Abstract
The Chronic Care Model (CCM) is a promising framework for improving population health, but little is known regarding the long-term impact of scalable, informatics-enabled interventions based on this model. To address this challenge, this study evaluated the long-term impact of implementing a scalable, electronic health record (EHR)- enabled, and CCM-based population health program to replace a labor-intensive legacy program in 18 primary care practices. Interventions included point-of-care decision support, quality reporting, team-based care, patient engagement, and provider education. Among 6,768 patients with diabetes receiving care over 4 years, hemoglobin A1c levels remained stable during the 2-year pre-intervention and post-intervention periods (0.03% and 0% increases, respectively), compared to a 0.42% increase expected based on A1c progression observed in the United Kingdom Prospective Diabetes Study long-term outcomes cohort. The results indicate that an EHR-enabled, team- based, and scalable population health strategy based on the CCM may be effective and efficient for managing population health.
Introduction
Chronic diseases such as diabetes mellitus are the leading cause of death in the United States,1 with almost half of all adults having at least one chronic illness.2 Therefore, there is an urgent need to identify and deploy effective and scalable population health strategies for chronic disease. The Chronic Care Model (CCM) is a promising approach for population health management that encompasses six elements designed to make the care of chronic conditions proactive, planned, and population-based rather than acute and reactive: delivery system design, clinical information systems, decision support, organizational support, self-management support, and community interaction.3,4
The CCM has been generally shown to be effective for improving process measures in published studies, but the CCM has not been found to be uniformly effective for improving clinical outcome measures.5 Similar promising, but non-uniform, results have been reported when various components of the CCM were implemented in various combinations.5 Moreover, under traditional fee-for-service payment models, the financial benefits of improved population health can accrue to insurers, while the costs of implementing improved population health management generally fall on providers.5 Therefore, cost-effective, scalable approaches are needed that do not require significant additional resources. For example, while Piatt et al. demonstrated that a CCM-based intervention can significantly improve process and outcome measures for diabetes management, this intervention required resources that would not be generally available to primary care practices, such as chart auditors to provide performance feedback reports and on-site diabetes educators supported by the investigators.6 Moreover, the increasing shift from “volume-based” to “value-based” payment models7 is making it imperative for health care providers to efficiently improve the health of their patient populations. To succeed in this new environment, health care providers must develop population health strategies that deliver greater value through improved outcomes and lower costs.
Given the promising, but non-uniform, results from previous evaluations of the CCM, as well as the need for further evaluations of pragmatic, scalable population health strategies based on this model, the present study was conducted. We evaluated a CCM-based population health program that was in operational clinical use at 18 primary care practices of the Duke University Health System from January 2009 until June 2011, when a new electronic health record (EHR) system was implemented. While the population health program encompassed several chronic conditions as well as preventive care, this study was focused on diabetes due to its inclusion in the population health program from the initial program deployment and the availability of a widely accepted physiological measure of disease control in the form of hemoglobin A1c (A1C) levels. A1C levels reflect average blood glucose levels over a three month period8 and are strongly correlated with adverse outcomes.9,10
Because diabetes is a progressive condition characterized by worsening insulin resistance, A1C levels typically increase over time despite treatment. For example, a literature review of the expected progression of type 2 diabetes found that A1C levels increase by an average of as much as 0.5% per year (absolute change) even with therapy.11 While such disease progression is managed through therapy intensification,12 including the addition of new therapeutic classes such as insulin, A1C levels are still generally expected to increase over time. For example, based on long-term data from the United Kingdom Prospective Diabetes Study (UKPDS), and as incorporated into the UKPDS Outcomes Model,13 an individual starting with an A1C of 7% is expected to experience an A1C increase of approximately 1.5% per year (relative change) despite therapy. Thus, while reducing A1C levels (e.g., to < 8% or < 7%)12 is an important clinical goal, maintaining or slowing the increase in A1C levels is also an important goal.
