Abstract
Anthracycline-based cardiotoxicity is concerning for women with breast cancer and portends a dose-dependent risk of developing left ventricular dysfunction. Overall, the prevalence of heart failure (HF) is ≈2% of the total US population; however, BRCA-deficient mice have shown increased HF. We evaluated for the inherent risk of HF in women with BRCA mutations to determine whether treatment with anthracycline-based therapy increased this risk. We obtained results on BRCA mutation carriers regarding cancer treatment and HF, identified through the BRCA patient advocacy organization Facing Our Risk for Cancer Empowered (FORCE) and the Moffitt-based Inherited Cancer Registry. In our patient group (232 BRCA1 and 159 BRCA2 patients; 10 with both mutations), 7.7% reported HF, with similar proportions in BRCA1 versus BRCA2 carriers (7.4% and 8.2%, respectively). These proportions are significantly higher than published rates (p < 0.001). There was no statistically significant difference in HF rates comparing anthracycline-treated versus anthracycline-naïve patients however (7.1% vs. 8.3%; p = 0.67). In addition, 9.1% of BRCA1 carriers and 8.2% of BRCA2 carriers reported arrhythmias. BRCA mutation carriers showed increased risk of cardiotoxicity versus the general population and an overall increased risk of cardiotoxicity from anthracycline-based therapy. Our study supports data that BRCA carriers have increased non-cancer mortality from cardiotoxicity. A prospective trial to determine HF and conduction abnormalities in this population is warranted.
Keywords: anthracycline-related cardiac toxicity, BRCA1 mutation, BRCA2 mutation, conduction abnormalities, heart failure
1. Introduction
Overall, approximately 5%–10% of breast and ovarian cancer cases are due to mutations in the high-penetrance genes BRCA1 and BRCA2 (BRCA) [1]. It is well-established that patients with mutations in the BRCA genes have increased mortality due to malignancy [2]; however, a recent study has also suggested a significant association between BRCA mutations and increased non-cancer mortality (p = 0.024) [3]. Mutation carriers have a 56%–87% risk of developing breast cancer by the age of 70 [2,4]. Lifetime risks for ovarian cancer are up to 44% and 27% for BRCA1 and BRCA2, respectively [1].
Prevalence of heart failure (HF) in the general population of American women varies and increases with age. According to American Heart Association data, this risk varies between 0.3% in women under 30 and 11.8% in the elderly [5]. The overall risk for the general US population including males and females is estimated at 2% [5]. It is well-established that anthracycline use can lead to heart failure. The cardiotoxic effects of anthracycline therapy are seen in a dose-dependent fashion. The risk of heart failure due to adriamycin at 450 mg/m2 is 3%–4% [6].
This exploratory study was performed to determine whether patients with BRCA mutations are at a higher inherent predisposition for cardiac disease. We also sought to determine whether mutation carriers were at a higher risk for toxicity from anthracycline therapy, which is one of the current standards of care for chemotherapy in this population. Third, we attempted to identify other possible causes responsible for increased mortality in these patients.
