Abstract
The ability to synthesize high-quality hierarchical core/shell nanocrystals from an efficient host lattice is important to realize efficacious photon upconversion for applications ranging from bioimaging to solar cells. Here, we describe a strategy to fabricate multicolor core @ shell α-NaLuF4:Yb3+/Ln3+@CaF2 (Ln = Er, Ho, Tm) upconversion nanocrystals (UCNCs) based on the newly established host lattice of sodium lutetium fluoride (NaLuF4). We exploited the liquid-solid-solution method to synthesize the NaLuF4 core of pure cubic phase and the thermal decomposition approach to expitaxially grow the calcium fluoride (CaF2) shell onto the core UCNCs, yielding cubic core/shell nanocrystals with a size of 15.6 ± 1.2 nm (the core ~9 ± 0.9 nm, the shell ~3.3 ± 0.3 nm). We showed that those core/shell UCNCs could emit activator-defined multicolor emissions up to about 772 times more efficient than the core nanocrystals due to effective suppression of surface-related quenching effects. Our results provide a new paradigm on heterogeneous core/shell structure for enhanced multicolor upconversion photoluminescence from colloidal nanocrystals.
Keywords: core/shell nanocrystals, liquid-solid-solution method, thermal decomposition, multicolor emissions
1. Introduction
Upconversion nanocrystals (UCNCs) are able to convert two or more long wavelength photons into short wavelength emissions through the use of real energy levels of trivalent lanthanide ions embedded in an inorganic host lattice [1]. Owing to the high physicochemical stability and intrinsic low phonon energy, fluoride-based UCNCs are able to minimize energy losses at the intermediate states of the incorporated lanthanide ions, thus generally exhibiting efficient upconversion (UC) luminescence efficiency [2]. Moreover, fluoride UCNCs also have superior features, such as low toxicity, non-blinking, non-photobleaching, absence of autofluorescence, and tissue-penetrable near-infrared (NIR) light excitation [3,4]. These superb attributes promise their applications in biological imaging [5,6,7,8], bio-detection [9,10], and three-dimensional display [11,12,13]. This motivation fuels a range of works to synthesize UCNCs with controlled size and morphology, as well as to prepare epitaxial core/shell UCNCs with enhanced efficiency and multifunction for theranostic applications, such as, NaYF4:Yb/Er@NaYF4 [14], (NaLuF4:Gd3+/Yb3+/Er3+)@NaLuF4:Yb3+ [15], and NaYbF4:Er@NaGdF4 core/shell nanostructures [16].
Sodium lutetium fluoride (NaLuF4) has recently emerged as a new type of efficient host lattice for photon upconversion, similar to the well-established host material of sodium yttrium fluoride (NaYF4). NaLuF4-based UCNCs have been demonstrated to exhibit bright upconversion luminescence (UCL) [17,18,19,20,21,22,23,24] and show efficient five- and four-photon ultraviolet emissions under continuous wave excitation at 980 nm [25,26,27]. Despite recent success in synthesizing NaLuF4 nanoplates or nanorods with size over ~30 nm using thermal decomposition method [28,29,30,31] or hydrothermal method [32,33,34,35,36,37,38], the ability to prepare uniform single crystal phase sub-10 nm NaLuF4 UCNCs remains elusive. Moreover, doping of a high concentration of inert Gd3+ ions (≥20%) was typically required to prepare small-sized monodisperse NaLuF4 UCNCs with single crystal phase previously [17,39], thus delivering traits actually from an entity of Lu-Gd alloyed host. On the other hand, small-sized UCNCs are important for single molecule imaging [40] and in vivo bioimaging with reduced toxicity, considering the renal clearance [41]. However, they always come at the sacrifice of UCL efficiency due to the increased size-induced surface-related surface quenching effect [42]. A core/shell geometric structure is therefore needed to eliminate or suppress this detrimental effect by spatial isolation of the core nanoparticle from the surrounding quenching sites. A straightforward approach is to grow a homogenous core/shell structure where the host of the shell is identical to the core [37,43]. However, the possible leaking of rare earth ions from the host lattice could possibly lead to diseases such as nephrogenic systemic fibrosis [44,45]. Compared with lanthanide fluorides, calcium fluoride (CaF2) has unique advantages owing to its superior biocompatibility and high optical transparency [46,47,48,49,50,51]. It has recently been demonstrated that CaF2 also has low lattice mismatch with NaReF4 nanocrystals, and can efficiently prevent rare-earth ions from leaking [46,50]. This implies that the growth of a CaF2 shell not only renders UCL enhancement, but also imparts biocompatibility with reduced leaking effect.
In this work, we describe our effort toward the controlled synthesis of single crystal phase sub-10 nm α-NaLuF4:Yb3+/Ln3+ (Ln = Er, Ho, or Tm) nanoparticles using a liquid-solid-solution method without the involvement of doping with a high concentration of Gd3+, and then utilize them as the core to epitaxially grow a high quality α-NaLuF4:Yb3+/Ln3+@CaF2 (Ln = Er, Ho, or Tm) core/shell UCNC via a thermal decomposition protocol. We found that the growth of a ~3 nm thin CaF2 shell layer was able to enhance the multicolour UCL of the core nanocrystals by up to ~772-fold.
