Abstract
[Purpose] No literature has described a suitable method for measuring muscle strength in a supine position during acute phase after stroke. This study investigated the feasibility and reliability of using a commercial handheld dynamometer to measure the muscle strengths of the hip flexor, knee extensor, and dorsiflexor in the supine position with a modified method for patients at a stroke intensive care center within 7 days of stroke onset. [Subjects and Methods] Fifteen persons with acute stroke participated in this cross-sectional study. For each patient, the muscle strengths of the hip flexors, knee extensors, and dorsiflexors were measured twice by two testers on the same day. Each patient was re-tested at the same time of day one day later. Inter-rater and test-retest reliability were then determined by the intraclass correlation coefficients (ICCs). [Results] For the three muscle groups, the inter-rater reliability ICCs were all 0.99 and the test-retest reliability ICCs were greater than 0.85. The investigated method thus has good inter-rater reliability and high agreement between the test-retest measurements, with acceptable measurement errors. [Conclusion] The modified method using a handheld dynamometer to test the muscle strength of acute stroke patients is a feasible and reliable method for clinical use.
Key words: Acute stroke, Reliability, Handheld dynamometer
INTRODUCTION
Muscle weakness arising from upper motor neuron inhibition1) is a common consequence of stroke and typically presents in the lower extremities2, 3). Muscle strength is an essential component for patients with stroke to perform daily activities4,5,6). A previous study showed that the severity of accompanying motor impairments due to muscle weakness after stroke as measured by Motricity Index scores, including the muscle strengths of hip flexors, knee extensors, and ankle dorsiflexors, is closely correlated with walking ability and the Barthel Index7).
Manual muscle testing (MMT) is a technique that is widely used to assess muscle strength in a clinical setting. The technique is inexpensive to perform and follows a standard protocol. One major limitation of this method, however, is that muscle strength is rated on an ordinal scale rather than a continuous scale, which makes subtle changes in muscle strength difficult to detect. In particular, medical interns and inexperienced physicians may find it difficult to identify subtle changes in muscle strength when using MMT8). This is of clinical importance because although improvements in post-stroke muscle strength may not be apparent on the MMT ordinal scale, subtle improvements in muscle strength may nonetheless be sufficient to alter the training plans or the goals set for stroke patients. For example, the hip flexors of patients who rate at level 3- but close to level 3 on the MMT scale may allow those patients to perform a different walking pattern than that performed by patients who rate at level 3- without being close to level 3; however, it is difficult to differentiate between such patients, as they are all graded the same, 3-, on the MMT scale.
Handheld dynamometry is another method that can be used to manually assess muscle strength. One major advantage of handheld dynamometry, compared with MMT, is that dynamometry is able to measure subtle differences in muscle strength. In addition, a previous study found that handheld dynamometry had better test-retest reliability in weaker patients compared with strong patients, thus making it more suitable for bedridden patients9). One difficulty faced in using handheld dynamometry to assess patients with acute stroke, however, is that the standard protocols10) and the test positions described by Bohannon11)are not always applicable. For example, when the muscle strengths of the hip flexor, knee extensor, and dorsiflexor are graded three or higher, they are often measured while the patient is sitting10). This position, however, is not easily achieved by some stroke patients, especially those residing in an intensive care unit (ICU) or those who have physiological limitations when sitting up12). At our facility, patients in the ICU are generally prescribed bed rest after stroke within 3 days to reduce neurological complications. Moreover, the sitting ability and endurance level of the given patient needs to be taken into consideration when measuring muscle strength in the sitting position. Some patients may not be able to remain in the sitting position long enough to complete the muscle strength tests. As these patients mostly lie supine during their stay in the ICU, it becomes important for clinicians to be able to objectively measure the muscle strength of patients in this position. Early and accurate testing procedures of muscle strength can help clinicians better determine rehabilitation goals, functional training approaches, and the need for assistive devices after discharge.
