Abstract
Background
Carbapenem antibiotics are considered the treatment of choice for serious extended-spectrum beta-lactamase (ESBL)-producing Gram-negative bacteria (GNB) infections. The study objectives were to evaluate efficacy and safety of de-escalation therapy to ertapenem for treatment of infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae.
Methods
We conducted a randomized controlled trial of adult patients with documented ESBL-producing Enterobacteriaceae infections who had received any group 2 carbapenem for less than 96 h. In the intervention group, the previously-prescribed group 2 carbapenem was de-escalated to ertapenem. In the control group, the group 2 carbapenem was continued.
Results
During June 2011–December 2014, 32 patients were randomized to the de-escalation group and 34 to the control group. Most common sites of infection were urinary tract infection (42%). Characteristics of both groups were comparable. By using a 15% predefined margin, ertapenem was non-inferior to control group regarding the clinical cure rate (%Δ = 14.0 [95% confidence interval: −2.4 to 31.1]), the microbiological eradication rate (%Δ = 4.1 [−5.0 to 13.4]), and the superimposed infection rate (%Δ = −16.5 [−38.4 to 5.3]). Patients in the de-escalation group had a significantly lower 28-day mortality rate (9.4% vs. 29.4%; P = .05), a significantly shorter median length of stay (16.5 days [4.0–73.25] vs. 20.0 days [1.0–112.25]; P = .04), and a significantly lower defined daily dose of carbapenem use (12.9 ± 8.9 vs. 18.4 ± 12.6; P = .05).
Conclusions
Ertapenem could be safely used as de-escalation therapy for ESBL-producing Enterobacteriaceae infections, once the susceptibility profiles are known. Future studies are needed to investigate ertapenem efficacy against ESBL-producing Enterobacteriaceae pneumonia to determine its applicability in life-threatening conditions.
Trial registration
ClinicalTrials.gov identifier: NCT01297842. Registered on 14 February 2011. First patient enrolled on 27 June 2011.
Keywords: ESBL, Enterobacteriaceae, Carbapenem, De-escalation, Ertapenem
Background
Empirical broad-spectrum antimicrobial therapy is highly recommended in patients with serious infections in aiming to improve patient’s outcomes [1]. However, overuse of broad spectrum antimicrobial therapy is an important risk factor for the emergence of bacterial resistance [2]. De-escalation therapy has been proposed as a strategy for balancing the advantages of adequate empirical therapy with the risk of emergence of bacteria resistance [3]. De-escalation therapy involves the initial use of empirical broad-spectrum antimicrobial therapy, which is then streamlined to more narrow-spectrum or targeted agents once culture and susceptibilities are available [4]. Efficacy of de-escalation therapy in patients with severe infections has been confirmed in many previous studies [5–7].
There are two carbapenem groups currently available worldwide: group 1 carbapenem (i.e.,ertapenem) and group 2 carbapenems (i.e., imipenem, meropenem, doripenem, and biapenem). Group 2 carbapenems are potent antibiotics that act against Gram-positive bacteria and Gram-negative bacteria, including extended-spectrum beta-lactamase-producing Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter spp. As compared to group 2 carbapenems, ertapenem is not active against P. aeruginosa and Acinetobacter spp. [8]. Nevertheless, a recent systematic review did not find a significant difference in resistant Enterobacteriaceae colonization between patients who received ertapenem and patients who received group 2 carbapenems [9].
Carbapenem antibiotics are considered the treatment of choice for serious infections caused by ESBL-producing Enterobacteriaceae [10]. Many studies have reported that ertapenem has promising efficacy as definitive therapy for these serious infections, but the majority of these studies were observational studies [11–13]. Based on results from a recent propensity score-matching cohort study, ertapenem appears as effective as group 2 carbapenems for empirical and definitive therapy of bloodstream infections caused by ESBL-producing Enterobacteriaceae [14].
