Abstract
African swine fever (ASF) is a devastating disease of domestic pigs. It is a socioeconomically important disease, initially described from Kenya, but subsequently reported in most Sub-Saharan countries. ASF spread to Europe, South America and the Caribbean through multiple introductions which were initially eradicated—except for Sardinia—followed by re‑introduction into Europe in 2007. In this study of ASF within the Democratic Republic of the Congo, 62 domestic pig samples, collected between 2005–2012, were examined for viral DNA and sequencing at multiple loci: C-terminus of the B646L gene (p72 protein), central hypervariable region (CVR) of the B602L gene, and the E183L gene (p54 protein). Phylogenetic analyses identified three circulating genotypes: I (64.5% of samples), IX (32.3%), and XIV (3.2%). This is the first evidence of genotypes IX and XIV within this country. Examination of the CVR revealed high levels of intra-genotypic variation, with 19 identified variants.
Keywords: African swine fever virus, outbreaks, Democratic Republic of Congo, swine, genotypes, molecular epidemiology, p72 gene, p54 gene, CVR
1. Introduction
African swine fever (ASF) is a complex and highly lethal haemorrhagic disease of domestic swine with mortality rates reaching 100%. ASF, which is threatening the world pig industry, is a notifiable disease by the World Organization for Animal Health (OIE) [1]. Since the recognition of ASF in Kenya in the 1920s [2], ASF has expanded to most sub-Saharan countries [3], and was exported outside Africa in 1957 to Portugal [4]. Subsequent exportations to Europe occurred in 1960 and 2007 [5,6]. Introduction of ASF has not been limited to Europe, as outbreaks with putative causal links to Spain have occurred in the Caribbean and South America [7].
Domestic pigs are most susceptible, with the disease course ranging from peracute, acute, subacute, chronic and unapparent and, mortality rates ranging from 100% to as little as 3% [8]. ASF is caused by African swine fever virus (ASFV), which is transmitted to swine through three main routes: (1) a sylvatic cycle involving wild swine and Ornithodoros ticks; (2) from the sylvatic cycle to domestic pigs; and (3) domestic pig cycle involving domesticated pig to pig transmission [3,9]. ASFV is a large arbovirus within the genus Asfivirus and is the sole member of the family Asfarviridae [10].
Due to the lack of treatment and vaccine, rapid and accurate diagnosis complemented by the genotyping of circulating ASFVs may contribute to timely improvement of prevention and control strategies. In order to identify and determine the heterogeneity of circulating ASFVs, a rapid method of polymerase chain reaction (PCR)-based sequencing of a 478 base pair (bp) fragment at the C-terminal end of the p72 gene has been commonly used to differentiate the different genotypes of ASFV [11]. Given the low level of genetic variation detected at the p72 locus among ASFVs recovered in domestic pig outbreaks, examination of more variable genomic regions such as the central hypervariable region (CVR) of the B602L gene [12] in combination with the p54 and p30 genes [13] were used to differentiate between isolates within a single outbreak.
Currently, there are 23 confirmed genotypes of ASFV based on the sequencing of the p72 gene [11], not all of which are known to be currently circulating. The most recent, genotype XXIII, was discovered in Ethiopia [14]. In Africa, further diagnostic analysis of suspected outbreaks shows genotype I continuing to circulate in Western and Central Africa [15,16]. Countries with continued presence of genotypes IX and X include Uganda and Kenya [17,18,19]. Genotype II has maintained its presence in Tanzania, Mozambique, Madagascar and Zambia, and is what led to the introduction of genotype II into Georgia in the Caucasus region in 2007 [6,20]. Since that time, ASFV has spread from Georgia and the Caucasus to the Baltic states (Estonia, Latvia and Lithuania), the Russian Federation, Ukraine, and Poland [21,22,23].
The Democratic Republic of the Congo (DRC) is the second largest country in Africa, with the central and western portions of the country being dominated by the second largest block of rainforest in the world, whereas the southern and eastern regions are characterized by savannas. The 9000-km perimeter of the DRC contacts nine countries and includes a number of lakes (e.g., Edward, Albert, Kivu, Tanganyika) and rivers (e.g., the Congo, Ubangi, Kasai, Semliki). These geographic elements, as well the presence of many trans-frontier tribes that inhabit the DRC and surrounding countries contribute to the semi-porous nature of the DRC’s political boundary.
In 2011, the DRC reported 84 outbreaks and a loss of 105,614 swine, leading African countries in both statistics [24]. Despite the prevalence of ASF in the DRC, the variety of genotypes reported in surrounding countries [9,19], and the large number of studies that have examined this disease, no in-depth study has focused on understanding the genetic diversity of ASFV within the DRC. The goal of this study was to improve the scientific community’s understanding of ASFV strains circulating in the DRC. To achieve this goal, we collected samples from swine that exhibited ASF clinical signs and pathological findings over a broad geographic and temporal range. We utilized a multi-locus genotyping approach to categorize gene sequences into genotypes, and used this data to improve the understanding of the natural history as well as the links between outbreaks of ASFV in the DRC.
2. Materials and Methods
2.1. Study Area and Samples
A total of 62 tissue samples (spleen, lymph node, kidney, lung, liver, heart and stomach) of ASF suspected cases were collected between 2005 and 2012 from 57 domestic pig carcasses and from 25 locations. Of these carcasses, 54 were collected in outbreaks and three were slaughtered in urban markets. Sampling localities were located in six provinces (Kinshasa, Equateur, Katanga, Orientale, Bas-Congo and Maniema) that contain the majority of the country’s domestic pig population. Tissue samples were transported to the laboratory, homogenized and supernatants were stored at −80 °C until use. Samples, collection localities, and other details are provided in Table 1.
Table 1.
Samples, details, and genotypes.