Prior to the introduction of the EHR-enabled, team-based, and scalable population health strategy in this study, a labor-intensive, disease registry-based approach had been in place for diabetes management at the study sites. The objective of this 4-year longitudinal study was to evaluate whether the more efficient and EHR-based population health strategy could maintain or potentially surpass the glycemic control achieved under the previous, laborintensive approach. A before-after study design was used in conjunction with an interrupted time series analysis.14
Methods
Study Site and Participants. The study was conducted at 18 primary care practices of Duke Primary Care (DPC), which is a community-based primary care system affiliated with the Duke University Health System and centered in Durham, North Carolina. DPC serves a seven-county area with internal medicine, family medicine, pediatrics, and urgent care services. DPC is comparable to a community primary care system, with no residents and some teaching provided for medical students, physician assistant students, and nurse practitioner students. During the intervention period, DPC was staffed by over 100 physicians and 15-20 physician assistants and nurse practitioners.
Study participants were adult patients with diabetes mellitus types I and II who were patients of DPC during the study period (2007-2010). Detailed inclusion criteria are as follows: (i) at least 18 years old as of January 1, 2007; (ii) had at least one ICD9CM diagnosis for diabetes as defined by the National Quality Forum15 in the first historical control year (2007) and also at least one diabetes diagnosis in the year prior (2006)16; and (iii) were seen at least once in the study practices in each of the four study years, as well as in the year prior (2006). The ICD9CM diagnosis codes used to identify diabetes were 250, 250.X, 250.XX, 357.2, 362.0, 362.0X, 366.41, 648.0, 648.00, and 648.0X. The study was approved by the Duke University Institutional Review Board (protocol # 00042607).
Study Period. The study period was January 2007 through December 2010, with the intervention rolled out across the study sites in January 2009. The study period was ended in 2010 because the EHR system was changed in 2011.
Interventions. The intervention was a Chronic Care Model (CCM)-based population health program for diabetes. This multi-faceted intervention included the use of an EHR system for pre-visit planning, the implementation of a point-of-care clinical decision support (CDS) system, the implementation of a quality reporting system, team-based care, active engagement of patients, and provider education. Care team development and patient engagement were integral to the intervention. The care team consisted of clinicians (physicians, physician assistants, and nurse practitioners), as well as certified medical assistants, licensed practical nurses, and registered nurses. Patient selfmanagement was supported through pre-visit planning and education from clinical support staff. The population health program was piloted on a small scale at the end of 2008 and rolled out across all sites in January 2009.
In order to promote external generalizability and scalability, the population health program was designed and implemented in a manner that could potentially be replicated widely. In particular, the need for labor-intensive tasks such as manual data entry was minimized, so that the approach could be sustained in typical clinical settings with existing clinical staff. Moreover, the point-of-care CDS system and quality reporting system were implemented using a software module that can be integrated with various EHR systems through a standard Health Level 7 (HL7) Web service interface.17 Consequently, the informatics approaches leveraged in this study could potentially be deployed to other institutions and information system environments.
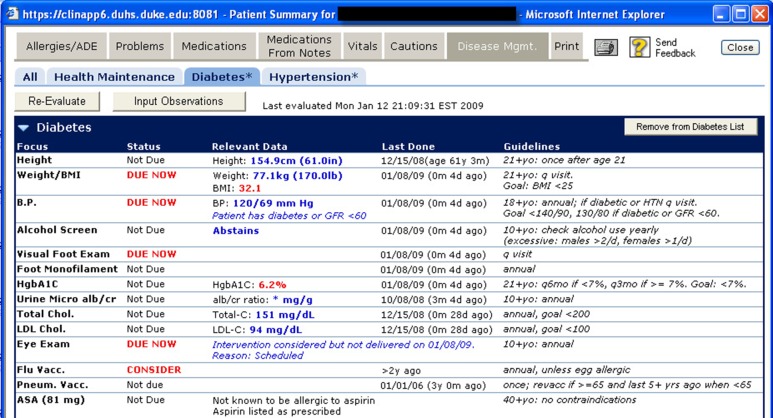
The details of the CDS system have been previously described.18 Briefly, the CDS system was available as a link within the EHR system and provided a dashboard for managing diabetes, including care metrics (e.g., appropriate laboratory monitoring), the patient’s current status on the metrics (e.g., not due, due now, almost due), relevant data (e.g., date and value of most recent laboratory test), and a summary of relevant guideline recommendations (Figures 1). This CDS system initially supported diabetes, hypertension, and health maintenance, and it was later enhanced to support other conditions including congestive heart failure, asthma, and chronic kidney disease. Diabetes care recommendations were based on American Diabetes Association guidelines.12 As noted earlier, this study was focused on diabetes a priori due to its inclusion in the population health program from initial deployment and the availability of a widely accepted physiological measure of disease control (A1C levels).