2. Results and Discussion
2.1. Synthesis of α-NaLuF4:Yb3+/Ln3+ (Ln = Er, Tm, Ho) or α-NaLuF4:Yb3+/Ln3+@CaF2 Core/Shell UCNCs
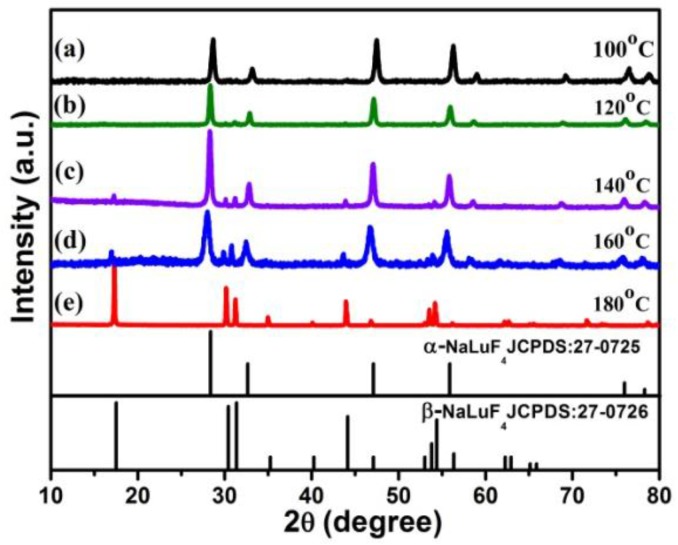
The crystal structure of NaLuF4 has two forms of the cubic (α-) and the hexagonal (β-) phase. To prepare high-quality α-NaLuF4@CaF2 core/shell NCs, we firstly controlled the synthesis of cubic (α) NaLuF4 core nanoparticles by varying the reaction temperature and the molar ratio of F−/Ln3+ (Ln = Lu + Yb + Er/Ho/Tm) precursor. Figure 1 shows X-ray diffraction (XRD) patterns of NaLuF4:Yb3+/Er3+ (Tm3+, Ho3+) NCs hydrothermally prepared at various temperatures with the molar ratio of F−/Ln3+ (Ln = Lu + Yb + Er/Ho/Tm) fixed at 4:1. Figure 1 reveals that the phase transition process (β → α or α → β) occurs at a reaction temperature of T = 100 or 120 °C. The sample obtained at low temperature (T = 100 or 120 °C) shows nearly pure α-phase (JCPDS No. 27-0725). The diffraction peaks of β-NaLuF4 appear at temperatures between 140 and 160 °C (See Figure 1c,d), while at 180 °C, only pure β-phase NaLuF4 exists. It could be concluded that low temperature favors the formation of pure α-NaLuF4 core NCs.
Figure 1.
The X-ray diffraction patterns of NaLuF4:Yb3+/Ln3+ nanocrystals synthesized at different hydrothermal temperature: (a) 100 °C; (b) 120 °C; (c) 140 °C; (d) 160 °C; (e) 180 °C. All samples were prepared at F−/Ln3+ = 4:1.
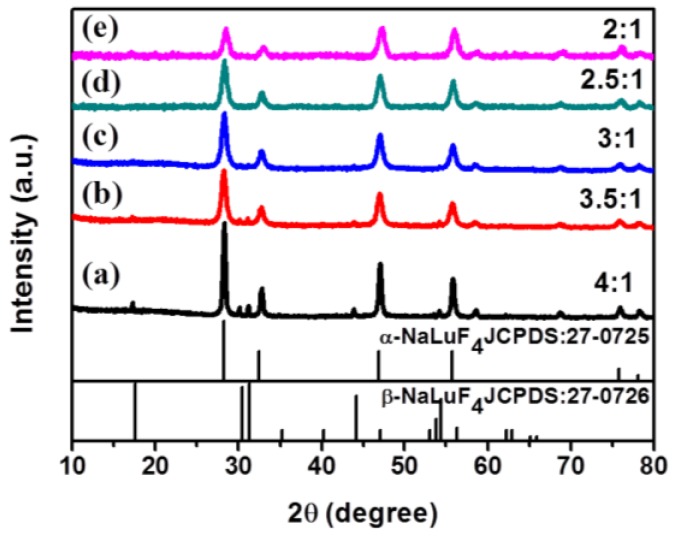
Next, we investigated the role of the molar ratio of F−/Ln3+ (Ln = Lu + Yb + Er/Ho/Tm) precursor on the crystal phase of our product when setting the synthesis temperature at 140 °C. As can be seen in Figure 2, when the molar ratio of F−/Ln3+ is fixed at 4:1, the sample shows a mixture of α-phase (JCPDS No. 27-0725) and β-phase (JCPDS No. 27-0726). As the F−/Ln3+ ratio is reduced to 3.5:1, the intensities of peaks of β-NaLuF4 decreases (Figure 2b). A pure α-phase NaLuF4 NCs can be produced at the ratio of F−/Re3+ below 3:1, and the diffraction peaks of α-NaLuF4 sample at F−/Re3+ = 3:1 show stronger intensity than at F−/Re3+ = 2.5:1 and 2:1. The average crystallite size of the nanocrystals was calculated according to Scherrer’s equation [51],
| (1) |
where K = 0.89, D represents the crystallite size (in nanometers), λ is the wavelength of the Cu Kα radiation, β is the corrected half-width of the diffraction peak, and θ is Bragg’s angle of the diffraction peak. According to Equation (1) and the half width of the main diffraction peak at 28° in Figure 2, the average size was calculated to be about 10 nm for F−/Re3+ = 3:1, in good agreement with the TEM result (Figure 3b). As a consequence, we selected α-NaLuF4 nanoparticles prepared at F−/Re3+ = 3:1 and T = 140 °C to epitaxially grow the α-NaLuF4:Yb/Ln@CaF2 core/shell structure.
Figure 2.
The X-ray diffraction patterns of NaLuF4:Yb3+/Er3+ nanocrystals synthesized with molar ratio of F−/Re3+ = (a) 4:1; (b) 3.5:1; (c) 3:1; (d) 2.5:1; (e) 2:1. (T = 140 °C).
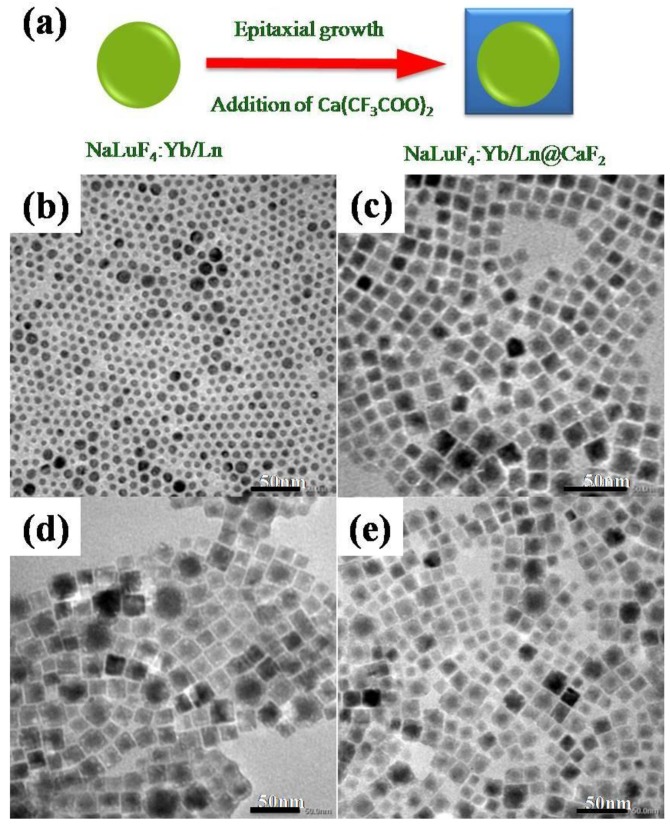
Figure 3.
