Abstract
Some of the bacterial cells in isogenic populations behave differently from others. We describe here how a new type of phenotypic heterogeneity relating to resistance to cationic antimicrobial peptides (CAMPs) is determinant for the pathogenic infection process of the entomopathogenic bacterium Photorhabdus luminescens. We demonstrate that the resistant subpopulation, which accounts for only 0.5% of the wild-type population, causes septicemia in insects. Bacterial heterogeneity is driven by the PhoPQ two-component regulatory system and expression of pbgPE, an operon encoding proteins involved in lipopolysaccharide (LPS) modifications. We also report the characterization of a core regulon controlled by the DNA-binding PhoP protein, which governs virulence in P. luminescens. Comparative RNAseq analysis revealed an upregulation of marker genes for resistance, virulence and bacterial antagonism in the pre-existing resistant subpopulation, suggesting a greater ability to infect insect prey and to survive in cadavers. Finally, we suggest that the infection process of P. luminescens is based on a bet-hedging strategy to cope with the diverse environmental conditions experienced during the lifecycle.
Phenotype switching, the ability to switch reversibly between phenotypic states, is a survival strategy commonly used by bacteria confronted with unpredictable environments1,2. Heterogeneity has been reported for various phenotypes in bacterial pathogens, including persistence in the presence of antibiotic treatment3, heterogeneous behavior within biofilms4 and the expression of motility determinants5,6,7. A textbook example in bacterial expression is the bistable expression of virulence genes in Salmonella Typhimurium8, resulting in phenotypically virulent and avirulent subpopulations. A division of labor has been demonstrated for these two subpopulations during S. Typhimurium infections9.
Photorhabdus luminescens (Enterobacteriaceae) is an entomopathogenic bacterium that lives in symbiotic association with the nematode Heterorhabditis. The complex invades insect larvae and the nematode regurgitates its bacterial symbiont directly into the hemolymph, the insect equivalent of blood. Once released from the nematode into the open circulatory system of the insect (the hemocoel), Photorhabdus interferes with the cellular immunity, which is mediated by circulating hemocytes that engulf the invading bacteria10. P. luminescens must also evade the peptide-mediated immune response, which involves antimicrobial peptides (AMPs)11. Recognition of Photorhabdus by the immune system is controlled by the Imd signaling pathway in Drosophila, which induces AMP synthesis12,13. Circulating P. luminescens bacteria are initially cleared from the insect hemolymph12,14, but bacterial septicemia then occurs. Virulence factors also play a role in the death of the insect10,15. Once the insect host is dead, the bacteria digest the content of the cadaver, which the nematode then uses as a food source to sustain several generations of reproduction16. Photorhabdus produces a number of antimicrobial molecules that eliminate bacteria that might antagonize the growth of the nematode partner and compete for nutrient resources10. Phenotypic switching is a frequent occurrence in the genus Photorhabdus16. Phenotypic variants generally emerge after prolonged in vitro culture of the wild-type strain collected from the nematode17. One particular phenotypic variant, the secondary variant, is characterized by changes to many of the traits of the wild-type form (the production of extracellular enzymes, antibiotics). Generally, both the wild-type and secondary variant forms are virulent in insect hosts, but only the wild-type supports nematode growth and development11. In the model strain, P. luminescens TT01, transcriptomic and proteomic analyses of the wild-type and secondary variants have suggested that phenotypic switching results in extensive cellular reprogramming mediated by transcriptional regulators14,18. However the emergence of secondary variants has been shown to be unrelated to genomic rearrangements19. Another source of phenotypic heterogeneity in P. luminescens is switching between the ON and OFF states of expression of the fimbrial maternal adhesion locus (mad genes), through an inversion of the promoter region. In the ON state, mad genes are transcribed and the bacteria are covered with fimbriae20. This form of Photorhabdus is called the M form (for “mutualistic”) and it is observed only during symbiosis with the nematode. When the mad promoter is in the OFF conformation, the bacteria have no fimbriae and are in P form (for “pathogenic”)21. Thus, phenotypic switching is required for the mutualistic relationship between Photorhabdus and its nematode host. We describe here a new heterogeneity phenomenon relating to resistance to cationic antimicrobial peptides (CAMPs) that has a determinant effect during the pathogenic phase of insect infection. CAMPs have antibacterial activity mediated by charge interactions with the anionic bacterial surface, predominantly through binding to the acidic lipid A moiety of LPS22. In some bacteria, LPS modifications are mediated by proteins encoded by genes regulated by the PhoPQ two-component system23. The addition of an aminoarabinose moiety to the lipid A core (a PbgPE-dependent LPS modification) decreases the initial electrostatic attraction by reducing the net negative charge on the outer membrane, thereby playing a role in bacterial virulence by increasing resistance to CAMPs24. In Salmonella, PhoP indirectly regulates the pbgPE operon (also called pmr or arn), which encodes the enzymes responsible for this lipid A modification, via another two-component system, PmrAB24. The pbgPE operon of Photorhabdus is required for virulence in G. mellonella insects25, but the genome of this bacterium contains no pmrAB genes26. The Photorhabdus phoP mutant results in a completely avirulent phenotype in lepidopterans and susceptibility to CAMPs, such as polymyxin B and cecropins A and B27. Furthermore, expression of the PhoP-regulated ail1 gene, which encodes an outer membrane protein, is dependent on Mg2+ concentration28. Mg2+ is also the main in vitro inducer responsible for activating PhoPQ in Salmonella29. We show here that the virulence strategy of P. luminescens involves the generation of a subpopulation of bacteria resistant to CAMPs that causes septicemia in insects. The resistant bacteria account for only 0.5% of the wild-type population in P. luminescens TT01 during in vitro culture. This bacterial heterogeneity depends on PhoP and pbgPE.
Results
A bacterial subpopulation in P. luminescens displays resistance to CAMPs dependent on the phoP and pbgPE genes
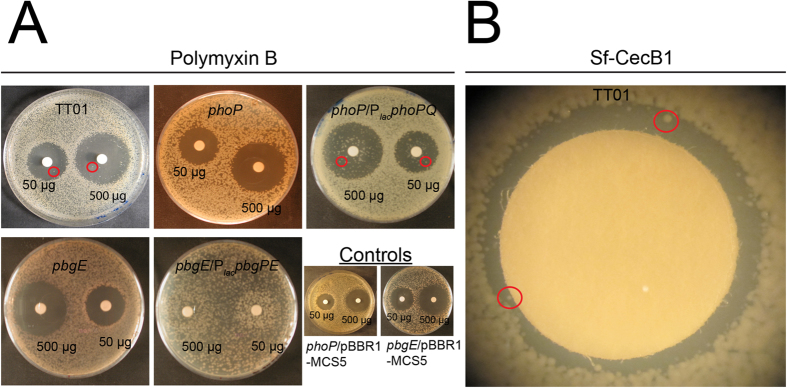
We analyzed the resistance profiles to CAMPs of the wild-type population TT01, a phoP mutant and its phoP/PlacphoPQ complemented strain, a pbgE mutant and its pbgE/PlacpbgPE complemented strain, by determining minimal inhibitory concentrations (MICs). As previously reported25, TT01 was resistant to high doses of CAMPs, whereas the phoP and pbgE mutant strains were susceptible to very low doses of antimicrobial compounds, such as polymyxin B (Table 1). The wild-type resistant phenotype was restored in the complemented strains (phoP/PlacphoPQ, pbgE/PlacpbgPE). The results of this bulk assay suggest that the wild-type population is fully resistant to CAMPs and that this resistance is dependent on the products of the pbgPE genes. Unlike MIC determination, agar disc diffusion assays can be used to analyze bacterial resistance to CAMPs at the individual CFU level (Fig. 1A). Surprisingly, only a few colonies of TT01 were able to grow in the halo containing a gradient of polymyxin B concentration, demonstrating that most of the cells of TT01 were susceptible to polymyxin B. By contrast, no resistant clones were observed in the halo for the phoP and pbgE mutant strains. The pbgE/PlacpbgPE complemented strain had a fully resistant phenotype, whereas the phoP/PlacphoPQ complemented strain was broadly susceptible, with a profile similar to that of TT01. We also assayed the resistance of TT01 to the synthetic Spodoptera frugiperda cecropin B (Sf-CecB1), to mimic the antimicrobial activities naturally found in the plasma of insect larvae challenged with bacteria30. A minor Sf-CecB1-resistant population of TT01 was also observed in the agar disc diffusion assay (Fig. 1B). However the high MIC of Sf-CecB1 (Table 1) made further experiments too costly, we therefore use polymyxin B throughout the study. Finally, the TT01 strain of P. luminescens is a mixed population of individuals, most of which are susceptible to CAMPs, with only a small number of bacterial cells displaying resistance dependent on the expression of phoP and pbgPE.