Group 2 carbapenems have to be administered parenterally at least twice a day, while ertapenem is generally administered once daily and can be given by intramuscular injection [8]. However, the maximum serum concentration of ertapenem is slightly lower after intramuscular administration compared with intravenous administration [15].
Therefore, some clinically stable patients could receive ertapenem as outpatient parenteral antibiotic therapy, which would have the effect of reducing both length of stay and hospital expenditure.
Given these considerations, an open-label randomized controlled trial was conducted in patients with documented ESBL-producing Enterobacteriaceae infections who had received group 2 carbapenems as empirical therapy. The aim was to compare the efficacy and safety of de-escalation therapy to ertapenem versus continuation of group 2 carbapenem antibiotics. Efficacy, resource use, and impact on emergence of bacterial resistance were investigated and determined.
Methods
Setting and study design
A single-center, open-label, randomized equivalence trial was conducted from June 2011 to December 2014 at Siriraj Hospital, a 2200-bed tertiary care university hospital in Bangkok, Thailand.
Study subjects
Eligible subjects were hospitalized patients aged 18 years or older who had documented ESBL-producing Enterobacteriaceae infections and who received group 2 carbapenems as empirical therapy. Exclusion criteria were, as follows: treatment with a group 2 carbapenem for longer than 96 h; having active P. aeruginosa co-infection; pregnancy; breast-feeding; having a history of carbapenem hypersensitivity; and having infection caused by carbapenem-resistant Enterobacteriaceae strain.
Randomization and treatment
Eligible subjects were 1:1 allocated to the intervention group or the control group by stratified randomization according to the site of infection (lower respiratory tract infection, urinary tract infection (UTI), and bacteremia). Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) surveillance definitions for specific types of healthcare-associated infections (HAIs) were used for diagnosis of each type of infection [16]. Randomization numbers were computer-generated with permuted blocks (block size of four).
For patients in the intervention group (de-escalation group), the previously prescribed group 2 carbapenem was de-escalated to ertapenem. For patients in the control group, the group 2 carbapenem was continued for the entire course of therapy. Carbapenem dosage was adjusted according to patient renal function, as shown in the products’ package insert. Renal function was calculated using Cockcroft-Gault formula. Each dose of either gr.1 or gr.2 carbapenem was given by infusion for 1 h. Extended infusion or continuous infusion of carbapenem was not allowed in this study. Duration of systemic antibiotic therapy was determined by each patient’s attending physicians. All other non-carbapenem antibiotics were given as needed.
Microbiological testing
Microbiological tests were routinely processed at our hospital’s microbiology laboratory. All tests were processed by a Vitek® 2 automated ID/AST instrument (bioMerieux SA, Marcy-l'Étoile, France). ESBL production was confirmed by double-disk method, according to the performance standards for antimicrobial susceptibility testing established by the Clinical and Laboratory Standards Institute (CLSI) [17].
Clinical specimens from the documented site of infection were obtained on day 3, day 7, day 14, and then at least once a week thereafter for microbiological culture. Specimen collection was discontinued if the culture result was negative or the course of antibiotic treatment was completed. To determine colonization by resistant Gram-negative pathogens, a stool culture or a rectal swab culture was obtained on day 0, at the end of therapy, and on day 28 of therapy if there were no contraindications. The target resistant Gram-negative pathogens were ESBL-producing Enterobacteriaceae, multidrug-resistant (MDR) P. aeruginosa, and MDR A. baumannii.
Data collection and outcome assessment
Baseline data, including demographic characteristics, underlying diseases, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, intensive care unit (ICU) admission, and previous antibiotic therapy were collected. Clinical outcomes were evaluated on a daily basis. Cost-related data, including length of stay (LOS) and amount of drug therapy for ESBL-producing Enterobacteriaceae were also collected. All adverse events were recorded for 28 days from the date of enrollment.
