Abstract
Neurodegeneration, the progressive death of neurons, loss of brain function, and cognitive decline is an increasing problem for senior populations. Its causes are poorly understood and therapies are largely ineffective. Neurons, with high energy and oxygen requirements, are especially vulnerable to detrimental factors, including age-related dysregulation of biochemical pathways caused by altered expression of multiple genes. GHK (glycyl-l-histidyl-l-lysine) is a human copper-binding peptide with biological actions that appear to counter aging-associated diseases and conditions. GHK, which declines with age, has health promoting effects on many tissues such as chondrocytes, liver cells and human fibroblasts, improves wound healing and tissue regeneration (skin, hair follicles, stomach and intestinal linings, boney tissue), increases collagen, decorin, angiogenesis, and nerve outgrowth, possesses anti-oxidant, anti-inflammatory, anti-pain and anti-anxiety effects, increases cellular stemness and the secretion of trophic factors by mesenchymal stem cells. Studies using the Broad Institute Connectivity Map show that GHK peptide modulates expression of multiple genes, resetting pathological gene expression patterns back to health. GHK has been recommended as a treatment for metastatic cancer, Chronic Obstructive Lung Disease, inflammation, acute lung injury, activating stem cells, pain, and anxiety. Here, we present GHK’s effects on gene expression relevant to the nervous system health and function.
Keywords: GHK, copper, dementia, Alzheimer’s disease, Parkinson’s disease, neurons, glial cells, DNA repair, anti-oxidant, anti-anxiety, anti-pain
1. Introduction
Age-related cognitive decline is a common problem for many elderly people, yet its cause is poorly understood. Over 99% of investigational drugs, participating in over 200 clinical trials, failed to receive approval for the treatment of Alzheimer’s disease [1]. Even the success of a few approved drugs provides only minimal patient improvement. There is a need for new, safe, and effective therapeutics with extensive safety and efficacy data that can be developed for use in humans within the next few years.
GHK (glycyl-l-histidyl-l-lysine) is a human plasma copper-binding peptide with a stunning array of actions that appear to counter aging-associated diseases and conditions. GHK was isolated in 1973 as an activity bound to human albumin that caused aged human liver tissue to synthesize proteins like younger tissue [2]. It has a strong affinity for copper and readily forms the complex GHK-Cu. It was first proposed that GHK-Cu functions by modulating copper intake into cells [3]. Since then, it has been established that the GHK peptide has stimulating and growth-promoting effects on many cells and tissues such as chondrocytes [4], liver cells and human fibroblasts [5]. It increases stemness and stimulates integrin secretion in human epidermal basal keratinocytes [6], as well as has a strong wound-healing and tissue-repairing effect [7]. GHK has also been shown to improve wound healing in controlled experiments using animals, such as rats, dogs, and rabbits [8,9,10].
In 2010, Hong et al. using the Broad Institute’s Connectivity Map (cMap), a compendium of transcriptional responses to compounds, identified GHK as the most active of 1309 bioactive substances, uniquely capable of reversing the expression of 54 genes in a metastatic-prone signature for aggressive early stage mismatch-repair colorectal cancer. GHK was active at a very low concentration of 1 µM [11].
Another study, which also used the cMap to identify genes affected by GHK, was conducted in 2012 when Campbell et al. identified 127 genes whose expression levels were associated with regional severity of chronic obstructive pulmonary disease (COPD). Emphysema and chronic bronchitis, the two main conditions of COPD, cause both small airway obstruction and significant loss of lung function over time. The cMap predicted that GHK would reverse the aberrant gene-expression signature associated with emphysematous destruction and induce expression patterns consistent with healing and repair. These finding were supported by laboratory experiments. GHK, at 10 nM, added to cultured fibroblasts from the affected lung areas of patients, changed gene expression patterns from tissue destruction to tissue repair. This led to the organization of the actin cytoskeleton, elevated the expression of integrin beta 1, and restored collagen contraction [12].
In addition to topping the list of 1309 biologically active molecules as the computer-recommended treatment for both human COPD (chronic obstructive pulmonary disease) and aggressive metastatic colon cancer, GHK has been recommended as a treatment for inflammations, acute lung injury, activation of stem cells, regeneration of aged skin, wound healing and tissue regeneration (skin, hair follicles, stomach and intestinal linings, hair growth, and boney tissue). It is also widely used in anti-aging skin products [13].
Even though it is not always possible to link gene expression data to biological actions, it is important to notice that GHK is highest in very healthy young people. Unfortunately, GHK declines with age. In studies at the University of California at San Francisco, young (age 20–25), male medical students were found to have about 200 nanograms/mL of GHK in their blood plasma, while the healthy, male medical school faculty (average age of 60) had only 80 nanograms/mL [7].
Our previous publication reviewed the biological effects of GHK relevant to neurodegeneration and cognitive health [14]. This paper will discuss the effect of GHK on gene expression relevant to nervous system functions and cognitive decline as well as review genetic and laboratory data relevant to nerve outgrowth, copper transport into cells, anxiety and pain, DNA repair, the ubiquitin proteasome system, the anti-oxidant system, changes in gene expression for glial cells, astrocytes, brain cells, dendrites, ganglia, motor neurons, Schwann cells, and sensory cells. It will also present possible methods for the use of therapeutic GHK in the treatment of nerve diseases.
2. Materials and Methods
The cMap was used to acquire the gene expression data. It is a large database that contains more than 7000 gene expression profiles of 5 human cell lines treated with 1309 distinct small molecules. Three GHK profiles are contained in this repository. The profiles are the result of cell lines treated with GHK at 1 micromolar which is around the concentration where many of GHK’s cellular effects occur [15]. These profiles were created using the GeneChip HT Human Genome U133A Array. Among the 5 cell lines used by the Connectivity Map only 2 were treated with GHK. Two of the profiles were created using the PC3 cell line - human prostate cancer cells, while the third used the MCF7 cell line – human breast cancer cells. Our studies utilized all three gene expression profiles.
GenePattern, a publicly available computational biology open-source software package developed for the analysis of genomic data, was used to analyze the gene data obtained from the cMap. The CEL files (data files used by Affymetrix software, used by the Broad Institute) were processed with MAS5 (Microarray Analysis Suite 5 software, Affymetrix, Santa Clara, CA, USA) and background correction. Files were then uploaded to the ComparativeMarkerSelectionViewer module in order to view fold changes for each probe set. Gene abbreviations appearing throughout the paper are consistent with the NCBI Gene database [16].
Due to multiple probe sets mapping to the same gene, the fold changes in m-RNA production produced by GenePattern were converted to percentages, and then all probe sets representing the same gene were averaged. It was determined that the 22,277 probe sets in the Broad data represent 13,424 genes. This ratio (1.66) was used to calculate the overall number of genes that affect GHK at various cutoff points (rather than probe sets).
The percentage of genes stimulated or suppressed by GHK with a change greater than or equal to 50% was estimated to be 31.2% [17]. Listed in the article are the gene expression effects of GHK on over 700 human genes associated with various nerve cell types. For well-defined systems where animal and cell cultures exist, such as anti-pain and anti-oxidation, relevant genes were manually chosen. For other systems, each gene’s Gene Ontology description was searched, using terms such as “neuron” or “glial”. The Gene Ontology consortium provides controlled vocabularies for the description of the molecular function, biological process, and cellular component of gene products. [18]. For most systems, gene expression numbers were given from 100% + or − and larger.
The cMap data was proven to be predictive of biological actions in most cases. In 2010, cMap predicted the anti-cancer actions of GHK. Subsequent work found GHK at 1 to 10 nanomolar reset the programmed cell death system on human nerve cancer cells and inhibited their growth in culture, while having the same effect on sarcoma cell growth in mice; it changed the gene expression of over 80 genes in an anti-growth manner [17]. Data from cMap also led to experiments that found GHK at 10 nanomolar caused human COPD-afflicted lung cells to switch cell expression from tissue destruction to repair and remodeling. For anti-oxidant actions, cMap has been very predictive of actions in mammals. However, gene expression numbers can vary widely at times and are not always predictable. For example, the cMap gives NGF (nerve growth factor) as a −243% decrease, yet in vivo rat studies have found NGF to be increased and two in vitro cell culture studies have found GHK to increase nerve outgrowth, an effect usually attributed to NGF.
Below, we cover GHK’s relationship with the following.
Nerve Outgrowth
Copper Lack in Nerve Diseases
Anti-Anxiety and Anti-Pain
Anti-Oxidant Biological and Gene Expression Data
DNA Repair Data and Gene Expression DNA Repair
Restoring Regeneration after Cortisone Treatment
Gene Expression—Clearing Damaged Protein with the Ubiquitin Proteasome System (UPS)
Gene Expression—Neurons
Gene Expression—Motor neurons
Gene Expression—Glial cells
Gene Expression—Astrocytes
Gene Expression—Schwann
Gene Expression—Myelin
Gene Expression—Dendrite
Gene Expression—Oligodendrocyte cells
Gene Expression—Schwann cells
Gene Expression—Spinal
Possible methods of therapeutic use of GHK for nerve disease
3. Results
3.1. Nerve Outgrowth
The lack of nerve outgrowth growth is considered a major factor in dementia [19,20,21].
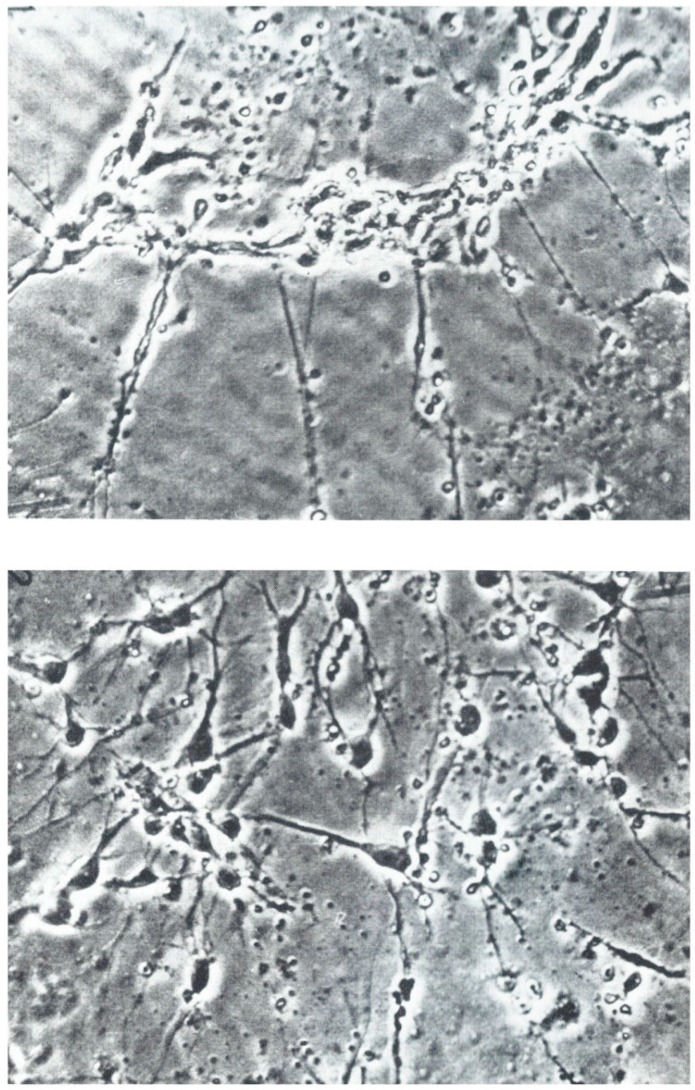
GHK was discovered in 1973 as a growth factor for cultured hepatocytes. In 1975, Sensenbrenner and colleagues reported that GHK induced the formation chick embryonic neurons while suppressing glial cells. See Figure 1 [22].
Figure 1.
(Top)—Control; (Bottom)—Addition of 200 ng/mL of GHK to culture media (Phase contrast ×250, photo micrographs used with permission of John Wiley and Sons).
Lindner and colleagues found that explants from chick embryo PNS (ganglion trigeminale) and from CNS of embryonal rats (hippocampus) and dissociated cells from chick embryo cerebral hemispheres that 0.01 microgram GHK per ml of medium stimulated the outgrowth of neuronal processes. Again, GHK promoted neuronal growth but not glial cells [23].
In studies of rats, severed sciatic nerves (axotomy) were inserted into a collagen prosthesis to which GHK was bonded. These were re-inserted into the rat, then removed after 10 days. GHK enhanced the production of trophic factors (Nerve Growth Factor, Neurotrophins 3 and 4) and recruited hematogenous cells and Schwann cells, which in turn help in the secretion of certain vital trophic and tropic factors essential for early regeneration. This improved nerve regeneration following axotomy [24]. Surprisingly, GHK’s gene expression data gives suppression of NGF (−243%) and NGFR (nerve growth factor receptor) (−132%). Thus, the biological system within wounded rat’s nervous tissue is more complex and probably due to other nerve stimulatory molecules.
3.2. Copper Deficiency, Dementia, and Nerve Dysfunction
Copper is an essential component of important anti-oxidant proteins such as SOD (copper zinc superoxide dismutase), ceruloplasmin, and Atox1 (human antioxidant protein 1). Copper deficiency has been postulated as a causative factor in a variety of gene diseases such as Alzheimer’s [25,26,27,28,29,30], myelopathy [31], motor neuron diseases and amyotrophic lateral sclerosis [32], Huntington’s [33], Lewy bodies and Creutzfeldt Jakob diseases [34].
More importantly, analysis of actual human brains from deceased patients with dementia has found the damaged areas to have very little cellular copper. In plaques from persons with Alzheimer’s disease, iron and aluminum appear to cause plaque formation while copper and zinc may be protective [26,27,28,35,36,37].
Copper deficiency caused by bariatric surgery or gastrointestinal bleeding led to myelopathy (human swayback), paralysis, blindness and behavioral and cognitive changes. Mice born and maintained on a copper deficient diet had 80% reduction in brain copper level at 6-8 weeks and had neuronal and glial changes typical for neurodegenerative disorders [25,31,38,39].
3.2.1. Supplying Copper to Nerve Cells
Though copper deficiency appears linked to major nerve diseases, the use of copper supplements as a treatment has been disappointing. A placebo-controlled study of 68 Alzheimer’s patients (34 control, 34 copper) with a treatment of 8 mgs of daily copper (a high level) for 1 year produced no negative findings. This seems to rule out excessive copper levels as a causative agent for the development of Alzheimer’s. The predictive protein marker, CSF Abeta42, is lower in persons developing Alzheimer’s. Subjects given extra copper supplementation maintained this protein at a higher level, a possible positive effect, but there was minimal improvement in the disease [40].
One small copper complex chelator, CuATSM (diacetyl-bis(4-methylthiosemicarbazonato)copper 2+), has given indications of ameliorating the effects of ALS (familial amyotrophic lateral sclerosis) in a strain of genetically modified mice that develop a form of ALS. CuATSM extends life in such mice by up to 25%. The motor neuron disease can be restarted and then stopped by controlling CuATSM treatment. The treatment increases the amount of active superoxide dismutase in the mice [41]. The safety of CuATSM is largely unknown. The safety data sheet states the following: “Material may be irritating to the mucous membranes and upper respiratory tract. May be harmful by inhalation, ingestion, or skin absorption. May cause eye, skin, or respiratory system irritation. To the best of our knowledge, the toxicological properties have not been thoroughly investigated.”
GHK-Copper 2+ increased superoxide dismutase (SOD) activity in mice as detailed below in Section 4 [42].
3.2.2. Albumin, GHK and Copper Transport
Both albumin and GHK transport copper 2+ to cells and tissues. However, in human blood, there are 700 albumin molecules for each GHK molecule, so albumin is the major source of copper for tissue use. GHK and albumin have high and very similar binding constants for copper 2+ (Albumin = pK binding log 10 |16.2|; GHK = pK binding log 10 |16.4|). Human plasma contains about 15 micromolar copper and 12% (1.8 micromolar) of this is bound to albumin. But GHK-Cu is maximally active on most cells around one nanomolar or less. Aqueous dialysis studies established that GHK can obtain copper 2+ from albumin. We assume that this also occurs in cell culture and within mammals and that GHK has adequate copper for biological actions.
Our studies over the past 39 years have indicated that virtually all biological GHK effects require the presence of copper 2+ chelated to the tripeptide. Strong copper chelators such as bathocuproine abolish GHK actions. GHK alone is often effective in murine wound healing or hair growth models, but GHK-Cu always produced much stronger responses. GHK attached to radioactive copper-64 increases copper uptake into cultured hepatoma cells [7].