(a) Schematic illustration of the epitaxial growth of CaF2 shell on α-NaLuF4:Yb3+/Ln3+ core nanoparticles (NPs); (b) TEM image of α-NaLuF4:Yb3+/Ln3+ core nanocrystals prepared at F−/Re3+ = 3:1 and T = 140 °C. TEM images of (c) NaLuF4:Yb3+/Er3+@CaF2 core/shell upconversion nanocrystals (UCNCs); (d) α-NaLuF4:Yb3+/Ho3+@CaF2 core/shell UCNCs; (e) α-NaLuF4:Yb3+/Ln3+@CaF2 core/shell UCNCs. Particles in (c–e) were synthesized by the thermal decomposition method.
The principle for the epitaxial growth of the core/shell structure is illustrated in Figure 3a, which involves an injection of the (CF3COO)2Ca solution into the growing solution containing pre-synthesized α-NaLuF4:Yb/Ln core nanocrystals prepared at F−/Re3+ = 3:1 and T = 140 °C. The morphologies and sizes of the α-NaLuF4:Yb3+/Ln3+ core and the resulting α-NaLuF4:Yb3+/Ln3+@CaF2 core/shell nanoparticles were examined by transmission electron microscopy (TEM), and the results are shown in Figure 3b. As one can see, the α-NaLuF4:Yb3+/Ln3+ core has sphere-like morphology with a size of 9 ± 0.9 nm, in good agreement with the XRD result in Figure 2c.
After growing the CaF2 shell, the core/shell NCs showed a cubic morphology with a size of 15.6 ± 1.2 nm (Figure 3c–e). The size difference indicated that a CaF2 shell with a thickness of about 3.3 ± 0.3 nm was grown on the surface of α-NaLuF4:Yb3+/Ln3+ core. Moreover, the formation of core/shell structure can be seen in the TEM images, shown in Figure 3c–e.
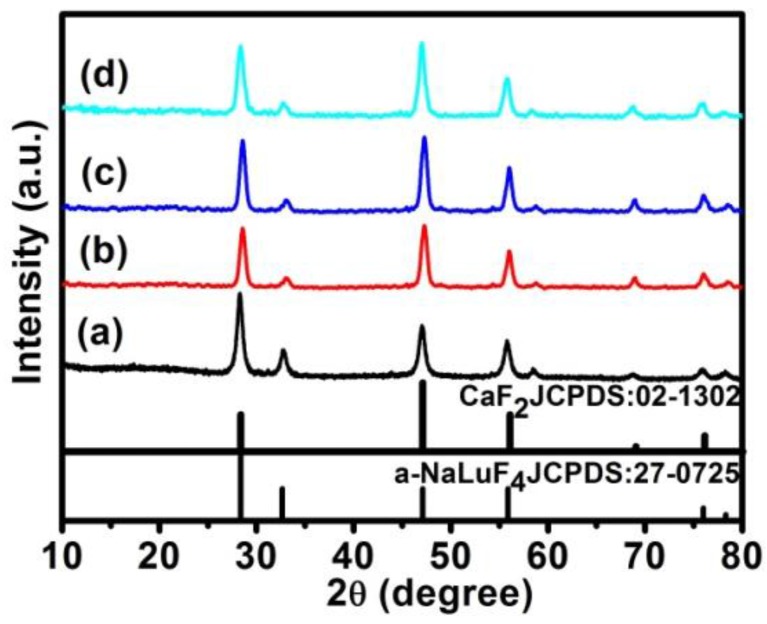
The XRD peaks of the α-NaLuF4:Yb3+/Ln3+ core, the α-NaLuF4:Yb3+/Er3+@CaF2 core/shell, the NaLuF4:Yb3+/Ho3+@CaF2 core/shell, and the NaLuF4:Yb3+/Tm3+@CaF2 core/shell UCNCs are presented in Figure 4. As one can see, the core and core/shell NCs have identical peak positions, agreeing well with the standard JCPDF27-0725 sample of cubic NaLuF4 and JCPDF 02-1302 sample of cubic CaF2. The well-defined peaks are indicative of the high crystallinity of both the core and the core/shell NCs. The narrower XRD peaks of the core/shell NCs compared to that of core NPs indicate the larger size of core/shell NCs, in accordance with the TEM results in Figure 3.
Figure 4.
The X-ray diffraction patterns of the (a) α-NaLuF4:Yb3+/Ln3+ NCs; (b) α-NaLuF4:Yb3+/Er3+@CaF2 core/shell UCNCs; (c) α-NaLuF4:Yb3+/Ho3+@CaF2 core/shell UCNCs; and (d) the α-NaLuF4:Yb3+/Tm3+@CaF2 core/shell UCNCs, in reference to the standard diffraction patterns of the α-phase NaLuF4 (JCPDS 27-0725) and cubic phase CaF2 (JCPDS 02-1302).
