Abstract
Information sharing among health practitioners, either for coordinated or unscheduled care, is necessary to guarantee care quality and patient safety. In most countries, nationwide programs have provided tools to support information sharing, from centralized care records to health information exchange between electronic health records (EHRs). The French personal medical record (DMP) is a centralized patient-controlled record, created according to the opt-in consent model. It contains the documents health practitioners voluntarily push into the DMP from their EHRs. Five years after the launching of the program in December 2010, there were nearly 570,000 DMPs covering only 1.5% of the target population in December 2015. Reasons for this poor level of adoption are discussed in the perspective of other countries’ initiatives. The new French governmental strategy for the DMP deployment in 2016 is outlined, with the implementation of measures similar to the US Meaningful Use.
Introduction
In 2000, the Institute of Medicine published a report about medical errors “To Err is Human: Building a Safer Health System”1 estimating that up to 98,000 deaths in the US may occur annually as a result of medical errors. This alarming number of deaths was debated, some people suggesting that it was over-estimated.2 In 2013, James3 updated the estimates about deaths caused by medical errors and reported that “the number of premature patient deaths associated with preventable harm could be as many as 400,000 per year”. No country is immune to the increasing number of avoidable premature patient deaths. Indeed, patient management has became critically complex. Patients have more and more often various pathologies and comorbities that require input from multiple healthcare providers accross many different care settings. If care is not adequatly coordinated among the different providers in charge of a given patient, the quality of care experience for her and for her family can be frustating at best, harmful at worst. Numerous studies have reported that quality improvement strategies focused on the coordination of care reduced hospital admissions among patients with chronic conditions other than mental illness and reduced emergency department visits among older patients. 4
The US AHRQ defines care coordination as “the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient’s care to facilitate the appropriate delivery of health care services”.5 However, care coordination goes beyond organized care activities. For instance, unscheduled patient encounters accross providers escape the organized care coordination framework. Non-deliberately organized care activities may indeed occur when patients move or travel, or in case of accidents or emergency situations. The management of patients in such situations requires that information about their medical condition (current and past) be available to any “new” healthcare provider to ensure care quality and patient safety, and guarantee the continuity of care, e.g. medication reconciliation. This is of primary importance in emergency situations where the lack of knowledge on a given patient condition could yield unexpected adverse events, especially when the patient herself cannot provide vital information or current medications during the medical interview (unconsciousness, mental disabilities).6 Thus, a key success factor to care coordination is sharing the same holistic view of a patient’s condition by all actors, including patient’s active diseases and current treatments.
A solution to improve medical information sharing is digitalization that makes health information “‘liquid’ enough” to flow accross healthcare institutions and providers.7 In most countries, policy makers have promoted the adoption and use of electronic health records (EHRs) to address health information sharing and care coordination. Medical information is thus available to healthcare providers of the same care team, which is usually the case of healthcare providers within the same institution, from a single practice to a large hospital, or to wider managed care consortia. Clinical information remains locally stored in original health information systems, but may be accessed on demand through data exchange protocols. This is the model adopted by the US which promote health information exchange (HIE) between different EHRs.8 The challenge of this model is to achieve interoperability between multiple EHRs. The US Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009 has offered substantial financial incentives for providers demonstrating meaningful use (MU) of EHRs. In this framework, virtually any patient data available in EHRs can be collected, whether it comes from affiliated physicians’ offices, hospitals, and clinics, or from completely disparate systems. In this way, clinical information follows patients as they move across different care settings, whether or not they share an organizational affiliation.