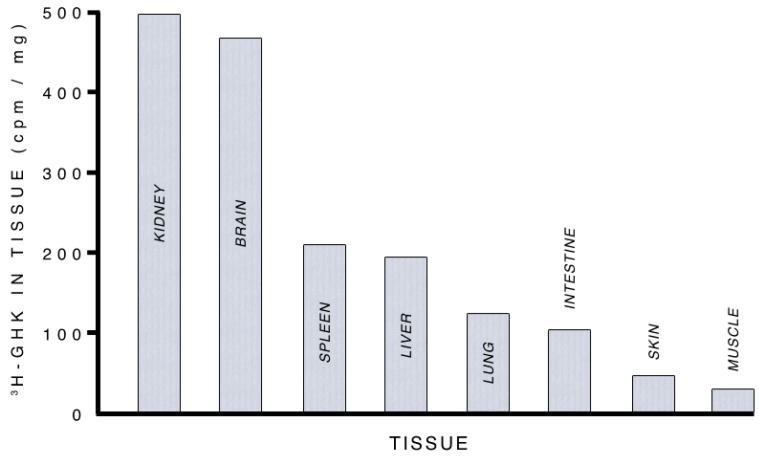
The intravenous injection of tritiated copper-free GHK into mice was found, after 4 h, to concentrate most densely within the animals’ kidneys and brain. See Figure 2 [43].
Figure 2.
Uptake of glycyl-l-histidyl-l-lysine (GHK) into various mouse tissues. (Reprinted from Pickart, L. [43]).
The best evidence that GHK can obtain copper 2+ from body fluids was from a study that used biotinylated GHK bound to collagen films placed over wounds in rats. The GHK pads raised the copper concentration by ninefold at the wound site when compared to non-GHK collagen films. Such biotinylated GHK collagen films also increased wound healing, cell proliferation, and increased the expression of antioxidant enzymes in the treated group [9].
Most importantly, GHK activates numerous regenerative and protective genes. Albumin will not mimic the GHK activated systems. So GHK must act through a separate pathway, not the albumin pathway. Albumin’s copper feeds cells; GHK’s copper activates regenerative and protective genes.
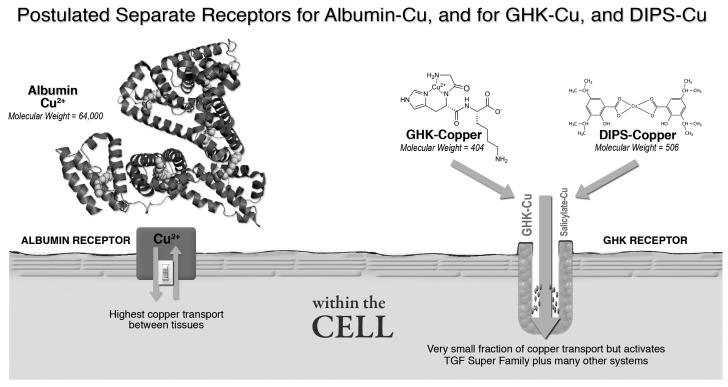
GHK-Cu’s regenerative and protective actions on tissue are very similar to those found by John R Sorenson throughout his 33 years of work on various copper salicylates. See Table 1. It appears that GHK-Copper and Sorenson’s DIPS-Cu (diisopropylsalicylate-copper 2+) both activate the same pathway, a pathway strongly associated with tissue health and repair. GHK-copper 2+ (molecular weight 404) and Sorenson’s DIPS-Cu (molecular weight 506) are both very small molecules while albumin is much larger (molecular weight 64,000). Hence, they are likely to use different cell receptor systems [44,45,46,47,48,49]. See Figure 3.
Table 1.
Similarity of Actions of GHK-Copper and Diisopropylsalicylate-Copper.
| Action | GHK-Copper 2+ | Diisopropylsalicylate-Copper 2+ |
|---|---|---|
| Wound Healing | Yes | Yes |
| Inhibit Cancer Growth | Yes | Yes |
| Anti-Ulcer | Yes | Yes |
| Anti-Pain | Yes | Yes |
| Improve Recovery After Radiation | Yes | Yes |
| Increase Stem Cell Activity | Yes | Yes |
Figure 3.
Proposed cell receptor for GHK-Cu.
3.3. Anti-Anxiety (Anxiolytic) and Anti-Pain
Anxiety and pain are serious issues in patients with dementia and other disabling mental conditions. Opiate peptides often possess both anti-pain and wound healing properties [50]. When healthy human males were fed a low copper diet (1 mg/day of copper) for 11 weeks, their plasma opiate levels dropped by 80%. As soon as copper was restored (with a diet containing 3 mg/day of copper), the levels returned to normal [51].
GHK has been found to possess analgesic and anxiolytic effects (anti-anxiety) in animal experiments. GHK reduced pain after thermal injury to rats at a dose of 0.5 milligrams/kg. Within 12 min after intraperitoneal injection, it also increased the amount of time the rats spent exploring more open areas of the maze and decreased time spent immobile (the freeze reaction), which indicated reduction of fear and anxiety. These effects were observed at 0.5 micrograms/kg [52,53].
These effects also prove that GHK rapidly affects the brain perception and function. This is an area where GHK could be used on patients today.
A manual search of genes affected by GHK found that seven anti-pain genes increased and two genes decreased. See Table 2 and Table 3.
Table 2.
Distribution of Genes Affected by GHK and Associated with Pain.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 0 | 0 |
| 100%–199% | 5 | 2 |
| 200%–299% | 1 | 0 |
| 300%–399% | 0 | 0 |
| 400%–499% | 0 | 0 |
| 500%+ | 1 | 0 |
| Total | 7 | 2 |
Table 3.
GHK and Genes Associated with Pain.
| UP | Gene | Percent Change in Gene Expression | Comments |
| 1 | OPRMI | 1294 | Opioid mu 1-High Affinity for enkephalins and beta-endorphins |
| 2 | OPRL1 | 246 | Receptor for neuropeptide nociceptin |
| 3 | CCKAR | 190 | Cholecystokinin A receptor, cholecystokinin affects satiety, release of beta-endorphin and dopamine |
| 4 | CNR1 | 172 | Cannabinoid receptor, pain-reducing |
| 5 | SIGMAR1 | 155 | Non-opioid receptor |
| 6 | PNOC | 150 | Prepronociceptin, complex interactions with pain and anxiety induction |
| 7 | OXT | 136 | Ocytocin, bonding protein—gene also increases human chorionic gonadotropin |
| DOWN | Gene | Percent Change in Gene Expression | Comments |
| 1 | AMPA 3/GRIA3 | −126.00% | Glutamate receptor, retrograde endocannaboid signaling, nervous system |
| 2 | OPRK1 | −119.00% | Reduced cocaine effects |
3.4. Antioxidant Activity of the GHK Peptide
High metabolic activity found in the brains of both humans and animals results in elevated oxygen consumption and constant production of reactive oxygen species (ROS) in mitochondria. At the same time, the brain tissue is rich in unsaturated fatty acids and transition metal ions, yet has relatively fewer antioxidants compared to other organs, creating favorable conditions for oxidative damage. Since the blood-brain barrier prevents many dietary antioxidants from entering the brain, it largely relays on endogenous antioxidants such as Cu and Zn dependent superoxide dismutase (Cu, Zn SOD1). This enzyme requires the metal ions copper and zinc in order to be active. Hence, copper deficiency can lead to reduced SOD activity and increased oxidative brain damage. When pregnant rats were fed a copper deficient diet, the embryos displayed low SOD activity, increased super oxide anion radical level, and higher incidence of DNA damage and malformations [54].
GHK has broad and powerful anti-oxidation properties in both mammals and cell culture, and it is known to increase anti-oxidant gene expression. Tissue oxidation has been postulated as a causative factor in Parkinson’s disease and other various nerve diseases of aging [55,56,57,58,59].
Diminished copper has been found in cells expressing SOD1 mutations postulated to cause ALS in mice and increase memory loss [60,61].
A peptidomimetic inhibitor (P6), based on GHK, interacts with amyloid beta (Aβ) peptide and its aggregates. P6 prevents the formation of toxic Aβ oligomeric species, fibrillar aggregates and DNA damage. It is a potential therapeutic candidate to ameliorate the multifaceted Aβ toxicity in Alzheimer’s [62].
3.4.1. GHK’s Anti-Oxidant Effects in Mammals and Cell Culture
The use of GHK-Cu in mice protected their lung tissue from lipopolysaccharide-induced acute lung injury (ALI). When GHK-Cu was used by mice with LPS-induced ALI, it attenuated related histological alterations in the lungs and suppressed the infiltration of inflammatory cells into the lung parenchyma. The GHK-Cu also increased superoxide dismutase (SOD) activity while decreasing TNF-α and IL-6 production through the suppression of the phosphorylation of NF-κB p65 and p38 MAPK in the nucleus of lung cells [42].
P38 mitogen-activated protein kinases are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis, and autophagy. NF-κB/RELA p65 activation has been found to be correlated with cancer development, suggesting the potential of RELA as a cancer biomarker. Specific modification patterns of RELA have also been observed in many cancer types.
Multiple antioxidant actions of GHK have been demonstrated in vitro and in animal wound healing studies. They include inhibiting the formation of reactive carbonyl species (RCS), detoxifying toxic products of lipid peroxidation such as acrolein, protecting keratinocytes from lethal UVB radiation, and preventing hepatic damage by dichloromethane radicals.
The ability of GHK to prevent oxidative stress was tested in vitro using Cu(2+)-dependent oxidation of low-density lipoproteins (LDL). LDL were treated with 5 μM Cu(2+) for 18 h in either phosphate buffered saline (PBS) or Ham’s F-10 medium. There was increased production of thiobarbituric acid reactive substances (TBARSs), which indicated increased oxidation. GHK and histidine “entirely blocked” (quoted from the article) the in vitro Cu(2+)-dependent oxidation of low-density lipoproteins (LDL). In comparison, superoxide dismutase (SOD1) provided only 20% reduction of oxidation [63].
Acrolein, a well-known carbonyl toxin, is produced by lipid peroxidation of polyunsaturated fatty acids. GHK effectively reduces the formation of both acrolein and another product of oxidation, 4-hydroxynonenal. GHK also blocks lethal ultraviolet radiation damage to cultured skin keratinocytes by binding and inactivating reactive carbonyl species such as 4-hydroxynoneal, acrolein, malondialdehyde, and glyoxal [64,65,66].
The intraperitoneal injection of 1.5 mg/kg of GHK into rats for five days before dichloromethane poisoning and five days thereafter provided protection of the functional activity of hepatocytes and immunological responsiveness. Dichloromethane is toxic to hepatic tissue via the formation of a dichloromethane free radical that induces acute toxic damage [67].
In rats with experimental bone fractures peptides, GHK (0.5 μg/kg), dalargin (1.2 μg/kg), and thymogen (0.5 μg/kg) were injected intraperitoneally. Within 10 days, there was a decrease of malonic dialdehyde and an increase of catalase activity in blood. There was also a marked increase of reparative activity. Each combination of peptides was more potent than any of the studied peptides injected separately. The synergetic action of peptides Gly-His-Lys, thymogen, and dalargin was proposed for stimulation of reparative osteogenesis [68].
GHK-Cu reduced iron release from ferritin by 87%. Iron has also been shown to have a direct role in the initiation of lipid peroxidation. An Fe(2+)/Fe(3+) complex can serve as an initiator of lipid oxidation. In addition, many iron complexes can catalyze the decomposition of lipid hydroperoxides to the corresponding lipid alkoxy radicals. The major storage site for iron in serum and tissue is ferritin. Ferritin in blood plasma can store up to 4500 atoms of iron per protein molecule, and superoxide anions can promote the mobilization of iron from ferritin. This free iron may then catalyze lipid peroxidation and the conversion of a superoxide anion to the more damaging hydroxyl radical [69].
3.4.2. Synthesis of GHK-Cu Analogs with Higher Anti-ROS Activity
GHK-Cu has, on a molar basis, about 1% to 3% of the activity of the Cu, Zn superoxide dismutase protein. By simple modifications to the peptide, it is possible to raise the SOD-mimetic activity up 223-fold. Given the broad range of the antioxidant actions of GHK, it is likely that modifications will increase its countering reactive species such as RCS and dichloromethane radicals. See Table 4 [70].
Table 4.
Superoxide Dismutase Mimetic Activity of GHK and Analogs.
| Molecule | Superoxide Dismutase Mimetic Activity |
|---|---|
| Gly-His-Lys:Cu(2+) | 100 |
| Lys-His-Gly-Amide:Cu(2+) | 21 |
| Gly-His-Lys-Ala-Phe-Ala:Cu(2+) | 561 |
| Ala-His-Lys:Cu(2+) | 563 |
| Gly-His-Lys-Octyl Ester:Cu(2+) | 810 |
| Gly-His-Caprolactam:Cu(2+) | 4500 |
| His-Gly-Lys:Cu(2+) | 22,300 |
3.4.3. Antioxidant Gene Expression Analysis
A manual search of antioxidant associated genes effected by GHK yielded 18 genes with significant antioxidant activity. See Table 5 and Table 6.
Table 5.
Distribution of Genes Affected by GHK with Antioxidant Activity.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 2 | 0 |
| 100%–199% | 7 | 1 |
| 200%–299% | 2 | 0 |
| 300%–399% | 1 | 0 |
| 400%–499% | 1 | 0 |
| 500%+ | 3 | 1 |
| Total | 16 | 2 |
Table 6.
GHK and Genes Associate with Antioxidant Activity.
| UP | Genes | Percent Change in Gene Expression | Comments |
| 1 | TLE1 | 762 | Inhibits the oxidative/inflammatory gene NF-κB [71]. |
| 2 | SPRR2C | 721 | This proline-rich, antioxidant protein protects outer skin cells from oxidative damage from reactive oxygen species (ROS). When the ROS level is low, the protein remains in the outer cell membrane, but when the ROS level is high, the protein clusters around the cell’s DNA to protect it [72,73]. |
| 3 | ITGB4 | 609 | Up-regulation of ITGB4 promotes wound repair ability and antioxidative ability [74]. |
| 4 | APOM | 403 | Binds oxidized phospholipids and increases the antioxidant effect of high-density lipoproteins (HDL) [75]. |
| 5 | PON3 | 319 | Absence of PON3 (paraoxonase 3) in mice resulted in increased rates of early fetal and neonatal death. Knockdown of PON3 in human cells reduced cell proliferation and total antioxidant capacity [76]. |
| 6 | IL18BP | 295 | The protein encoded by this gene is an inhibitor of the pro-inflammatory cytokine IL18. IL18BP abolished IL18 induction of interferon-gamma (IFN gamma), IL8, and activation of NF-κB in vitro. Blocks neutrophil oxidase activity [77]. |
| 7 | HEPH | 217 | Inhibits the conversion of Fe(2+) to Fe(3+). HEPH increases iron efflux, lowers cellular iron levels, suppresses reactive oxygen species production, and restores mitochondrial transmembrane potential [78]. |
| 8 | GPSM3 | 193 | Acts as a direct negative regulator of NLRP3. NLRP3 triggers the maturation of the pro-inflammatory cytokines IL-1β and IL-18 [79]. |
| 9 | FABP1 | 186 | Reduces intracellular ROS level. Plays a significant role in reduction of oxidative stress [80,81]. |
| 10 | AGTR2 | 171 | AGTR2 exerts an anti-inflammatory response in macrophages via enhanced IL-10 production and ERK1/2 phosphorylation, which may have protective roles in hypertension and associated tissue injury [82]. |
| 11 | PON1 | 149 | PON1 (paraoxonase 1) is a potent antioxidant and a major anti-atherosclerotic component of HDL [83]. |
| 12 | MT3 | 142 | Metallothioneins (MTs) display in vitro free radical scavenging capacity, suggesting that they may specifically neutralize hydroxyl radicals. Metallothioneins and metallothionein-like proteins isolated from mouse brain act as neuroprotective agents by scavenging superoxide radicals [84,85]. |
| 13 | PTGS2 | 120 | Produces cyclooxygenase-II (COX-II), which has antioxidant activities [86]. |
| 14 | SLC2A9 | 117 | The p53-SLC2A9 pathway is a novel antioxidant mechanism. During oxidative stress, SLC2A9 undergoes p53-dependent induction, and functions as an antioxidant by suppressing ROS, DNA damage, and cell death [87]. |
| DOWN | Genes | Percent Change in Gene Expression | Comments |
| 1 | IL17A | −1018 | This cytokine can stimulate the expression of IL6 and cyclooxygenase-2 (PTGS2/COX-2), as well as enhance the production of nitric oxide (NO). High levels of this cytokine are associated with several chronic inflammatory diseases including rheumatoid arthritis, psoriasis, and multiple sclerosis ([88]). |
| 2 | TNF | −115 | GHK suppresses this pro-oxidant TNF gene [89]. |
3.5. DNA Repair, Cell Culture, and Gene Expression
A lack of adequate DNA repair may be related to neurological degeneration in the aging population [90,91,92,93].
