Abstract
Silver nanoparticles (AgNP) are known to penetrate into the brain and cause neuronal death. However, there is a paucity in studies examining the effect of AgNP on the resident immune cells of the brain, microglia. Given microglia are implicated in neurodegenerative disorders such as Parkinson’s disease (PD), it is important to examine how AgNPs affect microglial inflammation to fully assess AgNP neurotoxicity. In addition, understanding AgNP processing by microglia will allow better prediction of their long term bioreactivity. In the present study, the in vitro uptake and intracellular transformation of citrate-capped AgNPs by microglia, as well as their effects on microglial inflammation and related neurotoxicity were examined. Analytical microscopy demonstrated internalization and dissolution of AgNPs within microglia and formation of non-reactive silver sulphide (Ag2S) on the surface of AgNPs. Furthermore, AgNP-treatment up-regulated microglial expression of the hydrogen sulphide (H2S)-synthesizing enzyme cystathionine-γ-lyase (CSE). In addition, AgNPs showed significant anti-inflammatory effects, reducing lipopolysaccharide (LPS)-stimulated ROS, nitric oxide and TNFα production, which translated into reduced microglial toxicity towards dopaminergic neurons. Hence, the present results indicate that intracellular Ag2S formation, resulting from CSE-mediated H2S production in microglia, sequesters Ag+ ions released from AgNPs, significantly limiting their toxicity, concomitantly reducing microglial inflammation and related neurotoxicity.
The widespread use of silver nanoparticles (AgNPs) in various consumer products, ranging from food packaging to antibacterial sprays and water purifiers1,2, has raised concerns regarding their potential adverse effects on human health. Indeed, AgNPs are able to induce cytotoxicity in human lung, skin and fibroblast cells3,4,5. In relation to the central nervous system (CNS), AgNPs have been shown to be able to cross the blood-brain barrier6,7 and accumulate in the brain following ingestion8 and inhalation9,10. Furthermore, a growing body of evidence indicates AgNPs are able to directly induce cytotoxicity in neurons in vitro11,12,13,14,15, and cause neurodegeneration in vivo following oral16,17, gastric18 or nasal administration19. However, the precise mechanisms of neurodegeneration are not fully understood. Therefore, the effects of AgNPs on other CNS cell types and their contribution to AgNP-induced neurodegeneration need to be more thoroughly examined.
Microglia are the brain’s resident immune cells, responsible for mounting protective inflammatory reactions to destroy invading pathogens20. However, excessive microglial inflammation is capable of inducing collateral neuronal damage through over-production of pro-inflammatory factors such as the pro-apoptotic protein tumour necrosis factor (TNF)-α, reactive oxygen species (ROS) and nitric oxide (NO), and is implicated in the chronic neuronal death seen in neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease21,22,23. Furthermore, nanomaterials have been shown to be able to induce brain inflammation and changes related to neurodegenerative diseases24,25,26,27. Therefore, it is important to examine the effect of AgNPs on microglial cell viability and inflammation to fully understand AgNP-induced neurodegeneration and whether AgNP-exposed microglia exacerbate this process.
In addition to mounting inflammatory reactions for pathogen destruction, microglia are responsible for phagocytosing foreign material20. Previous work within our group has shown microglia have the capacity to internalize and degrade nanosized materials28. Therefore, they are expected to be the principal cell type responsible for processing brain-penetrating AgNPs. Hence, understanding how microglia take up AgNPs and the mechanisms employed to process them will allow for the bioreactivity and biopersistence of AgNPs to be better predicted.
AgNP toxicity mainly arises from released Ag+ ions interacting with and damaging cell membranes, thiol protein groups and DNA29,30,31,32. Previous studies indicate silver nanowire toxicity may be limited by sequestration of released Ag+ ions through sulphiding reactions33. However, it is unknown whether Ag+ ion sulphidation is elicited by silver nanoparticles in microglial cells, and, if so, which mechanism is employed. With these questions in mind, this study employs the murine microglial N9 cell line and thoroughly characterized citrate-capped AgNPs to test the hypothesis that Ag+ ions released from AgNPs following endocytosis by microglial cells induce expression of H2S-synthesizing enzymes, leading to reprecipitation of silver ions as insoluble Ag2S, reducing the toxicity of the AgNPs. As H2S is a potent anti-inflammatory agent34, the effect of AgNPs on microglial inflammation is also examined by quantification of the pro-inflammatory factors ROS, NO and TNF-α. Furthermore, the dopaminergic neuronal cell line N27 is employed to examine whether modulation of microglial inflammation by AgNPs affects microglia-mediated neurotoxicity.
Results
Characterisation of AgNPs
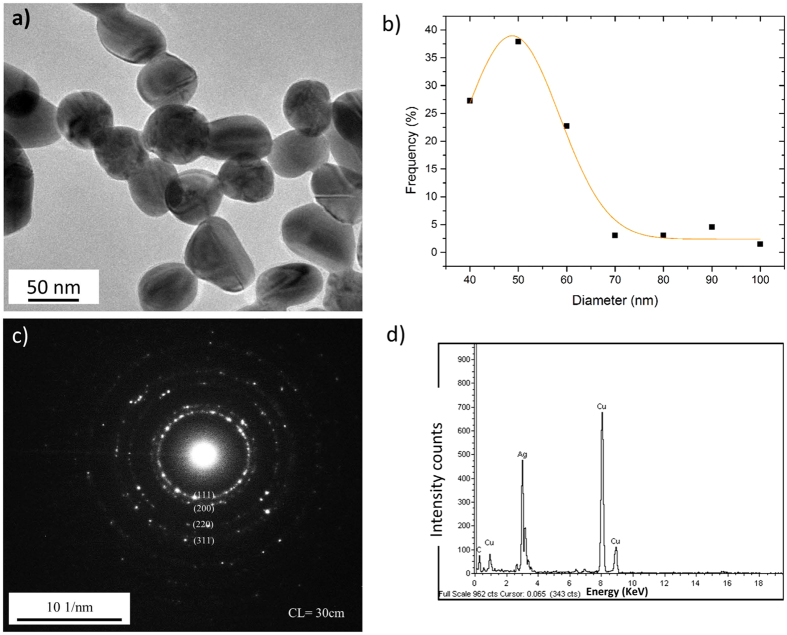
AgNPs were synthesised in-house and characterised using transmission electron microscopy (TEM), scanning transmission electron microscopy with energy-dispersive X ray spectroscopy (STEM-EDX), selected area electron diffraction (SAED), zeta (ζ)-potential and dynamic light scattering (DLS) analyses. Transmission electron microscopy showed AgNPs possessed uniform, spheroid morphologies, with a mean diameter of 49.7 ± 10.5 nm (Fig. 1a,b) (Table 1). SAED patterns (Fig. 1c) showed characteristic lattice spacings of 0.236 nm, 0.204 nm, and 0.145 nm, corresponding to the (111), (200) and (220) planes of metallic silver (see Table S2). EDX spectra acquired from the AgNPs (Fig. 1d) only identified silver from the sample, confirming both the successful removal of impurities following washing with DI water and that no adventitious sulphide was formed prior to cell exposure35. DLS measurements showed AgNPs aggregated into larger-sized particles after 1 hr incubation in RPMI cell culture medium (Table 2). The DLS measurement was consistent with ζ-potential measurements indicating AgNPs became less negative in RPMI at pH 7, compared to DI water (Table 2). The change in ζ-potential probably arises due to the screening effect of salts in cell culture medium on the surface charge of colloidal particles, which acts to decrease the electrostatic repulsive forces between particle surfaces, leading to aggregation of AgNPs36.
Figure 1. Characterization of the synthesised AgNPs.
(a) TEM image of synthesised AgNPs and (b) their size distribution measured through TEM. (c) Indexed selected area electron diffraction (SAED) patterns of AgNPs incubated in DI water, revealing characteristic lattice spacings of 0.236, 0.204 and 0.145 nm, corresponding to the (111), (200) and (220) planes of metallic silver. The selective area aperture size used was 100 nm in diameter. (d) EDX spectra of AgNPs. Data shown are representative of analysis of 200 particles.
Table 1. Summary of the physicochemical characteristics of AgNPs in DI water.
| Average Size (TEM) | 49.7 ± 10.5 nm in diameter |
|---|---|
| Capping Agent | Citrate |
| Surface Charge | −27.8 ± 0.1 mV |
Table 2. Hydrodynamic diameter and zeta potential of AgNPs in water and cell culture media.
| Solvent | Duration (h) | Average diameter (DLS), nm | Zeta potential, mV |
|---|---|---|---|
| Water | 0 | 61.5 | −27.8 ± 0.1 |
| RPMI | 1 | 379.9 | −23.0 ± 0.8 |
| DMEM | 24 | 853.0 | −18.5 ± 1.2 |
Cellular uptake of AgNPs
AgNP internalization by N9 microglia was quantified through ICP-AES. Following a 1hr pulse- 24 hr chase AgNP (50 μg/mL) treatment, microglia internalized 29.96 ± 0.77% of the initial dose, with each individual cell internalizing an average of 30.52 ± 0.79 ng of silver (Table 3).