To the best of our knowledge, there is no literature that describes a suitable method for measuring the muscle weakness of acute phase stroke patients in the supine position. Hence, there is a need to establish a feasible and reliable muscle testing protocol for patients in the acute stage after stroke. This study aimed to determine the feasibility and reliability of using a handheld dynamometer to measure the muscle strengths of the hip flexor, knee extensor, and dorsiflexor in the supine position for acute phase stroke patients in an ICU.
SUBJECTS AND METHODS
A total of 15 persons with acute stroke participated in this cross-sectional study. The inclusion criteria were as follows: (1) admittance to the National Taiwan University Hospital (NTUH) stroke ICU within 3 days of onset of the first stroke episode with a National Institutes of Health Stroke Scale (NIHSS) score ranges from 5 to 25; (2) activity of daily living-independent pre-stroke; (3) age between 40 and 80 years; (4) stroke with unilateral hemiparesis lesions confirmed by magnetic resonance imaging (MRI) or computed tomography (CT), with vascular lesions verified by magnetic resonance angiography (MRA); (5) a cortical or subcortical infarction or hemorrhage; (6) the ability to follow simple commands; (7) Brunnstrom’s stage of affected lower extremity > stage III (able to perform selective limb control); (8) no active inflammation or pathologic changes in the joints; (9) no other peripheral or central nervous system (CNS) dysfunction; and (10) no other active medical problems. The exclusion criteria were as follows: (1) medical conditions unrelated to the cerebrovascular accident but affect walking performance; and (2) cognitive, emotional, or behavioral impairments that resulted in insufficient comprehension, understanding, or collaboration.
This research was approved by the Institutional Review Board (IRB) of NTUH. Informed consent and all research procedures were conducted in accordance with the ethical standards of the IRB.
The principle investigator trained two licensed physical therapists regarding the standard testing position, stabilization techniques, and the appropriate use of the handheld dynamometer. Before the formal experiment, the therapists completed competency tests on two volunteers without disabilities and one chronic stroke patient to ensure that the testing procedures were standardized between the two raters.
After signing the informed consent, each patient was measured twice (once by the first rater, and once by the second rater) on the same day. One rater measured the three muscle groups in the morning, and the other tested the three muscle groups in the afternoon. All subjects were re-tested at the same time of day one day later by the same tester. It was assumed that the muscle strength would not vary within 24 hours. The muscle groups being tested were the hip flexors, knee extensors, and ankle dorsiflexors of the paralyzed side. Each muscle group was tested 3 times during one test session.
A MicroFET2 handheld dynamometer (HOGGAN Scientific LLC, Salt Lake City, UT, USA) was used to assess the isometric strengths of the hip flexors, knee extensors, and ankle dorsiflexors. Moreover, the method of make test, wihich resistance applied throughout the range is termed, was used when applying MMT with the device. This device could measure force output from 0.8 to 300 lbs, with a sensitivity of 0.1 lb. Evidence for the validity of a similar handheld dynamometer has been established in a previous study6).
