Abstract
The advance of replication forks to duplicate chromosomes in dividing cells requires the disassembly of nucleosomes ahead of the fork and the rapid assembly of parental and de novo histones at the newly synthesized strands behind the fork. Replication-coupled chromatin assembly provides a unique opportunity to regulate fork advance and stability. Through post-translational histone modifications and tightly regulated physical and genetic interactions between chromatin assembly factors and replisome components, chromatin assembly: (1) controls the rate of DNA synthesis and adjusts it to histone availability; (2) provides a mechanism to protect the integrity of the advancing fork; and (3) regulates the mechanisms of DNA damage tolerance in response to replication-blocking lesions. Uncoupling DNA synthesis from nucleosome assembly has deleterious effects on genome integrity and cell cycle progression and is linked to genetic diseases, cancer, and aging.
Keywords: DNA replication, chromatin assembly, DNA damage tolerance, replication fork stability, homologous recombination
1. Introduction
Chromosome duplication during cell division has to accurately maintain the genetic and epigenetic information written in the chromatin. This presents a major challenge for cells, as inferred by the number of mechanisms aimed at preventing and repairing problems generated during DNA replication [1,2]. Defective replication can lead to loss of cell fitness, developmental defects, genetic diseases, and cancer. Indeed, replication-associated genetic instability is linked to the early stages of cancer development [3], and replication stress promotes alterations in the pattern of histone-associated epigenetic marks and might fuel tumorigenesis [4,5]. A major source of genetic and epigenetic instability is generated during the advance of the replication fork, a dynamic nucleosome-free structure with single-stranded DNA (ssDNA) gaps and DNA ends susceptible to being aberrantly processed. These fragile structures have to deal with a number of obstacles such as DNA adducts, abasic sites, DNA-binding proteins and specific DNA and chromatin structures that hamper their advance and compromise their stability, and are therefore specifically controlled by the replication checkpoint to ensure their correct progression and stability.
Chromatin is built by regularly spaced nucleosomes, each of which consists of 146 bp of DNA wrapped around an octamer of two copies each of the core histones H3, H4, H2A, and H2B. The assembly of newly synthesized DNA into nucleosomes occurs during the S phase, with the deposition of an (H3/H4)2 tetramer followed by the addition of two H2A/H2B dimers at each side. A key feature of the process of chromatin assembly during DNA replication is that it occurs immediately behind DNA synthesis, with the first deposited nucleosome detected ~250 bp behind the replication fork [6]. The processes of DNA synthesis and nucleosome assembly are physically coupled and genetically co-regulated. In this review, we will focus on how histone dynamics affect replication fork progression and stability, under both unperturbed and stress conditions, and the genetic consequences that the uncoupling between DNA synthesis and histone dynamics at the fork have for genome integrity and cell cycle progression. Therefore, we will introduce only those aspects of replication-coupled nucleosome assembly that are relevant to understanding its connection with replication dynamics. We refer the readers to recent reviews for more detailed analyses of the mechanisms of chromatin assembly, with discussions on the biological functions of canonical and histone variants [7,8,9,10].
2. Building a Functional Replisome
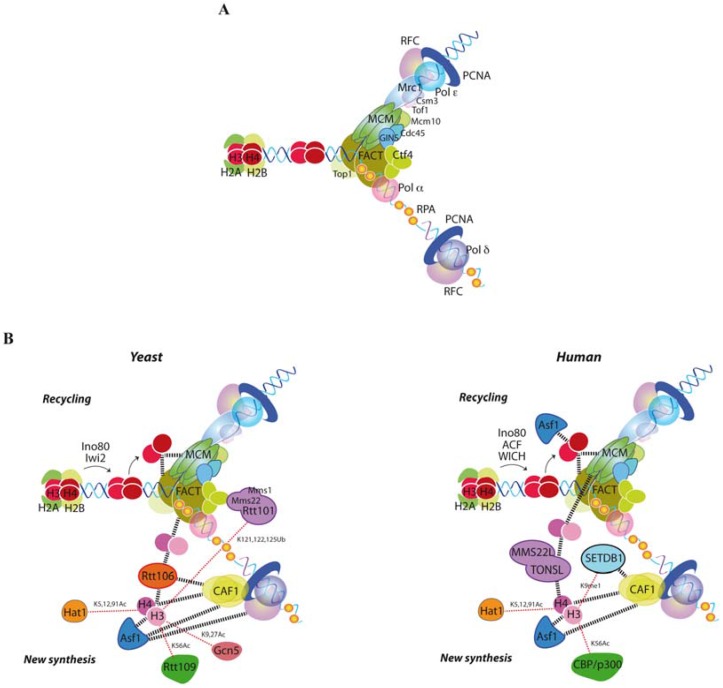
The whole genome has to be replicated once and only once during the cell cycle. This is achieved by temporally separating the loading of the replicative Mcm2-7 helicases onto the origin-recognition complexes (ORC) that mark the replication origins from their further activation. At the end of mitosis, reduction in the levels of cyclin-dependent kinase (CDK) allows the protein Cdc6 to load Mcm2-7 as a head-to-head double hexamer that embraces the double-stranded DNA (dsDNA) molecule. The lack of both CDK and the Dbf4-dependent kinase (DDK) Cdc7 at G1 maintains the helicase inactive until the S phase. During the transition between the G1 and S phase, rising levels of CDK prevent additional Mcm2-7 helicases from being loaded and, together with Cdc7, phosphorylate several subunits of Mcm2-7 and other accessory factors, which then help to recruit Cdc45 and GINS (Sld5/Psf1/Psf2/Psf3). These latter two factors and a single Mcm2-7 hexamer form the CMG (Cdc45/Mcm2-7/GINS) complex, which is the active helicase. At this stage, the two Mcm2-7 helicases are dissociated and encircle opposite ssDNA strands to promote bidirectional replication from each origin [11,12,13] (Figure 1A).
Figure 1.
Replication-coupled chromatin assembly. (A) Scheme of the eukaryotic replisome progression complex together with the replication processivity factor proliferating cell nuclear antigen (PCNA), its loader replication factor C (RFC), the single-strand (ss)DNA binding protein replication protein A (RPA) and the replicative polymerases. Only the yeast names are indicated here, though all factors are conserved in human [22]; (B) Histone modifiers, histone chaperones and histone deposition factors in yeast (left) and human (right). Dashed lines indicate physical interactions. See text for details.
Replication elongation is initiated by DNA unwinding mediated by CMG and requires the DNA synthesis activity of the three multi-subunit DNA polymerases Pol α, Pol δ and Pol ε. A primase-Pol α complex primes continuous DNA synthesis at the leading strand by Pol ε, and discontinuous synthesis of Okazaki fragments at the lagging strand by Pol δ [13,14]. The heterotrimeric ring of proliferating cell nuclear antigen (PCNA), which is loaded at the fork by the clamp loader replication factor C (RFC), is instrumental in this process due to its dual activity as a processivity factor for the polymerases and as a molecular platform that coordinates replication fork advance with chromatin assembly, sister cohesion, and DNA damage tolerance [15].
DNA unwinding and DNA synthesis need to be tightly coordinated to avoid the deleterious effects of an excess of ssDNA at the fork; indeed, ssDNA is coated and protected by the RPA (Replication protein A) complex, and RPA exhaustion causes replication fork collapse [16]. Coupling between the helicase and the polymerases is mediated by Mrc1 and Ctf4, which physically bridge Mcm2-7 with Pol ε, and GINS with Pol α, respectively [17,18], thereby regulating the rate of fork progression [19,20,21]. The replication progression complex comprises the helicase CMG and its activator Mcm10, as well as Ctf4, Mrc1, the replication fork–pausing proteins Tof1 and Csm3, the histone chaperone FACT (Facilitates chromatin transcription) and topoisomerase I [22]. Additional components are also required to replicate the genome, of which some travel continuously with the fork while others are temporarily recruited to overcome specific obstacles. Proteomic analysis of nascent chromatin has recently uncovered the presence of multiple factors involved in DNA repair, checkpoint, cohesion establishment and release of torsional stress [23,24,25].
3. Replication Fork Advance through Chromatin
Packaging DNA into nucleosomes could be expected to generate a physical barrier for replication fork advance. Indeed, the activation of the mammalian replication origins is associated with several rounds of abortive DNA synthesis that end up at the boundary of a nucleosome-free region [26], reflecting an early need for chromatin-disrupting activities during replication elongation. Paradoxically, however, the main mechanism required to help forks to progress through chromatin is the nucleosome assembly process itself (Figure 1B).
Chromatin assembly requires a huge amount of histones, which are supplied both by new synthesis and by recycling of parental histones. Subtly reducing the pool of new histones and consequently the degree of nucleosome occupancy accelerates the rate of both RNA synthesis [27] and DNA synthesis [28], and suggests that nucleosomes slow down replication fork advance. This increase in the speed of DNA synthesis, however, could also be associated with the changes in the composition of the parental chromatin that accompany the reduction of canonical histones, such as a drop in the levels of the histone variants H2A.X and H2A.Z and/or an increase in H3.3 [27].
Recycling parental histones not only saves resources but is also an essential mechanism of maintaining epigenetic information. Parental histones are removed from the nucleosomes ahead of the fork and distributed randomly between the sister chromatids [29]. Therefore, the mechanism of histone recycling provides a way to “clear” the road for the advancing replication fork.
The first step for histone recycling is the destabilization of nucleosomes ahead of the fork, which affects an average of two nucleosomes [30]. DNA unwinding by the CMG helicase activity during replication causes an accumulation of positive supercoiling ahead of the fork that might facilitate nucleosome disruption [31]. Whether or not assisted by topological activities, ATP-dependent chromatin remodeling complexes seem to be required for destabilizing the nucleosomes ahead of the fork. In yeast, Ino80 and Isw2 facilitate replication fork progression through a mechanism that becomes less dispensable as the forks move away from the replication origin [32,33,34]. These factors can be detected at stalled forks, although it is not known whether they travel with the fork or are recruited specifically in response to replication stress [32,33,34]. Ino80 is also required for replication fork progression in mammalian cells, in which it interacts with unperturbed forks via the tumor suppressor protein BAP1 and monoubiquitinated histone H2A [35,36]. Moreover, human ISWI facilitates replication elongation as part of the ACF1 and WICH complexes by a mechanism that appears to decondense chromatin [37,38], suggesting that it operates ahead of the fork by breaking higher-order chromatin structures.
A factor that might also connect nucleosome disruption and DNA synthesis at the fork is the histone fold motif-containing protein Dpb4. This protein is an integral component of Pol ε [39] and Iswi2/CHRAC, a yeast counterpart of human ACF [40]. Although its histone chaperone activity has not yet been demonstrated, Iswi2/CHRAC and Pol ε are required for heterochromatin silencing [40].
4. Replication Fork Control by Histone Recycling
The mechanisms of histone recycling are still poorly understood. However, it is well documented that the canonical histones H3 and H4 are transferred as a tetramer [29,41,42]. Recent crystal structure analyses have shown that the Mcm2 subunit of Mcm2-7, which contains a conserved region that is able to bind to the globular domain of histone H3 [43,44], can chaperone (H3-H4)2 tetramers as part of the helicase alone or with another Mcm2 [45,46]. The physiological relevance of the latter structure is unclear, as only one Mcm2-7 complex is present at the fork. However, we cannot discard that, at some particular regions and/or under specific conditions, the Mcm2-7 helicase at the fork chaperones (H3-H4)2 tetramers together with another one from the excess of Mcm2-7 molecules loaded in G1. In any case, these data open the possibility that Mcm2-H3-H4 complexes are intermediates in the process of tetramer transfer during DNA replication [45,46]. In agreement with this possibility, chromatin-bound Mcm2 associates with histone H3 trimethylated at lysine 9 (H3K9me3), a mark of parental chromatin in humans [46], and the histone-binding domain of yeast Mcm2 is required to pick up parental histones that have been released from chromatin [44].
Mcm2 mutations that disrupt its chaperone activity impair cell proliferation in human cells. Indeed, these mutations reduce the binding of Mcm2-7 to Cdc45, establishing a direct connection between histone recycling and replisome activity [46]. In yeast, Mcm2 mutants defective in histone binding are affected in heterochromatin silencing, a phenotype shared by many chromatin assembly mutants. However, they have no detectable defects in bulk replication, in part due to the fact that a second histone chaperone, Spt16, is able to pick up released histones cooperatively with Mcm2 [44]. Spt16 interacts with Pob3 to form FACT in yeast, a conserved complex with roles in transcription, repair, and replication [47]. FACT was identified as part of the yeast replisome progression complex [22], and accordingly, specific physical interactions have been reported between FACT and Pol α and between FACT and RPA in yeast [48,49], and between FACT and Mcm2-7 in yeast and humans [50,51]. In yeast, Spt16 ubiquitination by the ubiquitin ligase Rtt101 positively regulates its interaction with Mcm2-7 [51]. FACT is essential, and genetic analyses in yeast and human viable mutants have shown that it is required for replication elongation [50,52]. Notably, human FACT promotes the unwinding activity of the Mcm2-7 helicase on nucleosomal templates in vitro [50], suggesting that it could directly disassemble nucleosomes ahead of the fork to facilitate DNA synthesis during replication.
