Abstract
We are developing a new process of health information exchange supported collaboration that leverages the HL7 consolidated CDA standard through four document types (consultation note, history and physical, progress note and discharge summary). The focus of the present study is the C-CDA consultation note template that will be submitted from poison control centers (PCC)s to emergency departments (EDs) with treatment recommendations. Specifically, we aimed to (i) create computable mappings between a poison control center database and the C-CDA consultation note template; and (ii) assess the extent and nature of information types that successfully map to discrete data elements in a poison control center database. The resulting template and mappings can be used to implement standards-based health information exchange between PCCs and EDs in the U.S. This is a part of the first formal effort to leverage health information exchange standards to support PCC-ED collaboration.
Introduction
Poison Control Centers (PCCs) are responsible for handling poisoning cases all over the U.S.1. PCCs receive telephone calls from both the general public and healthcare facilities2, especially emergency departments (EDs). EDs ask for the consultations from PCCs via an iterative telephone call process, that carries a lot of inefficiencies and vulnerabilities that may greatly affect patient safety and the provided healthcare quality3. Therefore, electronic health information exchange (HIE) between EDs and PCCs is a promising and complementary alternative to the telephone-based approach4. The overall goal of our research is to enable standards-based HIE between PCCs and EDs to improve the efficiency and quality of care for poisoning cases.
Most PCCs use information systems that allow PCC staff to record information about and keep track of active poisoning cases. Although PCC information systems allow the Specialists in Poisoning Information (SPIs) to capture detailed information about poisoning cases, current PCC systems do not support standards-compliant HIE or implement document standards, such as the Health Level Seven (HL7) Consolidated Clinical Document Architecture (C-CDA) 5. In addition, existing HIE standards provide no specific guidance to support PCC-ED information exchange. In previous studies, we developed a model for PCC-ED HIE, including: (i) identification and analysis of information types exchanged between PCCs and EDs via telephone calls6; and (ii) mappings between PCC information types and C-CDA documents, sufficient to support communication and information requirements in a poisoning care episode (i.e., referral request, consultation note, progress report, discharge summary) 5,6.
The focus of the present study is the C-CDA consultation note that will be transmitted from PCCs to EDs. This document contains essential patient information along with poisoning case information and initial treatment recommendations. Specifically, we aimed to (i) create computable mappings between data elements in the Utah PCC system, an instance of the widely used PCC information system toxiCall®7, and the C-CDA consultation note specifications; and (ii) assess the percentage of poisoning communication information types that could be successfully mapped to discrete data elements in toxiCall®6,7. The resulting mappings can be used to guide the implementation of standards-based HIE for PCCs and EDs across the US. This is a part of the first formal effort to leverage HIE standards to support PCC-ED communication process.
Background and Significance
PCCs and their information systems
There are 55 PCCs that serve all 50 U.S. states and territories, 24 hours per day and 7 days per week1. The PCC staff who consult with the public and ED care providers on poison exposures are the SPIs, typically licensed pharmacists or nurses, and medical or clinical toxicologists. The American Association of Poison Control Centers (AAPCC) owns and manages the National Poison Data System (NPDS), to which all U.S. PCCs regularly upload a set of standard data elements describing human poison exposure cases8. These data are used for epidemiology and surveillance. AAPCC produces an annual report on the aggregate statistics of NPDS data, in addition to providing specialized reports to national agencies1,9. A PCC uses one of four specialized information systems to support the tasks of data entry, retrieval and data transmission to the NPDS10.
PCC-ED health information exchange
Although the management of poisoning cases is an information and communication intensive process that can benefit from HIE, little has been done to enable HIE between PCCs and EDs. Previously, we identified important legal, operational, and clinical considerations in relation to PCC-ED HIE4. We also analyzed telephone conversations between poisoning specialists and ED staff to (i) identify inefficiencies and vulnerabilities3; and (ii) to identify and analyze the information requirements for ED-PCC collaboration6. In a current study funded by the Agency for Healthcare Research and Quality (AHRQ), our goal is “to develop, implement, and evaluate a replicable, scalable infrastructure for HIE supported ED-PCC collaboration”11. The project started in September 2013 and is expected to complete in September 2018.
