Abstract
Millions of tons of wastewater containing both inorganic and organic pollutants are generated every day, leading to significant social, environmental, and economic issues. Herein, we designed a graphene foam/TiO2 nanosheet hybrid, which is able to effectively remove both chromium (VI) cations and organic pollutants simultaneously. This graphene foam/TiO2 nanosheet hybrid was synthesized via a facile single-step one-pot hydrothermal method. The structure of the hybrid was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The hybrid was evaluated for both chromium (VI) and organic pollutants (using methyl blue (MB) as an example) removal, and the removal mechanism was also investigated. During water treatment, graphene and TiO2 nanosheets function complimentarily, leading to a significant synergy. The hybrid exhibited outstanding chromium (VI) and MB removal capacity, much superior to the performance of the individual pure TiO2 sheets or pure graphene foam. The hybrid could also be easily separated after water treatment, and exhibited excellent recycle stability. Considering the very facile synthesis of this graphene foam/TiO2 nanosheet hybrid, and its excellent water treatment performance and recycle stability, such a hybrid is promising for large scale production for practical applications where both chromium (VI) cations and organic dyes are the main pollutants.
Environmental crisis has attracted much attention these decades. Millions of tons of wastewater are generated every day and discharges to the rivers and oceans. Under most circumstances, it contains both inorganic and organic pollutants1. One major part of inorganic pollutants is heavy metal ions, which tend to accumulate in living bodies and known to be toxic2,3,4. Some heavy metals such as cadmium, nickel and chromium, are considered to be carcinogenic5,6. For chromium ions, chromium (VI) is highly toxic while the chromium (III) is one of the basic elements we need in our bodies7. The US Environmental Protection Agency (EPA) recommends that the level of chromium in water should be lower than 0.1 mg/L8. It is important to remove the chromium (VI) in the water to the limit required.
There are several ways for heavy metal removal, including chemical precipitation, ion exchange, membrane filtration, electrochemical treatment, and adsorption2,9,10,11,12,13. Among these methods, adsorption is one of the most economical and facile approaches to remove heavy metal ions in the water14,15,16. Some of the adsorbents can be regenerated and recycled due to the reversible nature of adsorption. Chromium (VI) removal by adsorption has been widely studied. Lo et al. used activated carbon as the adsorbent to remove chromium (VI) in water through van der waals interactions17. Alternatively, Hu and coworkers adopted maghemite nanoparticles as the adsorbent to remove chromium (VI) under acid condition based on the electrostatic interactions between the adsorbent and chromium (VI) ions18. Cao et al. synthesized nitrogen doped magnetic carbon nano-adsorbents that can effectively remove chromium (VI) via electrostatic attraction and redox reaction19. Furthermore, Guo et al. removed chromium (VI) from water through reduction using magnetic carbon nanocomposites derived from cellulose20. All adsorbents can adsorb chromium (VI) ions on their surface and eventually become saturated. In comparison, a much higher chromium (VI) removal efficiency can be achieved if the adsorbent can reduce the chromium (VI) to chromium (III). In this way, the adsorbent would never be saturated with chromium (VI) and can remove chromium (VI) continuously. Titanium dioxide, one of the most widely used photo-catalysts, has the ability to reduce chromium (VI) to chromium (III) under UV radiation21,22. Moreover, TiO2 is desirable because of its high efficiency, low cost, nontoxicity, and high stability23,24,25,26. Nanoscale TiO2 with a high specific surface area would be a particularly ideal choice for chromium (VI) removal.
Waste water also contains varies of organic pollutants, including pesticides, herbicides, phenols, and dyes. Those pollutants could be removed by oxidation, ion exchange, electro-dialysis, electrolysis, adsorption, etc. ref. 27. Adsorption is considered one of the best methods to remove organic pollutants, because of its universality, as well as low cost and ease of operation. Carbon based materials (such as activated carbon, carbon nanotubes, etc.) have been used broadly as adsorbents owing to their high specific surface area28,29. Graphene is a single atomic layer of graphite, which is composed of sp2-hybridized carbon atoms. Graphene has gained significant attention due to its incredibly high specific surface area (theoretical value: 2620 m2g−1), high thermal conductivity, and outstanding electrical conductivity30,31. Most of the pollutants are aromatic, which contain benzene rings and can be easily adsorbed on graphene surface due to π-π stacking effect32. Zhao et al. synthesized sulfonated graphene that adsorbed aromatic pollutants efficiently33. TiO2 can help degrade many kinds of organics under UV light34,35,36. Degradation reaction would happen after organic pollutants are adsorbed on graphene if TiO2 is attached on graphene.