| Virus | Outbreak Date | Location | GPS | Ecological Profile | Tissues | Farming System | Genotype |
|---|---|---|---|---|---|---|---|
| drc49/05/p1a | May 2005 | Limete | 4°18 S/15°22 E | City | Spl | Commercial | I |
| drc49/05p1b | May 2005 | Limete | 4°18 S/15°22 E | City | Ln | Commercial | I |
| drc49/05/P2a | May 2005 | Limete | 4°18 S/15°22 E | City | Spl | Commercial | I |
| drc70/05/1 | 2005 | Limete | 4°18 S/15°22 E | City | Spl | Commercial | I |
| drc75/05/1 | 2005 | Maniema | 2°93 S/25°86 E | City | Spl | Commercial | I |
| drc99/05/a | 2005 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drcKG28110805 | Nov 2008 | Ngaliema | 4°21 S/15°21 E | City | Spl | Backyard | I |
| drcKG28040802 | Apr 2008 | Kasavubu | 4°20 S/15°20 E | City | Spl | Backyard | I |
| drc74/09/2 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Kd | Commercial | I |
| drc74/09/3 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Lg | Commercial | I |
| drc74/09/4 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Lv | Commercial | I |
| drc74/09/6 | 2009 | Nsele | 4°24 S/15°30 E | Peri-urban | Hrt | Commercial | I |
| drc94/09/2 | 2009 | Kintambo | 4°20 S/15°18 E | City | Ln | Commercial | I |
| drc35/10/1 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Spl | Backyard | I |
| drc35/10/5 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Ln | Backyard | I |
| drc35/10/4 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Ln | Backyard | I |
| drc51/10/23 | 2010 | Ndjili | 4°24 S/15°21 E | City | Spl | Commercial | I |
| drc73/10/2 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Kn | Backyard | I |
| drc73/10/3 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Lv | Backyard | I |
| drc73/10/4 | 2010 | Ngaliema | 4°21 S/15°05 E | City | Ln | Backyard | I |
| drc85/10/13 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drc85/10/12 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Hrt | Commercial | I |
| drc85/10/11 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lg | Commercial | I |
| drc85/10/27 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Hrt | Commercial | I |
| drc85/10/25 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc86/10/1 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Kd | Commercial | I |
| drc86/10/3 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc108/10/3 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc108/10/5 | 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drc27/11/1 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Spl | Commercial | I |
| drc27/11/2 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Ln | Commercial | I |
| drc27/11/3 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Stm | Commercial | I |
| drc27/11/5 | 2011 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lv | Commercial | I |
| drc65/11/2 | 2011 | Nsele | 4°24 S/15°30 E | Peri-urban | Kd | Commercial | I |
| drc65/11/3 | 2011 | Nsele | 4°24 S/15°30 E | Peri-urban | Spl | Commercial | I |
| drc65/11/4 | 2011 | Nsele | 4°24 S/15°30 E | Peri-urban | Lg | Commercial | I |
| drc96/12/1 | 2012 | Mayanda | 5°12 S/15°14 E | Rural | Lg | Village | I |
| drc96/12/2 | 2012 | Mayanda | 5°12 S/15°14 E | Rural | Spl | Village | I |
| drc96/12/3 | 2012 | Mayanda | 5°12 S/15°14 E | Rural | Hrt | Village | I |
| drc108/10/1 | Dec 2010 | Ngafula | 4°21 S/15°14 E | Peri-urban | Lg | Commercial | I |
| drc46/11/2 | Jun 2011 | Kinshasa | 4°20 S/15°18 E | City | Hrt | Backyard | I |
| drc20/07/19 | Apr 2007 | Mahagi * | 2° S/31° E | Rift Valley | Kd | Backyard | IX |
| drc20/07/20 | Apr 2007 | Mahagi * | 2° S/31° E | Rift Valley | Ln | Backyard | IX |
| drc25/08/3a | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Kd | Free range | IX |
| drc25/08/3 | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc25/08/42 | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Kd | Free range | IX |
| drc25/08/9 | Mar 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/1 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/13 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/P42 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/15 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/18 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Kd | Free range | IX |
| drc35/08/20 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc35/08/3 | Apr 2008 | Boende | 0°15 S/21°01 E | Forest | Spl | Free range | IX |
| drc66/07/43 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Lg | Free range | IX |
| drc66/07/48 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Spl | Free range | IX |
| drc66/07/491 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Spl | Free range | IX |
| drc66/07/492 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Ln | Free range | IX |
| drc66/07/50 | Nov 2007 | Yakoma | 4° S/22° E | Forest | Spl | Free range | IX |
| drcKG31208/3 | Dec 2008 | Lingwala | 4°20 S/15°19 E | City | Spl | Backyard | IX |
| drc35/10/3 | Apr 2010 | Ngaliema | 4°21 S/15°05 E | City | Kd | Backyard | XIV |
| drc21/07/22 | 2007 | Kipushi † | 12° S/28° E | City | Spl | Backyard | XIV |
drc, Democratic Republic of the Congo; Ln, lymph node; Hrt, heart; Spl, spleen; Kd, kidney; Lg, lung; Lv, liver; Stm, stomach; GPS, global positioning system; *, Uganda border; †, Zambia border.
2.2. African Swine Fever (ASFV) DNA by Polymerase Chain Reaction (PCR)
ASFV DNA was extracted from tissue samples using the QIAGEN blood and tissue extraction kit (Qiagen, Hilden, Germany) according to the manufacturers’ protocol. Each sample of extracted DNA was then tested by real-time PCR (qPCR), as described by King et al. [25] to confirm the presence of viral DNA for ASFV using the primers King forward (5′-CTGCTCATGGTATCAATCTTATCGA-3′), King reverse (5′-GATACCACAAGATCRGCCGT-3′), and the King probe (5′‑Fam‑CCACGGGAGGAATACCAACCCAGTG-Tam-3′).
2.3. Generation of ASFV Sequence Data
Samples that were positive by qPCR had each target gene fragments amplified separately using the PCR protocols outlined below. The C terminal end of the B646L (p72) gene was amplified using primers p72U (5′-GGCACAAGTTCGGACATGT-3′) and p72D (5′‑GTACTGTAACGCAGCACAG-3′) as recommended [11]. The CVR locus was amplified using primers, ORF9RLW_F (5′-AATGCGCTCAGGATCTGTTAAATCGG-3′) and ORF9RLW_R (5′‑TCTTCATGCTCAAAGTGCGTATACCT-3′) as described [26], The full E183L (p54) gene was amplified using primers P54F (5′-GCCTGCGGATTCTGAAGATA-3′), and P54R (5′‑AGGACGCAATTGCTTAAACG-3′) using a touchdown PCR protocol as follows, 95 °C for 5 min, followed by 15 cycles of 95 °C, 30 s, 60 °C, 30 s, 72 °C, 1 min, then 25 cycles of 95 °C, 30 s, 58 °C, 30 s, 72 °C, 1 min, with a final extension or 72 °C for 5 min. PCR products were purified using Wizard SV Gel and PCR Clean Up kit, according to the manufacturers’ protocol (Promega Corporation, Madison, WI, USA). Purified PCR products were submitted to LGC Genomics (Berlin, Germany) with amplification primers, for sequencing. Raw sequences were assembled and edited using Vector NTI 11.5 Software (Life Technologies, Carlsbad, CA, USA). Sequences were then aligned with GenBank reference sequences using MEGA (Version 6.0) or BioEdit (Version 7.2.3) using the ClustalW method. All nucleotide sequences were deposited in GenBank (Accession # KX121429‑KX121600).
2.4. Molecular Characterization of ASFV
Multiple sequence alignments of both the p54 and p72 genes were generated in MEGA (Version 6.0) [27] using default values of the ‘by codon’ option with the ClustalW algorithm with additional manual editing as needed. The p72 alignment was 404 bp in length and contained 120 sequences. Of these 120 sequences, 62 were generated for this study and 58 were reference sequences with at least one representing each of the known 23 genotypes. The p54 alignment was 657 bp in length and included 84 sequences; 34 were generated for this study, and 50 were reference sequences for 20 of the 23 known p72 genotypes. Published sequences for genotypes XI, XII, and XVIII were unavailable for examination. The most appropriate model of molecular evolution was determined by the corrected Akaike Information Criterion (AICc) using MEGA [27]. Maximum likelihood (ML) analyses with 1000 bootstrap replicates were performed using the program MEGA with the predetermined model of molecular evolution (GTR+I+G for the p72 dataset and HKY+G for the p54 dataset) using all sites. The intra-genotypic genetic distances for the p72 and p54 nucleotides sequences of DRC samples were calculated using MEGA [27].
2.5. Central Hypervariable Region (CVR) of B602L Gene
Grouping of amino acids into tetramers at this locus has been utilized by other researchers, therefore the coding of tetramers followed methods outlined previously [20,26,28,29,30]. The amino acid tetramer codes are provided in Table 2.
Table 2.