The primary outcome was clinical cure rate at the end of therapy. Secondary outcomes included microbiological eradication rate, superimposed infection rate during study treatment, 28-day mortality rate, and adverse drug reactions (ADRs). Prevalence of colonization with resistant pathogens and economic outcomes (LOS, antibiotic consumption, and hospital expenditure) were also evaluated. Study definitions are shown in Table 1.
Table 1.
Study definitions
| Terms | Definitions |
|---|---|
| Clinical cure | Resolution of or improvement in the index infection without need for further antibiotic therapy |
| Microbiological eradication | At least one negative culture of an index pathogen from a clinical specimen that was obtained during or after the course of antibiotic therapy |
| Superimposed infection | New onset of infection caused by any pathogens other than ESBL-producing Enterobacteriaceae during the course of antibiotic therapy |
| 28-day mortality | Death from any cause within 28 days after enrollment |
Statistical analysis
Based on data obtained from our pilot study [unpublished data], the estimated clinical cure rate of group 2 carbapenems in the treatment of ESBL-producing Enterobacteriaceae infection is approximately 90%. To test the equivalency hypothesis with a predefined margin of ±15% using a 2-sided confidence interval (CI) approach, the required sample size was calculated to be 100 patients or 50 patients for each of two groups. However, the study was terminated early due to slow recruitment of eligible subjects. As such, this study describes an interim analysis of 66 enrolled patients. Data are presented as n (%), mean ± standard deviation (SD), or median [range]. Treatment outcomes are reported as % difference (%Δ, % of the de-escalation group - % of the control group) and 95% CI. Intention to treat analysis was used for all analyses. All statistical calculations were performed using STATA version 14.0/IC (StataCorp LP, College Station, TX, USA).
Results
From June 2011 to December 2014, 484 patients with documented ESBL-producing Enterobacteriaceae infection were empirically treated with group 2 carbapenems at our medical center. Sixty-six of those patients satisfied the inclusion criteria and were enrolled in this study (Fig. 1). Thirty-two patients were randomized into the de-escalation group (intervention group) and 34 into the non-de-escalation group (control group). De-escalation group patients were de-escalated to ertapenem and non-de-escalation group patients were continued with their previously prescribed group 2 carbapenem. Demographic and clinical characteristics between groups were comparable (Table 2). Nearly 60% of patients were female, with a mean age of 64.8 years (67.5 ± 17.2 years in the de-escalation group and 62.2 ± 21.6 years in the non-de-escalation group).
Fig. 1.
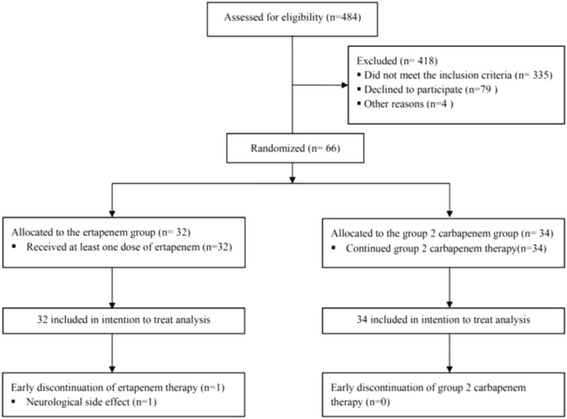
Study flow chart
Table 2.