Another factor involved in recycling parental histones is Asf1, a conserved histone chaperone first described in yeast for its function in heterochromatin silencing [53] and later purified from Drosophila as a replication-coupled assembly factor (RCAF) [54]. Asf1, which is essential in mammalian cells but not in yeast, has conserved roles in transcription, DNA repair, and replication [55]. Asf1 accumulates at replication foci in Drosophila [56] and interacts with RFC in yeast [57] and Mcm2-7 in human cells [58]. Importantly, the histone dimer H3-H4 that bridges Asf1 with Mcm2-7 is specifically modified with parental marks (H4K16Ac and H3K9me3) under hydroxyurea (HU) conditions that enable accumulation of replication forks, suggesting that Mcm2-H3-H4-Asf1 can be an intermediate in the process of parental histone assembly [58]. Interestingly, Asf1 binding to (H3-H4)2 splits the tetramer in vitro and binds to the dimer in a way that occludes the H3-H4 tetramerization interface [59,60], and accordingly, a crystal structure of a ternary complex with Asf1, Mcm2 and a dimer of H3-H4 has been solved [45,46]. These results raise the possibility that, under certain conditions, the parental (H3-H4)2 tetramer is transiently split during its transfer [61].
Depletion of human Asf1 affects DNA unwinding at replication sites and leads to a reduction in the amount of ssDNA at the fork, and a similar phenotype can be obtained by impairing Asf1 function through histone overexpression [58]. These results suggest that Asf1 might facilitate DNA unwinding at the fork through its capacity to transfer histones during chromatin assembly. Accordingly, Asf1 depletion causes cell cycle arrest in fly, chicken and human cells [56,58,62]. Intriguingly, a V94R Asf1 mutant, which lacks (H3-H4)2 splitting activity and cannot form Asf1-H3-H4-Mcm2-7 complexes [58,63], enhances rather than decreases DNA synthesis in a cell-free system of Xenopus egg extracts [63]. Asf1 may therefore play a more complex role in the fine-tuning regulation of replication fork progression.
5. Mechanisms of New Histone Assembly
In addition to recycled parental histones, chromatin assembly at the fork requires the deposition of newly synthesized histones. Expression of canonical histones is activated in late G1/early S phase to ensure a rapid supply of histones during replication, and it is repressed in early G1, G2, and mitosis to prevent the deleterious consequences of an unscheduled excess of histones for DNA metabolism [64,65]. Accordingly, mutations and inhibitors that impair DNA synthesis trigger a number of mechanisms that repress new histone synthesis and buffer from an excess of histones [66,67].
Newly synthesized histones are chaperoned from the cytoplasm to the nucleus and modified post-translationally to facilitate their transfer to the chromatin assembly factors at the replication fork (Figure 1). In particular, acetylation of the amino terminal tails of H3 and H4 plays redundant roles in chromatin assembly [68]. Acetylation of lysines 5 and 12 in histone H4 by the acetyltransferase Hat1 is conserved from yeast to humans [69,70], while the acetylation pattern of the amino terminal tail of H3 is less conserved. In budding yeast, lysines 9 and 27 are the main targets and are acetylated by the acetyltransferases Rtt109 and Gcn5 [71,72], whereas a fraction of mammalian H3 is acetylated at lysines 14 and 18 [5]. Equally important for replication-coupled chromatin assembly in yeast is the acetylation of H4K91 by Hat1 [73] and of H3K56 by Rtt109 [74,75,76,77]. Indeed, the vast majority of newly synthesized histones H3 is acetylated at lysine 56 in yeast [76]. In contrast, in humans this modification is present in less than 1.5% of histone H3, and marks such as H3.1K9 monomethylation by SETDB1 characterize newly synthesized histones [5].
The contribution of yeast H3K56 acetylation to the deposition of newly synthesized histones has been extensively studied. The chaperone Asf1 plays an instrumental role in this process by binding newly synthesized H3-H4 dimers and presenting them to Rtt109 for acetylation [54,77,78,79,80]. H3K56 acetylation increases the binding affinity of the dimer H3-H4 for the histone deposition factors CAF1 and Rtt106, and the binding of CAF1 to chromatin [78]. This process is facilitated by the Rtt101Mms1/22 complex (formed by Rtt101 and the putative adaptor proteins Mms1 and Mms22) or its human ortholog Cul4DDB1 [81]. Rtt101Mms1/22, which associates with the replication progression complex during S phase [82], binds and ubiquitinates new histone H3 acetylated at lysine 56. This modification weakens Asf1-H3-H4 interactions and facilitates H3-H4 transfer to downstream chromatin assembly factors including Rtt106 [81]. It is important to note that the lack of H3K56 acetylation is not essential, in part due to the Gcn5-mediated acetylation of the H3 amino-terminal tail, which also increases the binding affinity of the dimer H3-H4 for CAF1 and Rtt106 [72].
CAF1 is a highly conserved histone chaperone that operates in the deposition of newly synthesized histones through direct and independent interactions between its three subunits and the H3-H4 dimer [83,84,85,86,87]. Although CAF1 has also been proposed to operate in the recycling of parental histones [9], direct evidence for this hypothesis is still missing. CAF1 is recruited to replication forks through direct interactions with PCNA [88,89,90] and assembles nucleosomes by a mechanism that is stimulated by physical interactions with Asf1 [54,91,92,93]. This CAF1-Asf1 histone deposition complex associates with a single H3-H4 dimer [91], although the capacity of CAF1 to form homodimers might provide the second H3-H4 dimer required for the deposition of a (H3-H4)2 tetramer [94]. Moreover, analysis of in vitro interactions has shown that CAF1 can also bind (H3-H4)2 tetramers as a monomer [95]. Interestingly, these approaches have shown that H3-H4 binding to Asf1 stimulates the Asf1-CAF1 association, whereas H3-H4 binding to CAF1 is mutually exclusive of Asf1, suggesting a H3-H4 transfer process from Asf1 to DNA via CAF1 [93].
In yeast, CAF1 interacts with the histone chaperone Rtt106 [96]. Rtt106 can also form a homodimer that directly binds to new (H3-H4)2 tetramers through a double pleckstrin-homology domain, which interacts with the K56-containing region of histone H3. Indeed, Rtt106-H3 affinity is enhanced upon H3K56 acetylation [97,98]. CAF1 and Rtt106 have redundant roles in the deposition of new histones, and only the absence of both complexes affects the process, although not enough to significantly affect cell growth [78,99]. This points to the existence of additional chromatin assembly activities. Accordingly, FACT is also involved in the deposition of new histones, as supported by the recent purification of a yeast complex formed by FACT, CAF1, Rtt106 and H3K56Ac-H4 (but not Asf1); here, FACT appears to be linked to the complex by Rtt106-H3K56Ac-H4 [99]. The characterization of a histone-interacting defective Spt16 mutant (spt16-m) affected in the deposition of new histones has shown that FACT improves both CAF1 and Rtt106 histone deposition pathways; consistently, a triple mutant cac1∆ rtt106∆ spt16-m is extremely sick [99]. Newly synthesized histone H2B is also monoubiquitinated at chromatin by the Bre1 ubiquitin ligase at lysine 123, and this modification promotes their assembly or stability [100]. Moreover, H2BK123Ub1 stabilizes Spt16 on replicated DNA, suggesting that this modification cooperates with FACT in histone deposition [100].
In human cells, Mcm2 has been purified with H4K12Ac, suggesting that this chaperone is also involved in the deposition of new histones [46]. Mcm2-7, FACT and Asf1 interact with TONSL, a chaperone without a known homolog in yeast, and its partner MMS22L, the human homologue of Mms22 [101,102,103,104]. Indeed, newly synthesized H3-H4 dimers bridge the interactions between TONSL-MMS22L with Mcm2 and Asf1, suggesting that they form a large histone pre-deposition complex [105].
6. Replication Fork Progression and Stability by New Histone Assembly
The aforementioned studies have revealed that a key feature of chromatin assembly is the redundancy of histone deposition pathways. Indeed, impaired histone deposition in CAF1-depleted human cells can partially be compensated by the HIRA-dependent mechanism of replication-independent chromatin assembly, which incorporates the histone variant H3.3 instead of the canonical H3.1 [106]. The presence of these redundant and compensatory mechanisms highlights the importance of ensuring a timely supply of histones during replication-coupled nucleosome assembly.
As shown for the recycling of parental histones, the assembly of newly synthesized histones can strongly influence replication fork advance. In agreement with this, human CAF1 is essential for S-phase progression [107,108]. Likewise, a strong inhibition of histone biosynthesis causes severe defects in nucleosome occupancy and a concomitant reduction in DNA replication in mammalian cells [109,110,111,112,113,114]. This deficit of histones is initially accompanied by an accumulation of PCNA at the forks, suggesting that one way to couple DNA synthesis and histone deposition is by regulating the CAF1-interacting platform [113]. An inverse correlation between the amount of available histones and the length of the S phase has been reported in the fruit fly [115]. These results are consistent with the idea that DNA synthesis is coupled to histone deposition. Indeed, this coupling is actively regulated; for instance, the human protein codanin-1 forms a cytosolic complex with Asf1, H3-H4, and importin-4 in a mutually exclusive interaction with CAF1 and negatively regulates both the amount of Asf1 at chromatin and the rate of DNA synthesis [116]. Along the same line, the response to histone supply is signaled through the Tousled-like kinases, which phosphorylate Asf1 to increase its binding affinity to histones and CAF1 under conditions of limited histone availability, facilitating S-phase progression [117]. In contrast to mammalian cells, partial histone depletion causes only a minor delay in completing S phase in yeast [118]. Likewise, the lack of both H3K56Ac and H2BK123Ub1 only slightly affects completion of DNA replication [100,118,119,120]. An intermediate situation is observed in the fruit fly, which can complete bulk DNA replication in the absence of newly synthesized histones, albeit very slowly [115].
These results suggest that duplication of the more demanding mammalian genome is more sensitive to defects in histone supply. However, accumulation of DNA damage, recombinogenic lesions and gross chromosomal rearrangements (GCRs) in chromatin assembly mutants is observed from yeast to humans [72,74,75,101,103,104,108,113,114,121,122,123], suggesting that the maintenance of replication fork stability is a conserved function of nucleosome assembly. Indeed, replication in asf1∆ yeast mutants is highly sensitive to the absence of the replicative checkpoints [120]. However, chromatin disassembly/assembly factors are involved in DNA repair, as this process requires access to the lesion and a further restoration of the original chromatin environment, and accordingly, chromatin assembly mutants are sensitive to genotoxic agents [124]. Moreover, many chromatin disassembly/assembly factors operate in other processes that influence genome integrity, such as transcription [55,125]. Therefore, the accumulation of genetic instability in chromatin assembly mutants might stem from defects in the repair of spontaneous DNA lesions and/or DNA processes other than replication.
A connection between nucleosome assembly and replication fork stability is provided by the loss of replisome stability in yeast chromatin assembly mutants under conditions of dNTP depletion by HU as determined by chromatin immunoprecipitation (ChIP) analyses. Thus, Ino80, which accumulates at HU-stalled forks, is required for the stability of RPA, PCNA, and Pol α at the fork [33]. In addition, the lack of either H3K56 acetylation or H2BK123 monoubiquitination causes a drop in the amount of RFC, PCNA, and Pol α the fork [57,80,100]. The lack of H2BK123 monoubiquitination also affects the stability of the CMG helicase and Pol α [100], whereas the lack of H3K56 acetylation does not affect Mcm2-7 binding to the fork but leads to an increase in Pol α and an accumulation of Mcm2-7 ahead of the fork, suggesting that H3K56 acetylation is required to couple DNA unwinding and synthesis [57]. Altogether, these results suggest a role for the assembly of new histones in the stability of stalled replication forks.