Central hypervariable region (CVR) locus-based intra-genotype resolution.
| Strain | Location | Year | Genotype | Tetrameric Repeats | TRS |
|---|---|---|---|---|---|
| drc35/10/1 | Ngaliema * | 2010 | I | AAAAAAAAAAAAAAAAAAAAAAAAAAAAABNAB NBTDBNAAAAAAAAAAAF |
51 |
| drcKG28110805 | Ngaliema * | 2010 | I | AAAAAAAAABNABNBTABNAAAAAAAAAAAAAAAAA AAAAAAAAF |
45 |
| drc65/11/4 | Nsele * | 2011 | I | AAAAAABNABNBTDBNAAAAAAAAF | 25 |
| drc74/09/2 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc74/09/3 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc74/09/4 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc74/09/6 | Nsele * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc94/09/2 | Kintambo * | 2009 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc27/11/3 | Ngafula * | 2011 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drc27/11/5 | Ngafula * | 2011 | I | AAAAAAAAAAAAAAAAAAAAAAF | 23a |
| drcKG28040802 | Kasavubu * | 2008 | I | AAAAAAAAAAAAAAAF | 16 |
| drc96/12/1 | Mayanda | 2012 | I | AAAAAAAAAAAAAAAF | 16 |
| drc96/12/2 | Mayanda | 2012 | I | AAAAAAAAAAAAAAAF | 16 |
| drc96/12/3 | Mayanda | 2012 | I | AAAAAAAAAAAAAAAF | 16 |
| drc99/05a | Ngafula * | 2005 | I | AAAAAAAAAAAAAF | 14 |
| drc51/10/23 | Ndjili * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc85/10/13 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc85/10/27 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc86/10/3 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc86/10/1 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc108/10/1 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc108/10/3 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc108/10/5 | Ngafula * | 2010 | I | AAAAAAAAAAAF | 12 |
| drc85/10/12 | Ngafula * | 2010 | I | AAAAAAAAAAF | 11 |
| drc73/10/2 | Ngaliema * | 2010 | I | AAAAAAAAAAF | 11 |
| drc73/10/3 | Ngaliema * | 2010 | I | AAAAAAAAAAF | 11 |
| drc73/10/4 | Ngaliema * | 2010 | I | AAAAAAAAAAF | 11 |
| drc49/05/P2a | Limete * | 2005 | I | AAAAAAAAAF | 10 |
| drc85/10/25 | Ngafula * | 2010 | I | AAAAAAAAF | 9 |
| drc75/05/1 | Maniema | 2005 | I | AAAAAAAAF | 9 |
| drc49/05/p1b | Limete * | 2005 | I | AAAAAAAAF | 9 |
| drc65/11/3 | Nsele * | 2011 | I | AAAAAAAF | 8 |
| drc49/05/p1a | Limete * | 2005 | I | AAAAAF | 6 |
| drc70/05/1 | Limete * | 2005 | I | AAAAF | 5 |
| Con09/Ni16 | Congo 1 | 2009 | I | AAAAAAAAAF | 10 |
| Kat67 | DRC(Zaire) 2 | 1967 | I | AAAAAAAABNABTDBNAAAAAAA | 23 |
| Nig13_KAF_14 | Nigeria 3 | 2014 | I | ABNABNAAAAACBNAFA | 17 |
| drc66/07/491 | Yakoma | 2007 | IX | AAABBAABBNABBAABBNABNABA | 24a |
| drc66/07/43 | Yakoma | 2007 | IX | AAABNABBBNABBAABBNABNABA | 24b |
| drc66/07/50 | Yakoma | 2007 | IX | AAABNABBBNABBAABBNABNABA | 24b |
| drc66/07/492 | Yakoma | 2007 | IX | AAABNABBBNABBAABBNABNABA | 24b |
| drc66/07/48 | Yakoma | 2007 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/p42 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/18 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/13 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/3 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/20 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/1 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/15 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc35/08/3a | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc25/08/3 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc25/08/9 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drc25/08/42 | Boende | 2008 | IX | AAAABNABBNABBAABBNABNABA | 24c |
| drcKG31208/3 | Lingwala * | 2008 | IX | AAAABNABBNABBAAABNABNABA | 24d |
| drc20/07/19 | Mahagi | 2007 | IX | AAABNABBNABBAABBNABNABA | 23b |
| drc20/07/20 | Mahagi | 2007 | IX | AAABNABBNABBAABBNABNABA | 23b |
| drc86/10/2 | Ngafula * | 2010 | IX | AAAAAAAAAAAF | 12 |
| UG03H.1 | Uganda 4 | 2003 | IX | AAABNABBNABBAABBNABNABA | 23b |
| Ken06.B1 | Kenya 5 | 2006 | IX | AAABNABBNABBAABBNABNABA | 23b |
| drc35/10/3 | Ngaliema * | 2010 | XIV | AAAAAAAAAAAAAAAAAAAAAAAAAAAAABNABNBTDBN AAAAAAAAAAAF |
51 |
| drc21/07/p22 | Kipushi | 2007 | XIV | AVVOVAVVNBVOV | 13 |
| ETH/3 | Ethiopia 6 | 2011 | XXIII | ABNAAAAACBNABTDBNAFA | 20 |
Codes as labeled in previous studies: [20,26,28,29,30]. TRS, tetrameric repeat sequence number; *, indicates strains collected within Kinshasa Province. A=CAST, CVST, CTST, or CASI; B=CADT, CADI, CTDT, or CAGT; C = GAST or GANT; F = CANT or CAAT; N = NVDT, NVGT, NVDI, or NCDT; T = NVNT; H = RAST; S = SAST; O = NANI, NADI, or NASI; V = NAST, NAVT, NADT, or NANT; D = CASM; G = CTNT; M = NEDT; W = SADT or SVDT; U = NIDT or NTD. Additional sequences utilized in this table from previous studies: 1 [17]; 2 [26]; 3 [16]; 4 [13]; 5 [13]; 6 [14].
3. Results
3.1. Clinical Findings and African Swine Fever (ASF) Diagnosis
Field Identification and Description of Collecting Localities
Fifty-four out of the 57 sampled pigs exhibited many of the following signs or pathological findings: hemorrhagic edema; enlargement of spleen and some internal lymph nodes; hydropericardium and pericarditis; hydrothorax; ascites; as well as skin cyanosis and petechiae. The three remaining pigs sampled in the markets appeared superficially healthy, but presented with enlarged, congestive or hemorrhagic spleens and/or gastrohepatic lymph nodes. Massive mortality was documented in the 25 sampled localities. Four of the 25 sampling sites, including the cities of Yakoma, Boende, Mahagi and Kipushi, recorded indigenous pigs of local breeds, primarily free ranging to be the most commonly lost. The remaining 21 locations were commercial farms raising improved breeds of pigs in backyards or securely fenced areas, minimizing intermingling of wildlife and domestic swine.
3.2. Laboratory Diagnostics
Real time PCR identified 62 samples (100%) as positive for ASFV DNA and distributed per location as follows: Ngafula (n = 17) Boende (n = 11), Ngaliema (n = 8), Nsele (n = 7) Yakoma (n = 5) Limete (n = 4), Mayanda (n = 3), Mahagi (n = 2), Kasavubu (n = 1), Kintambo (n = 1), Kipushi (n = 1), Lingwala (n = 1), Maniema (n = 1) and Ndjili (n = 1). The p72 gene was sequenced for all 62 positive samples and 55 positive samples were sequenced for the p54 gene and 54 for the CVR (Table 1).