Characteristics of patients in the de-escalation group (intervention) and in the non-de-escalation group (control)
| Variables | All (n = 66) | De-escalation (n = 32) | Non-de-escalation (n = 34) | P-value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Mean age, years | 64.8 ± 19.6 | 67.5 ± 17.2 | 62.2 ± 21.6 | 0.28 |
| Female | 38 (57.6%) | 19 (59.4%) | 19 (55.9%) | 0.81 |
| Medicine ward | 58 (87.9%) | 26 (81.3%) | 32 (94.1%) | 0.11 |
| Underlying disease | ||||
| ● Hypertension | 37 (56.1%) | 19 (59.4%) | 18 (52.9%) | 0.60 |
| ● Cerebrovascular disease | 15 (22.7%) | 9 (28.1%) | 6 (17.7%) | 0.31 |
| ● Chronic lung disease | 10 (15.2%) | 2 (6.3%) | 8 (23.5%) | 0.08 |
| ● Cardiovascular disease | 22 (33.3%) | 11 (34.4%) | 11 (32.4%) | 0.86 |
| ● Diabetes mellitus | 25 (37.9%) | 11 (34.4%) | 14 (41.2%) | 0.57 |
| ● Chronic kidney disease | 23 (34.9%) | 12 (37.5%) | 11 (32.4%) | 0.66 |
| ● Chronic liver disease | 7 (10.6%) | 3 (9.4%) | 4 (11.8%) | 1.00 |
| ● Malignancy | 17 (26.6%) | 9 (28.1%) | 8 (23.5%) | 0.67 |
| ● Any immunocompromised conditiona | 16 (24.2%) | 7 (21.9%) | 9 (36.5%) | 0.66 |
| APACHE II score at enrollmentb | 12.4 ± 5.9 (n = 10) | 13.8 ± 8.2 (n = 5) | 11.0 ± 2.3 (n = 5) | 0.49 |
| Modified APACHE II score at enrollmentc | 8.9 ± 4.9 | 8.9 ± 5.1 | 8.9 ± 4.9 | 0.94 |
| ● History of organ insufficiency | 35 (53.9%) | 20 (62.5%) | 15 (45.5%) | 0.17 |
| ● Emergency surgery | 8 (12.1%) | 5 (15.6%) | 3 (8.8%) | 0.47 |
| ● Acute renal failure | 8 (12.1%) | 8 (25.0%) | 0 (0.0%) | 0.002 |
| ● ICU admission | 3 (4.6%) | 2 (6.3%) | 1 (2.9%) | 0.42 |
| Characteristics of infections | ||||
| Hospital-acquired infection | 32 (48.5%) | 12 (37.5%) | 20 (58.8%) | 0.08 |
| Site of infection at enrollment | 1.00 | |||
| ● Urinary tract infection | 27 (40.9%) | 13 (40.6%) | 14 (41.2%) | |
| ● Pneumonia | 11 (16.7%) | 5 (15.6%) | 6 (16.7%) | |
| ● Others | 29 (43.9%) | 14 (43.8%) | 15 (44.1%) | |
| ● Primary bacteremia | 26 (78.8%) | 14 (82.4%) | 12 (75.0%) | |
| ● Skin infection | 1 (1.5%) | 0 | 1 (2.9%) | |
| ● Reproductive tract infection | 1 (1.5%) | 0 | 1 (2.9%) | |
| ● Gastrointestinal tract infection | 1 (1.5%) | 0 | 1 (2.9%) | |
| Having bacteremia | 33 (50.0%) | 17 (53.1%) | 16 (47.1%) | 0.62 |
| Cause of bacteremiad | (n = 17) | (n = 16) | 0.57 | |
| ● Primary bacteremia | 26 (78.8%) | 14 (82.4%) | 12 (75.0%) | |
| ● Urinary tract infection | 5 (15.2%) | 3 (17.6%) | 2 (12.5%) | |
| ● Catheter-related infection | 2 (6.0%) | 0 | 2 (12.5%) | |
| Causative pathogene | ||||
| ● Escherichia coli | 46 (69.7%) | 24 (75.0%) | 22 (64.7%) | 0.36 |
| ● Klebsiella spp. | 21 (31.8%) | 8 (25.0%) | 13 (38.2%) | 0.25 |
| Mean time from onset of infection to enrollment, days | 4.3 ± 1.4 | 4.2 ± 1.6 | 4.5 ± 1.2 | 0.47 |
| Mean duration of pre-enrollment use of group 2 carbapenems, days | 2.8 ± 1.6 | 3.1 ± 1.5 | 2.6 ± 1.7 | 0.29 |
| Mean duration of pre-enrollment use of any antibiotics, days | 3.1 ± 5.8 | 3.3 ± 5.4 | 3.0 ± 6.3 | 0.82 |