2.2. Characterization of α-NaLuF4:Yb3+/Ln3+@CaF2 (Ln = Er, Tm, or Ho) Core/Shell UCNCs
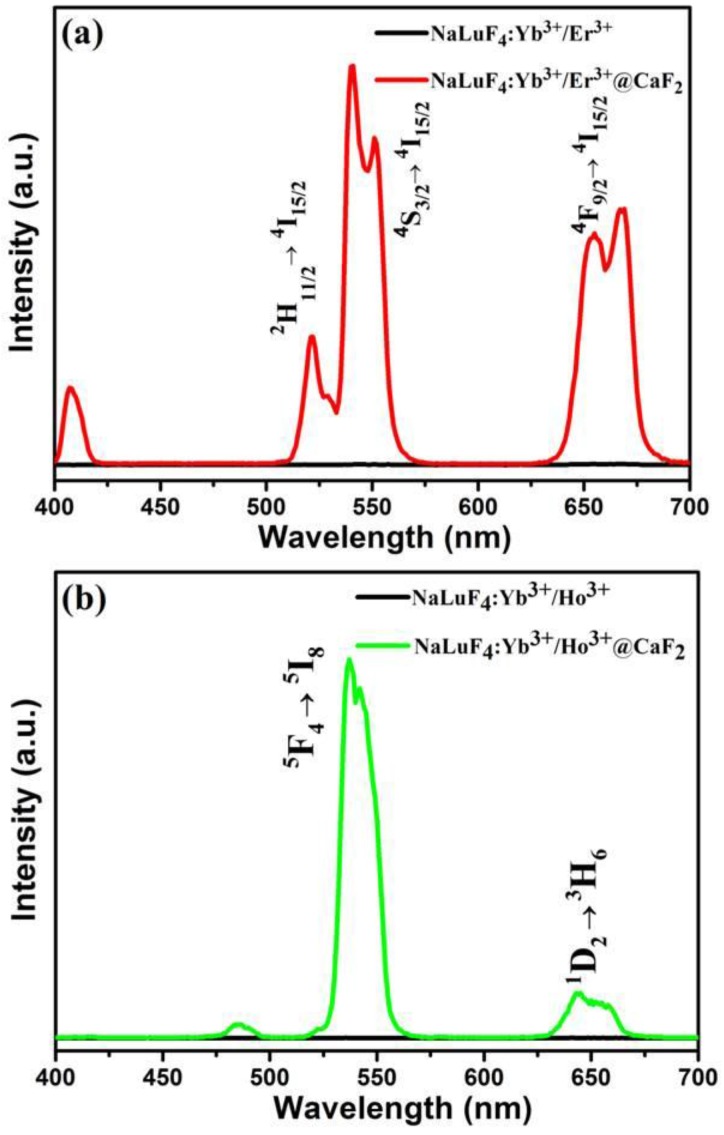
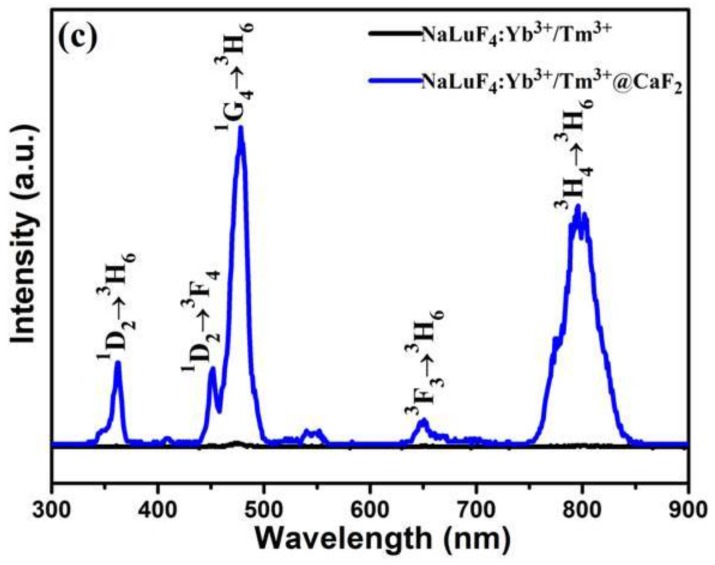
We compared the UCPL from the corresponding core NaLuF4:20%Yb3+/2%Ln3+ and the core/shell NaLuF4:20%Yb3+/2%Ln3+@CaF2 UCNCs (Ln = Er, Ho, and Tm) (Figure 5). As shown in Figure 5a, two UC bands with maxima at 525/540 nm and 660 nm were observed from both the NaLuF4:20%Yb3+/2%Er3+ core and the NaLuF4:20%Yb3+/2%Er3+@CaF2 core/shell UCNCs, corresponding to the 2H11/2/4S3/2 → 4I15/2 and the 4F9/2 → 4I15/2 transitions of Er3+ ions, respectively. Moreover, the intensity of UCPL from core/shell UCNCs is dramatically higher than that of core nanoparticles. The intensity for UCPL band at 540 nm from the NaLuF4:20%Yb3+/2%Er3+@CaF2 core/shell NCs were found to be about 656 times higher than that from the α-NaLuF4:Yb3+/Er3+ core nanocrystals. Moreover, according to Figure 5b,c, when compared with the α-NaLuF4:Yb3+/Ho3+ and α-NaLuF4:Yb3+/Tm3+ core nanocrystals, the intensity of UCPL from the corresponding core/shell UCNCs showed a 772- and 75-fold enhancement, respectively. Taken together, we conclude that the core/shell structure resulted in a significant UCPL enhancement of the core nanocrystals. Since the CaF2 is an inactive layer, such an impressive enhancement undoubtedly arises from the suppression of surface quenching effects on the surface of core nanoparticles. In fact, the ultrasmall size (9 nm) of the core UCNCs is able to expose most of the doped sensitizer (Yb3+) and the doped activator (Er3+/Ho3+/Tm3+) to surface quenching effects (surface defects, surface strains, ligand and solvent molecules with groups possessing high vibration energy) [42] due to the extremely high “surface-to-volume” ratio. The epitaxial growth of the thin CaF2 shell not only eliminates the quenching defects on the surface of the core, but also shields all the sensitizer and activator ions in the core from the environmental quenching factors.
Figure 5.
The upconversion luminescence spectra under excitation at 976 nm using a fiber-coupled laser diode: (a) the α-NaLuF4:Yb3+/Er3+ core and α-NaLuF4:Yb3+/Er3+@CaF2 core/shell NPs; (b) the α-NaLuF4:Yb3+/Ho3+ core and α-NaLuF4:Yb3+/Ho3+@CaF2 core/shell NPs; (c) the α-NaLuF4:Yb3+/ Tm3+ core and α-NaLuF4:Yb3+/Tm3+@CaF2 core/shell NPs. The concentration of Ln3+ in all samples was kept identical at about 0.5 mmol nanoparticles (i.e., nanoparticles formed by 0.5 mmol lanthanide precursors) per 10 mL cyclohexane.