The isometric strengths of the participants’ hip flexors, knee extensors, and ankle dorsiflexors were assessed13). Three test trials were performed on each muscle group at the Stroke Center within 7 days of onset of the stroke. The prevention of muscle fatigue was achieved by a 20-second recovery period after each test and 5 minutes of rest between the evaluations of each muscle group. One or two practice tests were conducted to allow the participants to familiarize themselves with the testing procedures before the actual strength measurements were taken. To ensure that the testing positions were consistent between the two raters, a wedge composed of two boards (each 68 cm long and 13 cm wide) was specifically designed. A movable joint was included between the two boards. Another movable joint was also included on one of the boards, such that one side was 38 cm long and the other was 30 cm long (Fig. 1). Using this wedge, each participant’s lower extremity could be placed in a position with a hip flexion of 45 degrees and a knee flexion of 90 degrees. A movable foam pillow was placed beneath the ankle so that the muscle strength was tested at 10 degrees of plantar flexion. In addition, the lower board of the wedge had five notches (Fig. 1) that could be used to adjust the angle formed by the two upper boards to accommodate for the patients’ leg lengths and ensuring required joint placement during measurements. Participants were asked to apply maximal force against the dynamometer held by the physical therapist. Before the test, the participants were given a standard instruction of “push as hard as you can.” Encouragement to apply maximal effort was also given during the test. Each participant was instructed to exert maximal strength for 3–5 seconds until the examiner instructed the patient to relax. The maximal isometric muscle strength was recorded in pounds for all the lower-extremity muscle groups. The standardized testing positions, including the joint angles, placements of applied resistance, and locations of stabilization are shown in Table 1. To test the hip flexors, knee extensors, and ankle dorsiflexors, the participants were placed in a supine position with the wedge placed below the knees, such that the knees were flexed at 90 degrees and the hips were at an angle of 45 degrees from the bed. To test the strength of the hip flexors, the handheld dynamometer was placed on the lower third of the given participant’s thigh and held in place on the wedge (Fig. 2-A). While holding the dynamometer in place, the tester told the participant to push his or her thigh upwards with maximal force for 3–5 seconds. To test the strength of the knee extensors, the handheld dynamometer was placed on the lower third of the participant’s tibia bone and held in place on the wedge (Fig. 2-B). The participant was then instructed to push his or her tibia upwards with maximal force for three to five seconds. To test the ankle dorsiflexors (Fig. 2-C), the handheld dynamometer was placed on the dorsal surface of the first metatarsal base and held in place on the wedge. The participant was then instructed to push his or her sole upwards with maximal force for three to five seconds. The testing protocol for each muscle group was repeated three times, with a 20-second rest period between each repetition. The participants were asked if they felt any discomfort and their vitals were monitored during the tests.
Fig. 1.
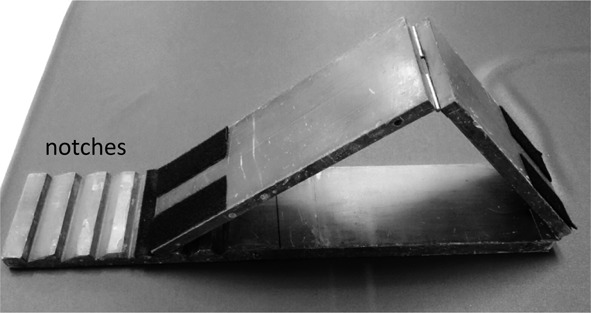
Two boards that were each 68 cm long and 13 cm wide and that could be combined to form a wedge were designed. A movable joint was included between the two boards. Another movable joint was included on the upper board, such that one side was 38 cm long and the other was 30 cm long.
Table 1. Description of muscle testing positions.
| Muscle | Gravity-Related Position | Position | Patient Position | Dynamometer Placement | Direction of Resistance |
|---|---|---|---|---|---|
| Hip flexor | Alternate against | Supine | Hip flexed 45° and knee flexed 90° | Lower 1/3 to anterior thigh | Hip extension |
| Knee extensor | Alternate against | Supine | Hip flexed 45° and knee flexed 90° | Lower 1/3 to anterior tibia | Knee flexion (as patient attempts to extend the knee) |
| Ankle dorsiflexor | Alternate against | Supine | Hip flexed 45° and knee flexed 90° and ankle in 10° plantarflexion | Dorsal surface of 1st metatarsal head | Plantarflexion (as patient attempts to maintain dorsiflexion) |
Fig. 2.