PCC-ED HIE and the C-CDA standard
C-CDA is an HL7 messaging standard that specifies a common structure and content for electronic clinical documents, according to document type, e.g., history and physical note vs. discharge summary. The ultimate goal of the C-CDA standard is to make a document that is both human and machine readable by combining narrative blocks with coded entries12. Extensible Markup Language (XML) is used to represent the document content in a structured, computable way. Viewed through a styling sheet (i.e., XSL file), this XML file is formatted for human reading, without visible XML markups13.
Generally, a C-CDA document is divided into two main parts, the document header and the document body. The C-CDA header contains metadata about the healthcare encounter and information necessary for document housekeeping and management. Specifically, the C-CDA header includes patient demographics, document author(s), document recipient, document type, patient encounter, and document type. The document body represents the clinically relevant information, structured within a set of sections that are specific according to the C-CDA document type.
The C-CDA standard is an implementation guide that includes specifications for 13 C-CDA document types12. The specification provides a library of templates that can be used to support various HIE use cases. The detailed constraints on document and section levels are provided within the C-CDA Implementation Guide12. In a previous study, we linked our proposed PCC-ED information exchange events to four C-CDA document types; and mapped poisoning information types identified in previous work to specific C-CDA sections. The four identified C-CDA document types are Consultation, History & Physical, Progress Note and Discharge Summary5. The present study builds on our previous work by providing physical mappings between a PCC information system (toxiCall®) and the C-CDA Consultation Note document type, which is a necessary step towards the interoperability between PCC and ED information systems.
Methods
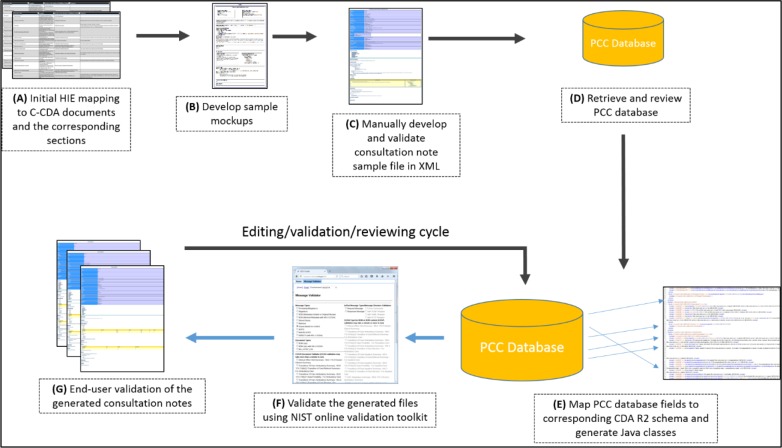
The approach used in this study consisted of three main steps. First, we developed consultation note mockups and sample documents containing the required information content, as determined in previous studies5,6. Second, we developed a computable mapping between the C-CDA schema (conforming to consultation note constrains) and the Utah PCC database, an instance of toxiCall®7. Last, we assessed the percentage and nature of information types that successfully mapped from the PCC database to the C-CDA. An overview of the steps and their sequence is depicted below (Figure 1) where each step is labelled by a letter and these letters are used in the following paragraphs.
Figure 1.
Mapping of PCC database fields to CDA R2, in the context of C-CDA consultation note development
Mockups and sample document
Mappings between the information types and consultation note sections were previously established (Step - A) 5. We elicited preferences for information content and presentation format from emergency medicine physicians at our collaborating sites. Based on the mappings and physician preferences, we developed two sample mockups using HotGloo prototyping tool (Step - B)14. The mockups were then validated by SPIs, ED clinicians and project members to ensure that the content of the consultation note meets their needs. We collaborated with these users to make iterative changes to the design, then manually developed a sample consultation note XML file, and validated that file according to the C-CDA R2.1 Implementation Guide and its schema (Step - C)12. This sample document was valuable as it identified the actual location of data within the document XML structure, especially for the document header part that was not represented within the initial mapping5.
PCC database and computational mapping
The Utah PCC provided us with access to a copy of their database, an instance of toxiCall®, filled with a small set of anonymized patient data. The data were anonymized by replacing all patient demographics, SPI related information and identifiers with fictitious information. Then, we identified tables that contain the required information for the C-CDA Consultation Note (Step - D); and created mappings between the PCC database and C-CDA Release 2.1 XML schema (Step - E)12, with the permission of Computer Automation Systems, Inc7. We created the mappings using Altova MapForce® version 2016, which allows mappings between different data sources and targets through a graphical user interface15. The mapping process consisted of “dragging and dropping” toxiCall® database fields to the corresponding elements and attributes in the C-CDA Release 2.1 XML schema. We used custom data transformations to map data from multiple source tables and columns to a single element within the XML structure and to represent them in a proper format.