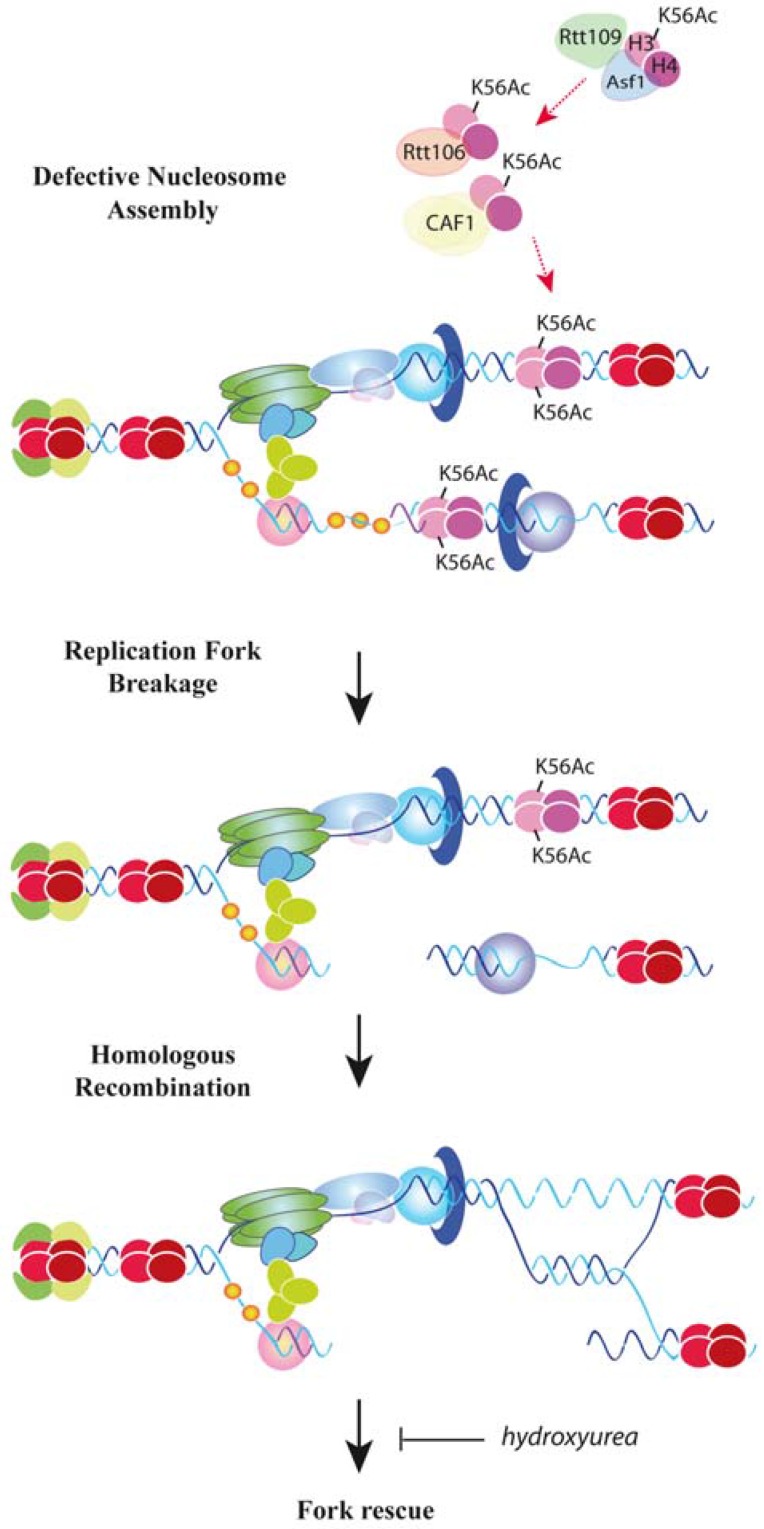
How important is chromatin assembly for the stability of advancing forks under unperturbed conditions? ChIP analyses require fork stalling by HU to detect sufficient signal. An alternative approach to avoid this limitation and assess the dynamic and stability of unperturbed advancing forks consists in following the accumulation of replication intermediates from a specific replication origin along a region in synchronized cultures by two-dimensional (2D) gel electrophoresis. Chromatin assembly mutants resulting from partial histone depletion or lack of either H3K56 acetylation (asf1∆, rtt109∆, H3K56R) or CAF1 and Rtt106 activities display a strong loss of replication intermediates that is not due to defects in replication initiation or checkpoint-mediated control of fork stability [118,119]. Importantly, the loss of replication intermediates and the subsequent accumulation of recombinogenic DNA lesions and checkpoint activation require the absence of both CAF1 and Rtt106, indicating that these two factors prevent fork collapse by redundant mechanisms [119]. Since cac1∆ rtt106∆ cells are not impaired in H3K56 acetylation [78], the loss of replication intermediates is due to defective chromatin assembly rather than the absence of H3K56Ac at chromatin. The loss of replication intermediates is similar in rtt109∆, asf1∆, cac1∆ rtt106, and asf1∆ cac1∆ rtt106∆ mutants, suggesting that the major role of H3K56Ac in fork stability is mediated by its function in nucleosome deposition [119] (Figure 2).
Figure 2.
Replication fork stability by chromatin assembly. Defective nucleosome assembly in yeast by a deficit in the pool of newly synthesized histones, or a lack of the H3K56Ac/CAF1/Rtt106 histone deposition pathway (symbolized by a dashed red arrow) causes replication fork breakage and its rescue by homologous recombination [118,119]. Uncoupling DNA synthesis from histone deposition might expose ssDNA fragments to nucleases, impair the process of Okazaki fragment maturation or alter the correct distribution of cohesins, leading to DNA breaks.
Replication fork instability in yeast cells lacking H3K56 acetylation or CAF1 and Rtt106 activities, or expressing low levels of histones is associated with the formation of recombinogenic structures, and is exacerbated in the absence of the essential recombination protein Rad52. In fact, replication and viability are strongly compromised in these mutants, indicating that defective nucleosome deposition causes fork breaks that are rescued by homologous recombination [118,119]. Accordingly, homologous recombination prevents the accumulation of GCRs induced by the double-strand break (DSB) repair process of non-homologous end joining in H3K56 acetylation mutants [123]. Cells lacking Asf1 are proficient in DSB-induced sister chromatid exchange, a type of event that they accumulate spontaneously [122], suggesting that broken forks in H3K56Ac mutants are repaired with the sister chromatid. Importantly, the loss of replication intermediates in these mutants is higher in the presence than in the absence of HU, except in cells lacking Rad52, suggesting that HU does not destabilize forks in chromatin assembly mutants but rather prevents the rescue of collapsed replication forks by exhausting the pool of dNTP required to resume DNA synthesis [119] (Figure 2). Therefore, nucleosome assembly of newly synthesized histones stabilizes advancing replication forks, and accordingly, yeast chromatin assembly mutants display a genome-wide accumulation of the DNA damage-associated phosphorylation of histone H2A at serine 129 [126].
Nucleosome assembly drives the correct maturation of Okazaki fragments at the lagging strand during replication [127], and yeast mutants defective in Okazaki fragment processing accumulate recombinogenic lesions and GCRs [128,129]. Thus, genetic instability in chromatin assembly mutants might be due to problems in Okazaki fragments’ maturation [130]. The lack of histone H3K56 acetylation in asf1∆ and rtt109∆ mutants does not alter the Okazaki fragment periodicity, as observed for mutants lacking CAF1 or Rtt106; however, asf1∆ and rtt109∆ share with cac1∆ and rtt106∆ cells the accumulation of longer unligated Okazaki fragments, likely due to a delay in histone delivery and nucleosome assembly [131]. This delay could facilitate the accumulation of ssDNA fragments at the lagging strand. In agreement with this, cells lacking Asf1 and Rtt109 accumulate two types of rearrangements that are associated with problems at the lagging strand: CAG/CTG contractions, linked to hairpin-like structures formed at ssDNA regions [132], and ribosomal DNA repeat expansions, likely initiated by fork breakage at the lagging strand [133]. Moreover, asf1 mutants are highly sensitive to mutations in Pol α and accumulate this polymerase at stalled forks [57,134]. An alternative and not mutually exclusive explanation could be that the loss of replication intermediates in chromatin assembly mutants is due to chromatin condensation, which can lead to breakage of fragile forks in yeast and humans [135,136]. Along the same line, cohesin activity is regulated at the forks by physical interactions between PCNA and yeast Eco1 (ESCO1/2 in humans), an acetyltransferase that modifies cohesins to close the ring and establish sister chromatid cohesion as the fork progresses [137]. Cohesins are required to establish and maintain nucleosome-free regions at intergenic regions [138], and aggravate chromatin defects in histone-depleted cells [139]. Moreover, H3K56 acetylation is required to maintain sister chromatid cohesion, establishing a functional connection between histone deposition and cohesin activity [140]. Further studies are required to understand how chromatin assembly protects replication forks, but it seems clear that DNA synthesis and its assembly into chromatin need to be physically and genetically coupled to maintain genome integrity.
7. DNA Damage Tolerance Control by Nucleosome Assembly
Duplicating chromosomes in the presence of DNA lesions that hamper the advance of the replication fork presents a major task for the cell. Cells are endowed with DNA damage tolerance (DDT) mechanisms to bypass the lesion and postpone its repair, thus ensuring timely completion of DNA synthesis. Replication fork lesion bypass occurs by different mechanisms that either directly copy the damaged template using specialized DNA polymerases or circumvent the lesion by switching to the intact sister chromatid template [141,142]. In the latter mechanisms, homologous recombination factors play important roles in facilitating the advance of the replication fork through the DNA lesions by not-yet understood functions [141,143,144,145,146,147,148]. In either case, these mechanisms take place in the context of nucleosome assembly, and it is therefore not surprising to find a growing amount of evidence that supports a direct role of this process in DDT regulation.
H3K56Ac is deacetylated at the end of S/G2 by the sirtuins Hst3 and Hst4, unless cells grow in the presence of DNA damage, which leads to the maintenance of the acetylation by checkpoint-mediated degradation of Hst3/Hst4 [76,140,149,150]. This suggests a role for this modification in the DNA damage response. Indeed, yeast cells defective in H3K56 acetylation are highly sensitive to DNA lesions that trigger the DDT response, namely to methyl-methane sulfonate (MMS)-induced alkylated bases and camptothecin (CPT)-induced Top1-DNA adducts [76]. Genetic analyses suggest that H3K56Ac operates upstream of the Rtt101Mms1/Mms22 ubiquitin ligase complex to resist these genotoxic agents [151,152]. As mentioned above, these sensitivities are partially due to the role of chromatin assembly factors in DNA repair processes such as base excision repair and nucleotide excision repair [124]. More direct evidence for a role of H3K56Ac in DDT comes from the specific requirement of Asf1/Rtt109/H3K56Ac and Rtt101Mms1/Mms22 to replicate MMS-damaged DNA [152,153,154]. This function is unlikely related to the role of chromatin assembly in fork stability, because cells lacking CAF1 and Rtt106 are much less sensitive to MMS and CPT than H3K56 acetylation mutants, even though they all display a similar loss of replication intermediates [119]. Likewise, mutants defective in the Asf1/Rtt109/H3K56Ac/Rtt101Mms1/22 pathway but not in CAF1 Rtt106 are lethal in the absence of the helicase Rrm3 [155], which is required to overcome nonhistone protein-DNA complexes [156]. Along the same line, cells expressing a histone H3K56E mutant are proficient in histone binding to CAF1 and Rtt106 but are sensitive to genotoxic agents [157].
Components of the Asf1/Rtt109/H3K56Ac/Rtt101Mms1/22 pathway are required for the recombinational repair of MMS and CPT-induced replicative DNA lesions but not of DSBs [122,152,153,158]. Consistently, mutants defective in this pathway have problems in checkpoint recovery after drug treatment [119,158]. This suggests a function for this pathway in the template switching mechanism of DDT. Notably, the sensitivity to genotoxic agents and the lethality in the absence of Rrm3 of Asf1/Rtt109/H3K56Ac/Rtt101Mms1/22 mutants can be suppressed by mutations in Ctf4, Mrc1, Dpb4, or Mcm6, which uncouple the CMG helicase from the DNA polymerases [82,155]. Moreover, the absence of Mrc1 restores MMS-induced recombination in cells lacking Rtt101Mms1/22 [82]. Therefore, H3K56Ac deposition appears to promote the ubiquitination of some unknown substrate to uncouple the replicative helicase from the polymerases as a prerequisite for the recombinational bypass of the blocking lesion (Figure 3, bottom). Consistent with this model, Rtt101Mms1/22 physically interacts with Ctf4 though the amino-terminal tail of Mms22, and this interaction is necessary for the function of H3K56Ac in tolerating replicative stress [82,155]. The targets and effects of the ubiquitination are unknown; Mrc1 and Ctf4 are putative targets, but they remain at the forks after DNA damage, indicating that, if targeted, ubiquitination does not lead to their degradation [82,155]. FACT is ubiquitinated by Rtt101 but in an Mms1/Mms22-independent manner [51]. Histones are also potential targets, as they are ubiquitinated in humans by CUL4DDB in response to UV-induced photodimers, and this modification weakens their interaction with DNA and facilitates the recruitment of repair proteins [159]. Finding the target of Rtt101Mms1/22/CUL4ADDB1 is therefore an essential task for the future for understanding how newly deposited histones facilitate fork progression through DNA lesions.