However, due to the challenges of standardization and interoperability, networking local EHRs through HIE to get a holistic view of a patient condition remains uncertain. An other solution to computerized medical information sharing is represented by a centralized framework where care records, centrally-stored in purpose-built platforms, are specifically created to serve information sharing. They usually do not contain the comprehensive information stored in all EHRs but only what is considered as necessary to support a coordinated management of the patient inside and outside care coordination practices. This information may be automatically extracted from EHRs or manually added. When a healthcare provider needs some medical information for a given patient, he may access the centralized care record with the consent of the patient. One of the most advanced example of such a centralized nationwide system is the electronic summary care record (SCR) implemented in the UK.9 In Scotland, the SCR contains elements such a patient’s name, address, age, allergies, current medications, diagnoses. It is connected to 100% of general practices, and is automatically updated from existing GP records.10 Its main objective is to be accessed in emergency and unscheduled care scenarios.11
Based on the UK experience, Eason and Waterson12 reported that the centralized model is less feasible than the distributed model. In addition, it relies on a classic “top-down” approach to e-health whereas a “middle-out” approach is more likely to succeed.7 Quantin et al.13 also advocated for a distributed model of connected EHRs on the basis of pragmatic and cost reasons. On the contrary, Lapsia et al.14 suggested that the centralized model for nationwide health information infrastructure scales better than the distributed model in terms of data availability and integrity, with lower failure rates. However, literature about centralized nationwide care records generaly reports poor actual adoption.11,15 The same difficulties in the adoption and deployment of HIE are reported with the distributed model despite MU incentives.16,8 A recent study reported that even among US HIT/EHR adopters, one third does not routinely receive the needed patient information for care coordination.17 Thus, sharing patient information from distributed locations through HIE has been questioned, and initiatives aiming at “recentralizing” the model have been launched. For instance, health record banking promotes the centralized storage of and access to care records fed by HIE. Such health record banks are not operated by governmental bodies and might rely on private organizations.18
Beyond digitalizing medical information, another solution to improve the sharing of the same holistic view of a patient’s condition is to count on patients and their caregivers to serve as care coordinators, filling information gaps between providers, addressing conflicting instructions, and managing redundant orders and medications during care transitions. This is possible only if patients are informed, involved, and engaged. The Blue Button project has become a way for many Americans to download their health records. A recent survey reported that one third of patients at the VA were Blue Button users.19 The most highly endorsed benefit was that it helped patients understand their health history and thus be active in their management.
In 2004, the French government decided to develop a “personal” nationwide care record, called DMP or “Dossier Médical Personnel”. First elaborated as a set of distributed region-wide records, the DMP was re-engineered in 2009 using the centralized model and was officially launched in December 2010. Though personally-controlled to promote patient empowerment and built according to the centralized model to avoid interoperability-related technical issues, the DMP has had a chaotic history. This article provides a description of the historical background of the DMP in France, the rates of adoption of the DMP nationwide, an analysis of the reasons why, five years after its launching, DMP adoption rates did not match expectations, and the new solutions to be implemented to re-launch the program.
Background
Ups and downs of the DMP
The French health system is a centralized public system controlled by the government (Ministry of Health) with a regional support (Regional Health Agencies in charge of the regional implementation of National health policies). The government health insurance (in French “Assurance Maladie”) funds most care expenses of the population, mainly from the social contributions of active workers. All workers (either currently active or retired) benefit from the government health insurance: they are namely insured. Their health care coverage includes both their own medical expenses and the care costs of their spouse, when unemployed, and children. Care is provided in medical institutions, either public or private, monitored by the French Ministry of Health. Outside health care facilities, private self-employed health practitioners are monitored by the government health insurance (patients used to pay their doctors for medical consultations and were reimbursed by the government health insurance, but now the government health insurance directly pays the medical consultations to doctors). Some National pay-for-performance programs such as ROSP (“Rémunération sur Objectifs de Santé Publique”) have been implemented by the government health insurance that offer financial incentives to private physicians for meeting certain performance measures (29 criteria). Adoption of an EHR is one of these measures and yields €525 (US $580) per year. When fulfilling all criteria, private doctors may earn an additional bonus up to €6,000 (US $6,650) per year.
The DMP has been created in France by the Act of August 13th, 2004 reforming the government health insurance. Only individuals namely insured by the government health insurance may have a DMP. The DMP was expected to improve the quality of care, and to reduce health costs by €3.5 billion (US $3.9 billion) yearly. In 2005, a public interest group, the GIP-DMP, was set up under the supervision of the Ministry of Health, in order to manage the design and the implementation of the DMP throughout the country. The first DMP was relying on a distributed model involving regional medical records. From 2006, several experiments were launched in different regions. These experiments permitted the mobilization of the different stakeholders (healthcare practitioners, patients, and medical software vendors) on the strengths and weaknesses of digitalized data, and the need for legal and technical safeguards to preserve privacy. In 2007, serious concerns about the safety and the confidentiality of personal medical data recorded in DMPs were raised and the program considered as potentially unethical was stopped.
After numerous audits to determine how the program could continue with appropriate security warranties, the DMP was re-designed in 2009 as a nationwide centralized personal medical record with a focus on the protection of patients rights. DMP regulations were written in the Law (Code of Public Health) as stated by the HPST Act (in French, “Hôpital, Patient, Santé et Territoires”) confirming the DMP as a National public health program to serve patient empowerment. A national agency for the promotion of shared health information systems (ASIP Santé) was created to develop and implement the tool. At that time, it was forecasted to get 2 millions of DMPs the launching year (2010), 5 millions the second year (2011), 9 millions the third year (2012), 11 millions the fourth year (2013), and finally 13 millions at the end of the first cycle (2014), thus covering one third of the ultimate target population (38 million of individuals namely insured by the government health insurance, out of the 66.6 million inhabitants of France). However, the adoption of DMP was very slow. In 2012, a report from The Court of Auditors (in French “Cour des Comptes”), an independant public body in charge of conducting financial and legislative audits of most public institutions, proclaimed that the DMP costed about €210 millions, for only 150,000 records (in July 2012), and severely criticized the management of the program. This report almost killed the DMP program.