Table 1. MICs* of four CAMPs for the P. luminescens strains.
| Strains | MIC (μg.ml−1) |
|||
|---|---|---|---|---|
| Colistin | Cecropin A | Cecropin B (S. frugiperda) | Polymyxin B | |
| TT01 | >10,000 | >25 | >50 | >250 |
| phoP | 20 | 0.8–1.6 | 6–12 | 1–3 |
| phoP/PlacPhoPQ | >10,000 | >25 | ND | >250 |
| pbgE | <10 | 12 | 6–12 | 1–2 |
| pbgE/PlacpbgPE | ND | >25 | >100 | >250 |
*MICs were determined by culturing bacterial strains for 48 h with various concentrations of insect (cecropins) and non-insect CAMPs (colistin and polymyxin B). All these experiments were performed at least three times.
Figure 1. Heterogeneity of CAMP resistance in the TT01 wild-type strain population.
(A) Bacteria were layered onto agar plates with paper disks loaded with 500 μg and 50 μg of polymyxin B. The antimicrobial resistance patterns of the various strains were determined in agar disk diffusion assays. Polymyxin B created a visible halo for all strains except for pbgE /PlacpbgPE, which was completely resistant. (B) Cecropin B (Sf cecB1) resistance patterns of TT01 in agar disk-diffusion assays. Red circles highlight colonies found within the halos. Experiments were performed at least twice.
CAMP resistance is a new reversible non-genetic phenotype in P. luminescens
While phenotypic variation phenomena have been described in Photorhabdus16,21, the variant trait (antimicrobial resistance) was not previously reported. Beside polymyxin resistance, we showed that all other tested phenotypes (dye adsorption, the production of extracellular lipases, hemolysins and antibiotics and the ability to generate bioluminescence) were the same as for the wild-type demonstrating a new type of phenotypic heterogeneity. We then quantified the proportions of resistant and susceptible cells in TT01 during bacterial growth in liquid medium, by spreading the bacteria on plates with and without polymyxin B supplementation (Supplementary Figure 1). About 0.5% of the wild-type population was found to be resistant to CAMPs. The reversion phenomenon was then studied from a single colony of polymyxin-resistant clones. After subculturing in broth medium without antibiotic, we yielded a rate of about 1% of resistant bacteria as observed for the wild type showing that the CAMP resistance profile of this subpopulation of cells was reversible in vitro. Such survival rate and reversion phenomenon suggest that resistance heterogeneity in P. luminescens is not governed by mutations alone. We confirmed the hypothesis of a non-genetic origin of CAMP resistance, by first comparing the sequences of the pbgPE and phoP loci between the wild-type population and the resistant subpopulation, using cultures established from the same inoculum. Sanger sequencing revealed no differences between the wild-type population and the resistant subpopulation. We also compared the whole-genome sequence of the polymyxin B-resistant subpopulation obtained with SMRT technology to that of the wild-type population; we found no major rearrangement or relevant mutation (Supplementary Table 1). In particular, no inversion of the promoter region of the fimbrial mad locus was detected showing that polymyxin-resistant clones are not the previously described M form variant21.
Single-cell analysis of the dynamics of resistance gene expression
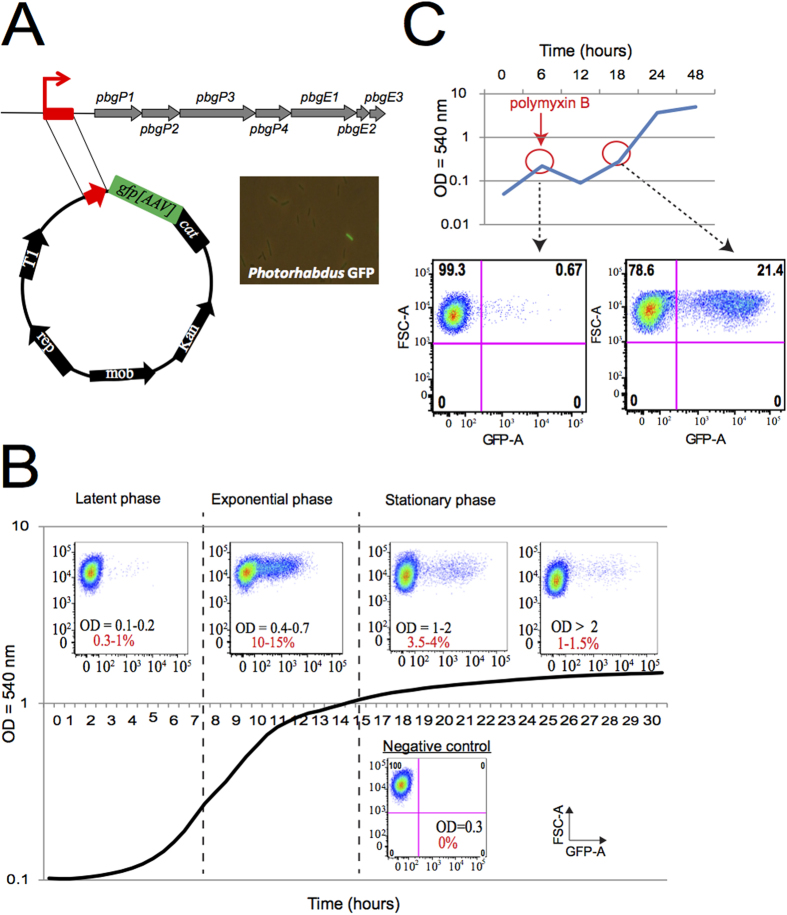
We investigated whether the emergence of the resistant subpopulation was correlated with higher levels of pbgPE expression in single cells, by constructing a transcriptional fusion between the promoter region of the pbgPE operon and a destabilized GFP-encoding gene named TT01/PpbgPE-gfp[AAV] reporter strain (Fig. 2A). In the absence of CAMP selection pressure (Fig. 2B), the fluorescence of individual cells harvested at various growth phases was heterogeneous, suggesting a lack of synchronization of pbgPE gene expression within the population during bacterial growth. The proportion of cells expressing pbgPE was highest during the exponential growth phase (10–15%). Cytometry analysis of a population of cells harboring a transcriptional fusion between the promoter of the pbgPE operon and a stable GFP-encoding gene (the PpbgPE-gfp[mut3]) revealed that 70 to 80% of the bacteria were GFP-positive during the exponential and post-exponential growth phases, whereas up to 87% of the bacteria in long-term cultures were GFP-positive (data not shown). These data illustrate that most cells were able to activate the pbgPE promoter during growth. We then used the same procedure with the destabilized GFP to determine the pattern of fluorescence for the bacterial population during the selection of a resistant subpopulation with polymyxin B (Fig. 2C). The addition of polymyxin B led to a 32-fold increase in the number of living cells producing GFP. The intensity of the GFP signal representing the pbgPE expression of the cell (x-axis) was not higher in LB than after polymyxin B treatment. These findings are consistent with the selection of a resistant subpopulation by CAMP.
Figure 2. Resistance gene expression after polymyxin B selection at the single-cell level.
(A) Representative scheme of the PpbgPE-gfp[AAV] transcriptional fusion between the pbgPE promoter and a destabilized GFP-encoding gene. A GFP-positive bacterium expressing resistance genes is shown. (B) TT01/PpbgPE-gfp[AAV]) was cultured in LB and samples taken over a time course were fixed with formaldehyde and analyzed in a FACS Canto II cytometer. Results are presented as dot plots of side scatter (FSC) against GFP fluorescence intensity. Each dot represents one bacterium. (C) TT01/PpbgPE-gfp[AAV] was first cultured in LB. Samples were collected at an OD540 of 0.3, before and after the addition of polymyxin B to the culture medium. The representation used here is as in panel B. Only live cells were considered, after treatment with eFluor 660. One representative experiment from more than three independent experiments is shown.