Since wastewater contains both inorganic and organic pollutants, it is ideal if they can be removed through a single process. To achieve this goal, herein we designed a nanostructured hybrid, graphene foam attached with TiO2 nanosheets (graphene foam/TiO2 nanosheet hybrid, abbreviated as G/TiO2 hybrid) via a single-step one-pot hydrothermal method. For chromium (VI) removal, TiO2 nanosheets can reduce hexavalent chromium to trivalent chromium. During this process, graphene can promote the electron mobility and render the light excited electron-hole pairs on TiO2 nanosheets to separate more efficiently37. As a result, photocatalytic efficiency would be increased and therefore chromium (VI) removal rate would be enhanced. Moreover, G/TiO2 hybrid is able to effectively remove organic pollutants by using TiO2 to degrade the ones adsorbed on graphene foam with the assistance of UV radiation. In addition to serving as a platform to help collect organic pollutants, graphene foams can improve electron transfer rate and thus expedite the degradation process. As such, graphene foam and TiO2 nanosheets are complimentary to each other during both organic and inorganic pollutants removal. Furthermore, by designing a foam based structure, the hybrid can maintain a high removal efficiency owing to the high transport nature of the porous foam structure, as well as be easily separated from wastewater after treatment, leading to a facile and effective recycling process.
Experimental
Materials
Graphite (Grafguard 160–50N) was obtained from GrafTech. Sulfuric acid (95.0–98.0%), phosphorus pentoxide (>98%), potassium persulfate (>99.0%), potassium permanganate (>99.0%), titanium n-butoxide (>99%), hydrofluoric acid (48–51%), and potassium dichromate (>99.0%) were purchased from Alfa Aesar and used as received without further purification.
Synthesis of graphene foam
A sample of 100 mg GO (made by the modified Hummers’ method38,39) was charged into a beaker with 20 mL DI water. After ultrasonication (Branson 8510R-MT, 250 W, 44 kHz) for 3 hours, a stable GO suspension was obtained. Then the GO suspension was transferred into a 50 mL Teflon® lined autoclave and heated at 180 °C for 24 hours. After hydrothermal treatment, the suspension was filtrated and washed with DI water for 3 times, then dried under vacuum at room temperature for 24 hours40,41.
Synthesis of TiO2 nanosheets
A sample of 1.6 mL titanium n-butoxide was added into a Teflon® beaker together with 0.2 mL HF acid (48–51%). After stirring for 3 minutes, the solution was transferred into a Teflon® lined autoclave and hydrothermally treated for 24 hours at 180 °C. After the hydrothermal treatment, the dispersion was filtrated and washed with DI water for 3 times, then dried under vacuum at room temperature for 24 hours42.
Synthesis of G/TiO2 hybrid
A sample of 100 mg GO (made by the modified Hummers’ method38,39) was charged into a Teflon® beaker with 20 mL DI water. After ultrasonication (Branson 8510R-MT, 250 W, 44 kHz) for 3 hours, 0.8 mL titanium n-butoxide and 0.1 mL HF acid (48–51%) were added into the GO dispersion during stirring. After stirring for 3 minutes, the dispersion was transferred into a Teflon® lined autoclave. Then, the hydrothermal treatment was conducted for 24 hours at 180 °C. After the hydrothermal process, the suspension was filtrated and washed with DI water for 3 times, then dried under vacuum at room temperature for 24 hours.
Characterization
The morphology and structure of the samples were characterized by scanning electron microscopy (SEM, JEOL JSM-6335F FESMs with an accelerating voltage of 10 kV) and transmission electron microscopy (TEM, FEI Tecnai T12 with an accelerating voltage of 120 kV).
Evaluation of chromium (VI) removal ability
In this work, we choose fluorescent light as the light source for sample treatment out of the consideration of future practical applications. The ultraviolet radiation in fluorescent light is able to initiate the photocatalytic reaction for the removal of pollutants. A sample of 30 mg G/TiO2 hybrid was added into 25.0 mL (400 μg/L) potassium dichromate solution. After 4 hours of vigorous shaking under fluorescent light (SYLVANIA T5 fluorescent lamp, 28 W, 2 meters distance between the sample and lamp), 10.0 mL solution was collected and mixed with 0.2 mL phosphoric acid (85 wt%) and 0.5 mL coloring agent (1,5-diphenylcarbazide, DPC, 2.0 g/L)43. The obtained solution was analyzed by a UV-Vis spectrophotometer (Varian Cary 5000 UV-Vis NIR) by recording the absorbance. The absorbance of the characteristic peak at 540 nm is proportional to the concentration of chromium (VI) ion44 according to the Lambert-Beer’s Law. After the first cycle of evaluation, the G/TiO2 hybrid was collected by 2 minutes of gravity settling and subsequently washed by DI water for 3 times and dried in an oven. The dried G/TiO2 hybrid was used for the next cycle of evaluation through the same procedures.
Evaluation of MB removal ability
A sample of 30 mg G/TiO2 hybrid was mixed with 25.0 mL (10 mg/L) methyl blue (MB) solution. After 4 hours of vigorous shaking under fluorescent light, 5.0 mL solution was collected and subsequently diluted by 10.0 mL DI water. The obtained solution was analyzed by a UV-Vis spectrometer (Varian Cary 5000 UV-Vis NIR) by recording the absorbance. The absorbance of characteristic peak at 664 nm is proportional to the concentration of MB45 according to the Lambert-Beer’s Law. After the first cycle of evaluation, the G/TiO2 hybrid was collected by gravity settling and subsequently washed by DI water for 3 times and dried in an oven. The dried G/TiO2 hybrid was used for the next cycle of evaluation through the same procedures.