Model and Functionalities of the DMP
The DMP is a centralized, nationally shared, widely accessible, patient-centered electronic medical record. It is optional and free for all the individual recipients of the government health insurance. DMPs are created by health practitioners only after the patient gave her informed consent (opt-in model). The creation of a DMP requires that both the health practitioner and the patient are “strongly” authenticated. Currently, this strong authentication is achieved by the electronic reading of the smart identity card of the health practitioner and of the health insurance card of the patient.
Patients may access their DMP via a web-accessible DMP portal (patient access). They have to identify themselves using the login-password they were given at the moment their DMP was created.
Health practitioners involved in the management of a given patient need to be authorized by the patient to read information recorded in the DMP. For accessing, reading, or pushing information in the DMP, health practitioners need to be strongly authenticated (health practitioner identity smart card or equivalent). However, unauthorized health practitioners may “break the glass” and then access the DMP without the patient’s consent only in case of emergency, when the patient is unable to give her consent. Similarly, practitionners must be strongly authenticated to break the glass.
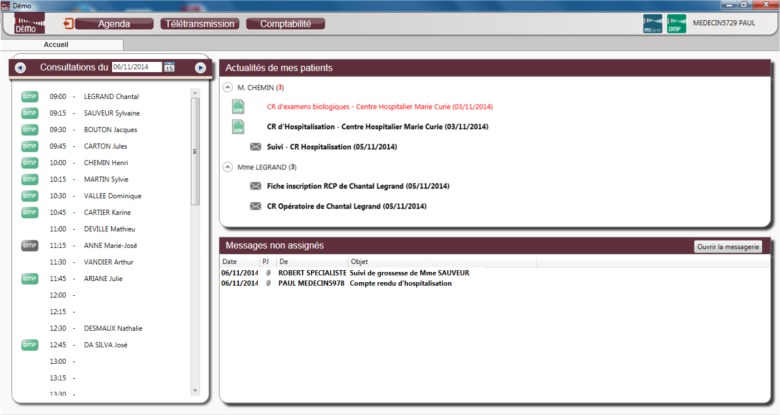
Health practitioners may access their patients’ DMP through their own EHR. When the EHR software is DMP-compatible, both records are interoperable and smoothly interconnected. DMP information appears in the EHR interface and documents are tagged according to their origin. Figure 1 shows a screenshot of an EHR with the display of the list of the patients of the day in the left window, with a green DMP logo when the health practitioner is authorized to access their DMP, a grey DMP logo when she is not authorized, and no logo when patients don’t have a DMP. In the upper right window, news concerning some patients (that may be either planned for encounter on the same day or not) are displayed: new documents may be available in the DMP (green DMP logo) or have been received by secure messaging (grey envelope). Abnormal results are in red. DMP-compatibilty relies on the interoperability framework defined by ASIP Santé and based on international standards (HL7 CDA r2).
Figure 1.
Screenshot of the home page of a DMP-compatible EHR for health practitioners (courtesy of ASIP Santé).
When the EHR is not DMP-compatible, health practitioners may use the DMP portal (professional access) to access, read, and post information into the DMP of their patients. Once again, they need to be strongly authenticated. However, in this case, they have to manage two different environments, their EHR on the one side, and the DMP on the other side, which is known to be less convenient.20
As opposed to the UK where patient information is automatically updated from GP records in the Emergency Care Summary,10 updating the DMP content is an active process: health practitioners are required to push into the DMP the documents they consider to be relevant for care continuity. With a DMP-compatible EHR, the list of the documents newly created for a given patient is displayed on the screen for the health practitioner to check if they should be shared in the DMP. She has then only to tick a box to select the documents to be sent to the DMP.
Because all the operations on a DMP require the operator to be strongly authenticated, the system logs each access to the DMP (name, date, time, break glass procedure, consultation, addition of a document, etc.) and this information is available to the patient as an audit log. The DMP owner and her primary care physician may see all DMP accesses. Thus, the patient can check that only authorized health practitioners accessed her DMP. Other health practitioners than her primary care physician only see their own traces. Unauthorized access is subject to criminal prosecution.
