Biosystems, Stockholm, Sweden) in a 96-well optical plate on a StepOnePlusTM Real-Time PCR system (Applied Biosystems). The reaction mixture consisting of 10 µL Syber Green master mix (Applied Biosystems), 2 µL primer and 1 µL cDNA was prepared according to the manufactures instructions. The cycling conditions were 40 cycles of 15 s at 94 °C, 30 s at 55 °C and 30 s at 68 °C. Predesigned genes specific primer sets (QuantiTect1 Primer Assays; Qiagen) were used for detecting the expression of interferon induced with helicase C domain 1 (IF1H1), 2′–5′-oligoadenylate synthetase 1 (OAS1), interferon-β (IFN-β), chemokine (C–X–C motif) ligand 10 (CXCL10), chemokine (C–C motif) ligand 5 (CCL5), insulin and amylase. The mRNA expression of each gene was normalized to the expression level of 18S and β-actin housekeeping gene using ΔCt method and is presented as 2−ΔCt values, where Ct is the cycle threshold. The gene expression of infected cells was compared with the one of the mock-infected cells. Melt curve analysis was used to verify the specificity of final PCR products.
2.5. Glucose Stimulation Test
Fifty hand-picked and size-matched islets from three donors were infected with E6 strains isolated in 1991 and 2012 or mock-infected as a control. Insulin secretion in response to glucose stimulation was assessed on day 3 of culture in a dynamic perifusion system (Suprafusion 1000; BRANDEL, Gaithersburg, MD, USA). Islets were perifused with two glucose concentrations (1.67 and 16.7 mmol/L and then 1.67 mmol/L again), fractions were collected at six-minute intervals for 120 min and insulin concentration was determined by enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden).
2.6. Immunohistochemistry
Explanted islets and exocrine cell clusters of three donors were either mock-infected or infected with the E6/11 strain. Viruses-infected and mock-infected islets and exocrine cells were transferred to glass tubes on day 3 post infection. They were washed in PBS, fixed in 4% buffered paraformaldehyde (PFA), dehydrated in graded ethanol and embedded in paraffin. The paraffin embedded cells were sectioned (5 mm) and dried on Superfrost glass slides (Menzel-Gläzer; Fischer Scientific, Braunschweig, Germany), followed by de-paraffinization and re-hydration in 99%–70% ethanol. Antigen retrieval was performed in pH 9 TE buffer (10 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid (EDTA); DAKO, Glostrup, Denmark) in a steam boiler and permeabilized in PBS containing 0.05% TWEEN 20 (Sigma–Aldrich, St. Louis, MO, USA). Endogenous peroxidase was blocked by the use of a ready-to use peroxidase blocker (DAKO). After rinsing with PBS for 10 min, they were stained at room temperature with monoclonal antibody against a broad reacting epitope on the structural HEV protein 1 (VP1). Incubation with this antibody was performed at room temperature for one hour and the visualization was achieved by the anti-mouse Envision-kit (DAKO), using 3,3′-diaminobenzidine (DAB) as substrate chromogen.
2.7. Electron Microscopy
Islets and exocrine cells from a representative donor were infected with the E6/11 strain. Virus-infected and mock-infected samples were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer supplemented with 0.1 M sucrose, followed by 1.5 h post fixation in 1% OsO4, dehydration and embedding in epoxy plastic agar 100 (Agar Aids, Ltd., Stansted, UK). Ultra-thin sections (50 nm) were placed on Formvar-coated Cu grids (Agar Aids, Ltd., Stansted, UK), contrasted with uranyl acetate and lead citrate and analyzed in a Tecnai 12 BIO Twin electron microscope (FEI Company, Hillsboro, OR, USA).
2.8. Statistical Analysis
All statistical analyses were performed using SPSS package version 23 (IBM Corp., Armonk, NY, USA). Data are presented as mean value ± standard deviation (SD). All the experiments included control and virus-infected samples from the same donor. Comparisons among multiple groups were evaluated by Bonferroni-adjusted one-way ANOVA test. Mann-Whitney U test was used for comparisons between two groups. A p-value <0.05 was considered as statistically significant, with a confidence interval of 95%.
2.9. Ethics Statement
The study was conducted in compliance with the principles expressed in the Declaration of Helsinki and in the European Council’s Convention on Human Rights and Biomedicine. All methods were carried out in accordance with relevant guidelines and regulations. Consent for organ donation (for clinical transplantation and for use in research) was obtained from the relatives of the deceased donors by the donor’s physicians and documented in the medical records of the deceased donor. All procedures were approved by ethics committees at Uppsala University (Permit number: Dnr 2009/043, 2009/371, Ups 02-577) and Lund University (Permit number: Dnr.173/207). Consents for the use of the stool samples were waived because only left-over samples were used.