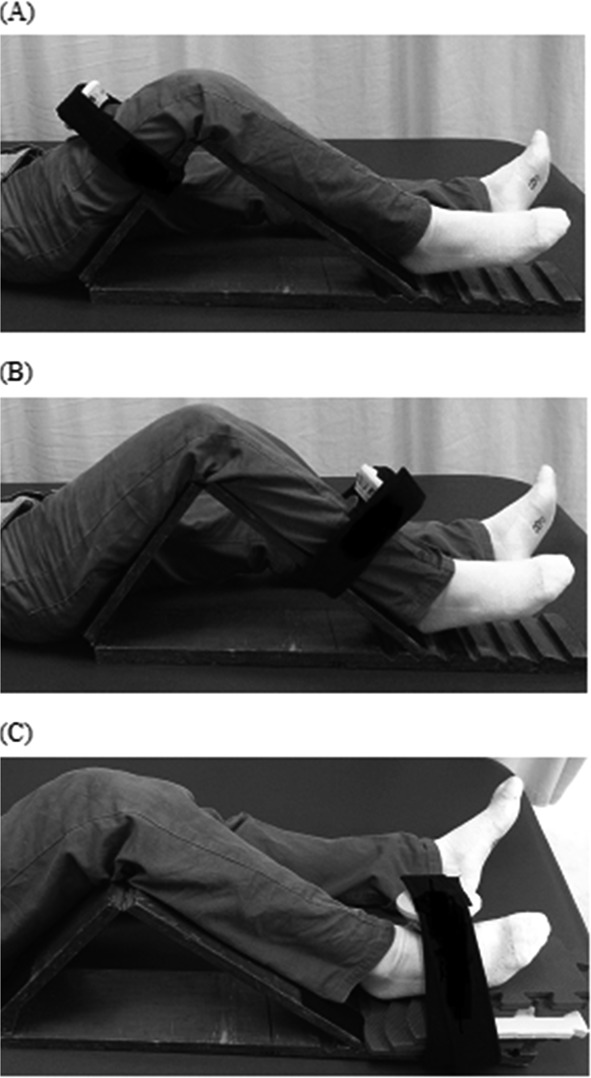
(A) testing the hip flexor using the handheld dynamometer at a time; (B) testing the knee extensor using the handheld dynamometer at a time; (C) testing the ankle dorsiflexor using the handheld dynamometer at a time.
Statistical analysis was carried out using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Participants’ basic information was presented using descriptive statistics. The following methods were used: (1) Inter-rater reliability for the two testers was determined by the intraclass correlation coefficients (ICCs) for the averages of three trials performed during the first test day. (2) ICCs were used examine the test-retest reliability for each tester; ICC (3,1)≥0.75 indicated good reliability and 0.4–0.75 indicated moderate reliability14). (3) ICCs were used to calculate the standard error of measurement (SEM)15) in order to determine test-retest error variation; under the assumption of normal distribution and constant participant abilities, 95% of observed values fell within the true score standard of 1.96. The SEM was calculated by pooled SD*sqrt(1-ICC), whereas the minimal detectable Change (MDC) was calculated by SEM*sqrt(2) *1.9616).
RESULTS
The participants in this study were recruited from National Taiwan University Hospital (NTUH), Taipei, Taiwan. Of the 15 participants, 10 were male and 5 were female. The average age was 56.6 ± 12.9 (mean ± SD) years old, and the average NIHSS score was 8.9 ± 6.1 (mean ± SD). In the 36 test trails for a subject, all subjects were able to be tested the muscle strength with the method. The mean of lower-extremity isometric muscle strength performed during the first and second test day for two testers using handheld dynamometry were showed in Table 2. The inter-rater reliability for the three muscle groups were showed excellent reliability for hip flexors (ICC=0.99, p<0.01), knee extensors (ICC=0.99, p<0.01), and ankle dorsiflexors (ICC=0.99, p<0.01). For the three muscle groups, the test-retest reliability ICCs for each tester were greater than 0.85, indicating high reliability (Table 3). The test-retest SEMs for hip flexors were 0.90 and 0.66, the SEMs for knee extensors were 1.67 and 1.19, and the SEMs for ankle dorsiflexors were 1.23 and 1.30 for two raters respectively (Table 3). The test-retest MDCs for hip flexors were 2.50 and 1.83, the MDCs for knee extensors were 4.60 and 3.27, and the MDC s for ankle dorsiflexors were 3.38 and 3.58 for two raters respectively (Table 3).