If the DMP is not expected to be reduced to a single patient summary as it is the case in UK with the SCR, it does not aim at being exhaustive and providing the complete picture of a given patient medical history. The DMP should contain only the relevant information a health practitioner considers necessary for other health practitioners in order to provide efficient and secure care, be it scheduled or not. A non-exhaustive list of documents that may be posted into DMPs according to best practices is provided. This list covers the main medical reports: updated patient care summary (elaborated by the primary care physician), current prescriptions of drugs and care, hospital discharge summaries, imaging reports, laboratory tests.
Currently, the DMP is essentially document-based with no structured medical data except some metadata (e.g. document type, date of production, author). Documents may have three different statuses. They may be either open, hidden, or sensitive. When they are open, they are accessible to all authorized health practitioners. When they are hidden, they are only accessible to the author of the document and to the primary care physician. In this later case, documents are “invisible” for all other authorized practitioners: they don’t even know that some documents they don’t have access to exist (the hiding of the document is masked). Sensitive documents are documents that are temporarily not accessible to the patient. This concerns for instance the anatomo and cytopathological reports that set a diagnosis of cancer or a laboratory report that evidences a diagnosis of HIV stated by the ELISA test. Patients cannot become aware of this kind of information by reading it alone in their DMP and they need to be assisted by their doctor. Once the medical consultation annoucing the sensitive information has occurred, documents are no more sensitive, and they become open, unless the patient decides to hide them.
The DMP is a personally controlled health record preserving patient rights: the patient should give her consent for a health practitioner to create her DMP, she decides what can be seen and what must be hidden in her record, as well as which health professionals are authorized to get access. The patient is provided with the journal of every access to check for illegal accesses. The DMP also provides a personal section where the patient has the liability to create various documents and record personal information she wants to share with her physicians.
Methods
After the publication in 2012 of the report from The Court of Auditors, the French Minister of Health ordered a study to analyze the reasons for the poor adoption of the DMP and the solutions that could be implemented to improve the situation. The question at this time was to know what was the best option between stopping the DMP project, redefining a totally new project, or going on with the existing project but improving it. ASIP Santé conducted a nation wide satisfaction survey with unstructured interviews and focus groups of patients and health professionnals. Within the Ministry of Health, the Department of Health Information Systems Strategy (DSSIS) (where one author, BS, is working part-time as DMP project leader) was in charge of working with the different key stakeholders to recommend the best stragegy to adopt.
The method applied was to build a working group to gather the different stakeholders and have the different opinions expressed regarding the DMP. We included all unions of general practitioners, the National Council of physicians, the National Council of pharmacists, the French Federation of Medical Specialties, the French Association of Emergency Physicians, ASIP Santé, the French National Authority for Health (HAS), the National federation of vendors (EHRs for hospitals and private practices), patient organizations, chief information officers of main university hospitals. A series of five meetings of the working group was organized in 2013 to discuss the future of the DMP project with a diagnosis step (what went wrong?), and a “therapeutic” step to collectively construct a consensual roadmap.
All quantitative results are given on the basis of data provided by the ASIP Santé (DMP adoption data monthly sent to the DSSIS) and the government health insurance.
Results
A Weak Adoption of the DMP
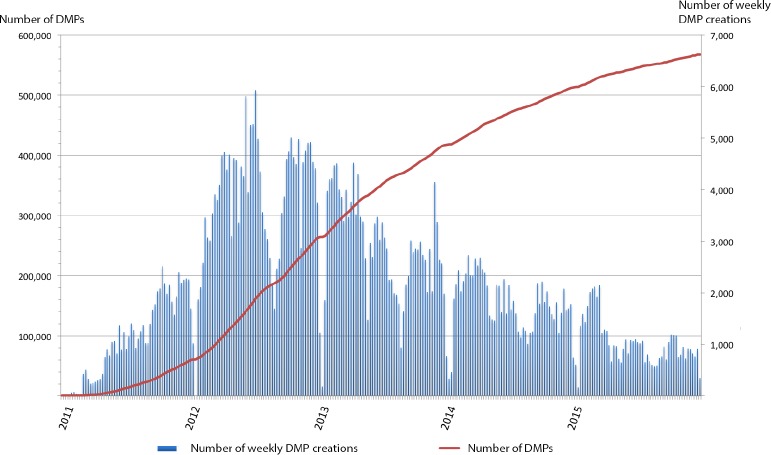
Consolidated data were obtained from December 2010, which is the date from which the DMP infrastructure was operational, until the end of December 2015. By this date, there were 567,888 DMPs at the national level. Figure 2 provides the distribution of DMP creations per week as well as the evolution of the number of DMPs on the study period. More than two thirds (72%) of all DMPs belong to patients older than 45 years (35% between 45 and 65, and 39% for older patients).