Table 3. Quantification of AgNP internalization into N9 microglia cells by ICP-AES following a 1 hr pulse/24 hr chase treatment (50 μg/mL).
| Silver internalization (% of initial dose ± SEM) | 29.96 ± 0.77 |
|---|---|
| Silver internalization per cell (pg ± SEM) | 30.52 ± 0.79 |
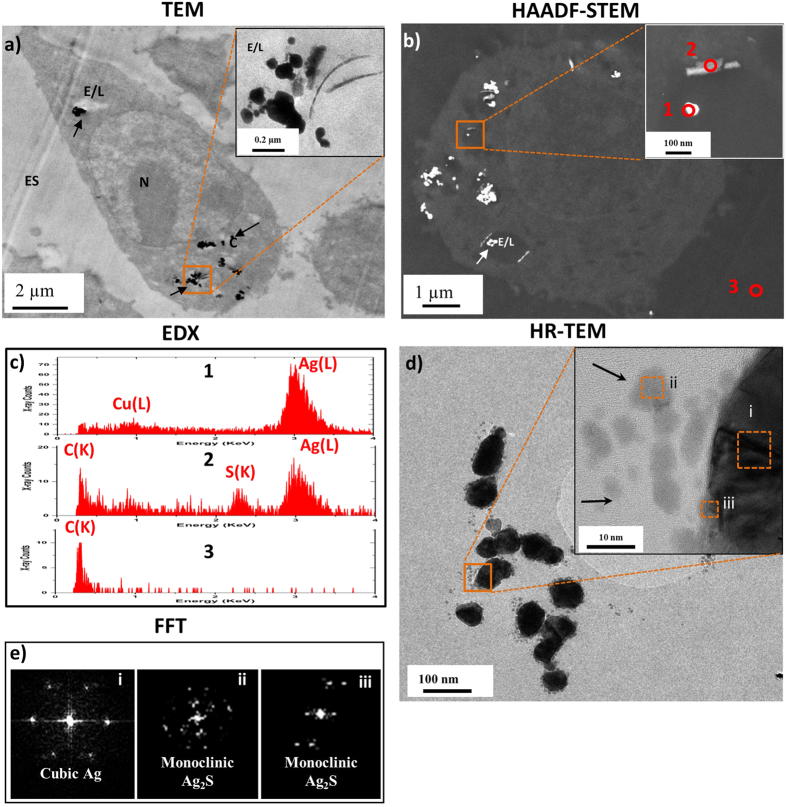
Bright field (BF) TEM images confirmed microglial uptake of AgNPs after a 1 hr pulse treatment followed by a 24 hr chase period (Fig. 2a,b). AgNPs were observed within endosome/lysosome-like vesicles as well as within the cytoplasm (Fig. 2a,b, arrows). Some AgNPs agglomerated inside vesicles (Fig. 2a, insert), possibly due to the lower pH of the intravesicular environment and thermodynamic driving force to minimize total AgNP surface energy37.
Figure 2. AgNP uptake and transformation by N9 microglia following a 1 hr pulse treatment (50 μg/mL) with 24 hrs chase period.
(a) TEM images showing the cellular uptake of AgNPs by microglia cells (N: nucleus; C: cytoplasm; E/L: endosome/lysosome; ES: extracellular space). (b) HAADF-STEM images showing the intracellular distribution of AgNPs and a corresponding higher magnification image of the boxed area. (c) STEM-EDX spectra acquired from corresponding areas 1–3 marked in (b). (d) TEM imaging of intracellular AgNPs with secondary particles in close poximity to, and partially coating the surface of AgNPs. (e) FFT patterns taken from corresponding areas (i–iii) marked in (d) reveal secondary particles are composed of Ag2S. Images are representative of >100 cells surveyed per experiment, from three independent experiments.
The chemical and morphological changes of intracellular AgNPs were analyzed using high angle annular dark field (HAADF)-STEM and STEM–EDX (Fig. 2b,c). STEM-EDX spectra (Fig. 2c) taken from AgNPs and precipitates surrounding the intracellular AgNPs in Fig. 2b show Ag(L) peaks and S(K) peaks, indicating association of sulphur on or around the AgNPs. Phase contrast high resolution (HR) TEM images were acquired to examine whether the surface of the AgNPs had been fully or partially sulphided. To ensure sample preparation did not alter the physicochemistry of AgNPs inside the cells, the AgNP-exposed cells were prepared by a combination of high pressure freezing (HPF) and freeze substitution (FS). These methods induce the formation of amorphous ice that is subsequently replaced by organic solvents at low temperatures38, ensuring close-to-native preservation of cellular structures, AgNP composition and morphology, and ion distribution39. Formation of sub-particles on the surfaces of AgNPs was observed 24 hrs post-exposure, as indicated by their rough surfaces in HR-TEM analysis (Fig. 2d). Furthermore, fast fourier transform (FFT) patterns (Fig. 2e) from selected areas40 (inset Fig. 2d) revealed the sub-particles were composed of silver sulphide (Ag2S), probably due to Ag+ ions binding to organic ligands on the surface and vicinity of AgNPs, supporting the STEM-EDX observations. In contrast, the bulk of the AgNPs remained as pure silver. The interplanar spacing of the material coating the AgNP surface was measured from phase contrast HR-TEM images taken at the surface and the core of AgNPs. The spacing between the spots in the FFT pattern and their lattice plane identifications are shown in SI (Table S1).
TEM/STEM-EDX analysis of AgNPs incubated in RPMI (24 hrs) in the absence of cells revealed no structural or chemical modifications (Fig. S2), indicating no significant dissolution of AgNP and reprecipitation of Ag2S prior to cell internalization. Importantly, incubation of AgNP in perchlorate buffer (pH 5, corresponding to the average pH of endosomes) led to an increase in free Ag+ ions in solution at both 4 hrs and 24 hrs (Fig. S1), suggesting that the AgNP dissolution and subsequent Ag+ ion sulphidation requires cellular internalization.
Effect of AgNPs internalization on H2S-synthesizing enzyme expression
Given the fact that H2S is a major sulphiding species for nanosilver41, and is an important regulator of cellular function, the effect of AgNPs on H2S synthesis was examined in order to elucidate the mechanism of AgNP sulphidation evidenced following internalization by microglia. H2S synthesis was indirectly measured by quantifying the expression of H2S-synthesizing enzymes.
Confocal Analysis
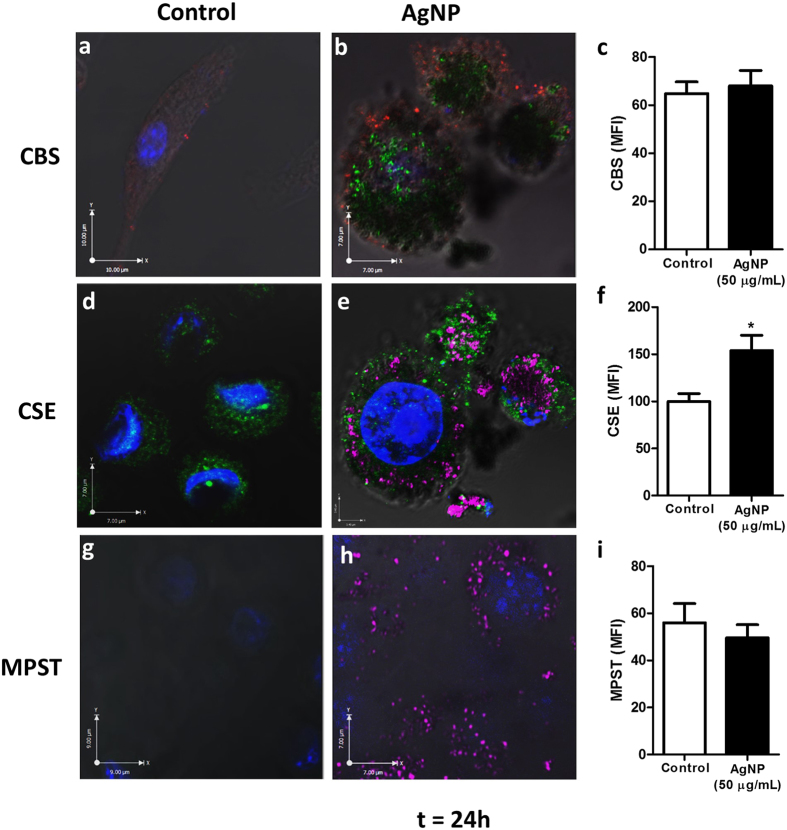
The expression of the H2S-synthesizing enzymes cystathionine-γ-lyase (CSE), cystathionine β-synthase (CBS) and mercaptopyruvate sulfurtransferase (MPST) was examined by confocal microscopy after a 1 hr pulse AgNP treatment followed by a 24 hrs chase period. Confocal microscopy images indicated baseline enzyme expression in control cells was stronger for CSE compared to CBS and MPST (Fig. 3a,d,g), suggesting CSE is a major H2S producer in microglial cells. A similar expression pattern was seen at an earlier time-point (4 hrs chase period) (Fig. S3).