3. Results
3.1. Primary Human Pancreatic Exocrine and Endocrine Cells Are Productively Infected by Echovirus 6
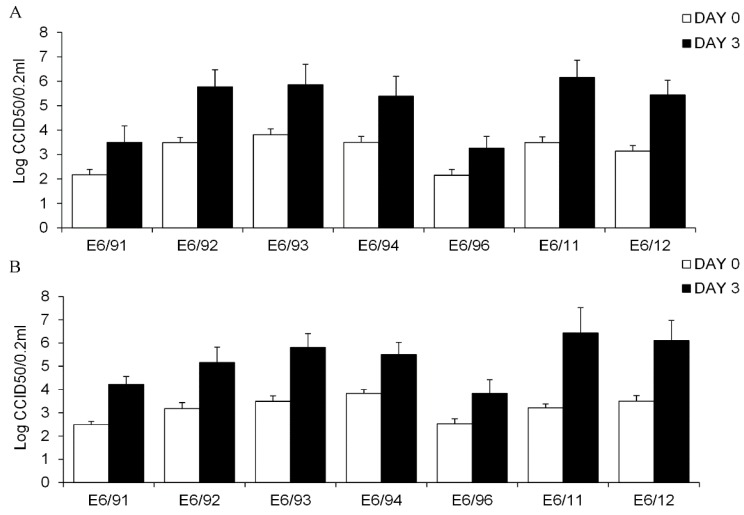
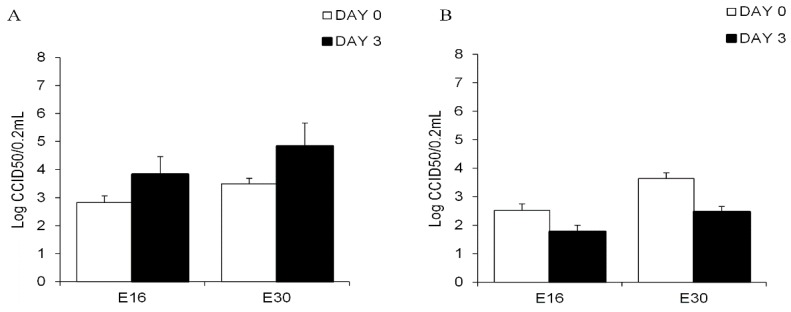
The infection with the seven different strains of E6 isolated during a period of 21 years, resulted in increased viral titer in both explanted human exocrine and endocrine cells after 72 h (Figure 1). In exocrine cells, the increase of virus titer during the three days post-infection ranged from 1.12 (E6/96) to 2.70 (E6/11) log10 CCID50/0.2 mL, 1.86 ± 0.59; mean ± SD (Figure 1A) whereas for endocrine cells ranged from 1.33 (E6/96) to 3.30 (E6/11) log10 CCID50/0.2 mL, 2.20 ± 0.64 mean ± SD (Figure 1B).
Figure 1.
Replication of field strains of echovirus 6 (E6) in primary human exocrine cells (A) and into the islets (B). Free-floating human exocrine cells derived from ten donors and islets from seven donors were infected with a 1000 50% cell culture infectious dose (CCID50)/0.2 mL of E6/91, E6/92, E6/93, E6/94, E6/96, E6/11 and E6/12. The samples were taken at day 0 (empty bar) and day 3 (filled bar) post-infections and were assayed for total infectivity by using the CCID50 titration method. Data are presented as log10 (CCID50/0.2 mL), means ± standard deviation (SD) from experiment performed in triplicate.
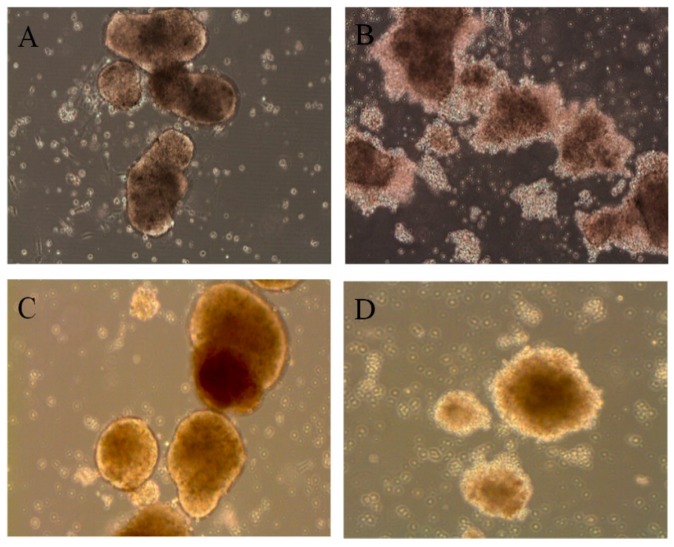
Bonferroni-adjusted ANOVA test for multiple comparisons showed that, among all the E6 strains, E6/11 infection resulted in significantly higher viral titer in exocrine (p = 0.006) and endocrine (p = 0.001) cells in comparison with that observed after the infection with the other E6 strains. However, the overall difference in virus titer increase was not statistically significant between islets and exocrine cells (p = 0.146). All the E6 strains induced similar morphologic alterations in exocrine (Figure 2A,B) and endocrine cells (Figure 2C,D), with cytopathic effects detectable 72 h post-infection.
Figure 2.
Virus-induced cytopathic effect in explanted human pancreatic exocrine cells and islets three days post-infection. (A) Mock-infected exocrine cells; (B) E6-infected exocrine cells; (C) Mock-infected islets; (D) E6-infected islets. The present results are a sample from different experiments with similar findings performed on islets from seven donors and exocrine cells from ten donors inoculated with the seven strains of E6.