Results and Discussion
Structure and morphology of G/TiO2 hybrid
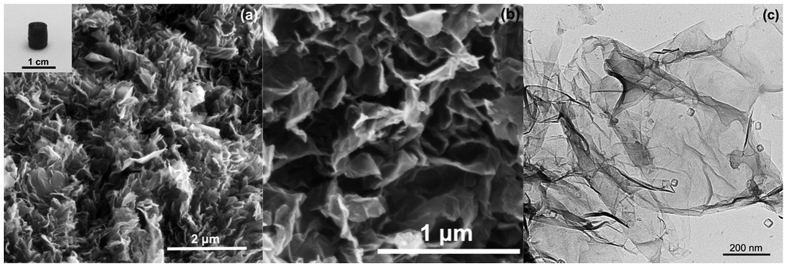
Graphene can be synthesized as a form of foam via hydrothermal treatment of graphene oxide suspension40,41. G/TiO2 hybrid was synthesized via a similar approach by incorporating TiO2 nanosheets. The morphology and structure of the G/TiO2 hybrid was characterized by SEM and TEM. As presented in Fig. 1a and b, the G/TiO2 hybrid shows a rough surface and a porous structure. After the hydrothermal reaction, the graphene oxide and TiO2 nanosheets were condensed to a small cylinder-shaped foam, as shown in the inset of Fig. 1a. Under TEM, square-shaped TiO2 nanosheets were uniformly dispersed on the surface of large graphene sheets, as shown in Fig. 1b. Graphene sheets also exhibited folds and wrinkles. The exact amount of graphene and TiO2 in the synthesized hybrid was determined by thermogravimetric analysis to be 23.6 and 6.4 mg, respectively.
Figure 1.
SEM (a and b) and TEM (c) images of G/TiO2 hybrid. The inset in (a) shows a digital image of the prepared G/TiO2 hybrid.
Hexavalent chromium removal ability of G/TiO2 hybrid
Figure 2 shows the chromium (VI) removal evaluation results by the G/TiO2 hybrid. Pure graphene foam and pure TiO2 nanosheets were also synthesized and evaluated as controls. The results show that G/TiO2 hybrid can remove chromium (VI) ions much more effectively compared to the two control samples. Pure graphene foam has virtually no adsorption ability, which is expected. This also indicates that TiO2 is the key component in the G/TiO2 hybrid for chromium (VI) removal. Meanwhile, the results clearly show that the performance of the pure TiO2 nanosheets is much inferior to that of the graphene/TiO2 foam that contains the same amount of TiO2 nanosheets, which is exactly as designed. This series of results show that the G/TiO2 hybrid exhibited the designed synergy between the graphene foam and TiO2 nanosheets. Graphene is a single layer of graphite composed of sp2-hybridized carbon atoms, which possesses superior electron conductivity31. Since the entire removal process is basically a photocatalytic reduction of chromium (VI) ions, the existence of graphene can expedite the separation rate of electron-hole pairs by rapidly conducting the light-excited electrons37. In this way, the photocatalytic efficiency is improved significantly, thus G/TiO2 hybrid demonstrates a much higher chromium (VI) removal rate than the pure TiO2 sheets.
Figure 2. UV-Vis spectra of 400 μg/L chromium (VI) solution after being treated by different samples.
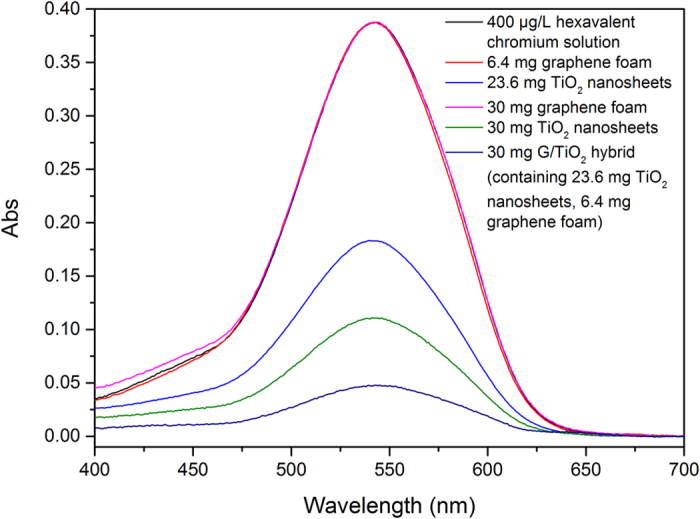
To further validate the efficiency of the G/TiO2 hybrid for chromium (VI) removal, it was also evaluated with the same mass amount of TiO2 nanosheets. As shown in Fig. 2, the chromium (VI) removal ability of 30 mg of G/TiO2 hybrid (containing 23.6 g TiO2 nanosheets) was even higher than that of 30 mg of pure TiO2 nanosheets. This result further suggests that the existence of graphene can effectively speed up the reduction of chromium (VI) ions, thus increasing the practical capacity of TiO2 nanosheets for chromium (VI) removal.