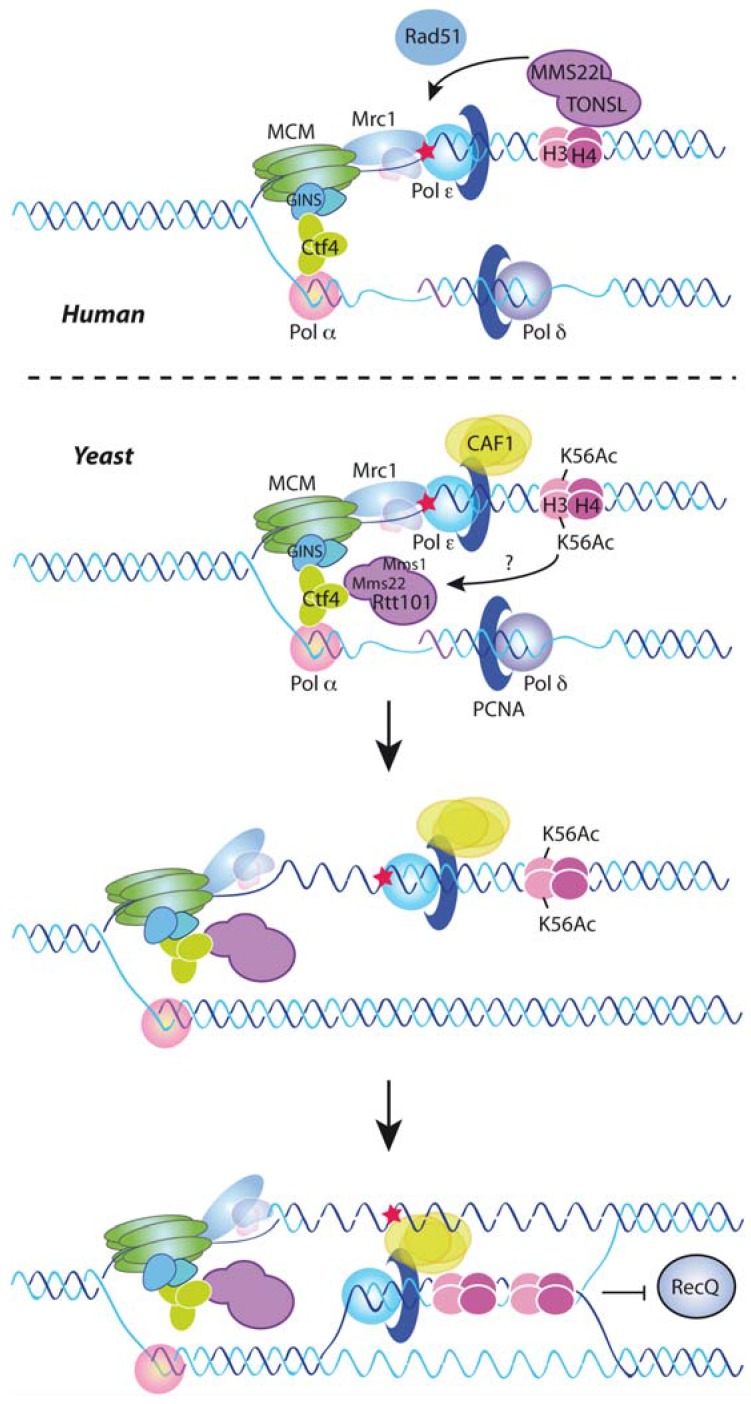
Figure 3.
DNA damage tolerance by chromatin assembly. Encounter of a replication fork with DNA lesions that hamper its advance triggers the DNA damage tolerance (DDT) response. In yeast, replication-coupled deposition of newly synthesized histones marks chromatin at the fork with acetylated H3K56, which in turn activates the ubiquitin ligase activity of the Rtt101Mms1/Mms22 complex through an unknown mechanism. Ubiquitination of an unknown factor appears to act upon Mrc1 and Ctf4 and uncouple the helicase CMG from the polymerases, facilitating the recombinational bypass of the blocking lesion [82,155]. Moreover, the chromatin assembly factor CAF1 interacts with and counteracts the D-loop dissociation activity of the RecQ helicase, either directly or by assembling nucleosomes onto the D-loop [161]. In human cells, deposition of newly synthesized histones is performed by MMS22L-TONSL, which also binds to unmethylated H3-H4K20 (H3-H4K20me0) and promotes the recruitment of Rad51 and the recombinational rescue of the forks [105].
The human MMS22L-TONSL complex displays remarkable functional similarities with the yeast Rtt101Mms1/22 complex. Cells lacking the MMS22L-TONSL complex are highly sensitive to CPT but not to ionizing irradiation-induced DSBs [101,102,103,104]. Moreover, the MMS22L-TONSL complex is required for CPT-induced Rad51 recruitment and homologous recombination, suggesting a role in the recombinational rescue of stressed replication forks [101,104]. Accordingly, the absence of the MMS22L-TONSL complex impairs replication fork progression in the presence of CPT [101,104]. Indeed, MMS22L-TONSL is also necessary for DNA synthesis in the absence of genotoxic agents [104], which likely reflects the requirement of homologous recombination for replication fork progression under unperturbed conditions in mammalian cells [148]. As previously mentioned, less than 1.5% of total histone H3 is acetylated at lysine 56 [5], and this amount does not change during the cell cycle [160]. However, human cells incorporate unmethylated H3-H4K20 histones (H3-H4K20me0) that become methylated in late G2/M [105]. Strikingly, MMS22L-TONSL is able to bind not only to newly synthesized soluble histones, as part of a pre-deposition complex with Mcm2 and Asf1, but also to H4K20me0 at nascent chromatin, where it accumulates in the presence of CPT and facilitates the recombinational response to replicative stress likely by recruiting/stabilizing Rad51 [105] (Figure 3, top).
A direct role for CAF1 in promoting Rad51-mediated replication fork bypass has been recently reported in yeast [161]. Template switching requires Rad51-mediated DNA strand invasion and strand-exchange, which lead to the formation of a D-loop structure that precedes a sister-chromatid junction [141]. D-loop formation and stability are negatively regulated by the dissociation activity of RecQ-type helicases to prevent unscheduled recombination events [162]. Remarkably, CAF1 interacts physically with the Schizosaccharomyces pombe RecQ helicase Rqh1 and promotes replication fork bypass by counteracting D-loop dissociation by Rqh1, suggesting that nucleosome assembly makes the D-loop refractory to the antirecombinogenic activity of Rqh1 [161] (Figure 3, bottom). The physical interaction is conserved between CAF1 and the RecQ-helicase Bloom in human cells, where both factors accumulate at centers of DNA replication in a manner that is stimulated by replicative stress and promotes survival [163].
8. Replication-Coupled Chromatin Assembly and Disease
Alterations in genome integrity and chromatin structure are something frequently observed in a large number of diseases including neurological and developmental disorders [164] and cancer [165], as well as during senescence and aging [166,167]. In this review, we provide strong evidence for concluding that chromatin assembly defects during replication can cause replication stress and DNA damage. Uncoupling of DNA replication and chromatin assembly in response to replicative stress can also trigger changes in the epigenome that might fuel cancer [4,5]. In agreement with these alterations in genetic and epigenetic information, enzymes involved in histone metabolism and chromatin assembly are frequently mutated in many diseases and tumors [8]. However, these enzymes play key roles in several cellular processes simultaneously, making it difficult to assess which process(es) are affected in these mutants that are directly involved in the development of each disease.
Key factors involved in chromatin assembly during replication, such as Asf1, CAF1, and FACT, are usually overexpressed (rather than absent or mutated) in tumors and are most likely required for tumor proliferation [168,169,170]. This feature strongly argues in favor of the idea that chromatin assembly during replication constitutes an essential process in which cells are unable to tolerate mutations that interfere with it. Thus, genome instability mediated through defects in nucleosome assembly during replication must arise from mutations that are able to produce minor or transient defects in this process. Here we will specifically highlight some diseases that may be directly linked to defects in replication-coupled nucleosome assembly.
Wolf-Hirschhorn syndrome (WHS) is one of the first examples in the literature of a genetic disorder that might be associated with a defect in chromatin replication. This clinically variable and complex genetic disorder is caused by a partial deletion of the distal part of chromosome 4 (4p16.3), which usually leads to a haploinsufficiency of stem-loop binding protein (SLBP) [171]. SLBP is a key factor involved in histone metabolism and is required for the efficient processing, transport, translation and stability of histone mRNAs [172]. Cell lines derived from WHS patients exhibit a delay in S-phase progression, fewer histones associated to DNA, and a higher amount of soluble histones associated to histone chaperones [171]. SLBP depletion recapitulates all these phenotypes observed in cell lines from WHS-patients [113]. This feature suggests that chromatin assembly defects during replication may be directly involved in the development of this syndrome. Interestingly, SLBP has quite recently been shown to be a target of arsenic, a common carcinogenic agent that promotes genome instability [173,174].
Viral infections are also strongly connected to changes in host chromatin and may trigger genome instability through a negative regulation of histone biosynthesis. Many viral pathogens need to change and modify chromatin structure in order to facilitate processes such as transcription, integration, replication, and latency. These viruses usually secrete proteins that either directly change the chromatin structure or that modify the function of host proteins, which will then accomplish this function [175]. Tax is a viral protein secreted by the human T-cell leukemia/lymphotropic virus type-I (HTLV-1) that acts as an oncogene and promotes adult T-cell leukemia/lymphoma (ATLL). Tax expression in cells leads to genome instability and promotes the formation of multinucleated giant cells, in a process that is thought to facilitate ATLL, but that remains poorly understood. Notably, Tax can target and decrease the amount of histones present in the cell during DNA replication and has been proposed to be an enhancer of genome instability upon HTLV-1 infection [176]. HIV infection also enhances genome instability and promotes the formation of several types of lymphomas [177]. Interestingly, a recent report from patients infected with HIV has inversely correlated the ability of this retrovirus to self-replicate with the levels of SLBP present in the cell [178].
Congenital dyserythropoietic anemia type I (CDAI) is another disease recently connected to replication-coupled chromatin assembly. This rare anemic disorder is caused by mutations in the gene that encodes codanin-1, CDAN1 [179]. Codanin-1 interacts with Asf1 in the cytoplasm through the same region as CAF1 and this interaction presumably regulates the amount of Asf1 at chromatin and the rate of DNA synthesis. Indeed, a conditional cell line expressing a codanin-1 R714 mutant, which is present in some CDAI patients, is unable to maintain Asf1 in the cytoplasm, providing a possible explanation for how mutations in CDAN1 promote this disease [116].
Recent evidence in the literature also links chromatin replication to telomere maintenance in cancer. Alternative lengthening of telomeres (ALT) constitutes an alternative pathway to telomerase reactivation present in approximately 10% of tumors and is thought to preserve telomere length through a recombination-dependent mechanism [180]. Asf1 depletion promotes ALT in a process that exclusively takes places in cells with long telomeres and that requires a functional intra-S-phase checkpoint. Uncoupling of chromatin assembly and DNA replication in the absence of Asf1 appears to impair replication through telomeres leading to fork collapse and hyper-recombination [181]. Asf1 depletion constitutes the first reported case of a direct induction of ALT in human cells, which strongly argues in favor of chromatin replication playing a central role in the generation of ALT. However, Asf1 mutations are rare in cancer suggesting either that ALT formation in tumors is not related to a defect in Asf1 function or that other mutations can target Asf1 function indirectly.
Finally, a connection between chromatin assembly and replicative senescence has been uncovered with the observation that cells, from yeast to human, induce histone depletion during telomere shortening [182,183,184]. In human cells, histone depletion is accompanied by down-regulation of SLBP, CAF1, and Asf1 and leads to a loss of nucleosome occupancy and epigenetic information at telomeres, which activates the DNA damage response and accelerates the program of replicative senescence [183]. Likewise, old yeast and quiescent skeletal muscle stem cells have reduced levels of canonical histones [185,186], which lead to transcriptional misregulation and genome instability [187].