aAny immunocompromised condition (e.g., HIV infection, receipt of immunosuppressive agents (steroid, cytotoxic agents, and/or chemotherapy))
bAPACHE II score was calculated using data from 10 patients with available arterial blood gas results
cModified APACHE II score was calculated in all patients. For those who did not have arterial blood gas results, the arterial pH was substituted by the level of serum bicarbonate
dAll patients with secondary bacteremia due to UTI (n = 5) were enrolled into the UTI stratum. Other cases of secondary bacteremia were due to cellulitis (n = 1) and spontaneous bacterial peritonitis (n = 1)
eOne patient in the group 2 carbapenems group had bacteremia caused by both Escherichia coli and Klebsiella spp
Abbreviation: APACHE II Acute Physiology and Chronic Health Evaluation II
Data are presented as n (%), mean ± SD; P-value < 0.05 indicates statistical significance
Twelve patients (37.5%) in the de-escalation group and 20 patients (58.8%) in the non-de-escalation group had a healthcare-associated infection (P = .08). Approximately 40% of patients in both groups had urinary tract as the site of ESBL-producing Enterobacteriaceae infection. Fourteen patients in the de-escalation group (43.8%) and 12 patients in the non-de-escalation group (35.3%) had primary bacteremia. The mean APACHE II score and mean modified APACHE II score were comparable between groups. Mean time from onset of infection to enrollment was 4.2 ± 1.6 days in the de-escalation group and 4.5 ± 1.2 days in the non-de-escalation group (P = .47). There was no significant difference in duration of pre-enrollment use of group 2 carbapenems (3.1 ± 1.5 days vs. 2.6 ± 1.7 days; P = .29) or duration of pre-enrollment use of any other antibiotic (3.3 ± 5.4 days vs. 3.0 ± 6.3; P = .82) between the de-escalation and non-de-escalation groups, respectively.
Clinical, microbiological and cost-related outcomes of patients in the de-escalation group (intervention) and in the non-de-escalation group (control) are shown in Table 3. This study failed to prove equivalency in all clinical outcomes, including clinical cure rate (%Δ = 14.0 [95% CI: -2.4 to 31.1]), microbiological eradication rate (%Δ = 4.1 [-5.0 to 13.4]), superimposed infection (%Δ = -16.5 [-38.4 to 5.3]), and 28-day mortality (%Δ = -20.0 [-39.3 to -0.8]). Although the equivalency hypothesis was not proven, the lower bounds of the CIs for difference in clinical cure and microbiological eradication rate were within the -15% margin. The upper bounds of the CIs for difference in 28-day mortality and superimposed infection were within the +15% margin. Therefore, non-inferiority of de-escalation was established for all primary and secondary outcomes. Additionally, the upper bound of the CI for difference in 28-day mortality was below zero, probably suggesting the superiority of de-escalation for 28-day mortality.
Table 3.