Figure 1.
Screenshot of point-of-care CDS system.
CDS recommendations could be obtained in a disease-specific manner (e.g., using the “Diabetes” tab in Figures 1), or for all supported conditions at once by selecting the “All” tab. This point-of-care CDS system was designed to align with the findings from a previous systematic review of CDS interventions, which found that computer-based CDS interventions that provided actionable recommendations automatically and at the appropriate point in clinical workflow significantly improved clinical practice in over 90% of randomized controlled trials.19
In a typical clinic visit, clinical staff used the CDS system to generate the care reminders, which were printed and provided to clinicians for use during the encounters. This approach worked well due to the routine use of a visit billing form to which the printed reminders were attached. Alternatively, clinicians retrieved the care reminders directly through the EHR. Furthermore, standing orders were in place for clinic staff to perform required interventions per protocol and as identified by these tools. For example, if the CDS system identified that a patient was in need of an A1C test, nurses were empowered to obtain the required test. This team-based care involved the use of existing clinical staff (i.e., clinicians, nurses, and medical assistants), rather than the addition of care providers who would generally not be available in a typical primary care practice (e.g., dedicated dietician, pharmacist, etc.). Most data points (e.g., laboratory test results, blood pressure values, vaccinations, eye exams) were retrieved from the EHR and did not require additional data entry. Clinic staff facilitated any supplemental data updates (e.g., to document that an eye exam was not indicated because of blindness), and the clinic staff also facilitated the completion of needed care interventions. Clinicians engaged patients through discussion of the care reminder summary and needed interventions.
The quality reporting system (Figures 2) provided the clinic managers and designated nurses of each clinic with monthly reports on performance measures, which were then used to recall patients to obtain necessary care. The goal of this intervention component was to identify patients who were outliers in relevant physiological measures (e.g., A1C levels), so that designated nurses could increase patient engagement in their care, identify and seek to address barriers, and schedule follow-up appointments. The quality reporting system was included in the intervention based on a previous randomized controlled trial that showed that adding a clinician feedback component increased the effectiveness of a CDS system.20 Implementation of this approach was challenging, however, due to the labor-intensive nature of the required follow-up and difficulty in reaching some patients.
Figure 2.
Quality reporting system with views for clinic group (top), clinic (middle), and clinician (bottom).
Pre-Intervention Population Health Management Approach. During the historical control period, usual care was provided using a labor-intensive approach centered around the use of a commercial disease registry tool known as DocSite. In our implementation, DocSite was a stand-alone disease registry separate from the EHR, with data imports available only for demographic data. Clinical staff were required to manually enter all other data (e.g., blood pressure values, immunization records, and laboratory values). In a typical clinical visit, a visit planner similar to that in Figures 1 was printed, which included information on what was due and what was done previously. A medical assistant would fill out information on needed data points (e.g., relevant laboratory results), and clinical staff would transcribe that data into the registry. The main differences of the study intervention compared to the historical control were (i) little or no manual data entry was required for the intervention, versus significant manual data entry required for the historical approach; (ii) integration of the population health management system with the EHR; and (iii) the availability of more sophisticated algorithms in some cases, for example for vaccination logic.
From an end-user perspective, the functionality of the DocSite system was quite similar to the study intervention except for the extensive need for manual data entry. Specifically, the DocSite system provided clinicians with diabetes care recommendations during clinic visits and outlier reports were available for identifying patients in need of targeted follow-up. Thus, even a demonstration of non-inferiority would show value, as the intervention significantly reduced the labor resources required for managing the health of patients with diabetes.
Evaluation Measures. The primary evaluation measure was the change in A1C levels among study participants during the intervention period compared to the historical control period. A potentially important confounder in the use of clinically measured A1C levels is that diabetes care guidelines recommend that A1C levels be measured more frequently among individuals with higher A1C levels.12 Pursuant to these guidelines, for example, the point-of-care CDS system reminded clinicians to test for A1C levels every 3 months if the last A1C level was >= 7%, and only every 6 months if the last level was < 7% (Figures 1). In order to address this potential source of confounding, as well as to reduce the impact of any random measurement errors, we estimated the A1C level of each patient on the 15th day of each month. If the patient had a test on the 15th, that value was used. Otherwise, the value was estimated through the interpolation of the nearest values (i.e., the latest test result from before the 15th and the earliest test result from after that date). This approach ensured that all patients had an equal number of A1C level estimates (48 values, one for each month of the four year study period).