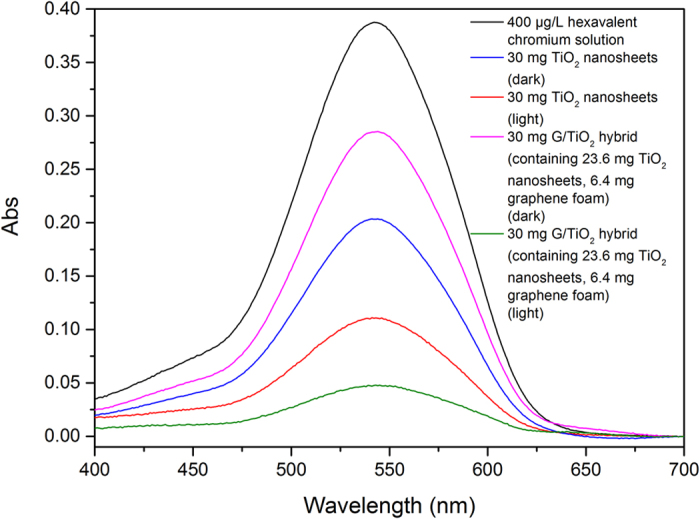
As discussed above, TiO2 nanosheets can barely adsorb chromium (VI) ions physically; the main mechanism for chromium (VI) removal by TiO2 nanosheets is photo-catalyzed reduction of hexavalent chromium to trivalent chromium under UV radiation21,22. As such, we further evaluated the chromium (VI) removal efficiency of the TiO2 sheets under both fluorescent light and dark. The TiO2 nanosheets only removed a small amount of chromium (VI) ions under the dark condition, while it exhibited a much higher adsorption under the fluorescent light, as shown in Fig. 3. This result is expected and is consistent with our initial hypothesis. Figure 3 also shows the chromium (VI) removal capacity of the G/TiO2 hybrid under fluorescent light and dark. The 30 mg G/TiO2 hybrid removed less chromium (VI) ions under the dark compared to the 30 mg of pure TiO2 nanosheets under the same conditions, which is probably because of the fact that there is only ca. 23.6 mg of TiO2 nanosheets in the hybrid, and such TiO2 nanosheets are less exposed to light, and meanwhile graphene cannot adsorb chromium (VI) ions. After applying room light, the removal capacity of the G/TiO2 hybrid (containing ca. 23.6 mg of TiO2 nanosheets) increased dramatically, even higher than that of the 30 mg of pure TiO2 nanosheets. This result further shows the promoting effect of graphene on TiO2 nanosheets for chromium (VI) ions removal, in which graphene can increase the electron mobility37. As such, graphene can improve the photocatalytic efficiency of TiO2 nanosheets and therefore chromium (VI) removal rate can be significantly enhanced.
Figure 3. UV-Vis spectra of 400 μg/L chromium (VI) solution after treatment with TiO2 sheets and G/TiO2 hybrid in fluorescent light and in dark.
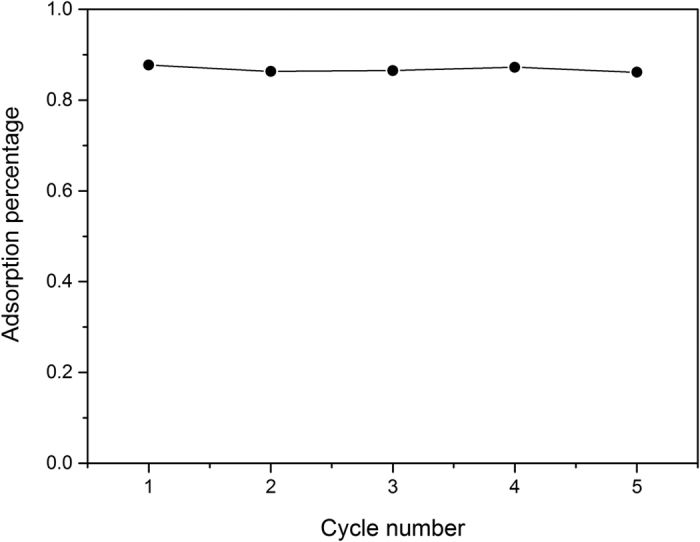
The recycle evaluation result in Fig. 4 shows that the G/TiO2 hybrid has an excellent recycle stability for chromium (VI) removal. The removal capacity in terms of percentage of chromium (VI) ions removed changed little after 5 cycles of operation. Since the adsorption mainly due to the photocatalytic degradation of chromium (VI) and limited physical adsorption of chromium (VI) ions on the G/TiO2 hybrid, it is able to maintain excellent recycle stability.