Figure 3. Cellular uptake and distribution of AgNPs combined with CBS, CSE and MPST enzymes expression in microglia.
N9 microglia were treated with AgNP (50 μg/mL) for 1 hr (pulse) followed by a 24 hrs chase period. Cells were immunostained and enzyme expression analysed through confocal microscopy. Confocal images of CBS (a,b), CSE (d,e) and MPST (g,h) enzymes in control (a,d,g) and AgNP-treated (b,e,h) microglial cells. For images (a,b), CBS = red; AgNP = green; DAPI = blue. For images (d,e,g,h) CSE/MPST = green, AgNP = magenta, DAPI = blue. White = colocalization of enzymes and AgNP. Images are representative of three independent experiments. The mean fluorescent intensity (MFI) of CBS (c), CSE (f) and MPST (i) immunoreactivity was measured from the confocal microscopy images using Image J analysis software. Results are displayed as mean ± SD of three independent experiments, quantifying 40 individual cells per experiment. *Denotes p < 0.05 vs. control, as assessed by a student’s t-test.
Treatment of microglia with AgNPs led to an increase in CSE signal intensity in the vicinity of AgNPs (Fig. 3d,e), without affecting CBS signal intensity (Fig. 3a,b). MPST was weakly detected in both control and AgNP-treated cells (Fig. 3g,h). Quantitative analysis of the confocal images revealed that the CSE enzyme level was significantly increased following exposure to AgNPs, with a 50% mean fluorescence intensity increase observed compared to control cells (Fig. 3f). However, neither CBS (Fig. 3c) nor MPST (Fig. 3i) enzyme levels were significantly affected by AgNP internalization.
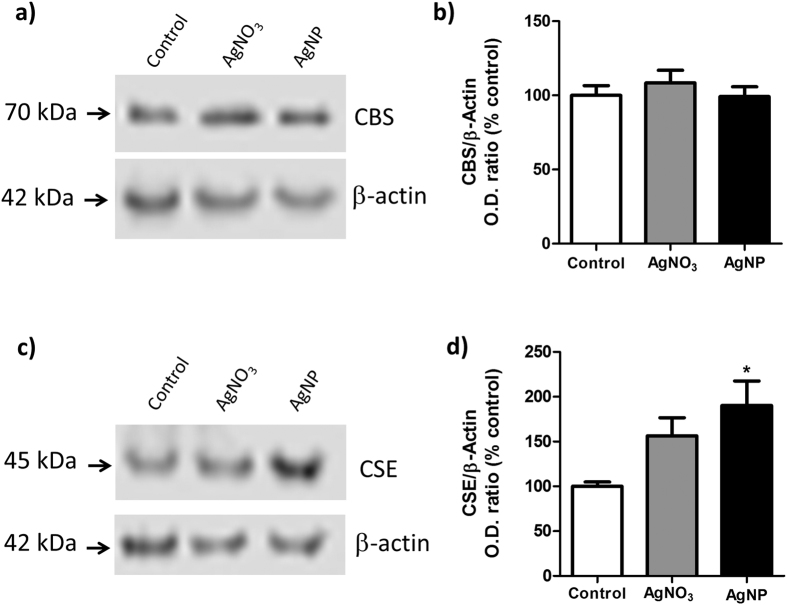
Western blot immunodetection
To confirm the enzyme expression results obtained through confocal microscopy analysis, CBS and CSE enzyme expression levels were quantified through Western blotting. MPST was omitted from the WB analysis due to its negligible detection through confocal microscopy for all conditions and time-points. As shown in Fig. 4, Western blot data indicated CBS expression remained unaltered following AgNP treatment (1 hr pulse exposure with 24 hrs chase) (Fig. 4a,b), while expression of CSE was significantly increased (Fig. 4c,d), consistent with the confocal analysis images. In order to verify the modulation of enzyme expression was due to Ag+ ions released from AgNPs, microglia were subjected to a similar pulse-chase treatment with silver nitrate (AgNO3) as a positive control for the presence of silver ions. Consistent with the effects of AgNP treatment, CBS expression was not affected by AgNO3 treatment (Fig. 4a,b). While expression of CSE appears upregulated by AgNO3 treatment, the change marginally failed to reach statistical significance (Fig. 4c,d).
Figure 4. Modulation of microglial CBS and CSE expression by AgNP or AgNO3 treatment.
N9 microglia were treated with AgNP (50 μg/mL) or AgNO3 (3.5 μM) for 1 hr (pulse) followed by a 24 hrs chase period. CBS/CSE protein expression was then quantified by immunostaining Western blots from whole cell lysates. Immunostaining for β-actin was used as loading control. Image in (a,c) is a representative Western blot from three independent experiments, from which enzyme expression was quantified by normalizing enzyme optical density against β-actin optical density (b,d). *Denotes p < 0.05 vs. control, as assessed by a one-way ANOVA. Results are displayed as mean ± SEM of three independent experiments.
Effect of AgNP treatment on microglial inflammation and cell viability
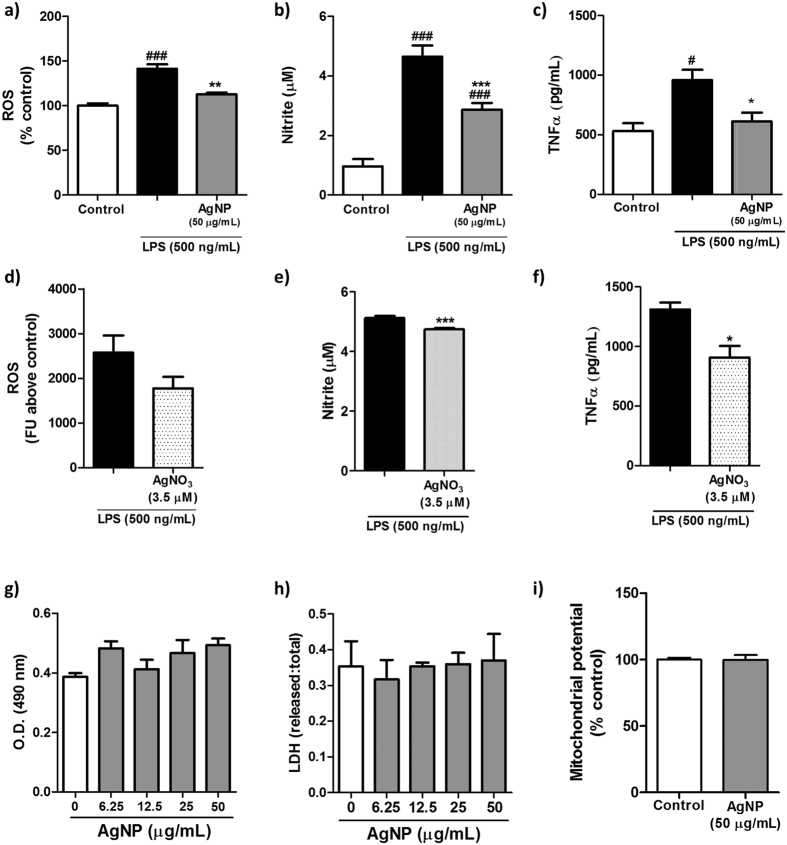
Given H2S is a known important modulator of inflammation, especially in microglia and macrophages42,43, the effect of AgNP treatment on microglial inflammation was examined by quantifying its effects on LPS-induced ROS production, nitrite synthesis and TNFα release. A simultaneous 1 hr treatment of microglia with LPS (500 ng/mL) and AgNPs followed by a 24 hrs chase period led to an overall reduction in pro-inflammatory microglial markers, as evidenced by a reduction in ROS production (Fig. 5a), nitrite synthesis (Fig. 5b) and TNFα release (Fig. 5c) compared to LPS treatment alone. Consistent with the CSE enzyme expression data, the anti-inflammatory effects of AgNPs were mirrored by a similar treatment with AgNO3 (Fig. 5d–f), however the effects were less pronounced, and in the case of ROS reduction did not reach statistical significance (Fig. 5d).
Figure 5. Modulation of microglial reactivity and cell viability by AgNP/AgNO3 treatment.