9. Concluding Remarks
An integrative view of DNA replication cannot be presented without taking into consideration the process of nucleosome assembly. The main players of DNA synthesis and histone deposition have been biochemically defined. We now describe several examples of the importance of chromatin assembly for replication fork progression and stability through undamaged and damaged templates. Although we have focused on how H3-H4 assembly influences replication fork progression and stability, it is highly likely that the dynamics of both H2A-H2B and histone variants also have a direct impact on the replication process. Indeed, we cannot establish to what extent the replication defects associated with mutations in FACT are related to its function as a chaperone of H2A-H2B dimers [47]. The same can be argued for the reported replicative defects in cells depleted of canonical histones [109,110,111,112,113,114,115,118].
Further advances in this field demand a direct approach to determine the mechanisms by which cells sense histone availability and signal this information to the replication apparatus, to adjust replication fork speed to histone supply. Nucleosome stability maintains the integrity of advancing replication forks, but the functional mechanisms remain elusive. Likewise, we are just starting to appreciate the complexity and relevance of newly assembled chromatin structures during DNA damage tolerance (DDT). In all these cases, global and local factors are likely to play important roles. Indeed, some aspects of the process of replication-dependent chromatin assembly have not been discussed here because there is still little evidence connecting them to the advance and stability of the replication forks. These include the timing of chromatin duplication (early, middle, or late S phase), the asymmetric inheritance of parental histones (and therefore of epigenetic information) at specific loci, the local chromatin states (e.g., euchromatin versus heterochromatin or loci-specific chromatin marks), and the physiological context (e.g., development) [10]. A detailed understanding of these processes may be particularly relevant to revealing the functions of nucleosome assembly in the replication of the more demanding genomes of higher eukaryotes. Finally, it is also worth mentioning that although we have focused on how chromatin assembly regulates DNA replication, the influence is reciprocal. For instance, replication stress generated by replisome impediments facilitates the formation of heterochromatin [188]. The combination of genetic and biochemical approaches with genome-wide analyses may help to unveil the kinetics of chromosome duplication under these different scenarios, and to understand the molecular basis of how defective replication-coupled chromatin assembly contributes to genetic diseases, cancer, and aging.
Acknowledgments
We thank Maria Gomez and Cristina Gonzalez-Aguilera for critical reading of the manuscript. Douglas Maya is a recipient of a postdoctoral grant from the Andalusian government, which funded this work together with the Spanish government (BFU2015-63698-P and P12-CTS-2270).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ciccia A., Elledge S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berti M., Vindigni A. Replication stress: Getting back on track. Nat. Struct. Mol. Biol. 2016;23:103–109. doi: 10.1038/nsmb.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halazonetis T.D., Gorgoulis V.G., Bartek J. An Oncogene-Induced DNA Damage Model for Cancer Development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 4.Jasencakova Z., Groth A. Replication stress, a source of epigenetic aberrations in cancer? Bioessays. 2010;32:847–855. doi: 10.1002/bies.201000055. [DOI] [PubMed] [Google Scholar]
- 5.Jasencakova Z., Scharf A.N.D., Ask K., Corpet A., Imhof A., Almouzni G., Groth A. Replication stress interferes with histone recycling and predeposition marking of new histones. Mol. Cell. 2010;37:736–743. doi: 10.1016/j.molcel.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Sogo J.M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J. Mol. Biol. 1986;189:189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- 7.Henikoff S., Smith M.M. Histone variants and epigenetics. Cold Spring Harb. Perspect. Biol. 2015 doi: 10.1101/cshperspect.a019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess R.J., Zhang Z. Histone chaperones in nucleosome assembly and human disease. Nat. Struct. Mol. Biol. 2013;20:14–22. doi: 10.1038/nsmb.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almouzni G., Cedar H. Maintenance of Epigenetic Information. Cold Spring Harb. Perspect. Biol. 2016 doi: 10.1101/cshperspect.a019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alabert C., Groth A. Chromatin replication and epigenome maintenance. Nat. Rev. Mol. Cell Biol. 2012;13:153–167. doi: 10.1038/nrm3288. [DOI] [PubMed] [Google Scholar]
- 11.Deegan T.D., Diffley J.F.X. MCM: One ring to rule them all. Curr. Opin. Struct. Biol. 2016;37:145–151. doi: 10.1016/j.sbi.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010;24:1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell S.P., Labib K. Chromosome Duplication in Saccharomyces cerevisiae. Genetics. 2016;203:1027–1067. doi: 10.1534/genetics.115.186452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stillman B. DNA Polymerases at the Replication Fork in Eukaryotes. Mol. Cell. 2008;30:259–260. doi: 10.1016/j.molcel.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moldovan G.-L., Pfander B., Jentsch S. PCNA, the Maestro of the Replication Fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Toledo L.I., Altmeyer M., Rask M.-B., Lukas C., Larsen D.H., Povlsen L.K., Bekker-Jensen S., Mailand N., Bartek J., Lukas J. ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell. 2013;155:1088–1103. doi: 10.1016/j.cell.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 17.Simon A.C., Zhou J.C., Perera R.L., van Deursen F., Evrin C., Ivanova M.E., Kilkenny M.L., Renault L., Kjaer S., Matak-Vinkovic D., et al. A Ctf4 trimer couples the CMG helicase to DNA polymerase α in the eukaryotic replisome. Nature. 2015;510:293–297. doi: 10.1038/nature13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou H., Komata M., Katou Y., Guan Z., Reis C.C., Budd M., Shirahige K., Campbell J.L. Mrc1 and DNA Polymerase ɛ Function Together in Linking DNA Replication and the S Phase Checkpoint. Mol. Cell. 2008;32:106–117. doi: 10.1016/j.molcel.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tourriere H., Versini G., Cordon-Preciado V., Alabert C., Pasero P. Mrc1 and Tof1 Promote Replication Fork Progression and Recovery Independently of Rad53. Mol. Cell. 2005;19:699–706. doi: 10.1016/j.molcel.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Gambus A., van Deursen F., Polychronopoulos D., Foltman M., Jones R.C., Edmondson R.D., Calzada A., Labib K. A key role for Ctf4 in coupling the MCM2-7 helicase to DNA polymerase alpha within the eukaryotic replisome. EMBO J. 2009;28:2992–3004. doi: 10.1038/emboj.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szyjka S.J., Viggiani C.J., Aparicio O.M. Mrc1 Is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell. 2005;19:691–697. doi: 10.1016/j.molcel.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Gambus A., Jones R.C., Sanchez-Diaz A., Kanemaki M., van Deursen F., Edmondson R.D., Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 2006;8:358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 23.Sirbu B.M., Couch F.B., Feigerle J.T., Bhaskara S., Hiebert S.W., Cortez D. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes Dev. 2011;25:1320–1327. doi: 10.1101/gad.2053211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Contreras A.J., Ruppen I., Nieto-Soler M., Murga M., Rodriguez-Acebes S., Remeseiro S., Rodrigo-Perez S., Rojas A.M., Mendez J., Munoz J., et al. A Proteomic Characterization of Factors Enriched at Nascent DNA Molecules. Cell Rep. 2013;3:1105–1116. doi: 10.1016/j.celrep.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alabert C., Bukowski-Wills J.-C., Lee S.-B., Kustatscher G., Nakamura K., de Lima Alves F., Menard P., Mejlvang J., Rappsilber J., Groth A. Nascent chromatin capture proteomics determines chromatin dynamics during DNA replication and identifies unknown fork components. Nat. Cell Biol. 2014;16:281–293. doi: 10.1038/ncb2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez M., Antequera F. Overreplication of short DNA regions during S phase in human cells. Genes Dev. 2008;22:375–385. doi: 10.1101/gad.445608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimeno-Gonzalez S., Payan-Bravo L., Munoz-Cabello A.M., Guijo M., Gutierrez G., Prado F., Reyes J.C. Defective histone supply causes changes in RNA polymerase II elongation rate and cotranscriptional pre-mRNA splicing. Proc. Natl. Acad. Sci. USA. 2015;112:14840–14845. doi: 10.1073/pnas.1506760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almeida R., SantaMaría C., Gomez M. (Centro de Biología Molecular “Severo Ochoa” (CBMSO/CSIC), Madrid, Spain). Personal communication. 2017.
- 29.Annunziato A. The Fork in the Road: Histone Partitioning During DNA Replication. Genes. 2015;6:353–371. doi: 10.3390/genes6020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasser R., Koller T., Sogo J.M. The stability of nucleosomes at the replication fork. J. Mol. Biol. 1996;258:224–239. doi: 10.1006/jmbi.1996.0245. [DOI] [PubMed] [Google Scholar]
- 31.Corless S., Gilbert N. Effects of DNA supercoiling on chromatin architecture. Biophys. Rev. 2016;8:1–14. doi: 10.1007/s12551-016-0242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent J.A., Kwong T.J., Tsukiyama T. ATP-dependent chromatin remodeling shapes the DNA replication landscape. Nat. Struct. Mol. Biol. 2008;15:477–484. doi: 10.1038/nsmb.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papamichos-Chronakis M., Peterson C.L. The Ino80 chromatin-remodeling enzyme regulates replisome function and stability. Nat. Struct. Mol. Biol. 2008;15:338–345. doi: 10.1038/nsmb.1413. [DOI] [PubMed] [Google Scholar]
- 34.Shimada K., Oma Y., Schleker T., Kugou K., Ohta K., Harata M., Gasser S.M. Ino80 Chromatin Remodeling Complex Promotes Recovery of Stalled Replication Forks. Curr. Biol. 2008;18:566–575. doi: 10.1016/j.cub.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 35.Lee H.-S., Lee S.-A., Hur S.-K., Seo J.-W., Kwon J. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat. Commun. 2014 doi: 10.1038/ncomms6128. [DOI] [PubMed] [Google Scholar]
- 36.Vassileva I., Yanakieva I., Peycheva M., Gospodinov A., Anachkova B. The mammalian INO80 chromatin remodeling complex is required for replication stress recovery. Nucleic Acids Res. 2014;42:9074–9086. doi: 10.1093/nar/gku605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poot R.A., Bozhenok L., van den Berg D.L.C., Steffensen S., Ferreira F., Grimaldi M., Gilbert N., Ferreira J., Varga-Weisz P.D. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat. Cell Biol. 2004;6:1236–1244. doi: 10.1038/ncb1196. [DOI] [PubMed] [Google Scholar]
- 38.Collins N., Poot R.A., Kukimoto I., Garcia-Jimenez C., Dellaire G., Varga-Weisz P.D. An ACF1–ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat. Genet. 2002;32:627–632. doi: 10.1038/ng1046. [DOI] [PubMed] [Google Scholar]