A secondary study measure was the proportion of encounters at study clinics by study participants which resulted in use of the CDS system for diabetes. The system was considered to have been used for the encounter if system use logs indicated that the diabetes module of the CDS system had been activated for the patient on the day of the encounter or up to three days prior to the encounter in preparation for the visit.
Estimate of Expected A1C Progression. We estimated the expected progression of average A1C levels in the study population over the study timeframe using an A1C progression equation in the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model© (https://www.dtu.ox.ac.uk/outcomesmodel/)13. This equation was developed based on the UKPDS long term outcomes data, with patient level A1C data from our study cohort used in the model as the input for time zero A1C. We did not use this estimate for hypothesis-testing purposes, but we used it to contextualize our findings in the absence of a comparison group.
Analysis Approach. The study cohort was identified using a guided database query tool known as DEDUCE, which provides a Web-based interface for identifying patient cohorts and for obtaining approved data from the Duke University Health System’s data warehouse.21 Most study data were obtained through this tool.21 Data on the utilization of the point-of-care CDS system was obtained from the Duke Clinical Data Repository.
A software program was developed in Java to prepare the data in the format required for statistical analyses. Statistical analyses were conducted using SAS Enterprise Guide Version 4.3 (SAS Institute, Inc., Cary, NC, USA). Usage rates for the point-of-care CDS system were summarized graphically and using descriptive statistics. To estimate the changes in A1C levels during the pre- and post-intervention periods, a mixed linear model analysis was performed using the PROC MIXED procedure with maximum likelihood estimation. In the analysis, the patient was the class, the number of years since the beginning of the evaluation period was the predictor, and the A1C level was the dependent variable. To evaluate whether the changes in A1C levels in the intervention and pre-intervention periods were different, the PROC MIXED procedure was used with maximum likelihood estimation, with the patient as the class and with the years before or after January 2009 as the predictors. For all analyses, indicator variables for the months of the year were included in the models to adjust for seasonal variation,22 and sensitivity analyses were conducted omitting these variables. To the best of the investigators’ knowledge, no other population health management or clinical practice changes believed to have influenced diabetes care and outcomes were undertaken during the study timeframe.
The sample was restricted by the number of patients meeting inclusion criteria seen at the 18 primary care clinics. With a sample of 6,768 subjects, for the primary measure, we estimated a power of >99% to detect an improvement in A1C levels during the intervention period at least 0.5% greater than the change during the control period. These calculations were based on a one sample t-test with a 2-sided type I error rate of 0.05.
Results
There were 6,768 individuals with diabetes mellitus who received care at the study sites, met the study inclusion criteria, and were included in the analysis. All patients meeting inclusion criteria were included. These individuals had an average of 8.7 distinct A1C test results on record during the four year study period. The average A1C level for this population during the study period was 7.2%, with a standard deviation of 1.4%.