Characterization of the PhoP regulon in P. luminescens
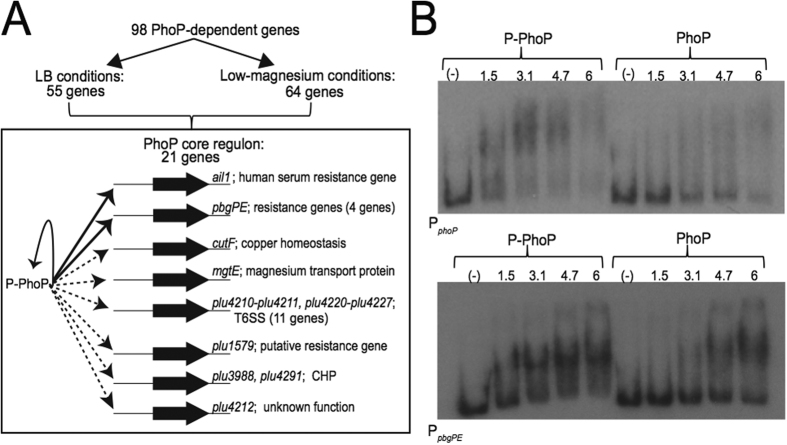
As PhoP was essential for the emergence of a CAMP-resistant subpopulation, we first characterized the PhoP regulon in P. luminescens at the population scale. Growth in the presence of low levels of Mg2+ is a triggering signal for PhoP regulon expression29. We therefore used RNAseq analysis to compare the gene expression profiles of TT01 and the phoP mutant grown either in LB or in a medium containing low Mg2+ levels, at mid-exponential growth phase (OD540 = 0.3). We used DESeq differential analysis to compare each annotated feature between transcriptomes. We observed significant differences in expression between the phoP mutant and TT01 for 55 coding sequences (|log2 fold change| ≥1; adjusted p-values ≤ 0.005) in LB medium and for 64 coding sequences in low-Mg2+ medium (Supplementary Table 2A,B). The PhoP regulatory network, then, consists of 98 genes that are differentially expressed between TT01 and the phoP mutant strain (Fig. 3A). Only 26 genes were downregulated by PhoP in both LB and low-Mg2+ conditions. We subsequently defined the core PhoP regulon in P. luminescens as the 21 differentially regulated genes (de facto over-expressed) in both sets of conditions out of the 98 PhoP-dependent genes (Fig. 3A) and six loci were validated by real-time RT-qPCR (Supplementary Table 2C). The core PhoP regulon includes genes involved in CAMP resistance (pbgP operon), resistance to host factors (ail1, plu1579) and genes encoding conserved hypothetical proteins of unknown function. We also found 11 genes belonging to a locus encoding putative components of a type 6 secretion system in TT01 (T6SS-4TT01: plu4198-plu4227) (Fig. 3A). For the entire PhoP regulon, we identified six additional genes from the T6SS-4TT01 locus and four genes from the T6SS-3TT01 locus (plu3259-3262) as differentially regulated (Supplementary Table 2A). T6SS are widely thought to be involved in cellular interactions between bacteria or between bacteria and eukaryotes. Their function in Photorhabdus has yet to be determined, but both these roles are consistent with the lifestyle of this bacterium16.
Figure 3. Model summarizing the core PhoP regulon in P. luminescens.
(A) Two sets of PhoP-dependent genes (55 LB and 64 low Mg2+) were identified after RNAseq analysis of the gene expression patterns of the TT01 and phoP strains in two different sets of growth conditions (LB medium or minimal M9 medium supplemented with 10 μM Mg2+). The intersection of the two lists identifies 21 gene markers as the core PhoP regulon (see box). Bold thin arrows indicate direct regulation by the phosphorylated form of PhoP (P-PhoP). Hatched thin arrows indicated that the binding profile is unknown. CHP: conserved hypothetical protein, T6SS: type 6 secretion system. (B) Electrophoretic mobility shift assays were carried out to test the binding of the PhoP protein activated in vitro with 10 mM acetyl phosphate (P-PhoP) or of the non-activated PhoP-His to the 206-bp phoP and 198-bp pbgPE promoter regions. The PhoP-His concentrations indicated are in micromoles per liter. We checked that binding was specific, by adding BSA and poly(dI-dC) to the binding buffer to saturate non-specific binding sites.
PhoP is a transcriptional regulator that mostly upregulates its network through direct or indirect interactions. The phosphorylated form of PhoP can bind directly to the promoter region of the ail1 gene28, a member of the core PhoP regulon. We investigated the architecture of the PhoP network further, by exploring the interactions of PhoP with the promoter sequences of the pbgPE and phoPQ operons, both of which are required for the emergence of the resistant subpopulation. We carried out electromobility shift assays (EMSA) to compare the interaction profiles of different amounts of PhoP protein with the pbgPE and phoP promoter regions. A shift was observed for both promoters after the addition of phosphorylated PhoP-His (Fig. 3B). No such shift was observed after incubation with unphosphorylated PhoP-His at low concentration (below 4.7 μM). This specific binding to the promoter regions of phoP and pbgPE confirmed that the phosphorylated form of PhoP is the active isoform and revealed that PhoP is involved in positive feedback control of its own expression (Fig. 3A).
Concomitant overexpression of genes involved in resistance, virulence and bacterial antagonism in the polymyxin B-resistant subpopulation
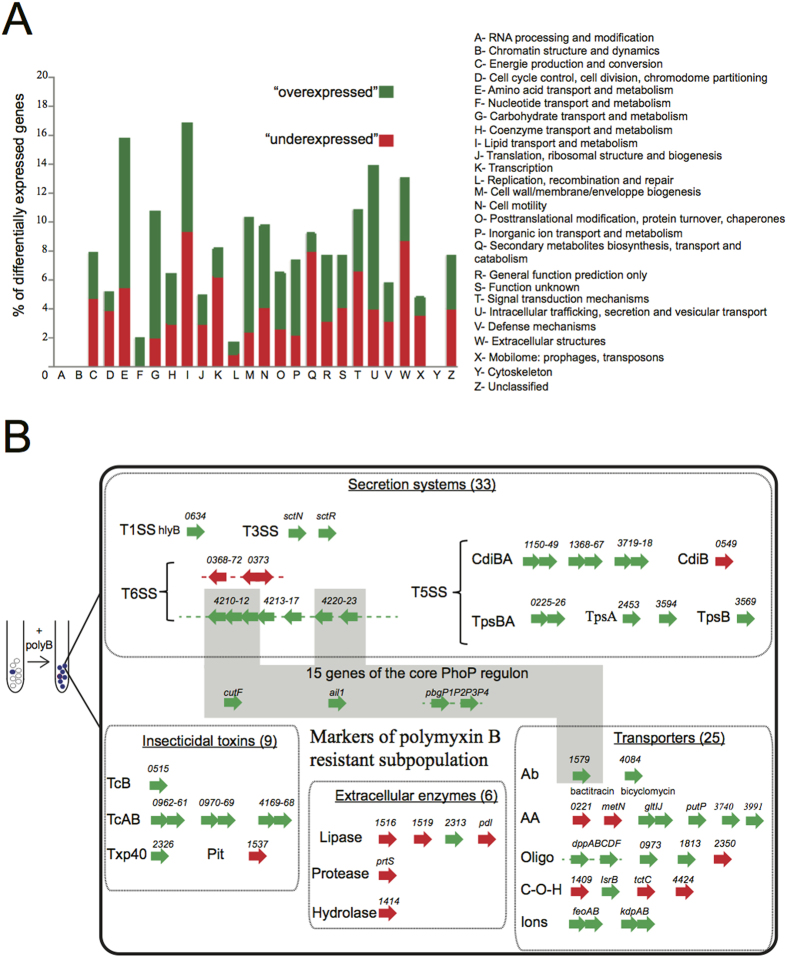
We investigated the gene expression profiles of the subpopulation defined on the basis of CAMP resistance, by extracting RNA from bacteria grown in LB medium and harvested at an OD540 of 0.3–0.4 before (control) and after the addition of polymyxin B (10–12 hours of exposure). We observed significant differences (|log2 fold change| ≥1; adjusted p-values ≤ 0.005) in expression between the two sets of conditions for 445 genes (Supplementary Table 3). We found that 208 genes were underexpressed and 237 genes were overexpressed in the resistant subpopulation relative to the control (i.e., the same clonal population before the addition of polymyxin B) (Supplementary Table 3B,C). COG annotations and representations of percentages by COG class in TT01 genome (Fig. 4A) indicated that the polymyxin B-selected regulon was not massively biased towards one specific COG class. Nevertheless, we observed a slight enrichment in genes encoding proteins involved in amino-acid transport and metabolism (E), lipid transport and metabolism (I), intracellular trafficking, secretion and vesicular transport (U) and extracellular structures (W). Interestingly, four large families of proteins previously described in P. luminescens26 were over-represented (Fig. 4B). First, components of secretion systems were frequently found to be differentially expressed and some of these systems have been described as bacterial contact-dependent delivery systems31. One remarkable marker of the polymyxin B-resistant subpopulation is the family of proteins secreted by the two-partner secretion (TPS) pathway (T5SS). Actually, six of the ten genes encoding TpsA (the exoproteins) and six of the eight genes encoding the TpsB (β-barrel protein pores) were differentially expressed. For tpsAB genes, the expression of phlAB, encoding a protein involved in secretion of the hemolysin PhlA in P. luminescens32, was similar in the presence and absence of polymyxin B, whereas most of the cdiAB genes (seven of eight cdi genes) encoding a new functional family of contact-dependent inhibition (CDI) system33 were representative markers of the polymyxin B subpopulation (Fig. 4B). Moreover, 12 PhoP-dependent genes encoding the T6SS-4TT01 were overexpressed markers in the resistant subpopulation, whereas six genes from another T6SS locus (T6SS-1TT01: plu0335-plu0373) were underexpressed in the resistant subpopulation (Fig. 4B). Similarly, 25 genes encoding components of transporters of small molecules were differentially expressed between the resistant subpopulation and the wild-type population. The other overrepresented proteins were large multimeric protein complexes from the insecticidal toxin complex (Tc) family34. Seven genes encoding TcA or TcB subunits were overexpressed in the resistant subpopulation, suggesting higher virulence for the resistant subpopulation in insects. Finally, five extracellular enzymes classically described as causal factors of insect cadaver degradation35 or enhancers of Tc toxins, were underexpressed in the resistant subpopulation. We also found that a large proportion of the genes of the PhoP regulon were overexpressed in the resistant subpopulation: 37 of the 55 PhoP-dependent genes in LB broth (Supplementary Table 3C) and 15 of the 21 genes from the core PhoP regulon (Fig. 4B; Supplementary Table 3C). Moreover we showed by RT-qPCR that the main signature markers (PhoP-dependent or not) are also present in polymyxin-resistant clones grown without polymyxin B (Supplementary Table 3D) demonstrating that the presence of the antibiotic is not required to the differential expression of signature markers. Thus, the polymyxin B-resistant subpopulation mainly displayed an upregulation of genes involved in CAMP resistance, virulence (such as tc) and bacterial antagonism (such as cdi), suggesting an enhanced ability to infect insects and to outcompete saprophytic bacteria in cadavers.