Figure 2.
Distribution of DMP creation per week and cumulative number of DMPs at the national level between December 2010 and December 2015.
On the basis of the 38 million people namely insured, which are the potential DMP owners, 1.5% of the target population has a DMP after 5 years of deployment. As mentionned in the historical background, in 2009, ASIP Santé prospectively delivered yearly expectations of DMPs for the next five years reaching 13 millions DMPs in 2014.
As for DMP contents, 42% of DMPs are empty. The 330,273 non-empty DMPs contain as a whole 2,080,171 documents, with an average of 6.3 documents per DMP. These include the 129,826 (6.2%) documents that have been added by the patients themselves in less than 5% of all DMPs. Among all documents, 1,616 have been hidden by patients (less than 0.1%) and 36,501 were made sensitive by physicians (less than 2%). Among the 34 types of documents stored in DMPs, the most frequent is the report of medical consultation which represents 34% of all documents. Table 1 lists the 13 main types of documents (each type represents more than 1% of all documents). It must be noted that documents stored by patients come in fourth position representing 6.2% of all documents and that drug prescriptions are in eighth position with 2.9%.
Table 1.
Distribution of main documents stored in DMPs sorted by frequency.
| Rank | Document type | # | % |
|---|---|---|---|
| 1 | Report of medical consultation | 703,817 | 34.1% |
| 2 | Hospital discharge summary | 291,941 | 14.1% |
| 3 | Imaging report | 254,284 | 12.3% |
| 4 | Patient-entered document | 129,350 | 6.2% |
| 5 | Biology report | 126,198 | 6.1% |
| 6 | Discharge letter | 116,437 | 5.6% |
| 7 | Document including non-DICOM images | 95,459 | 4.6% |
| 8 | Surgery report | 65,828 | 3.2% |
| 9 | Drug prescription | 60,287 | 2.9% |
| 10 | Diagnostic report | 58,237 | 2.8% |
| 11 | Clinical synthesis | 56,188 | 2.7% |
| 12 | Anatomo and cytopathology report | 29,594 | 1.4% |
| 13 | Emergency department report | 20,764 | 1.0% |
| … | … |
A DMP user is recognized as such once she had created, feeded, or consulted at least one DMP. 690 healthcare structures, including public and private hospitals and clinics, are DMP users on an estimated total of about 75,000 healthcare structures. 6,636 private healthcare professionals, including mostly GPs, are DMP users. As a baseline, medical doctors in the private sector are about 130,000, in 2015 and other private healthcare profesionals are 318,700 (less than 1%). Patients are DMP users when they logged at least one time into their DMP. They are 79,965 and represent 14.1% of all DMP owners and 24.2% of those for whom the DMP has been actually used and is not empty.
About one half of non-empty DMPs have been consulted (151,563), among which more than one half are also consulted by patients. Considered as a care coordination instrument, only 139,745 DMPs (42.3% of non empty DMPs) have been shared by 2 or more healthcare professionals.
Reasons for the weak adoption of the DMP
The fortune of national programs of HIT adoption varies according to the history of the countries. In France, unlike some countries, we were not starting from scratch, and it was much more difficult to change the components of the current system. From the different meetings of the working group gathered to elaborate the new DMP strategy, it appeared that the problem was cultural rather than technical although some technical aspects were not totally solved. Below are some reported issues:
No clear political support: there was no clear strong political support of the DMP program, and an incredibly noisy silence of political personalities on the subject. Because the DMP program crossed a lot of difficulties since its creation, successive Ministers of Health were not very keen on supporting it. After the report from The Court of Auditors in 2012, it took six months for the Minister of Health to say the DMP was not stopped, then six more months to announce the DMP2, the new version of the DMP, and another six months to advertize the program as part of the national health strategy.21 However, between these scarce interventions of the Minister of Health on the subject, there was no public information towards the different stakeholders. Suppliers of medical software stopped implementing DMP functionalities in their products. Health practitioners stopped investing in a tool which future seemed to be compromised, as illustrated by the decreasing rate of DMP creation (Figure 2).
No culture of medical information sharing among health practitioners: winning the bet of DMP adoption was relying on the assumption that doctors were ready and in demand of a tool to support the sharing of the medical information concerning their patients. But the patient-centered culture required to understand the benefits of sharing medical information was actually missing. Resistance came from health practitioners, especially from unions of physicians from private practice. If they could easily adhere to the concept of exchanging medical information, and were ready to move from paper-based letters to emails sent to known colleagues, they were reluctant to the concept of sharing, i.e. making relevant medical information available, for emergency and unscheduled care episodes, to practitioners unknown at the moment the information was delivered, but who could happen to manage the patient in the future. As opposed to the secure electronic messaging which is someting acceptable since it is “just” replacing a known paper-based practice by a numeric exchange, the DMP program was a new object to support new practices, i.e. sharing medical information for the sake of care continuity. This object did not exist previously in “paper” version, and the organization and practices underlying its use did not exist in a formalized way. Thus, the adoption of both the concept and the tool was a challenge.