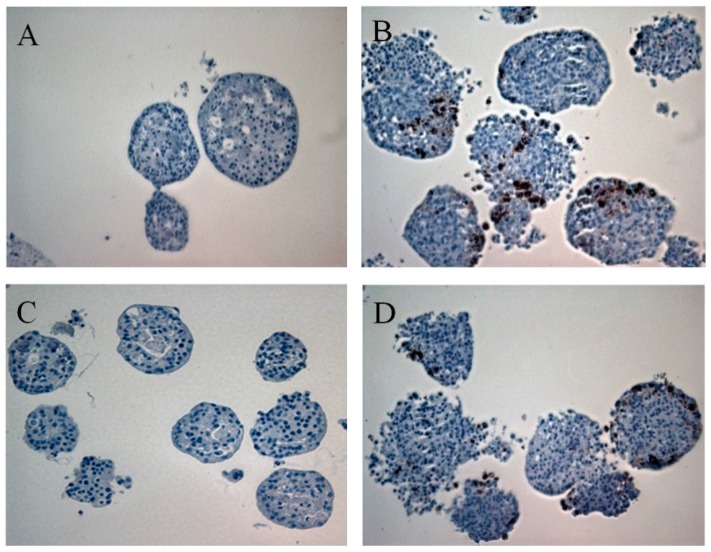
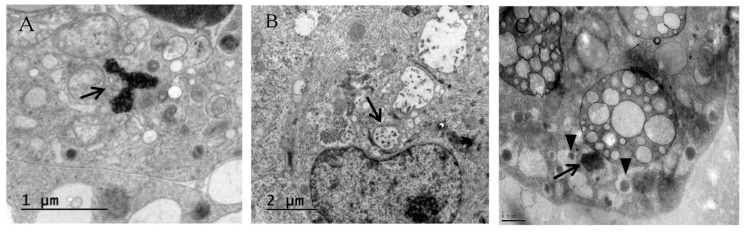
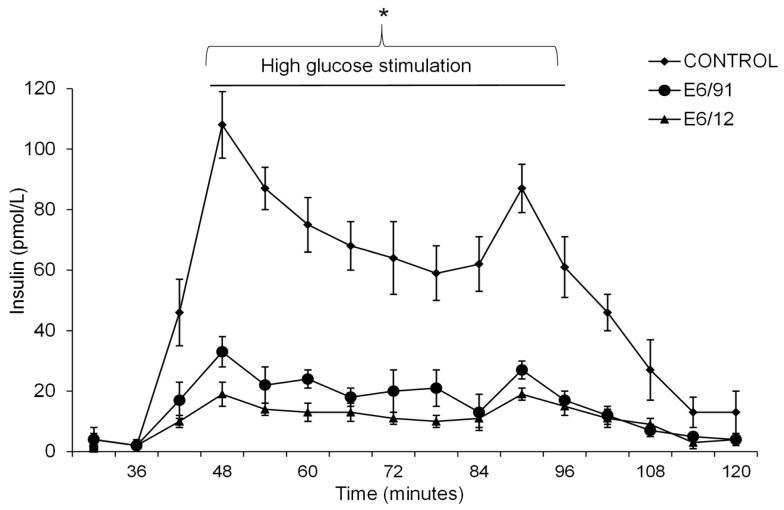
Acinar (Figure 3A,B) and islet (Figure 3C,D) cells infected with E6/11 strain were stained positive with antibody directed against the HEV capsid protein VP1. Virus particles were observed by electron microscopy in exocrine and endocrine cells infected with E6/11 strain (Figure 4). Aggregates of virus and membrane-bound vesicles (Figure 4A) were detected in the exocrine cell cytoplasm. Echovirus 6-infected acinar cells showed a massive degradation of cytoplasm along with an increased number of vesicular structures and vacuoles containing virus-like particles (Figure 4B). Echovirus 6-infected islets exhibited an accumulation of E6 particles in close proximity to insulin granules (Figure 4C). The glucose stimulated insulin secretion in E6/91 and E6/12 infected islets was significantly lower than that measured in mock-infected cells (p < 0.05, Figure 5).
Figure 3.
Immunostaining for human enteroviruses (HEV) protein VP1 in exocrine cells and islets three days after infection with E6/11. (A) Mock-infected exocrine cells; (B) E6-infected exocrine cells; (C) Mock-infected islets; (D) E6-infected islets. Representative example of three independent experiments.
Figure 4.
Electron microscopy of exocrine and endocrine cells infected with E6/11. (A) Virus particles (arrow) arranged in arrays in the cytoplasm of acinar cells, scale bar: 1 μm; (B) Portion of acinus showing degraded cytoplasm, membrane vesicles and vacuoles (arrow), scale bar: 2 μm; (C) Beta cells from islets infected with E6 containing virus particles arranged in a cluster (arrow) in close proximity to insulin granules (arrowhead), scale bar: 0.5 μm.
Figure 5.
Dynamic release of glucose-stimulated insulin secretion in E6-infected islets. Fifty hand-picked and size-matched islets from three donors were infected with E6/91 and E6/12. Mock-infected islets were left as a negative control. On day three after infection, the islets were perifused with glucose (1.67 mmol/L, 16.7 mmol/L, and 1.67 mmol/L). Fractions were collected at six-minute intervals and the secreted insulin was measured by enzyme-linked immunosorbent assay (ELISA). Data are presented as means ± SD and are based on observations from at least three donors. * p < 0.05 between groups (minutes 42 to 102).
3.2. Echovirus 16 or Echovirus 30 Can Replicate in Human Islets but Not in Human Exocrine Cells
Explanted human islets were productively infected with E16 and E30. The mean CCID50 titers increase in islet after three days of infection with E16 and E30 was 1 logCCID50 and 1.34 logCCID50, respectively (Figure 6A). In contrast, a titer decrease was observed when exocrine cells from all ten donors were inoculated with E16 or E30 (Figure 6B). These viruses did not induce cytopathic effect on exocrine cells for up to five days. Production of HEV capsid protein VP1 could not be detected in the acinar tissue. No structures resembling virus particles or hallmark for virus infection in exocrine cells inoculated with E16 and E30 or mock-infected acinar cells were observed by electron microscopy (data not shown).
Figure 6.
Viral titers of the epidemic strains of E16 and E30 in the culture medium of infected human islets (A) and exocrine cells (B). Free-floating human exocrine cells from ten donors and islets from seven donors were infected with a 1000 CCID50/0.2 mL of E16 and E30. Aliquots of the culture medium were withdrawn day 0 (empty bar) and day 3 (filled bar). Virus titers were obtained using the CCID50 titration method. Data are presented as log10 (CCID50/0.2 mL), means ± SD from experiment performed in triplicate.