3.3. Molecular Characterization of ASFV
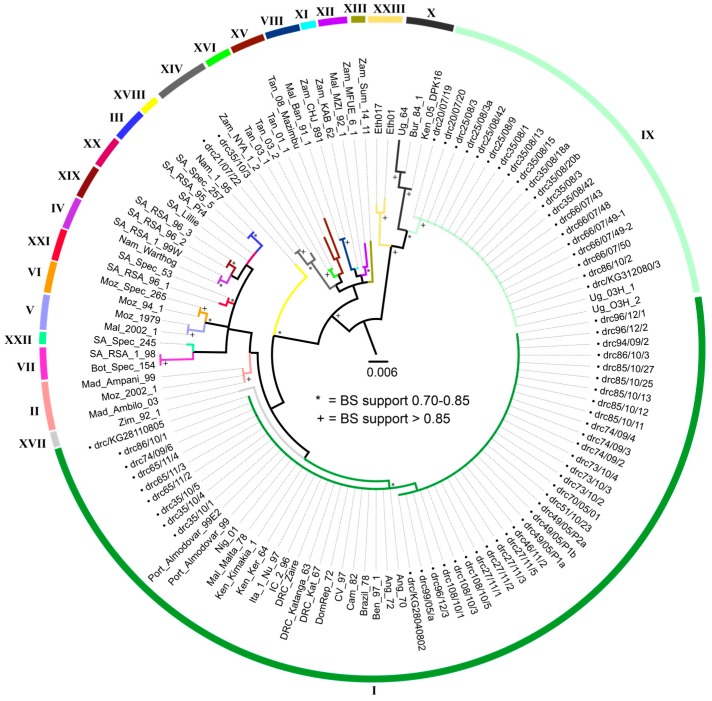
Phylogenetic analyses of the p72 gene revealed that the newly sequenced ASFV strains which circulated in the DRC from 2005 to 2012 clustered into three p72 genotypes: I, IX and XIV (Figure 1). Of the newly analyzed stains, 40 (64.5%) grouped with strains previously identified as belonging to genotype I, including three published strains from the DRC (Katanga63 (Genbank: AF301540) [11], Kat67 (Genbank: FJ174377) [13] and Zaire (Genbank: AY351515) [9]); 20 strains (32.3%) were recognized as genotype IX, and 2 (3.2%) belong to genotype XIV. This is the first report of genotypes IX and XIV circulating in the DRC. Although sequences generated herein grouped with high bootstrap support (>75%) with reference samples of their respective genotypes, support for both inter- and intra-genotypic relationships varied.
Figure 1.
Maximum likelihood tree depicting genetic relationships of strains examined in this study utilizing the p72 locus. Bootstrap support (BS) is indicated by * for values between 75% and 85%, and by + for values greater than 85% support. The scale bar indicates the number of nucleotide substitutions per site. Each color is a different genotype, indicated by the corresponding roman numerals. Labels for all strains generated by this study begin with •drc (Democratic Republic of the Congo). Strains are named in the following manner: Country_Strain Name. Acronyms used for countries of origin are as follows: Ang = Angola, Ben = Benin, Bot = Botswana, Bra = Brazil, Bur = Burundi, Cam = Cameroon, CV = Cape Verde, Dom Rep = Dominican Republic, the DRC = Democratic Republic of Congo, IC = Ivory Coast, Ita = Italy, Ken = Kenya, Mad = Madagascar, Mal = Malawi, Malt = Malta, Moz = Mozambique, Nam = Namibia, Nig = Nigeria, Port = Portugal, SA = South Africa, Tan = Tanzania, Ug = Uganda, Zam = Zambia, Zimb = Zimbabwe.
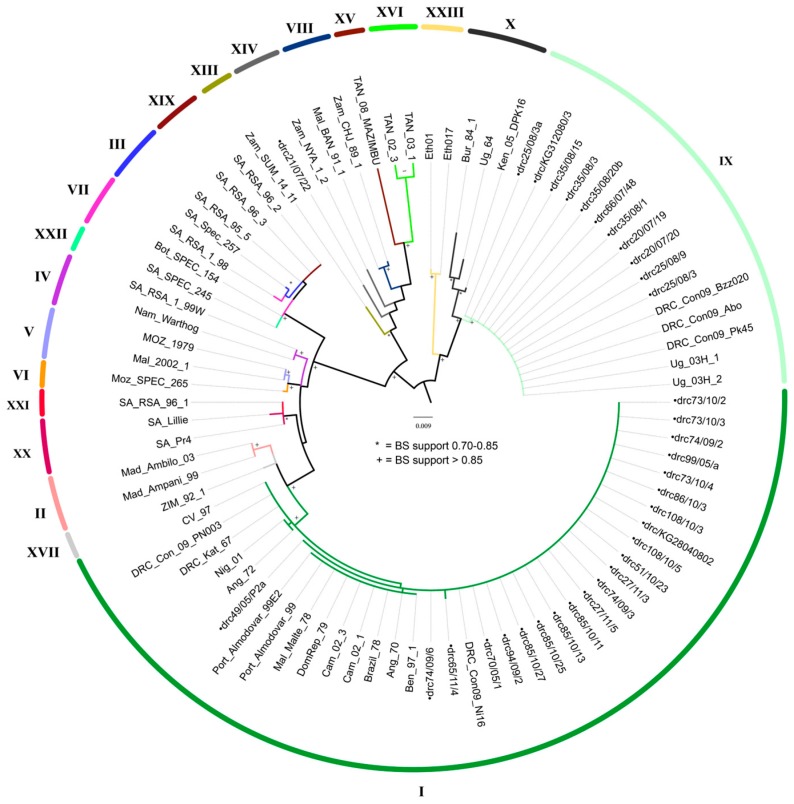
Phylogenetic analyses of the p54 gene recovered the same groupings of new sequences with respective genotypes as the p72 analyses with high bootstrap support (>83%) (Figure 2). The arithmetic means of nucleotide substitutions per site between the DRC ASFVs of each of the three genotypes (within group mean distance) were estimated using MEGA version 6. The within group mean distance for the p72 nucleotide sequences were 0.002 for genotype I and 0.0 for genotype IX and XIV. Likewise, for the p54 nucleotide sequences, the within group mean distance was 0.02 for the genotype I members, and 0.0 for genotype IX members. Only one isolate of genotype XIV was successfully amplified and sequenced for this gene.
Figure 2.
Maximum likelihood tree depicting genetic relationships of examined strains using the p54 locus (E183L gene). Bootstrap support values greater than 75% are shown. The scale bar indicates the number of nucleotide substitutions per site. The colored clades indicate genotypes represented by strains sequenced in this study. Labels for all strains generated by this study begin with •drc. Strains are named in the following manner: Country_Strain Name. Acronyms used for countries of origin are as follows: Ang = Angola, Ben = Benin, Bot = Botswana, Bra = Brazil, Bur = Burundi, Cam = Cameroon, CV = Cape Verde, Dom Rep = Dominican Republic, the DRC = Democratic Republic of Congo, IC = Ivory Coast, Ita = Italy, Ken = Kenya, Mad = Madagascar, Mal = Malawi, Malt = Malta, Moz = Mozambique, Nam = Namibia, Nig = Nigeria, Port = Portugal, RC = Republic of Congo, SA = South Africa, Tan = Tanzania, Ug = Uganda, Zam = Zambia, Zimb=Zimbabwe.