Figure 4. Identification of marker genes for the polymyxin B-resistant subpopulation.
(A) Classification by COG (cluster of orthologous class) annotation of the 445 genes displaying a change in expression after the addition of polymyxin B (polymyxin B gene set) according to the 2014 update (ftp://ftp.ncbi.nih.gov/pub/COG/COG2014/static/lists/homeCOGs.html). The percentage of genes from each COG class differentially expressed between the polymyxin B-resistant subpopulation and Photorhabdus luminescens TT01 genes is shown. (B) Four functional groups of representative marker genes for the polymyxin B-resistant subpopulation. The number of genes in each group is indicated in brackets. Genes are represented as arrows with their name or their label number. Overexpressed and underexpressed genes are indicated in green and red, respectively. The gray area corresponds to genes from the core PhoP regulon. AA: amino acids; C-O-H: carbohydrates; Ab: antibiotics; oligo: oligopeptides.
The resistant subpopulation is responsible for killing insects
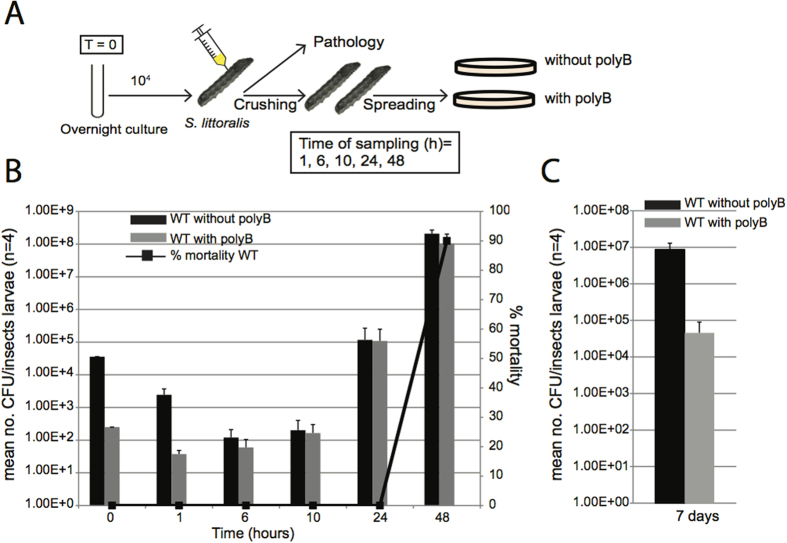
The observation of a Photorhabdus population containing cells in which virulence and resistance genes were pre-activated in vitro led us to study the fate of this resistant subpopulation in insects through studies of the in vivo growth kinetics of the P. luminescens TT01 strain. At various time points after injection, we quantified the cells of the resistant subpopulation by plating extracts of crushed insect larvae (Fig. 5A). As previously described14, the total number of living TT01 CFU decreased by two to three orders of magnitude during the first few hours after injection, corresponding to the clearance phase. Between 6 to 10 hours post infection (hpi), the total population of living cells consisted entirely of resistant bacteria (Fig. 5B). Spodoptera larvae synthesize AMPs 3 to 9 hours post injection36,37, a time period corresponding perfectly to the observed clearance phase. During the septicemia phase, when the insects were dying, the resistant subpopulation outcompeted the susceptible population, highlighting the dependence of septicemia on the multiplication of resistant bacteria. No insect death and septicemia was observed after the injection of the phoP or pbgE mutant strains (see Supplementary Figure 2) and as expected, no resistant bacteria were observed (data not shown). To confirm that the resistant subpopulation consisted of pre-adapted infectious cells, we injected a selected polymyxin B-resistant subpopulation into insects at a lower dose (103 bacteria per insect). The LT50 was reached four hours earlier than for the TT01 wild-type population (see Supplementary Figure 3). We also assessed the reversibility of the resistant phenotype in insects after long-term incubation in vivo. The proportion of resistant cells in the population present in the insect cadaver returned to its initial value after seven days (Fig. 5C).
Figure 5. The resistant subpopulation is the major population present during septicemia in insects.
(A) Representative diagram of the experimental procedure. (B) Bacterial growth and insect larval mortality following the injection of P. luminescens TT01 into Spodoptera littoralis. We injected 3 × 104 bacteria into each larva at time zero. The histogram shows the mean numbers of CFU recovered from four larvae per time point, after plating on nutrient agar (black bars) or nutrient agar supplemented with polymyxin B (gray bars). The error bars indicate the standard error of the mean. The lines indicate larval mortality rates (for 20 larvae per treatment). One representative experiment from more than three independent experiments carried out is shown. (C) The procedure and representation are as in panel B, except that the insect cadaver extracts were plated on agar seven days after injection.
Discussion
We identified phenotypic and transcriptional heterogeneity underlying individual variation in resistance to CAMPs in subpopulations of P. luminescens. The high rate with which resistance emerged and its rapid reversion in the CAMP-resistant subpopulation suggested that the variation was not genetic. Genome sequence analysis identified no causal mutation related to the polymyxinB-resistance phenotype, suggesting that the resistance was instead epigenetic. Differences between cells in isogenic sbacterial populations may reflect noise due to central cellular processes1. We have shown in the sister species of this entomopathogenic bacterium, Xenorhabdus5, that feedback loops in regulatory networks lead to bimodal gene expression or bistability, as described elsewhere38. We also found that the emergence of P. luminescens resistant individuals is driven by the PhoP-PhoQ two-component regulatory system and the pbgPE resistance genes encoding proteins involved in LPS modifications25. Consistent with previous reports27, we found that PhoP regulated its own transcription via a positive feedback loop and we observed direct binding of phosphorylated PhoP to the promoter region of the pbgPE operon. However, no conserved PhoP box consisting of a repeated hexameric sequence39 was identified in the 5′UTR of these two genes. In Salmonella enterica, the positive feedback loop of the phoPQ operon is required for a surge in the activity of the PhoP/PhoQ system40. In P. luminescens, the ectopic expression of phoPQ genes and bacterial growth in inducing conditions (low Mg2+ conditions) did not significantly increase the proportion of polymyxin B-resistant individuals in the total population. Furthermore, the feedback loop controlling phoP expression was unable to stabilize the noisy pattern of pbgP expression in the presence and absence of CAMP. Despite the lack of synchronization of PhoP-dependent pbgPE gene expression during the growth of P. luminescens, appropriate levels of expression, at the right time, can be achieved randomly in certain individuals, facilitating thereby the modifications to LPS involved in CAMP resistance. The mechanisms underlying the erratic expression of pbgPE genes have yet to be characterized. Finally, this non-genetic variation in P. luminescens displayed some degree of heritability, as the production of a sufficiently large progeny allowed colony formation in the presence of CAMP selection, suggesting a role for epigenetic variation. It is noteworthy that PhoP-mediated LPS modifications also occur in a subset of S. enterica during infection and this bacterial heterogeneity is sufficient to drive radically different host immune responses41. Another successful strategy of antibiotic resistance used by subpopulations of bacterial cells involves the generation of persister cells, which cannot multiply in the presence of antimicrobial compounds3. However, transient dormancy states cannot confer resistance to cationic peptides acting like surfactants. A recent study described an Enterobacter cloacae isolate harbouring a minor subpopulation highly resistant to colistin (polymyxin E)42. As shown here with polymyxin B, this subpopulation become predominant in colistin and returned to baseline after antibiotic removal and was also dependent on PhoQ. Authors proposed to refer to this resistance phenomenon described in E. cloacae as “clonal heteroresistance”. However, excepted a higher levels of the lipid A modification genes such as arnB (pbgP1 synonym), signature markers described in polymyxin-resistant subpopulation of Photorhabdus (such as T6SS or T5SS upregulation) were not found in colistin-resistant E. cloacae. Other clinical studies have also reported the preadaptation of certain bacterial cells to antibiotic challenge in the absence of mutation. So-called “adaptive” resistance typically involves transient changes in the expression of genes encoding porins or efflux pumps, increasing the chances of the isogenic bacterial population surviving antibiotic treatment43. For instance, single-cell heterogeneity in expression of the porin gene ompC confers adaptive resistance to kanamycin in Salmonella44. The resistance phenotype described here is a similar, transient phenomenon, but the mechanism involved is different. Moreover we also showed here that the emergence of polymyxin resistance in P. luminescens is a phenomenon different than phenotypic variation previously described in this genus.