No education of patients and citizens: another difficulty came from the fact that patients were not actively involved in the DMP program. There was no national publicity campaign towards patients, giving some space to a negative press exacerbating the DMP program’s political sensitivity, associated with a wider “surveillance state”, the risk on data confidentiality, and the lost of the physician-patient privilege (in French “medical secret”). In addition, despite the “P” of DMP was for “personal”, patients could not create their DMP by themselves, and had to ask their physicians to do it whereas most of them either were against the tool, or were thinking the program was stopped. In 2013, according to an opinion poll*, 85% of French people were in favor of the DMP, an exceptionally high adherence rate (40% even said they were “strongly in favor”), in a marked progression in three years (they were 74% to be favorable in December 2010). Thus, involving patients and making them compagnions of the program would surely have helped the adoption of DMP beyond the benefit of empowering patients.
Persistence of technical difficulties: although 100% of EHR commercial software available in France are DMP-compatible, not all vendors provide integrated user-friendly DMP-specific functionalities. Besides, numerous health practitioners did not use the last DMP-compatible version of their medical software. Without incentive to change, because of the cost of the new software, and of the risks that always exist to modify a functionnal equilibrium, sometimes acquired with difficulties, health practitioners kept on using the old non DMP-compatible versions of their system, keeping the DMP outside their usual workflow, and making the adoption of the tool much more difficult.
Discussion
By the end of 2015, five years after the launching of the program in 2010, only 1.5% of potential DMPs had been created with a total of about 570,000 DMPs, and only 27% of all DMPs have been accessed. Updated data report 584,000 DMPs six month later, at the beginning of July 2016. As of February 2016, UK’s Health & Social Care Information Centre reported that 55 million people in England, representing more than 90% of the population, have an SCR created from their GP records†. According to the same source, 2.5 million SCRs were accessed by authorised healthcare professionals. Such difference in nationwide care records adoption between France and UK could be explained by the opt-out model used in the UK, the automated extraction of medical information from GPs’ EHRs to update the SCR, and the authority exercised by the NHS on GPs. Another important difference is that though being patient-centered, the SCR is not a “personal” record belonging to the patient who cannot acces it, but a professional, medical, tool to be used by health practitioners in case of emergency. 11
The French DMP seems more comparable to the Australian personnally-controlled electronic health record (PCEHR). Although not built on the same architectural model, the functionalities of the PCEHR22 are similar to those of the DMP, with an initial opt-in model, a richer document-based medical content, and the on-line patient access. The 2013-2014 annual report on the PCEHR23 mentions that, on June 30th, 2014, 1.73 million people registered for a PCEHR, i.e. 7.4% of the population two years after its launching. However, PCEHRs are mostly accessed by patients (around 512,000 times), and weakly accessed by health practitioners (around 25,000 times). The comparison with the US is more difficult due to the decentralized model for sharing medical information distributed among multiple EHRs through HIE.8 The number of people having a “personal record” does not make sense, since most patients have many records distributed within the medical practices they visit. However, not all providers are equipped with an EHR. The US CDC reported that in 2013, 22% of office-based physicians still did not use any type of EHR.24 Nevertheless, all patients may potentially access and download their medical information distributed among EHRs using the “Blue Button” functionality.25 This functionality builds upon HIE. From the patient’s point of view, this functionality is similar to the DMP and the PCEHR, except that the patient has no control over the content and cannot add or push any personal information. Recently, Ford et al.26 reported a simulation-based study to forecast the level of adoption of personal health records in the US. They estimate that the MU stage 3 target of 50% of consumers having access to their entire health record could be reached by 2020.
Conclusion
A nationally shared, widely accessible, electronic care record has a powerful symbolic meaning; it may be perceived as improving the quality and safety of care or, alternatively, as threatening patient data confidentiality or the traditional role of doctors and nurses. 10,27,28 It is thus important to communicate about the nature and the aim of the tool. In France, the low adoption rate of the DMP highligths the complex socio-technical challenges of implementing HIT. In the DMP case, the most important difficulty comes from the cultural change DMP was involving. However, despite the reported poor level of DMP adoption, patients and most healthcare professionals seem now to be ready to use it, although some unions of private practice physicians are still against the program because they feel that it is the role of the GP (and not of the DMP) to be the hub of care coordination, without considering that one valuable aspect of the DMP is for non-coordinated and emergency care.