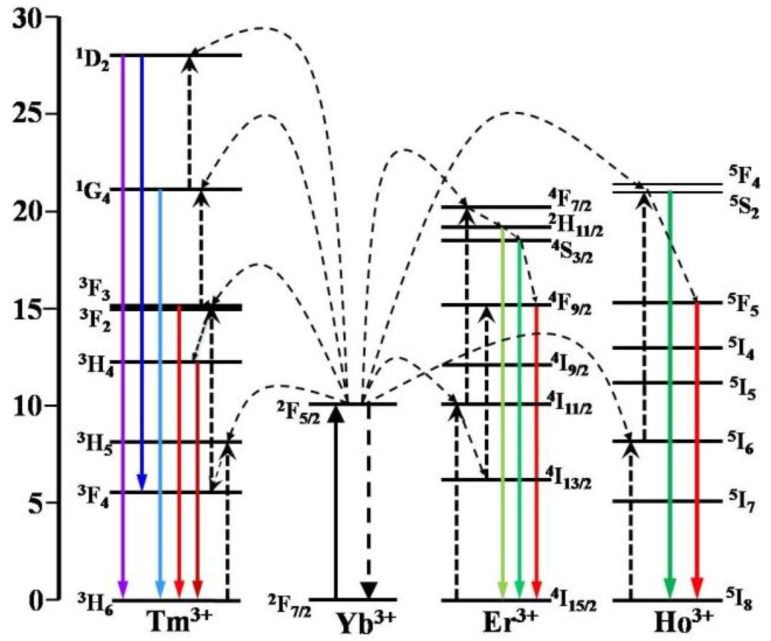
To illustrate the UC mechanisms of the NaLuF4:20%Yb3+/2%Ln3+@CaF2 (Ln3+ = Er3+, Ho3+, Tm3+) NCs, possible UC processes are schematically given in the energy level diagrams of Yb3+, Er3+, Ho3+, and Tm3+ ions in Figure 6. The observed green UC bands (2H11/2 → 4I15/2, 525 nm; 4S3/2 → 4I15/2, 540 nm) and red UC band (4F9/2 → 4I15/2, 660 nm) from Er3+ ions in NaLuF4:20%Yb3+/2%Er3+@CaF2 UCNCs may take place via the following process: Yb3+ ion absorbs one laser photon and gets excited from the ground 2F7/2 state to the exclusive excited 2F5/2 state. The Yb3+ ions in the excited state transfer their absorbed energy to neighboring Er3+ ions and excite them from the ground 4I15/2 state to the 4I11/2 state, then to the 4F7/2 state. Multiphonon assisted relaxations from the 4F7/2 state can decay nonradiatively to the lower 2H11/2 and 4S3/2 levels, emitting the 525 and 540 nm UCL, respectively. The red emission 660 nm originates from the 4F9/2 → 4I15/2 transition, and the 4F9/2 state can be populated either from nonradiative relaxations from the 4S3/2 level or the energy transfer from Yb3+ ions to the Er3+ ion at the 4I13/2 state. In addition, the processes for green (5F4 → 5I8, 537 nm) and red (5F5 → 5I8, 645 nm) UCL of Ho3+ ions involved two different centers—the sensitizer (Yb3+), the activator (Ho3+)—along with two successive transfers from Yb3+ ions to Ho3+ ions. In the first transfer, the Yb3+ ion absorbs the excitation photons through the ground state absorption and transfers its absorbed energy to the neighboring Ho3+ ion to populate to its intermediate (5I6 level). The energy difference between the two levels was abridged by the vibration energy of the host lattice. The second transfer is to promote the population from the intermediate 5I6 level to the emitting energy levels (5S2) by energy transfer (ET) from another excited Yb3+ ion [52]. Once the 5S2 level is populated, the excited electron can release its energy by emitting green emissions. The red emission at 645 nm can be produced by radiative decay to the ground 5F5 state. The blue UCL of Tm3+ occurs via a three-step ET from Yb3+ to Tm3+. First, the Tm3+ ion in the ground state 3H6 is excited to the state 3H5 via an ET from a neighboring excited Yb3+ ion. Subsequent nonradiative relaxation of 3H5/3F4 populates the 3F4 level. In the second-step excitation, the Tm3+ ion in the 3F4 state is excited to the 3F2,3 state via another ET from a neighboring excited Yb3+ ion. The populated 3F2 level may nonradiatively relax to the 3F3 level. When the Tm3+ ion at the 3F3 level decays to the ground state, a weak red emission (3F3 → 3H6) is produced. Additionally, the near-infrared UCL at 802 nm arises from the 3H4 → 3H6 transition, where the 3H4 state is populated by the efficient nonradiative relaxation from the 3F2,3 state. A third energy transfer from Yb3+ excites Tm3+ at the 3F3 level to the 1G4 level, from which the blue emission (1G4 → 3H6) occurs by radiative decay to the ground state. The fourth energy transfer from Yb3+ promotes the Tm3+ at the 1G4 level to the 1D2 level, from which a 360 nm ultraviolet UCL (1D2 → 3H6) is generated.
Figure 6.
The energy level diagrams of Yb3+, Er3+, Tm3+, and Ho3+ ions, showing the proposed upconversion mechanisms in the α-NaLuF4:Yb3+/Ln3+@CaF2 (Ln = Er, Ho, or Tm) core/shell UCNCs.
3. Materials and Methods
3.1. Materials
All Ln2O3 (99.9%, Ln = Lu, Yb, Er, Tm, Ho) were obtained from Jianfeng Rare-Earth Limited Company, Conghua, China. The basic chemical reagents, such as sodium hydroxide, oleic acid (OA), absolute ethyl alcohol, trifluoroacetic acid (TFA), calcium oxide, sodium fluoride and octadecene (ODE) were purchased from Sinopharm Chemical Reagent Co., Ltd., Beijing, China. All chemicals were of analytical grade and were used as received without further purification.
3.2. Hydrothermal Synthesis of α-NaLuF4:Yb3+/Ln3+ UCNCs
We synthesized α-NaLuF4:Yb3+/Ln3+ (Ln = Er, Tm, or Ho) nanocrystals by a hydro-thermal method adapted from the literature [53]. Typically, 0.6 g of NaOH, 3 mL of water, 14 mL of oleic acid (OA) (90 wt. %), and 10 mL (120 mmol) of ethanol were well-mixed at room temperature to yield a white viscous solution. Then, 1 mmol Ln(NO3)3 (Ln = Lu, Yb, Er, Tm, Ho, and Lu3+:Yb3+:Er3+/Tm3+/Ho3+ = 78%:20%:2%/2%/2%) was added into the above solution and kept vigorous stirring. After aging for 30 min, 4 mL (4 mmol) of NaF (F−/Ln3+ = 4:1) solution was added under vigorous stirring for 30 min. Subsequently, the mixture was transferred to a 50-mL Teflon-lined autoclave and heated at 140–180 °C for 24 h. After washing with ethanol, the final products were dispersed in cyclohexane.
3.3. Thermal Decomposition Synthesis of α-NaLuF4:Yb3+/Ln3+@CaF2 Core/Shell UCNCs
The core/shell nanoparticles were synthesized using the thermal decomposition method. Typically, 0.5 mmol CaO was first added to a 50 mL flask containing 5 mL deionized water and 5 mL trifluoroacetic acid (TFA). The solution was heated at 90 °C until the solution became transparent, and was then dried at this temperature with nitrogen purge to yield the shell precursor (CF3COO)2Ca. After obtaining the (CF3COO)2Ca powders, 10 mL of OA, 10 mL of ODE, and the pre-prepared α-NaLuF4:Yb3+/Ln3+ (0.5 mmol) in cyclohexane were added. The solution was then vacuum-degassed at 120 °C for 30 min to remove water, oxygen, and cyclohexane. Subsequently, the solution was heated to 300 °C at a rate of 15 K·min−1 under nitrogen protection. After maintaining at 300 °C for 30 min, the reaction was stopped and cooled down to room temperature. After washing with ethanol, the products were dispersed in cyclohexane for further use.