Once the mapping was completed, we used Altova MapForce® to automatically generate consultation notes for the anonymized case records. We validated these instances against the C-CDA schema and the NIST online validation toolkit, (Step - F)16. Also, a human validation was done by the study co-authors and collaborators from the Utah PCC and EDs (Step - G). For human validation, we transformed the documents into HTML using the Extensible Stylesheet Language (XSL) style sheet provided with the C-CDA R2 Implementation Guide12. Once validated, we used Altova MapForce® to automatically generate source code for the mappings in the Java programming language. The Java code was then wrapped with a RESTful Web services layer to be integrated with other systems.
Extent of structural mapping
For comparison with previous efforts, we calculated the percentage of information types that successfully mapped to the C-CDA from the Utah PCC database. For this calculation, the information type was considered to be present when it was available in a discrete (non-narrative) form within the PCC database, and not present otherwise. For data found to be not present, we identified potential alternative sources such as poisoning knowledge bases and additional data entry fields to be collected by SPIs. Details on alternative sources are subject for a future study. The information content to be used in the C-CDA header was not considered in this analysis as the focus was on clinical information requirements represented in the C-CDA body.
Results
Mockups and sample document
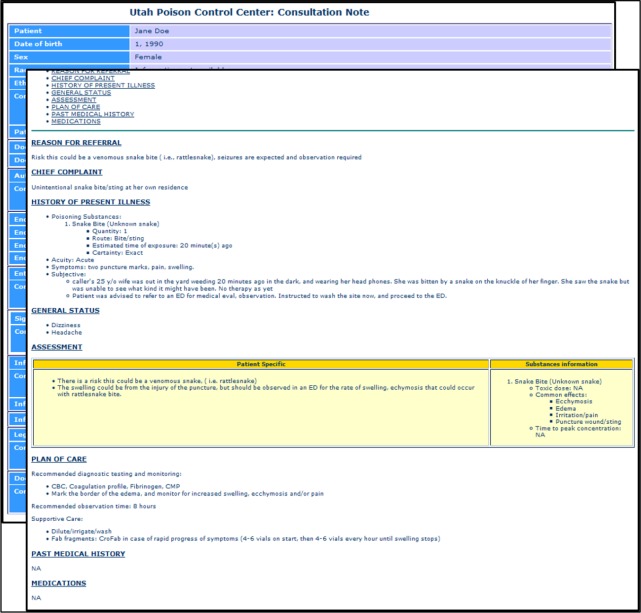
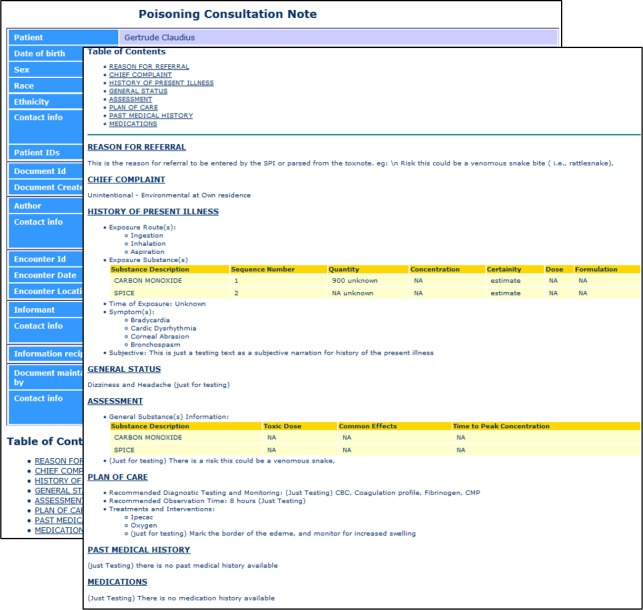
Figure 2 shows a sample mockup of a PCC consultation note for a poisoning exposure due to snake bite. This mock-up includes additional html formatting within sections, to implement ED user preferences related to information presentation. Figure 3 shows the manually developed XML consultation note, rendered in HTML format. The actual XML file is available at (https://github.com/alykhalifa/PCC-ED-C-CDA-Consultation-Notes). The header section contains metadata such as patient demographics, document author, intended recipient and encounter information (e.g., data and time of the call). The body contains 8 sections: (i) reason for referral; (ii) chief complaint; (iii) history of present illness; (iv) general status; (v) assessment; (vi) plan of care; (vii) past medical history; and (viii) medications. The history of present illness section contains information about the poisoning exposure such as poison information and observed symptoms. The assessment section consists of two main parts. The first part contains patient-specific information, while the second part contains general information about the poisoning exposure itself, such as its toxic dose, common effects and the time to peak concentration. The plan of care section consists of three main parts: (i) recommended diagnostic testing and monitoring; (ii) recommended observation time; and (iii) supportive care. The other sections contained standard information as defined in the C-CDA standard. The sample XML document was successfully validated with no errors using the C- CDA R2.1 schema and the NIST online validation toolkit16.