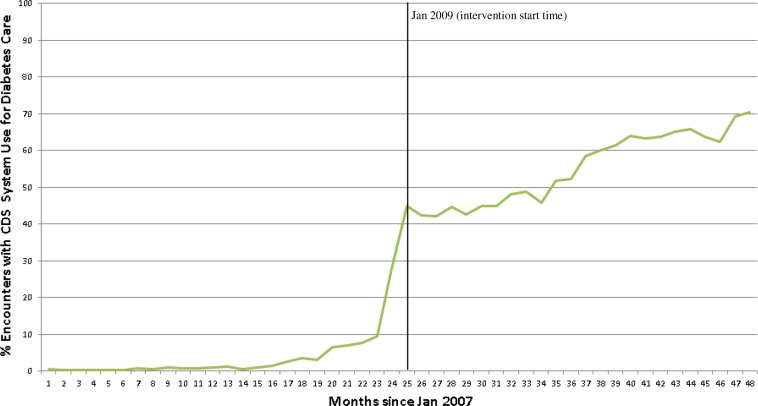
Usage of the Point-of-Care CDS System. Figures 3 shows the usage of the point-of-care CDS system for the study population for diabetes care. As noted, prior to the intervention start date (January 2009), there was limited usage of the CDS system for diabetes care for several months due to pilot use of the system by some sites. To roll out the population health intervention, the Duke Primary Care leadership notified each clinic of the switch-over from the manual data entry-driven approach to the more automated intervention approach, and basic on-site education and training were provided at each practice by clinic staff. Immediately following the start of the intervention period, usage rates increased to approximately 45%, and steadily increased to approximately 70% by the end of the study. Stratified at the clinic level, the highest usage rate in the final month of the study was 88% at one clinic; 5 clinics had usage rates ≥ 80%; 6 clinics had usage rates ≥ 70% and < 80%; 3 clinics had usage rates ≥ 60% and < 70%; and 4 clinics had usage rates < 60%. Usage therefore was high but not universal. During the intervention, usage of the tool was not monitored at an individual provider level. While we did not measure the time required to use the system during this intervention, there was significant manual work that was required for data entry in the preintervention period. Based on expert opinion (JBA, Duke Primary Care Chief Medical Officer), we estimate that in the post-intervention period there was a savings of at least an hour a day for the clinical support staff at each clinic, depending on the size of the practice and number of providers.
Figure 3.
% of encounters at study sites by study participants with CDS system use for diabetes care. Month 25 = January 2009.
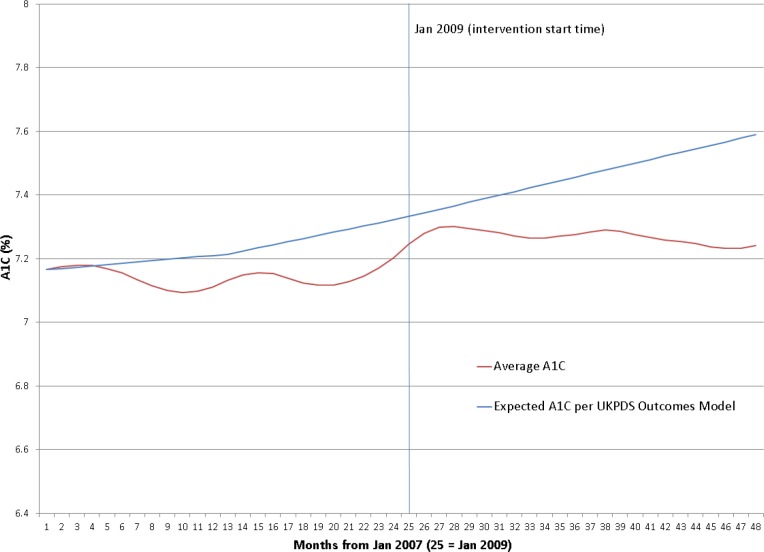
Hemoglobin A1c Levels. Figures 4 provides an overview of the study population’s A1C levels during the historical control and intervention periods. The A1C levels remained relatively unchanged during both the control and intervention periods. During the pre-intervention period, the A1C started at 7.17% in January 2007 and ended at 7.20% in December 2008. During the intervention period, the average A1C level remained essentially unchanged, starting and ending at 7.24%. Included in Figures 4 is the expected A1C progression from the UKPDS long term outcomes study,13 with an expected A1C level of 7.59% (0.42% absolute increase) by December 2010.
Figure 4.
Average A1C levels over time for study population (2007-2010)
Table 1 provides the model for the A1C level during the pre-intervention period, and Table 2 provides the model for the A1C level during the intervention period. As noted, the slope for the A1C level was 0.01%/year for the preintervention period and -0.02%/year for the intervention period. This difference in slope was statistically significant (p = 0.01). However, we do not consider this difference in slope to be clinically significant.
Table 1.