Figure 4. Recycle test of chromium (VI) removal using the G/TiO2 hybrid.
Methyl blue removal ability of G/TiO2 hybrid
Methyl blue (MB) was selected as a representative organic component to evaluate the organic pollutant removal capacity of the G/TiO2 hybrid. The MB removal capability of 30 mg of G/TiO2 hybrid (containing ca. 6.4 mg of graphene foam and 23.6 mg of TiO2 nanosheets) was proved to be much higher compared to 6.4 mg of pure graphene foam only or 23.6 mg of pure TiO2 nanosheets only, as shown in Fig. 5. Moreover, 30 mg of G/TiO2 hybrid removed more MB than 30 mg of pure graphene foam only or 30 mg of pure TiO2 sheets only, clearly showing the synergy between the two components.
Figure 5. UV-Vis spectra of 10 mg/L MB solution after treated by different samples.
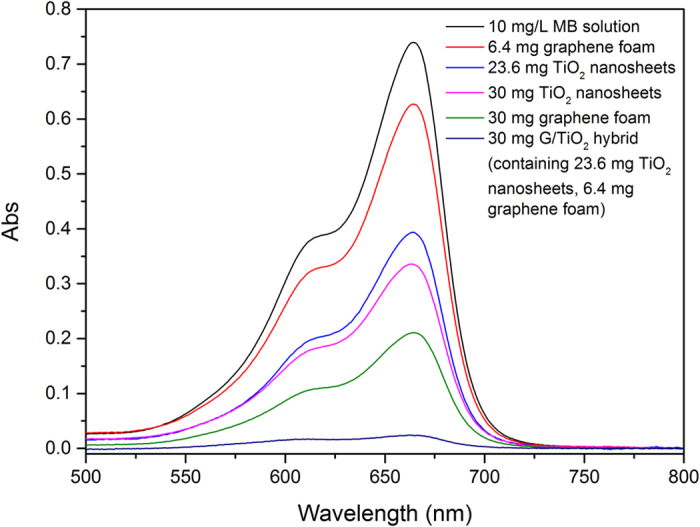
Graphene foam is composed of graphene sheets. Since graphene sheets can adsorb organics, especially the ones with benzene rings due to π-π stacking effect, MB could be physically adsorbed by graphene foams. TiO2 nanosheets can remove MB by photocatalytic degradation. This photocatalytic reaction requires photons to excite the electrons and further react with MB. The rate of the photocatalytic reaction could not meet the rate of physical adsorption of graphene. This explains why the pure graphene foam can remove more MB from solution compared to the pure TiO2 nanosheets (as shown in Fig. 5). The G/TiO2 hybrid contains TiO2 nanosheets attached on the surface of graphene. Under such a structure, graphene foam can adsorb MB to the surface and facilitate TiO2 nanosheets to be in contact with MB. As a result, photocatalytic reaction occurred and the adsorbed MB was degraded, which opens space for additional MB molecules to be adsorbed by graphene foam and subsequently degraded by TiO2 sheets. In addition, graphene can also help speed up the electron transfer rate in the photo-catalysis process37. Such coherent synergy between the physical adsorption by graphene sheets and the photo-catalyzed reduction by TiO2 nanosheets lead to very effective MB removal by the G/TiO2 hybrid.
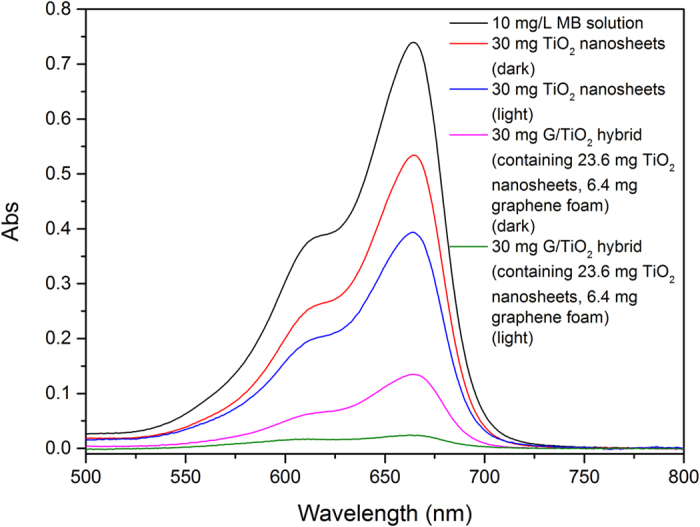
To further investigate the photo-catalyzed degradation, the MB removal reaction was conducted under both fluorescent light and dark. The results showed that TiO2 nanosheets can remove a small amount of MB even at dark (Fig. 6), which suggests that TiO2 nanosheets can remove MB by physical adsorption as well. However, the overall MB removal capacity is relatively low. The result of MB removal capacity of the G/TiO2 hybrid under room light and dark shows that a large percentage of MB molecules were removed by physical adsorption of both TiO2 nanosheets and graphene foam while not applying the light. This is mainly contributed by the high physical adsorption capacity of graphene foam. However, nearly all MB molecules were removed when applying light. This result again confirms that the MB removal by G/TiO2 hybrid is a combination of physical adsorption and photocatalytic degradation. TiO2 can degrade MB while graphene can physically adsorb MB to its surface until saturated. For graphene/TiO2 foam, the MB adsorbed on graphene foam can be degraded by TiO2 nanosheets. Thus, graphene surface would not be saturated and thus can adsorb MB continuously. Meanwhile, TiO2 nanosheets can degrade MB more rapidly with the help of graphene, since graphene can help improve electron transfer rate. As a result, there is an ideal synergistic effect between TiO2 and graphene, leading to highly effective removal of MB.