Table 2. The mean of lower-extremity isometric muscle strength performed during the first and second test day for two testers using handheld dynamometry.
| Patient | Tester I | Tester II | |||||
|---|---|---|---|---|---|---|---|
| Number | Hip flexor | Knee extensor | Ankle dorsiflexor | Hip flexor | Knee extensor | Ankle dorsiflexor | |
| 1 | Day 1 | 47.47 | 50.27 | 50.30 | 46.33 | 44.97 | 55.10 |
| Day 2 | 44.95 | 63.54 | 55.34 | 49.75 | 48.93 | 67.45 | |
| 2 | Day 1 | 41.00 | 35.67 | 7.00 | 38.67 | 35.67 | 7.47 |
| Day 2 | 48.32 | 28.67 | 13.48 | 43.28 | 41.38 | 17.86 | |
| 3 | Day 1 | 35.53 | 24.77 | 26.33 | 35.33 | 23.80 | 26.57 |
| Day 2 | 31.56 | 33.89 | 31.69 | 42.93 | 19.74 | 38.26 | |
| 4 | Day 1 | 42.10 | 66.23 | 41.80 | 42.53 | 66.80 | 42.53 |
| Day 2 | 37.11 | 79.63 | 50.12 | 44.31 | 61.16 | 47.82 | |
| 5 | Day 1 | 39.10 | 67.67 | 46.43 | 39.57 | 62.70 | 45.83 |
| Day 2 | 43.83 | 61.48 | 41.89 | 41.34 | 69.94 | 38.56 | |
| 6 | Day 1 | 23.47 | 34.30 | 11.63 | 20.50 | 34.43 | 11.43 |
| Day 2 | 27.34 | 39.12 | 8.12 | 24.38 | 33.83 | 18.66 | |
| 7 | Day 1 | 39.03 | 38.97 | 24.53 | 41.20 | 42.40 | 23.93 |
| Day 2 | 41.12 | 32.53 | 33.63 | 44.56 | 46.33 | 34.51 | |
| 8 | Day 1 | 21.87 | 35.70 | 24.40 | 22.47 | 39.07 | 23.50 |
| Day 2 | 23.35 | 44.71 | 28.27 | 17.82 | 47.32 | 19.97 | |
| 9 | Day 1 | 33.63 | 62.70 | 41.30 | 34.00 | 59.13 | 40.90 |
| Day 2 | 38.19 | 56.35 | 37.60 | 32.98 | 63.56 | 49.38 | |
| 10 | Day 1 | 38.13 | 39.30 | 52.53 | 40.10 | 41.10 | 53.80 |
| Day 2 | 43.12 | 45.97 | 51.80 | 42.12 | 44.56 | 60.02 | |
| 11 | Day 1 | 49.75 | 47.70 | 51.75 | 47.05 | 44.70 | 56.05 |
| Day 2 | 56.28 | 49.12 | 55.34 | 50.05 | 48.78 | 52.77 | |
| 12 | Day 1 | 36.25 | 26.65 | 27.75 | 36.50 | 24.20 | 27.60 |
| Day 2 | 32.73 | 23.47 | 25.11 | 31.22 | 28.11 | 33.61 | |
| 13 | Day 1 | 22.85 | 32.95 | 24.35 | 24.40 | 36.80 | 25.15 |
| Day 2 | 28.12 | 25.37 | 29.63 | 27.92 | 31.25 | 29.33 | |
| 14 | Day 1 | 40.55 | 40.10 | 51.80 | 40.85 | 41.05 | 51.55 |
| Day 2 | 50.77 | 52.79 | 54.22 | 43.52 | 44.98 | 64.72 | |
| 15 | Day 1 | 24.15 | 35.70 | 12.50 | 20.65 | 36.40 | 12.30 |
| Day 2 | 19.36 | 40.58 | 14.44 | 23.56 | 39.06 | 19.31 | |
Measure force output unit: lb; Day 1: first test day; day 2: second test day
Table 3. Test-retest reliability, standard error of measurement, and MDC of lower-extremity isometric muscle strength using handheld dynamometry.