N9 microglial cells were treated with LPS (500 ng/mL) with or without AgNPs/AgNO3 for a 1 hr pulse period followed by a 24 hrs chase period. Microglial inflammation was assessed through quantification of (a,d) ROS production, (b,e) nitrite production, and (c,f) TNFα release. Cell viability was assessed through (g) an MTS assay, (h) LDH release assay, and (i) mitochondrial potential quantification. Data is presented as mean ± SEM of three independent experiments; #, ### indicate p < 0.05, 0.005, respectively, vs. control; *, ** and *** indicate p < 0.05, 0.01, 0.005, respectively, vs. LPS treatment as assessed by a one-way ANOVA with Tukey’s post-hoc test (a,b,c,g,h) or a student’s t-test (d,e,f,i).
Importantly, the effects of AgNPs/AgNO3 on ROS production, TNFα release and nitrite synthesis were due to a bona fide anti-inflammatory effect and not a decrease in cell viability, as evidenced by a lack of a cytotoxic effect by either AgNPs (Fig. 5g–i) or AgNO3 (Fig. S4). Interestingly, a similar AgNP treatment of N27 dopaminergic neurons induced significant neurotoxicity (Fig. S5), supporting silver ion sulphidation as a detoxifying mechanism inside microglial cells.
Effect of AgNP treatment on microglia-mediated neurotoxicity
In order to examine whether the reduction of microglial inflammation by AgNP treatment translated into modulation of neurotoxicity, dopaminergic N27 neurons were incubated (48 hr) with microglial medium from control, LPS or LPS/AgNP-treated microglia. LPS treatment induced a significant neurotoxic microglial phenotype, as evidenced by a robust increase in LDH release from neurons incubated with medium derived from LPS-treated microglia, compared to neurons incubated in medium derived from control microglia (Fig. 6). Consistent with the reduction in microglial inflammation by AgNPs, co-treatment of N9 microglia with AgNP reduced the LPS-induced neurotoxicity, as evidenced by decreased LDH release from neurons incubated in medium derived from LPS/AgNP-treated microglia compared to neurons incubated in medium derived from microglia treated with LPS alone (Fig. 6). In order to corroborate the reduction of microglia-mediated neurotoxicity by AgNP treatment, neuronal viability was further assessed by quantifying metabolic activity through an MTS assay following microglia treatment with commercially acquired AgNP (see Supplementary Materials and methods). In agreement with the LDH release results, AgNP treatment of microglia abolished the LPS-induced microglia neurotoxicity, as evidenced by statistically equal levels of metabolic activity of neurons incubated with medium derived from control microglia vs. LPS/AgNP-treated microglia (Fig. S6a). Importantly, treatment of microglia with commercially acquired AgNP reduced LPS-induced microglial inflammation (Fig. S6b) similarly to the in-house synthezised AgNP.
Figure 6. Modulation of microglial neurotoxicity by AgNP treatment.
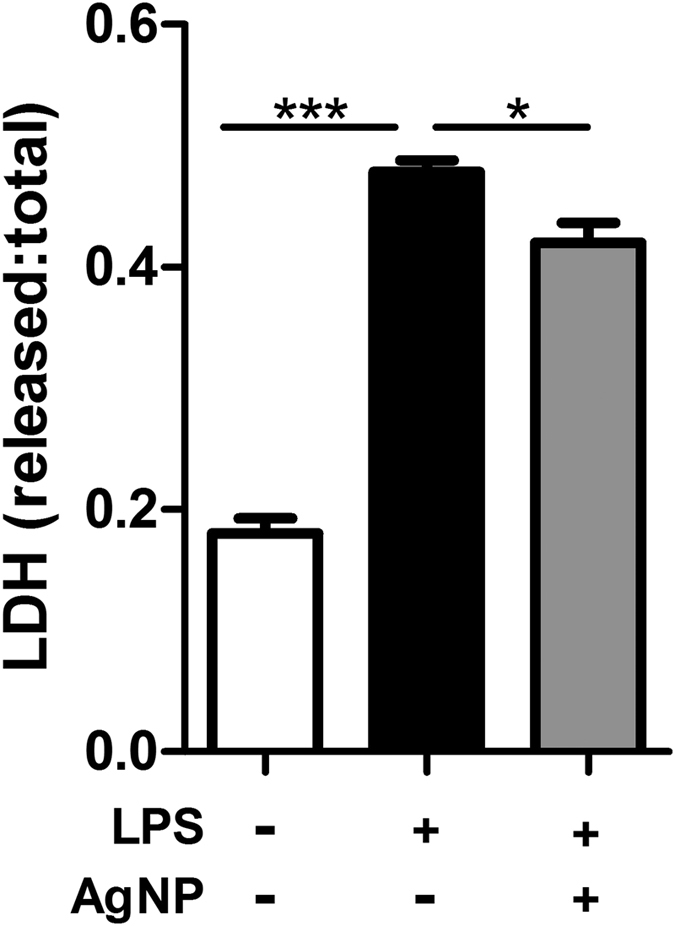
N9 microglia were treated for 1 hr (pulse) with AgNP (50 μg/mL) and/or LPS (500 ng/mL), followed by a 24 hrs chase period with only LPS present. The medium was then transferred to N27 neurons and incubated for 48 hrs, after which time-point cell viability was assessed through an LDH release assay. Data is presented as mean ± SEM of three independent experiments; * and *** indicate p < 0.05, 0.005, respectively, vs. corresponding groups, as assessed by a one-way ANOVA with Tukey’s post-hoc test.
Discussion
In the present study, we directly assessed the internalization of AgNPs into a mouse microglial cell line, examining the intracellular chemical and morphological transformation of the nanoparticles, as well as the effect of AgNP-treatment on microglial viability and LPS-induced microglial inflammation and related neurotoxicity.
ICP-AES and TEM imaging clearly showed AgNPs internalization by microglial cells following a 1 hr exposure. Furthermore, STEM-EDX spectra and FFT patterns indicated Ag2S complexes formed intracellularly in the vicinity of AgNPs, as well as partially coating the nanoparticles. As internalized AgNPs are not thermodynamically stable in the acidic and oxidising intracellular environment of endosomes, Ag+ ions are released from the AgNPs due to nanoparticle dissolution. The Ag+ ions react with sulphide species to form the evidenced Ag2S complexes. Since the solubility of Ag2S is extremely low (Ksp = 5.92 × 10−51), AgNP-surface coating by Ag2S complexes would modify the surface properties of AgNPs and inhibit further oxidative dissolution of AgNP33. The formation of Ag2S as a Ag+ sequestering mechanism is expected to occur in preference over formation of silver chloride and silver oxide, as their solubility is significantly higher than that of Ag2S (Ksp = 1.77 × 10−10 and 4 × 10−11, respectively)33, and therefore they will not be thermodynamically favoured.
AgNP cytotoxicity is mainly due to the release of Ag+ ions, which interact via several damaging mechanisms: Ag+ ions interact with cell membranes, leading to lower membrane integrity and increased permeability; they are able to bind thiol groups in proteins, causing improper protein function; and they bind to and damage DNA, resulting in cell apoptosis29,30,31,32. Recent studies indicate that the transformation of Ag+ ions to insoluble Ag2S reduces the potential toxicity of silver nanomaterials in the lung33. Therefore, the formation of Ag2S complexes around AgNPs may represent a Ag+-sequestering and detoxifying mechanism similarly present in microglial cells. Such a mechanism would explain the lack of AgNP-induced cell death evidenced in the current study, as a similar concentration of AgNPs induced significant toxicity to dopaminergic neurons in the present study, and has been shown to be toxic to other cell types14,44.
Cell type differences in H2S levels and/or in the ability to up-regulate H2S-synthesizing enzyme expression would affect the cell’s capacity to sulphide and sequester released Ag+ ions, hence may underlie the differing susceptibility of cells to AgNP-induced toxicity. H2S is produced mainly by the pyridoxal-5′-phosphate (PLP)-dependent enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) via the amino acids homocysteine, cysteine and cystathionine45,46, as well as by 3-mercaptopyruvate sulfurtransferase (MPST) via the cysteine catabolic pathway47. We have demonstrated the H2S-synthesizing enzyme CSE becomes up-regulated in response to the sulphur-sequestering capacity of Ag+, while the alternative H2S-synthesizing enzymes CBS and MPST remained unchanged. Indeed, the levels of H2S have been shown to be able to regulate both the expression and activity rate of CSE in mammalian cells and tissue48,49. Hence, a decrease in available H2S following Ag+ sequestration would prompt the up-regulation of CSE to maintain H2S homeostasis. This process might not be present in neurons, resulting in insufficient H2S levels necessary for efficient AgNP detoxification. Indeed, expression of hydrogen sulphide-producing enzymes has been shown to be higher in glial cells compared to neuronal cells50,51. Moreover, certain neuronal populations have been shown to lack CSE as a primary H2S synthesising enzyme52. Therefore, lack of CSE may restrict the ability of certain neuronal populations to rapidly counteract the decreasing H2S levels, preventing detoxification of Ag+. In addition, given that microglia are the sentinels of the CNS, they are programmed to actively respond to infiltrating material within their extracellular environment. Hence, the presence of intracellular AgNPs may elicit an active response involving localisation of CSE and H2S synthesis in the vicinity of the nanoparticles, as opposed to passive sulphiding of Ag+ ions by available H2S. Thus, Ag+ ions released within microglia will be sulphided and sequestered more efficiently in comparison to neurons, limiting their toxicity towards microglial cells. Speculatively, given CSE is expressed by a number of different tissues both centrally and peripherally53,54, its up-regulation by AgNPs might be a general mechanism of silver toxicity limitation in a wide range of tissues.