3.4. Thermal Decomposition Synthesis of α-NaLuF4:Yb3+/Ln3+@CaF2 Core/Shell UCNCs
The size and morphology of the α-NaLuF4:Yb3+/Ln3+ and α-NaLuF4:Yb3+/Ln3+@CaF2 core/shell nanocrystals were characterized by transmission electron microscopy (TEM) using a JEOL JEM-2010 microscope (JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 200 kV. The powder X-ray diffraction (XRD) patterns were recorded by a Siemens D500 diffractometer (Bruker Beijing Scientific Technology Co. Ltd, Beijing, China) using Cu Kα radiation (λ = 0.15418 nm). The 2θ angle of the XRD spectra was recorded at a scanning rate of 5°/min. The UCPL spectra were obtained using a Zolix monochoromator (Beijing Zolix Instruments CO., Ltd., Beijing, China) under excitation at 976 nm using a fiber-coupled laser diode (BWT Beijing Ltd., Beijing, China).
4. Conclusions
In summary, cubic phase α-NaLuF4:Yb/Ln cores can be precisely controlled through a simple variation of reaction temperature and the added amount of NaF in a hydrothermal method. Moreover, a seed-mediated growth protocol with selected parameters favorable for shell growth yields the α-NaLuF4:Yb/Ln@CaF2 (Ln = Er, Ho, Tm) core/shell structure NPs having a core size of ~9 nm and shell thickness of ~3.3 nm. Moreover, we found that the growth of the inert thin shell of CaF2 onto the α-NaLuF4:Yb/Ln (Ln = Er, Ho, Tm) core could enhance its multicolor UCL by up to 772-fold, being attributed to effective suppression of surface-related quenching effects via spatial isolation of the core from the surrounding environment. Small-sized α-NaLuF4:Yb/Ln@CaF2 (Ln = Er, Ho, Tm) UCNCs developed here have implication for uses in a range of biophotonic applications, such as bioimaging.
Acknowledgments
This work is supported in part by National Natural Science Foundation of China (51672061 and 51402071), National Natural Science Fund for Distinguished Young Scholars (51325201), and the Fundamental Research Funds for the Central Universities, China (AUGA5710052614, AUGA8880100415, and HIT. BRETIV.201503).
Author Contributions
Hui Li and Guanying Chen conceived and designed the experiments; Hui Li performed the experiments and drafted the manuscript; Chunhui Yang provided valuable suggestions; Guanying Chen and Shuwei Hao advised the work and edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Suyver J.F., Aebischer A., Biner D., Gerner P., Grimm J., Heer S., Krämer K.W., Reinhard C., Güdel H.U. Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Opt. Mater. 2005;27:1111–1130. doi: 10.1016/j.optmat.2004.10.021. [DOI] [Google Scholar]
- 2.Suyver J.F., Grimm J., van Veen M.K., Biner D., Kramer K.W., Gudel H.U. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+ J. Lumin. 2006;117:1–12. doi: 10.1016/j.jlumin.2005.03.011. [DOI] [Google Scholar]
- 3.Gnach A., Bednarkiewicz A. Lanthanide-doped up-converting nanoparticles: Merits and challenges. Nano Today. 2012;7:532–563. doi: 10.1016/j.nantod.2012.10.006. [DOI] [Google Scholar]
- 4.Naccache R., Rodríguez E.M., Bogdan N., Sanz-Rodríguez F., de la Cruz M.D.I., de la Fuente Á.J., Vetrone F., Jaque D., Solé J.G., Capobianco J.A. High resolution fluorescence imaging of cancers using lanthanide ion-doped upconverting nanocrystals. Cancers. 2012;4:1067–1105. doi: 10.3390/cancers4041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian G., Gu Z., Zhou L., Yin W., Liu X., Yan L., Jin S., Ren W.L., Xing G.M., Li S.J., et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012;24:1226–1231. doi: 10.1002/adma.201104741. [DOI] [PubMed] [Google Scholar]
- 6.Heer S., Koempe K., Guedel H.U., Haase M. Highly efficient multicolor upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004;16:2102–2105. doi: 10.1002/adma.200400772. [DOI] [Google Scholar]
- 7.Lim S.F., Riehn R., Ryu W.S., Khanarian N., Tung C.K., Tank D., Austin R.H. In vivo and scanning electron microscopy imaging of upconverting nanophosphors in Caenorhabditis elegans. Nano Lett. 2006;6:169–174. doi: 10.1021/nl0519175. [DOI] [PubMed] [Google Scholar]
- 8.Wang F., Liu X. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008;130:5642–5643. doi: 10.1021/ja800868a. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Yan R., Huo Z., Wang L., Zeng J., Bao J., Wang X., Peng Q., Li Y. Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. 2005;44:6054–6057. doi: 10.1002/anie.200501907. [DOI] [PubMed] [Google Scholar]
- 10.Yao L., Jing Z., Liu J., Wei F., Li F. Iridium-complex-modified upconversion nanophosphors for effective LRET detection of cyanide anions in pure water. Adv. Funct. Mater. 2012;22:2667–2672. doi: 10.1002/adfm.201102981. [DOI] [Google Scholar]
- 11.Miyazaki D., Lasher M., Fainman Y. Fluorescent volumetric display excited by a single infrared beam. Appl. Opt. 2005;44:5281–5285. doi: 10.1364/AO.44.005281. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J., Zheng X., Schartner E.P., Lonescu P., Zhang R., Nguyen T.L., Jin D., Ebendorff-Heidepriem H. Upconversion nanocrystal-doped glass: A new paradigm for photonic materials. Adv. Opt. Mater. 2016;4:1507–1517. doi: 10.1002/adom.201600296. [DOI] [Google Scholar]