DNA damage is a major problem in the life cycle of biological cells. Normal cellular metabolism releases compounds that damage DNA such as reactive oxygen species, reactive nitrogen species, reactive carbonyl species, lipid peroxidation products and alkylating agents, among others, while hydrolysis cleaves chemical bonds in DNA. It is estimated that each normally functioning cell in the human body suffers at least 10,000 DNA damaging incidents daily [94].
Radiation therapy is believed to stop cell replication by damaging cellular DNA. A study of cultured primary human dermal fibroblast cell lines from patients who had undergone radiation therapy for head and neck cancer found that the procedure slowed the population doubling times for the cells. But treatment with one nanomolar GHK-Cu restored population doubling times to normal. Irradiated cells treated with GHK-Cu also produced significantly more basic fibroblast growth factor and vascular endothelial growth factor than untreated irradiated cells [5].
GHK is primarily stimulatory for gene expression of DNA Repair genes (47 UP, 5 DOWN), suggesting an increased DNA repair activity. Here we searched the Gene Ontology descriptions for “DNA Repair”. See Table 7 and Table 8.
Table 7.
Distribution of Genes Affected by GHK and Associated with DNA Repair.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–100% | 41 | 4 |
| 100%–150% | 2 | 1 |
| 150%–200% | 1 | 0 |
| 200%–250% | 2 | 0 |
| 250%–300% | 1 | 0 |
| Total | 47 | 5 |
Table 8.
GHK and Genes Associate with DNA Repair.
| UP | Gene Title | Percent Change in Gene Expression |
| 1 | poly (ADP-ribose) polymerase family, member 3, PARP3 | 253 |
| 2 | polymerase (DNA directed), mu, POLM | 225 |
| 3 | MRE11 meiotic recombination 11 homolog A MRE11A | 212 |
| 4 | RAD50 homolog (S. cerevisiae), RAD50 | 175 |
| 5 | eyes absent homolog 3 (Drosophila), EYA3 | 128 |
| 6 | retinoic acid receptor, alpha, RARA | 123 |
| DOWN | Gene Title | Percent Change in Gene Expression |
| 1 | cholinergic receptor, nicotinic, alpha 4, CHRNA4 | −105 |
3.6. Restoring Regeneration After Cortisone Treatment
Steroid dementia syndrome describes the signs and symptoms of hippocampal and prefrontal cortical dysfunction, such as deficits in memory, attention, and executive function, induced by glucocorticoids. Dementia-like symptoms have been found in some individuals who have been exposed to glucocorticoid medication, often dispensed in the form of asthma, arthritis, and anti-inflammatory steroid medications. The condition reverses, but not always completely, within months after steroid treatment is stopped [95].
In the human body, cortisone and cortisol are easily interconvertible and have similar anti-inflammatory actions. They also profoundly inhibit tissue regeneration, such as wound repair. DHEA (dehydroepiandrosterone) is an androgenic hormone. It is a precursor for testosterone and the estrogens. DHEA antagonizes the effects of cortisol but decreases about 80% from age 20 to age 80 while cortisone/cortisol levels remain high. It has been proposed that many of the deleterious effects of aging are due to excessive cortisol that is not balanced by DHEA.
GHK-Cu, when administered systemically to mice, rats, and pigs, counters the wound healing inhibition of cortisone throughout the animal [96].
3.7. Gene Expression—Clearing Damaged Protein—Ubiquitin Proteasome System
The ubiquitin proteasome system (UPS) clears damaged proteins. Insufficient activity of this system is postulated to produce an accumulation of toxic protein oligomers which start the neurodegenerative process. During aging, there is decreased activity of the ubiquitin proteasome system. To date, no effective therapies have been developed that can specifically increase the UPS activity [97,98,99,100].
GHK strongly stimulates the gene expression of the UPS system with 41 genes increased and 1 gene suppressed. Here we searched gene title for “ubiquitin” or “proteasome”. See Table 9 and Table 10.
Table 9.
Distribution of Genes Affected by GHK and Associated with the Ubiquitin Proteasome System.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 31 | 1 |
| 100%–199% | 7 | 0 |
| 200%–299% | 0 | 0 |
| 300%–399% | 1 | 0 |
| 400%–499% | 1 | 0 |
| 500%+ | 1 | 0 |
| Total | 41 | 1 |
Table 10.
GHK and Genes Associated with the Ubiquitin Proteasome System.
| UP | Gene Title | Percent Change |
|---|---|---|
| 1 | ubiquitin specific peptidase 29, USP29 | 1056 |
| 2 | ubiquitin protein ligase E3 component n-recognin 2, UBR2 | 455 |
| 3 | gamma-aminobutyric acid (GABA) B receptor, 1 /// ubiquitin D, GABBR1 /// UBD | 310 |
| 4 | ubiquitin specific peptidase 34, USP34 | 195 |
| 5 | parkinson protein 2, E3 ubiquitin protein ligase (parkin), PARK2 | 169 |
| 6 | ubiquitin-conjugating enzyme E2I (UBC9 homolog, yeast), UBE2I | 150 |
| 7 | ubiquitin protein ligase E3 component n-recognin 4, UBR4 | 146 |
| 8 | ubiquitin protein ligase E3B, UBE3B | 116 |
| 9 | ubiquitin specific peptidase 2, USP2 | 104 |
| 10 | ubiquitin-like modifier activating enzyme 6, UBA6 | 104 |
3.8. Gene Expression—Neurons
Neurons are cells that carry messages between the brain and other parts of the body; they are the basic units of the nervous system.
GHK is primarily stimulatory for gene expression of neuron related genes. Here we searched the Gene Ontology descriptions for “Neuron”. See Table 11 and Table 12.
Table 11.
Distribution of Genes Affected by GHK and Associated with Neurons.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 230 | 80 |
| 100%–199% | 99 | 80 |
| 200%–299% | 45 | 35 |
| 300%–399% | 19 | 14 |
| 400%–499% | 9 | 10 |
| 500%+ | 6 | 11 |
| Total | 408 | 230 |
Table 12.
GHK and Genes Associated with Neurons.
| UP | Gene Title | Percent Change |
| 1 | opioid receptor, mu 1, OPRM1 | 1294 |
| 2 | tumor protein p73, TP73 | 938 |
| 3 | potassium voltage-gated channel, Shal-related subfamily, member 1, KCND1 | 845 |
| 4 | solute carrier family 8 (sodium/calcium exchanger), member 2, SLC8A2 | 737 |
| 5 | contactin associated protein-like 2, CNTNAP2 | 581 |
| 6 | stathmin-like 3, STMN3 | 500 |
| 7 | latrophilin 3, LPHN3 | 494 |
| 8 | angiopoietin 1, ANGPT1 | 487 |
| 9 | synapsin III, SYN3 | 478 |
| 10 | dipeptidyl-peptidase 6, DPP6 | 448 |
| 11 | somatostatin receptor 2, SSTR2 | 442 |
| 12 | G protein-coupled receptor, family C, group 5, member B, GPRC5B | 431 |
| 13 | sodium channel, voltage-gated, type III, alpha subunit, SCN3A | 423 |
| 14 | smoothened homolog (Drosophila), SMO | 415 |
| 15 | tryptophan hydroxylase 1, TPH1 | 409 |
| 16 | caspase 8, apoptosis-related cysteine peptidase, CASP8 | 399 |
| 17 | gamma-aminobutyric acid (GABA) A receptor, alpha 5 /// gamma-aminobutyric acid receptor subunit alpha-5-like, GABRA5 /// LOC100509612 | 392 |
| 18 | transcription factor 7 (T-cell specific, HMG-box), TCF7 | 372 |
| 19 | solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 6, SLC17A6 | 369 |
| 20 | doublecortin-like kinase 1, DCLK1 | 365 |
| 21 | p21 protein (Cdc42/Rac)-activated kinase 1, PAK1 | 363 |
| 22 | neurogenic differentiation 4, NEUROD4 | 362 |
| 23 | zinc finger protein 335, ZNF335 | 358 |
| 24 | wingless-type MMTV integration site family, member 3, WNT3 | 352 |
| 25 | ADAM metallopeptidase domain 8, ADAM8 | 352 |
| 26 | neuropeptide Y, NPY | 346 |
| 27 | potassium voltage-gated channel, Shaw-related subfamily, member 3, KCNC3 | 332 |
| 28 | EPH receptor B1, EPHB1 | 330 |
| 29 | LIM domain kinase 1, LIMK1 | 322 |
| 30 | myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila), MLL | 318 |
| 31 | growth associated protein 43, GAP43 | 305 |
| 32 | FBJ murine osteosarcoma viral oncogene homolog, FOS | 305 |
| 33 | sal-like 1 (Drosophila), SALL1 | 302 |
| 34 | synovial sarcoma, X breakpoint 2 /// synovial sarcoma, X breakpoint 2B, SSX2 /// SSX2B | 301 |
| 35 | inositol 1,4,5-triphosphate receptor, type 3, ITPR3 | 298 |
| 36 | bone morphogenetic protein receptor, type IB, BMPR1B | 298 |
| 37 | synuclein, gamma (breast cancer-specific protein 1), SNCG | 292 |
| 38 | calcium channel, voltage-dependent, P/Q type, alpha 1A subunit, CACNA1A | 286 |
| 39 | capping protein (actin filament) muscle Z-line, beta, CAPZB | 285 |
| 40 | plexin C1, PLXNC1 | 282 |
| 41 | nuclear factor I/B, NFIB | 279 |
| 42 | islet amyloid polypeptide, IAPP | 276 |
| 43 | nephroblastoma overexpressed gene, NOV | 275 |
| 44 | hyperpolarization activated cyclic nucleotide-gated potassium channel 4, HCN4 | 269 |
| 45 | calsyntenin 2, CLSTN2 | 268 |
| 46 | potassium intermediate/small conductance calcium-activated channel, subfamily N, member 1, KCNN1 | 266 |
| 47 | sodium channel, voltage-gated, type II, alpha subunit, SCN2A | 264 |
| 48 | neuroligin 1, NLGN1 | 261 |
| 49 | ELKS/RAB6-interacting/CAST family member 2, ERC2 | 261 |
| 50 | scratch homolog 1, zinc finger protein (Drosophila), SCRT1 | 252 |
| 51 | low density lipoprotein receptor-related protein 1, LRP1 | 249 |
| 52 | hypothetical protein LOC728392 /// NLR family, pyrin domain containing 1, LOC728392 /// NLRP1 | 249 |
| 53 | opiate receptor-like 1, OPRL1 | 246 |
| 54 | myosin, heavy chain 14, non-muscle, MYH14 | 243 |
| 55 | nitric oxide synthase 1 (neuronal), NOS1 | 240 |
| 56 | wingless-type MMTV integration site family, member 2B, WNT2B | 238 |
| 57 | glutamate receptor, metabotropic 1, GRM1 | 231 |
| 58 | glutamate receptor interacting protein 1, GRIP1 | 230 |
| 59 | myelin associated glycoprotein, MAG | 229 |
| 60 | chemokine (C-C motif) ligand 3 /// chemokine (C-C motif) ligand 3-like 1 /// chemokine (C-C motif) ligand 3-like 3, CCL3 /// CCL3L1 /// CCL3L3 | 228 |
| 61 | family with sequence similarity 162, member A, FAM162A | 228 |
| 62 | sphingosine-1-phosphate receptor 5, S1PR5 | 227 |
| 63 | protein tyrosine phosphatase, receptor type, R, PTPRR | 225 |
| 64 | IKAROS family zinc finger 1 (Ikaros), IKZF1 | 225 |
| 65 | potassium intermediate/small conductance calcium-activated channel, subfamily N, member 3, KCNN3 | 221 |
| 66 | solute carrier family 18 (vesicular monoamine), member 2, SLC18A2 | 219 |
| 67 | glutamate receptor, ionotropic, N-methyl d-aspartate 1, GRIN1 | 216 |
| 68 | v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian), SRC | 216 |
| 69 | jagged 1, JAG1 | 215 |
| 70 | adenylate cyclase activating polypeptide 1 (pituitary), ADCYAP1 | 215 |
| 71 | ATPase, Ca++ transporting, plasma membrane 2, ATP2B2 | 214 |
| 72 | tripartite motif-containing 2, TRIM2 | 213 |
| 73 | netrin 1, NTN1 | 212 |
| 74 | paired related homeobox 1, PRRX1 | 209 |
| 75 | purinergic receptor P2X, ligand-gated ion channel, 3, P2RX3 | 207 |
| 76 | inhibitor of DNA binding 4, dominant negative helix-loop-helix protein, ID4 | 203 |
| 77 | solute carrier family 5 (choline transporter), member 7, SLC5A7 | 202 |
| 78 | empty spiracles homeobox 1, EMX1 | 202 |
| 79 | muscle, skeletal, receptor tyrosine kinase, MUSK | 200 |
| 80 | GATA binding protein 2, GATA2 | 193 |
| 81 | cadherin 13, H-cadherin (heart), CDH13 | 192 |
| 82 | Rho/Rac guanine nucleotide exchange factor (GEF) 2, ARHGEF2 | 191 |
| 83 | anaplastic lymphoma receptor tyrosine kinase, ALK | 191 |
| 84 | cholecystokinin A receptor, CCKAR | 190 |
| 85 | GLI family zinc finger 2, GLI2 | 183 |
| 86 | cholinergic receptor, nicotinic, beta 1 (muscle), CHRNB1 | 182 |
| 87 | NK2 homeobox 2, NKX2-2 | 181 |
| 88 | purinergic receptor P2X, ligand-gated ion channel, 4, P2RX4 | 180 |
| 89 | gamma-aminobutyric acid (GABA) receptor, rho 2, GABRR2 | 179 |
| 90 | PDZ and LIM domain 5, PDLIM5 | 177 |
| 91 | plasminogen activator, urokinase, PLAU | 172 |
| 92 | cannabinoid receptor 1 (brain), CNR1 | 172 |
| 93 | chondrolectin, CHODL | 172 |
| 94 | neurexin 2, NRXN2 | 171 |
| 95 | parkinson protein 2, E3 ubiquitin protein ligase (parkin), PARK2 | 169 |
| 96 | calcium channel, voltage-dependent, L type, alpha 1F subunit, CACNA1F | 168 |
| 97 | neuregulin 1, NRG1 | 164 |
| 98 | zinc finger protein 536, ZNF536 | 162 |
| 99 | endothelin 3, EDN3 | 161 |
| 100 | paired box 7, PAX7 | 161 |
| 101 | calcium/calmodulin-dependent protein kinase II beta, CAMK2B | 161 |
| 102 | solute carrier family 30 (zinc transporter), member 3, SLC30A3 | 160 |
| 103 | ciliary neurotrophic factor /// zinc finger protein 91 homolog (mouse) /// ZFP91-CNTF readthrough transcript, CNTF /// ZFP91 /// ZFP91-CNTF | 159 |
| 104 | calcium channel, voltage-dependent, T type, alpha 1I subunit, CACNA1I | 156 |
| 105 | membrane associated guanylate kinase, WW and PDZ domain containing 2, MAGI2 | 155 |
| 106 | sigma non-opioid intracellular receptor 1, SIGMAR1 | 155 |
| 107 | leptin, LEP | 152 |
| 108 | microtubule-associated protein tau, MAPT | 150 |
| 109 | erythropoietin receptor, EPOR | 147 |
| 110 | frizzled homolog 8 (Drosophila), FZD8 | 147 |
| 111 | nuclear mitotic apparatus protein 1, NUMA1 | 147 |
| 112 | ninjurin 2, NINJ2 | 144 |
| 113 | probable transcription factor PML-like /// promyelocytic leukemia, LOC652346 /// PML | 144 |