Figure 2.
Mockup of a consultation note for a poisoning due to snake bite.
Figure 3.
Sample C-CDA Consultation Note rendered in HTML format using the XSL style sheet provided with the C-CDA R2.1 standard specification.
PCC database and computational mappings
Mappings between the C-CDA standard and the Utah PCC database, an instance of toxiCall®” involved 8 tables and over 250 columns in the PCC database. Out of 38 information types needed to create a C-CDA compliant consultation note that supports poisoning communication, 21 (55%) successfully mapped to discrete fields within the PCC database. Seventeen information types did not successfully map. We defined over 130 constant variables within Altova Mapforce® to support required elements and attributes according to the Consultation Note C-CDA R2.1 constraints.
Table 1 shows the information types that successfully mapped, along with associated data transformations, where “D” means retrieval of code label from the code dictionary table, and “MC” means collection of required information from multiple columns in the PCC database. Table 2 shows the information types that did not successfully map. A snapshot of the auto-generated consultation note is shown on Figure 4. The validation of the generated documents against the C-CDA R2 schema and through the NIST online toolkit16 showed no errors or warnings, and was validated by domain experts, with only minor, iterative adjustments. The actual mapping file and the generated Java code are available upon request, with the permission of Computer Automation Systems, Inc., as they contain some information proprietary for toxiCall®7, while the generated sample consultation notes are available at (https://github.com/alykhalifa/PCC-ED-C-CDA-Consultation-Notes).
Table 1.
Information types that were successfully mapped to the Toxicall® database and their data transformations
| Information Type | Description (Transformations if present*) |
|---|---|
| Exposure information | |
| Exposure type | General description of the poisoning case, e.g., unintentional food poisoning (D) |
| Certainty of formulation | Certainty level of reported poison substance formulation (D) |
| Chronicity | Exposure duration and repetition of the poisoning incident (D) |
| Establishing background/certainty | More details about the poisoning case such as symptoms, routes of exposure and exposure site (D) |
| Substance class | General category of the poisonous substance/product, e.g., sedative (D) |
| Substance name (generic) | Generic name of the poisonous substance/product |
| Substance amount | Quantity and units for the substance amount and concentration (D, MC) |
| Substance name (brand) | Brand name of the poisonous substance/product |
| Substance description | More details about the poisonous substance |
| Substance form, formulation and type | Physical form of the poisonous substance, e.g., gas and tablet, and more details about pharmaceutical nature, e.g., sustained release (D) |
| Substance-non-pharmacological | Names and descriptions for non-pharmacological substances/products |
| Time since ingestion | Duration since the time of poison exposure till the documentation/reporting time |
| Subjective and objective information | |
| Chief complaint/reason for visit | Explaining what is the patient suffers from and she/he need to visit an ED (D) |
| Caller reported symptoms | Symptoms mentioned by the one who reported for a poisoning incident over a telephone call (MC) |
| Unrelated symptoms | Symptoms reported and deemed unrelated to the current poisoning incident |
| PCC recommendations and toxicology information | |
| PCC recommendations for treatment and discharge parameters | Therapeutic advice on how to handle the poisoning case, in terms of therapies, labs and patient monitoring time (MC) |
| ED treatment/management information | |
| Diagnostic test results | Results of diagnostic test reported to PCC and stored within its database (MC) |
| Time laboratory test was performed/drawn | Reported time of conducting lab tests |
| Treatment performed (or recommended by the PCC) | Differentiating between treatments that were recommended, performed or both (MC) |
D, retrieve code label from code dictionary table in PCC database; MC, retrieve values from multiple columns in the PCC database
Table 2.