- 13.Deng R., Qin F., Chen R., Huang W., Hong M., Liu X. Temporal full-colour tuning through non-steady-state upconversion. Nat. Nanotechnol. 2015;10:237–242. doi: 10.1038/nnano.2014.317. [DOI] [PubMed] [Google Scholar]
- 14.Wang F., Liu X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009;38:976–989. doi: 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang J., Yin D., Cao X., Wang C., Song K., Liu B., Zhang L., Han Y., Wu W. Synthesis of NaLuF4-based nanocrystals and large enhancement of upconversion luminescence of NaLuF4:Gd, Yb, Er by coating an active shell for bioimaging. Dalton Trans. 2014;43:14001–14008. doi: 10.1039/C4DT00509K. [DOI] [PubMed] [Google Scholar]
- 16.Dong C., Korinek A., Blasiak A.B., Tomanek B., van Veggel F.C.J.M. Cation Exchange: A Facile Method to Make NaYF4:Yb,Tm-NaGdF4 Core-Shell Nanoparticles with a Thin, Tunable, and Uniform Shell. Chem. Mater. 2012;24:1297–1305. doi: 10.1021/cm2036844. [DOI] [Google Scholar]
- 17.Liu Q., Sun Y., Yang T., Feng W., Li C., Li F. Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in vivo. J. Am. Chem. Soc. 2011;133:17122–17125. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]
- 18.Hu S., Wu X., Chen Z., Hu P., Yan H., Tang Z., Xi Z., Liu Y. Uniform NaLuF4 nanoparticles with strong upconversion luminescence for background-free imaging of plant cells and ultralow power detecting of trace organic dyes. Mater. Res. Bull. 2016;73:6–13. doi: 10.1016/j.materresbull.2015.08.020. [DOI] [Google Scholar]
- 19.Yang T., Sun Y., Liu Q., Feng W., Yang P., Li F. Cubic sub-20 nm NaLuF4-based upconversion nanophosphors for high-contrast bioimaging in different animal species. Biomaterials. 2012;33:3733–3742. doi: 10.1016/j.biomaterials.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z., Wu X., Hu S., Hu P., Yan H., Tang Z., Liu Y. Multicolor upconversion NaLuF4 fluorescent nanoprobe for plant cell imaging and detection of sodium fluorescein. J. Mater. Chem. C. 2015;3:153–161. doi: 10.1039/C4TC01766H. [DOI] [Google Scholar]
- 21.Gao W., Dong J., Liu J.H., Yan X.W. Enhancement of red upconversion emission of cubic phase NaLuF4:Yb3+/Ho3+/Ce3+ nanocrystals. Mater. Res. Bull. 2016;80:256–262. doi: 10.1016/j.materresbull.2016.03.024. [DOI] [Google Scholar]
- 22.Liu Q., Feng W., Yang T.S., Yi T., Li F.Y. Upconversion luminescence imaging of cells and small animals. Nat. Protoc. 2013;114:2033–2044. doi: 10.1038/nprot.2013.114. [DOI] [PubMed] [Google Scholar]
- 23.Yang D., Dai Y., Ma P., Kang X., Cheng Z., Li C., Lin J. One-step synthesis of small-sized and water-soluble NaREF4 upconversion nanoparticles for in vitro cell imaging and drug delivery. Chem. Eur. J. 2013;19:2685–2694. doi: 10.1002/chem.201203634. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Huang L., Liu X. Unraveling epitaxial habits in the NaLnF4 system for color multiplexing at the single-particle level. Angew. Chem. Int. Ed. 2016;55:5718–5722. doi: 10.1002/anie.201511626. [DOI] [PubMed] [Google Scholar]
- 25.Shi F., Wang J., Zhai X., Zhao D., Qin W. Facile synthesis of β-NaLuF4:Yb/Tm hexagonal nanoplates with intense ultraviolet upconversion luminescence. CrystEngComm. 2011;13:3782–3787. doi: 10.1039/c1ce05092c. [DOI] [Google Scholar]
- 26.Wang L., Lan M., Liu Z., Qin G., Wu C., Wang X., Qin W., Huang W., Huang L. Enhanced deep-ultraviolet upconversion emission of Gd3+ sensitized by Yb3+ and Ho3+ in β-NaLuF4 microcrystals under 980 nm excitation. J. Mater. Chem. C. 2013;1:2485–2490. doi: 10.1039/c3tc00936j. [DOI] [Google Scholar]
- 27.Zheng K.Z., Liu Z.Y., Lv C.J., Qin W.P. Temperature sensor based on the UV upconversion luminescence of Gd3+ in Yb3+–Tm3+–Gd3+ codoped NaLuF4 microcrystals. J. Mater. Chem. C. 2013;1:5502–5507. doi: 10.1039/c3tc30763h. [DOI] [Google Scholar]
- 28.Wang Z., Zhang P., Yuan Q., Xu X., Lei P., Liu X., Su Y., Dong L., Feng J., Zhang H. Nd3⁺-sensitized NaLuF4 luminescent nanoparticles for multimodal imaging and temperature sensing under 808 nm excitation. Nanoscale. 2015;7:17861–17870. doi: 10.1039/C5NR04889C. [DOI] [PubMed] [Google Scholar]
- 29.Cui Y., Zhao S.L., Liang Z.Q., Han M., Xu Z. Optimized upconversion emission of NaLuF4:Er, Yb nanocrystals codoped with Gd3+ ions and its mechanism. J. Alloy. Compd. 2014;593:30–33. doi: 10.1016/j.jallcom.2014.01.051. [DOI] [Google Scholar]
- 30.Zhu W., Zhao S.L., Liang Z.Q., Yang Y.X., Zhang J.J., Xu Z. The color tuning and mechanism of upconversion emission from green to red in NaLuF4:Yb3+/Ho3+ nanocrystals by codoping with Ce3+ J. Alloy. Compd. 2016;659:146–151. doi: 10.1016/j.jallcom.2015.11.006. [DOI] [Google Scholar]
- 31.Zheng K.Z., He G.H., Song W.Y., Bi X.Q., Qin W.P. A strategy for enhancing the sensitivity of optical thermometers in β-NaLuF4:Yb3+/Er3+ nanocrystals. J. Mater. Chem. C. 2015;3:11589–11594. doi: 10.1039/C5TC02640G. [DOI] [Google Scholar]
- 32.Lin H., Xu D.K., Teng D.D., Yang S.H., Zhang Y.L. Shape-controllable synthesis and enhanced upconversion luminescence of Li+ doped β-NaLuF4:Yb3+,Ln3+ (Ln = Tm, Ho) microcrystals. New J. Chem. 2015;39:2565–2572. doi: 10.1039/C4NJ02257B. [DOI] [Google Scholar]
- 33.Lin H., Xu D.K., Li A.M., Teng D.D., Yang S.H., Zhang Y.L. Tuning of structure and enhancement of upconversion luminescence in NaLuF4:Yb3+, Ho3+ crystals. Phys. Chem. Chem. Phys. 2015;17:19515–19526. doi: 10.1039/C5CP02627J. [DOI] [PubMed] [Google Scholar]