Figure 6. UV-Vis spectra of 10 mg/L MB solution after treated with pure TiO2 sheets and G/TiO2 hybrid in fluorescent light and in dark.
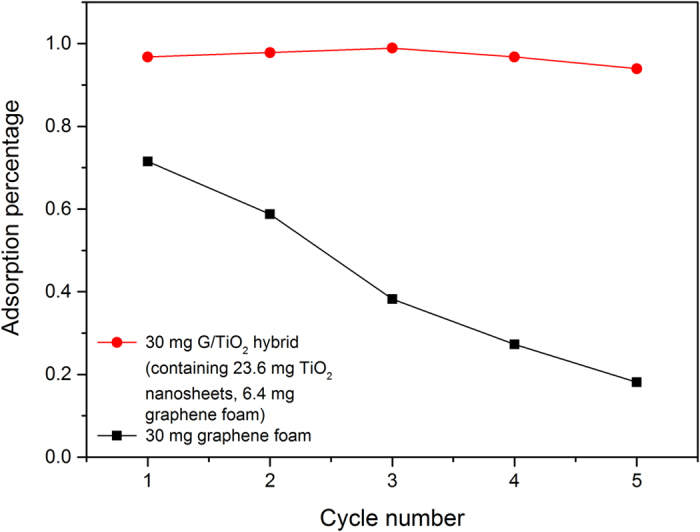
Recycling is essential for adsorbents. It would be ideal if adsorbents can be used for many times while the efficiency remains high. The G/TiO2 hybrid was designed to be used for multiple cycles because of its removal mechanism. Its overall foam structure also makes it easy to be separated from the water being treated.
The recycle testing result (Fig. 7) shows that the G/TiO2 hybrid has a very good recycle stability, while graphene foam lost part of adsorption ability after each cycle. This result is expected and can be explained by the different adsorption mechanisms of these two foams. Pure graphene foam can only physically adsorb MB to its surface, thus eventually will be saturated during the treatment. As a result, it will lose some MB removal ability after each cycle. For the G/TiO2 hybrid, it is never the case. TiO2 nanosheets can degrade the MB molecules adsorbed by graphene foam. In this way, graphene surface would always have open room for MB molecules, thus the adsorption process goes on and on.
Figure 7. Recycle test of MB removal using G/TiO2 hybrid and graphene foam only.
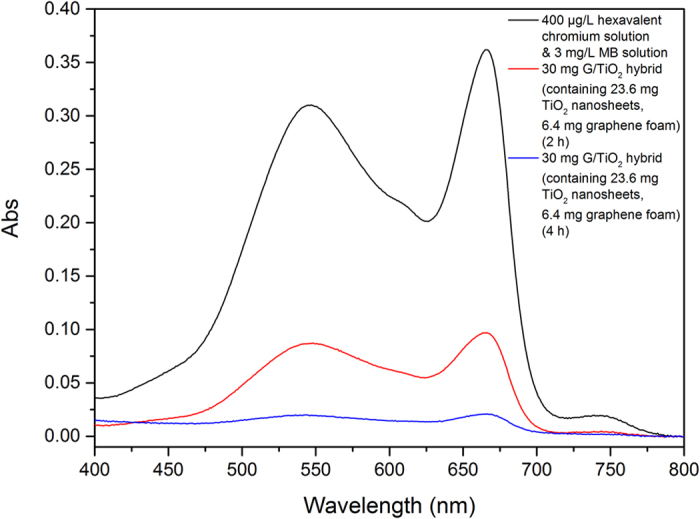
Hexavalent chromium ions & methyl blue mixture removal ability of G/TiO2 hybrid
As most waster water contains both inorganic and organic pollutants, the synthsized G/TiO2 hybrid was also evaluated for simultaneous removal of chromium (VI) ions and MB. Figure 8 displays the mixed adsorption of both pollutants by the G/TiO2 hybrid. The results show that the G/TiO2 hybrid can effectively remove both chromium (VI) ions and MB simultaneously and effectively. This results suggests that the G/TiO2 hybrid are promising for practical applications where both chromium (VI) ions and organic pollutants present.
Figure 8. UV-Vis spectra of a mixture of hexavalent chromium and MB after 2 and 4 hours of treatments by the G/TiO2 hybrid.