Outcomes of patients in the de-escalation group (intervention) and in the non-de-escalation group (control)
| Outcomes | De-escalation (n = 32) | Non-de-escalation (n = 34) | P-value |
|---|---|---|---|
| Clinical outcomes | |||
| Clinical cure rate | 30 (93.8%) | 24 (79.4%) | 0.09 |
| Microbiological eradication ratea | 20/20 (100.0%) | 23/24 (95.8%) | 0.36 |
| 28-day mortality rate | 3 (9.4%) | 10 (29.4%) | 0.05b |
| • ID-related mortality | 1 (3.1%) | 9 (26.5%) | 0.01b |
| • Non ID-related mortality | 1 (3.1%) | 1 (2.9%) | 1.00b |
| Superimposed infection rate | 6 (18.8%) | 12 (35.3%) | 0.13 |
| • Acinetobacter baumannii | 2 (6.3%) | 6 (17.3%) | 0.26b |
| • MRSA | 2 (6.3%) | 3 (8.8%) | 1.00b |
| • Candida spp. | 1 (3.1%) | 4 (11.8%) | 0.51b |
| • Pseudomonas aeruginosa | 2 (6.3%) | 2 (5.9%) | 1.00b |
| • Enterococcus spp. | 2 (6.3%) | 0 | 0.23b |
| Cost-related outcomes | |||
| Median length of stay, days | 16.5 (4.0–73.0) | 20.0 (1.0–112.0) | 0.04b |
| Median length of stay after enrollment, days | 9.5 (0–37.0) | 11.5 (1.0–102.0) | 0.11b |
| Mean duration of carbapenem use, days | 14.4 ± 6.0 | 16.5 ± 10.5 | 0.32 |
| Mean DDD of carbapenem | 12.9 ± 8.9 | 18.4 ± 12.6 | 0.05 |
| Stool colonization | N = 32 | N = 32c | |
| At baseline | |||
| • Any MDR bacteria | 20 (62.5%) | 21 (65.6%) | 1.0 |
| • Any ESBL-producing GNB | 19 (59.4%) | 20 (62.5%) | 1.0 |
| • MDR-Acinetobacter baumannii | 4 (12.5%) | 3 (9.4%) | 1.0b |
| • MDR-Pseudomonas aeruginosa | 0 | 1 (3.1%) | 1.0b |
| At the end of therapy | |||
| • Any MDR bacteria | 16 (50.0%) | 11 (34.4%) | 0.31 |
| • Any ESBL-producing GNB | 11 (34.4%) | 8 (25.0%) | 0.59 |
| • MDR-Acinetobacter baumannii | 6 (18.8%) | 4 (12.5%) | 0.73b |
| • MDR-Pseudomonas aeruginosa | 1 (3.1%) | 0 (0.0%) | 1.0b |
aMicrobiological eradication was calculated in patients who had a follow-up culture of the infection site
bNonparametric test
cTwo patients in the group 2 carbapenem group had a contraindication for rectal swab culture
Abbreviations: MRSA methicillin-resistant Staphylococcus aureus, DDD defined daily dose, MDR multi-drug resistant, ESBL extended-spectrum beta-lactamase enzyme, GNB Gram-negative bacteria
Data are presented as n (%), mean ± SD or median (range); P-value < 0.05 indicates statistical significance
Superimposed infection rate was slightly lower among patients in the de-escalation group (18.8% vs. 35.3%; p = 0.13). The three leading causative pathogens of superimposed infection were MDR A. baumannii, methicillin-resistant Staphylococcus aureus, and Candida spp. There was no significant difference in the distribution of causative pathogens between groups. Patients in the de-escalation group had a significantly shorter median length of hospital stay (16.5 days [4.0-73.0] vs. 20.0 days [1.0-112.0]; p = .04) and a significantly lower defined daily dose (DDD) of carbapenem antibiotic use (12.9 ± 8.9 vs. 18.4 ± 12.6; p = .05). There were no significant differences in stool colonization by MDR pathogens at enrollment or at the end of therapy.
Ertapenem was discontinued early in one patient due to the development of neurological side effects, including dizziness and headache. All neurological symptoms subsided after ertapenem was switched to meropenem. There was no early discontinuation in the non-de-escalation group that related to adverse drug reaction (ADRs). There was no statistical difference in ADRs between groups.
Discussion
The findings of this study indicate that de-escalation therapy to ertapenem is non-inferior to continuation of group 2 carbapenems for clinical cure rate, microbiological eradication rate, and superimposed infection rate. These findings confirm the efficacy of ertapenem and are consistent with the findings of previously documented in many observational cohort studies [11–14]. The slightly lower rate of superimposed infections among patients in the de-escalation group also supported the findings of previous studies that ertapenem has lower collateral impact. Further, the lower rate of superimposed infections may be a contributing factor to the significantly lower 28-day mortality rate among patients in the de-escalation group. Given that the patients in the de-escalation group were slightly older and sicker than those in the non-de-escalation group, the lower 28-day mortality among the de-escalation group was unlikely to be the results of unbalanced baseline characteristics.
