Abstract
The type II beta regulatory subunit of cAMP-dependent protein kinase (RII beta) has been hypothesized to play an important role in the growth inhibition and differentiation induced by site-selective cAMP analogs in human cancer cells, but direct proof of this function has been lacking. To address this issue, HL-60 human promyelocytic leukemia cells were exposed to RII beta antisense synthetic oligodeoxynucleotide, and the effects on cAMP-induced growth regulation were examined. Exposure of these cells to RII beta antisense oligodeoxynucleotide resulted in a decrease in cAMP analog-induced growth inhibition and differentiation without apparent effect on differentiation induced by phorbol esters. This loss in cAMP growth regulatory function correlated with a decrease in basal and induced levels of RII beta protein. Exposure to RII beta sense, RI alpha and RII alpha antisense, or irrelevant oligodeoxynucleotides had no such effect. These results show that the RII beta regulatory subunit of protein kinase plays a critical role in the cAMP-induced growth regulation of HL-60 leukemia cells.
Full text
PDF
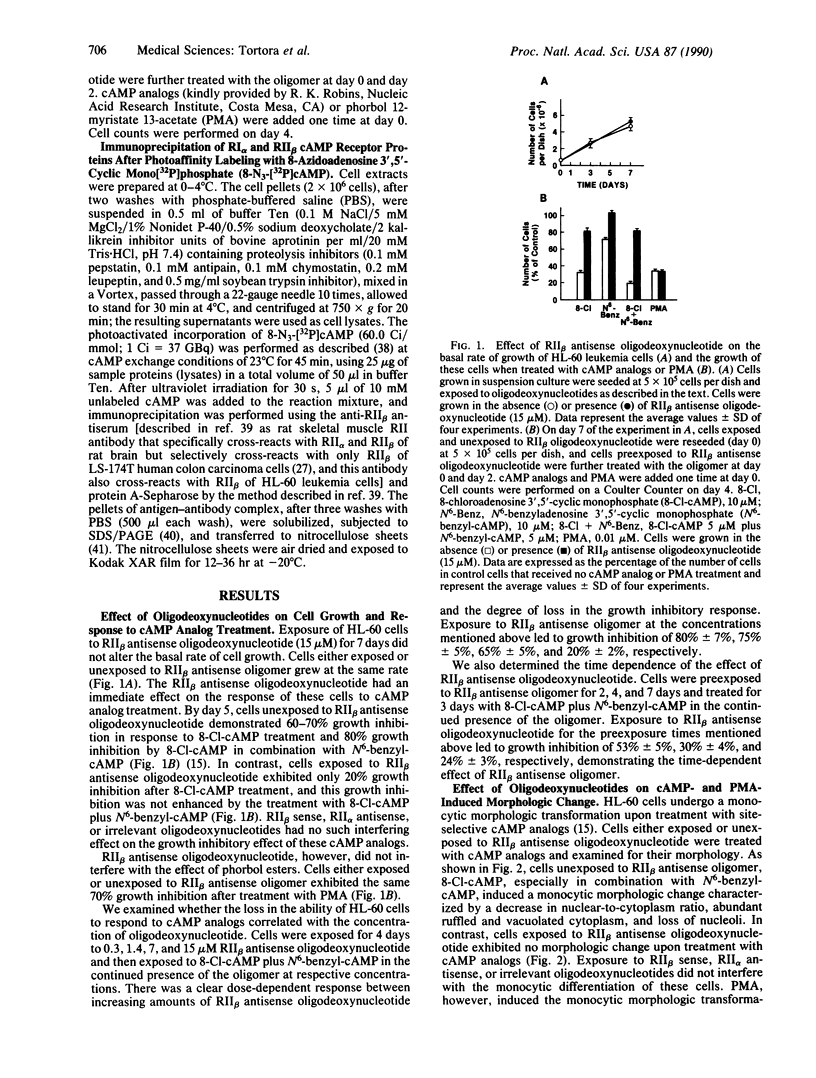
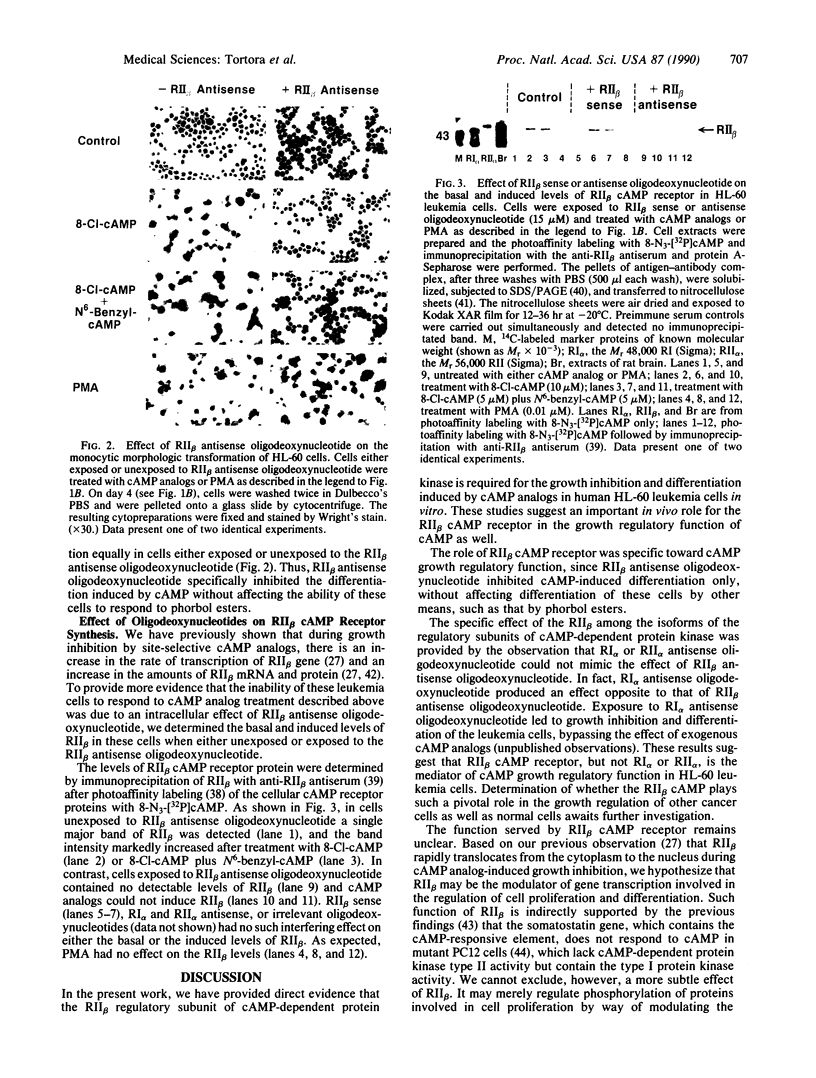

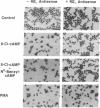
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ally S., Tortora G., Clair T., Grieco D., Merlo G., Katsaros D., Ogreid D., Døskeland S. O., Jahnsen T., Cho-Chung Y. S. Selective modulation of protein kinase isozymes by the site-selective analog 8-chloroadenosine 3',5'-cyclic monophosphate provides a biological means for control of human colon cancer cell growth. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6319–6322. doi: 10.1073/pnas.85.17.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlapowski F. J., Kelly L. A., Butcher R. W. Cyclic nucleotides in cultured cells. Adv Cyclic Nucleotide Res. 1975;6:245–338. [PubMed] [Google Scholar]
- Cho-Chung Y. S. Hypothesis. Cyclic AMP and its receptor protein in tumor growth regulation in vivo. J Cyclic Nucleotide Res. 1980;6(3):163–177. [PubMed] [Google Scholar]
- Cho-Chung Y. S. Site-selective 8-chloro-cyclic adenosine 3',5'-monophosphate as a biologic modulator of cancer: restoration of normal control mechanisms. J Natl Cancer Inst. 1989 Jul 5;81(13):982–987. doi: 10.1093/jnci/81.13.982. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Cadd G. G., McKnight G. S. Genetic characterization of a brain-specific form of the type I regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3703–3707. doi: 10.1073/pnas.85.