Model for A1C level during pre-intervention period (2007-2008)
| Solution for Fixed Effects Standard | |||
|---|---|---|---|
| Effect | Estimate | Error | P-value |
| Intercept | 7.1494 | 0.01773 | <.0001 |
| yearSinceJan2007 | 0.005152 | 0.003045 | 0.0907 |
| Jan | -0.00323 | 0.007964 | 0.6855 |
| Feb | 0.009910 | 0.007879 | 0.2084 |
| Mar | 0.01454 | 0.007801 | 0.0624 |
| Apr | 0.01267 | 0.007730 | 0.1013 |
| May | -0.00084 | 0.007668 | 0.9131 |
| Jun | -0.01552 | 0.007613 | 0.0415 |
| Jul | -0.02936 | 0.007566 | 0.0001 |
| Aug | -0.03907 | 0.007528 | <.0001 |
| Sep | -0.04232 | 0.007498 | <.0001 |
| Oct | -0.03694 | 0.007476 | <.0001 |
| Nov | -0.02196 | 0.007463 | 0.0033 |
| Dec | 0 | . | . |
Table 2.
Model for A1C level during intervention period (2009-2010)
| Solution for Fixed Effects Standard | |||
|---|---|---|---|
| Effect | Estimate | Error | P-value |
| Intercept | 7.2869 | 0.01703 | <.0001 |
| yearSinceJan2009 | -0.01916 | 0.002727 | <.0001 |
| Jan | -0.01099 | 0.007131 | 0.1232 |
| Feb | 0.01128 | 0.007055 | 0.1098 |
| Mar | 0.01992 | 0.006985 | 0.0044 |
| Apr | 0.01734 | 0.006922 | 0.0122 |
| May | 0.01172 | 0.006866 | 0.0879 |
| Jun | 0.005451 | 0.006817 | 0.4239 |
| Jul | 0.001153 | 0.006775 | 0.8649 |
| Aug | -0.00537 | 0.006740 | 0.4257 |
| Sep | -0.01145 | 0.006714 | 0.0882 |
| Oct | -0.01272 | 0.006694 | 0.0575 |
| Nov | -0.00885 | 0.006683 | 0.1855 |
| Dec | 0 | . | . |
The intercept was higher in the intervention period compared to the pre-intervention period. However, the overall trend in A1C was essentially flat throughout the study period, starting at 7.17% in January 2007, going to 7.24% in January 2009 (the first month of the intervention), and ending at 7.24% in December 2010.
Sensitivity analyses were also conducted in which indicator variables for the months of the year were removed. These sensitivity analyses without adjustment for seasonal variation resulted in similar findings as the primary analyses with seasonality adjustment.
Discussion
Summary of Findings. In this study, a pragmatic, scalable, and CCM-based population health strategy for diabetes care was evaluated in 18 primary care clinics using a before-after study design with an interrupted time series analysis. A key component of the intervention, the point-of-care CDS system, was well accepted, with usage rates rising to approximately 70% of primary care encounters by study participants by the end of the intervention period. Potential reasons for non-universal system use may have included chronic disease care being of secondary concern during clinic visits for acute medical conditions, as well as individual providers making limited use of the tool. Given a usage rate of 88% in one clinic by the end of the study period, and a rate exceeding 80% in five clinics, a system usage target of ≥ 80%, or perhaps even ≥ 90%, appears to be an attainable goal for this type of CDS tool in primary care.
After adjusting for seasonality, the rate of change in A1C levels during the intervention period was -0.02%/year, as compared to 0.01%/year during the historical control period (p = 0.01). However, we do not deem this difference to be clinically significant. Overall, the A1C levels remained remarkably stable during both the pre-intervention period (increasing from 7.17% in January 2007 to 7.20% in December 2008) as well as the intervention period (unchanged, at 7.24% in both January 2009 and December 2010). The overall change in A1C levels over the course of the entire study period, encompassing both the pre-intervention and intervention periods, was 0.07% (7.17% to 7.24%). This change was only 17% of the 0.42% increase expected based on the UKPDS long term outcomes data and the baseline A1C levels. Thus, the study results suggest that both labor-intensive, disease registry-based population health approaches as well as an EHR-enabled, CCM-based population health program are capable of slowing A1C progression and presumably the associated complications. As noted in the descriptions of these two population health approaches, however, the EHR-facilitated population health program required much less time and resources to operate compared to the stand-alone disease registry-based approach.