One of the most striking findings of this study is the selection of the transient resistant state during infection, rendering the subpopulation of cells resistant to CAMPs virulent in insect larvae. Indeed, P. luminescens kills insects through bacteremia, and the LT50 of Photorhabdus has been shown to be directly correlated with its rate of growth in vivo45. We found that the observed clearance of circulating bacteria from the insect hemolymph12,14 was consistent with the destruction of most of the bacterial cells susceptible to the CAMPs produced by the insect early in infection. In particular, attacin C and drosocin were strongly upregulated after Photorhabdus infection13. The subsequent growth of the CAMP-resistant population leads to septicemia and insect death. After the infection stage, reversibility of the resistant phenotype, with a return to the initial proportions of resistant cells was observed after long-term incubation in insect cadavers, consistent with reports that CAMPs may be degraded by proteases, produced by P. luminescens46. The reversible phenotypic variation in Photorhabdus, conferring an adaptive resistance phenotype on a minor subpopulation, may clearly be seen as a bet-hedging strategy47. Our findings are consistent with the hypothesis that bet-hedging is potentially advantageous in harsh and unpredictable environments48. One question that remains to be addressed is why the infection strategy is based on bet-hedging, rather than direct environmental sensing in P. luminescens. Indeed, Photorhabdus has to cope with very different environments imposed by the host immune system. The nematode hosts of these bacteria target a wide range of insects49. Each insect family has its own defense system, with different patterns and the production of different families of CAMPs (for review see50). We therefore suggest that P. luminescens survival is partly dependent on a non-specific mechanism of resistance to CAMPs, involving LPS modifications and that the expression of the resistance phenotype is noisy because the insect-host environmental signals are highly diverse and unpredictable due to the diversity of insect prey49. We also found that the selection mediated by polymyxin B in vitro or by insect CAMPs early in infection resulted in the generation of individuals strongly expressing antibiotic resistance genes. As expected, the overexpression of pbgPE and of the other main PhoP-dependent genes from the PhoP core regulon was identified as a marker of this selection. However, with the exception of two genes encoding putative transport proteins (Fig. 4), very few other antimicrobial peptide resistance genes were selected. In addition, our RNAseq data revealed no induction of regulon involved in stress responses. Interestingly, detailed analysis revealed enrichment in genes encoding type 5 and 6 secretion systems. Complex interplay has already been reported between LPS O-antigen structure in Pseudomonas aeruginosa and polymyxin B, a membrane-disrupting product, with the induction of T3SS and T6SS expression51,52. Like T6SS, the CdiAB family of TPS proteins mediates interbacterial competition in a contact-dependent manner53. Indeed, cdi genes are highly representative markers of the polymyxin B-resistant subpopulation. The CdiAB toxin family is overrepresented in the genus Photorhabdus33, but its function remains unknown. By analogy to other bacteria53, the overexpression of contact-dependent delivery systems in pre-adapted resistant P. luminescens may confer a substantial growth advantage in kin competition (i.e. with other nematode-associated bacterial strains) in cases of multiple nematode infestations. The cost of bet-hedging is related to the generation of types poorly adapted to the current conditions. The majority population of susceptible bacteria is probably avirulent in lepidopterans, as reported for pbgE and phoP mutants (Supplementary Fig. 2). This population may also display other impairments of the expression of secretion systems, transporters and insecticidal toxins. The CAMP-susceptible subpopulation is probably at an advantage under one indeterminate condition, and can outcompete CAMP-resistant individuals under a different set of conditions. Remarkably, it is likely that the majority population of susceptible bacteria produces larger amounts of extracellular enzymes than the resistant subpopulation. This susceptible population may, therefore, be better adapted to the next phase of the Photorhabdus life cycle, involving the degradation of insect cadavers to support nematode reproduction.
The phenotypic variation in P. luminescens underlies its virulence and the rapid transition from an exponentially growing pathogenic and resistant state to a post-exponential growth-stage form able to promote its own growth and that of its nematode host. Low nutrient concentration triggers the last phase of the Photorhabdus lifecycle: colonization of the nematode host and transmission to the next insect prey. The entry into stationary growth phase also coincides with the emergence of another variant form previously described21: the mutualistic form able to colonize the infective juvenile stage of the nematode. In conclusion, both studies clearly illustrate that phenotypic switching fully controls the pathogenic and mutualistic interactions between Photorhabdus and its invertebrate hosts.
Methods
Bacterial strains, plasmids, and growth conditions
The strains and plasmids used in this study are listed in Supplementary Table 4. P. luminescens strains were routinely grown at 28 °C in Luria-Bertani (LB) or Mueller-Hinton broth (Biokar), nutrient agar medium (Difco), or NBTA agar54. Photorhabdus was also grown in M9 liquid medium supplemented with 0.1% casamino acids, 0.41 mM nicotinic acid, 9.1 mM sodium pyruvate, 0.1 mM CaCl2 and 0.2% glycerol, with various concentrations of MgSO4 (10 μM for low Mg2+ conditions and 10 mM for high Mg2+ conditions). When required, antibiotics were used at the following final concentrations: polymyxin B (polyB), 100 mg.l−1; kanamycin, 20 mg.l−1; gentamicin, 15 mg.l−1; and erythromycin 15 mg.l−1.
Antibacterial activity
In vitro susceptibility tests to determine MICs were performed by the broth microdilution method, according to guidelines55. Stock solutions of colistin methane sulfonate (Sigma) and polymyxin B (Sigma) were diluted in sterile water to obtain concentrations of 20 and 50 mg.l−1, respectively. Stock solutions of 0.4 mg.l−1 cecropin A and B (Sigma) were prepared in sterile water. Synthetic S. frugiperda cecropin B (CecB1)30 was prepared in distilled water, to yield a concentration of 0.5 mg.l−1. Antibiotics were then added directly to 96-well microtiter plates in serial two-fold dilutions. We dispensed 104 bacteria grown to an OD540 of 0.6–0.8 into each microdilution well. MICs were determined by eye after incubation in microtiter plates containing Mueller-Hinton broth (Biokar) at 28 °C for 48 h.
Electrophoretic mobility shift assays (EMSA)
The promoters of phoP and pbgP1 were amplified by PCR from the genomic DNA of TT01 with the primers (Supplementary Table 5), and purified with the High Pure PCR Product Purification kit (ROCHE). The 5′ ends of the DNA were labeled with [γ-32P] ATP and T4 polynucleotide kinase (Promega). The PhoP-His protein was purified and phosphorylated in vitro by incubation with acetyl phosphate56. Radioactive DNA probe (2000 cpm.ml−1), 200 ng of poly(dI-dC)-poly(dI-dC) (SIGMA) and various amounts of PhoP-His were mixed in binding buffer (50 mM Tris-HCl pH 8, 50 mM KCl, 50 μg.ml−1 BSA), in a total volume of 20 μl, and incubated for 20 minutes at room temperature. The mixture was then loaded onto a native 6% (w/v) polyacrylamide TBE precast gel (Invitrogen) and subjected to electrophoresis in 1% TBE (Tris-borate-EDTA) buffer for 1 h at 100 V. Radioactive species were detected by autoradiography. PhoP-His was activated by in vitro phosphorylation with acetyl phosphate57.