2. Materials and Methods
This study received Institutional Review Board approval (number Pro00004774; 20/06/2014). Patients with BRCA1 or BRCA2 mutation status were offered enrollment in the patient advocacy groups known as Facing Our Risk for Cancer Empowered (FORCE) and Inherited Cancer Registry (ICARE). FORCE is a nonprofit organization devoted to patients and family members with BRCA mutations. This organization meets annually and has over 13,000 members nationwide. We also identified patients using the ICARE initiative at the Moffitt Cancer Center (see Appendix A). Through FORCE and ICARE, the surveys were available to over 13,000 patients. Eligible patients included females with confirmed BRCA1 or BRCA2 mutations of any age and any treatment history. Patients were invited to participate in an optional online or paper survey (see Supplementary Survey S1) regarding general adverse effects surrounding their breast cancer treatment and overall health. Patients were consented by voluntarily completing the survey and had the option to give additional consent to be contacted for follow-up questions. Four hundred one surveys were completed, and all were included for analysis. The survey consisted of 57 questions, including a thorough patient-reported review of the cardiovascular system and disease states. Specific information regarding baseline cardiac risk factors and medical history prior to therapy was also obtained. Age, prior or current tobacco history, diabetic status, cholesterol levels, and history of cardiac disease were assessed. Targeted questions included Number 8 (cardiac)—“Did you experience any of the following: irregular rhythm, prior heart attack, heart failure”—Number 25—“Was your heart function normal before starting treatment?”—Number 26—“Is your heart function normal now?”—Number 42b—“Have you been diagnosed with heart attack?”—and Number 42c—“Have you been diagnosed with heart failure?” The survey also included detailed questions to determine preexisting conditions and comorbidities. Data of patients who underwent treatment at Moffitt Cancer Center were reviewed. Attempts were made to contact patients treated at outside centers, with questionnaire responses suggestive of cardiovascular complications.
The next part of the study included assessing the cardiac risk profiles of patients who had specifically been treated with anthracycline chemotherapy, comparing these values to established historical data. We contacted patients who answered “Yes” to any of the targeted questions (see above) and gave permission to do so. Of the 31 patients with heart failure, only 8 agreed to be contacted. They were all able to provide proof of HF diagnosis via clinical history or echo report. The survey, including targeted cardiac questions, is included in Supplementary Survey S1.
Population characteristics were summarized using descriptive statistics: frequency and proportion for categorical variables and median and range for continuous variables. The exact binomial distribution was used to compute the 95% confidence interval (CI) for proportions and to compare those with the proportions of a well-established population control. The association between two categorical variables was evaluated by the chi-square test.
3. Results
3.1. Population Characteristics
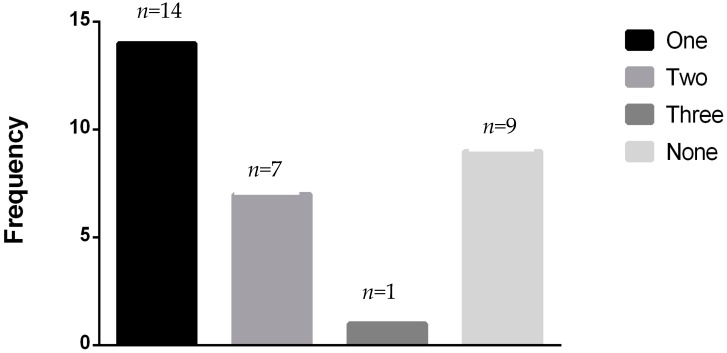
A total of 401 patients were entered into the study: 232 were BRCA1 carriers, and 159 were BRCA2 carriers (see Table 1 for patient characteristics). Ten patients carried both mutations. Patient accrual was initiated in April 2010 and concluded in November 2010 after 400 surveys were completed. All patients were female. Age ranged from 40 to 76 years. The patient cohort was generally healthy, and most patients with heart failure had 0–1 risk factor (see Figure 1).
Table 1.
Patient characteristics.
| Number of Patients (%) (n = 401) | |
|---|---|
|
BRCA status BRCA1 BRCA2 BRCA1 and BRCA2 |
232 (57.9) 159 (39.7) 10 (2.5) |
| Hypertension | 97 (24.2) |
| Diabetes | 18 (4.5) |
| Hyperlipidemia | 207 (51.6) |
| Tobacco use | 37 (9.2) |
| Age group 20–39 years 40–59 years 60–79 years No answer |
28 (7.0) 264 (65.8) 76 (19.0) 33 (8.2) |
Figure 1.
Number of confounding risk factors seen in the 31 BRCA patients with heart failure.
Thirty-one patients (7.3%) were diagnosed clinically with HF. Of these, 17 patients (7.3%) carried BRCA1 mutations and 13 patients (8.2%) carried BRCA2 mutations. One patient that carried both mutations was diagnosed with heart failure (Table 2).