- 39.Ohya T., Maki S., Kawasaki Y., Sugino A. Structure and function of the fourth subunit (Dpb4p) of DNA polymerase epsilon in Saccharomyces cerevisiae. Nucleic Acids Res. 2000;28:3846–3852. doi: 10.1093/nar/28.20.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iida T., Araki H. Noncompetitive counteractions of DNA polymerase epsilon and ISW2/yCHRAC for epigenetic inheritance of telomere position effect in Saccharomyces cerevisiae. Mol. Cell. Biol. 2004;24:217–227. doi: 10.1128/MCB.24.1.217-227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M., Long C., Chen X., Huang C., Chen S., Zhu B. Partitioning of Histone H3-H4 Tetramers During DNA Replication-Dependent Chromatin Assembly. Science. 2010;328:94–98. doi: 10.1126/science.1178994. [DOI] [PubMed] [Google Scholar]
- 42.Katan-Khaykovich Y., Struhl K. Splitting of H3-H4 tetramers at transcriptionally active genes undergoing dynamic histone exchange. Proc. Natl. Acad. Sci. USA. 2011;108:1296–1301. doi: 10.1073/pnas.1018308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishimi Y., Komamura Y., You Z., Kimura H. Biochemical function of mouse minichromosomemaintenance 2 protein. J. Biol. Chem. 1998;273:8369–8375. doi: 10.1074/jbc.273.14.8369. [DOI] [PubMed] [Google Scholar]
- 44.Foltman M., Evrin C., De Piccoli G., Jones R.C., Edmondson R.D., Katou Y., Nakato R., Shirahige K., Labib K. Eukaryotic Replisome Components Cooperate to Process Histones During Chromosome Replication. Cell Rep. 2013;3:892–904. doi: 10.1016/j.celrep.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 45.Richet N., Liu D., Legrand P., Velours C., Corpet A., Gaubert A., Bakail M., Moal-Raisin G., Guerois R., Compper C., et al. Structural insight into how the human helicase subunit MCM2 may act as a histone chaperone together with ASF1 at the replication fork. Nucleic Acids Res. 2015;43:1905–1917. doi: 10.1093/nar/gkv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H., Stromme C.B., Saredi G., Hodl M., Strandsby A., Gonzalez-Aguilera C., Chen S., Groth A., Patel D.J. A unique binding mode enables MCM2 to chaperone histones H3-H4 at replication forks. Nat. Struct. Mol. Biol. 2015;22:618–626. doi: 10.1038/nsmb.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Formosa T. The role of FACT in making and breaking nucleosomes. Biochim. Biophys. Acta. 2012;1819:247–255. doi: 10.1016/j.bbagrm.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanDemark A.P., Blanksma M., Ferris E., Heroux A., Hill C.P., Formosa T. The Structure of the yFACT Pob3-M Domain, Its Interaction with the DNA Replication Factor RPA, and a Potential Role in Nucleosome Deposition. Mol. Cell. 2006;22:363–374. doi: 10.1016/j.molcel.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Wittmeyer J., Formosa T. The Saccharomyces cerevisiae DNA polymerase alpha catalytic subunit interacts with Cdc68/Spt16 and with Pob3, a protein similar to an HMG1-like protein. Mol. Cell. Biol. 1997;17:4178–4190. doi: 10.1128/MCB.17.7.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan B.C.-M., Chien C.-T., Hirose S., Lee S.-C. Functional cooperation between FACT and MCM helicase facilitates initiation of chromatin DNA replication. EMBO J. 2006;25:3975–3985. doi: 10.1038/sj.emboj.7601271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han J., Li Q., McCullough L., Kettelkamp C., Formosa T., Zhang Z. Ubiquitylation of FACT by the Cullin-E3 ligase Rtt101 connects FACT to DNA replication. Genes Dev. 2010;24:1485–1490. doi: 10.1101/gad.1887310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlesinger M.B., Formosa T. POB3 is required for both transcription and replication in the yeast Saccharomyces cerevisiae. Genetics. 2000;155:1593–1606. doi: 10.1093/genetics/155.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le S., Davis C., Konopka J.B., Sternglanz R. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast. 1997;13:1029–1042. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1029::AID-YEA160>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Tyler J.K., Adams C.R., Chen S.R., Kobayashi R., Kamakaka R.T., Kadonaga J.T. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature. 1999;402:555–560. doi: 10.1038/990147. [DOI] [PubMed] [Google Scholar]
- 55.Mousson F., Ochsenbein F., Mann C. The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways. Chromosoma. 2006;116:79–93. doi: 10.1007/s00412-006-0087-z. [DOI] [PubMed] [Google Scholar]
- 56.Schulz L.L., Tyler J.K. The histone chaperone ASF1 localizes to active DNA replication forks to mediate efficient DNA replication. FASEB J. 2006;20:488–490. doi: 10.1096/fj.05-5020fje. [DOI] [PubMed] [Google Scholar]
- 57.Franco A.A., Lam W.M., Burgers P.M., Kaufman P.D. Histone deposition protein Asf1 maintains DNA replisome integrity and interacts with replication factor C. Genes Dev. 2005;19:1365–1375. doi: 10.1101/gad.1305005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groth A., Corpet A., Cook A.J.L., Roche D., Bartek J., Lukas J., Almouzni G. Regulation of Replication Fork Progression Through Histone Supply and Demand. Science. 2007;318:1928–1931. doi: 10.1126/science.1148992. [DOI] [PubMed] [Google Scholar]
- 59.Natsume R., Eitoku M., Akai Y., Sano N., Horikoshi M., Senda T. Structure and function of the histone chaperone CIA/ASF1 complexed with histones H3 and H4. Nature. 2007;446:338–341. doi: 10.1038/nature05613. [DOI] [PubMed] [Google Scholar]
- 60.English C.M., Adkins M.W., Carson J.J., Churchill M.E.A., Tyler J.K. Structural Basis for the Histone Chaperone Activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clement C., Almouzni G. MCM2 binding to histones H3-H4 and ASF1 supports a tetramer-to-dimer model for histone inheritance at the replication fork. Nat. Struct. Mol. Biol. 2015;22:587–589. doi: 10.1038/nsmb.3067. [DOI] [PubMed] [Google Scholar]
- 62.Sanematsu F., Takami Y., Barman H.K., Fukagawa T., Ono T., Shibahara K.-I., Nakayama T. Asf1 is required for viability and chromatin assembly during DNA replication in vertebrate cells. J. Biol. Chem. 2006;281:13817–13827. doi: 10.1074/jbc.M511590200. [DOI] [PubMed] [Google Scholar]
- 63.Ishikawa K., Ohsumi T., Tada S., Natsume R., Kundu L.R., Nozaki N., Senda T., Enomoto T., Horikoshi M., Seki M. Roles of histone chaperone CIA/Asf1 in nascent DNA elongation during nucleosome replication. Genes Cells. 2011;16:1050–1062. doi: 10.1111/j.1365-2443.2011.01549.x. [DOI] [PubMed] [Google Scholar]
- 64.Osley M.A. The regulation of histone synthesis in the cell cycle. Annu. Rev. Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- 65.Marzluff W.F., Duronio R.J. Histone mRNA expression: Multiple levels of cell cycle regulation and important developmental consequences. Curr. Opin. Cell Biol. 2002;14:692–699. doi: 10.1016/S0955-0674(02)00387-3. [DOI] [PubMed] [Google Scholar]
- 66.Maya D., Morillo-Huesca M., Delgado L., Chavez S., Munoz-Centeno M.-C. A Histone Cycle. In: Stuart D., editor. The Mechanisms of DNA Replication. InTech; Rijeka, Croatia: 2013. [Google Scholar]
- 67.Singh R.K., Liang D., Gajjalaiahvari U.R., Kabbaj M.-H.M., Paik J., Gunjan A. Excess histone levels mediate cytotoxicity via multiple mechanisms. Cell Cycle. 2010;9:4236–4244. doi: 10.4161/cc.9.20.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ling X., Harkness T.A., Schultz M.C., Fisher-Adams G., Grunstein M. Yeast histone H3 and H4 amino termini are important for nucleosome assembly in vivo and in vitro: Redundant and position-independent functions in assembly but not in gene regulation. Genes Dev. 1996;10:686–699. doi: 10.1101/gad.10.6.686. [DOI] [PubMed] [Google Scholar]
- 69.Ai X., Parthun M.R. The nuclear Hat1p/Hat2p complex: A molecular link between type B histone acetyltransferases and chromatin assembly. Mol. Cell. 2004;14:195–205. doi: 10.1016/S1097-2765(04)00184-4. [DOI] [PubMed] [Google Scholar]
- 70.Sobel R.E., Cook R.G., Perry C.A., Annunziato A.T., Allis C.D. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc. Natl. Acad. Sci. USA. 1995;92:1237–1241. doi: 10.1073/pnas.92.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fillingham J., Recht J., Silva A.C., Suter B., Emili A., Stagljar I., Krogan N.J., Allis C.D., Keogh M.C., Greenblatt J.F. Chaperone Control of the Activity and Specificity of the Histone H3 Acetyltransferase Rtt109. Mol. Cell. Biol. 2008;28:4342–4353. doi: 10.1128/MCB.00182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burgess R.J., Zhou H., Han J., Zhang Z. A Role for Gcn5 in Replication-Coupled Nucleosome Assembly. Mol. Cell. 2010;37:469–480. doi: 10.1016/j.molcel.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye J., Ai X., Eugeni E.E., Zhang L., Carpenter L.R., Jelinek M.A., Freitas M.A., Parthun M.R. Histone H4 Lysine 91 Acetylation. Mol. Cell. 2005;18:123–130. doi: 10.1016/j.molcel.2005.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J., Zhou H., Horazdovsky B., Zhang K., Xu R.M., Zhang Z. Rtt109 Acetylates Histone H3 Lysine 56 and Functions in DNA Replication. Science. 2007;315:653–655. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- 75.Driscoll R., Hudson A., Jackson S.P. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315:649–652. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masumoto H., Hawke D., Kobayashi R., Verreault A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–298. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- 77.Tsubota T., Berndsen C.E., Erkmann J.A., Smith C.L., Yang L., Freitas M.A., Denu J.M., Kaufman P.D. Histone H3-K56 Acetylation Is Catalyzed by Histone Chaperone-Dependent Complexes. Mol. Cell. 2007;25:703–712. doi: 10.1016/j.molcel.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Q., Zhou H., Wurtele H., Davies B., Horazdovsky B., Verreault A., Zhang Z. Acetylation of Histone H3 Lysine 56 Regulates Replication-Coupled Nucleosome Assembly. Cell. 2008;134:244–255. doi: 10.1016/j.cell.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Recht J., Tsubota T., Tanny J.C., Diaz R.L., Berger J.M., Zhang X., Garcia B.A., Shabanowitz J., Burlingame A.L., Hunt D.F., et al. Histone chaperone Asf1 is required for histone H3 lysine 56 acetylation, a modification associated with S phase in mitosis and meiosis. Proc. Natl. Acad. Sci. USA. 2006;103:6988–6993. doi: 10.1073/pnas.0601676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han J., Zhou H., Li Z., Xu R.-M., Zhang Z. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J. Biol. Chem. 2007;282:28587–28596. doi: 10.1074/jbc.M702496200. [DOI] [PubMed] [Google Scholar]
- 81.Han J., Zhang H., Zhang H., Wang Z., Zhou H., Zhang Z. A Cul4 E3 ubiquitin ligase regulates histone hand-off during nucleosome assembly. Cell. 2013;155:817–829. doi: 10.1016/j.cell.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buser R., Kellner V., Melnik A., Wilson-Zbinden C., Schellhaas R., Kastner L., Piwko W., Dees M., Picotti P., Maric M., et al. The Replisome-Coupled E3 Ubiquitin Ligase Rtt101Mms22 Counteracts Mrc1 Function to Tolerate Genotoxic Stress. PLoS Genet. 2016;12:e1005843. doi: 10.1371/journal.pgen.1005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufman P.D., Kobayashi R., Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 84.Smith S., Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-X. [DOI] [PubMed] [Google Scholar]