3.4. CVR of B602L Gene
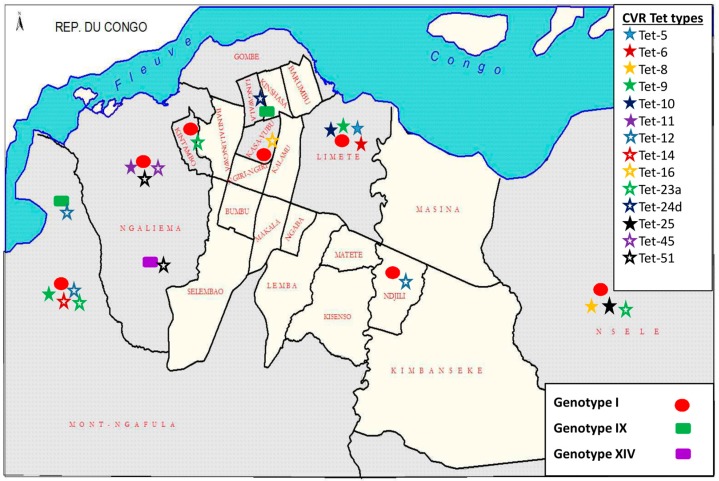
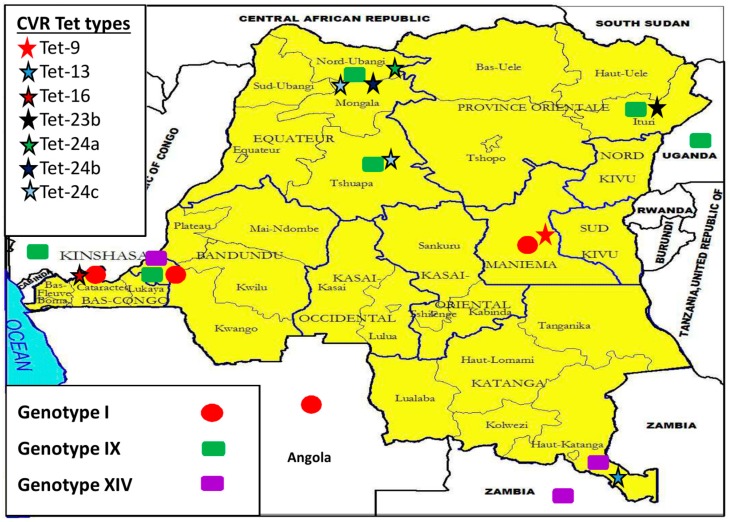
Sequence analysis of the CVR locus showed distinct variability in nucleotide sequence and recognized 19 unique nucleotide sequences, which were translated into amino acid sequences, and subsequently coded as amino acid tetramers (tet-types). Thirteen tet-types were detected within strains identified as belonging to p72 genotype I, six from strains belonging to genotype IX, and two within genotype XIV strains (Table 2). All strains grouped within a tet-type contained identical CVR nucleotide sequences; therefore, no resolution was lost by converting nucleotide sequences to tetrameric repeat sequences. Tet-12 and 51 were both detected in p72 genotype I strains, but tet-12 was also found in a p72 genotype IX strain (drc86/10/2), and tet-51 was also found in a p72 genotype XIV strain (drc35/10/3). All three p72 genotypes and 14 tet-types were identified throughout Kinshasa (Table 2, Figure 4). Regarding the five remaining provinces: Equateur presented four tet‑types (within genotype IX); Bas-Congo and Maniema each had a single tet-type (within genotype I); Katanga (within genotype XIV) and Oriental each contained a single tet‑type (within genotype IX), as well (Figure 3).
Figure 4.
Localization of ASFV p72 genotypes and the corresponding CVR tet-types in this study within the Kinshasa City Province.
Figure 3.
Provincial localization of revealed p72 genotypes and their corresponding central hypervariable region (CVR) tet-types within the DRC, as well as some historical African swine fever virus (ASFV) genotypes from neighboring countries.
3.5. Democratic Republic of the Congo (DRC) ASFV Genotypes Geographical Distribution
Different ASFV strains were identified in the provinces of Bas-Congo, Equateur, Katanga, Kinshasa, Maniema, and Orientale (Figure 3). Genotype I strains were detected in Bas-Congo (Localities-Mayanda), Kinshasa (Localities-Limete, Ngafula, Ngaliema, Kasavubu, Nsele, Kintambo, Ndjili, Nsele, Kinshasa) (Figure 4) and Maniema (Locality-Maniema), whereas genotype IX strains were recovered from Equateur (Localities-Boende, Yakuma), Kinshasa (Localities-Ngafula, Lingwala), and Oriental provinces (Locality-Mahagi). Genotype XIV was detected from Katanga (Locality-Kipushi) and Kinshasa (Locality-Ngaliema).
4. Discussion
4.1. Molecular Characterization
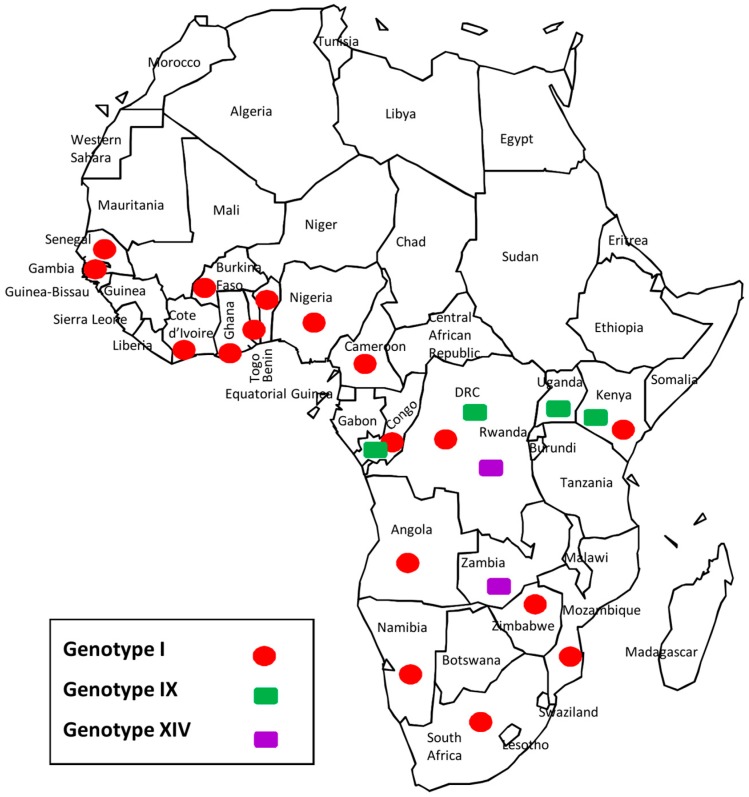
This is the first extensive molecular evaluation of circulating ASFV genotypes in the DRC. Previous knowledge was based on data from single samples submitted for ASF diagnosis [9,11,26,29,31,32], but little was known about the dynamics of circulating ASFV strains in the DRC. Analysis of the p72 gene identified three genotypes (I, IX and XIV) circulating in the DRC. Forty of 62 (64.5%) clustered with DRC historical strains (DRC_Kat63 and DRC_Kat67), as well as other strains from genotype I, confirming that genotype I is the most prevalent in the country. Additionally, DRC strains of this genotype exhibited limited genetic variability, which has been repeatedly documented and hypothesized to be predominantly a result of maintenance through the domestic pig cycle [9]. There were 19 p72 genotype IX strains (30.6%) with no genetic variation detected. Previously undocumented within the DRC, genotype IX is much more geographically restricted, as it is endemic to East and Central Africa (Figure 5), having been reported previously in Republic of Congo, Uganda, and Kenya (Figure 5) where it is involved in sylvatic and domestic cycles [9,17]. Genotype XIV was also not previously reported in the country prior to the two (3.2%) strains reported herein. Genotype XIV was previously only reported from Zambia (Figure 5), where it was originally isolated from a tick of the genus Ornithodoros in 1986 [9]. In regards to the distribution of ASFV within the DRC recovered in this study, all genotypes were found near the eastern and western borders, and confirmed localities for all three genotypes occurred in the southern half of the country; however, only genotype IX viruses were collected from localities in the northern DRC (Figure 3 and Figure 4). The presence of all three genotypes within the Kinshasa province is likely a result of pig shipments, as pork sold at markets in Kinshasa has been documented to originate from multiple regions of the country, including the provinces of Equateur, Bandundu, and Bas-Congo [33].