Table 2.
Patients diagnosed with heart failure.
| Patient Group | Heart Failure, No. of Patients (%) | No Heart Failure | Total | 95% Confidence Interval (%) | p Value Compared to 2% |
|---|---|---|---|---|---|
| BRCA1 | 17 (7.3%) | 215 | 232 | 4.3–11.5 | <0.0001 |
| BRCA2 | 13 (8.2%) | 146 | 159 | 4.4–13.6 | <0.0001 |
| BRCA1 and BRCA2 | 1 (10%) | 9 | 10 | 0.3–44.5 | 0.37 |
| Total | 31 (7.7%) | 370 | 401 | 5.3–10.8 | <0.0001 |
When compared with rates of heart failure in the overall population, this proportion in our patient population was significantly higher (7.7% vs. 2%; p < 0.001). When adjusted for age, BRCA carriers in the 40- to 59-year-old age group (15/264 patients) had a 5.7% risk (CI, 3.2–9.2) versus 0.8% in the general population of women (p < 0.0001), and patients in the 60- to 79-year-old age group (12/76 patients) had a 15.8% risk (CI, 8.4–26.0) versus 5.4% in the general population (p < 0.0001). Four patients could not be included in age stratification because they did not include their age on the survey.
There was no difference between risk in BRCA1 and BRCA2 carriers who had 7.3% and 8.2% incidence of heart failure, respectively (p = 0.76). Of the 7.7% of BRCA1 and BRCA2 mutation carriers who developed heart failure, 8.3% were anthracycline naïve (statistically significant at p ≤ 0.001) compared with the general population risk of 2%. This indicates that BRCA carriers may have a higher inherent risk of heart failure than the general population even when anthracycline use is excluded. In our patient group, 7.1% of mutation carriers developed heart failure after receiving anthracycline therapy with either adriamycin or epirubicin. When compared to the known risk of heart failure from anthracycline therapy of 3%, BRCA mutation carriers had a higher risk of cardiotoxicity from anthracycline therapy (p = 0.008) (Table 3). There was no statistically significant difference in the incidence of HF between the anthracycline-treated patients and those who were chemotherapy naïve (7.1% vs 8.3%; p = 0.67).
Table 3.
Heart failure risk in BRCA patients with anthracyline therapy.
| Number of Patients | % HF on Anthracycline | % HF Chemotherapy Naïve | ||||
|---|---|---|---|---|---|---|
| Received Anthracycline | Chemotherapy Naïve | HF on Anthracycline | HF Chemotherapy Naïve | |||
| BRCA1 | 106 | 126 | 6 | 11 | 5.7 | 8.7 |
| BRCA2 | 70 | 89 | 6 | 7 | 8.6 | 7.9 |
| Both | 7 | 3 | 1 | 0 | 14.2 | 0 |
| Total | 183 | 218 | 13 | 18 | 7.1 | 8.3 |
Abbreviations: HF, heart failure.
In addition, 21 of 232 (9.1%) BRCA1 carriers and 13 of 159 (8.2%) of BRCA2 carriers reported arrhythmias. Overall, 37 patients of 391 (9.2%) reported arrhythmias (Table 4).
Table 4.
Arrhythmias in BRCA patients.
| Patient Group | Irregular Rhythm, n | No Irregular Rhythm or Unknown, n | Total, n | Irregular Rhythm, % (95% CI) |
|---|---|---|---|---|
| BRCA1 | 21 | 211 | 232 | 9.1 (5.7–13.5) |
| BRCA2 | 13 | 146 | 159 | 8.2 (4.4–13.6) |
| BRCA1 and BRCA2 | 3 | 7 | 10 | 30.0 (6.7–65.3) |
| Total | 37 | 364 | 401 | 9.2 (6.6–12.5) |
4. Discussion
There is clear evidence of increased non-malignant mortality in BRCA patients [3]. In our study, we sought to determine whether this risk was related to cardiac mortality, namely, heart failure. A recent study of 81 patients showed no change in ejection fraction, but this may be due to a small sample size and a short duration of follow-up [7].