- 85.Smith S., Stillman B. Stepwise assembly of chromatin during DNA replication in vitro. EMBO J. 1991;10:971–980. doi: 10.1002/j.1460-2075.1991.tb08031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shibahara K., Verreault A., Stillman B. The N-terminal domains of histones H3 and H4 are not necessary for chromatin assembly factor-1-mediated nucleosome assembly onto replicated DNA in vitro. Proc. Natl. Acad. Sci. USA. 2000;97:7766–7771. doi: 10.1073/pnas.97.14.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaufman P.D., Kobayashi R., Kessler N., Stillman B. The p150 and p60 subunits of chromatin assembly factor I: A molecular link between newly synthesized histones and DNA replication. Cell. 1995;81:1105–1114. doi: 10.1016/S0092-8674(05)80015-7. [DOI] [PubMed] [Google Scholar]
- 88.Shibahara K., Stillman B. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell. 1999;96:575–585. doi: 10.1016/S0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- 89.Moggs J.G., Grandi P., Quivy J.P., Jónsson Z.O., Hübscher U., Becker P.B., Almouzni G. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol. Cell. Biol. 2000;20:1206–1218. doi: 10.1128/MCB.20.4.1206-1218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Z., Shibahara K., Stillman B. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature. 2000;408:221–225. doi: 10.1038/35048530. [DOI] [PubMed] [Google Scholar]
- 91.Tagami H., Ray-Gallet D., Almouzni G., Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/S0092-8674(03)01064-X. [DOI] [PubMed] [Google Scholar]
- 92.Tyler J.K., Collins K.A., Prasad-Sinha J., Amiott E., Bulger M., Harte P.J., Kobayashi R., Kadonaga J.T. Interaction between the Drosophila CAF-1 and ASF1 chromatin assembly factors. Mol. Cell. Biol. 2001;21:6574–6584. doi: 10.1128/MCB.21.19.6574-6584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu W.H., Roemer S.C., Port A.M., Churchill M.E.A. CAF-1-induced oligomerization of histones H3/H4 and mutually exclusive interactions with Asf1 guide H3/H4 transitions among histone chaperones and DNA. Nucleic Acids Res. 2012;40:11229–11239. doi: 10.1093/nar/gks906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quivy J.P., Grandi P., Almouzni G. Dimerization of the largest subunit of chromatin assembly factor 1: Importance in vitro and during Xenopus early development. EMBO J. 2001;20:2015–2027. doi: 10.1093/emboj/20.8.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu W.H., Roemer S.C., Zhou Y., Shen Z.-J., Dennehey B.K., Balsbaugh J.L., Liddle J.C., Nemkov T., Ahn N.G., Hansen K.C., et al. The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones. eLife. 2016;5:e18023. doi: 10.7554/eLife.18023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang S., Zhou H., Katzmann D., Hochstrasser M., Atanasova E., Zhang Z. Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc. Natl. Acad. Sci. USA. 2005;102:13410–13415. doi: 10.1073/pnas.0506176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su D., Hu Q., Li Q., Thompson J.R., Cui G., Fazly A., Davies B.A., Botuyan M.V., Zhang Z., Mer G. Structural basis for recognition of H3K56-acetylated histone H3-H4 by the chaperone Rtt106. Nature. 2012;482:104–107. doi: 10.1038/nature10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fazly A., Li Q., Hu Q., Mer G., Horazdovsky B., Zhang Z. Histone chaperone Rtt106 promotes nucleosome formation using (H3-H4)2 tetramers. J. Biol. Chem. 2012;287:10753–10760. doi: 10.1074/jbc.M112.347450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J., Zhang X., Feng J., Leng H., Li S., Xiao J., Liu S., Xu Z., Xu J., Di L., et al. The Histone Chaperone FACT Contributes to DNA Replication-Coupled Nucleosome Assembly. Cell Rep. 2016;14:1–37. doi: 10.1016/j.celrep.2015.12.096. [DOI] [PubMed] [Google Scholar]
- 100.Trujillo K.M., Osley M.A. A Role for H2B Ubiquitylation in DNA Replication. Mol. Cell. 2012;48:734–746. doi: 10.1016/j.molcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Donnell L., Panier S., Wildenhain J., Tkach J.M., Al-Hakim A., Landry M.-C., Escribano-Diaz C., Szilard R.K., Young J.T.F., Munro M., et al. The MMS22L-TONSL Complex Mediates Recovery from Replication Stress and Homologous Recombination. Mol. Cell. 2010;40:619–631. doi: 10.1016/j.molcel.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duro E., Lundin C., Ask K., Sanchez-Pulido L., MacArtney T.J., Toth R., Ponting C.P., Groth A., Helleday T., Rouse J. Identification of the MMS22L-TONSL Complex that Promotes Homologous Recombination. Mol. Cell. 2010;40:632–644. doi: 10.1016/j.molcel.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 103.O’Connell B.C., Adamson B., Lydeard J.R., Sowa M.E., Ciccia A., Bredemeyer A.L., Schlabach M., Gygi S.P., Elledge S.J., Harper J.W. A Genome-wide Camptothecin Sensitivity Screen Identifies a Mammalian MMS22L-NFKBIL2 Complex Required for Genomic Stability. Mol. Cell. 2010;40:645–657. doi: 10.1016/j.molcel.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piwko W., Olma M.H., Held M., Bianco J.N., Pedrioli P.G.A., Hofmann K., Pasero P., Gerlich D.W., Peter M. RNAi-based screening identifies the Mms22L-Nfkbil2 complex as a novel regulator of DNA replication in human cells. EMBO J. 2010;29:4210–4222. doi: 10.1038/emboj.2010.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saredi G., Huang H., Hammond C.M., Alabert C., Bekker-Jensen S., Forne I., Reverón-Gómez N., Foster B.M., Mlejnkova L., Bartke T., et al. H4K20me0 marks post-replicative chromatin and recruits the TONSL–MMS22L DNA repair complex. Nature. 2016;534:1–21. doi: 10.1038/nature18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ray-Gallet D., Woolfe A., Vassias I., Pellentz C., Lacoste N., Puri A., Schultz D.C., Pchelintsev N.A., Adams P.D., Jansen L.E.T., et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol. Cell. 2011;44:928–941. doi: 10.1016/j.molcel.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Hoek M., Stillman B. Chromatin assembly factor 1 is essential and couples chromatin assembly to DNA replication in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:12183–12188. doi: 10.1073/pnas.1635158100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ye X., Franco A.A., Santos H., Nelson D.M., Kaufman P.D., Adams P.D. Defective S phase chromatin assembly causes DNA damage, activation of the S phase checkpoint, and S phase arrest. Mol. Cell. 2003;11:341–351. doi: 10.1016/S1097-2765(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 109.Nelson D.M., Ye X., Hall C., Santos H., Ma T., Kao G.D., Yen T.J., Harper J.W., Adams P.D. Coupling of DNA synthesis and histone synthesis in S phase independent of cyclin/cdk2 activity. Mol. Cell. Biol. 2002;22:7459–7472. doi: 10.1128/MCB.22.21.7459-7472.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao X., McKillop-Smith S., Müller B. The human histone gene expression regulator HBP/SLBP is required for histone and DNA synthesis, cell cycle progression and cell proliferation in mitotic cells. J. Cell Sci. 2004;117:6043–6051. doi: 10.1242/jcs.01523. [DOI] [PubMed] [Google Scholar]
- 111.Wagner E.J., Berkow A., Marzluff W.F. Expression of an RNAi-resistant SLBP restores proper S-phase progression. Biochem. Soc. Trans. 2005;33:471–473. doi: 10.1042/BST0330471. [DOI] [PubMed] [Google Scholar]
- 112.Barcaroli D., Bongiorno-Borbone L., Terrinoni A., Hofmann T.G., Rossi M., Knight R.A., Matera A.G., Melino G., De Laurenzi V. FLASH is required for histone transcription and S-phase progression. Proc. Natl. Acad. Sci. USA. 2006;103:14808–14812. doi: 10.1073/pnas.0604227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mejlvang J., Feng Y., Alabert C., Neelsen K.J., Jasencakova Z., Zhao X., Lees M., Sandelin A., Pasero P., Lopes M., et al. New histone supply regulates replication fork speed and PCNA unloading. J. Cell Biol. 2014;204:29–43. doi: 10.1083/jcb.201305017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghule P.N., Xie R.L., Medina R., Colby J.L., Jones S.N., Lian J.B., Stein J.L., van Wijnen A.J., Stein G.S. Fidelity of Histone Gene Regulation Is Obligatory for Genome Replication and Stability. Mol. Cell. Biol. 2014;34:2650–2659. doi: 10.1128/MCB.01567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Günesdogan U., Jäckle H., Herzig A. Histone supply regulates S phase timing and cell cycle progression. eLife. 2014;3:e02443. doi: 10.7554/eLife.02443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ask K., Jasencakova Z., Menard P., Feng Y., Almouzni G.E.V., Groth A. Codanin-1, mutated in the anaemic disease CDAI, regulates Asf1 function in S-phase histone supply. EMBO J. 2012;31:2013–2023. doi: 10.1038/emboj.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klimovskaia I.M., Young C., Strømme C.B., Menard P., Jasencakova Z., Mejlvang J., Ask K., Ploug M., Nielsen M.L., Jensen O.N., et al. Tousled-like kinases phosphorylate Asf1 to promote histone supply during DNA replication. Nat. Comm. 2014;5:1–13. doi: 10.1038/ncomms4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clemente-Ruiz M., Prado F. Chromatin assembly controls replication fork stability. EMBO Rep. 2009;10:790–796. doi: 10.1038/embor.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clemente-Ruiz M., González-Prieto R., Prado F. Histone H3K56 acetylation, CAF1, and Rtt106 coordinate nucleosome assembly and stability of advancing replication forks. PLoS Genet. 2011;7:e1002376. doi: 10.1371/journal.pgen.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kats E.S., Albuquerque C.P., Zhou H., Kolodner R.D. Checkpoint functions are required for normal S-phase progression in Saccharomyces cerevisiae RCAF- and CAF-I-defective mutants. Proc. Natl. Acad. Sci. USA. 2006;103:3710–3715. doi: 10.1073/pnas.0511102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prado F., Aguilera A. Partial depletion of histone H4 increases homologous recombination-mediated genetic instability. Mol. Cell. Biol. 2005;25:1526–1536. doi: 10.1128/MCB.25.4.1526-1536.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prado F., Cortés-Ledesma F., Aguilera A. The absence of the yeast chromatin assembly factor Asf1 increases genomic instability and sister chromatid exchange. EMBO Rep. 2004;5:497–502. doi: 10.1038/sj.embor.7400128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Myung K., Pennaneach V., Kats E.S., Kolodner R.D. Saccharomyces cerevisiae chromatin-assembly factors that act during DNA replication function in the maintenance of genome stability. Proc. Natl. Acad. Sci. USA. 2003;100:6640–6645. doi: 10.1073/pnas.1232239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Polo S.E., Almouzni G. Chromatin dynamics after DNA damage: The legacy of the access-repair–restore model. DNA Repair. 2015;36:1–8. doi: 10.1016/j.dnarep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dahlin J.L., Chen X., Walters M.A., Zhang Z. Histone-modifying enzymes, histone modifications and histone chaperones in nucleosome assembly: Lessons learned from Rtt109 histone acetyltransferases. Crit. Rev. Biochem. Mol. Biol. 2015;50:31–53. doi: 10.3109/10409238.2014.978975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cabello-Lobato M. J., Prado F. (Centro Andaluz de Biología Molecular y Medicina Regenerativa (CABIMER/CSIC), Seville, Spain). Personal communication. 2017.