| Muscle group | ICC | 95% CI | SD | SEM | MDC | |
|---|---|---|---|---|---|---|
| Tester I | Hip flexor | 0.87 | 0.667–0.956 | 2.51 | 0.91 | 2.50 |
| Knee extensor | 0.85 | 0.609–0.947 | 4.31 | 1.67 | 4.60 | |
| Ankle dorsiflexor | 0.96 | 0.887–0.987 | 6.13 | 1.23 | 3.38 | |
| Tester II | Hip flexor | 0.94 | 0.830–0.979 | 2.71 | 0.66 | 1.83 |
| Knee extensor | 0.94 | 0.842–0.981 | 4.84 | 1.19 | 3.27 | |
| Ankle dorsiflexor | 0.93 | 0.815–0.977 | 4.91 | 1.30 | 3.58 |
ICC: intraclass correlation coefficient; SD: standard deviation; CI: confidence interval; SEM: standard error of measurement; MDC: minimal detectable change
DISCUSSION
The goal of this study was to determine the feasibility and reliability of modified manual muscle testing procedures for patients after acute stroke. Clinically, it has often been observed that patients at the acute stage of stroke have difficulty maintain in a sitting position due to poor sitting balance and endurance. To effectively measure muscle strength of the lower extremity, we specifically designed a wedge to ensure the standardization of the testing procedure in a supine position. Our results indicate that the inter-rater reliability of the 3 muscle groups was very good (ICC≥0.99). The test-retest reliability of the modified manual muscle testing procedures at the 3 muscle groups was high (ICC≥0.89). Previous literature has pointed out that handheld dynamometry has better test-retest reliability for weaker patients than for stronger patients9), and is more suitable for bedridden or disabled patients. Although the testing positions for lower extremity muscle groups used in this study differed from past literature11), the high test-retest reliability suggested that muscle strength testing in the supine position can be used for neurological patients who are unable to maintain a sitting position in ICU.
Besides, the study used the method of make test while applying MMT with handheld dynamometer. Make test requires participants to hold their limb in position and isometric contraction is most often used. At the other hand, the break test applies gradual resistance until participants are unable to hold the position and eccentric contraction is most often used. The study used the make test was based on considering poor neurological recovery level of stroke patients and protection their joint; eccentric contraction is more difficult and the break test may cause joint compression and soreness after stroke.
In establishing a method of measurement with good reliability, test-retest reliability is an important part. Test retest reliability is generally divided into relative and absolute reliability. Relative reliability, such as ICC, indicates the level of consistency between tests; absolute reliability, such as SEM, indicates the level of error in test results. In spite of the high ICC results in the study, the SEM data could additionally provide us absolute reliability information, which can strengthen the interpretations of the results whether the changes in the participants surpass the measurement error17). As all tools will have some measurement error, this study also sought to understand if the results were within the acceptable range of error18). Therefore, this study used both relative and absolute reliability to determine the test-retest reliability of the muscle strength testing methods for acute stroke patients. The results showed that the highest hip flexor SEM between two raters was 0.90, the highest knee extensor SEM was 1.67, and the highest ankle dorsiflexor SEM was 1.30. These three values thus represent the smallest change threshold that indicates a real improvement for a group of individuals. The SEMs results also indicating that there was only a significant clinical change when the difference in a participant’s handheld dynamometer results between the first and second tests was greater than these values; otherwise, the change may have been due to error15). The highest MDCs of the hip flexors was 2.5, the highest MDCs of the knee extensors was 4.6, and the highest MDCs of the dorsiflexor was 3.58. These three values thus represent the smallest change threshold that indicates a real improvement for a single individual. The SEMs and MDCs in this study can be used as a reference when determining actual changes in muscle strength for acute stroke patients in ICU using a handheld dynamometer.
There are some limitations in the study. This study is limited in that only 15 patients participated19). Nevertheless, our results provide a rationale for the investigated measurement design, which might be further investigated in a larger study sample. In addition, we were unable to validate the muscle strength measured in the supine position with that measured in a sitting position. Comparing the differences between these two testing positions may further verify the use of our proposed protocol. However, this approach in the study would allow the positive effects of training programs to be more accurately assessed and the detection of strength changes for stroke in the acute stage.