3.3. Pancreatic Exocrine and Endocrine Cells Express Inflammatory and Antiviral Genes in Response to Echovirus 6 Infection
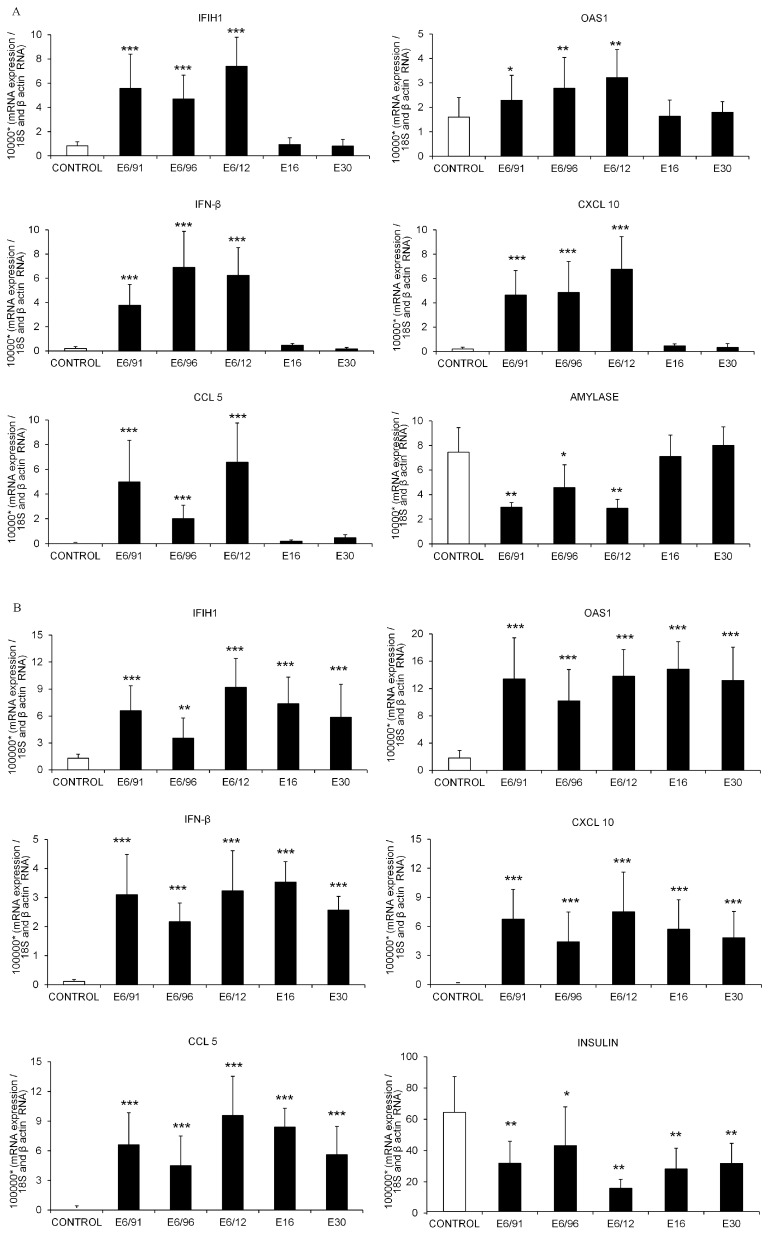
Gene expression was evaluated after infecting pancreatic exocrine cell clusters and islets with E16, E30 and three E6 isolates, representing a cross-section obtained at the beginning (E6/91), mid-stage (E6/96) and at the end (E6/12) of a 21-year endemic circulation of E6 in Cuba. Explanted human acinar cells inoculated with E16 or E30 and mock-infected cells exhibited high amylase gene expression; in contrast, amylase mRNA levels decreased significantly in exocrine cells infected with the three E6 strains (Figure 7A).
Figure 7.
Expression of genes related to virus recognition, antiviral response and amylase in mock-infected and virus-infected pancreatic acinar cells (A); Expression of genes related to virus recognition, antiviral response and islet hormone in mock-infected and virus-infected islets (B). Isolated human exocrine cell clusters and islets were mock-treated or infected with clinical strains of E6/91, E6/96, E6/12, E16 and E30 for 72 h. mRNA expression of interferon induced with helicase C domain 1 (IF1H1); 2′–5′-oligoadenylate synthetase 1 (OAS1); interferon-β (IFN-β); chemokine (C–X–C motif) ligand 10 (CXCL10); chemokine (C–C motif) ligand 5 (CCL5); amylase and insulin were assayed by real time reverse transcription-polymerase chain reaction (RT-PCR) and normalized by the house keeping gene 18S and β-actin using ΔCt method. Gene expression levels are presented as mRNA expression relative to the expression of the housekeeping gene (2−ΔCt). The expression level of each gene in virus-infected cells was compared to the one measured in mock-infected cells (control) from the same donors. Data are presented as means ± SD and were based on observations from at least three donors. Ct: cycle threshold. * p < 0.05, ** p < 0.01, *** p < 0.001.
High insulin gene expression was observed in mock-infected islets, this expression diminished after inoculation with E6, E16 and E30 (Figure 7B). Notably, E6-infected pancreatic exocrine cell clusters and pancreatic islets showed increased IF1H1, OAS1, IFN-β, CXCL10 and CCL5 mRNA expression in comparison with mock-infected cells (Figure 7A,B). Inoculation with E16 and E30 did not modify the expression of any of the tested genes in exocrine cell clusters (Figure 7A). However, gene expression levels of IF1H1, OAS1, IFN-β, CXCL10 and CCL5 were increased in islets following infection with E16 and E30 compared to the mock-infected control (Figure 7B). There were no differences in the level of expression of these genes between islets infected with E16, E30 or E6 (Figure 7B).
4. Discussion
The main finding of this study is that field isolates of E6, sampled over a 21-year lasting period in Cuba, are able to infect both pancreatic exocrine and endocrine cells and induce pro-inflammatory innate immune-responses in both cell types. This is the first report showing that explanted human pancreatic exocrine cells are permissive to E6 infection. In addition, we did not detect any virus replication in pancreatic exocrine cells clusters after infection with the closely related E16 and E30 serotypes, underlying remarkable differences in the tropism to exocrine cell among echovirus serotypes. All seven isolates replicated to high titers and induced a similar degree of cytopathic effect in acinar cells from ten different donors, indicating that no strain-specific variation in the tropism for the exocrine cells clusters was observed for endemic E6 strains.