BRCA1 is a human tumor suppressor gene that produces the breast cancer type 1 susceptibility protein. BRCA1 is expressed in cells of the breast and other tissues, where it functions to repair or destroy damaged DNA and protect cells against oxidative and genotoxic stress. Mutations in this gene alter function and allow for tumors to arise [8]. Thus, mutations in this gene could lead to pathologic pathways, predisposing to physiologic dysfunction caused by oxidative stress, particularly cardiovascular disease. In support of this theory, murine studies conducted with cardiac myocytes revealed that wild-type BRCA1 is essential to limiting apoptosis and improving cardiac function in response to genotoxic stress (doxorubicin) and oxidative stress (ischemia) [9]. Furthermore, heart-specific BRCA1 deletion has been shown to promote severe systolic dysfunction and limited survival in mice [9]. BRCA2 deletion has led to increased cardiomyocyte apoptosis after anthracycline therapy [10]. Follow-up studies reported an association between single nucleotide polymorphisms in BRCA1-associated protein and myocardial infarction risk in a large Japanese patient cohort with replication in additional Japanese and Taiwanese patient cohorts [11]. Even in women who do not receive anthracycline-based chemotherapy, BRCA protein deficiency can increase their susceptibility to any form of oxidative stress, whether it is from other types of cancer treatments, early surgical menopause, subclinical ischemia, or endothelial dysfunction from hypertension, hyperlipidemia, diabetes, or insulin resistance (which is known to be increased in the setting of BRCA protein deficiency) [12,13,14]. These mechanisms could play a role in the inherently higher risk for heart failure reported in those patients exposed to anthracyclines and those who were chemotherapy naïve.
Our study participants reported a significantly increased risk of heart failure after anthracycline therapy compared with the general population. The cardiotoxic effects of anthracyclines have been well-studied in cancer patients but have not been directly correlated to patients with BRCA mutations. Hypothesized mechanisms of anthracycline-induced cardiotoxicity include free radical damage to myocytes, which can occur non-enzymatically through direct interaction with iron [1] or enzymatically through interaction with cardiolipin. Anthracyclines act as reducing agents generating free radicals in both pathways and are shown to disrupt DNA synthesis by interference with topoisomerase, leading to apoptosis [1]. Because BRCA1 is associated with DNA repair, mutations may portend a higher risk of damage by anthracycline therapy.
Our secondary goal of this study was to identify other possible mechanisms to explain the increased non-cancer-related mortality in this population. By means of the encompassing survey, we also found an alarming number of otherwise healthy patients who reported arrhythmias. There are no well-established statistics for the overall rates of arrhythmias in women in the United States; therefore, our question regarding arrhythmias should have been more specific, so that more detailed statistical analysis could have been performed. However, the most common conduction abnormalities are first-degree atrio-ventricular blocks, with a 3% risk in black women and a 1.3% risk in white women, and atrial fibrillation, which ranges from 6.6 per 100,000 women per year for 15- to 44-year-olds and 1203.7 per 100,000 women per year for those ≥85 years of age [15,16]. These rates are much lower than the percentages that we found in our study. Because our survey was not initially formatted with detailed questions regarding arrhythmias, we did not have data as to specific types, durations, and treatments required for the arrhythmias found in our patient cohort.