The sulphiding of AgNPs is a complex process which may occur via two chemical pathways, depending on the sulphide concentration in the cellular environment. According to Liu et al., Ag sulphiding is dominated by an indirect reaction if the sulphide concentration is <0.025 μg/mL41. At higher sulphide concentrations, the mechanism switches to a direct reaction where the reaction rate increases with increasing sulphide concentration. The reactions below show the two competing chemical and transport pathways of AgNP sulphiding before transformation into the thermodynamically stable sulphide phases:
- Direct route (particle-fluid heterogeneous reaction) - AgNPs direct oxysulphidation reaction rate increases with increasing sulphide concentration.
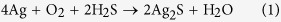
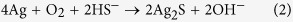
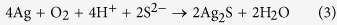
Indirect route (oxidative dissolution (Equation 4) and precipitation (Equations 5,6,7)) - dissolution of AgNPs is initiated by oxygen chemisorption accompanied by electron transfer37. The cooperative effect of both dissolved oxygen and protons by thermodynamic analysis can be represented in the following heterogeneous oxidation reaction stoichiometry:
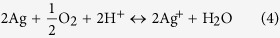 |
While Ag+ ions are released from AgNPs, they rapidly diffuse into the cellular environment and form sulphide by subsequent precipitation:
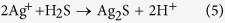 |
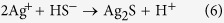 |
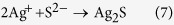 |
Initially, as intracellular sulphide concentration is maximum, sulphide species (e.g. H2S, HS− and S2−) may attack the outer layer of AgNPs surface via a direct particle-fluid reaction, leading to the formation of small Ag2S particles in the vicinity of the NPs surface, as observed through HR-TEM. Over time, released Ag+ ions will either deposit on the existing Ag2S nuclei to form larger-sized Ag2S particles or precipitate as new Ag2S particles. It will be interesting to examine how microglia process the accumulating Ag2S at longer time-points.
Nanomaterials have been shown to be able to induce cell death through a recently characterized non-apoptotic mechanism involving iron and lipid peroxidation termed ferroptosis55,56. While microglia in the current study are resistant to AgNP-induced ferroptosis as assessed by lack of membrane integrity and metabolic activity decrease, as well as the lack of ROS production increase (essential for the lipid-peroxidation mediated cell death), it will be of interest to examine whether AgNP are able to induce ferroptosis in neuronal cells and whether it is the main mechanism responsible for the differing susceptibilities of different cell types to AgNP-induced toxicity.
In the present study, increased expression of the H2S-synthesising enzyme CSE following AgNP treatment, in addition to correlating with Ag+ sulphide deposition and reduced AgNP toxicity, correlated with decreased microglial inflammation. Indeed, there is a growing body of evidence indicating H2S regulates inflammation42,57, and our results echo previous studies examining the role of H2S-synthesizing enzymes in modulation of microglial reactivity. For instance, treatment of microglia with the neurotoxic compound rotenone leads to an increase in microglial inflammation with a concomitant reduction in the levels of the H2S-synthesizing enzyme CBS58. Importantly, if CBS expression or H2S levels were enhanced, rotenone-induced microglial reactivity was decreased. Similarly, treatment of peripheral macrophages with oxidized low density lipoprotein (oxLDL) leads to a reduction in the expression levels of CSE with a concomitant increase in TNFα production which can be prevented by overexpression of CSE or exogenous H2S59. Importantly, CSE expression has also been detected in primary microglia60. Therefore, the present results demonstrate the increase in CSE expression to counterbalance the decreasing H2S levels due to Ag+ sequestration confers AgNPs with an anti-inflammatory effect. Furthermore, this anti-inflammatory effect translated into reduced microglia-mediated neurotoxicity. This finding has important consequences for AgNP-induced neurotoxicity, as microglial inflammation is known to contribute to neuronal toxicity, especially in cases of chronic neurodegeneration such as during PD61. Hence, from the results presented in this paper, it may be surmised that in vivo AgNP neurotoxicity is due to direct neuronal effects of AgNPs and not due to exacerbation of microglia-derived neurotoxic factors. Speculatively, highly controlled targeting of AgNP into microglia could decrease brain inflammation locally by inhibiting microglia reactivity through promotion of H2S synthesis, thereby reducing inflammatory injury to neighbouring neuronal cells. Additionally, since H2S has been shown to be a potent neuroprotective agent in models of neurodegenerative diseases62, induction of H2S synthesis by microglial targeted AgNPs could actually aid in decreasing chronic neurodegeneration. Interestingly, recent experiments have demonstrated a deficiency in CSE activity in human brain tissue derived from Huntington’s disease patients, which may underlie the progressive neurodegeneration seen in that disease63.
In summary, the present study proposes a AgNP-detoxifying mechanism in microglia involving the sulphiding of Ag+ ions and up-regulation of H2S-synthesizing enzymes. Importantly, this study demonstrates for the first time a correlation between AgNP-detoxifying mechanisms and a reduction in microglial inflammation which serves a neuroprotective role. In addition, it highlights the need for further characterisation of AgNPs at the cellular level inside brain tissue and other target organs to assess how AgNP transformation varies between relevant cell types and AgNP formats with varied physicochemistry, and thus how it affects AgNP bioreactivity and biopersistence. Future studies in experimental animal models may likewise benefit from the application of these methods to determine whether AgNP transformation processes occur in vivo. Characterisation of cellular uptake and transformation of AgNP may have important implications for understanding and ultimately providing guidelines to control the biopersistence and bioreactivity of AgNP which can be used to make informed decisions about how to design future classes of “safe” AgNPs.
Materials and Methods
Nanoparticle synthesis, characterisation and stability
Silver nanoparticle synthesis
Silver nanoparticles were synthesised via the chemical bath reduction method in which trisodium citrate (Na3C6H5O7) (Fisher Scientific, UK) serves a dual role as both reductant and stabilizer. Briefly, silver nitrite (AgNO3) (1.0 × 10−3 M) (Sigma-Aldrich, UK) solution was added to preheated de-ionized (DI) water (180 mL). Then, Na3C6H5O7 (1.0 × 10−3 M) solution was added dropwise to the AgNO3 solution as soon as boiling commenced. The color of the solution slowly turned grayish yellow, indicating the reduction of Ag+ ions. The solution was heated continuously for an additional 20 min, and then cooled to room temperature. In order to remove impurities and excess citrate, the AgNPs suspension was washed with DI water and centrifuged at a relative centrifugal force maximum value (RCFmax) of 10,000 g. This washing process was repeated three times to ensure no citrate remained in the solutions. Finally, citrate-coated AgNPs were suspended in DI water at the desired concentration, sealed and stored in the dark at 4 °C. The purity of synthesized AgNPs was confirmed using an energy dispersive X-ray (EDX) spectrometer mounted on a LEO Gemini 1525 field emission gun scanning electron microscope (FEGSEM), to ensure that AgNPs were not sulphided by adventitious contaminants in the atmosphere64 and that impurities, such as Na+ and Cl− ions had been removed by the washing process.
The size and surface charge of AgNPs in water, RPMI (1 hr) and DMEM (24 hrs) were measured using dynamic light scattering (DLS) and zeta potential analyses (Malvern Zetasizer Nano series), respectively.
Stability of AgNPs: Dissolution Kinetics
The amount of Ag dissolved from AgNPs in pH 5 perchlorate buffer solutions, corresponding to the pH found in intracellular endosomes, was analysed using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Non-interacting buffer solutions were used to minimise the effect of anions on the stability of Ag+ ions. The AgNP suspensions were incubated in perchlorate buffer solutions at 37 °C for 4 or 24 hrs followed by centrifugation with 2 kDa (<4 nm) filter tubes (Sartorius Stedim VIVACON 500) at 13,000 rpm to separate particles from the solutions. Chen et al.35 showed that dissolved Ag+ ions will reprecipitate as insoluble compounds in cell culture media (RPMI and DMEM), therefore ICP-AES cannot be employed to quantify the release of Ag+ ions in the medium.
Cell culture and treatments
N9 microglial cells
Microglia experiments were carried out on the immortalized embryonic mouse microglia N9 cell line, first developed by Dr. Ricciardi-Castagnoli et al.65 and given as a kind gift by Dr. Deanna Taylor, Imperial College London. N9 microglia reliably replicate cultured primary microglia with respect to NO production, cytokine synthesis and expression of cell surface markers66,67,68. N9 microglia were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, UK) with 5% Fetal Calf Serum (FCS, Sigma-Aldrich, UK), 8 mM L-glutamine (Sigma-Aldrich, UK) and 50 U/mL Penicillin and 50 μg/mL streptomycin (Sigma-Aldrich, UK) (termed full DMEM) at 37 °C in a humidified atmosphere with 5% CO2.