Human enteroviruses replication in islets and ductal—but not in acinar—cells has been demonstrated in earlier reports [3,18,19,32,33], suggesting that human exocrine pancreas may not represent a target of HEV. Accordingly, we previously showed by virus titration studies, immunoreactivity of HEV VP1 protein and mRNA expression of innate immunity genes that E16 and E30 can productively infect both primary human islets and β cell-derived lines [21,22]. The present study confirms this observation by revealing an increase in viral titer when islets were infected with E16 and E30.
In this study we detected some beta cells characteristically embedded in exocrine cells. This finding has already been reported in previous investigations [19,34] which concluded that, despite the careful handpicking under light microscope, this phenomenon cannot be totally prevented. However, the high viral titers observed at 72 h post-infection in the exocrine fraction indicate that acinar cells, rather than embedded endocrine islets, support a productive E6 infection. Conversely, the viral titers of the islets-tropic E16 and E30 strains decreased in exocrine cells and no VP1-positive cells were found in exocrine acini. Likewise, the expression of the gene encoding amylase, a digestive enzyme marker for acinar cells, significantly decreased in E6-infected exocrine cells in comparison with exocrine cells clusters infected with E16, E30, further supporting the observation that viral replication after E6 infection takes place in the exocrine cells, and did not result just from contaminating β cells.
The presence of immunoreactive HEV capsid protein VP1 in islets and exocrine cells confirmed that both cells types were productively infected by E6. The tropism of the E6 strains towards both human pancreatic islets and acinar cells is also supported by the ultrastructural observation of virus particles in the cytoplasm of both cell types. Additional ultrastructural changes, previously identified in CVB-infected pancreas as “compound membrane-vesicle complexes” [14], were observed both in islets and exocrine acini; these complexes resemble autophagosomes and autophagy-related vesicles and are thought to serve as a scaffold for replication [35]. The finding of accumulation of virus particles in close proximity to insulin granules in E6-infected islets is consistent with reports from ultrastructural analyses of CVB-infected islets and has been hypothesized to display a permissive effect on viral replication [19,36,37].
Moreover, the impairment of insulin secretory ability and the decreased expression of the insulin gene observed after E6 infection additionally supports an effective virus replication within insulin-producing β cells. Remarkably, the presence of insulin granules in the virus-infected beta cells, despite decreased expression of insulin mRNA and a decreased glucose-induced insulin secretion, might suggest that decrease in insulin secretion is due to an impairment of β cell function. While it is clear that E6 replicated extensively in islet β cells, we were unable to determine whether viral replication also occurred in other pancreatic endocrine cells. In any case, we have previously shown that beta cells rather than alfa cells are more likely to support an HEV infection [6,38]. This could be explained by the fact that alfa cells express a vigorous cell-autonomous immune response against viral infection, and thus be able to eradicate the virus more effectively than β cells [39].
Remarkably, both primary pancreatic islets and exocrine cells responded to E6 infection by upregulating the transcription of genes involved in viral RNA recognition (IF1H1), antiviral defense (OAS1 and IFN-β) and recruitment of immune cells (CXCL10 and CCL5). Since not all the cells in the clusters are infected at once, it cannot be discarded that the gene expression could be induced in non-infected cells via the release of chemokines or cytokines from infected neighboring cells. This finding not only indicates that acinar cells are well-equipped to recognize and respond to E6 infection, as described for islets, but also suggests that viral replication in acinar cells generates a pro-inflammatory environment potentially attracting immune cells into the pancreas.
This study did not aim to investigate factors potentially influencing the acinar cell tropism of E6 in human pancreas. However, it has been shown that the presence of appropriate virus receptors, the availability of cellular factors essential for virus replication and viral genomic regions capable of interacting with receptor and cellular factors result in more efficient viral infection and progeny production [40,41]. Our data show a serotype-specific difference to replicate and induce damage of acinar cells, which favor the view that distinctive features in echovirus structure might be critical for controlling virus infection. It has been shown that experimentally altered virulence maybe connected to the genetic variations in the region coding for the structural proteins involved in receptor binding. Several studies have indicated that even a single or few amino acid changes in the viral genome can influence the receptor usage and tissue tropism [33,42]. Remarkably, a single amino acid substitution in the capsid protein VP2 could control the expression of the decay-accelerating factor (DAF)-dependent phenotype [43]. It therefore seems likely that virus with different cell tropism could arise during infection in humans, possibly driven by the host immune response. Our study is important in this regard and has generated results that warrant further scrutiny. Further investigations are, therefore, required in order to elucidate determinants of E6 tropism toward endocrine and exocrine pancreas. It remains to be established whether other serotypes of echovirus than E6 also infect pancreatic exocrine cells.
Morphological changes in the acinar tissue, along with mild to moderate exocrine pancreatic insufficiency, were shown to represent early events in type 1 diabetes disease process [44,45,46,47]; notably, inflammatory processes have been detected in exocrine pancreas from patients with type 1 diabetes [48,49]. Furthermore, it has been recently hypothesized that β cell autoantibodies result from injuries to the exocrine pancreatic portion [50]. However, whether exocrine dysfunction in type 1 diabetes represents a primary damage induced by the same pathogenic event that led to islets destruction and beta cells loss, remains an unanswered question [51,52]. Our results suggest that the infection with HEV strains targeting pancreatic islets and exocrine cells may act as a common environmental trigger for endocrine and exocrine pancreatic dysfunction in patients with type 1 diabetes. It is plausible that the infection of exocrine cells represents the conduit for islets infection. Therefore, the acinar cells infection and the consequent innate immune-response activation may explain the pancreatic inflammation, which sets the stage for the development of autoimmunity in patients with type 1 diabetes [50,53].
It should be noted that although strains of E6 capable of damaging and inducing pro-inflammatory responses in pancreas have been endemic in Cuba over the last 21 years, the incidence of type 1 diabetes in this area has remained low and relatively constant in this timeframe [24,54,55]. In Finland, the country with the highest incidence of type 1 diabetes in the world, molecular epidemiological and evolutionary analysis of Finnish E6 strains revealed that this virus is also endemic in Finland [56]. Such a divergent scenario further highlights the complexity of tracing HEV-disease association in type 1 diabetes and other pancreas diseases.