Figure 5.
Continental distribution of the three p72 genotypes revealed by this study in the DRC. Position of symbols represents the presence of a genotype within the country, and is not indicative of specific geographic localities.
Although the p54 gene was previously determined to be a valuable locus for finer levels of discrimination [19,34,35], no additional resolution was gained for this dataset; however, the p54 locus did corroborate the topology generated by the p72 analysis. Given the sampling scheme of this study (domestic swine only), failure to achieve higher resolution may be due to the low genetic variation consistently detected within the domestic pig cycle, when compared to the higher levels of variation previously detected within the sylvatic cycle [9].
The CVR was capable of providing further resolution than either the p72 or p54 genes. The pattern of genetic variability varied dependent upon the p72 genotype examined. Within p72 genotype I strains, genetic variation was primarily a result of variability in the number of tetramers as previously reported [36], specifically the tetrameric amino acid repeats CAST and CVST. Also of interest within genotype I, is that tet-25, 45, and 51 (also found in one genotype XIV strain) contained a conserved sequence of 11 tetramers (tetrameric repeat sequence number (TRS) ABNABNBT[D/A]BN) not found in other tetrameric sequences within this genotype. This sequence was flanked by a variable number of ‘A’ coded tetramers. The historical isolate Kat67 genotype I CVR was tet-23, but had a similar sequence BNAxx. Additionally, BTDBN also flanked on each end by variable repeats of ‘A’ [26]. The new genotype XXIII from Ethiopia had one CVR sequence, ETH/3, which also has a similar motif with xxBNABTDBxx [14]. Nigerian sequences are of genotype I and also share part of this motif in the CVR region xxABNABNxx [16] (Table 2). CVR tet‑types of p72 genotype IX consisted primarily of a more complex sequence than genotype I strains, with the exception of tet-12 (common to genotype I strains) being documented in one genotype IX strain. Interestingly, CVR tet-23b which is present in two recent DRC genotype IX strains from 2007 is identical to two genotype IX CVR sequences from Uganda in 2003 and Kenya in 2006 [13] (Table 2). Of the two tet-types detected in genotype XIV strains, the first (tet-51) was identical to a tetrameric repeat sequence found in a genotype I strain, and the second was extremely different than any other tet-types reported herein (Table 2). Several CVR tet-types are discussed below in reference to the detection of multiple strains within outbreaks, and potential links between outbreaks based on this locus.
4.2. Disease Ecology, Molecular Epidemiology and Case Investigations
The higher resolution offered by the CVR allowed for the three genotypes to be further broken down into 19 variants. The high level of variability previously noted in the CVR [9,37,38] would suggest that highly similar/identical CVRs are more closely related than more divergent CVRs; however, given the nature of nucleotide repeat regions, such as the CVR, identical sequences can occur due to homoplasy. With these possibilities in mind, a number of putative outbreak connections are proposed and discussed below, as are details of co-circulation of multiple variants.
The detection of genotype I, tet-23 strains during the 2009 outbreaks at Nsele and Kintambo, as well as the 2011 outbreak in Ngafula may suggest that these geographically proximal outbreaks were caused by closely related strains, even though they occurred over a two-year time span which could be facilitated by the asymptomatic infection of domestic pigs [39] in the area. A second potential connection is suggested by analysis of sequences from the Mahagi outbreak in April 2007 (near the Ugandan border), as they were of identical p72 genotype IX and tet-23b to strains from Uganda (Ug03H.1, Genbank: GQ916933), and Kenya (Ken06.B1, Genbank: GQ916935 & Ken06.Bus, Genbank: GQ916940) examined previously [13]. A third connection stems from a strain from a clinically healthy pig sampled at the Kinshasa market (drcKG28040802) in 2008. This strain belonged to the same genotype I and tet-16 as samples from the 2012 outbreak in Mayanda, Bas-Congo province. The geographic proximity of these two provinces, in combination with the high level of commercial traffic between them, could easily result in the spread of ASF from one province to the other [1,20].
Again, either the ability of ASFV to persist in asymptomatic pigs (domestic or feral) or the sylvatic cycle (including ticks) could explain how closely related strains were responsible for outbreaks separated by four years, as Mayanda is a rural locality, where there is a high potential for interaction between domestic and feral swine. Another potential outbreak link was made apparent upon examination of genotype IX strains collected from outbreaks in Yakoma (April 2007) and Boende (March and April 2008). Tet-24c strains were detected in both outbreaks, which severely affected both feral and domestic pigs [40]. The Boende outbreak was estimated to have caused more than 4500 swine fatalities [40]. Both Yakoma and Boende are forested sites, located in North Ubangi and Tshuapa Districts of the Equateur Province, respectively. The forest environment and free ranging animal husbandry practices enable interaction between domestic and wild swine, potentially allowing for crossover between infection cycles and the occurrence of multiple strains.
Another instance of variation occurred in the Ngaliema 2010 outbreak, where tet-51 was found in two strains: drc35/10/1 (genotype I) and drc/35/10/3 (genotype XIV). Co-infection of different tet‑types was even documented within the same pig, when two genotype I strains, drc49/05/p1a (tet‑5) and drc49/05/p1b (tet-9), were extracted from the spleen and lymph node respectively, of a pig in 2005 from Kinshasa. In similar studies conducted in Mozambique and Nigeria, individual co‑infection was not observed [38,41].
Understanding the details of ASFV circulation across the DRC is clearly complex. Co-circulation of genotypes and tet-types and co-infection of multiple tet-types within a single pig suggests a high level of genetic variation and potential support of a previous hypothesis of recombination [42,43]. However, detection of conserved tet-types across large geographic areas, and over multiple years suggests that current markers can provide insight into the movement of ASFV strains. Unfortunately, connecting cases is not straight forward, as anthropogenic factors, such as trade of pigs and animal husbandry practices can play a role. Whole genome examination of these strains may provide a more definitive understanding of relationships.
As the DRC is the largest country in SSA, and borders nine countries, understanding the prevalence and distribution of ASFV genotypes within the DRC is an important step in better understanding large scale patterns of ASFV. Given the high degree of similarity, at all examined loci, between genotype IX strains collected in Mahagi, the DRC (presented herein), and Uganda strains of a previous study [13], it appears that the distribution of this strain spans the border between the DRC and Uganda, suggesting that this strain has been transmitted across boundaries by movement of either feral or domesticated swine. Two more potential cross-boundary transmissions of ASF into/out of the DRC are worth mentioning; however, their molecular evidence is less direct. The first possibility is that genotype XIV may have been transferred, between Zambia (Zam_NYA/12-(Genbank: AY351555), isolated in 1986) [9] and the DRC (drc21/07/22, strain from 2007) through Kipushi, as p72 sequences between these strains share 99.26% nucleotide sequence identity. Unfortunately, no CVR sequence for strain Zam_NYA/12 was available for a more detailed comparison. The next putative trans-boundary migration of ASF could have occurred between Brazzaville, Republic of Congo (Con09/Ni16-(Genbank: HQ645947), isolated 2009) [17] and the DRC (drc86/10/1, strain from in 2010). The p72 sequences were identical, and the CVR locus differed by the insertion of two amino acid tetramers coded as A (AAAAAAAAAF from the Brazzaville strain and AAAAAAAAAAAF in the DRC strain). Rapid mutation rates have been shown in vitro [44], therefore, given the highly variable nature of the CVR, it is difficult to omit the possible link between these outbreaks, especially as the boundary is a narrow aquatic border with high levels of human and animal traffic.