Microglial cells were plated either in 96 well plates, onto glass coverslips in 24-well plates, or 6 well plates (at 10,000, 50,000 or 500,000 cells per well, respectively) and incubated for 24 hrs before experimentation began to allow them to readopt a resting phenotype. Microglia were then washed with serum-free (SF) RPMI medium (Sigma-Aldrich, UK) (with 8 mM L-glutamine and 50 U/mL Penicillin and 50 μg/mL streptomycin; termed SF-RPMI) and incubated with AgNP alone, lipopolysaccharide (LPS, 500 ng/mL28) (Enzo Life Sciences, UK) alone or a combination of both in SF-RPMI for 1 hr (pulse) at 37 °C. The cells were then washed with SF-RPMI and incubated in SF-RPMI for a further hour. Finally, cells were incubated in full DMEM for 4 or 24 hrs (chase) before analysis (i.e. experimental end-point). SF-RPMI medium was used during AgNP exposure as AgNP do not dissolve or transform to Ag2S in this cell culture medium33. Cells were then incubated in full DMEM to ensure appropriate metabolic support.
N27 neuronal cells
Neuronal experiments were carried out on the immortalized foetal rat ventral mesencephalon dopaminergic N27 cell line, used extensively as an in vitro PD model69,70,71. N27 cells were a kind gift from Dr. Nabil Hajji, Imperial College London. Neuronal cells were cultured in full DMEM at 37 °C in a humidified atmosphere with 5% CO2. For experimentation, cells were removed from culture flask through trypsinization and plated in 12 well plates in full DMEM 24 hrs prior to experimentation to ensure cell attachment.
For microglia-mediated neurotoxicity studies, N9 microglia plated in 6 well plates were treated with LPS with or without AgNP as above (see ‘cell culture and treatment’ section). However, in order to ensure sufficient microglial activation to induce neuronal cell death, LPS was present in the cell culture medium throughout the 24 hrs chase period. At the experimental end-point, the medium was collected and centrifuged (5 min at × 1200 g) to clear floating cells. The supernatant was then transferred to the cultured N27 neurons and incubated for 48 hrs prior to cell viability quantification.
Cell viability assays
MTS assay
MTS is a chromogenic assay which involves the bioreduction of the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium by NADH in viable cells into a coloured formazan product. Therefore, the cellular metabolic activity can be quantified spectrophotometrically by measuring the absorbance of the formazan product.
At the experimental end-point, cells plated in 96 well plates were washed and incubated at 37 °C with fresh full DMEM (100 μL) containing MTS reagent (20 μL) (Promega, UK) for 2–3 hr before the optical density (at 490 nm) was quantified with a plate reader. Cell viability was determined by comparing the optical density of treated cells vs. control cells.
LDH assay
Lactate dehydrogenase (LDH) is a soluble cytosolic enzyme which catalyses the oxidation of lactate to pyruvate. Once cells lose their membrane integrity due to stress or injury, LDH is rapidly released into the extracellular medium. Thus, membrane damage and cytotoxicity can be assessed by the quantification of released LDH from cells.
At the experimental end-point, extracellular medium (50 μL) from cells plated in 24 well plates was incubated with LDH detection solution (50 μL) (Promega, UK). Following 10–20 min incubation (at 37 °C), the optical density (at 490 nm) was quantified with a plate reader to determine LDH concentration in the extracellular medium. To control for lower LDH in the extracellular medium due to a sparser cell population following cytotoxicity, total LDH (i.e. intracellular plus released LDH) was also quantified by lysing cells in the original culture medium (with 10 μL lysing buffer, Promega, UK) and the cell medium incubated with LDH reaction mixture as before. The amount of released LDH was then normalized against the total amount of LDH in the culture well.
Cellular uptake of AgNPs
N9 cells were exposed to 50 μg/mL AgNPs as above. This high dose has been shown to be toxic to neurons in vitro12,14, but showed no toxicity to microglia in the present study, therefore was suitable to provide evidence that Ag2S formation acts as a detoxifying mechanism for silver ions released from intracellular AgNPs.
Transmission electron microscopy
N9 microglia were rinsed with HEPES buffer and fixed in freshly prepared 2.5% glutaraldehyde for 1 hr at 4 °C. Each sample was dehydrated in a graded ethanol series (50%, 70%, 90% and dry100% volume ratio of ethanol to DI water) for 5 min × 3 times each, followed by dry acetronitrile (Sigma-Aldrich, UK) for an additional 10 min each, all at room temperature. After dehydration, samples were progressively infiltrated with 50% and 75% Quetol-based araldite resin/ethanol solutions (Agar scientific, UK) overnight and finally 100% resin for 4 days with fresh resin replaced daily. The specimens were then cured at 60 °C for 24 hrs before sectioning using an ultramicrotome (Leica ultracut UCT, Wien, Austria). Ultrathin cell monolayers (approximately 50–100 nm) were cut with a 35° diamond knife and each section was examined in a JEOL 2000 transmission electron microscope at an accelerating voltage of 80 kV. The use of heavy metal stains to enhance cell ultrastructure in the TEM can alter the morphology and accelerate the oxidation of AgNPs72, and therefore was avoided. Our previous published work has shown that the sample preparation procedure applied in this study does not alter the physicochemistry of the AgNPs in the cellular environment72.
Multiple cells (>100 per sample) from three different cell exposures were surveyed using an FEI Titan 80–300 scanning/transmission electron microscope operated at 80 kV, fitted with a Cs (image) corrector. HAADF-STEM was performed with a convergence semi-angle of 14 mrad and inner and outer collection semi-angles of 49 and 239 mrad, respectively. The probe diameter was <0.5 nm. STEM-EDX spectroscopy was performed using a SiLi EDX spectrometer (EDAX, Leicester UK).
High-pressure freezing
High-pressure freezing (HPS)/freeze substitution (FS) was employed to ensure preservation of cellular structures, AgNP composition and ion distribution. Samples were placed in specimen carriers with 200 μm deep cavities, mounted in a 20% (wt/vol) solution of bovine serum albumin (Sigma) in αMEM, and frozen using a Leica EM PACT 2 (Leica Microsystems) high-pressure freezer. FS was performed in a Leica electron microscope AFS2 freeze substitution device using an anhydrous solution of acetone containing a 3% (vol/vol) glutaldehyde, 1% (wt/vol) osmium tetraoxide, and 0.5% (wt/vol) uranyl acetate at −90 °C for 8 h. Samples were then gradually warmed to 0 °C at 5 °C/h, washed twice in acetone, brought to room temperature, and infiltrated with resin, as described in the TEM methodology section.
Inductively-coupled plasma atomic emission spectroscopy
The amount of silver internalized by N9 microglia following AgNP treatment (see above) was quantified through inductively-coupled plasma atomic emission spectroscopy (ICP-AES). At the experimental end-point, the cells were rinsed twice with 1 mL HBSS to remove all non-internalized silver. Following collection of the cells in 1 mL HBSS, cell number was quantified with the use of a haemocytometer. The cells were then pelleted by centrifugation (10,000 g × 10 mins) and resuspended in 200 μL HBSS. The cell suspension was digested with 800 μL aqua regia overnight and neutralized with 3 mL diH2O before ICP-AES silver quantification. AgNP internalization was calculated as a percentage of initial dose or total silver internalization per cell.
Microglial reactivity quantification
TNFα release
At the experimental end-point, TNFα release was measured in the cell culture medium using commercially available ELISA kits (Peprotech, UK) according to the manufacturer’s instruction.
Intracellular reactive oxygen species (ROS) production
Intracellular ROS levels were detected using the 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) assay (Sigma, UK). DCFH-DA is a lipophilic cell permeable compound that is deacetylated in the cytoplasm by cellular esterases to 2′, 7′-dichlorofluorescein (DCF), which is then oxidised by free radicals, including hydroxyl, alkoxyl, peroxyl, carbonate and nitrate radicals to produce a fluorescence signal.
For ROS quantification, N9 microglia were seeded in dark, flat transparent bottomed 96 well plates (10,000 cells per well) as above (see ‘cell culture and treatment’ section). At the experimental end-point, cell media was removed and replaced with 25 μM DCFDA diluted in full DMEM without phenol red for 45 min at 37 °C protected from light. Cells were then washed twice with PBS before measurement of fluorescence (at emission 485 nm and excitation 535 nm) in a microplate reader.