Following the announcements of the French Minister of Health in 2013, the DMP has been relaunched (again), and a DMP2 positioned as a professionnal tool to support care continuity and coordination for the benefit of patients has been advertized. It took then two years to publish the new French Health Act‡ (January 26th, 2016) that includes the promotion of DMP adoption and use (article 96). After considering that the name of the tool should be changed, it remained DMP but with “P” for “partagé” (shared) instead of “personal” to insist on its use to support care continuity among health professionals sharing the same relevant medical information. Instead on depending on busy doctors reluctant to perform administrative tasks, patients will be now able to create their own DMP which also helps to solve the problem of tracing the consent collection. Instead of ASIP Santé, it is now the responsability of the government health insurance to conduct the DMP program. Indeed the poor DMP adoption has been partly explained by a principled opposition of private doctors and by some technical difficulties private doctors may face in updating their EHR system. Thus having the governemnt health insurance that financially monitors private doctors and is already used to send computer technicians to doctors’ offices to check whether computerized billing tools are operational was a better candidate. In addition, the governemnt health insurance represents a significant counterpower towards the medical software vendors, which should also improve their reactivity to make the appropriate technical developments for interfacing their medical software with the DMP functionalities. Another important point is that the governement may push all health insurance claims information (including information about reimbursed medical consultations, reimbursed drugs, reimbursed biological exams, imaging prescriptions, among other clinical information), thus ensuring there would be no more empty DMPs. Finally, the government health insurance may use the ROSP program to offer financial incentives and make private doctors actually use (create, feed and read) the DMP in a kind of French Meaningful Use. The impact of this year’s new DMP initiative will have to be assessed and quantified.
Acknowledgments
Authors thank Dr Elie Lobel, from ASIP Santé, for the information he provided about the DMP program, as well as Philippe Burnel and Philippe Cirre from the DSSIS at the French Ministry of Health. Data and figures on DMP usage have been gracefully provided by ASIP Santé.
Footnotes
http://www.dmp.gouv.fr/newsletter/dmp-actu-17/newsletter/documents/barometre.pdf [accessed July 7th, 2016].
http://www.digitalhealth.net/shared_care_records/47161/scr-in-outpatients-and-police-rooms, dated Feb 9th 2016 [accessed June 7th, 2016].
https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000031912641 [accessed July 7th, 2016].
References
- 1.Kohn L, Corrigan J, Donaldson M. Washington: National Academy Press; 2000. To err is human: building a safer health system. [PubMed] [Google Scholar]
- 2.McDonald C, Weiner M, Hui S. Deaths due to medical errors are exaggerated in institute of medicine report. JAMA. 2000;284(1):93–5. doi: 10.1001/jama.284.1.93. [DOI] [PubMed] [Google Scholar]
- 3.James J. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9(3):122–8. doi: 10.1097/PTS.0b013e3182948a69. [DOI] [PubMed] [Google Scholar]
- 4.Tricco A, Antony J, Ivers N, Ashoor H, Khan P, Blondal E, et al. Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: a systematic review and meta-analysis. CMAJ. 2014;186(15):E568–78. doi: 10.1503/cmaj.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald K, Schultz E, Albin L, Pineda N, Lonhart J, Sundaram V, et al. Number 14-0037-EF in AHRQ Publication. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Jun, Care Coordination Atlas Version 4 (Prepared by Stanford University under subcontract to American Institutes for Research on Contract No. HHSA290-2010-00005I) [Google Scholar]
- 6.Boulain T, Runge I, Delorme N, Bouju A, Valéry A. Patients hospitalized in general wards via the emergency department: Early identification of predisposing factors for death or unexpected intensive care unit admission-a historical prospective. Emerg Med Int. 2014. 2014:203–747. doi: 10.1155/2014/203747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coiera E. Do we need a national electronic summary care record? Med J Aust. 2011;194(2):90–2. doi: 10.5694/j.1326-5377.2011.tb04176.x. [DOI] [PubMed] [Google Scholar]
- 8.Rudin RS, Motala A, Goldzweig C, Shekelle P. Usage and effect of health information exchange: a systematic review. Ann Intern Med. 2014;161(11):803–11. doi: 10.7326/M14-0877. [DOI] [PubMed] [Google Scholar]