Molecular techniques and RNA preparation
DNA manipulations were carried out as previously described58. Plasmids were introduced into E. coli WM3064 (Supplementary Table 4) by transformation and transferred to P. luminescens by filter mating32. All constructs were sequenced by Eurofins MWG Operon. The primers used (Eurogentec) are described in Supplementary Table 5. Total RNA was extracted and purified with the RNeasy miniprep kit (Qiagen), including a DNase I treatment step. For each RNA preparation, we assessed DNA contamination by carrying out a control PCR. The quantity and quality of RNA were assessed with an Agilent 2100 Bioanalyzer with the RNA 6000 Nano LabChip kit. RNA was prepared from a culture at an OD540 of 0.3–0.45 before and after the addition of polymyxin B to the medium (when the OD540 had returned to 0.3). Samples were differentially analyzed in the phoP mutant and the wild-type TT01 strain grown in LB or low Mg2+ conditions or before and after adding polymyxin B (six independent biological replicates per strain) and pooling equal amounts of total RNA from three replicates of the same strain together. We thus generated two biological samples per strain, which were subjected to two successive rounds of ribosomal RNA depletion with the Microbe Express kit (Ambion) before RNA Sequencing.
Single-molecule real-time (SMRT) DNA sequencing and mutation analysis
Genomic DNA was extracted from bacteria grown in LB or LB plus polymyxin B and harvested at an OD540 of 0.3–0.45 with the QIAamp DNA Mini Kit (Qiagen). An additional purification was performed with the DNA Clean-up kit (MoBio Laboratories). The DNA libraries were prepared according to PacBio guidelines and sequenced on two PacBio SMRT cells on a Pacific Biosciences RSII instrument (Genome Québec, Montréal, Canada). The raw data were processed with the PacBio SMRT Analysis Suite (version v2.3 p3). The reads were assembled de novo, with the high-quality Hierarchical Genome Assembly Process HGAP.3 algorithm. The assembled genomes were deposited in the Microscope platform database59. No major rearrangements were observed with progressiveMauve 2.1.0.a160. Conserved Synteny LinePlot revealed 100% conservation of synteny groups between the two genomes studied, with a synton size ≥3 and the P. luminescens TT01 NC_005126 genome as the reference (data not shown). We used two programs for the detection of SNPs, insertions and deletions: GATK v3.3.0 (The Genome Analysis Toolkit61) which was used to map PacBio reads onto the NC_005126 reference genome, and LAST v71262, which is based on the alignment of the NC_005126 reference genome and the two HGAP.3 assembled genomes. Regions of poor quality displaying homopolymers ≥4 nucleotides in length or discordant multiple alignments (i.e. mapping several times onto the reference genome, with one correct mapping and one (or several) displaying <100% homology) were considered irrelevant and discarded. With 20% of its genome consisting of repeats, P. luminescens TT01 NC_005126 has been identified as a genome with a particularly high level of repeat coverage.
RNA Sequencing
The RNA-seq libraries were prepared with the TruSeq® Stranded mRNA sample prep kit (Illumina). Samples depleted of rRNA were fragmented and reverse-transcribed with random hexamers, Superscript II (Life Technologies) and actinomycin D. During the generation of the second strand, dTTP was replaced with dUTP. Double-stranded cDNAs were adenylated at their 3′ ends before ligation with Illumina indexed adapters. Ligated cDNAs were amplified by 15 cycles of PCR and purified with AMPure XP Beads (Beckman Coulter Genomics). Libraries were validated with a DNA1000 chip (Agilent) and quantified with the KAPA Library quantification kit (Clinisciences). Twelve libraries were pooled in equimolar amounts in a single lane and were sequenced on a HiSeq2000 machine, with the single-read protocol (50 nt). Image analysis and base-calling were performed with Illumina HiSeq Control Software and the Real-Time Analysis component. Demultiplexing was performed with Illumina sequencing analysis software (CASAVA 1.8.2). Data quality was assessed with FastQC from the Babraham Institute and the sequencing analysis viewer (SAV) from Illumina software.
RNAseq analysis
High-throughput transcriptomic sequencing data were processed with a bioinformatic pipeline implemented at the Microscope platform59. The reads were mapped onto the P. luminescens subsp. laumondi TT01 genome sequence (EMBL accession number: BX470251) with BWA software (v. 0.7.4)63. We then used SAMtools (v.0.1.12)64 to lower the false-positive discovery rate and to extract reliable alignments from BAM-formatted files. The number of reads matching each genomic object harbored by the reference genome was then calculated with the Bioconductor-GenomicFeatures package65. For reads matching several genomic objects, the count number was weighted so as to keep the total number of reads constant. Finally, we used the Bioconductor-DESeq package66 with default parameters to analyze raw count data and to evaluate differential expression between conditions. In more details, we used the False positive Discovery Rate (FDR) method (variance estimate for each gene with the Negative Binomial distribution followed by a per gene Wald-test generating p-values that were adjusted by the method67. Between 11 and 20 million Illumina sequences (50-base reads) were obtained for each sample and between 15 and 40% of high-quality sequences mapped to at least one site in the reference genome. The complete dataset from this study has been deposited in the GEO database, under accession number GSE76559.
RT-qPCR analysis
RT-qPCR was performed as previously described5. The data for each sample are expressed relative to the level of recA, using REST software 200968. This method quantified the expression of a target gene relative to that of a reference gene, for comparisons of TT01 with the phoP mutant in different growth conditions and for comparisons of TT01 and the polymyxin B resistant subpopulation before and after the addition of polymyxin B.
Estimation of the size of the resistant subpopulation
Agar disk diffusion assays were performed as follows. An exponentially growing culture was diluted in Mueller-Hinton medium and 1 ml, corresponding to a total of 103 CFU, was spread on Mueller-Hinton agar plates. The excess medium was removed and the plates were dried for 5 to 10 minutes. We placed paper disks on the surface of the plates, and these disks were infiltrated with 500 μg or 50 μg of polymyxin B in a maximum of 10 μl. The plates were incubated at 28 °C and observed after incubation for 48 h. We also determined the proportion of the total population displaying resistance in vitro by counting CFUs on nutrient agar plates. Samples from the same culture of TT01 were diluted and at least three dilutions were spread on plates with nutrient agar or nutrient agar supplemented with polymyxin B (final concentration: 100 μg.ml−1) to isolate the resistant subpopulation. Samples were collected during bacterial growth, at each key point (lag, exponential and stationary phases). Colonies were counted 48 h after incubation at 28 °C.
Construction of plasmids expressing gfp[AAV] and gfp[mut3] under the control of the pbgPE promoter
We used a method similar to that reported in a previous study56 to construct plasmids expressing the reporter gene gfp[AAV] under the control of the pbgPE or lac promoter. We constructed PpbgPE-gfp[AAV] as follows. Briefly, a DNA fragment corresponding to the pbgPE promoter region (198 bp) was amplified by PCR from TT01 genomic DNA with primers containing a KpnI or XbaI restriction site (Supplementary Table 5). The PCR products were digested and inserted into the corresponding sites of pPROBE-gfp[AAV]. We constructed PpbgPE-gfp[mut3] by replacing the gfp[AAV] from PpbgPE-gfp[AAV] with the gfp mut3 gene (PCR amplification from pBB-KGFP). Finally, PpbgPE-gfp[AAV] and PpbgPE-gfp[mut3] were transferred into the TT01 wild-type strain by mating.
Flow cytometry analysis of pbgPE expression in individual bacterial cells
The cultures were standardized, with a starting OD540 of 0.05 in 100 ml of LB medium supplemented with kanamycin. For kinetic analyses, samples were washed once with PBS (without calcium and magnesium) and the bacteria were fixed by incubation in 2% formaldehyde in PBS for 15 minutes at room temperature. For analysis of the resistant subpopulation, samples were collected at an OD540 of 0.3 before the addition of 100 μg.ml−1 polymyxin B, and then again after 10 to 12 hours of growth in the presence of polymyxin B. For live-dead staining, samples were washed once and the bacteria were incubated with Hoechst 33342 stain (final concentration: 3 μg.ml−1) for 15 minutes, washed twice and then incubated with Fixable Viability Dye eFluor 660 (eBioscience), diluted 1:1,000 in PBS, at 4 °C for 30 minutes before fixation in formaldehyde. All samples were analyzed in a FACS Canto II flow cytometer (BD Bioscience)5 for GFP quantification and live-dead analysis. Data were captured for 30,000 bacteria per sample and the raw data were analyzed with FlowJo version 8.8.6 software (TreeStar). Compensation was performed, if required. Only live cells were counted in GFP analysis (Hoechst-positive and eFluor 660-negative bacteria).