In conclusion, this study showed that using handheld dynamometry to measure muscle for acute stroke patients in the designed supine position had high inter-rater and test-retest reliability. The findings of this study suggested the modified procedures of testing lower extremity muscle strength were feasible and reliable for clinical practice.
Conflict of interest
The authors have declared that no competing interests exist.
Acknowledgments
This study was supported by grants from National Taiwan University Hospital (NTUH.104-002879).
REFERENCES
- 1.Ng SS, Shepherd RB: Weakness in patients with stroke: implications for strength training in neurorehabilitation. Phys Ther Rev, 2000, 5: 227–238. [Google Scholar]
- 2.Bourbonnais D, Vanden Noven S: Weakness in patients with hemiparesis. Am J Occup Ther, 1989, 43: 313–319. [DOI] [PubMed] [Google Scholar]
- 3.Mayo NE: Epidemiology and recovery. Phys Med Rehabil State Art Rev, 1993, 7: 1–25. [Google Scholar]
- 4.Eng JJ, Chu KS, Dawson AS, et al. : Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke, 2002, 33: 756–761. [DOI] [PubMed] [Google Scholar]
- 5.Nadeau S, Arsenault AB, Gravel D, et al. : Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. Am J Phys Med Rehabil, 1999, 78: 123–130. [DOI] [PubMed] [Google Scholar]
- 6.Nollet F, Beelen A: Strength assessment in postpolio syndrome: validity of a hand-held dynamometer in detecting change. Arch Phys Med Rehabil, 1999, 80: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 7.Wade DT, Hewer RL: Functional abilities after stroke: measurement, natural history and prognosis. J Neurol Neurosurg Psychiatry, 1987, 50: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvir Z: Grade 4 in manual muscle testing: the problem with submaximal strength assessment. Clin Rehabil, 1997, 11: 36–41. [DOI] [PubMed] [Google Scholar]
- 9.Resnick JS, Mammel M, Mundale MO, et al. : Muscular strength as an index of response to therapy in childhood dermatomyositis. Arch Phys Med Rehabil, 1981, 62: 12–19. [PubMed] [Google Scholar]
- 10.Hislop HJ, Avers D, Brown M, et al. : Daniels and worthingham’s muscle testing: techniques of manual examination and performance testing. St. Louis: Elsevier, 2014. [Google Scholar]
- 11.Bohannon RW: Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil, 1997, 78: 26–32. [DOI] [PubMed] [Google Scholar]
- 12.Bernhardt J, Dewey H, Thrift A, et al. : A very early rehabilitation trial for stroke (AVERT): phase II safety and feasibility. Stroke, 2008, 39: 390–396. [DOI] [PubMed] [Google Scholar]
- 13.Collin C, Wade D: Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry, 1990, 53: 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiss JL: The design and analysis of clinical experiments. New York: Wiley, 1986. [Google Scholar]
- 15.Lexell JE, Downham DY: How to assess the reliability of measurements in rehabilitation. Am J Phys Med Rehabil, 2005, 84: 719–723. [DOI] [PubMed] [Google Scholar]
- 16.de Vet HC, Terwee CB, Knol DL, et al. : When to use agreement versus reliability measures. J Clin Epidemiol, 2006, 59: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson G, Nevill AM: Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med, 1998, 26: 217–238. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Hsieh CL, Liaw LJ, et al. : Test-retest reliability of the stroke rehabilitation assessment of movement (stream). Formos J Phys Ther, 2006, 31: 351–356. [Google Scholar]
- 19.Willemse L, Brehm MA, Scholtes VA, et al. : Reliability of isometric lower-extremity muscle strength measurements in children with cerebral palsy: implications for measurement design. Phys Ther, 2013, 93: 935–941. [DOI] [PubMed] [Google Scholar]