Nitric Oxide production
Due to its short half-life, production of nitric oxide (NO) was assessed through quantification of its stable metabolite nitrite (NO−2) using the Griess reaction73. At the experimental end-point, 75 μl of cell culture medium was transferred to 96 well plates and incubated with 75 μL of Griess reagent (sulfonilamide plus 1-(naphthyl)ethylenediamine) (Sigma-Aldrich, UK) at RT in the dark. Levels of nitrite were quantified by measuring absorbance at a wavelength of 540 nm after 5–10 min incubation. Optical density was converted to nitrite concentration using a sodium nitrite standard curve.
Mitochondria membrane potential
Mitochondrial membrane potential (MMP) was determined using the red-orange fluorescent dye tetramethylrhodamine ethyl ester perchlorate (TMRE) (Sigma, UK). This lipophilic, positively-charged dye enters cells and is sequestered by healthy mitochondria with a negative charge. Conversely, depolarized or inactive mitochondria lose their membrane potential, leading to a loss of their negative charge and ability to sequester TMRE. Therefore, the polarization state of mitochondria can be assessed by the amount of orange-red fluorescence inside cells.
At the experimental end-point, cells in 96 well plates were rinsed twice in PBS and incubated in 500 nM TMRE for 20 mins at 37 °C in the dark, followed by rinsing with 0.2% bovine serum albumin (BSA) in PBS prior to fluorescence measurement with excitation at 549 nm and emission at 575 nm using a microplate reader.
Enzyme expression quantification
Western Blotting
Protein expression was quantified through immunostaining of Western blots (WB) from N9 lysates. Cells seeded in 6 well plates were lysed with RIPA buffer (Sigma, UK) with 10% protein inhibitor cocktail (Aprotinin, Bestatin, E-64, Leupeptin, Pepstatin, AEBSF) (Sigma, UK) at the experimental end-point. The lysates were cleared by centrifugation and 20 μg of protein resolved by SDS-PAGE (10% gel) and transferred to PVDF membranes. After blocking with 5% BSA in TBS-T (2 hr at room temperature), the WB were stained with anti-CBS (40 ng/mL, SantaCruz, USA), anti-CSE (20 ng/mL, SantaCruz, USA), anti-MPST (200 ng/mL, SantaCruz, USA) or anti-β-actin (20 ng/mL, Abcam, UK) (all in 1% BSA/TBS-T) overnight at 4 °C. The WB was then incubated with horse radish peroxidase (HRP)-anti-mouse/rabbit secondary antibody (1:2000 in 1% BSA/TBS-T, Sigma, UK) for 2 hr at room temperature. Immunodetection was visualized through enhanced chemiluminescence (Biorad, UK).
Confocal microscopy
To assess AgNP colocalization with and expression of H2S-synthezising enzymes (CBS, CSE and MPST) in N9 microglia cells following AgNPs treatment, cells seeded on cover slips in 24 well-plates were washed with freshly prepared Dulbecco’s phosphate buffer saline (PBS) at the experimental end-point, followed by fixation using ice cold methanol (x5 mins). The cells were rinsed with PBS, and blocked (1% BSA in PBS, pH 7.4) for 30 min at room temperature before they were incubated with primary antibodies (anti-CBS, -CSE or –MPST, 1:200 dilution) in blocking solution at 4 °C overnight. Following rinsing with PBS, cells were incubated with fluorescently-labelled secondary antibodies diluted in blocking solution (1:200 dilution) for 1 hr at room temperature. Following further rinsing, the nuclei were counter stained with DAPI. The glass cover slips were mounted onto microscope slides with SlowFade® antifade reagent (Life Technologies, UK) and visualized using Leica SP5 inverted confocal microscope (Leica, Germany). As AgNPs have the capacity to reflect light, their uptake by cells can be monitored directly (i.e. without the use of fluorescent labelling) using confocal reflectance microscopy.
To quantify enzyme immunoreactivity, the fluorescent intensity of the enzyme in the cells was measured using Fiji (Image J) analysis software, and the data expressed as mean fluorescent intensity (MFI) ± standard deviation (SD). Forty individual cells were observed per sample, three independent samples were quantified per protein.
Statistical analysis
The data were analysed by one-way ANOVA with Tukey’s post-hoc test or student’s t-test using SPSS (IBM) and GraphPad Prism 5 software. Differences between means were considered statistically significant at p < 0.05. Data are presented as mean ± standard error of the mean (SEM) of at least three independent experiments, unless otherwise stated.
Additional Information
How to cite this article: Gonzalez-Carter, D. A. et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 7, 42871; doi: 10.1038/srep42871 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
An ERC starting investigator grant to AEP (CNTBBB). LBF acknowledges Dr. David McPhail for his support during her PhD studies.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.G.C., B.F.L., M.P.R. and A.E.P. design the study. D.G.C., B.F.L., P.R., A.E.G., S.C. carried out the experiments. D.G.C. and B.F.L. wrote the manuscript. All authors revised the manuscript.
References
- Liu J. Y., Sonshine D. A., Shervani S. & Hurt R. H. Controlled Release of Biologically Active Silver from Nanosilver Surfaces. Acs Nano 4, 6903–6913 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. & Schluesener H. J. Nanosilver: A nanoproduct in medical application. Toxicology Letters 176, 1–12 (2008). [DOI] [PubMed] [Google Scholar]
- Kim H. R., Kim M. J., Lee S. Y., Oh S. M. & Chung K. H. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutation Research-Genetic Toxicology and Environmental Mutagenesis 726, 129–135 (2011). [DOI] [PubMed] [Google Scholar]
- Arora S., Jain J., Rajwade J. M. & Paknikar K. M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicology Letters 179, 93–100 (2008). [DOI] [PubMed] [Google Scholar]
- Gliga A., Skoglund S., Odnevall W. I., Fadeel B. & Karlsson H. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Particle and fibre toxicology 11, 17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. Journal of nanoscience and nanotechnology 10, 5 (2010). [DOI] [PubMed] [Google Scholar]
- Tang J. et al. Distribution, Translocation and Accumulation of Silver Nanoparticles in Rats. Journal of Nanoscience and Nanotechnology 9, 4924–4932 (2009). [DOI] [PubMed] [Google Scholar]
- Loeschner K. et al. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Particle and fibre toxicology 8, 14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J. et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicological sciences 108, 10 (2009). [DOI] [PubMed] [Google Scholar]
- Ji J. et al. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhalation toxicology 19, 5 (2007). [DOI] [PubMed] [Google Scholar]
- Xu L. et al. Silver nanoparticles induce tight junction disruption and astrocyte neurotoxicity in a rat blood-brain barrier primary triple coculture model. International Journal of Nanomedicine 10, 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Piett C., Farkas S., Qazzaz M. & Syed N. I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Molecular Brain 6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. et al. Silver nanoparticles induced nerutoxicity through oxidative stress in rat cerebral astrocytes is distinct from the effects of silver ions. Neurotoxicology 52, 12 (2016). [DOI] [PubMed] [Google Scholar]
- Haase A. et al. Effects of silver nanoparticles on primary mixed nerual cell cultures: uptake, oxidatve stress and acute calcium responses. Toxicological sciences 126, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup N. et al. The similar neurotoxic effects of nanoparticulate and ionic silver in vivo and in vitro. Neurotoxicology 33, 416–423 (2012). [DOI] [PubMed] [Google Scholar]
- Bagheri-abassi F., Alavi H., Mohammadipour A., Motejaded F. & Ebrahimzadehbidesakan A. The effect of silver nanoparticles on apoptosis and dark neuron production in rat hippocampus. Iran Journal of Basic Medical Sciences 18, 5 (2015). [PMC free article] [PubMed] [Google Scholar]
- Skalska J., Forntczak-Baniewicz M. & Struzynska L. Synaptic degeneration in rat brain after prolonged oral exposure to silver nanoparticles. Neurotoxicology 46, 10 (2015). [DOI] [PubMed] [Google Scholar]
- Xu L. et al. Neurotoxicity of silver nanoparticles in rat brain after intragastric exposure. Journal of nanoscience and nanotechnology 15, 9 (2015). [DOI] [PubMed] [Google Scholar]
- Yin N., Yao X., Zhou Q., Faiola F. & Jian G. Vitamine E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochemical and Biophysical research communications 458, 6 (2015). [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Hanisch U.-K., Noda M. & Verkhratsky A. Physiology of Microglia. Physiological Reviews 91, 461–553 (2011). [DOI] [PubMed] [Google Scholar]
- Qian L. & Flood P. Microglial cells and Parkinson’s disease. Immunologic research 41, 12 (2008). [DOI] [PubMed] [Google Scholar]
- Mosher K. & Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer’s dsiease. biochemical Pharmacology 88, 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith C. M., Sullivan A. M. & Nolan Y. M. The influence of microglia on the pathogenesis of Parkinson’s disease. Progress in Neurobiology 89, 277–287 (2009). [DOI] [PubMed] [Google Scholar]
- Li X. et al. SiO2 nanoparticles change colour preference and cause Parkinson’s-like behaviour in zebrafish. Scientific Reports 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO(2) nanoparticles. Toxicology 254, 9 (2008). [DOI] [PubMed] [Google Scholar]