- 127.Smith D.J., Whitehouse I. Intrinsic coupling of lagging-strand synthesis to chromatin assembly. Nature. 2012;483:434–438. doi: 10.1038/nature10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen C., Kolodner R.D. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 1999;23:81–85. doi: 10.1038/12687. [DOI] [PubMed] [Google Scholar]
- 129.Tishkoff D.X., Filosi N., Gaida G.M., Kolodner R.D. A novel mutation avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell. 1997;88:253–263. doi: 10.1016/S0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 130.Prado F., Clemente-Ruiz M. Nucleosome assembly and genome integrity. BioArchitecture. 2012;2:6–10. doi: 10.4161/bioa.19737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yadav T., Whitehouse I. Replication-Coupled Nucleosome Assembly and Positioning by ATP-Dependent Chromatin- Remodeling Enzymes. Cell Rep. 2016;15:715–723. doi: 10.1016/j.celrep.2016.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang J.H., Freudenreich C.H. The Rtt109 histone acetyltransferase facilitates error-free replication to prevent CAG/CTG repeat contractions. DNA Repair. 2010;9:414–420. doi: 10.1016/j.dnarep.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Houseley J., Tollervey D. Repeat expansion in the budding yeast ribosomal DNA can occur independently of the canonical homologous recombination machinery. Nucleic Acids Res. 2011;39:8778–8791. doi: 10.1093/nar/gkr589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramey C.J., Howar S., Adkins M., Linger J., Spicer J., Tyler J.K. Activation of the DNA damage checkpoint in yeast lacking the histone chaperone anti-silencing function 1. Mol. Cell. Biol. 2004;24:10313–10327. doi: 10.1128/MCB.24.23.10313-10327.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hashash N., Johnson A.L., Cha R.S. Topoisomerase II- and Condensin-Dependent Breakage of MEC1ATR-Sensitive Fragile Sites Occurs Independently of Spindle Tension, Anaphase, or Cytokinesis. PLoS Genet. 2012;8:e1002978. doi: 10.1371/journal.pgen.1002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El Achkar E., Gerbault-Seureau M., Muleris M., Dutrillaux B., Debatisse M. Premature condensation induces breaks at the interface of early and late replicating chromosome bands bearing common fragile sites. Proc. Natl. Acad. Sci. USA. 2005;102:18069–18074. doi: 10.1073/pnas.0506497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ivanov D., Schleiffer A., Eisenhaber F., Mechtler K., Haering C.H., Nasmyth K. Eco1 is a novel acetyltransferase that can acetylate proteins involved in cohesion. Curr. Biol. 2002;12:323–328. doi: 10.1016/S0960-9822(02)00681-4. [DOI] [PubMed] [Google Scholar]
- 138.Lopez-Serra L., Kelly G., Patel H., Stewart A., Uhlmann F. The Scc2–Scc4 complex acts in sister chromatid cohesion and transcriptional regulation by maintaining nucleosome-free regions. Nat. Genet. 2014;46:1147–1151. doi: 10.1038/ng.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murillo-Pineda M., Cabello-Lobato M.J., Clemente-Ruiz M., Monje-Casas F., Prado F. Defective histone supply causes condensin-dependent chromatin alterations, SAC activation and chromosome decatenation impairment. Nucleic Acids Res. 2014;42:12469–12482. doi: 10.1093/nar/gku927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thaminy S., Newcomb B., Kim J., Gatbonton T., Foss E., Simon J., Bedalov A. Hst3 is regulated by Mec1-dependent proteolysis and controls the S phase checkpoint and sister chromatid cohesion by deacetylating histone H3 at lysine 56. J. Biol. Chem. 2007;282:37805–37814. doi: 10.1074/jbc.M706384200. [DOI] [PubMed] [Google Scholar]
- 141.Prado F. Homologous recombination maintenance of genome integrity during DNA damage tolerance. Mol. Cell. Oncol. 2014;1:e957039. doi: 10.4161/23723548.2014.957039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sale J.E., Lehmann A.R., Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell Biol. 2012;13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Minca E.C., Kowalski D. Multiple Rad5 Activities Mediate Sister Chromatid Recombination to Bypass DNA Damage at Stalled Replication Forks. Mol. Cell. 2010;38:649–661. doi: 10.1016/j.molcel.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.González-Prieto R., Muñoz-Cabello A.M., Cabello-Lobato M.J., Prado F. Rad51 replication fork recruitment is required for DNA damage tolerance. EMBO J. 2013;32:1307–1321. doi: 10.1038/emboj.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vázquez M.V., Rojas V., Tercero J.A. Multiple pathways cooperate to facilitate DNA replication fork progression through alkylated DNA. DNA Repair. 2008;7:1693–1704. doi: 10.1016/j.dnarep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 146.Alabert C., Bianco J.N., Pasero P. Differential regulation of homologous recombination at DNA breaks and replication forks by the Mrc1 branch of the S-phase checkpoint. EMBO J. 2009;28:1131–1141. doi: 10.1038/emboj.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Henry-Mowatt J., Jackson D., Masson J.-Y., Johnson P.A., Clements P.M., Benson F.E., Thompson L.H., Takeda S., West S.C., Caldecott K.W. XRCC3 and Rad51 modulate replication fork progression on damaged vertebrate chromosomes. Mol. Cell. 2003;11:1109–1117. doi: 10.1016/S1097-2765(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 148.Daboussi F., Courbet S., Benhamou S., Kannouche P., Zdzienicka M.Z., Debatisse M., Lopez B.S. A homologous recombination defect affects replication-fork progression in mammalian cells. J. Cell Sci. 2008;121:162–166. doi: 10.1242/jcs.010330. [DOI] [PubMed] [Google Scholar]
- 149.Maas N.L., Miller K.M., DeFazio L.G., Toczyski D.P. Cell Cycle and Checkpoint Regulation of Histone H3 K56 Acetylation by Hst3 and Hst4. Mol. Cell. 2006;23:109–119. doi: 10.1016/j.molcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 150.Celic I., Masumoto H., Griffith W.P., Meluh P., Cotter R.J., Boeke J.D., Verreault A. The Sirtuins Hst3 and Hst4p Preserve Genome Integrity by Controlling Histone H3 Lysine 56 Deacetylation. Curr. Biol. 2006;16:1280–1289. doi: 10.1016/j.cub.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 151.Collins S.R., Miller K.M., Maas N.L., Roguev A., Fillingham J., Chu C.S., Schuldiner M., Gebbia M., Recht J., Shales M., et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 152.Wurtele H., Kaiser G.S., Bacal J., St-Hilaire E., Lee E.H., Tsao S., Dorn J., Maddox P., Lisby M., Pasero P., et al. Histone H3 Lysine 56 Acetylation and the Response to DNA Replication Fork Damage. Mol. Cell. Biol. 2011;32:154–172. doi: 10.1128/MCB.05415-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luke B., Versini G., Jaquenoud M., Zaidi I.W., Kurz T., Pintard L., Pasero P., Peter M. The Cullin Rtt101p Promotes Replication Fork Progression through Damaged DNA and Natural Pause Sites. Curr. Biol. 2006;16:786–792. doi: 10.1016/j.cub.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 154.Zaidi I.W., Rabut G., Poveda A., Scheel H., Malmström J., Ulrich H., Hofmann K., Pasero P., Peter M., Luke B. Rtt101 and Mms1 in budding yeast form a CUL4DDB1-like ubiquitin ligase that promotes replication through damaged DNA. EMBO Rep. 2008;9:1034–1040. doi: 10.1038/embor.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luciano P., Dehé P.-M., Audebert S., Géli V., Corda Y. Replisome function during replicative stress is modulated by histone h3 lysine 56 acetylation through Ctf4. Genetics. 2015;199:1047–1063. doi: 10.1534/genetics.114.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ivessa A.S., Lenzmeier B.A., Bessler J.B., Goudsouzian L.K., Schnakenberg S.L., Zakian V.A. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past non histone protein-DNA complexes. Mol. Cell. 2003;12:1525–1536. doi: 10.1016/S1097-2765(03)00456-8. [DOI] [PubMed] [Google Scholar]
- 157.Erkmann J.A., Kaufman P.D. A negatively charged residue in place of histone H3K56 supports chromatin assembly factor association but not genotoxic stress resistance. DNA Repair. 2009;8:1371–1379. doi: 10.1016/j.dnarep.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Duro E., Vaisica J.A., Brown G.W., Rouse J. Budding yeast Mms22 and Mms1 regulate homologous recombination induced by replisome blockage. DNA Repair. 2008;7:811–818. doi: 10.1016/j.dnarep.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 159.Wang H., Zhai L., Xu J., Joo H.-Y., Jackson S., Erdjument-Bromage H., Tempst P., Xiong Y., Zhang Y. Histone H3 and H4 Ubiquitylation by the CUL4-DDB-ROC1 Ubiquitin Ligase Facilitates Cellular Response to DNA Damage. Mol. Cell. 2006;22:383–394. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 160.Tjeertes J.V., Miller K.M., Jackson S.P. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 2009;28:1878–1889. doi: 10.1038/emboj.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pietrobon V., Fréon K., Hardy J., Costes A., Iraqui I., Ochsenbein F., Lambert S.A.E. The Chromatin Assembly Factor 1 Promotes Rad51-Dependent Template Switches at Replication Forks by Counteracting D-Loop Disassembly by the RecQ-Type Helicase Rqh1. PLoS Biol. 2014;12:e1001968. doi: 10.1371/journal.pbio.1001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Branzei D., Foiani M. RecQ helicases queuing with Srs2 to disrupt Rad51 filaments and suppress recombination. Genes Dev. 2007;21:3019–3026. doi: 10.1101/gad.1624707. [DOI] [PubMed] [Google Scholar]
- 163.Jiao R., Bachrati C.Z., Pedrazzi G., Kuster P., Petkovic M., Li J.L., Egli D., Hickson I.D., Stagljar I. Physical and Functional Interaction between the Bloom’s Syndrome Gene Product and the Largest Subunit of Chromatin Assembly Factor 1. Mol. Cell. Biol. 2004;24:4710–4719. doi: 10.1128/MCB.24.11.4710-4719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ronan J.L., Wu W., Crabtree G.R. From neural development to cognition: Unexpected roles for chromatin. Nat. Rev. Genet. 2013;14:347–359. doi: 10.1038/nrg3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morgan M.A., Shilatifard A. Chromatin signatures of cancer. Genes Dev. 2015;29:238–249. doi: 10.1101/gad.255182.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prado F., Jimeno-Gonzalez S., Reyes J.C. Histone availability as a strategy to control gene expression. RNA Biol. 2016 doi: 10.1080/15476286.2016.1189071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.O’Sullivan R.J., Karlseder J. The great unravelling: Chromatin as a modulator of the aging process. Trends Biochem. Sci. 2012;37:466–476. doi: 10.1016/j.tibs.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gasparian A.V., Burkhart C.A., Purmal A.A., Brodsky L., Pal M., Saranadasa M., Bosykh D.A., Commane M., Guryanova O.A., Pal S., et al. Curaxins: Anticancer compounds that simultaneously suppress NF-κB and activate p53 by targeting FACT. Sci. Transl. Med. 2011 doi: 10.1126/scitranslmed.3002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Polo S.E., Theocharis S.E., Grandin L., Gambotti L., Antoni G., Savignoni A., Asselain B., Patsouris E., Almouzni G. Clinical significance and prognostic value of chromatin assembly factor-1 overexpression in human solid tumours. Histopathology. 2010;57:716–724. doi: 10.1111/j.1365-2559.2010.03681.x. [DOI] [PubMed] [Google Scholar]
- 170.Corpet A., De Koning L., Toedling J., Savignoni A., Berger F., Lemaître C., O’Sullivan R.J., Karlseder J., Barillot E., Asselain B., et al. Asf1b, the necessary Asf1 isoform for proliferation, is predictive of outcome in breast cancer. EMBO J. 2010;30:480–493. doi: 10.1038/emboj.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kerzendorfer C., Hannes F., Colnaghi R., Abramowicz I., Carpenter G., Vermeesch J.R., O’Driscoll M. Characterizing the functional consequences of haploinsufficiency of NELF-A (WHSC2) and SLBP identifies novel cellular phenotypes in Wolf-Hirschhorn syndrome. Hum. Mol. Genet. 2012;21:2181–2193. doi: 10.1093/hmg/dds033. [DOI] [PubMed] [Google Scholar]
- 172.Marzluff W.F., Wagner E.J., Duronio R.J. Metabolism and regulation of canonical histone mRNAs: Life without a poly(A) tail. Nat. Rev. Genet. 2008;9:843–854. doi: 10.1038/nrg2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martinez V.D., Vucic E.A., Becker-Santos D.D., Gil L., Lam W.L. Arsenic exposure and the induction of human cancers. J. Toxicol. 2011 doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brocato J., Chen D., Liu J., Fang L., Jin C., Costa M. A Potential New Mechanism of Arsenic Carcinogenesis: Depletion of Stem-Loop Binding Protein and Increase in Polyadenylated Canonical Histone H3.1 mRNA. Biol. Trace Element Res. 2015;166:72–81. doi: 10.1007/s12011-015-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Knipe D.M., Lieberman P.M., Jung J.U., McBride A.A., Morris K.V., Ott M., Margolis D., Nieto A., Nevels M., Parks R.J., et al. Snapshots: Chromatin control of viral infection. Virology. 2013;435:141–156. doi: 10.1016/j.virol.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bogenberger J.M., Laybourn P.J. Human T Lymphotropic Virus Type 1 protein Tax reduces histone levels. Retrovirology. 2008 doi: 10.1186/1742-4690-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grogg K.L., Miller R.F., Dogan A. HIV infection and lymphoma. J. Clin. Pathol. 2007;60:1365–1372. doi: 10.1136/jcp.2007.051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li M., Tucker L.D., Asara J.M., Cheruiyot C.K., Lu H., Wu Z.J., Newstein M.C., Dooner M.S., Friedman J., Lally M.A., et al. Stem-loop binding protein is a multifaceted cellular regulator of HIV-1 replication. J. Clin. Investig. 2016;126:3117–3129. doi: 10.1172/JCI82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dgany O., Avidan N., Delaunay J., Krasnov T., Shalmon L., Shalev H., Eidelitz-Markus T., Kapelushnik J., Cattan D., Pariente A., et al. Congenital Dyserythropoietic Anemia Type I Is Caused by Mutations in Codanin-1. Am. J. Hum. Genet. 2002;71:1467–1474. doi: 10.1086/344781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cesare A.J., Reddel R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 181.O’Sullivan R.J., Arnoult N., Lackner D.H., Oganesian L., Haggblom C., Corpet A., Almouzni G., Karlseder J. Rapid induction of alternative lengthening of telomeres by depletion of the histone chaperone ASF1. Nat. Struct. Mol. Biol. 2014;21:167–174. doi: 10.1038/nsmb.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Platt J.M., Ryvkin P., Wanat J.J., Donahue G., Ricketts M.D., Barrett S.P., Waters H.J., Song S., Chavez A., Abdallah K.O., et al. Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev. 2013;27:1406–1420. doi: 10.1101/gad.218776.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.O’Sullivan R.J., Kubicek S., Schreiber S.L., Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 2010;17:1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ivanov A., Pawlikowski J., Manoharan I., van Tuyn J., Nelson D.M., Rai T.S., Shah P.P., Hewitt G., Korolchuk V.I., Passos J.F., et al. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 2013;202:129–143. doi: 10.1083/jcb.201212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Feser J., Truong D., Das C., Carson J.J., Kieft J., Harkness T., Tyler J.K. Elevated Histone Expression Promotes Life Span Extension. Mol. Cell. 2010;39:724–735. doi: 10.1016/j.molcel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu L., Cheung T.H., Charville G.W., Hurgo B.M.C., Leavitt T., Shih J., Brunet A., Rando T.A. Chromatin Modifications as Determinants of Muscle Stem Cell Quiescence and Chronological Aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hu Z., Chen K., Xia Z., Chavez M., Pal S., Seol J.H., Chen C.C., Li W., Tyler J.K. Nucleosome loss leads to global transcriptional up-regulation and genomic instability during yeast aging. Genes Dev. 2014;28:396–408. doi: 10.1101/gad.233221.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nikolov I., Taddei A. Linking replication stress with heterochromatin formation. Chromosoma. 2016;125:1–11. doi: 10.1007/s00412-015-0545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]