- 34.Li C.X., Quan Z.W., Yang P.P., Huang S.S., Lian H.Z., Lin J. Shape-controllable synthesis and upconversion properties of lutetium fluoride (doped with Yb3+/Er3+) microcrystals by hydrothermal process. J. Phys. Chem. C. 2008;112:13395–13404. doi: 10.1021/jp802826k. [DOI] [Google Scholar]
- 35.Lin H., Xu D.K., Li A.M., Teng D.D., Yang S.H., Zhang Y.L. Morphology evolution and pure red upconversion mechanism of β-NaLuF4 crystals. Sci. Rep. 2016;6:28051. doi: 10.1038/srep28051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng S.J., Wang H.B., Lu W., Yi Z.G., Rao L., Liu H.R., Hao J.H. Dual-modal upconversion fluorescent/X-ray imaging using ligand-free hexagonal phase NaLuF4:Gd/Yb/Er nanorods for blood vessel visualization. Biomaterials. 2014;35:2934–2941. doi: 10.1016/j.biomaterials.2013.11.082. [DOI] [PubMed] [Google Scholar]
- 37.Liu J., Chen G.Y., Hao S.W., Yang C.H. Sub-6 nm hexagonal core/shell NaGdF4 nanocrystals with enhanced upconversion photoluminescence. Nanoscale. 2017;9:91–98. doi: 10.1039/C6NR08675F. [DOI] [PubMed] [Google Scholar]
- 38.Zhou N., Qiu P., Wang K., Fu H., Gao G., He R., Cui D. Shape-controllable synthesis of hydrophilic NaLuF4:Yb,Er nanocrystals by a surfactant-assistant two-phase system. Nanoscale Res. Lett. 2013;8:518. doi: 10.1186/1556-276X-8-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu S.G., Cao H.Y., Wu X.F., Zhan S.P., Wu Q.Y., Tang Z.J., Liu Y.X. Upconversion luminescence and magnetic turning of NaLuF4:Yb3+/Tm3+/Gd3+ nanoparticles and their application for detecting acriflavine. J. Nanomater. 2016;2016:63479. doi: 10.1155/2016/2463479. [DOI] [Google Scholar]
- 40.Gargas D.J., Chan E.M., Ostrowski A.D., Aloni S., Altoe M.V., Barnard E.S., Sanii B., Urban J.J., Milliron D.J., Cohen B.E., et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat. Nanotechnol. 2014;9:300–305. doi: 10.1038/nnano.2014.29. [DOI] [PubMed] [Google Scholar]
- 41.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Itty I.B., Bawendi M.G., Frangioni J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J.B., Lu Z.D., Yin Y.D., McRae C., Piper J.A., Dawes J.M., Jin D.Y., Goldys E.M. Upconversion luminescence with tunable lifetime in NaYF4:Yb, Er nanocrystals: Role of nanocrystal size. Nanoscale. 2013;5:944–952. doi: 10.1039/C2NR32482B. [DOI] [PubMed] [Google Scholar]
- 43.Su Y., Liu X.L., Lei P.P., Xu X., Dong L.L., Guo L.M., Yan X.X., Wang P., Song S.Y., Feng J., et al. Core–shell–shell heterostructures of α-NaLuF4: Yb/Er@NaLuF4:Yb@MF2 (M = Ca, Sr, Ba) with remarkably enhanced upconversion luminescence. Dalton Trans. 2016;45:11129–11136. doi: 10.1039/C6DT01005A. [DOI] [PubMed] [Google Scholar]
- 44.Grobner T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 45.Amuluru L., High W., Hiatt K.M., Ranville J., Shar S.V., Malik B., Swaminathan S. Metal Deposition in Calcific Uremic Arteriolopathy. J. Am. Acad. Dermatol. 2009;61:73–79. doi: 10.1016/j.jaad.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G.Y., Shen J., Ohulchanskyy T.Y., Patel N.J., Kutikov A., Li Z., Song J., Pandey R.K., Ågren H., Prasad P.N., et al. (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano. 2012;6:8280–8287. doi: 10.1021/nn302972r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao S.W., Yang L.M., Qiu H.L., Fan R.W., Yang C.H., Chen G.Y. Heterogeneous core/shell fluoride nanocrystals with enhanced upconversion photoluminescence for in vivo bioimaging. Nanoscale. 2015;7:10775–10780. doi: 10.1039/C5NR02287H. [DOI] [PubMed] [Google Scholar]
- 48.Shen J., Chen G., Ohulchanskyy T.Y., Kesseli S.J., Buchholz S., Li Z., Prasad P.N., Han G. Upconversion: Tunable Near Infrared to Ultraviolet Upconversion Luminescence Enhancement in (α-NaYF4:Yb,Tm)/CaF2 Core/Shell Nanoparticles for In situ Real-time Recorded Biocompatible Photoactivation. Small. 2013;9:3213. doi: 10.1002/smll.201370117. [DOI] [PubMed] [Google Scholar]
- 49.Prorok K., Bednarkiewice A., Cichy B., Gnach A., Misiak M., Sobczyk M., Strek W. The impact of shell host (NaYF4/CaF2) and shell deposition methods on the up-conversion enhancement in Tb3⁺, Yb3⁺ codoped colloidal α-NaYF4 core-shell nanoparticles. Nanoscale. 2014;6:1855–1864. doi: 10.1039/C3NR05412H. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y.F., Sun L.D., Xiao J.W., Feng W., Zhou J.C., Shen J., Yan C.H. Rare-earth Nanoparticles with enhanced upconverison emission and suppressed rare-earth-ion leakage. Chem. Eur. J. 2012;18:5558–5564. doi: 10.1002/chem.201103485. [DOI] [PubMed] [Google Scholar]
- 51.Chen G., Ohulchanskyy T.Y., Kumar R., Ågren H., Prasad P.N. Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano. 2010;4:3163–3168. doi: 10.1021/nn100457j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nadort A., Zhao J.B., Goldys E.M. Lanthanide upconversion luminescence at the nanoscale: Fundamentals and optical properties. Nanoscale. 2016;8:13099–13130. doi: 10.1039/C5NR08477F. [DOI] [PubMed] [Google Scholar]
- 53.Wang X., Zhuang J., Peng Q., Li Y.D. Heavy equipment operator training via virtual modeling technologies. Nature. 2005;437:121–124. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]