In conclusions, this study demonstrates that field isolates of E6 infect both the exocrine and endocrine pancreatic cells, whereas E16 and E30 strains have selective tropism for endocrine cells. Thus, it could be argued that while endocrine cells are permissible to a wide range of HEV serotypes, human pancreatic exocrine cells support productive infection of certain HEV serotypes. The determinants underlying the tropism of E6 for human exocrine and endocrine cells, and whether there is a link with the development of pancreatitis and type 1 diabetes in humans, warrant further investigations.
Acknowledgments
This work was supported by grants from the Swedish Research Council, Barndiabetesfondens (The Swedish Child Diabetes Foundation), the European Union’s Seventh Framework Programme PEVNET under grant agreement number 261441, and the Crafoord Foundation. The authors wish to thank the Nordic Network for Clinical Islet transplantation, the tissue isolation teams and Human Tissue Laboratory within EXODIAB/Lund University Diabetes Centre. We also thank Jeanette Arvastsson, Oskar Skog and Emmy Borgmästars for their assistance.
Author Contributions
L.S. conceived and designed the study, performed lab work, drafted the manuscript and approved the final version. G.F. and C.M.C. contributed to the study design, acquired data, revised and edited the intellectual content of the article and approved the final version. M.A., M.H. and E.N. collected and interpreted data, revised the intellectual content of the article and approved the final version. I.B. and E.C.-R. contributed to the study design, revised and edited the intellectual content of the article and approved the final version. L.S. and C.M.C. are responsible for the integrity of the work as a whole.
Conflicts of Interest
The authors declare no competing financial interests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huber S., Ramsingh A.I. Coxsackievirus-induced pancreatitis. Viral Immunol. 2004;17:358–369. doi: 10.1089/vim.2004.17.358. [DOI] [PubMed] [Google Scholar]
- 2.Yeung W.C., Rawlinson W.D., Craig M.E. Enterovirus infection and type 1 diabetes mellitus: Systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ylipaasto P., Klingel K., Lindberg A.M., Otonkoski T., Kandolf R., Hovi T., Roivainen M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47:225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 4.Ylipaasto P., Kutlu B., Rasilainen S., Rasschaert J., Salmela K., Teerijoki H., Korsgren O., Lahesmaa R., Hovi T., Eizirik D.L., et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia. 2005;48:1510–1522. doi: 10.1007/s00125-005-1839-7. [DOI] [PubMed] [Google Scholar]
- 5.Elshebani A., Olsson A., Westman J., Tuvemo T., Korsgren O., Frisk G. Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes. Virus Res. 2007;124:193–203. doi: 10.1016/j.virusres.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Anagandula M., Richardson S.J., Oberste M.S., Sioofy-Khojine A.B., Hyöty H., Morgan N.G., Korsgren O., Frisk G. Infection of human islets of Langerhans with two strains of Coxsackie B virus serotype 1: Assessment of virus replication, degree of cell death and induction of genes involved in the innate immunity pathway. J. Med. Virol. 2014;86:1402–1411. doi: 10.1002/jmv.23835. [DOI] [PubMed] [Google Scholar]
- 7.Ozsvár Z., Deákm J., Pap A. Possible role of Coxsackie-B virus infection in pancreatitis. Int. J. Pancreatol. 1992;11:105–108. doi: 10.1007/BF02925981. [DOI] [PubMed] [Google Scholar]
- 8.Arnesjö B., Eden T., Ihse I., Nordenfelt E., Ursing B. Enterovirus infections in acute pancreatitis. A possible etiological connection. Scand. J. Gastroenterol. 1976;11:645–649. [PubMed] [Google Scholar]
- 9.Gooby Toedt D.M., Byrd J.C., Omori D. Coxsackievirus-associated pancreatitis mimicking metastatic carcinoma. South. Med. J. 1996;89:441–443. doi: 10.1097/00007611-199604000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Akuzawa N., Harada N., Hatori T., Imai K., Kitahara Y., Sakurai S., Kurabayashi M. Myocarditis, hepatitis, and pancreatitis in a patient with coxsackievirus A4 infection: A case report. Virol. J. 2014;11:3. doi: 10.1186/1743-422X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y.F., Deng H.L., Fu J., Zhang Y., Wei J.Q. Pancreatitis in hand-foot-and-mouth disease caused by enterovirus 71. World J. Gastroenterol. 2016;22:2149–2152. doi: 10.3748/wjg.v22.i6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mena I., Fischer C., Gebhard J.R., Perry C.M., Harkins S., Whitton J.L. Coxsackievirus infection of the pancreas: Evaluation of receptor expression, pathogenesis, and immunopathology. Virology. 2000;271:276–288. doi: 10.1006/viro.2000.0332. [DOI] [PubMed] [Google Scholar]
- 13.Ramsingh A.I. Coxsackieviruses and pancreatitis. Front. Biosci. 1997;2:e53–e62. doi: 10.2741/A227. [DOI] [PubMed] [Google Scholar]
- 14.Burch G.E., Harb J.M. Electron microscopic studies of viral pancreatitis in coxsackie B4 virus infected mice. Exp. Mol. Pathol. 1979;31:23–35. doi: 10.1016/0014-4800(79)90004-2. [DOI] [PubMed] [Google Scholar]
- 15.Caggana M., Chan P., Ramsingh A. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J. Virol. 1993;67:4797–4803. doi: 10.1128/jvi.67.8.4797-4803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vella C., Easton A.J., Eglin R.P., Brown C.L., Perry L. Coxsackie virus B4 infection of the mouse pancreas. Detection of virus-specific RNA in the pancreas by in situ hybridisation. J. Med. Virol. 1991;35:46–49. doi: 10.1002/jmv.1890350110. [DOI] [PubMed] [Google Scholar]