In vivo pathogenicity assays
The common cutworm, Spodoptera littoralis, was reared with a photoperiod of 12 h on an artificial diet at 23 °C. Fifth-instar larvae were selected and surface-sterilized with 70% (vol/vol) ethanol before injections. To monitor survival curves over time, we injected 103 CFU of exponentially growing bacteria from cultures supplemented with antibiotics if necessary (polymyxin B 100 μg.ml−1) into groups of 20 larvae. Treated larvae were incubated individually for up to 96 h, and the time at which the insects died was recorded. Bacterial concentrations were determined by counting CFUs after the plating of dilutions on nutrient agar. Statistical analysis was performed to compare survival experiments. Signed-rank tests (Wilcoxon test) were used to compare mortality patterns, as previously described69. For the analysis of bacterial growth kinetics and clearance in insect larvae, we injected more bacteria than previously used for the pathogenicity assays (about 5 × 104 bacteria from overnight cultures) into each of 60 fifth-instar larvae. We used 40 larvae to monitor bacterial growth in hemocoel, respectively. Four groups of two larvae for each time point were surface-sterilized with 70% (vol/vol) ethanol, crushed in 3 ml LB medium with a TissueLyzer II (Qiagen) and centrifuged at 400 × g for two minutes to remove larval debris. The extract was then suitably diluted and the density of bacteria present was determined by counting CFUs for dilutions plated on NBTA nutrient agar supplemented with 15 μg.ml−1 erythromycin in order to avoid the growth of other bacteria than Photorhabdus that is naturally EryR. We evaluated the concentration of bacteria in the resistant subpopulation by adding 15 μg.ml−1 erythromycin and 100 μg.ml−1 polymyxin B to the plates.
Additional Information
How to cite this article: Mouammine, A. et al. An antimicrobial peptide-resistant minor subpopulation of Photorhabdus luminescens is responsible for virulence. Sci. Rep. 7, 43670; doi: 10.1038/srep43670 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Nadège Ginibre for assistance with the pathology analysis and Dr V. Molle and Dr M. Canova for help with EMSA manipulations. Image acquisition and analysis were performed on the workstations of the Montpellier RIO Imaging facility of the IGMM. We also thank Christophe Duperray and Myriam Boyer from the Montpellier RIO Imaging facility of the IRB and IGMM institutes for technical assistance with cell sorting manipulations and Philippe Clair from the Montpellier GenomiX facility for expert technical assistance with real-time PCR. A.M. was supported by a PhD grant from INRA and A.G. was supported by grant numbers 2011_1333_03 and 2015_1333 from INRA (http://www.spe.inra.fr/en/).
Footnotes
The authors declare no competing financial interests.
Author Contributions A.M. performed experiments and wrote the draft manuscript; S.P. performed experiments; A.L. performed experiments; S. G. analysed the data and interpretation; G.J. conceptualization; M.B. data curation; S.C. data curation; E.D. data curation; D.R. data curation; L. L. data analysis; J. B. analysed the data and interpretation A.G. writing (review and editing) and design of the work. All authors have read and approved the manuscript for publication.
References
- Smits W. K., Kuipers O. P. & Veening J. W. Phenotypic variation in bacteria: the role of feedback regulation. Nature reviews. Microbiology 4, 259–271, doi: 10.1038/nrmicro1381 (2006). [DOI] [PubMed] [Google Scholar]
- Avery S. V. Microbial cell individuality and the underlying sources of heterogeneity. Nature reviews. Microbiology 4, 577–587, doi: 10.1038/nrmicro1460 (2006). [DOI] [PubMed] [Google Scholar]
- Balaban N. Q., Merrin J., Chait R., Kowalik L. & Leibler S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625, doi: 10.1126/science.1099390 (2004). [DOI] [PubMed] [Google Scholar]
- Carcamo-Oyarce G., Lumjiaktase P., Kummerli R. & Eberl L. Quorum sensing triggers the stochastic escape of individual cells from Pseudomonas putida biofilms. Nature communications 6, 5945, doi: 10.1038/ncomms6945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelin G. et al. FliZ is a global regulatory protein affecting the expression of flagellar and virulence genes in individual Xenorhabdus nematophila bacterial cells. PLoS genetics 9, e1003915, doi: 10.1371/journal.pgen.1003915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobkova E., Emonet T., Vilar J. M., Shimizu T. S. & Cluzel P. From molecular noise to behavioural variability in a single bacterium. Nature 428, 574–578, doi: 10.1038/nature02404 (2004). [DOI] [PubMed] [Google Scholar]
- Saini S. et al. FliZ induces a kinetic switch in flagellar gene expression. Journal of bacteriology 192, 6477–6481, doi: 10.1128/JB.00751-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S., Ellermeier J. R., Slauch J. M. & Rao C. V. The role of coupled positive feedback in the expression of the SPI1 type three secretion system in Salmonella. PLoS pathogens 6, e1001025, doi: 10.1371/journal.ppat.1001025 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M. et al. Self-destructive cooperation mediated by phenotypic noise. Nature 454, 987–990, doi: 10.1038/nature07067 (2008). [DOI] [PubMed] [Google Scholar]
- Nielsen-LeRoux C., Gaudriault S., Ramarao N., Lereclus D. & Givaudan A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Current opinion in microbiology 15, 220–231, doi: 10.1016/j.mib.2012.04.006 (2012). [DOI] [PubMed] [Google Scholar]
- Waterfield N. R., Ciche T. & Clarke D. Photorhabdus and a host of hosts. Annual review of microbiology 63, 557–574, doi: 10.1146/annurev.micro.091208.073507 (2009). [DOI] [PubMed] [Google Scholar]
- Aymeric J. L., Givaudan A. & Duvic B. Imd pathway is involved in the interaction of Drosophila melanogaster with the entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus luminescens. Molecular immunology 47, 2342–2348, doi: 10.1016/j.molimm.2010.05.012 (2010). [DOI] [PubMed] [Google Scholar]
- Castillo J. C. et al. Drosophila anti-nematode and antibacterial immune regulators revealed by RNA-Seq. BMC genomics 16, 519, doi: 10.1186/s12864-015-1690-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanois A. et al. Transcriptional analysis of a Photorhabdus sp. variant reveals transcriptional control of phenotypic variation and multifactorial pathogenicity in insects. Applied and environmental microbiology 77, 1009–1020, doi: 10.1128/AEM.01696-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. P. et al. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cellular microbiology 4, 329–339 (2002). [DOI] [PubMed] [Google Scholar]
- Clarke D. J. The genetic basis of the symbiosis between Photorhabdus and its invertebrate hosts. Advances in applied microbiology 88, 1–29, doi: 10.1016/B978-0-12-800260-5.00001-2 (2014). [DOI] [PubMed] [Google Scholar]
- Akhurst R. J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J. Gen. Microbiol 121, 303–309 (1980). [Google Scholar]
- Turlin E. et al. Proteome analysis of the phenotypic variation process in Photorhabdus luminescens. Proteomics 6, 2705–2725, doi: 10.1002/pmic.200500646 (2006). [DOI] [PubMed] [Google Scholar]
- Gaudriault S. et al. Plastic architecture of bacterial genome revealed by comparative genomics of Photorhabdus variants. Genome biology 9, R117, doi: 10.1186/gb-2008-9-7-r117 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somvanshi V. S., Kaufmann-Daszczuk B., Kim K. S., Mallon S. & Ciche T. A. Photorhabdus phase variants express a novel fimbrial locus, mad, essential for symbiosis. Molecular microbiology 77, 1021–1038, doi: 10.1111/j.1365-2958.2010.07270.x (2010). [DOI] [PubMed] [Google Scholar]
- Somvanshi V. S. et al. A single promoter inversion switches Photorhabdus between pathogenic and mutualistic states. Science 337, 88–93, doi: 10.1126/science.1216641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V. I. & Weiss D. S. Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria. Antibiotics 4, 18–41, doi: 10.3390/antibiotics4010018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Reynolds C. M., Trent M. S. & Bishop R. E. Lipid A modification systems in gram-negative bacteria. Annual review of biochemistry 76, 295–329, doi: 10.1146/annurev.biochem.76.010307.145803 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J. S. et al. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Molecular microbiology 27, 1171–1182 (1998). [DOI] [PubMed] [Google Scholar]
- Bennett H. P. & Clarke D. J. The pbgPE operon in Photorhabdus luminescens is required for pathogenicity and symbiosis. Journal of bacteriology 187, 77–84, doi: 10.1128/JB.187.1.77-84.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaud E. et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nature biotechnology 21, 1307–1313, doi: 10.1038/nbt886 (2003). [DOI] [PubMed] [Google Scholar]