This first, in-depth examination of ASFV in the DRC, has provided evidence of (1) circulation of multiple genotypes previously not reported within the DRC; (2) putative links between both geographically and temporally separated outbreaks; (3) potential movement of ASFV strains across borders between the DRC and Uganda, Zambia, Congo; (4) co-circulation of multiple ASFV genotypes within outbreaks and (5) a pig co-infected with two tet-types. These data, in combination with examination of genotype relationships, will be useful for the optimization of current prevention and control strategies at the regional level given the location and size of the DRC in relation to the rest of the continent and those countries also dealing with ASF.
Acknowledgments
This work was supported by the International Atomic Energy Agency (IAEA) project “Improvement of Veterinary Laboratory Capacities in Sub-Saharan African Countries”. We pay gratitude to the Wellcome Trust (WT) for its support of this research through two grants, WT075813/C/04/Z and, WT087546MAthat allowed advanced field investigations.
Author Contributions
L. K. Mulumba-Mfumu, C. Saegerman, C. E. Lamien and A. Diallo conceived and designed the experiments. J. E. Achenbach, E. Blanco, N. Moreno, G. Tshilenge, and E. Thiry performed the experiments. L. K. Mulumba-Mfumu, J. E. Achenbach, M. R. Mauldin, and C. E. Lamien analyzed the data. L. K. Dixon, A. Diallo contributed reagents/materials/analysis tools. J. E. Achenbach and M. R. Mauldin performed critical revision of the manuscript. L. K. Mulumba-Mfumu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Sánchez-Vizcaíno J.M., Mur L., Sánchez-Matamoros A., Martínez-López B. African swine fever: New challenges and measures to prevent its spread; Proceedings of the 82nd General Session World Assembly of Delegates of the World Organisation for Animal Health (OIE); Paris, France. 25–30 May 2014. [Google Scholar]
- 2.Montgomery R.E. On a form of swine fever occurring in British East Africa (Kenya Colony) J. Comp. Pathol. Ther. 1921;34:159–191. doi: 10.1016/S0368-1742(21)80031-4. [DOI] [Google Scholar]
- 3.Costard S., Mur L., Lubroth J., Sanchez-Vizcaino J.M., Pfeiffer D.U. Epidemiology of African swine fever virus. Virus Res. 2013;173:191–197. doi: 10.1016/j.virusres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Detray D.E. African swine fever. Adv. Vet. Sci. 1963;8:299–333. [PubMed] [Google Scholar]
- 5.Sanchez-Vizcaino J.M., Mur L., Martinez-Lopez B. African swine fever: An epidemiological update. Transbound. Emerg. Dis. 2012;59(Suppl. 1):27–35. doi: 10.1111/j.1865-1682.2011.01293.x. [DOI] [PubMed] [Google Scholar]
- 6.Rowlands R.J., Michaud V., Heath L., Hutchings G., Oura C., Vosloo W., Dwarka R., Onashvili T., Albina E., Dixon L.K. African swine fever virus isolate, Georgia, 2007. Emerg. Infect Dis. 2008;14:1870–1874. doi: 10.3201/eid1412.080591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plowright W., Thomson G.R., Neser J.A. African swine fever. Infect. Dis. Livest. Spec. Ref. S. Afr. 1994;1:568–599. [Google Scholar]
- 8.Hess W.R., Endris R.G., Haslett T.M., Monahan M.J., McCoy J.P. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet. Parasitol. 1987;26:145–155. doi: 10.1016/0304-4017(87)90084-7. [DOI] [PubMed] [Google Scholar]
- 9.Lubisi B.A., Bastos A.D., Dwarka R.M., Vosloo W. Molecular epidemiology of African swine fever in East Africa. Arch. Virol. 2005;150:2439–2452. doi: 10.1007/s00705-005-0602-1. [DOI] [PubMed] [Google Scholar]
- 10.Dixon L.K., Abrams C.C., Bowick G., Goatley L.C., Kay-Jackson P.C., Chapman D., Liverani E., Nix R., Silk R., Zhang F. African swine fever virus proteins involved in evading host defence systems. Vet. Immunol. Immunopathol. 2004;100:117–134. doi: 10.1016/j.vetimm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Bastos A.D., Penrith M.L., Cruciere C., Edrich J.L., Hutchings G., Roger F., Couacy-Hymann E., Thomson R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003;148:693–706. doi: 10.1007/s00705-002-0946-8. [DOI] [PubMed] [Google Scholar]
- 12.Phologane S.B., Bastos A.D., Penrith M.L. Intra- and inter-genotypic size variation in the central variable region of the 9RL open reading frame of diverse African swine fever viruses. Virus Genes. 2005;31:357–360. doi: 10.1007/s11262-005-3254-z. [DOI] [PubMed] [Google Scholar]
- 13.Gallardo C., Mwaengo D.M., Macharia J.M., Arias M., Taracha E.A., Soler A., Okoth E., Martin E., Kasiti J., Bishop R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54, p72, and pB602L (CVR) genes. Virus Genes. 2009;38:85–95. doi: 10.1007/s11262-008-0293-2. [DOI] [PubMed] [Google Scholar]
- 14.Achenbach J.E., Gallardo C., Nieto-Pelegrín E., Rivera-Arroyo B., Degefa-Negi T., Arias M., Jenberie S., Mulisa D.D., Gizaw D., Gelaye E., et al. Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia. Transbound. Emerg. Dis. 2016 doi: 10.1111/tbed.12511. [DOI] [PubMed] [Google Scholar]
- 15.Kouakou K.V., Michaud V., Biego H.G., Gnabro H.P., Kouakou A.V., Mossoun A.M., Awuni J.A., Minoungou G.L., Aplogan G.L., Awoumé F.K., et al. African and classical swine fever situation in Ivory-Coast and neighboring countries, 2008–2013. Acta Trop. 2017;166:241–248. doi: 10.1016/j.actatropica.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Luka P.D., Achenbach J.E., Mwiine F.N., Lamien C.E., Shamaki D., Unger H., Erume J. Genetic Characterization of Circulating African Swine Fever Viruses in Nigeria (2007–2015) Transbound. Emerg. Dis. 2016 doi: 10.1111/tbed.12553. [DOI] [PubMed] [Google Scholar]
- 17.Gallardo C., Anchuelo R., Pelayo V., Poudevigne F., Leon T., Nzoussi J., Bishop R., Pérez C., Soler A., Nieto R., et al. African swine fever virus p72 genotype IX in domestic pigs, Congo, 2009. Emerg. Infect. Dis. 2011;7:1556–1558. doi: 10.3201/eid1708.101877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atuhaire D.K., Afayoa M., Ochwo S., Mwesigwa S., Okuni J.B., Olaho-Mukani W., Ojok L. Molecular characterization and phylogenetic study of African swine fever virus isolates from recent outbreaks in Uganda (2010–2013) Virol. J. 2013 doi: 10.1186/1743-422X-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallardo C., Ademun A.R., Nieto R., Nantima N., Arias M., Martín E., Pelayo V., Bishop R.P. Genotyping of African swine fever virus (ASFV) isolates associated with disease outbreaks in Uganda in 2007. Afr. J. Biotechnol. 2011;10:3488–3497. [Google Scholar]