| 114 | fasciculation and elongation protein zeta 1 (zygin I), FEZ1 | 143 |
| 115 | ribonucleotide reductase M1, RRM1 | 142 |
| 116 | retinoic acid receptor, beta, RARB | 142 |
| 117 | metallothionein 3, MT3 | 142 |
| 118 | vascular endothelial growth factor A, VEGFA | 141 |
| 119 | glycoprotein M6A, GPM6A | 140 |
| 120 | runt-related transcription factor 1, RUNX1 | 136 |
| 121 | cholinergic receptor, nicotinic, delta, CHRND | 135 |
| 122 | testis specific, 10, TSGA10 | 135 |
| 123 | growth hormone secretagogue receptor, GHSR | 135 |
| 124 | guanine nucleotide binding protein (G protein), beta polypeptide 3, GNB3 | 134 |
| 125 | glycine receptor, beta, GLRB | 132 |
| 126 | runt-related transcription factor 1; translocated to, 1 (cyclin D-related), RUNX1T1 | 131 |
| 127 | synaptotagmin V, SYT5 | 131 |
| 128 | bridging integrator 1, BIN1 | 130 |
| 129 | general transcription factor IIi, GTF2I | 128 |
| 130 | mitogen-activated protein kinase kinase 7, MAP2K7 | 127 |
| 131 | peroxisome proliferator-activated receptor gamma, coactivator 1 alpha, PPARGC1A | 126 |
| 132 | v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian), ERBB4 | 125 |
| 133 | retinoic acid receptor, alpha, RARA | 123 |
| 134 | baculoviral IAP repeat-containing protein 1-like /// NLR family, apoptosis inhibitory protein, LOC100510692 /// NAIP | 123 |
| 135 | myosin VA (heavy chain 12, myoxin), MYO5A | 122 |
| 136 | heat shock protein 90kDa alpha (cytosolic), class B member 1, HSP90AB1 | 121 |
| 137 | voltage-dependent anion channel 1, VDAC1 | 120 |
| 138 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase), PTGS2 | 120 |
| 139 | spectrin, beta, non-erythrocytic 1, SPTBN1 | 120 |
| 140 | tubulin, beta 2A /// tubulin, beta 2B, TUBB2A /// TUBB2B | 119 |
| 141 | misshapen-like kinase 1, MINK1 | 119 |
| 142 | neural cell adhesion molecule 1, NCAM1 | 119 |
| 143 | kelch-like 1 (Drosophila), KLHL1 | 119 |
| 144 | sperm associated antigen 9, SPAG9 | 118 |
| 145 | gonadotropin-releasing hormone 1 (luteinizing-releasing hormone), GNRH1 | 116 |
| 146 | cholinergic receptor, nicotinic, beta 3, CHRNB3 | 115 |
| 147 | neuralized homolog (Drosophila), NEURL | 115 |
| 148 | SRY (sex determining region Y)-box 14, SOX14 | 115 |
| 149 | purinergic receptor P2X, ligand-gated ion channel, 1, P2RX1 | 112 |
| 150 | transcription factor 4, TCF4 | 112 |
| 151 | lysozyme, LYZ | 111 |
| 152 | MYC associated factor X, MAX | 111 |
| 153 | synaptojanin 1, SYNJ1 | 108 |
| 154 | ret proto-oncogene, RET | 108 |
| 155 | cadherin 2, type 1, N-cadherin (neuronal), CDH2 | 108 |
| 156 | AXL receptor tyrosine kinase, AXL | 108 |
| 157 | ataxia telangiectasia mutated, ATM | 107 |
| 158 | parvalbumin, PVALB | 107 |
| 159 | glyceraldehyde-3-phosphate dehydrogenase, GAPDH | 107 |
| 160 | Rap guanine nucleotide exchange factor (GEF) 1, RAPGEF1 | 106 |
| 161 | protein kinase C, gamma, PRKCG | 106 |
| 162 | neurofibromin 2 (merlin), NF2 | 105 |
| 163 | serrate RNA effector molecule homolog (Arabidopsis), SRRT | 105 |
| 164 | syntaxin 3, STX3 | 105 |
| 165 | X-box binding protein 1, XBP1 | 104 |
| 166 | potassium large conductance calcium-activated channel, subfamily M, beta member 2, KCNMB2 | 104 |
| 167 | chemokine (C-X3-C motif) receptor 1, CX3CR1 | 104 |
| 168 | aldehyde dehydrogenase 1 family, member A2, ALDH1A2 | 103 |
| 169 | drebrin 1, DBN1 | 103 |
| 170 | UDP glycosyltransferase 8, UGT8 | 103 |
| 171 | achaete-scute complex homolog 1 (Drosophila), ASCL1 | 103 |
| 172 | POU class 4 homeobox 3, POU4F3 | 102 |
| 173 | neurofibromin 1, NF1 | 102 |
| 174 | steroidogenic acute regulatory protein, STAR | 101 |
| 175 | histamine receptor H3, HRH3 | 101 |
| 176 | nuclear receptor subfamily 2, group F, member 6, NR2F6 | 100 |
| 177 | transforming growth factor, beta 1, TGFB1 | 100 |
| 178 | homeobox D3, HOXD3 | 100 |
| DOWN | Gene Title | Percent Change |
| 81 | 5-hydroxytryptamine (serotonin) receptor 3A, HTR3A | −100 |
| 82 | neuroligin 3, NLGN3 | −101 |
| 83 | aquaporin 1 (Colton blood group), AQP1 | −101 |
| 84 | SH3 and multiple ankyrin repeat domains 2, SHANK2 | −102 |
| 85 | neurochondrin, NCDN | −102 |
| 86 | astrotactin 1, ASTN1 | −102 |
| 87 | mitogen-activated protein kinase 8 interacting protein 2, MAPK8IP2 | −103 |
| 88 | limbic system-associated membrane protein, LSAMP | −103 |
| 89 | calcium binding protein 1, CABP1 | −106 |
| 90 | integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12), ITGB1 | −107 |
| 91 | discs, large (Drosophila) homolog-associated protein 2, DLGAP2 | −108 |
| 92 | doublecortin, DCX | −108 |
| 93 | colony stimulating factor 3 (granulocyte), CSF3 | −108 |
| 94 | advanced glycosylation end product-specific receptor, AGER | −108 |
| 95 | corticotropin releasing hormone receptor 1, CRHR1 | −109 |
| 96 | neuropeptides B/W receptor 2, NPBWR2 | −109 |
| 97 | even-skipped homeobox 1, EVX1 | −110 |
| 98 | retinoid X receptor, gamma, RXRG | −110 |
| 99 | cytoplasmic polyadenylation element binding protein 3, CPEB3 | −112 |
| 100 | alpha tubulin acetyltransferase 1, ATAT1 | −113 |
| 101 | paralemmin, PALM | −115 |
| 102 | tumor necrosis factor, TNF | −115 |
| 103 | fatty acid binding protein 7, brain, FABP7 | −118 |
| 104 | olfactory marker protein, OMP | −118 |
| 105 | Amphiregulin, AREG | −118 |
| 106 | opioid receptor, kappa 1, OPRK1 | −119 |
| 107 | calbindin 2, CALB2 | −119 |
| 108 | phosphodiesterase 10A, PDE10A | −121 |
| 109 | early growth response 1, EGR1 | −121 |
| 110 | cell cycle exit and neuronal differentiation 1, CEND1 | −123 |
| 111 | 5-hydroxytryptamine (serotonin) receptor 3B, HTR3B | −123 |
| 112 | synaptosomal-associated protein, 23kDa, SNAP23 | −123 |
| 113 | sodium channel, voltage-gated, type XI, alpha subunit, SCN11A | −124 |
| 114 | growth arrest-specific 7, GAS7 | −124 |
| 115 | contactin 1, CNTN1 | −125 |
| 116 | neuroligin 4, X-linked, NLGN4X | −128 |
| 117 | gamma-aminobutyric acid (GABA) A receptor, alpha 1, GABRA1 | −130 |
| 118 | leucine zipper, putative tumor suppressor 1, LZTS1 | −130 |
| 119 | mesenchyme homeobox 2, MEOX2 | −131 |
| 120 | TYRO3 protein tyrosine kinase, TYRO3 | −131 |
| 121 | synaptophysin, SYP | −132 |
| 122 | coiled-coil domain containing 64, CCDC64 | −132 |
| 123 | leucine-rich, glioma inactivated 1, LGI1 | −132 |
| 124 | nerve growth factor receptor, NGFR | −132 |
| 125 | cholinergic receptor, nicotinic, beta 4, CHRNB4 | −135 |
| 126 | 5-hydroxytryptamine (serotonin) receptor 2A, HTR2A | −135 |
| 127 | myocyte enhancer factor 2C, MEF2C | −138 |
| 128 | cholinergic receptor, nicotinic, alpha 4, CHRNA4 | −139 |
| 129 | prodynorphin, PDYN | −142 |
| 130 | discs, large homolog 2 (Drosophila), DLG2 | −142 |
| 131 | neurexin 1, NRXN1 | −144 |
| 132 | secretin, SCT | −148 |
| 133 | serpin peptidase inhibitor, clade F (alpha-2 antiplasmin, pigment epithelium derived factor), member 1, SERPINF1 | −148 |
| 134 | tachykinin receptor 3, TACR3 | −150 |
| 135 | Ras homolog enriched in brain, RHEB | −150 |
| 136 | PARK2 co-regulated, PACRG | −153 |
| 137 | glutamate receptor, ionotropic, kainate 5, GRIK5 | −159 |
| 138 | bone morphogenetic protein 2, BMP2 | −159 |
| 139 | choline O-acetyltransferase, CHAT | −160 |
| 140 | sodium channel, voltage-gated, type I, alpha subunit, SCN1A | −162 |
| 141 | TOX high mobility group box family member 3, TOX3 | −163 |
| 142 | gastric inhibitory polypeptide, GIP | −164 |
| 143 | corticotropin releasing hormone receptor 2, CRHR2 | −165 |
| 144 | kinesin family member 1A, KIF1A | −165 |
| 145 | RAB35, member RAS oncogene family, RAB35 | −166 |
| 146 | protein kinase C, theta, PRKCQ | −167 |
| 147 | cell adhesion molecule with homology to L1CAM (close homolog of L1), CHL1 | −171 |
| 148 | unc-51-like kinase 4 (C. elegans), ULK4 | −172 |
| 149 | wingless-type MMTV integration site family, member 4, WNT4 | −175 |
| 150 | thyroid stimulating hormone receptor, TSHR | −175 |
| 151 | potassium voltage-gated channel, Shal-related subfamily, member 3, KCND3 | −175 |
| 152 | contactin 2 (axonal), CNTN2 | −180 |
| 153 | glutamate receptor, ionotropic, N-methyl D-aspartate 2A, GRIN2A | −180 |
| 154 | fibronectin leucine rich transmembrane protein 1, FLRT1 | −183 |
| 155 | gamma-aminobutyric acid (GABA) A receptor, gamma 3, GABRG3 | −186 |
| 156 | calcium/calmodulin-dependent protein kinase IG, CAMK1G | −187 |
| 157 | interleukin 6 receptor, IL6R | −190 |
| 158 | calsyntenin 3, CLSTN3 | −191 |
| 159 | vesicle-associated membrane protein 1 (synaptobrevin 1), VAMP1 | −193 |
| 160 | promyelocytic leukemia, PML | −196 |
| 161 | ATPase, H+ transporting, lysosomal accessory protein 2, ATP6AP2 | −209 |
| 162 | mitogen-activated protein kinase 8 interacting protein 3, MAPK8IP3 | −209 |
| 163 | estrogen receptor 2 (ER beta), ESR2 | −216 |
| 164 | cytochrome b-245, beta polypeptide, CYBB | −217 |
| 165 | purinergic receptor P2Y, G-protein coupled, 11 /// PPAN-P2RY11 readthrough, P2RY11 /// PPAN-P2RY11 | −219 |
| 166 | sonic hedgehog, SHH | −220 |
| 167 | growth differentiation factor 11, GDF11 | −221 |
| 168 | protein tyrosine phosphatase, receptor type, D, PTPRD | −221 |
| 169 | ELK1, member of ETS oncogene family, ELK1 | −224 |
| 170 | regulating synaptic membrane exocytosis 1, RIMS1 | −225 |
| 171 | hairy/enhancer-of-split related with YRPW motif-like, HEYL | −228 |
| 172 | neurotrophic tyrosine kinase, receptor, type 3, NTRK3 | −230 |
| 173 | potassium voltage-gated channel, Shab-related subfamily, member 2, KCNB2 | −233 |
| 174 | regulator of G-protein signaling 6, RGS6 | −235 |
| 175 | glycine receptor, alpha 3, GLRA3 | −235 |
| 176 | potassium voltage-gated channel, shaker-related subfamily, beta member 1, KCNAB1 | −235 |
| 177 | guanine nucleotide binding protein (G protein), alpha transducing activity polypeptide 1, GNAT1 | −242 |
| 178 | proprotein convertase subtilisin/kexin type 2, PCSK2 | −242 |
| 179 | nerve growth factor (beta polypeptide), NGF | −243 |
| 180 | corticotropin releasing hormone, CRH | −243 |
| 181 | laminin, alpha 1, LAMA1 | −245 |
| 182 | cyclic nucleotide gated channel alpha 3, CNGA3 | −249 |
| 183 | glutamate receptor, ionotropic, kainate 1, GRIK1 | −254 |
| 184 | lin-28 homolog A (C. elegans), LIN28A | −259 |
| 185 | empty spiracles homeobox 2, EMX2 | −260 |
| 186 | cyclin-dependent kinase 5, regulatory subunit 1 (p35), CDK5R1 | −260 |
| 187 | agrin, AGRN | −264 |
| 188 | T-box, brain, 1, TBR1 | −272 |
| 189 | stathmin-like 2, STMN2 | −274 |
| 190 | microcephalin 1, MCPH1 | −275 |
| 191 | ELAV (embryonic lethal, abnormal vision, Drosophila)-like 4 (Hu antigen D), ELAVL4 | −282 |
| 192 | mitogen-activated protein kinase 8 interacting protein 1, MAPK8IP1 | −289 |
| 193 | calcium channel, voltage-dependent, N type, alpha 1B subunit, CACNA1B | −290 |
| 194 | FEZ family zinc finger 2, FEZF2 | −295 |
| 195 | dopamine receptor D4, DRD4 | −296 |
| 196 | zinc finger E-box binding homeobox 1, ZEB1 | −300 |
| 197 | T-cell leukemia homeobox 1, TLX1 | −311 |
| 198 | sterile alpha motif domain containing 4A, SAMD4A | −315 |
| 199 | opioid binding protein/cell adhesion molecule-like, OPCML | −333 |
| 200 | fibroblast growth factor receptor 2, FGFR2 | −337 |
| 201 | SRY (sex determining region Y)-box 1, SOX1 | −337 |
| 202 | neurogenin 1, NEUROG1 | −345 |
| 203 | PTK2B protein tyrosine kinase 2 beta, PTK2B | −348 |
| 204 | somatostatin receptor 5, SSTR5 | −353 |
| 205 | myelin basic protein, MBP | −361 |
| 206 | EPH receptor A7, EPHA7 | −365 |
| 207 | G protein-coupled receptor 173, GPR173 | −373 |
| 208 | S100 calcium binding protein A5, S100A5 | −374 |
| 209 | acyl-CoA synthetase long-chain family member 6, ACSL6 | −384 |
| 210 | family with sequence similarity 107, member A, FAM107A | −407 |
| 211 | Kv channel interacting protein 1, KCNIP1 | −413 |
| 212 | Fas apoptotic inhibitory molecule 2, FAIM2 | −416 |
| 213 | bradykinin receptor B1, BDKRB1 | −426 |
| 214 | discs, large homolog 4 (Drosophila), DLG4 | −452 |
| 215 | adenylate cyclase 10 (soluble), ADCY10 | −460 |
| 216 | cyclin-dependent kinase 5, regulatory subunit 2 (p39), CDK5R2 | −481 |
| 217 | EPH receptor A3, EPHA3 | −485 |
| 218 | phosphodiesterase 1A, calmodulin-dependent, PDE1A | −485 |
| 219 | chemokine (C-X-C motif) receptor 4, CXCR4 | −496 |
| 220 | membrane metallo-endopeptidase, MME | −540 |
| 221 | paired-like homeodomain 3, PITX3 | −541 |
| 222 | notch 3, NOTCH3 | −547 |
| 223 | discs, large (Drosophila) homolog-associated protein 1, DLGAP1 | −547 |
| 224 | slit homolog 1 (Drosophila), SLIT1 | −553 |
| 225 | bassoon (presynaptic cytomatrix protein), BSN | −563 |
| 226 | cadherin, EGF LAG seven-pass G-type receptor 1 (flamingo homolog, Drosophila), CELSR1 | −647 |
| 227 | calcium channel, voltage-dependent, beta 4 subunit, CACNB4 | −672 |
| 228 | necdin homolog (mouse), NDN | −729 |
| 229 | endothelin receptor type B, EDNRB | −768 |
| 230 | cholinergic receptor, muscarinic 2, CHRM2 | −1049 |
3.9. Motor Neurons
Motor neurons are nerve cells forming part of a pathway along which impulses pass from the brain or spinal cord to a muscle or gland.