- 17.Foulis A.K., Farquharson M.A., Cameron S.O., McGill M., Schönke H., Kandolf R. A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia. 1990;33:290–298. doi: 10.1007/BF00403323. [DOI] [PubMed] [Google Scholar]
- 18.Richardson S.J., Willcox A., Bone A.J., Foulis A.K., Morgan N.G. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 19.Hodik M., Lukinius A., Korsgren O., Frisk G. Tropism analysis of two Coxsackie B5 strains reveals virus growth in human primary pancreatic islets but not in exocrine cell clusters in vitro. Open Virol. J. 2013;7:49–56. doi: 10.2174/1874357901307010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarmiento L., Galvan J.A., Cabrera-Rode E., Aira L., Correa C., Sariego S., Fonseca M., Cubas-Dueñas I., Hung L.H., Resik S., et al. Type 1 diabetes associated and tissue transglutaminase autoantibodies in patients without diabetes and coeliac disease with confirmed viral infections. J. Med. Virol. 2012;84:1049–1053. doi: 10.1002/jmv.23305. [DOI] [PubMed] [Google Scholar]
- 21.Sarmiento L., Frisk G., Anagandula M., Cabrera-Rode E., Roivainen M., Cilio C.M. Expression of innate immunity genes and damage of primary human pancreatic islets by epidemic strains of echovirus: Implication for post-virus islet autoimmunity. PLoS ONE. 2013;8:e77850. doi: 10.1371/journal.pone.0077850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarmiento L., Medina A., Aziz K., Anagandula M., Cabrera-Rode E., Fex M., Frisk G., Cilio C.M. Differential effects of three echovirus strains on cell lysis and insulin secretion in beta cell derived lines. J. Med. Virol. 2015;8:971–978. doi: 10.1002/jmv.24438. [DOI] [PubMed] [Google Scholar]
- 23.Otonkoski T., Roivainen M., Vaarala O., Dinesen B., Leipälä J.A., Hovi T., Knip M. Neonatal Type I diabetes associated with maternal echovirus 6 infection: A case report. Diabetologia. 2000;43:1235–1238. doi: 10.1007/s001250051518. [DOI] [PubMed] [Google Scholar]
- 24.Sarmiento L. Enteroviral meningitis and emergence of rare enterovirus types: Cuban experience. In: Strong P.V., editor. Focus on Meningitis Research. Nova; New York, NY, USA: 2004. pp. 1–14. [Google Scholar]
- 25.Cabrera-Rode E., Sarmiento L., Tiberti C., Molina G., Barrios J., Hernández D., Díaz-Horta O., Di Mario U. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia. 2003;46:1348–1353. doi: 10.1007/s00125-003-1179-4. [DOI] [PubMed] [Google Scholar]
- 26.Cabrera-Rode E., Sarmiento L., Molina G., Pérez C., Arranz C., Galvan J.A., Prieto M., Barrios J., Palomera R., Fonseca M., et al. Islet cell related antibodies and type 1 diabetes associated with echovirus 30 epidemic: A case report. J. Med. Virol. 2005;76:373–377. doi: 10.1002/jmv.20368. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . WHO Polio Laboratory Manual. 4th ed. World Health Organization; Geneva, Switzerland: 2004. Isolation and Identification of Polioviruses. [Google Scholar]
- 28.Lennette E.H. General principles underlying laboratory diagnosis of viral and rickettsial infections. In: Lennette E.H., Schmidt N.J., editors. Diagnostic Procedures for Viral and Rickettsial Infections. American Public Health Association; New York, NY, USA: 1969. pp. 1–63. [Google Scholar]
- 29.Oberste M.S., Maher K., Kilpatrick D.R., Flemister M.R., Brown B.A., Pallansch M.A. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 1999;37:1288–1293. doi: 10.1128/jcm.37.5.1288-1293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Human Tissue Laboratory. [(accessed on 6 may 2016)]. Available online: http://www.ludc.med.lu.se/platforms/human-tissue-laboratory/
- 31.Goto M., Eich T.M., Felldin M., Foss A., Källen R., Salmela K., Tibell A., Tufveson G., Fujimori K., Engkvist M., et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78:1367–1375. doi: 10.1097/01.TP.0000140882.53773.DC. [DOI] [PubMed] [Google Scholar]
- 32.Sane F., Caloone D., Gmyr V., Engelmann I., Belaich S., Kerr-Conte J., Pattou F., Desailloud R., Hober D. Coxsackievirus B4 can infect human pancreas ductal cells and persist in ductal-like cell cultures which results in inhibition of Pdx1 expression and disturbed formation of islet-like cell aggregates. Cell. Mol. Life Sci. 2013;70:4169–4180. doi: 10.1007/s00018-013-1383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smura T., Natri O., Ylipaasto P., Hellman M., Al-Hello H., Piemonti L., Roivainen M. Enterovirus strain and type-specific differences in growth kinetics and virus-induced cell destruction in human pancreatic duct epithelial HPDE cells. Virus Res. 2015;210:188–197. doi: 10.1016/j.virusres.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Skog O., Korsgren S., Wiberg A., Danielsson A., Edwin B., Buanes T., Krogvold L., Korsgren O., Dahl-Jørgensen K. Expression of human leukocyte antigen class in endocrine and exocrine pancreatic tissue at onset of type 1 diabetes. Am. J. Pathol. 2015;185:129–138. doi: 10.1016/j.ajpath.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Kemball C.C., Alirezaei M., Flynn C.T., Wood M.R., Harkins S., Kiosses W.B., Whitton J.L. Coxsackievirus infection induces autophagy like vesicles and megaphagosomes in pancreatic acinar cells in vivo. J. Virol. 2010;84:12110–12124. doi: 10.1128/JVI.01417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roivainen M., Rasilainen S., Ylipaasto P., Nissinen R., Ustinov J., Bouwens L., Eizirik D.L., Hovi T., Otonkoski T. Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells. J. Clin. Endocrinol. Metab. 2000;85:432–440. doi: 10.1210/jcem.85.1.6306. [DOI] [PubMed] [Google Scholar]