- 20.Misinzo G., Magambo J., Masambu J., Yongolo M.G., Van D.J., Nauwynck H.J. Genetic characterization of African swine fever viruses from a 2008 outbreak in Tanzania. Transbound. Emerg. Dis. 2011;58:86–92. doi: 10.1111/j.1865-1682.2010.01177.x. [DOI] [PubMed] [Google Scholar]
- 21.Gogin A., Gerasimov V., Malogolovkin A., Kolbasov D. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 2013;173:198–203. doi: 10.1016/j.virusres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Bosch J., Rodríguez A., Iglesias I., Muñoz M.J., Jurado C., Sánchez-Vizcaíno J.M., de la Torre A. Update on the Risk of Introduction of African Swine Fever by Wild Boar into Disease-Free European Union Countries. Transbound. Emerg. Dis. 2016 doi: 10.1111/tbed.12527. [DOI] [PubMed] [Google Scholar]
- 23.Gallardo C., Fernández-Pinero J., Pelayo V., Gazaev I., Markowska-Daniel I., Pridotkas G., Nieto R., Fernández-Pacheco P., Bokhan S., Nevolko O., et al. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 2014;20:1544–1547. doi: 10.3201/eid2009.140554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Sawalhy A., Soumaré B., Nouala S., Mukanda B., Wamwayi H., Ahmed I.G. Pan African Animal Health Yearbook. Interafrican Bureau for Animal Resources, African Union; Nairobi, Kenya: 2011. African Swine fever; pp. 16–17. [Google Scholar]
- 25.King D.P., Reid S.M., Hutchings G.H., Grierson S.S., Wilkinson P.J., Dixon L.K., Bastos A.D., Drew T.W. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods. 2003;107:53–61. doi: 10.1016/S0166-0934(02)00189-1. [DOI] [PubMed] [Google Scholar]
- 26.Nix R.J., Gallardo C., Hutchings G., Blanco E., Dixon L.K. Molecular epidemiology of African swine fever virus studied by analysis of four variable genome regions. Arch. Virol. 2006;151:2475–2494. doi: 10.1007/s00705-006-0794-z. [DOI] [PubMed] [Google Scholar]
- 27.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boshoff C.I., Bastos A.D., Gerber L.J., Vosloo W. Genetic characterisation of African swine fever viruses from outbreaks in southern Africa (1973–1999) Vet. Microbiol. 2007;121:45–55. doi: 10.1016/j.vetmic.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Lubisi B.A., Bastos A.D., Dwarka R.M., Vosloo W. Intra-genotypic resolution of African swine fever viruses from an East African domestic pig cycle: A combined p72-CVR approach. Virus Genes. 2007;35:729–735. doi: 10.1007/s11262-007-0148-2. [DOI] [PubMed] [Google Scholar]
- 30.Misinzo G., Kwavi D.E., Sikombe C.D., Makange M., Peter E., Muhairwa A.P., Madege M.J. Molecular characterization of African swine fever virus from domestic pigs in northern Tanzania during an outbreak in 2013. Trop. Anim. Health Prod. 2014;46:1199–1207. doi: 10.1007/s11250-014-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekue N.F., Wilkinson P.J. Comparison of genomes of African swine fever virus isolates from Cameroon, other African countries and Europe. Rev. Elev. Méd. Vét. Pays Trop. 2000;53:229–238. [Google Scholar]
- 32.Mulumba-Mfumu L.K., Goatley L.C., Saegerman C., Takamatsu H.H., Dixon L.K. Immunization of African Indigenous Pigs with Attenuated Genotype I African Swine Fever Virus OURT88/3 Induces Protection Against Challenge with Virulent Strains of Genotype I. Transbound. Emerg. Dis. 2016;63:e323–e327. doi: 10.1111/tbed.12303. [DOI] [PubMed] [Google Scholar]
- 33.Praet N., Kanobana K., Kabwe C., Maketa V., Lukanu P., Lutumba P., Polman K., Matondo P., Speybroeck N., Dorny P. Taenia solium cysticercosis in the Democratic Republic of Congo: How does pork trade affect the transmission of the parasite. PLoS Negl. Trop. Dis. 2010;4:e817. doi: 10.1371/journal.pntd.0000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atuhaire D.K., Ochwo S., Afayoa M., Mwesigwa S., Mwiine F.N., Okuni J.B., Olaho-Mukani W., Ojak L. Molecular characterization of African swine fever virus in apparently healthy domestic pigs in Uganda. Afr. J. Biotechnol. 2014;13:2491–2499. [Google Scholar]
- 35.Giammarioli M., Gallardo C., Oggiano A., Iscaro C., Nieto R., Pellegrini C., Dei G.S., Arias M., De Mia G.M. Genetic characterisation of African swine fever viruses from recent and historical outbreaks in Sardinia (1978–2009) Virus Genes. 2011;42:377–387. doi: 10.1007/s11262-011-0587-7. [DOI] [PubMed] [Google Scholar]
- 36.Goller K.V., Malogolovkin A.S., Katorkin S., Kolbasov D., Titov I., Hoper D., Beer M., Keil G.M., Portugal R., Blome S. Tandem repeat insertion in African swine fever virus, Russia, 2012. Emerg. Infect. Dis. 2015;21:731–732. doi: 10.3201/eid2104.141792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irusta P.M., Borca M.V., Kutish G.F., Lu Z., Caler E., Carrillo C., Rock D.L. Amino acid tandem repeats within a late viral gene define the central variable region of African swine fever virus. Virology. 1996;220:20–27. doi: 10.1006/viro.1996.0281. [DOI] [PubMed] [Google Scholar]
- 38.Bastos A.D., Penrith M.L., Macome F., Pinto F., Thomson G.R. Co-circulation of two genetically distinct viruses in an outbreak of African swine fever in Mozambique: No evidence for individual co-infection. Vet. Microbiol. 2004;103:169–182. doi: 10.1016/j.vetmic.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Gallardo C., Soler A., Nieto R., Cano C., Pelayo V., Sanchez M.A., Pridotkas G., Fernandez-Pinero J., Briones V., Arias M. Experimental Infection of Domestic Pigs with African Swine Fever Virus Lithuania 2014 Genotype II Field Isolate. Transbound. Emerg. Dis. 2017;64:300–304. doi: 10.1111/tbed.12346. [DOI] [PubMed] [Google Scholar]
- 40.Central Veterinary Laboratory (CVL) Rapport Annuel D’Activites (Kinshasa/RDC) CVL; Kinshasa, Democratic Republic of Congo: 2010. pp. 1–52. [Google Scholar]
- 41.Owolodun O.A., Bastos A.D., Antiabong J.F., Ogedengbe M.E., Ekong P.S., Yakubu B. Molecular characterisation of African swine fever viruses from Nigeria (2003–2006) recovers multiple virus variants and reaffirms CVR epidemiological utility. Virus Genes. 2010;41:361–368. doi: 10.1007/s11262-009-0444-0. [DOI] [PubMed] [Google Scholar]
- 42.Smith G.P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976;191:528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- 43.Blasco R., de la Vega I., Almazan F., Aguero M., Vinuela E. Genetic variation of African swine fever virus: Variable regions near the ends of the viral DNA. Virology. 1989;173:251–257. doi: 10.1016/0042-6822(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Barreno B., Sanz A., Nogal M.L., Vinuela E., Enjuanes L. Monoclonal antibodies of African swine fever virus: Antigenic differences among field virus isolates and viruses passaged in cell culture. J. Virol. 1986;58:385–392. doi: 10.1128/jvi.58.2.385-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]