Here we searched Gene Ontology descriptions for “motor neuron”. See Table 13 and Table 14.
Table 13.
Distribution of Genes Affected by GHK and Associated with Motor Neurons.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 9 | 5 |
| 100%–199% | 2 | 0 |
| 200%–299% | 2 | 1 |
| 300%–399% | 0 | 0 |
| 400%–499% | 0 | 2 |
| 500%+ | 0 | 1 |
| Total | 13 | 9 |
Table 14.
GHK and Genes Associate with Motor Neurons.
| UP | Gene Title | Percent Change |
| 1 | calcium channel, voltage-dependent, P/Q type, alpha 1A subunit, CACNA1A | 286 |
| 2 | plexin C1, PLXNC1 | 282 |
| 3 | GLI family zinc finger 2, GLI2 | 183 |
| 4 | NK2 homeobox 2, NKX2-2 | 181 |
| DOWN | Gene Title | Percent Change |
| 1 | slit homolog 1 (Drosophila), SLIT1 | −553 |
| 2 | chemokine (C-X-C motif) receptor 4, CXCR4 | −496 |
| 3 | EPH receptor A3, EPHA3 | −485 |
| 4 | sonic hedgehog, SHH | −220 |
3.10. Gene Expression—Glial Cells
Glial cells are non-neuronal cells that maintain homeostasis, form myelin, and provide support and protection for neurons in the central and peripheral nervous systems.
Here we searched Gene Ontology descriptions for “glial”. See Table 15 and Table 16.
Table 15.
Distribution of Genes Affected by GHK and Associated with Glial Cells.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 11 | 4 |
| 100%–199% | 7 | 3 |
| 200%–299% | 4 | 4 |
| 300%–399% | 2 | 1 |
| 400%–499% | 0 | 1 |
| 500%+ | 0 | 2 |
| Total | 24 | 15 |
Table 16.
GHK and Genes Associated with Glial Cells.
| UP | Gene Title | Percent Change |
| 1 | neurogenic differentiation 4, NEUROD4 | 362 |
| 2 | growth associated protein 43, GAP43 | 305 |
| 3 | nuclear factor I/B, NFIB | 279 |
| 4 | caspase 1, apoptosis-related cysteine peptidase (interleukin 1, beta, convertase), CASP1 | 257 |
| 5 | Kruppel-like factor 15, KLF15 | 238 |
| 6 | adenylate cyclase activating polypeptide 1 (pituitary), ADCYAP1 | 215 |
| 7 | neuregulin 1, NRG1 | 164 |
| 8 | versican, VCAN | 134 |
| 9 | protein kinase C, eta, PRKCH | 124 |
| 10 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4, SMARCA4 | 107 |
| 11 | chemokine (C-X3-C motif) receptor 1, CX3CR1 | 104 |
| 12 | achaete-scute complex homolog 1 (Drosophila), ASCL1 | 103 |
| 13 | neurofibromin 1, NF1 | 102 |
| DOWN | Gene Title | Percent Change |
| 1 | necdin homolog (mouse), NDN | −729 |
| 2 | insulin-like growth factor 1 (somatomedin C), IGF1 | −522 |
| 3 | forkhead box D4 /// forkhead box D4-like 1, FOXD4 /// FOXD4L1 | −498 |
| 4 | PTK2B protein tyrosine kinase 2 beta, PTK2B | −348 |
| 5 | pleiomorphic adenoma gene 1, PLAG1 | −276 |
| 6 | lin-28 homolog A (C. elegans), LIN28A | −259 |
| 7 | sonic hedgehog, SHH | −220 |
| 8 | forkhead box E1 (thyroid transcription factor 2), FOXE1 | −204 |
| 9 | allograft inflammatory factor 1, AIF1 | −144 |
| 10 | GDNF family receptor alpha 2, GFRA2 | −141 |
| 11 | chondroitin sulfate proteoglycan 4, CSPG4 | −113 |
3.11. Astrocyte
Astrocytes are characteristic star-shaped glial cells in the brain and spinal cord. The astrocyte proportion varies by region and ranges from 20% to 40% of all glial cells. They perform many functions, including biochemical support of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, and a role in the repair and scarring process of the brain and spinal cord following traumatic injuries.
Here we searched Gene Ontology descriptions for “astrocyte”. See Table 17 and Table 18.
Table 17.
Distribution of Gene Affected by GHK and Associated with Astrocytes.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 8 | 3 |
| 100%–199% | 5 | 2 |
| 200%–299% | 2 | 1 |
| 300%–399% | 0 | 0 |
| 400%–499% | 0 | 0 |
| 500%+ | 0 | 0 |
| Total | 15 | 6 |
Table 18.
GHK and Genes Associated with Astrocytes.
| UP | Gene Title | Percent Change |
| 1 | chemokine (C-C motif) ligand 3 /// chemokine (C-C motif) ligand 3-like 1 /// chemokine (C-C motif) ligand 3-like 3, CCL3 /// CCL3L1 /// CCL3L3 | 228 |
| 2 | inhibitor of DNA binding 4, dominant negative helix-loop-helix protein, ID4 | 203 |
| 3 | NK2 homeobox 2, NKX2-2 | 181 |
| 4 | metallothionein 3, MT3 | 142 |
| 5 | bridging integrator 1, BIN1 | 130 |
| 6 | matrix metallopeptidase 14 (membrane-inserted), MMP14 | 114 |
| 7 | neurofibromin 1, NF1 | 102 |
| DOWN | Gene Title | Percent Change |
| 1 | neurotrophic tyrosine kinase, receptor, type 3, NTRK3 | −230 |
| 2 | contactin 2 (axonal), CNTN2 | −180 |
| 3 | bone morphogenetic protein 2, BMP2 | −159 |
3.12. Schwann Cells
Schwann cells are cells of the peripheral nervous system that wrap around a nerve fiber, jelly-roll fashion, forming the myelin sheath.
Here we searched Gene Ontology descriptions for “Schwann”. See Table 19 and Table 20.
Table 19.
Distribution of Genes Affected by GHK and Associated with Schwann Cells.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 5 | 1 |
| 100%–199% | 2 | 0 |
| 200%–299% | 0 | 0 |
| 300%–399% | 1 | 1 |
| 400%–499% | 0 | 0 |
| 500%+ | 0 | 0 |
| Total | 8 | 2 |
Table 20.
GHK and Genes Associated with Schwann Cells.
| UP | Gene Title | Percent Change |
| 1 | Mediator complex subunit 12, MED12 | 393 |
| 2 | neurofibromin 2 (merlin), NF2 | 105 |
| 3 | neurofibromin 1, NF1 | 102 |
| DOWN | Gene Title | Percent Change |
| 1 | cytochrome P450, family 11, subfamily A, polypeptide 1, CYP11A1 | −393 |
3.13. Myelin
Myelin is a mixture of proteins and phospholipids that form a whitish insulating sheath around many nerve fibers, increasing the speed at which impulses are conducted.
Here we searched Gene Ontology descriptions for “myelin”. See Table 21 and Table 22.
Table 21.
Distribution of Genes Affected by GHK and Associated with Myelin.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 24 | 5 |
| 100%–199% | 8 | 8 |
| 200%–299% | 4 | 0 |
| 300%–399% | 0 | 3 |
| 400%–499% | 0 | 2 |
| 500%+ | 0 | 0 |
| Total | 36 | 18 |
Table 22.
GHK and Genes Associated with Myelin.
| UP | Gene Title | Percent Change |
| 1 | inositol 1,4,5-triphosphate receptor, type 3, ITPR3 | 298 |
| 2 | sodium channel, voltage-gated, type II, alpha subunit, SCN2A | 264 |
| 3 | myelin associated glycoprotein, MAG | 229 |
| 4 | inhibitor of DNA binding 4, dominant negative helix-loop-helix protein, ID4 | 203 |
| 5 | aspartoacylase, ASPA | 195 |
| 6 | probable transcription factor PML-like /// promyelocytic leukemia, LOC652346 /// PML | 144 |
| 7 | retinoic acid receptor, beta, RARB | 142 |
| 8 | retinoic acid receptor, alpha, RARA | 123 |
| 9 | myosin VA (heavy chain 12, myoxin), MYO5A | 122 |
| 10 | neurofibromin 1, NF1 | 102 |
| 11 | histamine receptor H3, HRH3 | 101 |
| 12 | transforming growth factor, beta 1, TGFB1 | 100 |
| DOWN | Gene Title | Percent Change |
| 1 | chemokine (C-X-C motif) receptor 4, CXCR4 | −496 |
| 2 | gap junction protein, gamma 2, 47kDa, GJC2 | −428 |
| 3 | lethal giant larvae homolog 1 (Drosophila), LLGL1 | −393 |
| 4 | myelin basic protein, MBP | −361 |
| 5 | chromosome 11 open reading frame 9, C11orf9 | −342 |
| 6 | promyelocytic leukemia, PML | −196 |
| 7 | myelin protein zero, MPZ | −180 |
| 8 | contactin 2 (axonal), CNTN2 | −180 |
| 9 | toll-like receptor 2, TLR2 | −169 |
| 10 | laminin, alpha 2, LAMA2 | −150 |
| 11 | retinoid X receptor, gamma, RXRG | −110 |
| 12 | integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12), ITGB1 | −107 |
| 13 | thyroglobulin, TG | −100 |
3.14. Gene Expression—Dendrites
Dendrites are short branched extensions of a nerve cell, along which impulses received from other cells at synapses are transmitted to the cell body.
Here we searched Gene Ontology descriptions for “dendrite”. See Table 23 and Table 24.
Table 23.
Distribution of Genes Affected by GHK and Associated with Dendrites.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 47 | 14 |
| 100%–199% | 19 | 31 |
| 200%–299% | 11 | 15 |
| 300%–399% | 8 | 3 |
| 400%–499% | 0 | 3 |
| 500%+ | 2 | 2 |
| Total | 87 | 68 |
Table 24.
GHK and Genes Associated with Dendrites.
| UP | Gene Title | Percent Change |
| 1 | potassium voltage-gated channel, Shal-related subfamily, member 1, KCND1 | 845 |
| 2 | contactin associated protein-like 2, CNTNAP2 | 581 |
| 3 | leukocyte specific transcript 1, LST1 | 395 |
| 4 | gamma-aminobutyric acid (GABA) A receptor, alpha 5 /// gamma-aminobutyric acid receptor subunit alpha-5-like, GABRA5 /// LOC100509612 | 392 |
| 5 | chemokine (C-C motif) ligand 19, CCL19 | 378 |
| 6 | doublecortin-like kinase 1, DCLK1 | 365 |
| 7 | p21 protein (Cdc42/Rac)-activated kinase 1, PAK1 | 363 |
| 8 | potassium voltage-gated channel, Shaw-related subfamily, member 3, KCNC3 | 332 |
| 9 | EPH receptor B1, EPHB1 | 330 |
| 10 | gamma-aminobutyric acid (GABA) B receptor, 1 /// ubiquitin D, GABBR1 /// UBD | 310 |
| 11 | calcium channel, voltage-dependent, P/Q type, alpha 1A subunit, CACNA1A | 286 |
| 12 | nephroblastoma overexpressed gene, NOV | 275 |
| 13 | obscurin-like 1, OBSL1 | 263 |
| 14 | neuroligin 1, NLGN1 | 261 |
| 15 | low density lipoprotein receptor-related protein 1, LRP1 | 249 |
| 16 | glutamate receptor, ionotropic, kainate 3, GRIK3 | 246 |
| 17 | RNA binding protein, fox-1 homolog (C. elegans) 2, RBFOX2 | 245 |
| 18 | glutamate receptor, metabotropic 1, GRM1 | 231 |
| 19 | glutamate receptor interacting protein 1, GRIP1 | 230 |
| 20 | glutamate receptor, ionotropic, N-methyl d-aspartate 1, GRIN1 | 216 |
| 21 | MCF.2 cell line derived transforming sequence, MCF2 | 202 |
| 22 | purinergic receptor P2X, ligand-gated ion channel, 4, P2RX4 | 180 |
| 23 | synapsin I, SYN1 | 170 |
| 24 | Abl-interactor 2, ABI2 | 168 |
| 25 | calcium channel, voltage-dependent, L type, alpha 1F subunit, CACNA1F | 168 |
| 26 | membrane associated guanylate kinase, WW and PDZ domain containing 2, MAGI2 | 155 |
| 27 | ubiquitin-conjugating enzyme E2I (UBC9 homolog, yeast), UBE2I | 150 |
| 28 | nuclear mitotic apparatus protein 1, NUMA1 | 147 |
| 29 | glutamate receptor, ionotropic, N-methyl d-aspartate 2C, GRIN2C | 146 |
| 30 | probable transcription factor PML-like /// promyelocytic leukemia, LOC652346 /// PML | 144 |
| 31 | fasciculation and elongation protein zeta 1 (zygin I), FEZ1 | 143 |
| 32 | glutamate receptor, metabotropic 7, GRM7 | 140 |
| 33 | acetylcholinesterase, ACHE | 131 |
| 34 | retinoic acid receptor, alpha, RARA | 123 |
| 35 | misshapen-like kinase 1, MINK1 | 119 |
| 36 | kelch-like 1 (Drosophila), KLHL1 | 119 |
| 37 | neuralized homolog (Drosophila), NEURL | 115 |
| 38 | protein kinase C, gamma, PRKCG | 106 |
| 39 | drebrin 1, DBN1 | 103 |
| 40 | neurofibromin 1, NF1 | 102 |
| DOWN | Gene Title | Percent Change |
| 1 | bassoon (presynaptic cytomatrix protein), BSN | −563 |
| 2 | membrane metallo-endopeptidase, MME | −540 |
| 3 | adenylate cyclase 10 (soluble), ADCY10 | −460 |
| 4 | discs, large homolog 4 (Drosophila), DLG4 | −452 |
| 5 | Kv channel interacting protein 1, KCNIP1 | −413 |
| 6 | EPH receptor A7, EPHA7 | −365 |
| 7 | PTK2B protein tyrosine kinase 2 beta, PTK2B | −348 |
| 8 | sterile alpha motif domain containing 4A, SAMD4A | −315 |
| 9 | dopamine receptor D4, DRD4 | −296 |
| 10 | FEZ family zinc finger 2, FEZF2 | −295 |
| 11 | calcium channel, voltage-dependent, N type, alpha 1B subunit, CACNA1B | −290 |
| 12 | mitogen-activated protein kinase 8 interacting protein 1, MAPK8IP1 | −289 |
| 13 | regulator of G-protein signaling 11, RGS11 | −266 |
| 14 | cyclin-dependent kinase 5, regulatory subunit 1 (p35), CDK5R1 | −260 |
| 15 | glutamate receptor, ionotropic, kainate 1, GRIK1 | −254 |
| 16 | thyroid hormone receptor, alpha (erythroblastic leukemia viral (v-erb-a) oncogene homolog, avian), THRA | −253 |
| 17 | cyclic nucleotide gated channel alpha 3, CNGA3 | −249 |
| 18 | adenylate cyclase 2 (brain), ADCY2 | −247 |
| 19 | proprotein convertase subtilisin/kexin type 2, PCSK2 | −242 |
| 20 | Rho guanine nucleotide exchange factor (GEF) 15, ARHGEF15 | −230 |
| 21 | potassium voltage-gated channel, Shal-related subfamily, member 3, KCND3 | −224 |
| 22 | protein tyrosine phosphatase, receptor type, D, PTPRD | −221 |
| 23 | cytochrome b-245, beta polypeptide, CYBB | −217 |
| 24 | GABA(A) receptors associated protein like 3, pseudogene, GABARAPL3 | −197 |
| 25 | neutrophil cytosolic factor 1C pseudogene, NCF1C | −196 |
| 26 | promyelocytic leukemia, PML | −196 |
| 27 | C-reactive protein, pentraxin-related, CRP | −182 |
| 28 | glutamate receptor, ionotropic, N-methyl d-aspartate 2A, GRIN2A | −180 |
| 29 | tubby like protein 1, TULP1 | −176 |
| 30 | Mitogen-activated protein kinase 8 interacting protein 3, MAPK8IP3 | −174 |
| 31 | cell adhesion molecule with homology to L1CAM (close homolog of L1), CHL1 | −171 |
| 32 | choline O-acetyltransferase, CHAT | −160 |
| 33 | glutamate receptor, ionotropic, kainate 5, GRIK5 | −159 |
| 34 | glutamate receptor, ionotropic, kainate 4, GRIK4 | −155 |
| 35 | 5-hydroxytryptamine (serotonin) receptor 6, HTR6 | −150 |
| 36 | tachykinin receptor 3, TACR3 | −150 |
| 37 | 5-hydroxytryptamine (serotonin) receptor 5A, HTR5A | −149 |
| 38 | protease, serine, 12 (neurotrypsin, motopsin), PRSS12 | −141 |
| 39 | cholinergic receptor, nicotinic, alpha 4, CHRNA4 | −139 |
| 40 | 5-hydroxytryptamine (serotonin) receptor 2A, HTR2A | −135 |
| 41 | leucine zipper, putative tumor suppressor 1, LZTS1 | −130 |
| 42 | neuroligin 4, X-linked, NLGN4X | −128 |
| 43 | glutamate receptor, ionotrophic, AMPA 3, GRIA3 | −126 |
| 44 | glutamate receptor, metabotropic 6, GRM6 | −120 |
| 45 | paralemmin, PALM | −115 |
| 46 | copine VI (neuronal), CPNE6 | −114 |
| 47 | cytoplasmic polyadenylation element binding protein 3, CPEB3 | −112 |
| 48 | corticotropin releasing hormone receptor 1, CRHR1 | −109 |
| 49 | doublecortin, DCX | −108 |
| 50 | regulator of G-protein signaling 14, RGS14 | −108 |
| 51 | apolipoprotein E, APOE | −107 |
| 52 | calcium binding protein 1, CABP1 | −106 |
| 53 | mitogen-activated protein kinase 8 interacting protein 2, MAPK8IP2 | −103 |
| 54 | neurochondrin, NCDN | −102 |
3.15. Gene Expression—Oligodendrocytes
Oligodendrocytes are glial cells similar to astrocytes, but with fewer protuberances, which are concerned with the production of myelin in the central nervous system.