Similar to results from a recent systematic review, our study did not find any significant difference in stool colonization by MDR pathogens. However, our baseline rate of colonization with resistant GNB pathogen was relatively high (>60%), compared with the studies included in another systematic review [9].
Patients in the de-escalation group also had a significantly shorter length of hospital stay, as well as a significantly lower defined daily dose (DDD) of carbapenem use. This can be simply explained by the practice of once-daily dosing of ertapenem [8], whereby some clinically stable patients could be discharged early and treated with parenteral antimicrobial therapy as outpatients.
This study has several inherent strengths. While most previous studies were observational, this was a randomized controlled trial. Randomization ensured a balance of measured and unmeasured confounders between the intervention and control groups. In addition to the evaluation of clinical outcomes, other important parameters, including stool colonization with resistant GNB and cost-related outcomes were evaluated.
This study also has some mentionable limitations. First, participation bias and observer bias may have occurred because the study design was open-label. However, the microbiological cure rate and 28-day mortality rate are objective measures, so observer bias would have been minimal. Second, the majority of patients in this study had UTI or primary bacteremia, with only a small proportion having pneumonia. Thus, we are not able to conclude that de-escalation to ertapenem is effective against ESBL-producing Enterobacteriaceae pneumonia. Third, the study was early terminated before achieving the target sample size, therefore all statistically significant findings are less reliable. Lastly, the study was designed to stratify only on the site of infection but did not stratify on other clinically significant factors such as age and APACHE score. Nevertheless, these two important factors were comparable between two groups.
Conclusions
Based on the results of this study, ertapenem can be safely used as de-escalation therapy for ESBL-producing Enterobacteriaceae infections, especially UTIs and primary bacteremia. Future studies are needed to investigate ertapenem efficacy against ESBL-producing Enterobacteriaceae pneumonia to determine its applicability in life-threatening conditions. Additionally, a study in the cost-effectiveness of de-escalation to ertapenem would be useful for healthcare policymakers.
Acknowledgements
This work was presented in part at the 26th European Congress of Clinical Microbiology and Infectious Diseases; Amsterdam, Netherlands, 2016 (oral presentation on 10 April 2016). The authors gratefully acknowledge Miss Luksame Wattanamongkolsil and Mr. Teerawit Tangkosakulfor their assistance with coordination of this study.
Funding
This work was supported by the Merck Investigator Studies Program (MSP), Merck, Sharp & Dohme Corp. and the Health Systems Research Institute (Thailand). All authors were supported by a Chalermphrakiat Grant, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. The study sponsors were not involved in the study design, data collection and interpretation, or writing of the manuscript.
Availability of data and materials
Use of data will be confined to the study team, but potential collaborators or request for data can be submitted at visanu.tha@mahidol.ac.th.
Authors’ contributions
PR was involved in study design, data collection, statistical analysis and writing the manuscript. PW, AJ and PK were involved in data collection and writing the manuscript. VT was involved in the conception of the study, study design, writing the manuscript. VT had full access to the data and responsibility for the decision to submit the manuscript for publication. All authors read and approved the final manuscript.
Competing interests
PR, PK and VT have received lecture honoraria from Merck, Sharp & Dohme Corp. All other authors report no potential conflicts.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Siriraj Institutional Review Board (SIRB), Mahidol University and written informed consent was obtained from all participants or their legal representatives.
Abbreviations
- APACHE II
Acute physiology and chronic health evaluation II
- CDC/NHSN
Centers for disease control and prevention/National healthcare safety network
- CI
confidence interval
- CLSI
Clinical and laboratory standards institute
- DDD
defined daily dose
- ESBL
extended-spectrum beta-lactamase
- GNB
Gram-negative bacteria
- HAIs
Healthcare-associated infections
- ICU
intensive care unit
- LOS
length of stay
- MDR
multidrug-resistant
- UTI
urinary tract infection
Contributor Information
Pinyo Rattanaumpawan, Email: pinyo.rat@mahidol.ac.th.