- 37.Yin H., Berg A.K., Westman J., Hellerström C., Frisk G. Complete nucleotide sequence of a Coxsackievirus B-4 strain capable of establishing persistent infection in human pancreatic islet cells: Effects on insulin release, proinsulin synthesis, and cell morphology. J. Med. Virol. 2002;68:544–557. doi: 10.1002/jmv.10236. [DOI] [PubMed] [Google Scholar]
- 38.Hodik M., Skog O., Lukinius A., Isaza-Correa J.M., Kuipers J., Giepmans B.N., Frisk G. Enterovirus infection of human islets of Langerhans affects β-cell function resulting in disintegrated islets, decreased glucose stimulated insulin secretion and loss of Golgi structure. BMJ Open Diabetes Res. Care. 2016;4:e000179. doi: 10.1136/bmjdrc-2015-000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marroqui L., Lopes M., dos Santos R.S., Grieco F.A., Roivainen M., Richardson S.J., Morgan N.G., de Beeck A.O., Eizirik D.L. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic α and β cells. eLife. 2015;4:e06990. doi: 10.7554/eLife.06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvala H., Kalimo H., Dahllund L., Santti J., Hughes P., Hyypiä T., Stanway G. Mapping of tissue tropism determinants in coxsackievirus genomes. J. Gen. Virol. 2002;83:1697–1706. doi: 10.1099/0022-1317-83-7-1697. [DOI] [PubMed] [Google Scholar]
- 41.Marjomäki V., Turkki P., Huttunen M. Infectious Entry Pathway of Enterovirus B Species. Viruses. 2015;7:6387–6399. doi: 10.3390/v7122945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paananen A., Ylipaasto P., Smura T., Lempinen M., Galama J., Roivainen M. A single amino acid substitution in viral VP1 protein alters the lytic potential of clone-derived variants of echovirus 9 DM strain in human pancreatic islets. J. Med. Virol. 2013;87:1267–1273. doi: 10.1002/jmv.23574. [DOI] [PubMed] [Google Scholar]
- 43.Pan J., Narayanan B., Shah S., Yoder J.D., Cifuente J.O., Hafenstein S., Bergelson J.M. Single amino acid changes in the virus capsid permit coxsackievirus B3 to bind decay-accelerating factor. J. Virol. 2011;85:7436–7443. doi: 10.1128/JVI.00503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 45.Lankisch P.G., Manthey G., Otto J., Koop H., Talaulicar M., Willms B., Creutzfeldt W. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25:211–216. doi: 10.1159/000198833. [DOI] [PubMed] [Google Scholar]
- 46.Hardt P.D., Hauenschild A., Nalop J., Marzeion A.M., Jaeger C., Teichmann J., Bretzel R.G., Hollenhorst M., Kloer H.U., S2453112/S2453113 Study Group High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. A multicenter study screening fecal elastase 1 concentrations in 1021 diabetic patients. Pancreatology. 2003;3:395–402. doi: 10.1159/000073655. [DOI] [PubMed] [Google Scholar]
- 47.Creutzfeldt W., Gleichmann D., Otto J., Stöckmann F., Maisonneuve P., Lankisch P.G. Follow-up of exocrine pancreatic function in type 1 diabetes mellitus. Digestion. 2005;72:71–75. doi: 10.1159/000087660. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar S.A., Lee C.E., Victorino F., Nguyen T.T., Walters J.A., Burrack A., Eberlein J., Hildemann S.K., Homann D. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes. 2012;61:436–446. doi: 10.2337/db11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Calvo T., Ekwall O., Amirian N., Zapardiel-Gonzalo J., von Herrath M.G. Increased immune cell infiltration of the exocrine pancreas: A possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–3890. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiberg A., Granstam A., Ingvast S., Härkönen T., Knip M., Korsgren O., Skog O. Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin. Exp. Immunol. 2015;182:278–288. doi: 10.1111/cei.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell-Thompson M., Rodriguez-Calvo T., Battaglia M. Abnormalities of the Exocrine Pancreas in Type 1 Diabetes. Curr. Diabetes Rep. 2015;15:79. doi: 10.1007/s11892-015-0653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battaglia M., Atkinson M.A. The streetlight effect in type 1 diabetes. Diabetes. 2015;64:1081–1090. doi: 10.2337/db14-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider D.A., von Herrath M.G. Potential viral pathogenic mechanism in human type 1 diabetes. Diabetologia. 2014;57:2009–2018. doi: 10.1007/s00125-014-3340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarmiento L., Cabrera-Rode E., Lekuleni L., Cuba I., Molina G., Fonseca M., Heng-Hung L., Borroto A., Gonzalez P., Mas P., et al. Occurrence of enterovirus RNA in serum of children with newly diagnosed type 1 diabetes and islet cell autoantibody-positive subjects in a population with a low incidence of type 1 diabetes. Autoimmunity. 2007;40:540–545. doi: 10.1080/08916930701523429. [DOI] [PubMed] [Google Scholar]
- 55.Sarmiento L., Cubas-Dueñas I., Cabrera-Rode E. Evidence of association between exposure to enterovirus and type 1 diabetes in Cuban children and adolescents. MEDICC Rev. 2013;15:29–32. doi: 10.1590/S1555-79602013000100007. [DOI] [PubMed] [Google Scholar]
- 56.Smura T., Kakkola L., Blomqvist S., Klemola P., Parsons A., Kallio-Kokko H., Savolainen-Kopra C., Kainov D.E., Roivainen M. Molecular evolution and epidemiology of echovirus 6 in Finland. Infect. Genet. Evol. 2013;16:234–247. doi: 10.1016/j.meegid.2013.02.011. [DOI] [PubMed] [Google Scholar]