In summary, this study contributes insights on the long-term clinical outcomes of an EHR-enabled, team-based, and scalable CCM approach to population health management. Additionally, this study provides validation for the enterprise-level use of standards-based CDS Web services to enable population health management, including for point-of-care CDS and feedback reporting.17
Limitations and Strengths of Approach. A limitation of our study is the lack of a concurrent control group, with its associated potential for confounding and the difficulty of definitively attributing the patient outcomes to the study intervention. While clinical leaders were not aware of any other major interventions or changes in clinical practice related to diabetes care during the study period, other confounding factors may have impacted the A1C trends that we observed. To contextualize our findings in the absence of a control group, we estimated A1C progression in the study population using the A1C equation embedded in the UKPDS Outcomes Model. We recognize that this equation is based on the UKPDS long-term outcomes population and has not been validated in a US cohort.
However, we are not aware of published data on long-term A1C progression in patients treated per usual care in the US that we could have used to estimate A1C progression. Another limitation of our approach is the inability to distinguish between the impacts of different components of our multi-faceted intervention. Our inclusion criteria also required patients to be seen at least once a year at the study sites during the study period; therefore, our findings may not be generalizable to patients with diabetes who do not consistently receive primary care. As another limitation, our estimate of time savings from the intervention is based on expert opinion rather than explicit measurement. Also, due to our focus on improving clinical outcomes, we did not evaluate for process measures (e.g., guideline compliance rates), which could have provided more insights into the mechanisms underlying the observed clinical outcomes. Finally, we used A1C results collected as a part of the routine care process, rather than A1C results collected specifically for the study, and at set time points. However, to account for this final limitation, we used interpolation to ensure that all patients had the same number of observations to contribute to the analysis.
A strength is the use of a large sample size, which increases the precision of our findings. In addition, our evaluation encompasses four years of data, which allows for a long-term assessment of the impact of the population health strategies under study. Third, the use of a historical control period provides some level of protection against confounding. Fourth, our system and analyses are population-based and not limited to a subset of poorly controlled patients who might experience substantial A1C improvement with disease management. Finally, the intervention evaluated was pragmatic and scalable, with clear benefits compared to a labor-intensive approach to population health management.
Implications and Future Directions. Overall glycemic control in the study population was quite good both before and after the intervention, with average A1C levels very close to 7%, which is the target goal for many individuals with diabetes.12 Moreover, A1C levels increased by only 0.07% during the entire evaluation period and remained unchanged during the intervention period, compared to an expected increase of 0.42%. Thus, an important implication of this study is that a team-based, EHR-enabled population health program based on the CCM is capable of effectively managing a population of patients with diabetes. Key components of this population health strategy include the empowerment of care team members, e.g., to order needed laboratory tests by protocol; standard care protocols that harness the efforts of the entire care team, such as the removal of shoes by patients with diabetes during the rooming process to facilitate recommended foot exams; and providing feedback to clinicians to reaffirm the importance of their population health management efforts. While our study design was not capable of teasing out the impact of these various facets of the intervention, our empiric observation is that each of these various facets of the intervention played an important role in the overall effectiveness of the population health program.
Another important implication of our study is that an automated approach to population health management can be just as effective as a highly labor-intensive approach. Indeed, by leveraging the data captured in EHR systems as a part of routine clinical care, an EHR-enabled population health program may be pursued in a highly automated fashion with minimal need for duplicate, manual data entry.
Looking into the future, we anticipate that the increasing adoption of EHR systems,23 combined with increasing financial pressures to more efficiently and effectively manage chronic conditions,24 will result in continually greater use of EHR-facilitated, team-oriented, and CCM-based approaches to improving population health. This study contributes to this increasingly important area of biomedical informatics research - the leveraging of information technology to more efficiently manage chronic conditions and improve population health. In the future, we anticipate that many more studies will be conducted by ourselves and others to evaluate how best to improve population health using these technology-enabled and team-based approaches.
Conclusion
An EHR-enabled, team-based, and scalable population health strategy based on the CCM was associated with limited A1C progression among patients receiving outpatient diabetes care. These benefits were comparable to the limited A1C progression associated with a labor-intensive population health management approach requiring extensive manual data entry.
Acknowledgements
The intervention implementation was supported in part by NIH grants R41 LM009051 and R42 LM009051. The authors would like to thank the individuals who contributed to the study, including David E. Shields.