- 9.Cresswell K, Sheikh A. The nhs care record service (nhs crs): recommendations from the literature on successful implementation and adoption. Inform Prim Care. 2009;17(3):153–60. doi: 10.14236/jhi.v17i3.730. [DOI] [PubMed] [Google Scholar]
- 10.Greenhalgh T, Morris L, Wyatt J, Thomas G, Gunning K. Introducing a nationally shared electronic patient record: case study comparison of Scotland, England, Wales and Northern Ireland. Int J Med Inf. 2013;82(5):e125–38. doi: 10.1016/j.ijmedinf.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Greenhalgh T, Hinder S, Stramer K, Bratan T, Russell J. Adoption, non-adoption, and abandonment of a personal electronic health record: case study of healthspace. BMJ. 2010;341:c5814. doi: 10.1136/bmj.c5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eason K, Waterson P. The implications of e-health system delivery strategies for integrated healthcare: lessons from england. Int J Med Inf. 2013;82(5):e96–106. doi: 10.1016/j.ijmedinf.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Quantin C, Jaquet-Chiffelle D, Coatrieux G, Benzenine E, Auverlot B, Allaert F. Medical record: systematic centralization versus secure on demand aggregation. BMC Med Inform Decis Mak. 2011;11:18. doi: 10.1186/1472-6947-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapsia V, Lamb K, Yasnoff W. Where should electronic records for patients be stored? Int J Med Inf. 2012;81(12):821–7. doi: 10.1016/j.ijmedinf.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 15.de Lusignan S, Ross P, Shifrin M, Hercigonja-Szekeres M, Séroussi B. A comparison of approaches to providing patients access to summary care records across old and new europe: an exploration of facilitators and barriers to implementation. In: Lehmann CU, Ammenwerth E, Nøhr C, editors. MEDINFO, (vol192) of Stud Health Technol Inform. IOS Press; 2013. pp. 397–401. [PubMed] [Google Scholar]
- 16.Kierkegaard P, Kaushal R, Vest J. How could health information exchange better meet the needs of care practitioners? Appl Clin Inform. 2014;5(4):861–77. doi: 10.4338/ACI-2014-06-RA-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao C, King J, Hing E, Simon A. The role of health information technology in care coordination in the united states. Med Care. 2015;53(2):184–90. doi: 10.1097/MLR.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasnoff W, Shortliffe E. Lessons learned from a health record bank start-up. Methods Inf Med. 2014;53(2):66–72. doi: 10.3414/ME13-02-0030. [DOI] [PubMed] [Google Scholar]
- 19.Turvey C, Klein D, Fix G, Hogan T, Woods S, Simon S, et al. Blue Button use by patients to access and share health record information using the department of Veterans Affairs’ online patient portal. J Am Med Inform Assoc. 2014;21(4):657–63. doi: 10.1136/amiajnl-2014-002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765–72. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Touraine M. Health inequalities and France’s national health strategy. Lancet. 2014;383(9923):1101–2. doi: 10.1016/S0140-6736(14)60423-2. [DOI] [PubMed] [Google Scholar]
- 22.Pearce C, Bainbridge M. A personally controlled electronic health record for Australia. J Am Med Inform Assoc. 2014;21(4):707–13. doi: 10.1136/amiajnl-2013-002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Personally Controlled Electronic Health Record System Operator. Annual Report - 1 july 2013 to 30 june 2014. Australian Government, Department of Health 2014 [PCEHR-System-Operater-Annual-Report13-14.pdf, accessed March 11th, 2015]
- 24.Hsiao C, Hing E. NCHS data brief, no 143. Hyattsville, MD: National Center for Health Statistics; 2014. Use and characteristics of electronic health record systems among office-based physician practices: United states, 2001-2013. [PubMed] [Google Scholar]
- 25.Hogan T, Nazi K, Luger T, Amante D, Smith B, Barker A, et al. Technology-assisted patient access to clinical information: an evaluation framework for blue button. JMIR Res Protoc. 2014;3(1):e18. doi: 10.2196/resprot.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford W, Hesse W, Huerta R. Personal health record use in the united states: Forecasting future adoption levels. J Med Internet Res. 2016;18(3):e73. doi: 10.2196/jmir.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwaanswijk M, Ploem M, Wiesman F, Verheij R, Friele R, Gevers J. Understanding health care providers’ reluctance to adopt a national electronic patient record: an empirical and legal analysis. Med Law. 2013;32(1):13–31. [PubMed] [Google Scholar]
- 28.Andrews L, Gajanayake R, Sahama T. The Australian general public’s perceptions of having a personally controlled electronic health record (PCEHR) Int J Med Inf. 2014;83(12):889–900. doi: 10.1016/j.ijmedinf.2014.08.002. [DOI] [PubMed] [Google Scholar]