Here we searched Gene Ontology descriptions for “oligodendrocyte”. See Table 25 and Table 26.
Table 25.
Distribution of Genes Affected by GHK and Associated with Oligodendrocytes.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 6 | 4 |
| 100%–199% | 6 | 3 |
| 200%–299% | 3 | 1 |
| 300%–399% | 0 | 1 |
| 400%–499% | 0 | 1 |
| 500%+ | 1 | 0 |
| Total | 16 | 10 |
Table 26.
GHK and Genes Associated with Oligodendrocytes.
| UP | Gene Title | Percent Change |
| 1 | tumor protein p73, TP73 | 938 |
| 2 | adenylate cyclase activating polypeptide 1 (pituitary), ADCYAP1 | 215 |
| 3 | gelsolin, GSN | 214 |
| 4 | inhibitor of DNA binding 4, dominant negative helix-loop-helix protein, ID4 | 203 |
| 5 | aspartoacylase, ASPA | 195 |
| 6 | NK2 homeobox 2, NKX2-2 | 181 |
| 7 | dopamine receptor D3, DRD3 | 164 |
| 8 | histone deacetylase 11, HDAC11 | 105 |
| 9 | achaete-scute complex homolog 1 (Drosophila), ASCL1 | 103 |
| 10 | neurofibromin 1, NF1 | 102 |
| DOWN | Gene Title | Percent Change |
| 1 | chemokine (C-X-C motif) receptor 4, CXCR4 | −496 |
| 2 | chromosome 11 open reading frame 9, C11orf9 | −342 |
| 3 | sonic hedgehog, SHH | −220 |
| 4 | zinc finger protein 287, ZNF287 | −143 |
| 5 | early growth response 1, EGR1 | −121 |
| 6 | apolipoprotein E, APOE | −107 |
3.16. Gene Expression—Sensory Nerve cells
Sensory neurons are nerves that transmit sensory information (sight, sound, feeling, etc.). They are activated by sensory input and send projections to other elements of the nervous system, ultimately conveying sensory information to the brain or spinal cord.
Here we searched Gene Ontology descriptions for “sensory”. See Table 27 and Table 28.
Table 27.
Distribution of Genes Affected by GHK and Associated with Sensory Nerve Cells.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 45 | 25 |
| 100%–199% | 24 | 36 |
| 200%–299% | 18 | 6 |
| 300%–399% | 7 | 1 |
| 400%–499% | 1 | 3 |
| 500%+ | 2 | 4 |
| Total | 97 | 75 |
Table 28.
GHK and Gene Associate with Sensory Nerve Cells.
| UP | Gene Title | Percent Change |
| 1 | opioid receptor, mu 1, OPRM1 | 1294 |
| 2 | T-box 1, TBX1 | 553 |
| 3 | adrenergic, beta-1-, receptor, ADRB1 | 477 |
| 4 | gamma-aminobutyric acid (GABA) A receptor, alpha 5 /// gamma-aminobutyric acid receptor subunit alpha-5-like, GABRA5 /// LOC100509612 | 392 |
| 5 | calcium channel, voltage-dependent, L type, alpha 1D subunit, CACNA1D | 372 |
| 6 | olfactory receptor, family 2, subfamily W, member 1, OR2W1 | 370 |
| 7 | guanine nucleotide binding protein (G protein), alpha activating activity polypeptide, olfactory type, GNAL | 366 |
| 8 | olfactory receptor, family 2, subfamily B, member 6, OR2B6 | 345 |
| 9 | cyclic nucleotide gated channel beta 1, CNGB1 | 330 |
| 10 | EPH receptor B1, EPHB1 | 330 |
| 11 | inositol 1,4,5-triphosphate receptor, type 3, ITPR3 | 298 |
| 12 | olfactory receptor, family 7, subfamily A, member 17, OR7A17 | 285 |
| 13 | nuclear factor I/B, NFIB | 279 |
| 14 | islet amyloid polypeptide, IAPP | 276 |
| 15 | opiate receptor-like 1, OPRL1 | 246 |
| 16 | potassium voltage-gated channel, KQT-like subfamily, member 4, KCNQ4 | 245 |
| 17 | myosin, heavy chain 14, non-muscle, MYH14 | 243 |
| 18 | taste receptor, type 2, member 13, TAS2R13 | 237 |
| 19 | olfactory receptor, family 2, subfamily F, member 2, OR2F2 | 232 |
| 20 | glutamate receptor, metabotropic 1, GRM1 | 231 |
| 21 | chemokine (C-C motif) ligand 3 /// chemokine (C-C motif) ligand 3-like 1 /// chemokine (C-C motif) ligand 3-like 3, CCL3 /// CCL3L1 /// CCL3L3 | 228 |
| 22 | polycystic kidney disease 2-like 1, PKD2L1 | 225 |
| 23 | glutamate receptor, ionotropic, N-methyl d-aspartate 1, GRIN1 | 216 |
| 24 | adenylate cyclase activating polypeptide 1 (pituitary), ADCYAP1 | 215 |
| 25 | ATPase, Ca++ transporting, plasma membrane 2, ATP2B2 | 214 |
| 26 | olfactory receptor, family 7, subfamily C, member 1, OR7C1 | 207 |
| 27 | purinergic receptor P2X, ligand-gated ion channel, 3, P2RX3 | 207 |
| 28 | neuropeptide Y receptor Y1, NPY1R | 201 |
| 29 | family with sequence similarity 38, member B, FAM38B | 193 |
| 30 | olfactory receptor, family 1, subfamily A, member 1, OR1A1 | 189 |
| 31 | taste receptor, type 2, member 14, TAS2R14 | 181 |
| 32 | purinergic receptor P2X, ligand-gated ion channel, 4, P2RX4 | 180 |
| 33 | receptor accessory protein 2, REEP2 | 174 |
| 34 | endothelin receptor type A, EDNRA | 173 |
| 35 | cannabinoid receptor 1 (brain), CNR1 | 172 |
| 36 | melanocortin 1 receptor (alpha melanocyte stimulating hormone receptor), MC1R | 164 |
| 37 | olfactory receptor, family 12, subfamily D, member 3 /// olfactory receptor, family 5, subfamily V, member 1, OR12D3 /// OR5V1 | 163 |
| 38 | odorant binding protein 2A /// odorant binding protein 2B, OBP2A /// OBP2B | 162 |
| 39 | prepronociceptin, PNOC | 150 |
| 40 | phospholipase C, beta 2, PLCB2 | 148 |
| 41 | glutamate receptor, metabotropic 7, GRM7 | 140 |
| 42 | oxytocin, prepropeptide, OXT | 136 |
| 43 | WD repeat domain 1, WDR1 | 127 |
| 44 | olfactory receptor, family 1, subfamily D, member 4 (gene/pseudogene) /// olfactory receptor, family 1, subfamily D, member 5, OR1D4 /// OR1D5 | 125 |
| 45 | UDP glucuronosyltransferase 2 family, polypeptide A1 /// UDP glucuronosyltransferase 2 family, polypeptide A2, UGT2A1 /// UGT2A2 | 121 |
| 46 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase), PTGS2 | 120 |
| 47 | taste receptor, type 2, member 4, TAS2R4 | 118 |
| 48 | lysozyme, LYZ | 111 |
| 49 | protein kinase C, gamma, PRKCG | 106 |
| 50 | collagen, type XI, alpha 1, COL11A1 | 103 |
| 51 | POU class 4 homeobox 3, POU4F3 | 102 |
| 52 | nuclear receptor subfamily 2, group F, member 6, NR2F6 | 100 |
| DOWN | Gene Title | Percent Change |
| 1 | taste receptor, type 2, member 9, TAS2R9 | −1494 |
| 2 | endothelin receptor type B, EDNRB | −768 |
| 3 | necdin homolog (mouse), NDN | −729 |
| 4 | membrane metallo-endopeptidase, MME | −540 |
| 5 | EPH receptor A3, EPHA3 | −485 |
| 6 | arachidonate lipoxygenase 3, ALOXE3 | −461 |
| 7 | bradykinin receptor B1, BDKRB1 | −426 |
| 8 | gap junction protein, beta 4, 30.3kDa, GJB4 | −317 |
| 9 | nerve growth factor (beta polypeptide), NGF | −243 |
| 10 | guanine nucleotide binding protein (G protein), alpha transducing activity polypeptide 1, GNAT1 | −242 |
| 11 | olfactory receptor, family 3, subfamily A, member 1, OR3A1 | −234 |
| 12 | apelin receptor, APLNR | −230 |
| 13 | olfactory receptor, family 2, subfamily F, member 1 /// olfactory receptor, family 2, subfamily F, member 2, OR2F1 /// OR2F2 | −212 |
| 14 | olfactory receptor, family 12, subfamily D, member 3, OR12D3 | −201 |
| 15 | olfactory receptor, family 6, subfamily A, member 2, OR6A2 | −199 |
| 16 | cholecystokinin B receptor, CCKBR | −198 |
| 17 | carbonic anhydrase VI, CA6 | −192 |
| 18 | olfactory receptor, family 5, subfamily I, member 1, OR5I1 | −191 |
| 19 | collagen, type XI, alpha 2, COL11A2 | −186 |
| 20 | olfactory receptor, family 10, subfamily H, member 3, OR10H3 | −182 |
| 21 | glutamate receptor, ionotropic, N-methyl D-aspartate 2A, GRIN2A | −180 |
| 22 | protein phosphatase, EF-hand calcium binding domain 2, PPEF2 | −178 |
| 23 | sodium channel, nonvoltage-gated 1 alpha, SCNN1A | −175 |
| 24 | trace amine associated receptor 5, TAAR5 | −168 |
| 25 | gastric inhibitory polypeptide, GIP | −164 |
| 26 | olfactory receptor, family 2, subfamily H, member 1, OR2H1 | −156 |
| 27 | olfactory receptor, family 2, subfamily J, member 2, OR2J2 | −155 |
| 28 | otoferlin, OTOF | −155 |
| 29 | discs, large homolog 2 (Drosophila), DLG2 | −142 |
| 30 | cholinergic receptor, nicotinic, alpha 4, CHRNA4 | −139 |
| 31 | 5-hydroxytryptamine (serotonin) receptor 2A, HTR2A | −135 |
| 32 | tectorin alpha, TECTA | −126 |
| 33 | sodium channel, voltage-gated, type XI, alpha subunit, SCN11A | −124 |
| 34 | olfactory receptor, family 7, subfamily C, member 2, OR7C2 | −120 |
| 35 | taste receptor, type 2, member 16, TAS2R16 | −120 |
| 36 | glutamate receptor, metabotropic 6, GRM6 | −120 |
| 37 | opioid receptor, kappa 1, OPRK1 | −119 |
| 38 | ATPase, H+ transporting, lysosomal 56/58kDa, V1 subunit B1, ATP6V1B1 | −118 |
| 39 | olfactory marker protein, OMP | −118 |
| 40 | contactin 5, CNTN5 | −116 |
| 41 | cysteinyl leukotriene receptor 2, CYSLTR2 | −113 |
| 42 | olfactory receptor, family 2, subfamily H, member 2, OR2H2 | −110 |
| 43 | rhodopsin, RHO | −108 |
| 44 | interleukin 10, IL10 | −107 |
| 45 | olfactory receptor, family 11, subfamily A, member 1, OR11A1 | −107 |
| 46 | polymeric immunoglobulin receptor, PIGR | −107 |
| 47 | guanine nucleotide binding protein (G protein), gamma 13, GNG13 | −106 |
| 48 | tubby homolog (mouse), TUB | −101 |
| 49 | glutamate receptor, metabotropic 8, GRM8 | −101 |
| 50 | cystatin S, CST4 | −101 |
3.17. Spinal Nerve Cells
Spinal nerve cells transfer information, which travels down the spinal cord, as a conduit for sensory information in the reverse direction, and finally as a center for coordinating certain reflexes.
Here we searched Gene Ontology descriptions for “spinal”. See Table 29 and Table 30.
Table 29.
Distribution of Genes Affected by GHK and Associated with Spinal Nerve Cells.
| Percent Change in Gene Expression | Genes UP | Genes DOWN |
|---|---|---|
| 50%–99% | 8 | 6 |
| 100%–199% | 9 | 3 |
| 200%–299% | 1 | 2 |
| 300%–399% | 0 | 1 |
| 400%–499% | 1 | 0 |
| 500%+ | 1 | 1 |
| Total | 20 | 13 |
Table 30.
GHK and Genes Associated with Spinal Nerve Cells.
| UP | Gene Title | Percent Change |
| 1 | tumor protein p73, TP73 | 938 |
| 2 | smoothened homolog (Drosophila), SMO | 415 |
| 3 | calcium channel, voltage-dependent, P/Q type, alpha 1A subunit, CACNA1A | 286 |
| 4 | GATA binding protein 2, GATA2 | 193 |
| 5 | GLI family zinc finger 2, GLI2 | 183 |
| 6 | NK2 homeobox 2, NKX2-2 | 181 |
| 7 | dopamine receptor D3, DRD3 | 164 |
| 8 | paired box 7, PAX7 | 161 |
| 9 | slit homolog 3 (Drosophila), SLIT3 | 154 |
| 10 | polycystic kidney disease 1 (autosomal dominant), PKD1 | 137 |
| 11 | achaete-scute complex homolog 1 (Drosophila), ASCL1 | 103 |
| 12 | neurofibromin 1, NF1 | 102 |
| DOWN | Gene Title | Percent Change |
| 1 | slit homolog 1 (Drosophila), SLIT1 | −553 |
| 2 | SRY (sex determining region Y)-box 1, SOX1 | −337 |
| 3 | growth differentiation factor 11, GDF11 | −221 |
| 4 | sonic hedgehog, SHH | −220 |
| 5 | glutamate receptor, ionotropic, N-methyl d-aspartate 2A, GRIN2A | −180 |
| 6 | even-skipped homeobox 1, EVX1 | −110 |
| 7 | aquaporin 1 (Colton blood group), AQP1 | −101 |
4. Possible Methods of Therapeutic Use of GHK for Nerve Diseases
4.1. Mode of Administering GHK-Cu to Patients
4.1.1. Skin Cream or Patch
GHK-Cu has an unexpectedly rapid passage through skin’s stratum corneum. When tested by Howard Maibach’s group (Univerisity of California at San Francisco), 0.68% GHK-Cu was applied to dermatomed skin. Over 48 h, 136 micrograms of GHK-Cu passed through the skin per centimeter squared. This is a significant amount of GHK-Cu, and a transdermal patch of a several centimeters squared may pass therapeutically effective amounts throughout the human body [101].