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Ekanger R., Sand T. E., Ogreid D., Christoffersen T., Døskeland S. O. The separate estimation of cAMP intracellularly bound to the regulatory subunits of protein kinase I and II in glucagon-stimulated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3393–3401. [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen T., Hedin L., Kidd V. J., Beattie W. G., Lohmann S. M., Walter U., Durica J., Schulz T. Z., Schiltz E., Browner M. Molecular cloning, cDNA structure, and regulation of the regulatory subunit of type II cAMP-dependent protein kinase from rat ovarian granulosa cells. J Biol Chem. 1986 Sep 15;261(26):12352–12361. [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Katsaros D., Tortora G., Tagliaferri P., Clair T., Ally S., Neckers L., Robins R. K., Cho-Chung Y. S. Site-selective cyclic AMP analogs provide a new approach in the control of cancer cell growth. FEBS Lett. 1987 Oct 19;223(1):97–103. doi: 10.1016/0014-5793(87)80517-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Carmichael D. F., Krebs E. G., McKnight G. S. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3608–3612. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. O., Oyen O., Sandberg M., Taskén K., Eskild W., Hansson V., Jahnsen T. Molecular cloning, complementary deoxyribonucleic acid structure and predicted full-length amino acid sequence of the hormone-inducible regulatory subunit of 3'-5'-cyclic adenosine monophosphate-dependent protein kinase from human testis. Mol Endocrinol. 1988 Dec;2(12):1364–1373. doi: 10.1210/mend-2-12-1364. [DOI] [PubMed] [Google Scholar]
- Marcus-Sekura C. J. Techniques for using antisense oligodeoxyribonucleotides to study gene expression. Anal Biochem. 1988 Aug 1;172(2):289–295. doi: 10.1016/0003-2697(88)90447-2. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Ogreid D., Ekanger R., Suva R. H., Miller J. P., Sturm P., Corbin J. D., Døskeland S. O. Activation of protein kinase isozymes by cyclic nucleotide analogs used singly or in combination. Principles for optimizing the isozyme specificity of analog combinations. Eur J Biochem. 1985 Jul 1;150(1):219–227. doi: 10.1111/j.1432-1033.1985.tb09010.x. [DOI] [PubMed] [Google Scholar]
- Oyen O., Myklebust F., Scott J. D., Hansson V., Jahnsen T. Human testis cDNA for the regulatory subunit RII alpha of cAMP-dependent protein kinase encodes an alternate amino-terminal region. FEBS Lett. 1989 Mar 27;246(1-2):57–64. doi: 10.1016/0014-5793(89)80253-4. [DOI] [PubMed] [Google Scholar]
- Oyen O., Scott J. D., Cadd G. G., McKnight G. S., Krebs E. G., Hansson V., Jahnsen T. A unique mRNA species for a regulatory subunit of cAMP-dependent protein kinase is specifically induced in haploid germ cells. FEBS Lett. 1988 Mar 14;229(2):391–394. doi: 10.1016/0014-5793(88)81163-3. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Pomerantz A. H., Rudolph S. A., Haley B. E., Greengard P. Photoaffinity labeling of a protein kinase from bovine brain with 8-azidoadenosine 3',5'-monophosphate. Biochemistry. 1975 Aug 26;14(17):3858–3862. doi: 10.1021/bi00688a019. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Differentiation of neuroblastoma cells in culture. Biol Rev Camb Philos Soc. 1975 May;50(2):129–165. doi: 10.1111/j.1469-185x.1975.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Robinson-Steiner A. M., Corbin J. D. Probable involvement of both intrachain cAMP binding sites in activation of protein kinase. J Biol Chem. 1983 Jan 25;258(2):1032–1040. [PubMed] [Google Scholar]
- Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Res. 1987 Apr 15;47(8):1981–1986. [PubMed] [Google Scholar]
- Sandberg M., Taskén K., Oyen O., Hansson V., Jahnsen T. Molecular cloning, cDNA structure and deduced amino acid sequence for a type I regulatory subunit of cAMP-dependent protein kinase from human testis. Biochem Biophys Res Commun. 1987 Dec 31;149(3):939–945. doi: 10.1016/0006-291x(87)90499-2. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Zoller M. J., Uhler M. D., Helfman D. M., McKnight G. S., Krebs E. G. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showers M. O., Maurer R. A. A cloned bovine cDNA encodes an alternate form of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Dec 15;261(35):16288–16291. [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Strife A., Clarkson B. Biology of chronic myelogenous leukemia: is discordant maturation the primary defect? Semin Hematol. 1988 Jan;25(1):1–19. [PubMed] [Google Scholar]
- Tagliaferri P., Katsaros D., Clair T., Neckers L., Robins R. K., Cho-Chung Y. S. Reverse transformation of Harvey murine sarcoma virus-transformed NIH/3T3 cells by site-selective cyclic AMP analogs. J Biol Chem. 1988 Jan 5;263(1):409–416. [PubMed] [Google Scholar]
- Tortora G., Clair T., Katsaros D., Ally S., Colamonici O., Neckers L. M., Tagliaferri P., Jahnsen T., Robins R. K., Cho-Chung Y. S. Induction of megakaryocytic differentiation and modulation of protein kinase gene expression by site-selective cAMP analogs in K-562 human leukemic cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2849–2852. doi: 10.1073/pnas.86.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G., Tagliaferri P., Clair T., Colamonici O., Neckers L. M., Robins R. K., Cho-Chung Y. S. Site-selective cAMP analogs at micromolar concentrations induce growth arrest and differentiation of acute promyelocytic, chronic myelocytic, and acute lymphocytic human leukemia cell lines. Blood. 1988 Jan;71(1):230–233. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., Carmichael D. F., Lee D. C., Chrivia J. C., Krebs E. G., McKnight G. S. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., Chrivia J. C., McKnight G. S. Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Nov 25;261(33):15360–15363. [PubMed] [Google Scholar]
- Van Buskirk R., Corcoran T., Wagner J. A. Clonal variants of PC12 pheochromocytoma cells with defects in cAMP-dependent protein kinases induce ornithine decarboxylase in response to nerve growth factor but not to adenosine agonists. Mol Cell Biol. 1985 Aug;5(8):1984–1992. doi: 10.1128/mcb.5.8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]