KK is or has been a consultant on CDS for the U.S. Office of the National Coordinator for Health IT, ARUP Laboratories, McKesson InterQual, ESAC, Inc., JBS International, Inc., Inflexxion, Inc., Intelligent Automation, Inc., Partners HealthCare, Mayo Clinic, and the RAND Corporation. KK receives royalties for a Duke University-owned CDS technology for infectious disease management known as CustomID that he helped develop. KK was formerly a consultant for Religent, Inc. and a co-owner and consultant for Clinica Software, Inc., both of which provide commercial CDS services, including through use of a CDS technology known as SEBASTIAN that KK developed, and which was used by the point-of-care CDS system described in this manuscript. KK no longer has a financial relationship with either Religent or Clinica Software. KK also has received grant support from Hitachi. KK has no competing interest with any specific product or intervention evaluated in this manuscript. HB receives grant funding from Takeda, Johnson & Johnson, Improved Patient Outcome, MeadWestVaco and Sanofi. HB also has received consulting funds from Sanofi, Regeron, CVS, Walgreens, and Blue Cross/Blue Shield of Arkansas. HB has no competing interest with any specific product or intervention evaluated in this manuscript. The other authors have no potential competing interests to declare.
References
- 1.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008 Apr 24;56(10):1–120. [PubMed] [Google Scholar]
- 2.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Preventing chronic disease. 2014;11(E62) doi: 10.5888/pcd11.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002 Oct 16;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 4.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002 Oct 9;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 5.Coleman K, Austin BT, Brach C, Wagner EH. 1. Vol. 28. Health Aff (Millwood); 2009. Jan-Feb. Evidence on the Chronic Care Model in the new millennium. pp. 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piatt GA, Orchard TJ, Emerson S, et al. Translating the chronic care model into the community: results from a randomized controlled trial of a multifaceted diabetes care intervention. Diabetes Care. 2006 Apr;29(4):811–7. doi: 10.2337/diacare.29.04.06.dc05-1785. [DOI] [PubMed] [Google Scholar]
- 7.Burwell SM. Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015 Mar 5;372(10):897–9. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 8.US National Library of Medicine. A1C test. Available from: https://www.nlm.nih.gov/medlineplus/ency/article/003640.htm. [DOI] [PubMed]
- 9.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study.; Diabetes Care; 2010. May, pp. 1090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000 Aug 12;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009 Nov;(32 Suppl 2):S151–6. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009. Jan, pp. S13–61. [DOI] [PMC free article] [PubMed]
- 13.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747–59. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002 Aug;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 15.National Quality Forum. Measure 0575 - diabetes HgbA1c control measure. Available from. http://www.Qualitvforum.org/Proiects/e-g/eMeasures/Electronic Quality Measures.aspx.
- 16.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what’s the optimal approach? Am J Med Qual. 2004 Sep-Oct;19(5):201–6. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto K, Lobach DF. Design, implementation, use, and preliminary evaluation of SEBASTIAN, a standards-based Web service for clinical decision support.; AMIA Annu Symp Proc.; 2005. pp. 380–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Lobach DF, Kawamoto K, Anstrom KJ, et al. Development, deployment and usability of a point-of-care decision support system for chronic disease management using the recently-approved HL7 Decision Support Service standard; Medinfo; 2007. pp. 861–5. [PubMed] [Google Scholar]
- 19.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765–8. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobach DF. Electronically distributed, computer-generated, individualized feedback enhances the use of a computerized practice guideline.; AMIA Annu Symp Proc.; 1996. pp. 493–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Horvath MM, Winfield S, Evans S, et al. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011 Apr;44(2):266–76. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng CL, Brimacombe M, Xie M, et al. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol. 2005 Mar 15;161(6):565–74. doi: 10.1093/aje/kwi071. [DOI] [PubMed] [Google Scholar]
- 23.DesRoches C. Progress and challenges in electronic health record adoption: findings from a national survey of physicians. Ann Intern Med. 2015 Mar 3;162(5) doi: 10.7326/L15-5060. [DOI] [PubMed] [Google Scholar]
- 24.Milani RV, Lavie CJ. Health care 2020 reengineering health care delivery to combat chronic disease. Am J Med. 2015 Apr;128(4):337–43. doi: 10.1016/j.amjmed.2014.10.047. [DOI] [PubMed] [Google Scholar]