Information types that were unsuccessfully mapped to the Toxicall® database.
| Information Type | Description |
|---|---|
| Exposure information | |
| Substance information | Pharmacology and pharmacokinetics information such as time to peak concentration and biological pathways |
| Substance identification rationale | Description of how the SPI identify the poisoning substance/product |
| Patient health history | |
| Medical history | Patient medical history that may be relevant to the poison exposure and treatment |
| Patient medications | Current and past medications of the patient |
| Subjective and objective information | |
| Absence of clinical effects | Absence of some of the expected symptoms for the poisoning incidence |
| Mental status | E.g., dizziness, hallucination… |
| Physical exam findings | Clinical findings reported to the PCC and stored within its database |
| Vital signs | Times and measures of reported vital signs |
| PCC recommendations and toxicology information | |
| Toxic dose | Toxicity threshold for the poisoning substance/product |
| Toxicity levels | Toxicity level of the poisoning substance for this specific poisoning incidence |
| Clinical effects of substance | Expected clinical symptoms for the poisoning substance/product |
| Worst case scenario | The risks and worst clinical effects that may occur in relation to the poisoning incidence |
| ED treatment/management information | |
| Confirmation that treatment was given | Indication if PCC recommendation were applied to the patient at the ED |
| Patient discharge medications | Discharge medications prescribed by the ED clinicians |
| Patient status | Patient status at the ED for certain date and time |
| Plan of care | Plan of care designed by ED clinicians |
| Time next laboratory tests will be ordered | Future laboratory tests timing as specified by ED clinicians |
Figure 4.
Auto generated Consultation Note rendered in HTML format using the XSL style sheet provided with the C-CDA R2.1 standard specification.
One of the challenges faced during the mapping process is that data for a single information type may be present over multiple tables and columns. For example, recommended therapies and interventions are scattered over 68 columns, where each column represents a single therapy/intervention that may be set to certain numeric values if recommended, or left as null otherwise. A user-defined transformation function was developed using Altova MapForce® to identify columns with numeric values, parse their column name (i.e., the treatment name), and instantiate a corresponding treatment instance in the C-CDA R2.1 format.
Discussion
In this study, we mapped a large proportion of the information content necessary to support poisoning communication from a PCC database to the HL7 C-CDA consultation note. The mappings are an important step towards enabling standards-based HIE between a proprietary system (i.e., toxiCall®) used by most PCCs in the US and various EHRs available at emergency departments. These mappings serve two main functions (i) identify existing data sources to automatically populate the consultation note, and (ii) identify informational gaps that need alternative input sources (e.g., data entry fields and /or a poison knowledge base). The Utah PCC information system (like most) was primarily designed to support the public health mission of poison control centers, namely case documentation and collection of NPDS data elements for the purpose of surveillance and epidemiology. PCC information systems are not designed for interoperability or communication with healthcare facilities. Therefore, much of the information needed by the ED clinicians is either orally conveyed through telephone calls and/or documented within a combined narrative field of the PCC database.
This clinically relevant information includes the expected common effects of the poison, time to peak concentration of the poison, more detailed plan of care, history of past illness, and past/current medications taken by the patient. Also, some information currently collected by this PCC lacks adequate detail of structure for the C-CDA. For example, patient age is stored within the PCC database, but the C-CDA requires date of birth. In anticipation of HIE, the Utah PCC has managed to solve these issues via workarounds. For example, date of birth is being collected in a generic PCC database field. However, a more standard approach to collecting information essential to HIE processes, such as date of birth, is preferred. Furthermore, some of the successfully mapped information types (e.g., clinical effects) may need to be presented in a more expressive form in order to be sufficiently meaningful for clinical use. In this case, some potential alternative sources (e.g., additional data entry fields and a poison knowledge base) may be used to provide this intended level of expressiveness, which is the same approach proposed for handling unsuccessfully mapped information types. As a solution, we are currently developing software that enables poison centers to create and send CCDAs using both template narrative data and coded data contained in the Utah PCC database. That software is currently under development and testing.