Conclusions
In this work, a G/TiO2 hybrid was designed and synthesized through a single-step one-pot hydrothermal method. The hybrid exhibited excellent chromium (VI) and methyl blue (MB) removal ability compared to pure TiO2 sheets or pure graphene foam. During water treatment, graphene foam and TiO2 nanosheets function complimentarily, leading to a significant synergy. The hybrid could also be easily separated after water treatment, and exhibited excellent recycle stability. Considering the very facile synthesis of this G/TiO2 hybrid, and its excellent water treatment performance and recycle stability, such a hybrid is promising for large scale production for practical applications where both chromium (VI) cations and organic dyes are the main pollutants.
Additional Information
How to cite this article: Wang, W. et al. Single-step One-pot Synthesis of Graphene Foam/TiO2 Nanosheet Hybrids for Effective Water Treatment. Sci. Rep. 7, 43755; doi: 10.1038/srep43755 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research is sponsored by the Air Force Office of Scientific Research (FA9550-12-1-0159) and an Open Fund of the Key Laboratory for Ultrafine Materials of the Ministry of Education (East China University of Science and Technology). W.W. acknowledges the China Scholarship Council for offering her a scholarship to conduct research at University of Connecticut.
Footnotes
The authors declare no competing financial interests.
Author Contributions W.W., Z.Z., and L.S. conceived the idea and designed the research. Z.W. participated in the project design. W.W. conducted the experiments. J.L. conducted microscopy imaging of the samples. W.W. and L.S. wrote the first draft of the manuscript, and all authors contributed to revise the manuscript.
References
- Sayari A., Hamoudi S. & Yang Y. Applications of Pore-Expanded Mesoporous Silica. 1. Removal of Heavy Metal Cations and Organic Pollutants from Wastewater. Chemistry of Materials. 17, 212–216 (2005). [Google Scholar]
- Fu F. & Wang Q. Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management. 92, 407–418 (2011). [DOI] [PubMed] [Google Scholar]
- Duruibe J., Ogwuegbu M. & Egwurugwu J. Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences. 2, 112–118 (2007). [Google Scholar]
- Chowdhury B. A. & Chandra R. K. Biological and health implications of toxic heavy metal and essential trace element interactions. Progress in food & nutrition science. 11, 55–113 (1986). [PubMed] [Google Scholar]
- Stohs S. J. & Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine. 18, 321–336 (1995). [DOI] [PubMed] [Google Scholar]
- Waalkes M. P. Cadmium carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 533, 107–120 (2003). [DOI] [PubMed] [Google Scholar]
- Olad A. & Farshi Azhar F. A study on the adsorption of chromium (VI) from aqueous solutions on the alginate-montmorillonite/polyaniline nanocomposite. Desalination and Water Treatment. 52, 2548–2559 (2014). [Google Scholar]
- Environmental Pollution Control Alternatives. (Environmental Protection Agency, Cincinnati, 1990).
- Matlock M. M., Howerton B. S. & Atwood D. A. Chemical precipitation of heavy metals from acid mine drainage. Water Research. 36, 4757–4764 (2002). [DOI] [PubMed] [Google Scholar]
- Dąbrowski A., Hubicki Z., Podkościelny P. & Robens E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere. 56, 91–106 (2004). [DOI] [PubMed] [Google Scholar]
- Juang R.-S. & Shiau R.-C. Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. Journal of Membrane Science. 165, 159–167 (2000). [Google Scholar]
- Hunsom M., Pruksathorn K., Damronglerd S., Vergnes H. & Duverneuil P. Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction. Water Research. 39, 610–616 (2005). [DOI] [PubMed] [Google Scholar]
- Demirbas A. Heavy metal adsorption onto agro-based waste materials: A review. Journal of Hazardous Materials. 157, 220–229 (2008). [DOI] [PubMed] [Google Scholar]
- O’Connell D. W., Birkinshaw C. & O’Dwyer T. F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresource Technology. 99, 6709–6724 (2008). [DOI] [PubMed] [Google Scholar]
- Bailey S. E., Olin T. J., Bricka R. M. & Adrian D. D. A review of potentially low-cost sorbents for heavy metals. Water Research. 33, 2469–2479 (1999). [Google Scholar]
- Wan Ngah W. S. & Hanafiah M. A. K. M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresource Technology. 99, 3935–3948 (2008). [DOI] [PubMed] [Google Scholar]
- Hu Z., Lei L., Li Y. & Ni Y. Chromium adsorption on high-performance activated carbons from aqueous solution. Separation and Purification Technology. 31, 13–18 (2003). [Google Scholar]
- Hu J., Chen G. & Lo I. M. C. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Research. 39, 4528–4536 (2005). [DOI] [PubMed] [Google Scholar]