- Derzelle S. et al. The PhoP-PhoQ two-component regulatory system of Photorhabdus luminescens is essential for virulence in insects. Journal of bacteriology 186, 1270–1279 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouammine A. et al. Ail and PagC-related proteins in the entomopathogenic bacteria of Photorhabdus genus. PloS one 9, e110060, doi: 10.1371/journal.pone.0110060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Vescovi E., Soncini F. C. & Groisman E. A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84, 165–174 (1996). [DOI] [PubMed] [Google Scholar]
- Duvic B. et al. Cecropins as a marker of Spodoptera frugiperda immunosuppression during entomopathogenic bacterial challenge. Journal of insect physiology 58, 881–888, doi: 10.1016/j.jinsphys.2012.04.001 (2012). [DOI] [PubMed] [Google Scholar]
- Hayes C. S., Aoki S. K. & Low D. A. Bacterial contact-dependent delivery systems. Annual review of genetics 44, 71–90, doi: 10.1146/annurev.genet.42.110807.091449 (2010). [DOI] [PubMed] [Google Scholar]
- Brillard J., Duchaud E., Boemare N., Kunst F. & Givaudan A. The PhlA hemolysin from the entomopathogenic bacterium Photorhabdus luminescens belongs to the two-partner secretion family of hemolysins. Journal of bacteriology 184, 3871–3878 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaston J. M. et al. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: convergent lifestyles from divergent genomes. PloS one 6, e27909, doi: 10.1371/journal.pone.0027909 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen D. et al. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280, 2129–2132 (1998). [DOI] [PubMed] [Google Scholar]
- Cabral C. M., Cherqui A., Pereira A. & Simoes N. Purification and characterization of two distinct metalloproteases secreted by the entomopathogenic bacterium Photorhabdus sp. strain Az29. Applied and environmental microbiology 70, 3831–3838, doi: 10.1128/AEM.70.7.3831-3838.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang K., Park S., Yoo J. Y. & Cho S. Characterization and expression of attacin, an antibacterial protein-encoding gene, from the beet armyworm, Spodoptera exigua (Hubner) (Insecta: Lepidoptera: Noctuidae). Molecular biology reports 39, 5151–5159, doi: 10.1007/s11033-011-1311-3 (2012). [DOI] [PubMed] [Google Scholar]
- Girard P. A. et al. X-tox: an atypical defensin derived family of immune-related proteins specific to Lepidoptera. Developmental and comparative immunology 32, 575–584, doi: 10.1016/j.dci.2007.09.004 (2008). [DOI] [PubMed] [Google Scholar]
- Dubnau D. & Losick R. Bistability in bacteria. Molecular microbiology 61, 564–572, doi: 10.1111/j.1365-2958.2006.05249.x (2006). [DOI] [PubMed] [Google Scholar]
- Lejona S., Aguirre A., Cabeza M. L., Garcia Vescovi E. & Soncini F. C. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. Journal of bacteriology 185, 6287–6294 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Lee E. J., Huang H. & Groisman E. A. A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science 314, 1607–1609, doi: 10.1126/science.1134930 (2006). [DOI] [PubMed] [Google Scholar]
- Avraham R. et al. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell 162, 1309–1321, doi: 10.1016/j.cell.2015.08.027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V. I. et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nature microbiology 1, 16053, doi: 10.1038/nmicrobiol.2016.53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L. & Hancock R. E. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clinical microbiology reviews 25, 661–681, doi: 10.1128/CMR.00043-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Romero M. A. & Casadesus J. Contribution of phenotypic heterogeneity to adaptive antibiotic resistance. Proceedings of the National Academy of Sciences of the United States of America 111, 355–360, doi: 10.1073/pnas.1316084111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. J. & Dowds B. C. Virulence mechanisms of Photorhabdus sp. strain K122 toward wax moth larvae. Journal of invertebrate pathology 66, 149–155 (1995). [Google Scholar]
- Caldas C., Cherqui A., Pereira A. & Simoes N. Purification and characterization of an extracellular protease from Xenorhabdus nematophila involved in insect immunosuppression. Applied and environmental microbiology 68, 1297–1304 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening J. W., Smits W. K. & Kuipers O. P. Bistability, epigenetics, and bet-hedging in bacteria. Annual review of microbiology 62, 193–210, doi: 10.1146/annurev.micro.62.081307.163002 (2008). [DOI] [PubMed] [Google Scholar]
- Olofsson H., Ripa J. & Jonzen N. Bet-hedging as an evolutionary game: the trade-off between egg size and number. Proceedings. Biological sciences/The Royal Society 276, 2963–2969, doi: 10.1098/rspb.2009.0500 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey L. A. et al. Insect pathogens as biological control agents: Back to the future. Journal of invertebrate pathology 132, 1–41, doi: 10.1016/j.jip.2015.07.009 (2015). [DOI] [PubMed] [Google Scholar]
- Vilcinskas A. Evolutionary plasticity of insect immunity. Journal of insect physiology 59, 123–129, doi: 10.1016/j.jinsphys.2012.08.018 (2013). [DOI] [PubMed] [Google Scholar]
- Augustin D. K. et al. Presence or absence of lipopolysaccharide O antigens affects type III secretion by Pseudomonas aeruginosa. Journal of bacteriology 189, 2203–2209, doi: 10.1128/JB.01839-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B. T., Basler M. & Mekalanos J. J. Type 6 secretion system-mediated immunity to type 4 secretion system-mediated gene transfer. Science 342, 250–253, doi: 10.1126/science.1243745 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe Z. C., Low D. A. & Hayes C. S. Bacterial contact-dependent growth inhibition. Trends in microbiology 21, 230–237, doi: 10.1016/j.tim.2013.02.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel B., Givaudan A., Lanois A., Akhurst R. J. & Boemare N. Fast and accurate identification of Xenorhabdus and Photorhabdus species by restriction analysis of PCR-amplified 16S rRNA genes. Applied and environmental microbiology 63, 574–580 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetru C. & Bulet P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. Methods in molecular biology 78, 35–49, doi: 10.1385/0-89603-408-9:35 (1997). [DOI] [PubMed] [Google Scholar]
- Mouammine A. Caractérisation de l’hétérogénéité bactérienne chez la bactérie pathogène d’insectes Photorhabdus - Rôle dans la résistance aux peptides antimicrobiens. PhD thesis, Université Montpellier 2, (2014).
- Tang Y. T. et al. Inhibition of bacterial virulence: drug-like molecules targeting the Salmonella enterica PhoP response regulator. Chemical biology & drug design 79, 1007–1017, doi: 10.1111/j.1747-0285.2012.01362.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel L. J., Bieganowska K. D. & Hafler D. A. Cross-reactivity of T-cell clones specific for altered peptide ligands of myelin basic protein. Cellular immunology 193, 99–107, doi: 10.1006/cimm.1998.1447 (1999). [DOI] [PubMed] [Google Scholar]
- Vallenet D. et al. MicroScope–an integrated microbial resource for the curation and comparative analysis of genomic and metabolic data. Nucleic acids research 41, D636–647, doi: 10.1093/nar/gks1194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A. E., Mau B. & Perna N. T. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PloS one 5, e11147, doi: 10.1371/journal.pone.0011147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research 20, 1297–1303, doi: 10.1101/gr.107524.110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielbasa S. M., Wan R., Sato K., Horton P. & Frith M. C. Adaptive seeds tame genomic sequence comparison. Genome research 21, 487–493, doi: 10.1101/gr.113985.110 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760, doi: 10.1093/bioinformatics/btp324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079, doi: 10.1093/bioinformatics/btp352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. et al. Software for computing and annotating genomic ranges. PLoS computational biology 9, e1003118, doi: 10.1371/journal.pcbi.1003118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Genome biology 11, R106, doi: 10.1186/gb-2010-11-10-r106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser 57, 289–300 (1995). [Google Scholar]
- Pfaffl M. W., Horgan G. W. & Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic acids research 30, e36 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubelin G. et al. Studies of the dynamic expression of the Xenorhabdus FliAZ regulon reveal atypical iron-dependent regulation of the flagellin and haemolysin genes during insect infection. Environmental microbiology 13, 1271–1284, doi: 10.1111/j.1462-2920.2011.02427.x (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.