The PCC database consists almost entirely of NPDS data elements, data elements collected by all U.S. PCCs, and all of the database fields that successfully mapped to the C-CDA represent NPDS data elements. Given the public health oriented nature of PCC data, it is not surprising that many of the information types necessary to support communication and collaboration in the context of a poison exposure cannot be mapped. However, the capability to partially construct a C-CDA document using the PCC database greatly lowers the manual effort required for data entry on the part of PCC specialists. This would save a lot of the time needed by the SPI to generate the consultation note, and ensure its validity and accuracy, directly supporting rapid communication in these emergency situations and improving usability for the SPIs. Moreover, since the mappings correspond entirely to NPDS data elements, they may potentially be adapted for use by any U.S. poison control center. The Utah PCC database is a local instance of one vendor solution for poison control centers, toxiCall®. U.S. PCCs use one of four different information systems. However, all U.S. poison control center information systems collect NPDS data elements and support the same essential clinical workflow/domain, and so there is some de facto standardization and similarity among different poison control center information systems. The AAPCC, the owner of NPDS, specifies the required data elements and has the authority to add or modify data elements. However, the purpose of NPDS is to support surveillance and epidemiology, and AAPCC hasn’t yet addressed interoperability in support of direct patient care.
This study has three main strengths that support generalizability of the results. First, we involved the intended users of the consultation note during design, development, and validation. Second, we validated the generated consultation notes using both human (i.e., intended users) and machine approaches. Third, the PCC database is an instance of toxiCall®, a widely used system among U.S. PCCs, and all mappings involved NPDS data elements. All U.S. PCC systems are NPDS complaint, so the developed mapping can be implemented or adapted for implementation at most PCCs with minor adjustments, providing an important building block for a nationwide PCC-ED HIE. A limitation of the mapping is the absence of coded entries in the generated consultation note. We conducted the mapping using the CDA level 2 conformance12, which does not specify these coded entries, and therefore limits computation for purposes such as clinical decision support and analytics. Nevertheless, the mappings are a critical step towards enabling HIE between PCCs and EDs to support the care of patients exposed to poisoning.
The current telephone-based ED-PCC collaboration process contains safety vulnerabilities and inefficiencies3 and there is an evident need for better access to PCC consultation information by other healthcare providers, which is not limited to US PCCs17. U.S. PCCs are not alone in facing challenges related to interoperability. An identified top barrier for achieving meaningful use stage 3 criteria18,19 among primary care practices is suboptimal HIE capabilities20. Where a main challenge for realizing effective HIE is the lagging effort in deploying HIE standards21. This project created an important building block to achieving HIE for U.S. PCCs by bridging a gap between PCC data and existing HIE standards. Ongoing efforts are now focused on: (i) mapping free-text entries and NPDS codes to standard terminologies (e.g., SNOMED-CT, RxNorm); and (ii) integrating the generated mapping as a module within software that supports the creation, sending, and receipt of HL7 C-CDA documents.
Conclusion
As a step toward standards-based HIE between PCCs and EDs, we mapped fields within a PCC database to corresponding fields of the C-CDA consultation note, according to previously defined information requirements. Much of the data was readily available in a discrete form within the PCC database, while other data were available only as part of a combined narrative field, or not at all. We validated the generated notes for their conformance to the C-CDA R2 standard and fulfillment of ED and PCC clinical information needs. The resulting mappings are an important step towards national adoption of HIE between PCCs and EDs. Ongoing efforts include integration of the mappings as a module within software that supports the creation, sending, and receipt of HL7 C-CDA documents. That software, implemented within an HIE framework, will enable HIE between poison control centers and emergency departments.
Acknowledgements
This study was funded by the U.S. Agency for Healthcare Research & Quality (4R01HS021472- 03). Clinicians from Intermountain Healthcare and the Utah Poison Control Center provided assistance. Thanks to Computer Automation Systems, Inc. for permission to create a mapping to the Utah PCC instance of toxiCall®.