The major limitation of this exploratory study was the inability to contact all participants for further information that was not listed in the primary survey. This was an online survey reaching patients across multiple institutions. Because this was an anonymous survey, we were unable to further discuss specific survey responses and obtain objective data from a large percentage of participants, unless they provided consent and contact information for further communication. The data collected also consisted of patients’ self-reporting their diagnoses and was limited to the questions addressed in the survey. Moreover, objective confirmatory data were unavailable in most cases. Of the contacted patients who reported heart failure, echocardiogram reports did confirm low ejection fraction when available. However, we considered the diagnosis valid since heart failure is a clinical diagnosis and the targeted questions were specific. Among the patients who reported arrhythmias, electrocardiograms confirmed the lack of sinus rhythm when available. An additional weakness was that these data were compared with historical controls, allowing different biases to be introduced, including selection bias given the inherent differences in the populations compared. Finally, the use of trastuzumab was not addressed in this study. However, the use of this therapy is less common in BRCA breast cancer patients, as most have triple negative tumors [17]. This may need to be addressed in the future if these data are confirmed with prospective studies.
5. Conclusions
Prior studies in humans have sought to determine why BRCA carriers have increased non-cancer mortality. Previous studies have indicated that this may be due to cardiotoxicity. The findings of our cross-sectional study design suggest this as well. We consider this an interesting hypothesis-generating observation; however, it remains important to conduct larger studies to test this hypothesis, ideally with prospective follow-up and objective record verification. A more in-depth review of cardiovascular effects in this patient population is warranted and currently underway. The prospective study will compare ejection fraction in BRCA patients with wild-type counterparts before and after treatment with anthracycline.
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance. We thank Sue Freidman, the director of FORCE, for posting our research survey on the FORCE website. Our study also received valuable assistance from the Survey Methods Core at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center, supported under NIH grant P30-CA76292.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4425/8/2/59/s1, S1: Cancer therapy and side effects in BRCA patients.
Appendix A
ICARE: The ICARE initiative, based at Moffitt, is an inherited cancer registry and database (grant funded) and initiated by Dr. Tuya Pal. Christina Bittner, a Board-Certified Genetic Counselor (a member of the ICARE team), contacts patients with resources that may be of interest to the BRCA patient.
FORCE: The FORCE organization was established in 1999 and offers expert-reviewed research information, resources, advocacy, and peer support for individuals and families affected by hereditary cancers. FORCE efforts include an annual conference, print and electronic newsletters, a comprehensive website, a toll-free helpline, webinars and webcasts, regional support groups, educational brochures, and chat rooms through which individuals can access the latest information on treatment, risk-management, prevention, psychosocial and quality-of-life issues, and research studies, as well as discuss and share the cancer-related issues that they are facing.
FORCE is the only international, nonprofit organization devoted to improving the lives of individuals and families affected by hereditary breast and ovarian cancer. Their mission includes providing up-to-date information, resources, peer support, and advocacy, raising awareness, and promoting research specific to hereditary cancer. FORCE maintains a print newsletter mailing list of about 13,000 individuals and an e-mailing list of approximately 10,000 individuals, growing at a rate of approximately 100 contacts monthly. FORCE has collaborations with major cancer centers and cancer advocacy groups. FORCE is a partner member of the Ovarian Cancer National Alliance (OCNA) and the Allied Support Group for Ovarian Cancer (organized by SGO).