Russian studies reported that 0.5 micrograms/kg reduced anxiety in rats. Scaled up for a human weight of 70 kg, this would be 35 micrograms in a human [52]. Our studies on activation of systemic healing in mice, rats, and pigs suggest that about 50 milligrams of GHK-Cu would be effective throughout the human body, although dose-ranging to determine the minimum active dosage was never performed.
4.1.2. Liposomal Encapsulated Oral Tablet
Alternately, the use of encapsulated liposomal GHK-Cu would allow its oral administration at relatively high dosages. Some sellers of an encapsulated liposomal tripeptide glutathione claim that 60% of the orally administrated peptide enters the human blood stream [102]. Direct administration in a regular pill form is unlikely to work because of GHK’s extreme sensitivity to breakdown by intestinal carboxypeptidase [103].
GHK-Cu costs about $8/gram in kilogram amounts. For a 50 mg dosage, the GHK-Cu would cost about $0.40. It is possible that GHK alone would be effective in humans and be able to obtain sufficient amounts of copper 2+ from albumin. If so, this would simplify its therapeutic use. The minimum effective dosage of GHK-Cu for various uses is unknown since such studies were never performed.
GHK-Cu does lower blood pressure, but the LD50 (Lethal Dose for 50% of mice) for such effects would be about a single dosage of 23,000 mgs of GHK-Cu in a 70 kg human. In GHK-Cu’s long history of use in cosmetics, no health issues have ever arisen. We were never able to find an LD 50 for GHK without copper.
In our studies, equimolar mixtures of GHK-Cu and GHK (no copper) are often used to avoid any release of loosely bound copper. Also, copper chelators such as penicillamine have been reported to cause psychosis in humans [104].
5. Conclusions
Given all the failed attempts to develop effective treatment methods for nerve degeneration, it is suggested that researchers must take a very broad view of the possible factors causing neurodegenerative diseases and not focus on limited possible causes. It is sensible to concentrate research efforts on the reversion of affected tissues to a healthier condition more characteristic of younger humans. GHK gene studies have increasingly led to the conclusion that the conditions and diseases of aging cannot be scientifically treated without understanding the extensive changes in overall gene activity during aging.
There are three sources of evidence on GHK actions:
The best data is in vivo mammalian data, including human clinical studies. As reviewed in this paper, these studies give overwhelming evidence of GHK’s effects on cells and tissue growth, as well as anti-cancer, anti-oxidant, wound-healing, anti-inflammation, anti-pain, anti-anxiety and skin regeneration actions.
A second form of data is in vitro cell culture and organ culture results. Culture results give evidence about the effect of GHK on cellular production of collagen and other structural proteins, the effect on stem cell function, the recovery of cellular function after anticancer radiation or ultraviolet radiation, and sensitivity of cells to oxidative molecules.
A third source of data is in Human Gene expression. Data analysis found that GHK induces a 50% or greater (plus or minus) change of expression in 31.2% of human genes, affecting genes linked to multiple biochemical pathways in many organs and tissue, including the nervous system.
Many studies highlight gene expression effects of various molecules. Given today’s advances in computer modeling, it is not that difficult to find substances which affect gene expression in one way or another. However, in most cases, computer-based predictions do not have the same supporting evidence of in vivo and in vitro laboratory data as GHK has. Also, in many cases, the safety and cost of the proposed treatments are a big concern. GHK is safe, inexpensive, and can be used in humans today.
The future research should be focused on further making sense of the very extensive gene data, which has to be paralleled with laboratory and clinical studies. GHK has a wealth of biological data in the areas of wound healing, hair and skin regeneration, intestinal tract and bone repair. However, there is a surprising lack of GHK research in the area of neurodegeneration and cognitive health. We hope that our gene data will encourage researchers to take a better look at biological actions and significance of GHK in connection with cognitive health and nervous system function.
The best administration method, in our opinion, would be GHK-Cu incorporated into liposomes, then administered as an enteric capsule for oral use. A dosage of 10 mgs per dose would be a good starting point, at least for safety studies, but inducing positive actions will most likely require a higher dosage.
Acknowledgments
We would like to thank Germaine Emilie Pugh and Cassia McClain for their invaluable work in the manuscript preparation.
Author Contributions
The authors have equally contributed to the writing and revision of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cummings J.L., Morstorf T., Zhong K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickart L. Ph.D. Thesis. University of California; San Francisco, CA, USA: 1973. A Tripeptide from Human Serum Which Enhances the Growth of Neoplastic Hepatocytes and the Survival of Normal Hepatocytes. [Google Scholar]
- 3.Pickart L., Freedman J.H., Loker W.J., Peisach J., Perkins C.M., Stenkamp R.E., Weinstein B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature. 1980;288:715–717. doi: 10.1038/288715a0. [DOI] [PubMed] [Google Scholar]
- 4.Pesakova V., Novotna J., Adam M. Effect of the tripeptide glycyl-l-histidyl-l-lysine on the proliferation and synthetic activity of chick embryo chondrocytes. Biomaterials. 1995;16:911–915. doi: 10.1016/0142-9612(95)93115-T. [DOI] [PubMed] [Google Scholar]
- 5.Pollard J.D., Quan S., Kang T., Koch R.J. Effects of copper tripeptide on the growth and expression of growth factors by normal and irradiated fibroblasts. Arch. Facial Plast. Surg. 2005;7:27–31. doi: 10.1001/archfaci.7.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Kang Y.A., Choi H.R., Na J.I., Huh C.H., Kim M.J., Youn S.W., Kim K.H., Park K.C. Copper-GHK increases integrin expression and p63 positivity by keratinocytes. Arch. Dermatol. Res. 2009;301:301–306. doi: 10.1007/s00403-009-0942-x. [DOI] [PubMed] [Google Scholar]
- 7.Pickart L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008;19:969–988. doi: 10.1163/156856208784909435. [DOI] [PubMed] [Google Scholar]
- 8.Gul N.Y., Topal A., Cangul I.T., Yanik K. The effects of topical tripeptide copper complex and helium-neon laser on wound healing in rabbits. Vet. Dermatol. 2008;19:7–14. doi: 10.1111/j.1365-3164.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 9.Arul V., Gopinath D., Gomathi K., Jayakumar R. Biotinylated GHK peptide incorporated collagenous matrix: A novel biomaterial for dermal wound healing in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73:383–391. doi: 10.1002/jbm.b.30246. [DOI] [PubMed] [Google Scholar]
- 10.Swaim S.F., Vaughn D.M., Kincaid S.A., Morrison N.E., Murray S.S., Woodhead M.A., Hoffman C.E., Wright J.C., Kammerman J.R. Effect of locally injected medications on healing of pad wounds in dogs. Am. J. Vet. Res. 1996;57:394–399. [PubMed] [Google Scholar]
- 11.Hong Y., Downey T., Eu K.W., Koh P.K., Cheah P.Y. A “metastasis-prone” signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin. Exp. Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 12.Campbell J.D., McDonough J.E., Zeskind J.E., Hackett T.L., Pechkovsky D.V., Brandsma C.A., Suzuki M., Gosselink J.V., Liu G., Alekseyev Y.O., et al. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med. 2012;4:67. doi: 10.1186/gm367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickart L., Vasquez-Soltero J.M., Margolina A. GHK peptide as a natural modulator of multiple cellular pathways in skin regeneration. Biomed. Res. Int. 2015;2015:648108. doi: 10.1155/2015/648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickart L., Vasquez-Soltero J.M., Margolina A. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: Implications for cognitive health. Oxid. Med. Cell. Longev. 2012;2012:324832. doi: 10.1155/2012/324832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickart L., Vasquez-Soltero J.M., Margolina A. Resetting Skin Genome Back to Health Naturally with GHK. In: Farage M.A., Miller K.W., Maibach H.I., editors. Textbook of Aging Skin. Springer; Berlin/Heidelberg, Germany: 2015. pp. 1–19. [Google Scholar]
- 16.National Center of Biotechnology Information Gene. [(accessed on 14 February 2017)]; Available online: https://www.ncbi.nlm.nih.gov/gene.
- 17.Pickart L., Vasquez-Soltero J.M., Pickart F.D., Majnarich J. GHK, the human skin remodeling peptide, induces anti-cancer expression of numerous caspase, growth regulatory, and DNA repair genes. J. Anal. Oncol. 2014;3:79–87. doi: 10.6000/1927-7229.2014.03.02.2. [DOI] [Google Scholar]
- 18.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuszynski M.H., Yang J.H., Barba D., U H.S., Bakay R.A., Pay M.M., Masliah E., Conner J.M., Kobalka P., Roy S., et al. Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer disease. JAMA Neurol. 2015;72:1139–1147. doi: 10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Ma Q., Yang W., Qi X., Yao Z., Liu Y., Liang L., Wang X., Ma C., Huang L., et al. Recombinant DNA vaccine against neurite outgrowth inhibitors attenuates behavioral deficits and decreases Abeta in an Alzheimer’s disease mouse model. Neuropharmacology. 2013;70:200–210. doi: 10.1016/j.neuropharm.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Hu S., Cui W., Mak S., Xu D., Hu Y., Tang J., Choi C., Lee M., Pang Y., Han Y. Substantial neuroprotective and neurite outgrowth-promoting activities by bis(propyl)-cognitin via the activation of alpha7-nAChR, a promising anti-Alzheimer’s dimer. ACS Chem. Neurosci. 2015;6:1536–1545. doi: 10.1021/acschemneuro.5b00108. [DOI] [PubMed] [Google Scholar]
- 22.Sensenbrenner M., Jaros G.G., Moonen G., Mandel P. Effects of synthetic tripeptide on the differentiation of dissociated cerebral hemisphere nerve cells in culture. Neurobiology. 1975;5:207–213. [PubMed] [Google Scholar]
- 23.Lindner G., Grosse G., Halle W., Henklein P. The effect of a synthetic tripeptide nervous tissue cultured in vitro. Z Mikrosk Anat Forsch. 1979;93:820–828. [PubMed] [Google Scholar]
- 24.Ahmed M.R., Basha S.H., Gopinath D., Muthusamy R., Jayakumar R. Initial upregulation of growth factors and inflammatory mediators during nerve regeneration in the presence of cell adhesive peptide-incorporated collagen tubes. J. Peripher. Nerv. Syst. 2005;10:17–30. doi: 10.1111/j.1085-9489.2005.10105.x. [DOI] [PubMed] [Google Scholar]
- 25.Zucconi G.G., Cipriani S., Scattoni R., Balgkouranidou I., Hawkins D.P., Ragnarsdottir K.V. Copper deficiency elicits glial and neuronal response typical of neurodegenerative disorders. Neuropathol. Appl. Neurobiol. 2007;33:212–225. doi: 10.1111/j.1365-2990.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 26.Exley C. Aluminium and iron, but neither copper nor zinc, are key to the precipitation of beta-sheets of Abeta_{42} in senile plaque cores in Alzheimer’s disease. J. Alzheimers Dis. 2006;10:173–177. doi: 10.3233/jad-2006-102-305. [DOI] [PubMed] [Google Scholar]
- 27.Exley C., House E., Polwart A., Esiri M.M. Brain burdens of aluminum, iron, and copper and their relationships with amyloid-beta pathology in 60 human brains. J. Alzheimers Dis. 2012;31:725–730. doi: 10.3233/JAD-2012-120766. [DOI] [PubMed] [Google Scholar]
- 28.Mold M., Ouro-Gnao L., Wieckowski B.M., Exley C. Copper prevents amyloid-beta(1–42) from forming amyloid fibrils under near-physiological conditions in vitro. Sci. Rep. 2013;3:1256. doi: 10.1038/srep01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J., Begley P., Church S.J., Patassini S., McHarg S., Kureishy N., Hollywood K.A., Waldvogel H.J., Liu H., Zhang S., et al. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: Metabolic basis for dementia. Sci. Rep. 2016;6:27524. doi: 10.1038/srep27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajonk F.G., Kessler H., Supprian T., Hamzei P., Bach D., Schweickhardt J., Herrmann W., Obeid R., Simons A., Falkai P., et al. Cognitive decline correlates with low plasma concentrations of copper in patients with mild to moderate Alzheimer’s disease. J. Alzheimers Dis. 2005;8:23–27. doi: 10.3233/jad-2005-8103. [DOI] [PubMed] [Google Scholar]
- 31.Kumar N., Gross J.B., Jr., Ahlskog J.E. Copper deficiency myelopathy produces a clinical picture like subacute combined degeneration. Neurology. 2004;63:33–39. doi: 10.1212/01.WNL.0000132644.52613.FA. [DOI] [PubMed] [Google Scholar]
- 32.Weihl C.C., Lopate G. Motor neuron disease associated with copper deficiency. Muscle Nerve. 2006;34:789–793. doi: 10.1002/mus.20631. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Lazcano J.C., Montes S., Sanchez-Mendoza M.A., Rodriguez-Paez L., Perez-Neri I., Boll M.C., Campos-Arroyo H.D., Rios C., Perez-Severiano F. Sub-chronic copper pretreatment reduces oxidative damage in an experimental Huntington’s disease model. Biol. Trace Elem. Res. 2014;162:211–218. doi: 10.1007/s12011-014-0127-0. [DOI] [PubMed] [Google Scholar]
- 34.Deloncle R., Guillard O. Is brain copper deficiency in Alzheimer’s, Lewy body, and Creutzfeldt Jakob diseases the common key for a free radical mechanism and oxidative stress-induced damage? J. Alzheimers Dis. 2015;43:1149–1156. doi: 10.3233/JAD-140765. [DOI] [PubMed] [Google Scholar]
- 35.Zou J., Kajita K., Sugimoto N. Cu(2+) Inhibits the Aggregation of Amyloid beta-Peptide(1–42) in vitro. Angew. Chem. Int. Ed. Engl. 2001;40:2274–2277. doi: 10.1002/1521-3773(20010618)40:12<2274::AID-ANIE2274>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Davies K.M., Bohic S., Carmona A., Ortega R., Cottam V., Hare D.J., Finberg J.P., Reyes S., Halliday G.M., Mercer J.F., Double K.L. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol. Aging. 2014;35:858–866. doi: 10.1016/j.neurobiolaging.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 37.Montes S., Rivera-Mancia S., Diaz-Ruiz A., Tristan-Lopez L., Rios C. Copper and copper proteins in Parkinson’s disease. Oxid. Med. Cell. Longev. 2014;2014:147251. doi: 10.1155/2014/147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazemi A., Frazier T., Cave M. Micronutrient-related neurologic complications following bariatric surgery. Curr. Gastroenterol. Rep. 2010;12:288–295. doi: 10.1007/s11894-010-0120-5. [DOI] [PubMed] [Google Scholar]
- 39.Naismith R.T., Shepherd J.B., Weihl C.C., Tutlam N.T., Cross A.H. Acute and bilateral blindness due to optic neuropathy associated with copper deficiency. Arch. Neurol. 2009;66:1025–1027. doi: 10.1001/archneurol.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessler H., Pajonk F.G., Bach D., Schneider-Axmann T., Falkai P., Herrmann W., Multhaup G., Wiltfang J., Schäfer S., Wirths O., et al. Effect of copper intake on CSF parameters in patients with mild Alzheimer’s disease: A pilot phase 2 clinical trial. J. Neural Transm. 2008;115:1651–1659. doi: 10.1007/s00702-008-0136-2. [DOI] [PubMed] [Google Scholar]