References
- 1.About AAPCC [Internet] [cited 2015 Mar 11]. Available from: http://www.aapcc.org/about/
- 2.Mowry JB, Spyker DA, Cantilena LR, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol. 2014 Dec 1;52(10):1032–283. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummins MR, Crouch B, Gesteland P, Wyckoff A, Allen T, Muthukutty A, et al. Inefficiencies and vulnerabilities of telephone-based communication between U. S. poison control centers and emergency departments. Clin Toxicol. 2013 May 23;51(5):435–43. doi: 10.3109/15563650.2013.801981. [DOI] [PubMed] [Google Scholar]
- 4.Cummins MR, Crouch BI, Gesteland P, Staggers N, Wyckoff A, Wong BG. Electronic information exchange between emergency departments and poison control centers: A Delphi study. Clin Toxicol. 2012 May 22;50(6):503–13. doi: 10.3109/15563650.2012.693183. [DOI] [PubMed] [Google Scholar]
- 5.Fiol GD, Crouch BI, Cummins MR. Data standards to support health information exchange between poison control centers and emergency departments. J Am Med Inform Assoc. 2014 Oct 23; doi: 10.1136/amiajnl-2014-003127. amiajnl - 2014-003127. [DOI] [PubMed] [Google Scholar]
- 6.Cummins MR, Crouch BI, Del Fiol G, Mateos B, Muthukutty A, Wyckoff A. Information requirements for health information exchange supported communication between emergency departments and poison control centers. AMIA Annu Symp Proc AMIA Symp AMIA Symp. 2014. 2014:449–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Computer Automation Systems, Inc. [Internet] [cited 2016 Mar 4]. Available from: http://main.toxicall.com/
- 8.National Poison Data System [Internet] [cited 2016 Feb 16]. Available from: http://www.aapcc.org/data-system/
- 9.Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol Phila Pa. 2015 Dec;53(10):962–1147. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 10.NPDS Coding Users’ Manual© Version 3.1. American Association of Poison Control Centers; 2014. [Google Scholar]
- 11.Electronic Exchange of Poisoning Information (Utah) AHRQ National Resource Center; Health Information Technology: Best Practices Transforming Quality, Safety, and Efficiency [Internet] [cited 2015 Apr 16]. Available from: http://healthit.ahrq.gov/ahrq-funded-projects/electronic-exchange-poisoning-information.
- 12.HL7 Standards Product Brief. HL7 Implementation Guide for CDA® Release 2: IHE Health Story Consolidation, Release 1.1 - US Realm [Internet] [cited 2016 Feb 12] Available from: http://www.hl7.org/implement/standards/product_brief.cfm?product_id=258.
- 13.Boone KW. London: Springer London; 2011. The CDA book [Internet] [cited 2016 Feb 23] Available from: http://link.springer.com/10.1007/978-0-85729-336-7. [Google Scholar]
- 14.HotGloo Wireframe UX Prototyping Tool [Internet] [cited 2016 Feb 16] Available from: http://www.hotgloo.com/
- 15.Altova MapForce. Graphical Data Mapping, Conversion, and Integration Tool [Internet] [cited 2016 Feb 12]. Available from: http://www.altova.com/mapforce.html.
- 16.XDS Toolkit [Internet] [cited 2016 Feb 12]. Available from: http://transport-testing.nist.gov/ttt/
- 17.Kabata PM, Waldman W, Sein Anand J. [Toxicological consultation data management system based on experience of Pomeranian Center of Toxicology] Med Pr. 2015;66(5):635–44. doi: 10.13075/mp.5893.00248. [DOI] [PubMed] [Google Scholar]
- 18.Meaningful Use Definition and Meaningful Use Objectives of EHRs. Providers & Professionals | HealthIT.gov [Internet] [cited 2016 Mar 2]. Available from: https://www.healthit.gov/providers-professionals/meaningful-use-definition-objectives.
- 19.Office of the National Coordinator for Health Information Technology (ONC), Department of Health and Human Services (HHS) 2015 Edition Health Information Technology (Health IT) Certification Criteria, 2015 Edition Base Electronic Health Record (EHR) Definition, and ONC Health IT Certification Program Modifications. Final rule. Fed Regist. 2015 Oct 16;80(200):62601–759. [PubMed] [Google Scholar]
- 20.Cohen GR, Adler-Milstein J. Meaningful use care coordination criteria: Perceived barriers and benefits among primary care providers. J Am Med Inform Assoc JAMIA. 2015 Nov 13; doi: 10.1093/jamia/ocv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams C, Mostashari F, Mertz K, Hogin E, Atwal P. From the Office of the National Coordinator: the strategy for advancing the exchange of health information. Health Aff Proj Hope. 2012 Mar;31(3):527–36. doi: 10.1377/hlthaff.2011.1314. [DOI] [PubMed] [Google Scholar]