Author Contributions
M. Sajjad drafted the manuscript; M. Fradley reviewed and edited the manuscript; W. Sun performed data entry and survey collection; J. Kim participated in the design of the study and performed statistical analysis; X. Zhao performed statistical analysis; T. Pal provided access and assistance with genetic information through ICARE; R. Ismail-Khan conceived the study, participated in its design and coordination, and helped draft the manuscript; all authors read, approved, and provided input on all drafts, including the final draft.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Claus E.B., Schildkraut J.M., Thompson W.D., Risch N.J. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77:2318–2324. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2318::AID-CNCR21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Struewing J.P., Hartge P., Wacholder S., Baker S.M., Berlin M., McAdams M., Timmerman M.M., Tucker M.A. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 3.Mai P.L., Chatterjee N., Hartge P., Tucker M., Brody L., Struewing J.P., Wacholder S. Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS ONE. 2009;4:e4812. doi: 10.1371/journal.pone.0004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford D., Easton D.F., Stratton M., Narod S., Goldgar D., Devilee P., Bishop D.T., Weber B., Lenoir G., Chang-Claude J., et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minow R.A., Benjamin R.S., Lee E.T., Gottlieb J.A. Adriamycin cardiomyopathy—Risk factors. Cancer. 1977;39:1397–1402. doi: 10.1002/1097-0142(197704)39:4<1397::AID-CNCR2820390407>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Barac A., Lynce F., Smith K.L., Mete M., Shara N.M., Asch F.M., Nardacci M.P., Wray L., Herbolsheimer P., Nunes R.A., et al. Cardiac function in BRCA1/2 mutation carriers with history of breast cancer treated with anthracyclines. Breast. Cancer Res. Treat. 2016;155:285–293. doi: 10.1007/s10549-016-3678-2. [DOI] [PubMed] [Google Scholar]
- 8.Murphy C.G., Moynahan M.E. BRCA gene structure and function in tumor suppression: A repair-centric perspective. Cancer J. 2010;16:39–47. doi: 10.1097/PPO.0b013e3181cf0204. [DOI] [PubMed] [Google Scholar]
- 9.Shukla P.C., Singh K.K., Lovren F., Pan Y., Leong-Poi H., Erret L., Verma S. BRCA1 as an essential regulator of cardiac function. Nat. Commun. 2011;20 doi: 10.1016/j.jamcollsurg.2009.06.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh K.K., Shukla P.C., Quan A., Desjardins J.F., Lovren F., Pan Y., Garg V., Gosal S., Garg A., Szmitko P.E., et al. BRCA2 protein deficiency exaggerates doxorubicin-induced cardiomyocyte apoptosis and cardiac failure. J. Biol. Chem. 2012;287:6604–6614. doi: 10.1074/jbc.M111.292664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozaki K., Sato H., Inoue K., Tsunoda T., Sakata Y., Mizuno H., Lin T.H., Miyamoto Y., Aoki A., Onouchi Y., et al. SNPs in BRAP associated with risk of myocardial infarction in Asian populations. Nat. Genet. 2009;41:329–333. doi: 10.1038/ng.326. [DOI] [PubMed] [Google Scholar]
- 12.Nozynski J.K., Konecka-Mrowka D., Zakliczynski M., Zembala-Nozynska E., Lange D., Zembala M. BRCA1 Reflects Myocardial Adverse Remodeling in Idiopathic Dilated Cardiomyopathy. Transplant. Proc. 2016;48:1746–1750. doi: 10.1016/j.transproceed.2015.12.141. [DOI] [PubMed] [Google Scholar]
- 13.Bordeleau L., Lipscombe L., Lubinski J., Ghadirian P., Foulkes W.D., Neuhausen S., Ainsworth P., Pollak M., Sun P., Narod S.A. Hereditary Breast Cancer Clinical Study Group. Diabetes and breast cancer among women with BRCA1 and BRCA2 mutations. Cancer. 2011;117:1812–1818. doi: 10.1002/cncr.25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Westerop L.L., Arts-de Jong M., Hoogerbrugge N., de Hullu J.A., Maas A.H. Cardiovascular risk of BRCA1/2 mutation carriers: A review. Maturitas. 2016;91:135–139. doi: 10.1016/j.maturitas.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Vitelli L.L., Crow R.S., Shahar E., Hutchinson R.G., Rautaharju P.M., Folsom A.R. Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am. J. Cardiol. 1998;81:453–459. doi: 10.1016/S0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 16.Miyasaka Y., Barnes M.E., Bailey K.R., Cha S.S., Gersh B.J., Seward J.B., Tsang T.S. Mortality trends in patients diagnosed with first atrial fibrillation: A 21-year community-based study. J. Am. Coll. Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Lakhani S.R., Van De Vijver M.J., Jacquemier J., Anderson T.J., Osin P.P., McGuffog L., Easton D.F. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002;20:2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.