- 41.Williams J.R., Trias E., Beilby P.R., Lopez N.I., Labut E.M., Bradford C.S., Roberts B.R., McAllum E.J., Crouch P.J., Rhoads T.W., et al. Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD(G93A) mice co-expressing the Copper-Chaperone-for-SOD. Neurobiol. Dis. 2016;89:1–9. doi: 10.1016/j.nbd.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J.R., Lee H., Kim S.I., Yang S.R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget. 2016;7:58405–58417. doi: 10.18632/oncotarget.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickart L. The biological effects and mechanism of action of the plasma peptide glycyl-l-histidyl-l-lysine. Lymphokines. 1983;8:425–446. [Google Scholar]
- 44.Sorenson J.R. Antiinflammatory, analgesic, and antiulcer activities of copper complexes suggest their use in a physiologic approach to treatment of arthritic diseases. Basic Life Sci. 1988;49:591–594. doi: 10.1007/978-1-4684-5568-7_92. [DOI] [PubMed] [Google Scholar]
- 45.Sorenson J.R., Soderberg L.S., Chidambaram M.V., de la Rosa D.T., Salari H., Bond K., Kearns G.L., Gray R.A., Epperson C.E., Baker M.L. Bioavailable copper complexes offer a physiologic approach to treatment of chronic diseases. Adv. Exp. Med. Biol. 1989;258:229–234. doi: 10.1007/978-1-4613-0537-8_20. [DOI] [PubMed] [Google Scholar]
- 46.Sorenson J.R. Copper complexes offer a physiological approach to treatment of chronic diseases. Prog. Med. Chem. 1989;26:437–568. doi: 10.1016/s0079-6468(08)70246-7. [DOI] [PubMed] [Google Scholar]
- 47.Soderberg L.S., Barnett J.B., Baker M.L., Salari H., Sorenson J.R. Postirradiation treatment with copper(II)2(3,5-diisopropylsalicylate)4 enhances radiation recovery and hemopoietic regeneration. Exp. Hematol. 1990;18:801–805. [PubMed] [Google Scholar]
- 48.Sorenson J.R., Soderberg L.S., Chang L.W. Radiation protection and radiation recovery with essential metalloelement chelates. Proc. Soc. Exp. Biol. Med. 1995;210:191–204. doi: 10.3181/00379727-210-43939a. [DOI] [PubMed] [Google Scholar]
- 49.Soderberg L.S., Barnett J.B., Baker M.L., Chang L.W., Salari H., Sorenson J.R. Copper(II)2(3,5-diisopropylsalicylate)4 stimulates hemopoiesis in normal and irradiated mice. Exp. Hematol. 1988;16:577–580. [PubMed] [Google Scholar]
- 50.Stein C., Kuchler S. Targeting inflammation and wound healing by opioids. Trends Pharmacol. Sci. 2013;34:303–312. doi: 10.1016/j.tips.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Bhathena S.J., Recant L., Voyles N.R., Timmers K.I., Reiser S., Smith J.C.J., Powell A.S. Decreased plasma enkephalins in copper deficiency in man. Am. J. Clin. Nutr. 1986;43:42–46. doi: 10.1093/ajcn/43.1.42. [DOI] [PubMed] [Google Scholar]
- 52.Bobyntsev I.I., Chernysheva O.I., Dolgintsev M.E., Smakhtin M.Y., Belykh A.E. Anxiolytic effects of Gly-His-Lys peptide and its analogs. Bull. Exp. Biol. Med. 2015;158:726–728. doi: 10.1007/s10517-015-2847-3. [DOI] [PubMed] [Google Scholar]
- 53.Bobyntsev I.I., Chernysheva O.I., Dolgintsev M.E., Smakhtin M.Y., Belykh A.E. Effect of Gly-His-Lys peptide and its analogs on pain sensitivity in mice. Eksp Klin Farmakol. 2014;78:13–15. [PubMed] [Google Scholar]
- 54.Hawk S.N., Lanoue L., Keen C.L., Kwik-Uribe C.L., Rucker R.B., Uriu-Adams J.Y. Copper-deficient rat embryos are characterized by low superoxide dismutase activity and elevated superoxide anions. Biol. Reprod. 2003;68:896–903. doi: 10.1095/biolreprod.102.009167. [DOI] [PubMed] [Google Scholar]
- 55.Shukla V., Mishra S.K., Pant H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011;2011:572634. doi: 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viader A., Sasaki Y., Kim S., Strickland A., Workman C.S., Yang K., Gross R.W., Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886–898. doi: 10.1016/j.neuron.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miotto M.C., Rodriguez E.E., Valiente-Gabioud A.A., Torres-Monserrat V., Binolfi A., Quintanar L., Zweckstetter M., Griesinger C., Fernandez C.O. Site-specific copper-catalyzed oxidation of alpha-synuclein: Tightening the link between metal binding and protein oxidative damage in Parkinson’s disease. Inorg. Chem. 2014;53:4350–4358. doi: 10.1021/ic4031377. [DOI] [PubMed] [Google Scholar]
- 59.Nunomura A., Tamaoki T., Motohashi N., Nakamura M., McKeel D.W., Jr., Tabaton M., Lee H.G., Smith M.A., Perry G., Zhu X. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J. Neuropathol. Exp. Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourassa M.W., Brown H.H., Borchelt D.R., Vogt S., Miller L.M. Metal-deficient aggregates and diminished copper found in cells expressing SOD1 mutations that cause ALS. Front. Aging Neurosci. 2014;6:110. doi: 10.3389/fnagi.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murakami K., Murata N., Noda Y., Tahara S., Kaneko T., Kinoshita N., Hatsuta H., Murayama S., Barnham K.J., Irie K., et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajasekhar K., Madhu C., Govindaraju T. Natural Tripeptide-Based Inhibitor of Multifaceted Amyloid beta Toxicity. ACS Chem. Neurosci. 2016;7:1300–1310. doi: 10.1021/acschemneuro.6b00175. [DOI] [PubMed] [Google Scholar]
- 63.Thomas C.E. The influence of medium components on Cu(2+)-dependent oxidation of low-density lipoproteins and its sensitivity to superoxide dismutase. Biochim. Biophys. Acta. 1992;1128:50–57. doi: 10.1016/0005-2760(92)90256-U. [DOI] [PubMed] [Google Scholar]
- 64.Beretta G., Arlandini E., Artali R., Anton J.M., Maffei Facino R. Acrolein sequestering ability of the endogenous tripeptide glycyl-histidyl-lysine (GHK): Characterization of conjugation products by ESI-MSn and theoretical calculations. J. Pharm. Biomed. Anal. 2008;47:596–602. doi: 10.1016/j.jpba.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Beretta G., Artali R., Regazzoni L., Panigati M., Facino R.M. Glycyl-histidyl-lysine (GHK) is a quencher of alpha,beta-4-hydroxy-trans-2-nonenal: A comparison with carnosine. insights into the mechanism of reaction by electrospray ionization mass spectrometry, 1H NMR, and computational techniques. Chem. Res. Toxicol. 2007;20:1309–1314. doi: 10.1021/tx700185s. [DOI] [PubMed] [Google Scholar]
- 66.Cebrian J., Messeguer A., Facino R.M., Garcia Anton J.M. New anti-RNS and -RCS products for cosmetic treatment. Int. J. Cosmet. Sci. 2005;27:271–278. doi: 10.1111/j.1467-2494.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 67.Smakhtin M., Konoplia A.I., Sever’ianova L.A., Shveinov I.A. Pharmacological correction of immuno-metabolic disorders with the peptide Gly-His-Lys in hepatic damage induced by tetrachloromethane. Patol. Fiziol. Eksp. Ter. 2003;2:19–21. [PubMed] [Google Scholar]
- 68.Cherdakov V.Y., Smakhtin M.Y., Dubrovin G.M., Dudka V.T., Bobyntsev I.I. Synergetic antioxidant and reparative action of thymogen, dalargin and peptide Gly-His-Lys in tubular bone fractures. Exp. Biol. Med. 2010;4:15–20. [Google Scholar]
- 69.Miller D.M., DeSilva D., Pickart L., Aust S.D. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Adv. Exp. Med. Biol. 1990;264:79–84. doi: 10.1007/978-1-4684-5730-8_11. [DOI] [PubMed] [Google Scholar]
- 70.Pickart L. Anti-Oxidative and Anti-Inflammatory Metal: Peptide Complexes and Uses Thereof. 511,866,5. U.S. Patent. 1992
- 71.Mariappan N., Elks C.M., Sriramula S., Guggilam A., Liu Z., Borkhsenious O., Francis J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc. Res. 2010;85:473–483. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vermeij W.P., Florea B.I., Isenia S., Alia A., Brouwer J., Backendorf C. Proteomic identification of in vivo interactors reveals novel function of skin cornification proteins. J. Proteome Res. 2012;11:3068–3076. doi: 10.1021/pr300310b. [DOI] [PubMed] [Google Scholar]
- 73.Vermeij W.P., Alia A., Backendorf C. ROS quenching potential of the epidermal cornified cell envelope. J. Investig. Dermatol. 2011;131:1435–1441. doi: 10.1038/jid.2010.433. [DOI] [PubMed] [Google Scholar]
- 74.Liu C., Liu H.J., Xiang Y., Tan Y.R., Zhu X.L., Qin X.Q. Wound repair and anti-oxidative capacity is regulated by ITGB4 in airway epithelial cells. Mol. Cell. Biochem. 2010;341:259–269. doi: 10.1007/s11010-010-0457-y. [DOI] [PubMed] [Google Scholar]
- 75.Elsoe S., Ahnstrom J., Christoffersen C., Hoofnagle A.N., Plomgaard P., Heinecke J.W., Binder C.J., Bjorkbacka H., Dahlback B., Nielsen L.B. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis. 2012;221:91–97. doi: 10.1016/j.atherosclerosis.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 76.Kempster S.L., Belteki G., Licence D., Charnock-Jones D.S., Smith G.C. Disruption of paraoxonase 3 impairs proliferation and antioxidant defenses in human A549 cells and causes embryonic lethality in mice. Am. J. Physiol. Endocrinol. Metab. 2012;302:E103–E107. doi: 10.1152/ajpendo.00357.2011. [DOI] [PubMed] [Google Scholar]
- 77.Novick D., Kim S.H., Fantuzzi G., Reznikov L.L., Dinarello C.A., Rubinstein M. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/S1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 78.Song N., Wang J., Jiang H., Xie J. Ferroportin1 and hephaestin overexpression attenuate iron-induced oxidative stress in MES23.5 dopaminergic cells. J. Cell. Biochem. 2010;110:1063–1072. doi: 10.1002/jcb.22617. [DOI] [PubMed] [Google Scholar]
- 79.Giguere P.M., Gall B.J., Ezekwe E.A., Jr., Laroche G., Buckley B.K., Kebaier C., Wilson J.E., Ting J.P., Siderovski D.P., Duncan J.A. G Protein signaling modulator-3 inhibits the inflammasome activity of NLRP3. J. Biol. Chem. 2014;289:33245–33257. doi: 10.1074/jbc.M114.578393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong Y., Wang G., Gong Y., Yan J., Chen Y., Burczynski F.J. Hepatoprotective role of liver fatty acid binding protein in acetaminophen induced toxicity. BMC Gastroenterol. 2014;14:44. doi: 10.1186/1471-230X-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang G., Gong Y., Anderson J., Sun D., Minuk G., Roberts M.S., Burczynski F.J. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 82.Dhande I., Ma W., Hussain T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertens. Res. 2015;38:21–29. doi: 10.1038/hr.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenblat M., Karry R., Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: Relevance to diabetes. Atherosclerosis. 2006;187:74–81. doi: 10.1016/j.atherosclerosis.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 84.Hussain S., Slikker W., Jr., Ali S.F. Role of metallothionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem. Int. 1996;29:145–152. doi: 10.1016/0197-0186(95)00114-X. [DOI] [PubMed] [Google Scholar]
- 85.Viarengo A., Burlando B., Ceratto N., Panfoli I. Antioxidant role of metallothioneins: A comparative overview. Cell. Mol. Biol. 2000;46:407–417. [PubMed] [Google Scholar]
- 86.Henry G.E., Momin R.A., Nair M.G., Dewitt D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002;50:2231–2234. doi: 10.1021/jf0114381. [DOI] [PubMed] [Google Scholar]
- 87.Itahana Y., Han R., Barbier S., Lei Z., Rozen S., Itahana K. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene. 2015;34:1799–1810. doi: 10.1038/onc.2014.119. [DOI] [PubMed] [Google Scholar]
- 88.National Center of Biotechnology Information IL17A interleukin 17A. [(accessed on 14 February 2017)]; Available online: http://www.ncbi.nlm.nih.gov/gene/3605.
- 89.Finkley M.B., Appa Y., Bhandarkar S. Copper Peptide and Skin. In: Elsner P., Maibach H.I., editors. Cosmeceuticals and Active Cosmetics: Drugs vs. Cosmetics. Marcel Dekker; New York, NY, USA: 2005. pp. 549–563. [Google Scholar]
- 90.Canugovi C., Misiak M., Ferrarelli L.K., Croteau D.L., Bohr V.A. The role of DNA repair in brain related disease pathology. DNA Repair. 2013;12:578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bucholtz N., Demuth I. DNA-repair in mild cognitive impairment and Alzheimer’s disease. DNA Repair. 2013;12:811–816. doi: 10.1016/j.dnarep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Jonson I., Ougland R., Larsen E. DNA repair mechanisms in Huntington’s disease. Mol. Neurobiol. 2013;47:1093–1102. doi: 10.1007/s12035-013-8409-7. [DOI] [PubMed] [Google Scholar]
- 93.Kwiatkowski D., Czarny P., Toma M., Jurkowska N., Sliwinska A., Drzewoski J., Bachurska A., Szemraj J., Maes M., Berk M., et al. Associations between DNA Damage, DNA Base Excision Repair Gene Variability and Alzheimer’s Disease Risk. Dement. Geriatr. Cogn. Disord. 2016;41:152–171. doi: 10.1159/000443953. [DOI] [PubMed] [Google Scholar]
- 94.De Bont R., van Larebeke N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 95.Wolkowitz O.M., Lupien S.J., Bigler E.D. The “steroid dementia syndrome”: A possible model of human glucocorticoid neurotoxicity. Neurocase. 2007;13:189–200. doi: 10.1080/13554790701475468. [DOI] [PubMed] [Google Scholar]
- 96.Pickart L. Method of Using Copper(II) Containing Compounds to Accelerate Wound Healing. 516,436,7. U.S. Patent. 1992 Nov 17;
- 97.Fecto F., Gorrie G., Zhai H., Liu E., Deng H., Sidd T. Impaired activity of the ubiquitin–proteasome system in transgenic mice expressing ALS/dementia-linked mutant UBQLN2 (P02.170) Neurology. 2013;80:P02–170. [Google Scholar]
- 98.Cheng S.Y., Hung H.L.C., Chang R.C.C. Differential roles of the ubiquitin-proteasome system and autophagy in experimental models of Alzheimer’s Disease; Proceedings of the 2016 Neuroscience Symposium and Annual Scientific Conference of the Hong Kong Society of Neurosciences; The University of Hong Kong, Hong Kong, China. 18 May 2016. [Google Scholar]
- 99.Opattova A., Cente M., Novak M., Filipcik P. The ubiquitin proteasome system as a potential therapeutic target for treatment of neurodegenerative diseases. Gen. Physiol. Biophys. 2015;34:337–352. doi: 10.4149/gpb_2015024. [DOI] [PubMed] [Google Scholar]
- 100.McKinnon C., Tabrizi S.J. The ubiquitin-proteasome system in neurodegeneration. Antioxid. Redox Signal. 2014;21:2302–2321. doi: 10.1089/ars.2013.5802. [DOI] [PubMed] [Google Scholar]
- 101.Hostynek J.J., Dreher F., Maibach H.I. Human skin retention and penetration of a copper tripeptide in vitro as function of skin layer towards anti-inflammatory therapy. Inflamm. Res. 2010;59:983–988. doi: 10.1007/s00011-010-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erdem S., Türkoglu M. Glycyl-l-Histidyl-l-Liysine-Cu(2+) loaded liposome formulations. Marmara Pharm. J. 2010;14:91–97. doi: 10.12991/201014455. [DOI] [Google Scholar]
- 103.Schlesinger D.H., Pickart L., Thaler M.M. Growth-modulating serum tripeptide is glycyl-histidyl-lysine. Experientia. 1977;33:324–325. doi: 10.1007/BF02002806. [DOI] [PubMed] [Google Scholar]
- 104.McDonald L.V., Lake C.R. Psychosis in an adolescent patient with Wilson’s disease: Effects of chelation therapy. Psychosom. Med. 1995;57:202–204. doi: 10.1097/00006842-199503000-00013. [DOI] [PubMed] [Google Scholar]