- Cao Y. et al. One-pot melamine derived nitrogen doped magnetic carbon nanoadsorbents with enhanced chromium removal. Carbon. 109, 640–649 (2016). [Google Scholar]
- Qiu B. et al. Cr (VI) removal by magnetic carbon nanocomposites derived from cellulose at different carbonization temperatures. Journal of Materials Chemistry A. 3, 9817–9825 (2015). [Google Scholar]
- Tuprakay S. & Liengcharernsit W. Lifetime and regeneration of immobilized titania for photocatalytic removal of aqueous hexavalent chromium. Journal of Hazardous Materials. 124, 53–58 (2005). [DOI] [PubMed] [Google Scholar]
- Kajitvichyanukul P., Ananpattarachai J. & Pongpom S. Sol–gel preparation and properties study of TiO2 thin film for photocatalytic reduction of chromium(VI) in photocatalysis process. Science and Technology of Advanced Materials. 6, 352–358 (2005). [Google Scholar]
- Lang X., Ma W., Chen C., Ji H. & Zhao J. Selective Aerobic Oxidation Mediated by TiO2 Photocatalysis. Accounts of Chemical Research. 47, 355–363 (2014). [DOI] [PubMed] [Google Scholar]
- Lu S.-y. et al. Photocatalytic decomposition on nano-TiO2: Destruction of chloroaromatic compounds. Chemosphere. 82, 1215–1224 (2011). [DOI] [PubMed] [Google Scholar]
- Li W. et al. Highly Thermal Stable and Highly Crystalline Anatase TiO2 for Photocatalysis. Environmental Science & Technology. 43, 5423–5428 (2009). [DOI] [PubMed] [Google Scholar]
- Kazuhito H., Hiroshi I. & Akira F. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Japanese Journal of Applied Physics. 44, 8269 (2005). [Google Scholar]
- Ali I., Asim M. & Khan T. A. Low cost adsorbents for the removal of organic pollutants from wastewater. Journal of Environmental Management. 113, 170–183 (2012). [DOI] [PubMed] [Google Scholar]
- Li L., Quinlivan P. A. & Knappe D. R. U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon. 40, 2085–2100 (2002). [Google Scholar]
- Pan B. & Xing B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environmental Science & Technology. 42, 9005–9013 (2008). [DOI] [PubMed] [Google Scholar]
- Stankovich S. et al. Graphene-based composite materials. Nature. 442, 282–286 (2006). [DOI] [PubMed] [Google Scholar]
- Zhu Y. et al. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Advanced Materials. 22, 3906–3924 (2010). [DOI] [PubMed] [Google Scholar]
- Tan L.-L., Chai S.-P. & Mohamed A. R. Synthesis and Applications of Graphene-Based TiO2 Photocatalysts. ChemSusChem. 5, 1868–1882 (2012). [DOI] [PubMed] [Google Scholar]
- Zhao G. et al. Sulfonated Graphene for Persistent Aromatic Pollutant Management. Advanced Materials. 23, 3959–3963 (2011). [DOI] [PubMed] [Google Scholar]
- Fan Y. et al. Ce-/S-codoped TiO2/Sulfonated graphene for photocatalytic degradation of organic dyes. Journal of Materials Chemistry A. 2, 13565–13570 (2014). [Google Scholar]
- Kumar S. G. & Devi L. G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. The Journal of Physical Chemistry A. 115, 13211–13241 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis. Applied Catalysis B: Environmental. 201, 470–478 (2017). [Google Scholar]
- Liu B. et al. Highly dispersive {001} facets-exposed nanocrystalline TiO2 on high quality graphene as a high performance photocatalyst. Journal of Materials Chemistry. 22, 7484–7491 (2012). [Google Scholar]
- Hummers W. S. & Offeman R. E. Preparation of Graphitic Oxide. Journal of the American Chemical Society. 80, 1339–1339 (1958). [Google Scholar]
- Xu Y., Bai H., Lu G., Li C. & Shi G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. Journal of the American Chemical Society. 130, 5856–5857 (2008). [DOI] [PubMed] [Google Scholar]
- Xu Y., Sheng K., Li C. & Shi G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano. 4, 4324–4330 (2010). [DOI] [PubMed] [Google Scholar]
- Wei W. et al. 3D Graphene Foams Cross-linked with Pre-encapsulated Fe3O4 Nanospheres for Enhanced Lithium Storage. Advanced Materials. 25, 2909–2914 (2013). [DOI] [PubMed] [Google Scholar]
- Jiang B. et al. Enhanced Photocatalytic Activity and Electron Transfer Mechanisms of Graphene/TiO2 with Exposed {001} Facets. The Journal of Physical Chemistry C. 115, 23718–23725 (2011). [Google Scholar]
- Apha A. WPCF, Standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA 1995). [Google Scholar]
- Ghosh P. K. Hexavalent chromium [Cr(VI)] removal by acid modified waste activated carbons. Journal of Hazardous Materials. 171, 116–122 (2009). [DOI] [PubMed] [Google Scholar]
- Almeida C. A. P., Debacher N. A., Downs A. J., Cottet L. & Mello C. A. D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. Journal of Colloid and Interface Science. 332, 46–53 (2009). [DOI] [PubMed] [Google Scholar]