- An L., Liu S., Yang Z. & Zhang T. Cognitive impairment in rats induced by nano-CuO and its possible mechanisms. Toxicological letters 213, 8 (2012). [DOI] [PubMed] [Google Scholar]
- Ren C., Hu X., Li X. & Zhou Q. Ultra-trace graphene oxide in a water environment triggers Parkinson’s disease-like symptoms and metabolic disturbance in zebrafish larvae. Biomaterials 93, 12 (2016). [DOI] [PubMed] [Google Scholar]
- Goode A. E. et al. High resolution and dynamic imaging of biopersistence and bioreactivity of extra and intracellular MWNTs exposed to microglial cells. Biomaterials 70, 57–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis V. et al. Negligible particle-specific toxicity mechanism of silver nanoparticles: the role of Ag+ ion release in the cytosol. Nanomedicine 11, 8 (2015). [DOI] [PubMed] [Google Scholar]
- Xiu Z. M., Zhang Q. B., Puppala H. L., Colvin V. L. & Alvarez P. J. J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Letters 12, 4271–4275 (2012). [DOI] [PubMed] [Google Scholar]
- Stoehr L. C. et al. Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Particle and Fibre Toxicology 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstjerna E., Johansson F., Klefbohm B. & Johansson U. E. Gold- and Silver Nanoparticles Affect the Growth Characteristics of Human Embryonic Neural Precursor Cells. Plos One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. et al. Sulfidation of silver nanowires inside human alveolar epithelial cells: a potential detoxification mechanism. Nanoscale 5, 9839–9847 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarajah A. et al. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 31, 8 (2009). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. High-Resolution Analytical Electron Microscopy Reveals Cell Culture Media-Induced Changes to the Chemistry of Silver Nanowires. Environmental Science & Technology 47, 13813–13821 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatisson J., Quevedo I. R., Wilkinson K. J. & Tufenkji N. Physicochemical characterization of engineered nanoparticles under physiological conditions: Effect of culture media components and particle surface coating. Colloids and Surfaces B-Biointerfaces 91, 198–204 (2012). [DOI] [PubMed] [Google Scholar]
- Leo B. F. et al. The Stability of Silver Nanoparticles in a Model of Pulmonary Surfactant. Environmental Science & Technology 47, 11232–11240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald K. L. & Auer M. High-pressure freezing, cellular tomography, and structural cell biology. Biotechniques 41, 137-+ (2006). [DOI] [PubMed] [Google Scholar]
- Budka D., Mesjasz-Przybylowicz J., Tylko G. & Przybylowlicz W. J. Freeze-substitution methods for Ni localization and quantitative analysis in Berkheya coddii leaves by means of PIXE. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms 231, 338–344 (2005). [Google Scholar]
- Leblanc G. A. et al. The Influence of Speciation on the Toxicity of Silver to Fathead Minnow (Pimephales-Promelas). Environmental Toxicology and Chemistry 3, 37–46 (1984). [Google Scholar]
- Liu J., Pennell K. G. & Hurt R. H. Kinetics and Mechanisms of Nanosilver Oxysulfidation. Environmental Science & Technology 45, 7345–7353 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufton N., Natividad J., Verdu E. F. & Wallace J. L. Hydrogen sulfide and resolution of acute inflammation: A comparative study utilizing a novel fluorescent probe. Scientific Reports 2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. et al. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacological research 84, 13 (2014). [DOI] [PubMed] [Google Scholar]
- Vrcek I. et al. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environemtnal toxicology (2014). [DOI] [PubMed] [Google Scholar]
- Lee S. W. et al. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54, 116–124 (2006). [DOI] [PubMed] [Google Scholar]
- Bianca R.d.E.d.V. et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proceedings of the National Academy of Sciences of the United States of America 106, 4513–4518 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O., Vitvitsky V., Xie P. & Banerjee R. The Quantitative Significance of the Transsulfuration Enzymes for H2S Production in Murine Tissues. Antioxidants & Redox Signaling 15, 363–372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Guo Z. & Wang S. The effect of certain conditions in the regulation of cystathionine gamma-lyase by exogenous hydrogen sulfide in mammalian cells. Biochem Genet 51, 503–513 (2013). [DOI] [PubMed] [Google Scholar]
- Wang M., Guo Z. & Wang S. Cystathionine gamma-lyase expression is regulated by exogenous hydrogen peroxide in the mammalian cells. Gene expression 15, 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido Y. et al. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. Faseb Journal 19, 1854-+ (2005). [DOI] [PubMed] [Google Scholar]
- Miyamoto R., Otsuguro K.-I., Yamaguchi S. & Ito S. Neuronal regulation of expression of hydrogen sulfide-producing enzyme cystathionine beta-synthase in rat spinal cord astrocytes. Neuroscience research 97, 52–59 (2015). [DOI] [PubMed] [Google Scholar]
- Miyamoto R., Otsuguro K.-i., Yamaguchi S. & Ito S. Contribution of cysteine aminotransferase and mercaptopyruvate sulfurtransferase to hydrogen sulfide production in peripheral neurons. Journal of Neurochemistry 130, 29–40 (2014). [DOI] [PubMed] [Google Scholar]
- Levonen A., Lapatto R., Saksela M. & Raivio K. Human cystathionine gamma lyase: developmental and in vitro expression of two isoforms. Biochemical Journal 347, 5 (2000). [PMC free article] [PubMed] [Google Scholar]
- Kraus J. et al. Cystathionine gamma lyase: clinical, metabolic, genetic and structural studies. Molecular genetics and metabolism 97, 10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. et al. Ferroptosis: An iron-dependent form of non-apoptotic cell death. Cell 149, 13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotecnol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde A. & Bhatia M. Hydrogen sulfide in inflammation: friend or foe? Inflammatory and allergy drug targets 10, 5 (2011). [DOI] [PubMed] [Google Scholar]
- Du C. et al. Downregulation of cystathionine beta-synthase/hydrogen sulfide contributes to rotenone-induced microglia polarization toward M1 type. Biochemical and Biophysical Research Communications 451, 239–245 (2014). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Dysregulation of CSE/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cellular Signalling 25, 8 (2013). [DOI] [PubMed] [Google Scholar]
- Lee S. et al. Hydrogen sulphide regulates calcium homeostatsis in microglial cells. Glia 54, 9 (2006). [DOI] [PubMed] [Google Scholar]
- Rogers J., Mastroeni D., Leonard B., Joyce J. & Grover A. Neurofinlammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder? International review of neurobiology 82, 12 (2007). [DOI] [PubMed] [Google Scholar]
- Kida K. et al. Inhaled Hydrogen Sulfide Prevents Neurodegeneration and Movement Disorder in a Mouse Model of Parkinson’s Disease. Antioxidants & Redox Signaling 15, 343–352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. et al. Cystathionine gamma-lyase deficiency mediates neurodegeneratio in Huntington’s disease. Nature 509, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levard C., Hotze E. M., Lowry G. V. & Brown G. E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environmental Science & Technology 46, 6900–6914 (2012). [DOI] [PubMed] [Google Scholar]
- Righi M. et al. Monokine production by microglial cell clones. European journal of immunology 19, 1443–1448 (1989). [DOI] [PubMed] [Google Scholar]
- Bulgarelli I. et al. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils. Journal of neuroscience research 87, 2718–2727 (2009). [DOI] [PubMed] [Google Scholar]
- Bureau G., Longpre F. & Martinoli M. G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. Journal of neuroscience research 86, 403–410 (2008). [DOI] [PubMed] [Google Scholar]
- Chang L. C. et al. Inhibition of nitric oxide production by the carbazole compound LCY-2-CHO via blockade of activator protein-1 and CCAAT/enhancer-binding protein activation in microglia. Biochemical pharmacology 76, 507–519 (2008). [DOI] [PubMed] [Google Scholar]
- Kaul S., Kanthasamy A., Kitazawa M., anantharam V. & Kanthasamy A. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates MPP+ -induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration.. European journal of neuroscience 18, 15 (2003). [DOI] [PubMed] [Google Scholar]
- Anantharam V., Kitazawa M., Latchoumycandane C., Kanthasamy A. & Kanthasamy A. Blockade of PKC delta proteolytic activation by loss of function mutants rescues mesencephalic dopaminergic neurons from methylcyclopentadienyl manganase tricarbonyl (MMT)-induced apoptotic cell death. Annals of the New York academy of sciences 1035, 19 (2004). [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C., Anantharam V., Jin H., Kanthasamy A. & Kanthasamy A. Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCdelta in cell culture and animal models of Parkinson’s disease. Toxicology and applied pharmacology 256, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. et al. Avoiding artefacts during electron microscopy of silver nanomaterials exposed to biological environments. Journal of microscopy 261, 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K., Espey M. & Wink D. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5, 10 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.