Peerawong Werarak, Email: peerawong.wek@mahidol.ac.th.
Anupop Jitmuang, Email: anupop.jit@mahidol.ac.th.
Pattarachai Kiratisin, Email: pattarachai.kir@mahidol.ac.th.
Visanu Thamlikitkul, Phone: (66) 2-412-5994, Email: visanu.tha@mahidol.ac.th.
References
- 1.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Shlaes DM, Gerding DN, John JF, Jr, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584–99. doi: 10.1086/513766. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli M, Mercurio G, Di Nunno S, Recchioni G, Deangelis G. De-escalation antimicrobial chemotherapy in critically III patients: pros and cons. J Chemother. 2001;13(1):218–23. doi: 10.1179/joc.2001.13.Supplement-2.218. [DOI] [PubMed] [Google Scholar]
- 4.Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149–62. doi: 10.1016/j.ccc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014;40(10):1399–408. doi: 10.1007/s00134-014-3411-8. [DOI] [PubMed] [Google Scholar]
- 6.Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41–9. doi: 10.1007/s00134-013-3148-9. [DOI] [PubMed] [Google Scholar]
- 7.Lew KY, Ng TM, Tan M, et al. Safety and clinical outcomes of carbapenem de-escalation as part of an antimicrobial stewardship programme in an ESBL-endemic setting. J Antimicrob Chemother. 2015;70(4):1219–25. doi: 10.1093/jac/dku479. [DOI] [PubMed] [Google Scholar]
- 8.Zhanel GG, Johanson C, Embil JM, et al. Ertapenem: review of a new carbapenem. Expert Rev Anti-Infect Ther. 2005;3(1):23–39. doi: 10.1586/14787210.3.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Falagas ME, Tansarli GS, Kapaskelis A, Vardakas KZ. Ertapenem use and antimicrobial resistance to group 2 carbapenems in Gram-negative infections: a systematic review. Expert Rev Anti-Infect Ther. 2013;11(1):69–78. doi: 10.1586/eri.12.149. [DOI] [PubMed] [Google Scholar]
- 10.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 11.Teng CP, Chen HH, Chan J, Lye DC. Ertapenem for the treatment of extended-spectrum beta-lactamase-producing Gram-negative bacterial infections. Int J Antimicrob Agents. 2007;30(4):356–9. doi: 10.1016/j.ijantimicag.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Berg ML, Crank CW, Philbrick AH, Hayden MK. Efficacy of ertapenem for consolidation therapy of extended-spectrum beta-lactamase-producing gram-negative infections: a case series report. Ann Pharmacother. 2008;42(2):207–12. doi: 10.1345/aph.1K365. [DOI] [PubMed] [Google Scholar]
- 13.Collins VL, Marchaim D, Pogue JM, et al. Efficacy of ertapenem for treatment of bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2012;56(4):2173–7. doi: 10.1128/AAC.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez-Gutierrez B, Bonomo RA, Carmeli Y, et al. Ertapenem for the treatment of bloodstream infections due to ESBL-producing Enterobacteriaceae: a multinational pre-registered cohort study. J Antimicrob Chemother. 2016;71(6):1672–80. doi: 10.1093/jac/dkv502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musson DG, Majumdar A, Birk K, et al. Pharmacokinetics of intramuscularly administered ertapenem. Antimicrob Agents Chemother. 2003;47(5):1732–5. doi: 10.1128/AAC.47.5.1732-1735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement: M100-S21: Wayne; Clinical Lab Standards Institute. 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Use of data will be confined to the study team, but potential collaborators or request for data can be submitted at visanu.tha@mahidol.ac.th.


